Abstract
The SARS‐Cov2 may have impaired care trajectories, patient overall survival (OS), tumor stage at initial presentation for new colorectal cancer (CRC) cases. This study aimed at assessing those indicators before and after the beginning of the pandemic in France. In this retrospective cohort study, we collected prospectively the clinical data of the 11.4 million of patients referred to the Greater Paris University Hospitals (AP‐HP). We identified new CRC cases between 1 January 2018 and 31 December 2020, and compared indicators for 2018‐2019 to 2020. pTNM tumor stage was extracted from postoperative pathology reports for localized colon cancer, and metastatic status was extracted from CT‐scan baseline text reports. Between 2018 and 2020, 3602 and 1083 new colon and rectal cancers were referred to the AP‐HP, respectively. The 1‐year OS rates reached 94%, 93% and 76% for new CRC patients undergoing a resection of the primary tumor, in 2018‐2019, in 2020 without any Sars‐Cov2 infection and in 2020 with a Sars‐Cov2 infection, respectively (HR 3.78, 95% CI 2.1‐7.1). For patients undergoing other kind of anticancer treatment, the percentages are 64%, 66% and 27% (HR 2.1, 95% CI 1.4‐3.3). Tumor stage at initial presentation, emergency level of primary tumor resection, delays between the first multidisciplinary meeting and the first anticancer treatment did not differ over time. The SARS‐Cov2 pandemic has been associated with less newly diagnosed CRC patients and worse 1‐year OS rates attributable to the infection itself rather than to its impact on hospital care delivery or tumor stage at initial presentation.
Keywords: colorectal neoplasms, COVID‐19, delivery of health care, health services research, quality of health care
What's new?
The SARS‐CoV‐2 pandemic caused reallocation of healthcare resources that led to delays in diagnosis and treatment of cancer. Here, the authors conducted a retrospective cohort to assess how the pandemic affected care trajectories for colorectal cancer (CRC) cases. They assessed overall survival and tumor stage at initial presentation for patients diagnosed between January 2018 and December 2020. The results show a lower overall survival rate for CRC patients diagnosed in 2020, and that decrease is a result of SARS‐CoV‐2 infection itself rather than lack of access to treatment.
Abbreviations
- AJCC
American Joint Committee on Cancer
- AP‐HP
Assistance Publique—Hôpitaux de Paris (Greater Paris University Hospitals)
- ARC
Association pour la recherche contre le cancer (ARC Foundation for Cancer Research)
- ATC
Anatomical Therapeutic Chemical
- CCAM
Classification Commune des Actes Médicaux
- CDW
Clinical Data Warehouse
- CNIL
Commission nationale de l'informatique et des libertés (French Regulatory Agency)
- CRC
colorectal cancer
- ED
emergency department
- EHR
electronic health records
- ICD‐10
International Classification of Diseases
- ICD‐O
International Classification of Diseases for Oncology
- LOINC
Logical Observation Identifiers Names and Codes
- MDM
multidisciplinary meeting
- MI‐CLAIM
Minimum Information about Clinical Artificial Intelligence Modeling checklist
- OS
overall survival
- PMSI‐MCO
Programme de Médicalisation des Systèmes d'Information en Médecine, Chirurgie, Obstétrique et Odontologie
- RECORD
REporting of studies Conducted using Observational Routinely‐collected health Data
- STROBE
STrengthening the Reporting of OBservational studies in Epidemiology
1. INTRODUCTION
Since the start of the Sars‐Cov2 pandemic, iterative campaigns of social distancing occurred worldwide to limit the spread of the virus and hospital crowding. Following guidelines from scientific societies, policymakers interrupted national cancer screening programs during the first lockdown in 2020, including those related to colorectal cancer (CRC). 1 , 2 Several studies have shown a significant decrease in both the number of CRC cases newly referred to tertiary hospitals and related colectomy procedures performed during national lockdowns with no sequential catching‐up. 3 , 4 , 5 , 6 Infection waves led to three lockdown periods in France (17 March to 11 May 2020; 30 October to 15 December 2020 and 3 April to 3 May 2021). Scientific societies issued guidelines for adapting care strategies to the situation of the pandemic. 7 , 8 The reallocation of doctors from various specialties to temporary units dedicated to Sars‐Cov2 patients and the modifications to treatment pathways could have disrupted the timelines of cancer care and the course of treatment chosen for patients. 9 , 10 , 11
This situation raised multiple hypotheses about the impact of the Sars Cov2 pandemic on CRC care. Previous evidence shows that the outbreak‐related policies have induced delays in CRC diagnostic procedures, but these policies may have also increased time‐to‐treatment after diagnosis. Patients with a new CRC diagnosed after the beginning of the Sars‐Cov2 outbreak period may be more likely to suffer from more advanced tumors at the initial clinical presentation, and to experience poorer outcomes. 12 , 13 , 14 , 15 , 16 Local extension of CRC may induce life‐threatening complications such as bowel obstruction, intestinal perforation and/or digestive hemorrhage requiring high‐risk surgical procedures being performed with no delay. Diagnosis delays for new CRC cases may also have increased the rate of unresectable tumors. 17 This may decrease patients' overall survival (OS) rates as the complete resection of tumor sites, including metastases, is the gold standard of CRC management. 18 Modeling studies anticipate increased mortality but little empirical evidence is available to assess standards of CRC care and patient outcomes during the pandemic. 19 , 20
This study aimed at assessing the impact of the Sars‐Cov2 pandemic and related public health policies on patient OS, initial tumor stage and hospital care trajectories of new CRC cases, in the Paris region during and after the outbreak of the Sars Cov2 epidemic in early 2020.
2. MATERIALS AND METHODS
We reported the study according to the REporting of studies Conducted using Observational Routinely‐collected health Data (RECORD) statement, the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement and to the Minimum Information about Clinical Artificial Intelligence Modeling checklist (MI‐CLAIM). 21 , 22 , 23 The completed checklists are available in the Supplementary Methods.
We conducted a retrospective cohort study using the Greater Paris University Hospitals (AP‐HP) Clinical Data Warehouse (CDW) integrating routinely collected medical and administrative data of 11.4 million patients. 24
2.1. Study participants
We included the 27 AP‐HP teaching hospitals for which data were available since 2016 (Supplementary Methods). We selected the study participants using diagnoses recorded during the hospital stay according to the 10th Edition of the International Classification of Diseases (ICD‐10) for billing purposes (PMSI‐MCO: Programme de Médicalisation des Systèmes d'Information en Médecine, Chirurgie, Obstétrique et Odontologie). We focused on patients with a CRC identified using the following list of ICD‐10 codes: C18, C19, D01.0, D01.1, D37.3, and D37.4 for colon; C20, D01.2, and D37.5 for rectal cancers, respectively. A patient was considered to have a newly diagnosed CRC if having a CRC‐related ICD‐10 code and no recorded CRC‐related ICD‐10 code in her discharge summaries within the 2 previous years. We excluded the patients having a medical history of a previous cancer in the two previous years before CRC diagnosis, except for patients coded for both an upper rectal cancer and a colon cancer, which we classified as patients having a colon cancer.
2.2. Data collection and analysis
Tumor stage of the CRC cases is available in patients' unstructured electronic health records (EHRs) only. We estimated the rates of missing text reports (CT scans and pathology) within patients' EHRs which were stable over time (Supplementary Methods and Figures S4 and S5). We extracted the pathological tumor stage (according to the AJCC 8th edition) for cases with upfront resection of a primary localized colon cancer. 25 To do so, we developed and implemented a regular expression algorithm on the first related postoperative pathology report within the EHR of each patient. We classified the tumor stage according to the risk of relapse: low and high risk defined as pT0‐T4N0 and pTxN1‐2, respectively.
We extracted the metastatic status of CRC cases at initial presentation from the available imaging text reports within the EHRs of each patient. To that aim, we identified CT‐scans between 90 days before and 45 days after the CRC diagnosis date. Among them, staging CT‐scans were identified using machine learning algorithms. We developed and implemented another regular expression algorithm to assess the tumor metastatic status on these selected reports. The development (training set) and validation (test set) steps of the implemented algorithms are described in the Supplementary Methods and Figures S7 and S8.
We identified the surgical resections of primary and secondary tumors, and the local liver destruction with radiofrequency ablation and intraarterial anticancer procedures through the CCAM Medical Procedures catalog (Appendix S1). Systemic anticancer chemotherapy and radiation therapy sessions were identified using the Z51.1 and Z51.01 ICD‐10 codes, respectively.
For patients resected from their primary CRC tumor, we defined three healthcare trajectories according to the level of emergency of the surgical procedure: (a) “major emergency”: patients operated without any preoperative referral to hospital (ie, no cancer‐related encounter before emergency CRC surgery); (b) “minor emergency”: patients operated on promptly after a visit to the emergency department (ED), but with previous CRC‐related encounters at hospital; (c) “no emergency”: patients operated in an preplanned context, that is, a surgical procedure being performed with no immediately preceding visit to an ED.
A positive PCR, serologic test, or one of the U071 ICD‐10 codes within 1 year from the date of cancer diagnosis defined Sars‐Cov2 infection.
2.3. Statistical methods
We modeled the number of prepandemics annual new CRC cases with Poisson distributions (λ 1 = 1274 for colon cancer and λ 2 = 378 for rectal cancers). The parameter of each distribution was calculated using the average of 2018‐2019 and 95% confidence intervals (CIs) were assessed with a χ 2‐based method. One‐year OS rates were calculated for patients diagnosed with a CRC between 1 January 2018 and 31 December 2019, and between 1 January and 31 December 2020, from the date of CRC diagnosis to the date of patients' death or last follow‐up up to 31 May 2021. Kaplan‐Meier curves were implemented, and a log‐rank test was performed between both groups (2018‐19 vs 2020). Hazard ratios (HR) for OS probability were assessed with 95% CIs. A χ 2 test was performed to test the difference in the repartition of low‐ vs high‐risk tumors.
We identified the dates of multidisciplinary meetings (MDMs) within patients' EHRs using the structured information of MDMs' reports and assessed the delay between MDMs and first CRC‐related therapeutic strategy. We estimated the consistency of available MDM reports within the CDW which was stable over time (Supplementary Methods and Figure S6).
We compared the indicators in 2020 with those recorded in 2018 and in 2019 and with the average number recorded between 2018 and 2019, and we performed aggregated comparisons between those periods. The analyses were performed with the 3.7 version of Python on JupyterLab.
3. RESULTS
3.1. Characteristics of the patient population and clinical outcomes over time
Between 1 January 2018 and 31 December 2020, 4685 patients with a unique new diagnosis of CRC were referred to the Greater Paris AP‐HP, 3602 with a colon cancer and 1083 with a rectal cancer (Table 1 and Figure S2). Figures 1A,B, and S3A,B summarize the cumulative monthly number of new cases. Compared to the average of 2018‐2019, 220 (95% CI [151‐292]) and 52 (95% CI [15‐92]) new cases were missing in 2021. Between January and May 2021, there was no catching up in the number of new cases (data not shown). The median duration of patients' follow‐up reached 16.3 months (interquartile 6.3‐26.0).
TABLE 1.
Characteristics of the patient' population undergoing an anticancer therapeutic strategy between 2018 and 2020, at the Greater Paris University Hospitals teaching hospital
| Colon cancer | Rectal cancer | |
|---|---|---|
| Overall population (N) | 3602 | 1083 |
| New cases with anticancer treatment (N) | 2908 | 737 |
| Female, N (%) | 1339 (46%) | 284 (38%) |
| Age (y, median, IQR) | 69 (58‐78) | 65 (56‐73) |
| Primary tumor resection, N (%) | 1906 (65%) | 557 (75%) |
| Exclusive primary tumor resection | 1325 | 394 |
| With perioperative chemotherapy | 359 | 56 |
| Secondary tumor resection, N (%) | 265 (9%) | 71 (9%) |
| Exclusive palliative chemotherapy (excluding primary and/or secondary tumor resection), N (%) | 497 (17%) | 97 (13%) |
| Intraarterial anticancer treatment, N (%) | 8 (<1%) | 3 (<1%) |
| Radiofrequency ablation, N (%) | 83 (2%) | 21 (2%) |
Abbreviation: IQR, interquartile range.
FIGURE 1.
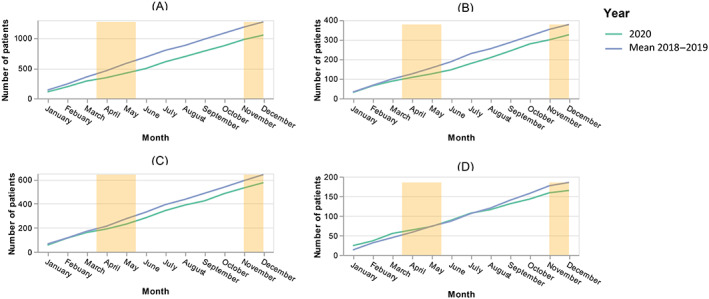
Evolution of the cumulative monthly number of new cancer cases of colon (A) and rectum (B), primary tumor resections for colon (C) and rectum (D) over time referred to Greater Paris University Hospitals teaching hospital, in 2018‐2019 and 2020, respectively [Color figure can be viewed at wileyonlinelibrary.com]
Among the 2463 cases with a primary tumor resection, 1740 and 723 patients were diagnosed in 2018‐2019 and 2020. Among the 1181 cases with any other kind of anticancer treatment, 833 and 348 patients were diagnosed in 2018‐2019 and 2020, respectively. The Sars‐Cov2 infection rates in patients diagnosed in 2018‐2019 vs patients diagnosed in 2020 were 1% vs 13% and 1% vs 15% across those categories, respectively.
The 1‐year OS rates reached 94%, 93% and 76% for new CRC patients undergoing a resection of the primary tumor, in 2018‐2019, in 2020 without any Sars‐Cov2 infection and in 2020 with a Sars‐Cov2 infection, respectively. For patients diagnosed in 2020, an infection related to the Sars‐Cov2 virus was associated with a worse OS rate (HR 3.78, 95% CI 2.1‐7.1) (Figure 2A). For new CRC patients undergoing other kind of anticancer treatment, the corresponding percentages are 64%, 66% and 27%. For patients diagnosed in 2020, an infection related to the Sars‐Cov2 virus was associated with a worse OS rate (HR 2.1, 95% CI 1.4‐3.3) (Figure 2B).
FIGURE 2.
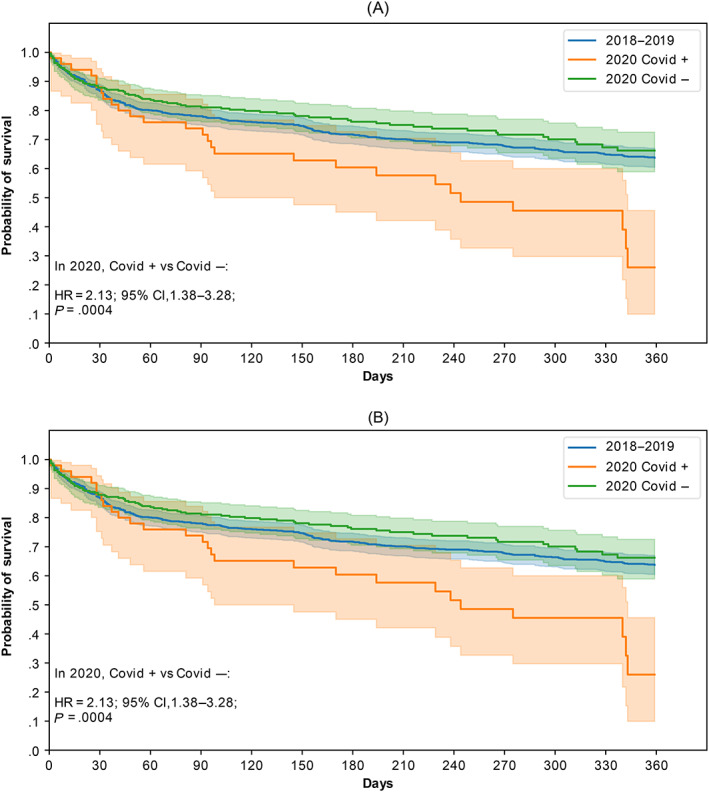
One‐year overall survival rates of patients undergoing an anticancer treatment at the AP‐HP hospital for a CRC between 2018 and 2019 (blue), and in 2020 (orange) with a resection of the primary tumor (A) and without any tumor resection (B), according to the occurrence of a Sars Cov2 infection [Color figure can be viewed at wileyonlinelibrary.com]
3.2. Tumor stage at the time of diagnosis
The algorithm of pTNM identification resulted in a sensitivity and a positive predictive value of 98% and 96% on the test set computed on the available text reports. Table 2 summarizes the pathological tumor stage of 929 localized colon cancers after initial primary tumor resection. No statistical difference was observed between both risk classes, including in 2021 (data not shown).
TABLE 2.
Pathological tumor stage after initial primary tumor resection in the first available related postoperative pathology report for 929 localized colon cancers, and metastatic status at initial diagnosis on available CT‐scan text reports for 782 CRC cases, according to the year of referral to Greater Paris University Hospitals
| Year of diagnosis | 2018 | 2019 | 2020 |
|---|---|---|---|
| Pathological tumor stage after initial primary tumor resection | |||
| Low risk, N (%): pT0/T1/T2/T3 and pN0 | 122 (53%) | 132 (49%) | 156 (53%) |
| High risk, N (%): pT4 or pN1/N2 | 107 (47%) | 137 (51%) | 136 (47%) |
| P‐values vs the average of 2018‐2019 | .56 | ||
| Metastatic status at initial diagnosis | |||
| Colon cancers | |||
| Stade I‐III, N (%) | 122 (74%) | 104 (63%) | 98 (68%) |
| Stade IV, N (%) | 43 (26%) | 62 (37%) | 47 (33%) |
| P‐values vs the average of 2018‐2019 | .97 | ||
| Rectal cancers | |||
| Stade I‐III, N (%) | 31 (74%) | 32 (80%) | 24 (77%) |
| Stade IV, N (%) | 11 (26%) | 8 (20%) | 7 (23%) |
| P‐values vs the average of 2018‐2019 | .85 | ||
The identification algorithm of staging CT‐scans among the initial imaging reports resulted in a sensitivity and a positive predictive value of 73% and 89% on the test set. The algorithm of metastatic status assessed resulted in a sensitivity and a positive predictive value of 73% and 73% on the test set. Table 2 summarizes the initial metastatic tumor status of 597 CRC cases, according to the year of CRC diagnosis. No statistical difference was observed between both risk classes, including in 2021 (data not shown).
Additional performance measures of the algorithms are described in the Supplementary Results.
3.3. Care trajectories
Among the overall population, 3645 patients underwent an anticancer treatment for their CRC, and 2463 patients underwent a surgical resection of the primary tumor (Table 1, Figures 1C,D and S3C,D). For those patients, the first therapeutic strategy was tumor resection, secondary tumor resection, chemotherapy, and radiation therapy for 2157, 49, 185, and 72 patients, respectively.
Figure 3 summarizes the care pathways of 2463 patients newly referred for a CRC according to the level of primary tumor resection emergency. Overall, 750 (31%), 57 (2%) and 1656 (67%) patients were operated in a context of “major emergency,” “minor emergency” and “no emergency,” respectively. This repartition did not appear to differ significantly over time, including during lockdown periods (Figure 4).
FIGURE 3.
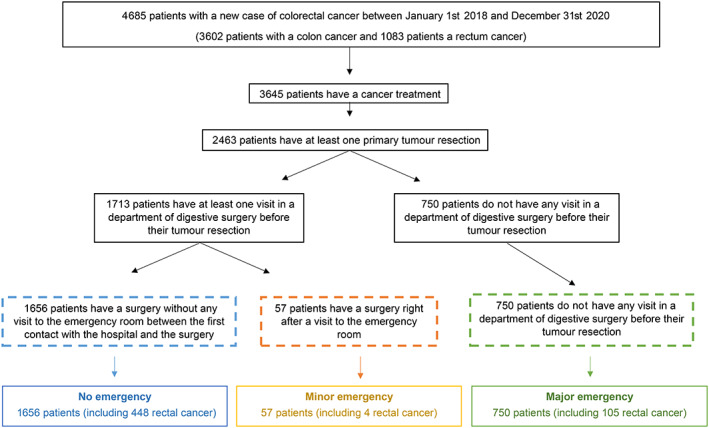
Flowchart of care trajectories for patients with a surgical resection of their colorectal primary tumor, according to the level of surgery emergency between 2018 and 2020 at the Greater Paris University Hospitals teaching hospital [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 4.
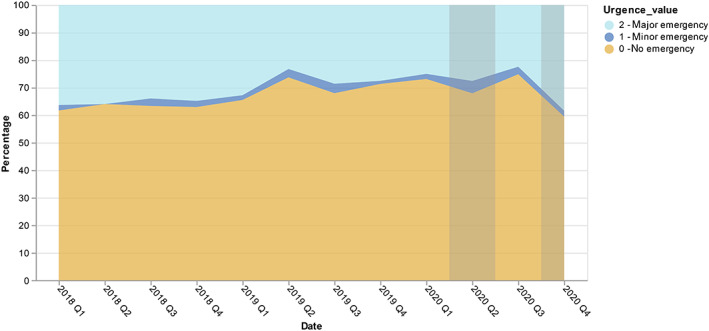
The rate of patients operated from their colorectal primary tumor, according to the level of surgery emergency between 2018 and 2020 at the Greater Paris University Hospitals (gray zones refer to the French lockdowns time periods) [Color figure can be viewed at wileyonlinelibrary.com]
3.4. Delays between MDMs and tumor resections
Among the 2572 CRC cases with a tumor resection (primary or secondary), 2289 (89%) were associated with an MDM report: 1331 cases had at least one MDM report before any therapeutic procedure while 958 cases had no MDM report before the first anticancer treatment. For both patient populations, the median delay between the MDM report and the first anticancer therapeutic strategy did not differ over time, including during the lockdown‐related time periods (Figure S1A‐D).
4. DISCUSSION
Less patients were diagnosed and treated for newly detected CRC at AP‐HP after the outbreak of Sars Cov2 in 2020. The poorer OS rates for patients diagnosed in 2020 compared to 2018‐2019 were related to the Sars Cov2 infection itself. Our study did not show significant changes in tumor stage at initial presentation, nor did it reveal significant changes in treatment trajectories (rate of emergency surgeries, MDM‐to‐treatment delays). Poorer outcomes are attributable to the pandemic itself rather than to its impact on hospital cancer care delivery.
We found that the backlog of patients not seen during the pandemic had not been compensated at the end of year 2020, along with other studies. 4 , 6 , 26 , 27 The study results do not show difference in OS rates for patients newly referred for a CRC in 2020 without any Sars Cov2 infection compared to 2018‐2019. Among the new cases of 2020, a Sars Cov2 infection impaired significantly the OS rates for patients undergoing any kind of anticancer treatment. Evidence has shown that active cancer has been a poor prognostic factor for the evolution of Sars Cov2 infections, especially for the postoperative settings of CRC resections. 28 , 29 What remains unknown is other factors such as the organization of care delivery and delays in diagnosis and treatment, affecting patient outcomes. In this study, there was no increase in the percentage of patients presenting with more advanced tumor stages at initial presentation after the outbreak of the pandemic. Studies elsewhere have yielded contrasted results. Single site studies in Romania and Brazil and a larger studies in France, Australia and New Zealand found significantly more advanced new CRC cases after the pandemic broke out, but a single‐site South‐Korean study found no statistically significant difference in pTNM stage distribution after Covid19. 13 , 14 , 16 , 30 , 31 Single‐site studies cannot exclude the fact that patients could have gone to another provider for treatment, whereas our study includes the AP‐HP teaching hospitals in Paris region.
The carcinogenesis steps of the CRC development are long‐term events, and the timeline of social distancing may not be significant compared to the timescale of the natural evolution of CRC, despite evidence suggesting that delays to treatment negatively affect outcomes. 12 , 32 A case‐control study on 10 000 patients showed that a delay reaching up to 1 year between the first CRC‐related symptom onset and the initial medical visit did not impair the subsequent patient' CRC‐related mortality rates. 33 Other observational published data showed no statistical relationship between delays between diagnosis and treatment of CRC, and patient' clinical outcomes—excluding emergency cases. 34 The pattern of carcinogenesis and metastasis evolution in CRC is also still under debate, some authors arguing for a metastatic stage occurring before any tumor detection. 35 On the other hand, the present study may have been conducted too soon to identify any impact of the CRC diagnosis delays on the initial tumor stage of patients with a new CRC.
Aside from initial tumor stage, the emergency with which primary tumor is resected could be a factor to explain the lower OS. Published studies demonstrated that elective surgeries were associated with better patient' clinical outcomes than emergency CRC primary tumor resections, even with local complications. 36 , 37 , 38 Our results showed that the rate of elective primary tumor resections reached around 70% over time and did not decrease after 2020. A study in Australia and New Zealand and a single‐site Japanese study found a significantly larger share of urgent CRC cases during the pandemic. 31 , 39 The different definitions of “urgent” or “emergency” cases used between studies do not allow direct comparisons.
Another possible cause for the higher mortality could be a change in treatment pathways, with changes in treatment strategies or longer time‐to‐treatment. The optimal care trajectory of a patient newly diagnosed with a CRC starts with a MDM, by law in France since 2007. 40 In the present study, almost half of CRC new cases were operated before the first MDM and this rate did not vary across time (data not shown). The clinical added value of MDMs in cancer pathways in under debate in the literature. 41 MDMs may benefit more to advanced stage CRCs to improve late‐stage tumor resectability. 18 , 42 , 43 Interestingly, the delays between MDMs and first anticancer therapeutic procedure did not vary over time in the present study, showing that potential hospital resource reallocations did not impact the quality of CRC‐oriented routine practices for new cases.
These results suggest that the statistically significant decrease in OS noticed between new CRC patients undergoing any kind of anticancer treatment in 2020 compared to the two previous years is explained by Sars Cov2 infection more than by the impact of the pandemic on CRC care delivery. This is consistent with English data showing no significant difference in mortality risk after major surgeries between 2019 and 2020, but a significantly higher risk of mortality for patients with Sars Cov2 infection. 44 Previous modeling studies focusing on CRC‐related mortality have anticipated a 15 000 quality‐adjusted life years loss in the UK and between 1445 and 1563 extra deaths due to the pandemic restrictions in the 5 years after diagnosis. 20 , 45
Among the study strengths, this is the largest published study showing the clinical impact of the Sars Cov2 outbreak on patients newly referred for a CRC. From this database of 11.4 million patients sharing a common unique identifier, we included solely the care sites for which the clinical data were available over time. The use of natural language processing algorithms on patient text reports extracted key clinical patient characteristics such as tumor stage at initial presentation.
Among the study weaknesses, most of patients reside in Paris' region. Nonetheless, our results on new cancer cases in Paris region mirror a national independent analysis of CRC care during the pandemic. 4 Those results should be interpreted in the context of routinely collected medical data with relevant information being missing such as the surgery quality assessment. 46 We included tertiary care centers with high‐volume cancer surgeries which is associated with better patients' outcomes. 47 No particular method was implemented to account for missing textual data. Yet, the rates of missing text reports were stable over time. Records were frequently reviewed manually to ensure that the data were complete and that their treatment was reliable, according to existing guidelines. 48 When automated data extraction from free‐text records was used, algorithms were carefully validated. Although coding errors may be prevalent, their incidence is unlikely to have changed significantly over the period of study and to have affected the longitudinal analysis of data. 49
5. CONCLUSION
In France, patients undergoing an anticancer treatment of any kind for a new CRC after 2020 were less likely to survive due to the Sars Cov2 infection itself. The Sars Cov2 outbreak and related disruptions in care practices does not seem to have impaired the quality of hospital care trajectories for the management of patients newly referred for a CRC, neither the tumor stage at initial presentation. Additional data on prehospital patient care trajectories would inform the diminution of newly referred CRC cases during the lockdown.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
Emmanuelle Kempf: Conceptualization, methodology, formal analysis, data curation, writing—original draft, project administration. Guillaume Lamé: Conceptualization, methodology, formal analysis, writing—original draft. Sonia Priou: Conceptualization, methodology, formal analysis, data curation, writing—original draft. Gilles Chatellier: Conceptualization, methodology, formal analysis, writing—original draft, supervision, project administration. Romain Bey: Conceptualization, methodology, resources. Stéphane Bréant: Conceptualization, methodology, resources. Gilles Galula: Conceptualization, methodology, supervision. Yazid Belkacemi: Conceptualization, methodology, formal analysis. Daniele Sommacale: Conceptualization, methodology, formal analysis. Namik Taright: Conceptualization, methodology, formal analysis, supervision. Rémi Flicoteaux: Conceptualization, methodology, formal analysis. Xavier Tannier: Conceptualization, methodology, writing—original draft. Bastien Rance: Conceptualization, methodology, writing—original draft. François Hemery: Conceptualization, methodology, resources, supervision. Etienne Audureau: Conceptualization, methodology, supervision. Christel Daniel: Conceptualization, methodology, formal analysis, resources, writing—original draft, supervision, project administration. Christophe Tournigand: Conceptualization, methodology, formal analysis, writing—original draft, supervision.
ETHICS STATEMENT
All structured and unstructured data used in the study have been pseudonymized. This study has been approved by the institutional review board (authorization number IRB 00011591) of AP‐HP's CDW (ref CSE 20‐55_COVONCO‐AP). The CDW was itself authorized by a decision of the French Regulatory Agency (CNIL Number 1980120). Subjects that objected to the reuse of their data were excluded from this study in accordance with the French legislation. Final data extraction was performed on July 26th 2021. No linkage was made with other databases.
Supporting information
Data S1 Supporting information.
ACKNOWLEDGMENTS
We thank Mrs Patricia Serre, Mrs Cécile Poret, Mr Ariel Cohen, Mr Thomas Petit‐Jean, Mr Alexandre Mouchet and Mr Stéphane Bréant for their help in the data access, quality assessment and analysis. This research was supported by the teams in charge of the Clinical Data Warehouse of Greater Paris University Hospitals (AP‐HP). The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Kempf E, Priou S, Lamé G, et al. Impact of two waves of Sars‐Cov2 outbreak on the number, clinical presentation, care trajectories and survival of patients newly referred for a colorectal cancer: A French multicentric cohort study from a large group of university hospitals. Int. J. Cancer. 2022;150(10):1609‐1618. doi: 10.1002/ijc.33928
Funding information Fondation ARC pour la Recherche sur le Cancer, Grant/Award Number: COVID202001343
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- 1. American Society of Clinical Oncology . Cancer Screening, Diagnosis, Staging, and Surveillance . Updated June 22, 2020. https://www.asco.org/asco-coronavirus-resources/care-individuals-cancer-during-covid-19/cancer-screening-diagnosis-staging2. Accessed July 13, 2020.
- 2. Ministère des Solidarités et de la Santé . Continuité des activités des Centres régionaux de coordination des dépistages des cancers (CRCDC). Boulogne Billancourt, France: Institut National Du Cancer; 2020. [Google Scholar]
- 3. Meyer A, Drouin J, Zureik M, Weill A, Dray‐Spira R. Colonoscopy in France during the COVID‐19 pandemic. Int J Colorectal Dis. 2021;36(5):1073‐1075. doi: 10.1007/s00384-020-03816-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Challine A, Lazzati A, Dousset B, Voron T, Parc Y, Lefevre JH. Colorectal screening: we have not caught up. A surge of colorectal cancer after the coronavirus disease 2019 (COVID‐19) pandemic? Surgery. 2021;169(4):991‐993. doi: 10.1016/J.SURG.2020.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kempf E, Lamé G, Layese R, et al. New cancer cases at the time of SARS‐Cov2 pandemic and related public health policies: a persistent and concerning decrease long after the end of national lockdown. Eur J Cancer. 2021;150:260‐267. doi: 10.1016/j.ejca.2021.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morris EJA, Goldacre R, Spata E, et al. Impact of the COVID‐19 pandemic on the detection and management of colorectal cancer in England: a population‐based study. Lancet Gastroenterol Hepatol. 2021;6:199‐208. doi: 10.1016/S2468-1253(21)00005-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Curigliano G, Banerjee S, Cervantes A, et al. Managing cancer patients during the COVID‐19 pandemic: an ESMO multidisciplinary expert consensus. Ann Oncol. 2020;31(10):1320‐1335. doi: 10.1016/J.ANNONC.2020.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jazieh AR, Chan SL, Curigliano G, et al. Delivering cancer care during the COVID‐19 pandemic: recommendations and lessons learned from ASCO global webinars. JCO Global Oncol. 2020;6:1461‐1471. doi: 10.1200/GO.20.00423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nab M, Vehmendahl R v, Somers I, Schoon Y, Hesselink G. Delayed emergency healthcare seeking behaviour by Dutch emergency department visitors during the first COVID‐19 wave: a mixed methods retrospective observational study. BMC Emerg Med. 2021;21(1):1‐9. doi: 10.1186/S12873-021-00449-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Adelhoefer S, Berning P, Solomon SB, et al. Decreased public pursuit of cancer‐related information during the COVID‐19 pandemic in the United States. Cancer Causes Control. 2021;32(6):577‐585. doi: 10.1007/s10552-021-01409-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blay JY, Boucher S, Le VB, et al. Delayed care for patients with newly diagnosed cancer due to COVID‐19 and estimated impact on cancer mortality in France. ESMO Open. 2021;6(3):100134. doi: 10.1016/J.ESMOOP.2021.100134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hanna TP, King WD, Thibodeau S, et al. Mortality due to cancer treatment delay: systematic review and meta‐analysis. BMJ. 2020;371:m4087. doi: 10.1136/bmj.m4087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thierry AR, Pastor B, Pisareva E, et al. Association of COVID‐19 lockdown with the tumor burden in patients with newly diagnosed metastatic colorectal cancer. JAMA Netw Open. 2021;4(9):e2124483. doi: 10.1001/JAMANETWORKOPEN.2021.24483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Radulovic RS, Cuk VV, Juloski JT, et al. Is colorectal cancer stage affected by COVID‐19 pandemic? Chirurgia (Bucur). 2021;116(3):331‐338. doi: 10.21614/chirurgia.116.3.331 [DOI] [PubMed] [Google Scholar]
- 15. Hartman HE, Sun Y, Devasia TP, et al. Integrated survival estimates for cancer treatment delay among adults with cancer during the COVID‐19 pandemic. JAMA Oncol. 2020;48109:1‐9. doi: 10.1001/jamaoncol.2020.5403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aguiar S, Riechelmann RP, de Mello CAL, et al. Impact of COVID‐19 on colorectal cancer presentation. Br J Surg. 2021;108(2):e81‐e82. doi: 10.1093/bjs/znaa124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jonge L d, Worthington J, Wifferen F v, et al. Impact of the COVID‐19 pandemic on faecal immunochemical test‐based colorectal cancer screening programmes in Australia, Canada, and The Netherlands: a comparative modelling study. Lancet Gastroenterol Hepatol. 2021;6(4):304‐314. doi: 10.1016/S2468-1253(21)00003-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Osterlund P, Salminen T, Soveri L‐M, et al. Repeated centralized multidisciplinary team assessment of resectability, clinical behavior, and outcomes in 1086 Finnish metastatic colorectal cancer patients (RAXO): a nationwide prospective intervention study. Lancet Reg Heal Eur. 2021;3:100049. doi: 10.1016/J.LANEPE.2021.100049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ho KMA, Banerjee A, Lawler M, Rutter MD, Lovat LB. Predicting endoscopic activity recovery in England after COVID‐19: a national analysis. Lancet Gastroenterol Hepatol. 2021;6(5):381‐390. doi: 10.1016/S2468-1253(21)00058-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maringe C, Spicer J, Morris M, et al. The impact of the COVID‐19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population‐based, modelling study. Lancet Oncol. 2020;21(8):1023‐1034. doi: 10.1016/S1470-2045(20)30388-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kwakkenbos L, Imran M, Mccall SJ, et al. CONSORT extension for the reporting of randomised controlled trials conducted using cohorts and routinely collected data ( CONSORT‐ROUTINE ): checklist with explanation and elaboration. BMJ. 2021;373:n857. doi: 10.1136/bmj.n857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Norgeot B, Quer G, Beaulieu‐Jones BK, et al. Minimum information about clinical artificial intelligence modeling: the MI‐CLAIM checklist. Nat Med. 2020;26(9):1320‐1324. doi: 10.1038/s41591-020-1041-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Elm E v, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ Br Med J. 2007;335(7624):806‐808. doi: 10.1136/BMJ.39335.541782.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Daniel C, Salamanca E. Hospital databases. AP‐HP Clinical DataWarehouse. Healthcare and Artificial Intelligence; New York City: Springer, Cham; 2020:57‐67. [Google Scholar]
- 25. Weiser MR. AJCC 8th edition: colorectal cancer. Ann Surg Oncol. 2018;25(6):1454‐1455. doi: 10.1245/S10434-018-6462-1 [DOI] [PubMed] [Google Scholar]
- 26. O'Leary MP, Choong KC, Thornblade LW, Fakih MG, Fong Y, Kaiser AM. Management considerations for the surgical treatment of colorectal cancer during the global Covid‐19 pandemic. Ann Surg. 2020;272(2):e98‐e105. doi: 10.1097/SLA.0000000000004029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. London JW, Fazio‐Eynullayeva E, Palchuk MB, Sankey P, McNair C. Effects of the COVID‐19 pandemic on cancer‐related patient encounters. JCO Clin Cancer Informatics. 2020;4:657‐665. doi: 10.1200/cci.20.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee AJX, Purshouse K. COVID‐19 and cancer registries: learning from the first peak of the SARS‐CoV‐2 pandemic. Br J Cancer. 2021;124(11):1777‐1784. doi: 10.1038/s41416-021-01324-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lock JF, Köhler F, Germer CT, Flemming S, Wiegering A. Impact of COVID‐19 on elective and emergency colorectal surgery. Chirurg. 2021;13:924‐928. doi: 10.1007/s00104-021-01464-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Choi JY, Park IJ, Lee HG, et al. Impact of the COVID‐19 pandemic on surgical treatment patterns for colorectal cancer in a tertiary medical Facility in Korea. Cancer. 2021;13(9):2221. doi: 10.3390/CANCERS13092221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Williams E, Kong JC, Singh P, Prabhakaran S, Warrier SK, Bell S. The impact of the COVID‐19 pandemic on colorectal cancer diagnosis and management: a binational colorectal cancer audit study. ANZ J Surg. 2021;91:2091‐2096. doi: 10.1111/ANS.17071 [DOI] [PubMed] [Google Scholar]
- 32. Simon K. Colorectal cancer development and advances in screening. Clin Interv Aging. 2016;11:967‐976. doi: 10.2147/CIA.S109285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pruitt SL, Harzke AJ, Davidson NO, Schootman M. Do diagnostic and treatment delays for colorectal cancer increase risk of death? Cancer Causes Control. 2013;24(5):961‐977. doi: 10.1007/S10552-013-0172-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roder D, Karapetis CS, Olver I, et al. Time from diagnosis to treatment of colorectal cancer in a south Australian clinical registry cohort: how it varies and relates to survival. BMJ Open. 2019;9(9):e031421. doi: 10.1136/BMJOPEN-2019-031421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hu Z, Curtis C. Looking backward in time to define the chronology of metastasis. Nat Commun. 2020;11(1):1‐4. doi: 10.1038/s41467-020-16995-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rosander E, Holm T, Sjövall A, Hjern F, Weibull C, Nordenvall C. Emergency resection or diverting stoma followed by elective resection in patients with colonic obstruction due to locally advanced cancer: a national cohort study. Color Dis. 2021;23(9):2387‐2398. doi: 10.1111/codi.15785 [DOI] [PubMed] [Google Scholar]
- 37. Nilssen Y, Eriksen MT, Guren MG, Møller B. Factors associated with emergency‐onset diagnosis, time to treatment and type of treatment in colorectal cancer patients in Norway. BMC Cancer. 2021;21(1):757. doi: 10.1186/s12885-021-08415-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guidolin K, Withers R, Shariff F, Ashamalla S, Nadler A. Quality of colon cancer care in patients undergoing emergency surgery. Curr Oncol. 2021;28(3):2079‐2086. doi: 10.3390/curroncol28030192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mizuno R, Ganeko R, Takeuchi G, et al. The number of obstructive colorectal cancers in Japan has increased during the COVID‐19 pandemic: a retrospective single‐center cohort study. Ann Med Surg. 2020;60:675‐679. doi: 10.1016/J.AMSU.2020.11.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. French Republic Official Journal . Article D6124‐131, Public Health Code ; 2007.
- 41. Pillay B, Wootten AC, Crowe H, et al. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: a systematic review of the literature. Cancer Treat Rev. 2016;42:56‐72. doi: 10.1016/j.ctrv.2015.11.007 [DOI] [PubMed] [Google Scholar]
- 42. Fernando C, Frizelle F, Wakeman C, Frampton C, Robinson B. Colorectal multidisciplinary meeting audit to determine patient benefit. ANZ J Surg. 2017;87(11):E173‐E177. doi: 10.1111/ans.13366 [DOI] [PubMed] [Google Scholar]
- 43. Peng D, Cheng YX, Cheng Y. Improved overall survival of colorectal cancer under multidisciplinary team: a meta‐analysis. Biomed Res Int. 2021;2021:1‐7. doi: 10.1155/2021/5541613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Deputy M, Rao C, Worley G, et al. Effect of the SARS‐CoV‐2 pandemic on mortality related to high‐risk emergency and major elective surgery. Br J Surg. 2021;20:754‐759. doi: 10.1093/BJS/ZNAB029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gheorghe A, Maringe C, Spice J, et al. Economic impact of avoidable cancer deaths caused by diagnostic delay during the COVID‐19 pandemic: a national population‐based modelling study in England, UK. Eur J Cancer. 2021;152:233‐242. doi: 10.1016/j.ejca.2021.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Panagiotou OA, Heller R. Inferential challenges for real‐world evidence in the era of routinely collected health data: many researchers, many more hypotheses, a single database. JAMA Oncol. 2021;9:1605. doi: 10.1001/JAMAONCOL.2021.3537 [DOI] [PubMed] [Google Scholar]
- 47. Sheetz KH, Chhabra KR, Smith ME, Dimick JB, Nathan H. Association of Discretionary Hospital Volume Standards for high‐risk cancer surgery with patient outcomes and access, 2005‐2016. JAMA Surg. 2019;154(11):1005‐1012. doi: 10.1001/JAMASURG.2019.3017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kohane IS, Aronow BJ, Avillach P, et al. What every reader should know about studies using electronic health record data but may be afraid to ask. J Med Internet Res. 2021;23(3):e22219. doi: 10.2196/22219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Burns EM, Rigby E, Mamidanna R, et al. Systematic review of discharge coding accuracy. J Public Health (Bangkok). 2012;34(1):138‐148. doi: 10.1093/PUBMED/FDR054 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 Supporting information.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


