Abstract
In addition to the highly variable clinical presentation of acute COVID‐19 infection, it can also cause various postacute signs and symptoms. This study aimed to evaluate patients with postacute COVID‐19 over 12 weeks of follow‐up. The study included 151 patients who were diagnosed with COVID‐19 by real‐time polymerase chain reaction of a nasopharyngeal swab 1 month earlier, had radiologic findings consistent with COVID‐19 pneumonia, and presented to the post‐COVID‐19 outpatient clinic between May and August 2021. The patients were divided into three groups based on COVID‐19 severity: nonsevere pneumonia (Group 1), severe pneumonia (Group 2), and severe pneumonia requiring intensive care (Group 3). Evaluation of laboratory parameters at 4 and 12 weeks showed that Group 3 had a higher lactose dehydrogenase (LDH) level and a lower mean platelet volume than the other groups at both time points (p = 0.001 for all). Group 3 also had lower percent predicted forced vital capacity (FVC%), percent predicted forced expiration volume in 1 s (FEV1%), and percent predicted diffusion capacity of the lungs for carbon monoxide divided by alveolar volume (DLCO/VA%) compared to Groups 1 and 2 at Week 4 (p = 0.001, 0.004, 0.001, respectively) and compared to Group 1 at 12 weeks (p = 0.002, 0.03, 0.001, respectively). Patients with persistent dyspnea at 12 weeks had significantly lower FEV1%, FVC%, DLCO/VA%, and saturation levels in room air and significantly higher LDH, pro‐BNP, D‐dimer, and heart rate compared to those without dyspnea (p = 0.001 for all). Although the lungs are most commonly affected after COVID‐19 infection, vascular and endothelial damage also causes multisystem involvement. Our study indicates that laboratory values, radiological signs, and pulmonary functional capacity improved in most patients after 12 weeks of follow‐up.
Keywords: postacute COVID‐19, pulmonary function test, radiological sign
Highlights
In the present study, we aimed to examine changes in clinical symptoms, laboratory parameters, and pulmonary function tests over 12 weeks of follow‐up in patients presenting to our COVID‐19 outpatient clinic due to postacute COVID‐19 syndrome.
The results of our study demonstrated significant regression in patients' postacute symptoms and radiological signs at 12 weeks. In addition, we observed that the decrease in pulmonary function parameters at 4 weeks in patients who developed macrophage activation syndrome and respiratory failure largely resolved and approached normal limits with follow‐up alone or methylprednisolone therapy administered based on individual patient assessment.
1. INTRODUCTION
There have been over 200 hundred million confirmed cases of novel coronavirus disease 2019 (COVID‐19) since the disease first appeared in December 2019, and this figure continues to rise. While many infected individuals are asymptomatic or have mild symptoms such as fatigue, muscle and joint pain, decreased appetite, and loss of smell and taste, severe illness can occur, particularly in advanced age, in the presence of comorbidities such as hypertension, diabetes mellitus, or chronic kidney disease, in states of immunosuppression or immunocompromise, and in pregnancy. 1 , 2
The most common severe clinical manifestations of COVID‐19 are acute respiratory failure and macrophage activation syndrome. Both are characterized by excessive cytokine release, which can cause endothelial and vascular dysfunction in many vital organs, especially the lungs. 3 The lungs are primarily affected in COVID‐19 patients due to alveolar epithelial destruction, capillary damage/hemorrhage, hyaline membrane formation, alveolar septal fibrous proliferation, and pulmonary consolidation. 4 Furthermore, persistent signs of damage in many organs and tissues after the active infection may necessitate long‐term medical treatment. The term “postacute COVID‐19 syndrome” has been used to describe symptoms and abnormalities that persist up to 12 weeks after the onset of acute COVID‐19 and cannot be attributed to alternative diagnoses. 5
Extrapulmonary effects in patients with postacute COVID‐19 syndrome include symptoms such as chest pain, myocardial dysfunction, venous thromboembolism, myalgia, weight loss, asthenia, hair loss, diarrhea, anosmia or parosmia, posttraumatic stress, depression, and anxiety. The main pulmonary signs and symptoms consist of dyspnea, cough, chronic oxygen dependence, dysfunctional breathing, and radiological sequelae. 6 In a study evaluating COVID‐19 patients after discharge, greater disease severity was associated with lower diffusion capacity, whereas forced expiratory volume in the first second (FEV1) and forced vital capacity (FVC) did not differ significantly between patients who had mild, moderate, and severe pneumonia. 7 In addition, in our previous study evaluating the relationship between laboratory parameters and pulmonary function tests during active COVID‐19 infection, we observed that FEV1 and FVC levels increased in correlation with the decline in C‐reactive protein and fibrinogen levels. 4
In the present study, we aimed to examine changes in clinical symptoms, laboratory parameters, and pulmonary function tests over 12 weeks of follow‐up in patients presenting to our COVID‐19 outpatient clinic due to postacute COVID‐19 syndrome.
2. MATERIALS AND METHODS
This prospective study was conducted in the post‐COVID outpatient clinic of Atatürk University Faculty of Medicine Hospital, Department of Chest Diseases. The study included 151 patients who had a confirmed diagnosis of COVID‐19 pneumonia and presented to the post‐COVID outpatient clinic of Atatürk University Faculty of Medicine Hospital between May and August 2021. We performed clinical, laboratory, and radiological follow‐up of these patients for 3 months and evaluated the results. The study was approved by the Erzurum Atatürk University Faculty of Medicine Ethics Committee. Before starting the study, participants were informed about the study purpose, methods, and time investment needed for their participation in the study. The participants were also informed that the study carried no risk, their participation was completely voluntary, and they could withdraw from the study at any time.
2.1. Inclusion criteria
The study included patients with and without comorbidities who were diagnosed with COVID‐19 by real‐time polymerase chain reaction test of a nasopharyngeal swab 1 month earlier, had persistent symptoms associated with postacute COVID‐19, and met the following additional criteria:
-
1.
Were over 18 years of age.
-
2.
Had radiological findings consistent with COVID‐19 pneumonia.
-
3.
Did not need intubation and mechanical ventilation.
-
4.
Agreed to attend follow‐up appointments within the 12‐week study period.
2.2. Exclusion criteria
Patients who violated the inclusion criteria or met any of the following criteria were excluded:
-
1.
Any potential contraindications to pulmonary function testing (recent myocardial infarction, pulmonary embolism, cerebral aneurysm, active hemoptysis, pneumothorax, nausea/vomiting, recent thoracic, abdominal, or ocular surgery).
-
2.
Mental disability or lack of cooperation.
-
3.
Previously known or newly detected lung pathology.
2.3. Study group
The patients included in the study were divided into three groups according to COVID‐19 severity and follow‐up. Patients in Group 1 had nonsevere pneumonia (n = 83), patients in Group 2 had severe pneumonia but did not require intensive care due to respiratory failure or macrophage activation syndrome (n = 34), and patients in Group 3 had severe pneumonia and were admitted to the intensive care unit due to respiratory failure or macrophage activation syndrome (n = 34). Severe pneumonia was defined as meeting any of the following criteria: respiratory rate of 30 breaths/min or higher, oxygen saturation of 92% or lower, and more than 50% lung infiltration.
2.4. Study procedure
Patients presenting to the post‐COVID outpatient clinic were registered and their history was obtained. Their smoking history was ascertained and the number of pack‐years was calculated. The route of COVID‐19 transmission was noted. Physical examination was performed and the patients were asked about common symptoms such as fever, shortness of breath, cough, fatigue, loss of taste/smell, joint/muscle pain, headache, sore throat, diarrhea, hair loss, and psychiatric disorders. Complete blood count was performed in the biochemistry laboratory. At 4 and 12 weeks after testing positive for COVID‐19, routine hemogram and biochemical tests were requested and pulmonary function tests including diffusion capacity of the lungs for carbon monoxide (DLCO) were performed.
2.5. Pulmonary function testing
Patients were instructed to wear light clothing and abstain from smoking for 24 h, alcohol for 4 h, heavy meals for 2 h, and strenuous exercise for 30 min before testing. Pulmonary function tests were performed by the same technician using a Plusmed MIR Spirolab III device with body temperature and pressure saturated with water vapor correction according to room air and barometric pressure. Testing was done in negative‐pressure room using protective equipment to prevent COVID‐19 transmission. The patients' age, height, and weight were recorded and the technician explained the expected maneuver to the patients. Three acceptable spirograms were obtained and those that met the 2019 American Thoracic Society/European Respiratory Society reproducibility and acceptability criteria were included in the study. 8 The lower limits of the normal range determined for the healthy population according to the criteria specified in the same report were also calculated and presented by the spirometry device.
2.6. Evaluation of radiologic findings
Posterior–anterior chest X‐rays were obtained from all patients in the study at 4‐ and 12‐week follow‐up and the findings were classified as follows:
Complete resolution: Chest X‐ray was normal or returned to pre‐COVID‐19 state.
Major improvement: Resolution of more than 50% of pulmonary opacities that developed during acute COVID‐19.
Minor improvement or no change: Resolution of less than 50% of pulmonary opacities that developed during acute COVID‐19.
Worsening: Increased alveolar opacities or development of pulmonary fibrosis despite resolution of alveolar opacities that developed during acute COVID‐19.
For patients with increased parenchymal consolidations during treatment and follow‐up, thoracic computed tomography was performed to confirm whether their current condition could be contributed to non‐COVID‐19 causes. Tomographic evaluation was not included in routine follow‐up for all patients.
2.7. Medical treatment during follow‐up
Patients who presented for post‐acute COVID‐19 with complaints of myalgia, malaise, diarrhea, loss of taste/smell, and brain fog were followed without treatment. Patients in Group 3, who presented due to cough and dyspnea, exhibited diffusion values lower than 80% on pulmonary function test or oxygen saturation of 92% or lower in room air. All of these patients were treated with methylprednisolone starting at a dose of 0.5 mg/kg/day and reduced according to clinical and laboratory parameters until discontinuation at 12 weeks. Long‐term homeoxygen therapy was recommended for patients with partial pressure of oxygen below 55 mmHg or oxygen saturation below 88% with or without hypercapnia. Patients presenting with cough and limited diffusion without oxygen desaturation but with forced expiratory flow at 25% and 75% of pulmonary volume (FEF25‐75%) lower than 80% were given low‐dose steroid inhaler or montelukast 10 mg/day. For patients with palpitations, the cardiology department was consulted to evaluate for cardiac involvement.
2.8. Statistical analysis
Analyses were performed using IBM SPSS version 20.0 software (IBM Corp.). Data were presented as mean, standard deviation, number, and percentage. Shapiro–Wilk test and Kolmogorov–Smirnov test were used to determine whether continuous variables were normally distributed. Independent samples t‐test was used to compare two independent groups, and Wilcoxon test was used to analyze dependent variables. Independent samples t‐test was used to compare two independent groups. Continuous variables were compared between more than two independent groups using analysis of variance (ANOVA) if normally distributed and Kruskal–Wallis test if nonnormally distributed. Post hoc tests after ANOVA were performed using Tukey's test when variances were homogeneous and Tamhane's T2 test when variances were not homogeneous. Post hoc analysis after Kruskal–Wallis test was performed using the Kruskal–Wallis one‐way ANOVA (k samples) test. Relationships between two quantitative variables were examined using Pearson correlation analysis if normally distributed and Spearman correlation analysis if non‐normally distributed. p values <0.05 were considered statistically significant.
3. RESULTS
The mean age of the participants was 48 ± 12.4 years. The mean ages in Groups 1, 2, and 3 were 46.4 ± 14.2, 51.8 ± 6.5, and 48.1 ± 11.5 years, respectively (p = 0.09). Eighty‐seven (57.6%) of the patients were men. The proportion of men was 42.1% in Group 1, 64.7% in Group 2, and 88.2% in Group 3. The difference in sex distribution between the groups was statistically significant (p = 0.001).
COVID‐19 transmission occurred from family in 107 patients (70.8%), the workplace in 26 (17.2%), public transportation in 9 (6%), and was unknown in 9 patients (6%). Fifty‐three patients had at least one comorbidity, while the other 98 patients had no comorbidities. Hypertension was present in 27 patients, diabetes in 22, coronary artery disease in 3, hyperthyroidism in 1, hypothyroidism in 4, and chronic renal failure in 3 patients.
Within‐group and between‐group comparisons of laboratory parameters at Weeks 4 and 12 are shown in Table 1. Patients in Group 3 had higher ferritin, lactose dehydrogenase (LDH), alanine transaminase (ALT), and aspartate transaminase (AST) levels than those in Group 1 (p = 0.002, <0.001, and <0.001, respectively). White blood cell count, platelet count, and AST level were higher and mean platelet volume (MPV) was lower in Group 3 than in Group 2 (p ≤ 0.001 and 0.001, respectively). Group 3 had higher white blood cell count, neutrophil count, and LDH level than Group 1 (p ≤ 0.001, <0.001, and 0.003, respectively). MPV was lower in Group 3 than in Groups 1 and 2 (p = 0.001).
Table 1.
Comparison of laboratory parameters at 4 and 12 weeks in patients with postacute COVID‐19 syndrome
| Group 1 (n = 83) | Group 1 (n = 83) | Group 2 (n = 34) | Group 2 (n = 34) | Group 3 (n = 34) | Group 3 (n = 34) | |||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||
| Week 4 | Week 12 | Week 4 | Week 12 | Week 4 | Week 12 | p* | p** | |
| WBC (/µl) | 7313.6 ± 2017.3 | 7388.6 ± 2012.1 | 8369.4 ± 2403.7 | 8356.9 ± 2472.4 | 10089.4 ± 3552.6b* | 10771.7 ± 3755.1a,b** | <0.001 | <0.001 |
| Lymphocytes (/µl) | 2233.3 ± 611.1 | 2276.1 ± 653.7 | 2347.9 ± 771.1 | 2570.4 ± 634.5 | 2833.5 ± 1387.8 | 2611.7 ± 1076.9 | 0.46 | 0.146 |
| Lymphocyte% | 31.3 ± 8.6 | 32.3 ± 12.2 | 47.1 ± 54.1 | 31.1 ± 8.6 | 41.1 ± 77.4 | 24.9 ± 8.2 a** | 0.2 | 0.002 |
| Neutrophils (/µl) | 4349.2 ± 1766.2 | 4420.9 ± 1781.9 | 5238.8 ± 2299.8 | 4951.5 ± 1963.8 | 6203.5 ± 2497.1 a* | 7095.5 ± 2826.3 a,b** | <0.001 | <0.001 |
| Neutrophil% | 58.1 ± 9.2 | 58.5 ± 9.6 | 60.7 ± 10.1 | 59.2 ± 7.6 | 58.5 ± 13.9 | 60.2 ± 17.1 | 0.43 | 0.186 |
| Platelets (/µl) | 282096.4 ± 92743.2 | 272389.8 ± 70765.5 | 221757.6 ± 48717.9 | 267615.4 ± 37999.1 | 296911.8 ± 113484.1 b* | 262137.9 ± 72925.9 | <0.001 | 0.43 |
| MPV (fl) | 9.9 ± 1.3 | 10.1 ± 0.9 | 10.2 ± 0.8 | 9.9 ± 0.6 | 9.3 ± 0.6b* | 9.3 ± 0.7a,b** | 0.001 | 0.001 |
| ALT (U/L) | 33.2 ± 24 | 31.2 ± 20.9 | 55.8 ± 34.6 a* | 32.2 ± 17.2 | 57.2 ± 30.5 a* | 33.8 ± 18.4 | <0.001 | 0.42 |
| AST (U/L) | 25.1 ± 10.1 | 23.8 ± 8.7 | 26.4 ± 8.9 | 22.1 ± 7.3 | 33.1 ± 11.5 a,b* | 22.2 ± 7.1 | <0.001 | 0.64 |
| Albumin (g/dl) | 4.2 ± 0.4 | 4.2 ± 0.3 | 4.2 ± 0.3 | 4.1 ± 0.6 | 4.2 ± 0.3 | 4.1 ± 0.4 | 0.31 | 0.21 |
| Fibrinogen (ng/ml) | 362.1 ± 131.1 | 355.8 ± 142.1 | 376.8 ± 92.5 | 321.4 ± 55.2 | 385.8 ± 103.4 | 360.7 ± 67.5 | 0.2 | 0.08 |
| Procalcitonin (ng/ml) | 0.04 ± 0.03 | 0.04 ± 0.03 | 0.05 ± 0.04 | 0.04 ± 0.03 | 0.05 ± 0.03 | 0.03 ± 0.03 | 0.38 | 0.09 |
| D‐dimer (ng/ml) | 395.6 ± 344.3 | 369.1 ± 243.8 | 403.2 ± 261.3 | 327.1 ± 171.7 | 444.4 ± 314.5 | 314.4 ± 176.8 | 0.91 | 0.6 |
| CRP (mg/dl) | 6.9 ± 9.6 | 7.7 ± 10.6 | 16.9 ± 41.3 | 7.1 ± 2.2 | 9.3 ± 12.4 | 7.2 ± 4.3 | 0.12 | 0.09 |
| LDH (U/L) | 216.7 ± 55.2 | 221 ± 63.5 | 288.5 ± 77.3 a* | 214.9 ± 23.3 | 282.4 ± 58.1 a* | 261.6 ± 58.1 a,b** | <0.001 | 0.003 |
| BUN (mg/dl) | 13.4 ± 4.1 | 13.3 ± 3.9 | 14.5 ± 4.3 | 14.9 ± 4.6 | 14.3 ± 6.7 | 16.1 ± 5.6a** | 0.66 | 0.01 |
| Ferritin (ng/ml) | 128.7 ± 161.2 | 134.3 ± 154.6 | 143.8 ± 102.7 | 137.1 ± 115.9 | 283.6 ± 358.6 a* | 223.2 ± 229.7 | 0.002 | 0.36 |
| Troponin‐I (ng/dl) | 2.2 ± 3.3 | 2.2 ± 3.8 | 2.1 ± 1.6 | 1.3 ± 1.5 | 3.2 ± 5.4 | 2.6 ± 4.9 | 0.52 | 0.1 |
| Pro‐BNP (pg/ml) | 74.6 ± 101.9 | 56.2 ± 84.9 | 45.3 ± 47.9 | 43.7 ± 83.4 | 100.4 ± 91.9 | 80.8 ± 64.4 | 0.12 | <0.001 |
Note: Wilcoxon test was used for within‐group comparisons of laboratory parameters and Weeks 4 and 12 (bold indicate statistically significant); Kruskal–Wallis test was used for between‐group comparisons of Week 4 and Week 12 data (p*: comparison of Week 4 data, p**: comparison of Week 12 data, p a: comparison with Group 1, p b: comparison between Groups 2 and 3).
Abbereviations: ALT, alanine transaminase; AST, aspartate transaminase; BUN, blood urea nitrogen; CRP, C‐reactive protein; LDH, lactose dehydrogenase; MPV, mean platelet volume; SD, standard deviation; WBC, white blood cells.
Comparison of pulmonary function test parameters, saturation, and heart rate values of the patients at Weeks 4 and 12 are shown in Table 2. At Week 4, Group 3 had lower percent predicted forced vital capacity (FVC%), percent predicted forced expiration volume in 1 s (FEV1%), and percent predicted DLCO divided by alveolar volume (DLCO/VA%), and oxygen saturation in room air than Groups 1 and 2 (p = 0.001, 0.004, 0.001, and 0.001, respectively) and significantly higher heart rate than in Group 1 (p = 0.001). At Week 12 of follow‐up, Group 3 still had lower FVC%, FEV1%, DLCO/VA%, and room air saturation values compared to Group 1 (p = 0.002, 0.03, 0.001, and 0.02, respectively) and higher heart rate compared to group 1 (p = 0.001).
Table 2.
Comparison of pulmonary function test results, saturation levels, and heart rate in postacute COVID‐19 patients at 4 and 12 weeks
| Group 1 (n = 83) | Group 1 (n = 83) | Group 2 (n = 34) | Group 2 (n = 34) | Group 3 (n = 34) | Group 3 (n = 34) | |||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||
| Week 4 | Week 12 | Week 4 | Week 12 | Week 4 | Week 12 | p* | p** | |
| FVC% | 111 ± 22.8 | 114.3 ± 17.4 | 109.1 ± 15.5 | 111.9 ± 15.2 | 91.3 ± 21.4 a,b* | 97.4 ± 22.7 a,b** | <0.001 | 0.002 |
| FEV1% | 106.5 ± 21.1 | 108.1 ± 20.3 | 104.3 ± 23.3 | 104.5 ± 16.1 | 91.6 ± 19.1 a,b* | 96 ± 21.2 a** | 0.004 | 0.03 |
| FEF25‐75% | 81.47 ± 29.3 | 84.1 ± 27.9 | 88.3 ± 37.2 | 89.2 ± 30.9 | 81 ± 23.5 | 86.1 ± 24.9 | 0.73 | 0.35 |
| DLCO/VA% | 115.1 ± 23.8 | 120.2 ± 18.8 | 111.7 ± 18.1 | 112.8 ± 16.5 | 89.2 ± 21.1 a,b* | 103.9 ± 13.6 a** | <0.001 | <0.001 |
| SO2 (%) | 93.1 ± 4.5 | 94 ± 2.7 | 92.3 ± 3.8 | 93.1 ± 1.3 | 87.1 ± 6.4 a,b* | 91.3 ± 4.7 a** | <0.001 | 0.02 |
| Heart rate (beats/min) | 90.6 ± 11.5 | 88.9 ± 12.5 | 98.5 ± 10.5 | 90.1 ± 8.6 | 104.2 ± 11.3a* | 104.1 ± 11.5a,b** | <0.001 | <0.001 |
Note: Wilcoxon test was used for within‐group comparisons of laboratory parameters and Weeks 4 and 12 (bold indicate statistically significant); Kruskal–Wallis test was used for between‐group comparisons of Week 4 and Week 12 data (p*: comparison of Week 4 data, p**: comparison of Week 12 data, p a: comparison with group 1, p b: comparison between groups 2 and 3).
Abbreviations: %, percent predicted; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s; DLCO/VA, diffusing capacity divided by the alveolar volume.
Comparison of laboratory and pulmonary function test parameters between patients with and without postacute COVID‐19 syndrome at Week 12 of follow‐up is shown in Table 3. Patients with postacute COVID‐19 syndrome had significantly higher white blood cell count, neutrophil count, LDH, D‐dimer, and pro‐BNP levels, and heart rate (p = 0.001, 0.001, 0.002, 0.001, 0.001, and 0.01, respectively) and significantly lower MPV, FEV1%, FVC%, DLCO/VA%, and oxygen saturation in room air (p = 0.01, 0.001, 0.001, 0.001, and 0.001, respectively).
Table 3.
Comparison of laboratory parameters differing significantly in patients with and without persistent postacute COVID‐19 syndrome at Week 12 of follow‐up
| Persistent postacute COVID‐19 syndrome at Week 12 | |||
|---|---|---|---|
| No | Yes | ||
| (n = 101) | (n = 50) | ||
| Mean ± SD | Mean ± SD | p | |
| WBC (/µl) | 7104.3 ± 1884.5 | 8420.2 ± 2210.4 | <0.001 |
| Neutrophils (/µl) | 3118.1 ± 1612.8 | 5052.4 ± 2004.1 | <0.001 |
| MPV (fl) | 10.2 ± 0.6 | 9.5 ± 0.6 | 0.01 |
| LDH (U/L) | 216 ± 60.1 | 245.7 ± 48.6 | 0.002 |
| D‐dimer (ng/ml) | 303.2 ± 136.5 | 381.2 ± 150.4 | <0.001 |
| Pro‐BNP (pg/ml) | 50.1 ± 66.6 | 75.9 ± 43.2 | <0.001 |
| FVC% | 115.5 ± 15.1 | 98.2 ± 18.9 | <0.001 |
| FEV1% | 110.1 ± 17.4 | 99.2 ± 17.2 | <0.001 |
| DLCO/VA% | 125.3 ± 16.4 | 96.4 ± 26.7 | <0.001 |
| SO2 (%) | 95 ± 1.5 | 91.4 ± 1.8 | <0.001 |
| Heart rate (beats/min) | 88.8 ± 14.6 | 92.1 ± 9.4 | 0.01 |
Abbreviations: %, percent predicted; DLCO/VA, diffusing capacity for carbon monoxide divided by alveolar volume; FEV1: forced expiratory volume in 1 s; FVC, forced vital capacity; LDH, lactose dehydrogenase; MPV, mean platelet volume; SO2, oxygen saturation; WBC, white blood cells.
Symptom frequency at Weeks 4 and 12 is shown in Figure 1. At Week 4, dyspnea was reported more frequently by patients in Group 3 than patients in Groups 1 and 2 (p < 0.05). Myalgia, fatigue, and cough were more common in Groups 2 and 3 compared to Group 1, while hair loss and brain fog were more common in Group 2 compared to Groups 1 and 3 (p < 0.05 for all). At Week 12 of follow‐up, dyspnea and myalgia were more frequent in Group 3 compared to Groups 1 and 2, while fatigue was more common in Groups 2 and 3 compared to Group 1 (p < 0.05 for all).
Figure 1.
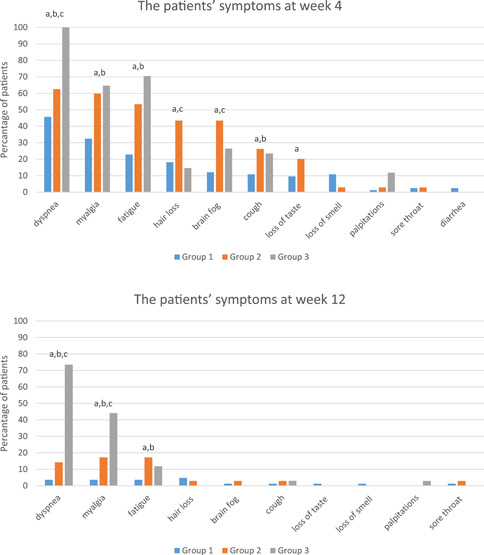
Symptoms reported by postacute COVID‐19 patients during follow‐up
The change in radiological findings by week is shown in Figure 2. At 4 weeks, 63 patients were classified as complete resolution, 64 as major improvement, 23 as minor improvement or no change, and 1 patient's condition had worsened. At 12 weeks, 106 patients were classified as complete resolution, 39 showed major improvement, and 6 showed minor improvement or no change. In total, 96.1% of the patients had partial or complete resolution of radiological findings at 12 weeks of follow‐up.
Figure 2.
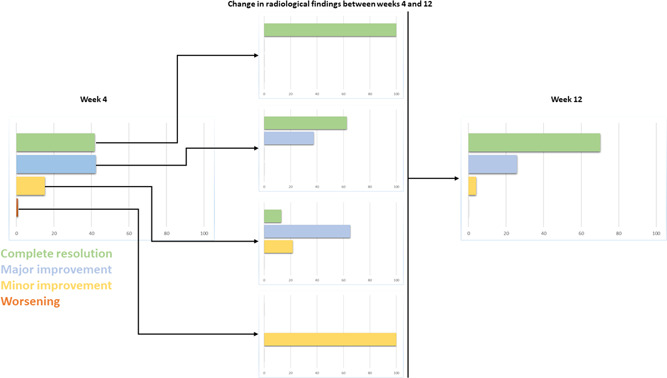
Radiological findings observed in postacute COVID‐19 patients during follow‐up (shown as percentage of patients)
The analysis of laboratory and pulmonary function parameters according to postacute COVID‐19 symptoms at Week 4 revealed no significant differences. However, persistent complaints of dyspnea at Week 12 were associated with significant differences in FEV1%, FVC%, and DLCO/VA%, as well as LDH, pro‐BNP, D‐dimer, oxygen saturation in room air, and heart rate compared to those in patients without dyspnea (p = 0.001 for all) (Figure 3).
Figure 3.
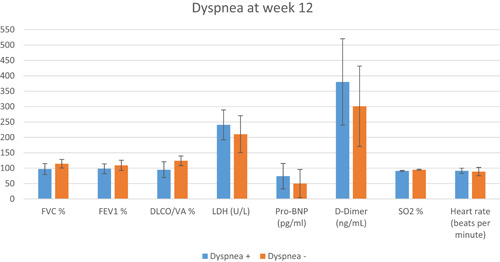
Comparison of pulmonary function and laboratory parameters between postacute COVID‐19 patients with and without dyspnea at Week 12 of follow‐up
4. DISCUSSION
In this study, we observed that men comprised a significantly higher proportion of patients who developed macrophage activation syndrome or respiratory failure. In addition, hypertension and diabetes were found to be the most common comorbidities in patients who were hospitalized for COVID‐19. Consistent with previous reports that ferritin and LDH have prognostic significance in COVID‐19, our comparison of laboratory parameters showed that patients recovering from severe illness still had higher ferritin and LDH levels at 4 weeks and persistent LDH elevation at 12 weeks. MPV, which is used a marker of active inflammatory processes, was low at both 4 and 12 weeks of follow‐up in patients recovering from severe COVID‐19. Although FVC%, FEV1%, and DLCO/VA% levels in patients with the most severe disease (Group 3) were increased at Week 12 compared to Week 4, they were still lower than in patients with less severe disease.
The most common symptoms reported by patients at 4 and 12 weeks were dyspnea and myalgia. Symptoms mostly resolved by Week 12 in patients in groups 1 and 2, whereas persistent dyspnea and myalgia were reported by many patients in group 3. Radiological follow‐up demonstrated complete resolution or major improvement in 96.1% of patients at 12 weeks.
Before COVID‐19, postacute syndromes were also identified after many other inflammatory pathologies such as Epstein–Barr virus, dengue fever, tick‐borne encephalitis, influenza, West Nile virus, Zika virus, and Ross River virus disease. 9 , 10 , 11 , 12 , 13 In a study evaluating COVID‐19 patients at 1 year after discharge, more than half of the patients had persistent fatigue and sleep disturbance. 14 In another study evaluating SARS‐CoV and MERS‐CoV patients who were treated in the intensive care unit and discharged, it was observed that 11%–45% of the patients continued to show limited diffusion in pulmonary function tests performed after 12 months. 15 , 16
The etiology of postacute symptoms after COVID‐19 may be attributable to multifactorial causes. The neuroinvasive property of the SARS‐CoV‐2 virus has been proposed as a factor contributing to symptoms such as loss of taste and smell and the brain fog observed after COVID‐19. 17 , 18 It has also been emphasized that cytokine storm, the term for extreme pro‐inflammatory cytokine production that occurs frequently in COVID‐19 patients, may be responsible for symptoms in the postacute period. Another possibility is that the virus localizes to immunologically privileged sites and cannot be completely eradicated by the immune system. 6 In postmortem studies of COVID‐19 patients, the presence of severe endothelial damage, thrombosis, and microangiopathy in the peripheral lung tissue are the main histopathologic signs explaining the subsequent organ damage. 19
A radiological study of COVID‐19 patients after discharge showed that ground glass opacities with areas of consolidation and subpleural fibrotic bands persisted at 3‐month follow‐up, while a substantial proportion of patients had fibrotic areas with or without parenchymal destruction at 6‐month follow‐up. 20 In another study, it was reported that FEV1 and FVC values were not correlated with disease severity in postdischarge pulmonary function tests, while DLCO decreased with greater disease severity. 7
In our study, comparison of laboratory data at 4 weeks revealed that patients with severe disease had elevated LDH levels as well as liver function tests. We believe this can be mainly attributed to hypoxia and the endothelial damage resulting from more exaggerated cytokine release in patients with severe illness relative to the other groups. Moreover, we consider the increase in white blood cell, neutrophil, and platelet counts in these patients as being secondary to methylprednisolone therapy administered for macrophage activation syndrome and respiratory failure that occurred during hospitalization. Studies on rheumatologic diseases involving intense cytokine discharge have shown that MPV levels may decrease secondary to platelet consumption in symptomatic systemic lupus erythematosus and rheumatoid arthritis. We also observed that patients with severe disease (Group 3) had low MPV levels, suggesting the predominance of low‐volume platelets in the peripheral blood due to consumption as reported in previous studies, and this decrease persisted at Week 12 of follow‐up. In the laboratory data obtained at Week 12, patients in Group 3 had higher LDH levels compared to other groups, and white blood cell and neutrophil counts were also increased. As mentioned above, we attribute this primarily to the methylprednisolone therapy administered to patients in Group 3 during follow‐up.
Comparison of pulmonary function test parameters between the groups showed that FEV1%, FVC%, and DLCO/VA% were lower in Group 3 compared to Group 1 at 4 weeks and were increased but still low relative to Group 1 at the end of 12 weeks. This finding suggests that in a significant proportion of patients with severe COVID‐19, pulmonary function test parameters improved at 12 weeks with medical treatment administered as necessary. Symptoms of dyspnea, fatigue, myalgia, and hair loss were common complaints at 4‐week follow‐up and regressed considerably at 12 weeks both with follow‐up only and with medical treatment for dyspnea when necessary. We observed that dyspnea was more frequent among patients in Groups 1 and 3 at 4‐week follow‐up, whereas at 12 weeks dyspnea was much more common in Group 3 compared to Groups 1 and 2. These results may indicate that in addition to the lung parenchyma damage in patients with severe COVID‐19, neuromuscular involvement in patients with mild COVID‐19 can also cause shortness of breath independent of pulmonary involvement.
Although many explanations for hair loss in COVID‐19 have been proposed, the greatest focus has been on inflammatory/anti‐inflammatory imbalance and genetic background. As observed in our patients, the frequency of hair loss independent of the clinical severity of illness suggests that genetic predisposition may be a factor in addition to inflammation.
In the patients' radiological evaluations at 4 and 12 weeks, we observed that 96.1% of the patients had radiological regression with follow‐up or medical treatment when necessary. Patients with persistent postacute COVID‐19 symptoms at Week 12 of follow‐up had low pulmonary function parameters and elevated levels of laboratory parameters with prognostic significance for COVID‐19. This may be related to delayed recovery of pulmonary function at 12 weeks in patients who had severe COVID‐19. The patients' desaturation, high pro‐BNP and LDH levels, and low MPV may have been a result of impaired pulmonary function and diffusion capacity.
Important limitations of this study were the small patient sample and nonhomogeneous sex distribution. However, this limitation occurred as a result of the more severe disease course in men and the presentation of postacute COVID‐19 patients with different variants during the study period.
In conclusion, it seems that after 2 years of dominating the public health stage, COVID‐19 will continue to challenge us with postacute manifestations. The results of our study demonstrated significant regression in patients' postacute symptoms and radiological signs at 12 weeks. In addition, we observed that the decrease in pulmonary function parameters at 4 weeks in patients who developed macrophage activation syndrome and respiratory failure largely resolved and approached normal limits with follow‐up alone or methylprednisolone therapy administered based on individual patient assessment. Evaluation of respiratory function parameters in patients with persistent dyspnea and high LDH, pro‐BNP, and D‐dimer levels at the end of the postacute period may guide follow‐up and treatment.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Conceptualization, methodology, software, validation, and formal analysis: Buğra Kerget, Ferhan Kerget, Eda Çelikda, and Alperen Aksakal; investigation, resources, and data curation: Ferhan Kerget, Buğra Kerget, and Metin Akgün; writing—original draft, writing—review and editing: Buğra Kerget and Ömer Araz; visualization, supervision, and project administration: Buğra Kerget and Elif Yılmazel Uçar.
Kerget B, Çelik E, Kerget F, et al. Evaluation of 3‐month follow‐up of patients with postacute COVID‐19 syndrome. J Med Virol. 2022;94:2026‐2034. 10.1002/jmv.27579
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Yuki K, Fujiogi M, Koutsogiannaki S. COVID‐19 pathophysiology: a review. Clin Immunol. 2020;215:108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Qiao J. What are the risks of COVID‐19 infection in pregnant women? Lancet. 2020;395(10226):760‐762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kerget B, Kerget F, Kocak AO, et al. Are serum interleukin 6 and surfactant protein D levels associated with the clinical course of COVID‐19? Lung. 2020;198(5):777‐784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kerget B, Aksakal A, Kerget F. Evaluation of the relationship between laboratory parameters and pulmonary function tests in COVID‐19 patients. Int J Clin Pract. 2021;75:e14237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Montani D, Savale L, Beurnier A, et al. Multidisciplinary approach for post‐acute COVID‐19 syndrome: time to break down the walls. Eur Respir J. 2021;58:(1):2101090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nalbandian A, Sehgal K, Gupta A, et al. Post‐acute COVID‐19 syndrome. Nature Med. 2021;27(4):601‐615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mo X, Jian W, Su Z, et al. Abnormal pulmonary function in COVID‐19 patients at time of hospital discharge. Eur Respir J. 2020;55(6):2001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Graham BL, Steenbruggen I, Miller MR, et al. Standardization of spirometry 2019 update. An official American thoracic society and European respiratory society technical statement. Am J Respir Crit Care Med. 2019;200(8):e70‐e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Behan PO, Behan W. Postviral fatigue syndrome. Crit Rev Neurobiol. 1988;4(2):157‐178. [PubMed] [Google Scholar]
- 10. Lidbury BA. Ross River Virus immune evasion strategies and the relevance to post‐viral fatigue, and myalgic encephalomyelitis onset. Front Med. 2021;8:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Seet RC, Quek AM, Lim EC. Post‐infectious fatigue syndrome in dengue infection. J Clin Virol. 2007;38(1):1‐6. [DOI] [PubMed] [Google Scholar]
- 12. Garcia MN, Hause AM, Walker CM, Orange JS, Hasbun R, Murray KO. Evaluation of prolonged fatigue post–West Nile virus infection and association of fatigue with elevated antiviral and proinflammatory cytokines. Viral Immunol. 2014;27(7):327‐333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hotchin N, Read R, Smith D, Crawford D. Active Epstein‐Barr virus infection in post‐viral fatigue syndrome. J Infect. 1989;18(2):143‐150. [DOI] [PubMed] [Google Scholar]
- 14. Amenta EM, Spallone A, Rodriguez‐Barradas MC, El Sahly HM, Atmar RL, Kulkarni PA. Postacute COVID‐19: an overview and approach to classification. Paper presented at: open Forum Infectious Diseases; 2020. [DOI] [PMC free article] [PubMed]
- 15. Ahmed H, Patel K, Greenwood DC, et al. Long‐term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: a systematic review and meta‐analysis. J Rehabil Med. 2020;52(5):00063. [DOI] [PubMed] [Google Scholar]
- 16. Park WB, Jun KI, Kim G, et al. Correlation between pneumonia severity and pulmonary complications in Middle East respiratory syndrome. J Korean Med Sci. 2018;33(24):169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Karimi‐Galougahi M, Naeini AS, Raad N, Mikaniki N, Ghorbani J. Vertigo and hearing loss during the COVID‐19 pandemic–is there an association? Acta Otorhinolaryngol Ital. 2020;40(6):463‐465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lamontagne SJ, Winters MF, Pizzagalli DA, Olmstead MC. Post‐acute sequelae of COVID‐19: evidence of mood & cognitive impairment. Brain Behav Immun Health. 2021;17:100347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Menter T, Haslbauer JD, Nienhold R, et al. Postmortem examination of COVID‐19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77(2):198‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Salehi S, Reddy S, Gholamrezanezhad A. Long‐term pulmonary consequences of coronavirus disease 2019 (COVID‐19): what we know and what to expect. J Thorac Imaging. 2020;35(4):W87‐W89. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


