Abstract
We aimed to perform meta‐analyses to summarize the overall effectiveness of the mRNA‐1273 vaccine against COVID‐19 caused by the Delta variant from real‐world studies. A systematic literature search with no language restriction was performed in electronic databases to identify eligible observational studies that reported the effectiveness of the mRNA‐1273 vaccine to prevent reverse transcription‐polymerase chain reaction (RT‐PCR) confirmed COVID‐19 caused by Delta variant of SARS‐CoV‐2 (B.1.617.2). A random‐effects meta‐analysis model was used to estimate the pooled odds ratio (OR) at a 95% confidence interval (CI), and the vaccine effectiveness was indicated as (pooled OR − 1)/OR. Five studies were included for this systematic review and meta‐analysis. The meta‐analysis revealed that the administration of mRNA‐1273 vaccine protected against RT‐PCR confirmed COVID‐19 caused by Delta variant ≥21 days post first dose, with pooled vaccine effectiveness of 66% (95% CI: 65%–67%), as well as ≥14 days after the second dose, with pooled vaccine effectiveness of 91% (95% CI: 84%–95%). In conclusion, the mRNA‐1273 vaccine offers a substantial protection rate against RT‐PCR confirmed COVID‐19 caused by the Delta variant upon full vaccination, although with slightly reduced effectiveness relative to other strains of SARS‐CoV‐2.
Keywords: coronavirus, disease control, pathogenesis, respiratory tract, vaccines/vaccine strains, virus classification
Highlights
Administration of mRNA‐1273 vaccine is 66% effective against COVID‐19 caused by Delta variant 21 days or more after the first dose.
Administration of mRNA‐1273 vaccine is 91% effective against COVID‐19 caused by Delta variant 14 days or more after the second dose.
1. INTRODUCTION
Delta variant, also known as B.1.617.2, belongs to a viral lineage of SARS‐CoV‐2 first identified in India in December 2020 and has since become the most prevalent SARS‐CoV‐2 variant in several other countries. 1 The lineage has been linked to a resurgence of COVID‐19 cases in many parts of the world, including those with robust vaccination drives. Therefore, there have been concerns that the currently available COVID‐19 vaccines may not adequately protect against COVID‐19 caused by the Delta variant. This paper aims to summarize through meta‐analyses the overall effectiveness of the mRNA‐1273 vaccine against COVID‐19 caused by the Delta variant from large real‐world studies.
2. METHODS
Two investigators (C.S.K. and S.S.H.) independently conducted a systematic literature search in multiple electronic databases, including PubMed, Google Scholar, Scopus, Web of Science, and medRxiv, in October 2021. The search strategy was designed to identify all publications, which reported the effectiveness of the mRNA‐1273 vaccine to prevent reverse transcription‐polymerase chain reaction (RT‐PCR) confirmed COVID‐19 caused by Delta variant of SARS‐CoV‐2 (B.1.617.2). We applied various combinations of Boolean operators for the following keywords during our search: [(SARS‐Cov‐2 OR 2019‐nCOv OR COVID‐19 OR coronavirus) AND (vaccine or vaccination) AND (variant)].
Studies were eligible for inclusion in our systematic review and meta‐analysis if they (1) were observational studies (of any design, e.g., case‐control, cohort, case series); (2) reported the effectiveness of the mRNA‐1273 vaccine to prevent RT‐PCR confirmed COVID‐19 caused by Delta variant of SARS‐CoV‐2 (B.1.617.2); (3) compared vaccine effectiveness between vaccinated and unvaccinated individuals or between pre‐ and postvaccination; and (4) reported adjusted effectiveness estimates. For two or more studies that utilized the same data source for their investigations on vaccine effectiveness, we included the study that performed analysis on the latest data cut‐off date. We excluded studies that utilized surrogate measures of vaccine effectiveness against COVID‐19 caused by Delta variant of SARS‐CoV‐2 by reporting vaccine effectiveness during delta predominance period, studies that reported unadjusted effectiveness estimates, and studies that reported the effectiveness of the vaccine to prevent COVID‐19‐related mortality or COVID‐19‐related hospitalization.
The outcome of interest, namely vaccine effectiveness, was defined as a relative risk reduction in RT‐PCR confirmed COVID‐19 caused by Delta variant in vaccinated individuals (postvaccination) compared with unvaccinated individuals (prevaccination). 2 All relevant information from the eligible studies was extracted and recorded in a predetermined data collection table. Newcastle–Ottawa Scale was used for critical appraisal of the quality of included observational studies. Two investigators (C.S.K. and S.S.H.) independently evaluated the quality of studies with the Newcastle–Ottawa Scale, and a Newcastle–Ottawa Scale of at least 8, indicating high quality. 3 Consensus discussions between the two investigators (C.S.K. and S.S.H.) with involvement of the third investigator (D.S.R) when necessary, were carried out to resolve disagreements on the inclusion of studies, extraction of study characteristics, and quality appraisal.
A random‐effects model was used to estimate the pooled odds ratio (OR) for the occurrence of COVID‐19 caused by Delta variants between vaccinated and unvaccinated individuals at 95% confidence intervals (CIs). We examined the heterogeneity between studies using the I 2 statistics and the χ 2 test, with 50% and p < 0.10, respectively, were considered as an indication of the presence of heterogeneity. The vaccine effectiveness was indicated as (pooled OR − 1)/OR, together with a 95% CI. All analyses were performed using Meta XL, version 5.3 (EpiGear International).
3. RESULTS
Our literature search yielded 4441 records. After deduplication and application of eligibility criteria, 14 relevant articles were shortlisted for inclusion through full‐text examination. Of these, nine studies were excluded since they utilized surrogate measures of vaccine effectiveness against COVID‐19 caused by Delta variant of SARS‐CoV‐2 by reporting vaccine effectiveness during Delta predominance period, reported the effectiveness of vaccines other than vaccines BNT162b2 mRNA vaccine, or reported unadjusted effectiveness estimates (Figure 1). Eventually, five studies were included in this systematic review and meta‐analysis; all included studies were of retrospective case‐control design. 4 , 5 , 6 , 7 , 8 The study characteristics are depicted in Table 1. The included studies are deemed moderate‐to‐high quality with a Newcastle–Ottawa Scale of 8 (Table 1).
Figure 1.
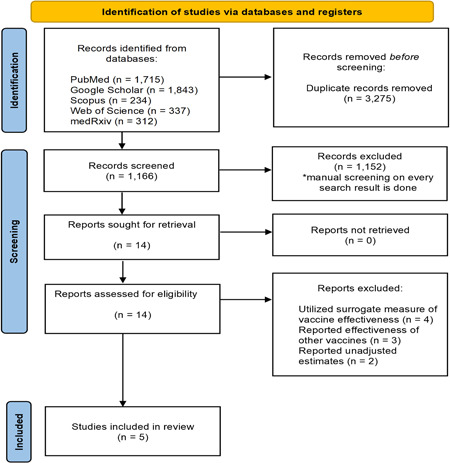
PRISMA flow diagram for study selection
Table 1.
Characteristics of included studies
| First author (year), country | Study design | Sample | Total number of participants | Incidence/frequency of COVID‐19 | NOS | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unvaccinated | ≥14 days after dose 1 | Adjusted estimate | Unvaccinated | ≥21 days after dose 1 | Adjusted estimate | Unvaccinated | ≥7 days after dose 2 | Adjusted estimate | Unvaccinated | ≥14 days after dose 2 | Adjusted estimate | ||||||
| Andrews et al. (2021), England a | Retrospective, test‐negative, case‐control | Individuals aged ≥16 years who had reported symptoms and were tested for SARS‐CoV‐2 within 10 days after symptom onset in England | 5 233 372 | – | – | – | 337 142/806 829 (41.8%) | 21 535/57 509 (37.4%) | OR = 0.34 (0.33–0.35) | – | – | – | 337 142/806 829 (41.8%) | 24 328/124 934 (19.5%) | OR = 0.05 (0.04–0.06) | 8 | |
| Nasreen et al. (2021), Canada b | Retrospective, test‐negative, case‐control | Community‐dwelling Ontarians aged ≥16 years who had symptoms consistent with or a severe outcome attributable to COVID‐19, and who were tested for SARS‐CoV‐2 | 352 531 | – | – | OR = 0.30 (0.24–0.36) | – | – | OR = 0.31 (0.25–0.38) | – | – | OR = 0.05 (0.03–0.09) | – | – | OR = 0.06 (0.03–0.10) | 8 | |
| Tang et al. (2021), Qatar c | Retrospective, test‐negative, case‐control | Resident population of Qatar | 3660 | n = 1629/3218 (50.6%) | n = 11/62 (17.7%) | OR = 0.20 (0.11–0.39) | – | – | – | – | – | – | n = 1644/3175 (34.5%) | n = 22/157 (14.0%) | OR = 0.14 (0.09–0.22) | 8 | |
| Skowronski et al. (2021), Canada d | Retrospective, test‐negative, case‐control | Adults aged 50–69 years in British Columbia, Canada | 68 074 | – | – | – | n = 50/12 244 (0.4%) | n = 2/1322 (0.2%) | OR = 0.27 (0.06–1.14) | – | – | – | – | – | – | 8 | |
| Bruxvoort et al. (2021), USA e | Retrospective, test‐negative, case‐control | Individuals aged 18 years who were tested for SARS‐CoV‐2 in Kaiser Permanente Southern California | 12 162 | – | – | – | – | – | – | – | – | – | 1795/7342 (24.4%) | 232/4820 (20.8%) | OR = 0.13 (0.11–0.16) | 8 | |
Abbreviations: NOS, Newcastle‐Ottawa scale; OR, odds ratio; PCR, polymerase chain reaction.
Adjusted for age, sex, index of multiple deprivations, ethnic group, care home residence status, geographic region, period (calendar week), health and social care worker status, clinical risk group, clinically extremely vulnerable group.
Adjusted for age, sex, public health unit region, period of test, number of SARS‐CoV‐2 tests in the 3 months before December 14, 2020, presence of any comorbidity that increases the risk of severe COVID‐19, receipt of 2019/2020 and/or 2020/2021 influenza vaccination, Census dissemination area‐level quintiles of household income, proportion of persons employed as nonhealth essential workers, persons per dwelling, proportion of self‐identified visible minorities.
Adjusted for age, sex, nationality, reason for PCR testing, and calendar week of COVID‐19 test.
Adjusted for age, sex, epidemiological week, location of health authority.
Adjusted for smoking status, Charlson comorbidity score, frailty index, liver disease, pregnancy, history of COVID‐19 diagnosis, number of outpatient and virtual visits, preventive care, medical center area, month of specimen collection, specimen type.
The meta‐analysis of three studies 4 , 5 , 6 revealed significant protective effect against RT‐PCR confirmed COVID‐19 caused by Delta variant 21 days or more after the first dose of mRNA‐1273 vaccine (pooled OR = 0.34; 95% CI: 0.33–0.35; I 2 = 0%; p = 0.66; Figure 2A), where pooled estimate indicates vaccine effectiveness of 66% (95% CI: 65%–67%). In addition, the meta‐analysis of four studies 4 , 5 , 7 , 8 revealed even higher significant protective effect against RT‐PCR confirmed COVID‐19 caused by Delta variant 14 days or more after the second dose of mRNA‐1273 vaccine (pooled OR = 0.09; 95% CI: 0.05–0.16; I 2 = 94%; p = 0.01; Figure 2B), where pooled estimate indicates vaccine effectiveness of 91% (95% CI: 84%–95%).
Figure 2.
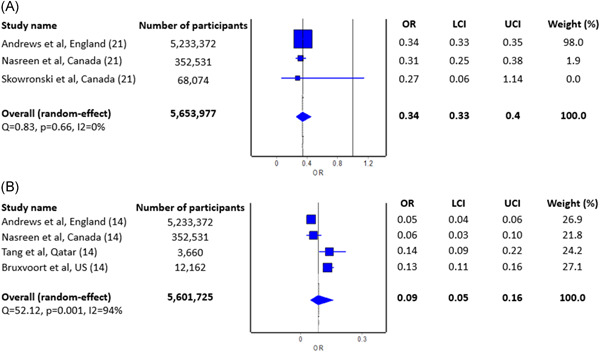
Pooled odds ratio (OR) of the incidence of COVID‐19 caused by Delta variant 21 days after the first dose of vaccine (A) and 14 days after the second dose of vaccine (B) relative to no vaccination
4. DISCUSSION
On the basis of the findings, it appears that the mRNA‐1273 vaccine still offers substantial protection against RT‐PCR confirmed COVID‐19 caused by the Delta variant in the real‐world settings in which partial vaccination (21 days or more after the first dose) reduced the risk of acquisition of COVID‐19 caused by the Delta variant by 66%, while full vaccination (14 days or more after the second dose) reduced the risk of acquisition of COVID‐19 caused by the Delta variant by 91%. Nevertheless, the protection rate was slightly lower than that previously reported in phase 3 randomized controlled trial 9 conducted before the Delta predominance period; 93.2% versus 91% upon full vaccination.
While our systematic review and meta‐analysis were limited by the inclusion of studies of the retrospective design, we believe it is of utmost importance to disseminate our findings at this stage to alleviate the concerns of practitioners surrounding the protection rate of the mRNA‐1273 vaccine amid the delta predominance period. In addition, our findings can offer valuable insights to the policymakers regarding the urgency to administer booster vaccine doses, which may further stretch the global vaccine supply.
In conclusion, the mRNA‐1273 vaccine offers a substantial protection rate against RT‐PCR confirmed COVID‐19 caused by the Delta variant upon full vaccination. Therefore, measures should be taken to hasten the global vaccination efforts, preferably with mRNA vaccines, to curb COVID‐19 transmission, which may drive the future emergence of variants of concern.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Kow CS, Ramachandram DS, Hasan SS. The effectiveness of mRNA‐1273 vaccine against COVID‐19 caused by Delta variant: A systematic review and meta‐analysis. J Med Virol. 2022;94:2269‐2274. 10.1002/jmv.27568
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article.
REFERENCES
- 1. Mlcochova P, Kemp SA, Dhar MS, et al. SARS‐CoV‐2 B.1.617.2 Delta variant replication and immune evasion. Nature. 2021;599:114‐119. 10.1038/s41586-021-03944-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weinberg GA, Szilagyi PG. Vaccine epidemiology: efficacy, effectiveness, and the translational research roadmap. J Infect Dis. 2010;201(11):1607‐1610. [DOI] [PubMed] [Google Scholar]
- 3. Wells G, Shea B, O'Connell D, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Accessed September 28, 2021. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 4. Andrews N, Tessier E, Stowe J, et al. Vaccine effectiveness and duration of protection of Comirnaty, Vaxzevria and Spikevax against mild and severe COVID‐19 in the UK. medRxiv. 2021. [Google Scholar]
- 5. Nasreen S, He S, Chung H, et al. Effectiveness of COVID‐19 vaccines against variants of concern in Ontario, Canada. medRxiv. 2021. [Google Scholar]
- 6. Skowronski DM, Setayeshgar S, Zou M, et al. Comparative single‐dose mRNA and ChAdOx1 vaccine effectiveness against SARS‐CoV‐2, including early variants of concern: a test‐negative design, British Columbia, Canada. medRxiv. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tang P, Hasan MR, Chemaitelly H, et al. BNT162b2 and mRNA‐1273 COVID‐19 vaccine effectiveness against the Delta variant in Qatar. Nat. Med. 2021;27(12):2136‐2143. 10.1038/s41591-021-01583-4 [DOI] [PubMed] [Google Scholar]
- 8. Bruxvoort K, Sy LS, Qian L, et al. Effectiveness of mRNA‐1273 against Delta, mu, and other emerging variants of SARS‐CoV‐2: test negative case‐control study. BMJ. 2021:e068848. 10.1136/bmj-2021-068848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. El Sahly HM, Baden LR, Essink B, et al. Efficacy of the mRNA‐1273 SARS‐CoV‐2 vaccine at completion of blinded phase. N Engl J Med. 2021;385(19):1774‐1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.


