Abstract
Background
We previously reported the efficacy of the adjuvanted-protein COVID-19 vaccine candidate S-Trimer (SCB-2019) in adults who showed no evidence of previous exposure to SARS-CoV-2. In this study, we aimed to investigate the extent of protection afforded by previous exposure to SARS-CoV-2 on subsequent COVID-19 infection, as well as the efficacy, safety, and reactogenicity of SCB-2019 in participants who were enrolled in the Study evaluating Protective-Efficacy and safety of Clover's Trimeric Recombinant protein-based and Adjuvanted COVID-19 vaccine (SPECTRA) trial who had already been exposed to SARS-CoV-2 before vaccination.
Methods
In a phase 2 and 3 multicentre, double-blind, randomised, placebo-controlled trial (SPECTRA) done at 31 sites in five countries, participants were randomly assigned 1:1 using the Cenduit Interactive Response Technology system (IQVIA, Durham, NC, USA), with a block size of six, to receive two doses of either SCB-2019 or placebo 21 days apart. The primary outcomes of the SPECTRA trial were vaccine efficacy, measured by real-time PCR (rtPCR)-confirmed COVID-19 of any severity, with onset from 14 days after the second vaccine dose, as well as the safety and solicited local and systemic adverse events in the phase 2 subset. Here, we present secondary analyses to calculate the protective efficacy due to previous exposure to SARS-CoV-2 against reinfection with COVID-19 according to severity in SPECTRA participants who had evidence of exposure to SARS-CoV-2 at baseline, including efficacy against identified viral variants, as well as efficacy of SCB-2019 vaccination in this population.
Findings
We enrolled 30 174 participants between March 24, 2021, and Aug 10, 2021. In the 14 670 participants who were randomly assigned to receive placebo, there were 418 (2·8%) confirmed cases of COVID-19; 65 (0·9%) of 7339 SARS-CoV-2-exposed participants, and 353 (4·8%) of 7331 SARS-CoV-2-naive participants (attack rates of 5·5 cases per 100 person-years for SARS-CoV-2-exposed participants and 32·4 cases per 100 person-years for SARS-CoV-2-naive participants). Protective efficacy due to previous exposure to SARS-CoV-2 was 83·2% (95% CI 78·0–87·3) against any COVID-19, 92·5% (82·9–97·3) against moderate-to-severe COVID-19, and 100% (59·3–100) against severe COVID-19; no SARS-CoV-2-exposed participants had hospitalisation associated with COVID-19. Protective efficacy against variants were 100% for alpha (B.1.1.7) and lambda (C.37) variants, 88·6% (14·9–99·7) for B.1.623, 93·6% (80·1–98·7) for gamma (P.1), and 92·4% (81·2–97·6) for mu (B.1.621) variants, and lowest against beta (B.1.351; 72·2% [33·1–89·9]) and delta (B.1.617.2; 77·2% [61·3–87·2]) variants. In addition, one dose of SCB-2019 had 49·9% (1·5–75·6) efficacy against any symptomatic COVID-19, and two doses had 64·2% (26·5–83·8) efficacy. SCB-2019 was well tolerated in SARS-CoV-2-exposed participants, but was associated with higher rates of injection site pain (89 [33·8%] of 263 participants) than placebo (16 [6·7%] of 239 participants). Rates of solicited systemic adverse events, severe adverse events, and serious adverse events were similar between vaccine and placebo groups, and with rates in SARS-CoV-2-naive vaccine recipients.
Interpretation
Previous exposure to SARS-CoV-2 decreased the risk and severity of subsequent COVID-19 infection, even against newly emerging variants. Protection is further enhanced by one or two doses of SCB-2019.
Funding
Clover Biopharmaceuticals, The Coalition for Epidemic Preparedness Innovations (CEPI).
Introduction
Despite the availability of several authorised vaccines for SARS-CoV-2, there is still a need for novel vaccines to combat the ongoing global COVID-19 pandemic. As of April 2, 2022, WHO estimated that 64·5% of the global population had received at least one vaccination, with only 14·5% of people in low-income countries having received one dose.1 There is also increasing demand for a third (booster) dose in countries that face a new wave of infections due to newly emerging variants.2 During the pandemic, the SARS-CoV-2 virus evolved such that almost all current cases of COVID-19 are due to infection with these novel variants. The most notable variant of concern throughout most of 2021, the delta (B.1.617.2) variant, has itself been displaced by the omicron (B.1.1.529) variant.3 Most of the authorised vaccines in use, or experimental formulations in development, have focused on the spike protein (S protein) of the SARS-CoV-2 virus,4 which is an essential component for viral cell entry.5 The Clover Biopharmaceuticals vaccine candidate consists of a recombinant SARS-CoV-2 S protein that has been stabilised in the native pre-fusion trimeric conformation using proprietary Trimer-Tag technology. The formulation selected for clinical development, S-Trimer (SCB-2019), contains two adjuvants: the toll-like receptor agonist CpG-1018 and alum.6
Research in context.
Evidence before this study
The global COVID-19 pandemic has resulted in an unprecedented global research effort to investigate effective vaccines for COVID-19 in preclinical and clinical studies, as well as investigations of the consequences of SARS-CoV-2 infections and emerging viral variants. We used an efficacy trial of one such vaccine to assess the effect of previous exposure to SARS-CoV-2 on reinfection. An unrestricted PubMed search of all studies published in English on Jan 8, 2022, with the terms “prior covid-19 infection” AND “reinfection” AND “protection” produced 46 results. We refined the list to human studies, which included some reviews, meta-analyses, and studies on medical records, but there were no controlled clinical trials that had included participants with evidence of previous exposure to SARS-CoV-2 and who were specifically monitored for occurrence of real-time PCR (rtPCR) confirmed COVID-19. One study used a National Electronic Data Surveillance System (for Kentucky state) to monitor individuals who had a previous positive PCR test for SARS-CoV-2 for a re-occurrence of a positive test from 90 days after the first test and found a protective effect of 80·3% (95% CI 78·2–82·2) in people aged 20–59 years, which is a similar result to that observed in our study population.
Added value of this study
The main objective of the SPECTRA study has been reported previously; two doses of the S protein subunit vaccine against SARS-CoV-2 (SCB-2019) had 100% efficacy (95% CI 25·3–100) against severe COVID-19 or hospitalisations due to COVID-19 in people with no evidence of previous exposure to the virus, and a vaccine efficacy of 78·7% (57·3–90·4) against any COVID-19 severity due to the delta variant and 91·8% (44·9–99·8) against any COVID-19 due to the gamma variant. However, almost half of the 31 201 people recruited for the study were not eligible for the per protocol analysis of vaccine efficacy as they showed previous exposure to SARS-CoV-2 at baseline. This provided us with the population for the reported analyses of the protection afforded by such previous exposure against a subsequent reinfection, as well as allowing some measurement of the additional benefits of vaccination for this population. Although we showed that previous exposure to SARS-CoV-2 provides substantial protection, the landscape of SARS-CoV-2 has changed since the first vaccines were assessed, with the emergence of variants such as delta, gamma, and mu, as noted in this study and, subsequently, omicron, which was detected after our monitoring ceased. Our previous report showed efficacy with SCB-2019 against each of these variants, while these new analyses show that previous exposure to SARS-CoV-2 provides variable protection against the new variants, which can be complemented by vaccination with SCB-2019.
Implications of all the available evidence
As the epidemiology of the COVID-19 pandemic changes with rapid emergence of new variants, such as delta and omicron, it is comforting to note that previous infection provides some protection against reinfection, but more importantly, vaccination of those with previous exposure to SARS-CoV-2 provides a further increment of protection with little or no reactogenicity or safety concern. This finding applies only to the SCB-2019 vaccine used in our investigation, and so needs confirmation with other vaccines for primary vaccination, homologous, or heterologous revaccination. If new variants continue to emerge, it will be important to ensure that future vaccination campaigns can be effective and be done safely and with little or no reactogenicity, which is supported by our observations. This might be more important in low-income and middle-income countries, which are currently lagging in their vaccination campaigns and so might be expected to have higher levels of natural infection (rather than naive serology) than high-income countries due to increased vaccination rates in high-income countries. SCB-2019 could be a suitable vaccine candidate for such countries because of potentially simpler logistics associated with the less demanding cold-chain logistics requirements.
Two doses of SCB-2019 administered 3 weeks apart have been shown to elicit a robust viral neutralising antibody response with titres that persist over baseline levels for at least 6 months.6, 7 The neutralising response to SCB-2019 compares well with the responses to the two authorised mRNA COVID-19 (BNT162b2 and mRNA-1273) vaccines in widespread use in high-income countries that have been shown to have clinical efficacies of over 90% against COVID-19 due to the original prototype SARS-CoV-2 virus.8 When assessed in over 30 000 adults in the phase 2 and 3 SPECTRA trial,9 the efficacy of SCB-2019 against COVID-19 of any severity 2 weeks after the second dose was 67·2% (95·72% CI 54·3–76·8) in initially SARS-CoV-2 naive adults, 83·7% (97·86% CI 55·9–95·4) against moderate-to-severe COVID-19, and 100% (95·72% CI 25·3–100) against severe COVID-19. There were no deaths or COVID-19-associated hospitalisations in the vaccine group. The study, which was conducted in five countries (Belgium, Brazil, Colombia, the Philippines, and South Africa), was complicated by its timing because it occurred when about half the enrolled participants were found to have a history of previous COVID-19 or serologic evidence of SARS-CoV-2 exposure. Although baseline seropositivity was not an exclusion criterion, individuals who were exposed to SARS-CoV-2 were excluded from the per protocol efficacy analysis, which was only estimated in participants who were seronegative at baseline with no history of COVID-19. Therefore, because of the importance of assessing the impact of COVID-19 vaccines in those who have had previous experience of SARS-CoV-2 infection, we aimed to investigate the extent of protection afforded by previous exposure to SARS-CoV-2 on subsequent COVID-19 infection, as well as the efficacy, safety, and reactogenicity of SCB-2019 in participants who were enrolled in the SPECTRA trial who had already been exposed to SARS-CoV-2 before vaccination.
Methods
Study design
This randomised, double-blinded, placebo-controlled, phase 2 and 3 trial was conducted at 31 sites in five countries (Belgium, Brazil, Colombia, The Philippines and South Africa). Data cutoff was defined in the protocol as the time when a sufficient number of people (150 people) had been detected for the per-protocol analysis. The study protocol was approved by the regulatory authorities and institutional ethics committees in the participating countries, and the study design was discussed with regulatory authorities in Brazil, China, Europe, the Philippines, and the UK. The trial was registered on EudraCT (2020-004272-17) and ClinicalTrials.gov (NCT04672395), and was done in accordance with the guidelines of the Declaration of Helsinki and the International Council for Harmonisation and Good Clinical Practice.
Participants
Eligible participants were adults aged 18 years or older who were in good health or had a stable chronic health condition. Data from a cohort of adolescents aged 12–17 years, which was added after a protocol amendment during the study, is still ongoing and is not presented here. The main patient exclusion criteria were pregnancy, any ongoing immunosuppressive therapy, a history of anaphylaxis to any vaccine component, or a previous receipt of any other COVID-19 vaccine. The inclusion of individuals with a previous history of COVID-19 was allowed unless it had occurred within the 14 days before recruitment. Detailed inclusion or exclusion criteria are provided in the appendix (pp 2–3). All participants supplied written informed consent at enrolment.
Randomisation and masking
Following screening, participants were enrolled and randomly allocated 1:1 to receive two doses of either SCB-2019 or saline placebo 21 days apart using the Cenduit Interactive Response Technology system (IQVIA, Durham, NC, USA) with a block size of six, as previously described.9 Randomisation schedules were stratified by study site, age (<65 years and ≥65 years), absence or presence of comorbidities that are associated with a high risk of severe COVID-19 (eg, cancer, chronic kidney disease, chronic obstructive pulmonary disease, obesity, and type 2 diabetes), and known history of COVID-19. Blocks were dynamically assigned to each site for each stratum from a central block pool on the first participant enrolment into the stratum. Subsequent participants enrolled into the site strata were allocated to the next available treatment group in the randomisation block. The randomisation lists were created by an unmasked statistician who had no further role in the endpoint analyses. These lists then informed the investigators of the assignation of each participant as they were enrolled via the interactive online system.
Procedures
For this study, the investigational vaccine was supplied in three separate containers that contained 720 μg of SCB-2019 in a 1·0 mL pre-filled syringe, CpG-1018 (Dynavax Technologies) in a 2·0 ml vial containing 12 mg/mL of a 22-mer phosphorothioate oligodeoxynucleotide in Tris buffered saline (24 mg per vial), and vials of 10 mg/mL aluminium hydroxide (Alhydrogel, Croda Health Care). The placebo was 0·9% sodium chloride for injection, which was supplied in 10 mL ampoules from local manufacturers. SCB-2019 and CpG-1018 were stored at temperatures between 2°C and 8°C before use. The final vaccine formulation contains 30 μg SCB-2019 with 1·5 mg CpG-1018 and 0·75 mg alum in each 0·5 mL dose. Two doses of either SCB-2019 or placebo were given 21 days apart to participants. The vaccines were prepared within 8 h of use, according to the pharmacy manual by trained unmasked vaccine administrators who administered the vaccines by intramuscular injection in the upper deltoid of the non-dominant arm. Other than the vaccine administrators, who had no further part in the study after giving the injections, all study staff and administrators were masked to the study material that had been administered.
Outcomes
The co-primary per protocol outcomes of the SPECTRA study have already been reported;9 in the embedded phase 2 study, the per protocol outcome was the reactogenicity of SCB-2019 in all vaccine recipients compared with placebo, and in the phase 3 study the per protocol outcome was the efficacy of SCB-2019 against COVID-19 in participants with no previous exposure to SARS-CoV-2. As almost half of the enrolled population presented evidence of previous exposure to SARS-CoV-2 and were excluded from these analyses, this provided a cohort in which we were able to do secondary analyses of vaccine efficacy in this population, as well as to conduct an exploratory assessment of the impact of previous exposure of SARS-CoV-2 on subsequent infection. We also assessed the safety and reactogenicity of SCB-2019 in participants with evidence of pre-exposure to SARS-CoV-2.
Participants were monitored for 30 min after each injection for immediate reactions. An embedded phase 2 study included the first 1600 participants enrolled; 800 participants in each the vaccine and placebo groups to assess reactogenicity. Participants in the phase 2 cohort completed electronic diaries (ePro, Signant Health, London, UK), which collected solicited local reactions and systemic adverse events for 7 days after each injection. Any unsolicited adverse events were reported up to day 43. All participants who received at least one dose of vaccine or placebo were included in the safety set and were required to report to their study centre whether they had any serious adverse event, any adverse event of special interest, or any medically attended adverse event at any time during the study. Participants were reminded of these commitments during telephone contacts after vaccination (telephone contacts occurred weekly, up to day 43).
Statistical analysis
We have previously reported the primary objective of the study, namely vaccine efficacy in the per-protocol population, which consisted of participants without evidence of previous SARS-CoV-2 infection.9 The secondary endpoints reported here include vaccine efficacy with 95% CIs (calculated by the Newcombe method) against COVID-19 of any severity, moderate-to-severe COVID-19, severe COVID-19, and COVID-19-associated hospitalisation, as defined in the appendix (pp 4–5), and COVID-19 associated hospitalisation in participants with evidence of previous SARS-CoV-2 infection. We also did a post-hoc estimation of protective efficacy due to previous exposure to SARS-CoV-2 against COVID-19 of any severity, moderate-to-severe COVID-19, severe COVID-19, COVID-19-associated hospitalisation, and against any severity COVID-19 according to identified virus lineage. Previous exposure was evidenced by having a medical history of COVID-19 or being seropositive for SARS-CoV-2 receptor-binding domain of the S protein at baseline. Vaccine efficacy was calculated as 100 × (1 – incidence rate ratio).12 The incidence rate was the number of participants with any real-time PCR (rtPCR)-confirmed COVID-19 of any severity, divided by cumulative follow-up person time in all participants at risk. For secondary endpoints, against moderate-to-severe and severe COVID-19, the predefined criterion for vaccine efficacy was if the lower limit of adjusted CI for vaccine efficacy was greater than 0% if the primary endpoint had been met, in accordance with the US Food and Drug Administration (FDA) recommendations.12 The protective efficacy provided by previous exposure to SARS-CoV-2, evidence by medical history of COVID-19 or seropositivity for SARS-CoV-2 at baseline, was calculated post hoc in the same manner as vaccine efficacy. The number needed to vaccinate to prevent one more case of COVID-19 was the reciprocal of the absolute risk reduction, which was the difference between attack rates. The 95% CI for the number needed to vaccinate was derived from the CI for the absolute risk reduction, which was calculated by Newcombe's method. Safety and reactogenicity data are presented according to protocol as percentages of each study group with any adverse event and specific solicited adverse events, presented according to the highest severity. Statistical analyses were done using SAS version 9.4.
Role of the funding source
Authors who are employees of the sponsor (IS, HHH, PL, CB, and CV) and scientific advisors for the study (RC, SACC, DA, PR, GS, and FR) participated in design and development of the protocol, as well as data analysis and interpretation. The Coalition for Epidemic Preparedness Innovations (CEPI) reviewed the protocol. IS, HHH, PL, FR, and RC worked with a medical writer, who was financed by Clover Biopharmaceuticals, to prepare a first draft of the manuscript. This manuscript was then reviewed and revised by all authors to create the final draft.
Results
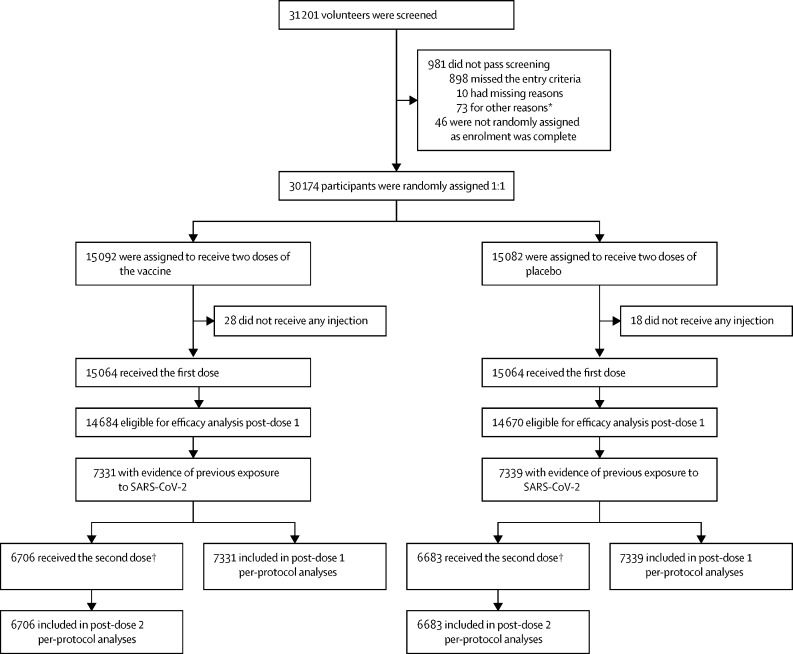
After screening 31 201 volunteers, a total of 30 174 adult participants were enrolled into the study between March 24, 2021, and July 19, 2021, and randomly assigned 1:1 to receive SCB-2019 vaccine (n=15 092) or placebo (n=15 082; figure 1 ). Of these participants, 14 757 (48·9%) were SARS-CoV-2 exposed at baseline, including 1602 (5·3%) with a previous medical history of COVID-19. After excluding 65 participants who had either developed COVID-19, received other COVID-19 vaccines, or who had been unmasked within 14 days after the first dose, 14 692 (99·6%) participants with evidence of exposure to SARS-CoV-2 at baseline were included in the full analysis set, 7353 participants were included in the vaccine group, and 7339 participants were included in the placebo groups (figure 1). The number of participants who had been exposed to SARS-CoV-2 at baseline varied between countries: 86 (12·6%) of 683 participants in Belgium, 2383 (30·6%) of 7776 participants in Brazil, 3050 (47·4%) of 6438 participants in Colombia, 8675 (64·9%) of 13 376 participants in the Philippines, and 498 (46·1%) of 1081 participants in South Africa. Demographics of these participants were balanced across the two groups with regard to sex, age, race, and presence of comorbidities, which increased the risk of severe COVID-19. These groups were also similar to participants in the SARS-CoV-2-naive group (who received placebo), except for the racial composition. This difference was because of the higher number of SARS-CoV-2-naive participants recruited from Belgium and Brazil than the Philippines (table 1 ).
Figure 1.
Study flow chart for participants with evidence of previous exposure to SARS-CoV-2 at baseline who were included in the secondary analyses
*Other reasons included administrative reasons, contraception requirements being unacceptable, personal medical reasons, withdrawal by volunteers before receiving dose one, etc. †Includes only participants who had received their second dose at the time of data cutoff.
Table 1.
Demographics SARS-CoV-2-exposed participants at baseline and SARS-CoV-2-naive placebo recipients in the post-dose 1 full analysis set
|
SARS-CoV-2-exposed individuals* |
SARS-CoV-2-naive individuals | ||
|---|---|---|---|
| SCB-2019 (n=7353) | Placebo (n=7339) | Placebo (n=7331) | |
| Sex | |||
| Male | 3775 (51·3%) | 3763 (51·3%) | 4059 (55·4%) |
| Female | 3578 (48·7%) | 3576 (48·7%) | 3272 (44·6%) |
| Age group | |||
| 18–64 years | 7224 (98·2%) | 7209 (98·2%) | 7255 (99·0%) |
| 65–74 years | 113 (1·5%) | 116 (1·6%) | 67 (<1%) |
| ≥75 years | 16 (<1%) | 14 (<1%) | 9 (<1%) |
| Mean age ± SD | 32·6±11·6 | 32·4±11·5 | 31·5±10·8 |
| High risk of severe COVID-19† | |||
| Yes | 1386 (18·8%) | 1366 (18·6%) | 1243 (17·0%) |
| No | 5967 (81·2%) | 5973 (81·4%) | 6088 (83·0%) |
| Race | |||
| American Indian† or Alaskan native | 1488 (20·2%) | 1509 (20·6%) | 1631 (22·2%) |
| Asian | 4316 (58·7%) | 4361 (59·4%) | 2359 (32·2%) |
| Black or African American | 666 (9·1%) | 594 (8·1%) | 840 (11·5%) |
| White | 735 (10·0%) | 742 (10·1%) | 2256 (30·8%) |
| Other | 32 (<1%) | 27 (<1%) | 56 (<1%) |
| Unknown or not reported | 114 (1·6%) | 106 (1·4%) | 186 (2·5%) |
| Baseline SARS-CoV-2 status | |||
| Negative | 56 (<1%) | 64 (<1%) | 7331 (100%) |
| Positive | 7297 (99·2%) | 7275 (99·1%) | 0 |
| Known history of COVID-19 at baseline | |||
| No | 6561 (89·2%) | 6554 (89·3%) | 7331 (100%) |
| Yes | 789 (10·7%) | 784 (10·7%) | 0 |
| Missing | 3 (<1%) | 1 (<1%) | 0 |
| Country | |||
| Belgium (three sites) | 47 (<1%) | 39 (<1%) | 303 (4·1%) |
| Brazil (five sites) | 1214 (16·5%) | 1169 (15·9%) | 2712 (37·0%) |
| Colombia (nine sites) | 1516 (20·6%) | 1534 (20·9%) | 1682 (22·9%) |
| The Philippines (ten sites) | 4314 (58·7%) | 4361 (59·4%) | 2334 (31·8%) |
| South Africa (four sites) | 262 (3·6%) | 236 (3·2%) | 300 (4·1%) |
All participants were either seropositive at baseline or had a medical history of COVID-19.
Risk due to presence of known comorbidities.
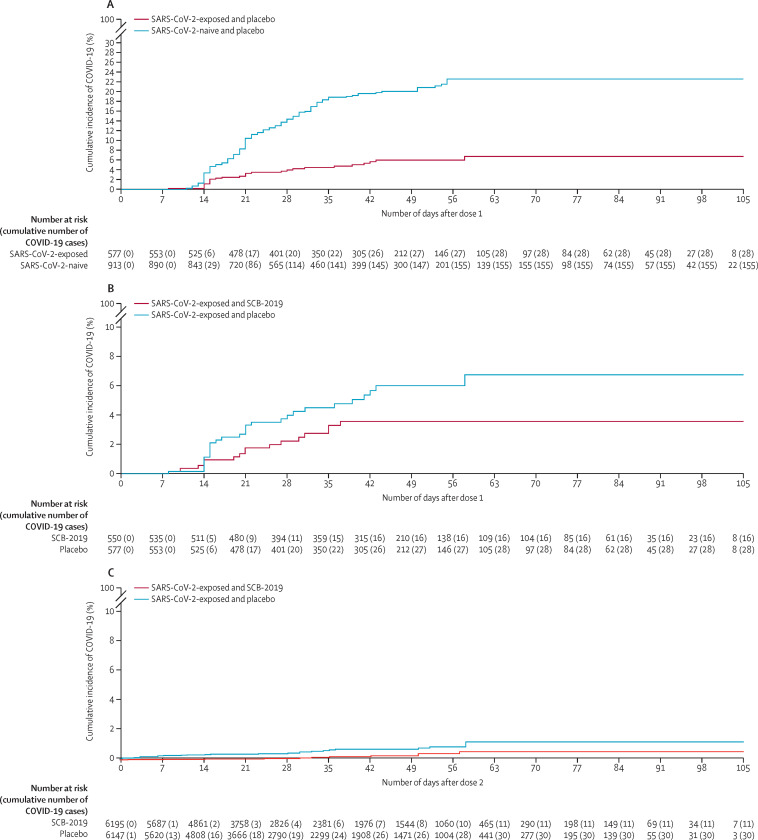
To assess the protection afforded by previous exposure to SARS-CoV-2, we compared COVID-19 attack rates in SARS-CoV-2 naive patients (n=7331) and SARS-CoV-2 exposed eligible placebo recipients (n=7339) (figure 2 ). 418 COVID-19 cases were confirmed by rtPCR in all placebo recipients; 65 (0·9%) in the 7339 participants who had evidence of previous exposure to SARS-CoV-2 for an attack rate of 5·5 cases per 100 person-years, and 353 (4·8%) cases in 7331 baseline SARS-CoV-2-naive participants with an attack rate of 32·4 cases per 100 person-years (table 2 ). These data equated to previous exposure having a protective efficacy of 83·2% (95% CI 78·0–87·3) against any severity of COVID-19. Further, previous exposure provided greater protection against moderate-to-severe disease (92·5% [82·9–97·3]) and severe disease (100% [59·3–100]); there were no cases of severe disease or COVID-19-associated hospitalisation reported in participants who had evidence of previous exposure.
Figure 2.
Kaplan-Meier plots of any severity of rtPCR-confirmed COVID-19
Protection afforded by previous exposure or vaccine in participants who had evidence of previous exposure to SARS-CoV-2. Protective efficacy due to previous exposure after the first dose of placebo in participants with and without evidence of previous exposure to SARS-CoV-2 (A); the additional efficacy of one dose of vaccine in participants who had evidence of previous exposure to SARS-CoV-2 (B); the additional efficacy of two doses of vaccine in participants who had evidence of previous exposure to SARS-CoV-2 (C).
Table 2.
Protective efficacy due to natural exposure to SARS-CoV-2
|
SARS-CoV-2-naive individuals (n=7331) |
SARS-CoV-2-exposed individuals (n=7339) |
Protective efficacy (95% CI)* | ||||
|---|---|---|---|---|---|---|
| Cumulative follow-up in person-years† | Number of patients with an event | Cumulative follow-up in person-years† | Number of patients with an event | |||
| Any severity rtPCR-confirmed COVID-19‡ | 1088·9 | 353 | 1192·5 | 65 | 83·2% (78·0 to 87·3) | |
| Moderate-to-severe rtPCR-confirmed COVID-19 | 1088·9 | 73 | 1192·5 | 6 | 92·5% (82·9 to 97·3) | |
| Severe rtPCR-confirmed COVID-19‡ | 1088·9 | 10 | 1192·5 | 0 | 100% (59·3 to 100) | |
| Protective efficacy endpoints against rtPCR-confirmed COVID-19 due to specific identified variants | ||||||
| Alpha variant (B.1.1.7) | 1088·9 | 19 | 1192·5 | 0 | 100% (80·4 to 100) | |
| Beta variant (B.1.351, B.135.2, B.1.351.3) | 1088·9 | 23 | 1192·5 | 7 | 72·2% (33·1 to 89·9) | |
| Delta variant (B.1.617.2) | 1088·9 | 72 | 1192·5 | 18 | 77·2% (61·3 to 87·2) | |
| Gamma variant (P.1; P.1.1; P.1.2) | 1088·9 | 43 | 1192·5 | 3 | 93·6% (80·1 to 98·7) | |
| Mu variant (B.1.621) | 1088·9 | 60 | 1192·5 | 5 | 92·4% (81·2 to 97·6) | |
| Lambda variant (C.37) | 1088·9 | 7 | 1192·5 | 0 | 100% (36·7 to 100) | |
| B.1.623 variant | 1088·9 | 8 | 1192·5 | 1 | 88·6% (14·9 to 99·7) | |
| Other | 1088·9 | 121 | 1192·5 | 31 | 76·6% (65·1 to 84·8) | |
95% CI for protective efficacy was calculated using the Clopper-Pearson method, which was based on conditional binomial distribution.
Cumulative follow-up was calculated for all participants at risk within each group using the time period from 1 day after the first dose to analysis cutoff on Aug 10, 2021.
Protective efficacy due to natural immunity in placebo recipients with evidence of previous exposure to SARS-CoV-2.
When data for the infective virus was available, the lineage was identified in 266 (63·6%) of the 418 cases. Numerically, the most important of variants identified were the beta (30 [11·3%] of 266 cases identified), delta (90 cases [33·8%]), mu (65 cases [24·4%]), and gamma (46 cases [17·3%]) variants; table 2). Protective efficacy due to previous exposure to SARS-CoV-2 against individual variants was 100% against alpha and lambda variants, 88·6% (14·9–99·7) against B.1.623, 93·6% (80·1–98·7) against gamma (P.1), and 92·4% (81·2–97·6) against mu (B.1.621) variants, and was lowest against the beta (72·2% [33·1–89·9]) and delta (77·2% [61·3–87·2]) variants.
Information about the strain of previous exposure was not available. However, the strain distribution in a country before the start of the study might provide some approximation. In Belgium, the dominant variant both before recruitment in the study and during the study was alpha, exposure to which provided high protection against subsequent infection with the homologous strain (100% [95% CI –1032 to 100]). In South Africa, exposure to beta, the dominant variant before recruitment, provided 94·3% (78·0 to 99·3) protection against the heterologous delta variant, which predominated during the study period. A similar situation was observed in Brazil, where the gamma variant predominated before the study, and previous exposure to SARS-CoV-2 provided 87·2% (60·4 to 97·4) protection against subsequent COVID-19 episodes, mostly due to the gamma variant. In Colombia, where the alpha, gamma, and lambda variants were co-circulating before the study and the mu variant was emerging, protection was 78·8% (66·5 to 87·2) against heterogeneous SARS-CoV-2 lineages. In the Philippines, the alpha lineage, which predominated before the study, was rapidly replaced by the beta, theta, and delta variants, but previous infection still provided 83·5% (76·1 to 88·8) protection against a subsequent episode (table 3 ).
Table 3.
Protective efficacy against any severity of COVID-19 due to previous SARS-CoV-2 exposure in placebo recipients, by country
|
Predominant variants |
SARS-CoV-2-naive patients |
SARS-CoV-2-exposed patients |
Protective efficacy (95% CI)* | ||||
|---|---|---|---|---|---|---|---|
| Before the study† | During the study | Cumulative follow-up in person-years‡ | Number of individuals with an event | Cumulative follow-up in person-years‡ | Number of individuals with an event | ||
| Belgium | Alpha | Alpha | 65·0 | 4 | 8·7 | 0 | 100% (−1032 to 100) |
| Brazil | Gamma | Gamma (57%); delta (13%) | 290·7 | 53 | 128·1 | 3 | 87·2% (60·4 to 97·4) |
| Colombia | Alpha, gamma, mu, lambda | Mu (49%); gamma (10%); B.1.623 (7%); lambda (6%) | 199·5 | 123 | 168·4 | 22 | 78·8% (66·5 to 87·2) |
| Philippines | Alpha | Delta 29%); beta (15%); alpha (14%); theta (3%) | 486·8 | 132 | 847·3 | 38 | 83·5% (76·1 to 88·8) |
| South Africa | Beta | Delta (61%); beta (7%) | 46·9 | 41 | 40·0 | 2 | 94·3% (78·0 to 99·3) |
| Overall | .. | .. | 1088·9 | 353 | 1192·5 | 65 | 83·2% (78·0 to 87·3) |
95% CI for protective efficacy was calculated using Clopper-Pearson method, which was based on conditional binomial distribution.
Sourced from Nextstrain and the Philippines Genome center .
Cumulative follow-up was calculated in all participants at risk within each group using the time period from 14 days after the first dose to analysis cutoff on Aug 10, 2021.
On top of the protection afforded by previous exposure to SARS-CoV-2, vaccination with SCB-2019 also conferred some protection. There were 6195 SARS-CoV-2-exposed recipients who received two doses of vaccine, as specified in the protocol, and 381 who received only one dose of vaccine. The effect of vaccination in pre-exposed individuals was calculated by comparing attack rates in vaccine recipients who had previous exposure to SARS-CoV-2 (14 [3·3%] of 420 cases per 100 person-years for one dose, and 11 [2·0%] of 551 cases per 100 person-years for two doses) with the attack rate in placebo recipients who had previous exposure to SARS-CoV-2 (6·6 cases per 100 person-years for one dose, and 5·6 cases per 100 person-years for two doses). After a mean follow-up of 84 days, a single vaccine dose had an efficacy of 49·9% (95% CI 1·5–75·6) against any severity of COVID-19 (table 4 ), whereas two doses according to the schedule (ie, 21 days apart), with mean follow-up of 54·5 days, had an efficacy of 64·2% (26·5–83·8; table 5 ). The cumulative effect of vaccinating participants with evidence of previous exposure to SARS-CoV-2 was 89·7% (82·5–94·4) after one dose, and 93·8% (88·9–97·0) if two doses were administered.
Table 4.
Vaccine efficacy of one dose of SCB-2019 in participants with evidence of previous exposure to SARS-CoV-2.
|
SCB-2019 (n=7353) |
Placebo (n=7339) |
Vaccine efficacy (95% CI)* | |||||
|---|---|---|---|---|---|---|---|
| Number of individuals at risk | Cumulative follow-up in person-years† | Number of individuals with event | Number of individuals at risk | Cumulative follow-up in person-years† | Number of individuals with event | ||
| Any severity rtPCR-confirmed COVID-19 | 7325 | 419·9 | 14 | 7305 | 421·1 | 28 | 49·9% (1·5 to 75·6) |
| Moderate-to-severe rtPCR-confirmed COVID-19 | 7325 | 419·9 | 3 | 7305 | 421·1 | 3 | −0·3% (−649·0 to 86·6) |
| Severe rtPCR-confirmed COVID-19 | 7325 | 419·9 | 0 | 7305 | 421·1 | 0 | − |
Vaccine efficacy endpoints (from 14 days after the first dose up to second dose) of the cut-off data for efficacy analysis. Participants included in the analysis population were considered at risk only if they were followed ≥14 days after the first or second dose in the corresponding vaccine efficacy analysis period.
95% CI for protective efficacy was calculated using the Clopper-Pearson method, which was based on conditional binomial distribution.
Cumulative follow-up was calculated for all participants at risk within each group using the time period from 1 day after the first dose to analysis cutoff on Aug 10, 2021.
Table 5.
Vaccine efficacy of two doses of SCB-2019 in participants with evidence of previous exposure to SARS-CoV-2 from 14 days post-dose 2
|
SCB-2019 (n=6706) |
Placebo (n=6683) |
Vaccine efficacy (95% CI)* | |||||
|---|---|---|---|---|---|---|---|
| Number of individuals at risk | Cumulative follow-up in person-years† | Number of individuals with event | Number of individuals at risk | Cumulative follow-up in person-years† | Number of individuals with event | ||
| Any severity rtPCR-confirmed COVID-19 | 6195 | 551·0 | 11 | 6147 | 537·8 | 30 | 64·2% (26·5 to 83·8) |
| Moderate-to-severe rtPCR-confirmed COVID-19 | 6195 | 551·0 | 1 | 6147 | 537·8 | 3 | 67·5% (−305 to 99·4) |
| Severe rtPCR-confirmed COVID-19 | 6195 | 551·0 | 0 | 6147 | 537·8 | 0 | − |
Vaccine efficacy endpoints (from 14 days after the first dose up to second dose) of the cutoff data for efficacy analysis. Participants included in the analysis population were considered at risk only if they were followed ≥14 days after the first or second dose in the corresponding vaccine efficacy analysis period.
95% CI for protective efficacy was calculated using the Clopper-Pearson method, which was based on conditional binomial distribution.
Cumulative follow-up was calculated for all participants at risk within each group using the time period from 1 day after the first dose to analysis cutoff on Aug 10, 2021.
The number needed to vaccinate to prevent one case of COVID-19 for a single dose was 30 (95% CI 15·2–556), and the number needed to vaccinate for two doses was 28 (16·3–84). This vaccine effect was most prominent against the delta variant, with efficacy of 67·1% (13·3–89·3) for one dose and 79·1% (25·1–96·1) after two doses. The number needed to vaccinate to prevent one case of the delta variant after two doses was 53 (26–500).
The embedded phase 2 study included 502 participants who were pre-exposed to SARS-CoV-2 at baseline; 263 vaccine recipients and 239 placebo recipients. Rates of solicited local reactions and systemic adverse events reported by these two groups are shown in table 6 . There were proportionally more local reactions in the vaccine group than placebo group: 91 (34·6%) of 263 participants had local reactions after the first dose in the vaccine group compared with 18 (7·5%) of 239 participants in the placebo group, and 43 (17·7%) of 243 participants had local reactions after the second dose in the vaccine group compared with 19 (8·7%) of 218 participants in the placebo group. As shown in the appendix (p 7), this increase was due to more reports of mild-to-moderate local injection site pain following vaccine administration than was reported in the placebo group, other solicited local reactions being rare or absent. Solicited systemic adverse events were reported at similar rates in vaccine and placebo groups after the first dose (74 [28·1%] of 263 participants in the vaccine group vs 71 [29·7%] of 239 participants in the placebo group) and second dose (35 [14·4%] of 243 participants in the vaccine group vs 38 [17·4%] of 218 participants in the placebo group) doses. The most frequent systemic adverse events were fatigue, headache, and myalgia in both groups (appendix p 7). Severe adverse events were infrequent and occurred in both groups. All solicited adverse events were transient and resolved within 2–3 days. The frequency and severity of solicited adverse events in participants who were previously exposed to SARS-CoV-2 were similar with those observed in SARS-CoV-2-naive participants who received SCB-2019 (table 6).
Table 6.
Reactogenicity in the phase 2 study and safety in the phase 3 safety set
|
SARS-CoV-2-exposed participants |
SARS-CoV-2-naive participants | |||
|---|---|---|---|---|
| SCB-2019+CpG and alum | Placebo | SCB-2019+CpG and alum | ||
| Phase 2 safety set | ||||
| First dose | ||||
| Any solicited local adverse event | 91 (34·6% [28·9–40·7]) of 263 participants | 18 (7·5% [4·5–11·6]) of 239 participants | 186 (38·4% [34·1–42·9]) of 484 participants | |
| Any solicited systemic adverse event | 74 (28·1% [22·8–34·0]) of 263 participants | 71 (29·7% [24·0–35·9]) of 239 participants | 203 (41·9% [37·5–46·5]) of 484 participants | |
| Second dose | ||||
| Any solicited local adverse event | 43 (17·7% [24·8–31·6]) of 243 participants | 19 (8·7% [5·30–13·3]) of 218 participants | 144 (35·5% [30·8–40·3]) of 406 participants | |
| Any solicited systemic adverse event | 35 (14·4% [10·2–19·5]) of 243 participants | 38 (17·4% [12·6–23·1]) of 218 participants | 124 (30·5% [26·1–35·3]) of 406 participants | |
| Phase 3 safety set | ||||
| Adverse events after any dose | ||||
| Unsolicited adverse event | 1039 events; 731 (9·9% [9·2–10·6]) of 7378 participants | 1066 events; 737 (10·0% [9·3–10·7]) of 7379 participants | 1676 events; 1101 (14·8% [14·0–15·7]) of 7439 participants | |
| Related | 416 events; 279 (3·8% [3·4–4·2]) of 7378 participants | 271 events; 204 (2·8% [2·4–3·2]) of 7379 participants | 600 events; 404 (5·4% [4·9–6·0]) of 7439 participants | |
| Severe adverse event | 19 events; 13 (<1% [0·1–0·3]) of 7378 participants | 19 events; 14 (<1% [0·1–0·3]) of 7379 participants | 28 events; 21 (<1% [0·2–0·4]) of 7439 participants | |
| Serious adverse event | ||||
| Any | 30 events; 24 (<1% [0·2–0·5]) of 7378 participants | 26 events; 15 (<1% [0·1–0·3]) of 7379 participants | 31 events; 25 (<1% [0·2–0·5]) of 7439 participants | |
| Related | 3 events; 3 (<1% [0·0–0·1]) of 7378 participants | 0 events; 0 (<1% [0·0–0·1]) of 7379 participants | 1 events; 1 (<1% [0·0–0·1]) of 7439 participants | |
| Medically attended adverse event | 422 events; 320 (4·3% [3·9–4·8]) of 7378 participants | 485 events; 350 (4·7% [4·3–5·3]) of 7379 participants | 451 events; 331 (4·5% [4·0–5·0]) of 7439 participants | |
| Adverse event of special interest | 22 events; 19 (<1% [0·2–0·4]) of 7378 participants | 35 events; 24 (<1% [0·2–0·5]) of 7379 participants | 260 events; 164 (2·2% [1·9–2·6]) of 7439 participants | |
| Adverse event leading to early withdrawal | 2 events; 2 (<1% [0·0–0·1]) of 7378 participants | 7 events; 5 (<1% [0·0–0·2]) of 7379 participants | 3 events; 3 (<1% [0·0–0·1]) of 7439 participants | |
| Death | 7 events; 2 (<1% [0·0–0·1]) of 7378 participants | 5 events; 3 (<1% [0·0–0·1]) of 7379 participants | 1 events; 1 (<1% [0·0–0·1]) of 7439 participants | |
In participants who were previously exposed to SARS-CoV-2, rates of unsolicited adverse events and medically-attended adverse events were similar to those observed in the vaccinated SARS-CoV-2-naive cohort. Adverse events of special interest were observed at a higher rate in the SARS-CoV-2-naive cohort (164 [2·2%] of 7439 participants) than in participants who were previously exposed to SARS-CoV-2 (19 [0·3%] of 7378 participants) due to the reporting of anosmia and ageusia in the SARS-CoV-2-naive group. In the pre-exposed cohort, vaccine recipients reported slightly more unsolicited adverse events than placebo recipients, 416 events reported by 279 (3·8%) of 7378 participants versus 271 events reported by 204 (2·8%) of 7379 placebo recipients. Of these, only 19 events in each group were considered to be related to the study injections (table 5). Serious adverse events were reported by 24 vaccine recipients and 15 placebo recipients, of which three events were considered to be related, all of which were in vaccine recipients. Two serious adverse events were hypersensitivity reactions after vaccination; one after the first dose and one 3 days after the second vaccination, both of which resolved the day after the occurrence. The third serious adverse event was a spontaneous abortion 31 days after the first vaccination, pregnancy being noted at the study visit on day 29 for the second vaccination, which was not administered.
Discussion
The availability of data from 14 757 (49%) participants who had been pre-exposed to SARS-CoV-2 from the SPECTRA study population allowed us to make the current analysis and show that previous exposure to SARS-CoV-2 affords 83·2% (95% CI 78·0–87·3) protection against any severity of rtPCR-confirmed COVID-19 and 100% protection against severe illness due to COVID-19 or COVID-19-associated-hospitalisation. On top of this protection, there were further incremental effects of efficacy from one dose (49·9% [1·5–75·6]) or two doses (64·2% [26·5–83·8]) of SCB-2019 vaccine. The protection due to previous exposure to SARS-CoV-2 varied in the different countries in which it was assessed, from 78·8% in Colombia to 100% in Belgium, although there were only few cases in Belgium. Of more significance for public health is the observation that protection due to previous exposure was more than 72% against all variants detected, notably the major variants of concern (at the time of the study): beta, delta, gamma, and mu variants. Doing the study in different countries allowed us to assess this efficacy against different epidemiologic backgrounds of infection. Protective efficacy in Colombia, where the mu variant first emerged and now predominates, was 78·8%, whereas in South Africa it was 94·3% against a background of the delta variant, which was the predominant variant, and in Brazil it was 87·2% against the gamma variant, which was the predominant variant.
The per-protocol analysis of vaccine efficacy in the SPECTRA study, which involved over 30 000 adult participants, was done according to the US FDA recommendations (ie, in those who were initially seronegative for SARS-CoV-2 and with no medical history of COVID-19).12 As the study was done after the peak of the first wave of COVID-19, approximately half of the volunteers had to be excluded because they displayed evidence of previous exposure to SARS-CoV-2. However, the composition of our study cohort reflects an updated reality during 2021–22, in which a substantial proportion of the global population has already been exposed to SARS-CoV-2. This study provides evidence that there is value in vaccinating people who have had previous exposure to SARS-CoV-2; a result that could become more important as novel variants of concern emerge, such as the omicron (B.1.1.529) variant, which has rapidly spread across the globe to become the predominant strain,13, 14 and which data from South Africa indicate is more likely to be responsible for reinfection than earlier variants.15 However, data from South Africa and the UK suggest that such reinfections are likely to be associated with lower rates of severe disease than earlier variants.16, 17
Several reports have indicated waning vaccine efficacy occurring several months after the completion of the original primary two dose vaccine schedules, which is exacerbated by the emergence of novel variants.17, 18, 19, 20 This finding might be associated with waning of vaccine-induced neutralising antibodies21 and lower cross-reactivity against the new variants.22 Other studies have shown that natural immunity due to previous infection might be more effective against the new variants than vaccine-induced immunity, but that such protection can be enhanced by additional vaccination.23, 24, 25, 26 Our data, which show that natural immunity due to previous exposure to SARS-CoV-2 provided 83·2% protection against any COVID-19 reinfection, provides further support for these observations. Moreover this immunity was sufficient to prevent any severe cases of COVID-19, especially COVID-19-associated-hospitalisations, which will be important for national health resources to have the capacity to deal with future waves of infection. A report that used the Kentucky National Electronic Diseases Surveillance System to determine the reoccurrence of a PCR-positive test for SARS-CoV-2 more than 90 days after an initial positive test estimated a protective effect of previous infection of 80·3% (95% CI 78·2–82·2) in people aged 20–59 years and 67·4% (62·8–71·4) in people aged over 60 years.27 Systematic reviews of studies have reported protection due to previous infection. An analysis of ten studies28 found a weighted risk reduction of 90·4% (SD 7·7%), and a meta-analysis of 1096 cases of reinfection derived from 19 studies29 found that a previous infection provided a protection rate against symptomatic reinfection of 87·2% (83·1–90·3). Our report, from a single clinical study, is unique, and is a consequence of the rapid spread of the pandemic, which resulted in half the study population having evidence of previous exposure to SARS-CoV-2 and being faced with newly emerged variants which, in April, 2022, reflects the current reality in many countries around the world. Furthermore, we showed that one dose of SCB-2019 was able to add further protection such that overall protection in the cohort of people with evidence of previous exposure to SARS-CoV-2 was 89·7% (82·5–94·4) after one dose, and 93·8% (88·9–97·0) if two doses were administered. This so-called hybrid immunity (or super immunity) in COVID-19 has been observed by other groups.30 With the underlying attack rate as described, the number needed to be vaccinated with two doses to prevent one additional symptomatic case of COVID-19 was 28 (16·3–84), and with one dose was 30 (15·2–556). In comparison, the number needed to vaccinate in the SARS-CoV-2-naive cohort was 5 (4·2–7·4) with one dose. Comparing numbers needed to vaccinate could provide guidance to policy makers on where best to allocate vaccines when supply is restricted.
Our data were obtained before the emergence of the omicron variant in November, 2021, and early data indicate that reinfection is more likely with omicron than with previous variants.15 Further surveillance will be required to confirm whether the data for protection and vaccine efficacy hold true for omicron and other potential variants, but data (as of March, 2022) show the potential for future vaccination programmes. Our results were achieved with minimal reactogenicity and no safety issues, the vaccine being generally well tolerated in pre-exposed participants, except for a higher rate of short-lived, mild-to-moderate injection site pain in SARS-CoV-2-exposed recipients (89 [33·8%] of 263 participants) than in the placebo group (16 [6·7%] of 239 participants). Rates of solicited systemic adverse events were similar in pre-exposed vaccine and placebo groups, and rates observed after SCB-2019 were similar to those with or without evidence of previous exposure to SARS-CoV-2. In participants with previous exposure to SARS-CoV-2, rates after a second vaccination were lower than those observed in the SARS-CoV-2-naive participants. There were few severe or serious adverse events and no safety concerns were raised. The reactogenicity profile of SCB in SARS-CoV-2-exposed individuals was not different from that observed in participants who were SARS-CoV-2-naive, obviating the necessity of screening for mass vaccination campaigns in the event of future waves of variants.
Our study has some limitations. First, we lacked knowledge about which viral strain (or strains) participants were exposed to. Second, we also did not have specific information regarding the timing or the nature of these exposures, as well as whether a participant had asymptomatic infection or a mild case of COVID-19 that was unrecognised and, as such, was believed to be another general illness. Third, our estimations against asymptomatic infections were preliminary and require further investigation. In common with our report of the efficacy of SCB-2019, this study was affected by only including participants from a limited range of ages. This limitation occurred because it was difficult to recruit older participants, who were the first to receive other COVID-19 vaccine as they became available.
To conclude, our study, which included data from the large cohort of participants with previous exposure to SARS-CoV-2 in the SPECTRA efficacy trial of the recombinant SARS-CoV-2 S protein vaccine candidate SCB-2019, showed that pre-exposure to SARS-CoV-2 provided over 83% protection against reinfection with COVID-19 and 100% protection against severe consequences of COVID-19. This protection was further enhanced by vaccination with SCB-2019 candidate vaccine, which can be safely used for vaccination in the event of new waves of COVID-19.
For more on Nextstrain see https://nextstrain.org
For more on the Philippine Genome Center see https://pgc.up.edu.ph/
Data sharing
Once the study is completed, the datasets, including the redacted study protocol, redacted statistical analysis plan, and individual participants data supporting the results reported in this article, will be available 3 months from initial request to researchers who provide a methodologically sound proposal. All data sharing will be done at the discretion of the company governing body. The data will be provided after it is de-identified, in compliance with applicable privacy laws, data protection, and requirements for consent and anonymisation.
Declaration of interests
IS, HHH, PL, CB, CV, and JL are full-time employees of Clover Biopharmaceuticals. FR is a statistical adviser, and SACC, RC, DA, PR, and GS are scientific advisers for Clover Biopharmaceuticals.
Acknowledgments
Acknowledgments
We wish to thank the participants in the trial, the members of the SPECTRA Study Group, and Dr Yung Huang, Hui-Ling Chen, Pilar Rubio, Carole Verhoeven, Haijing Qin, Vincent Mwangi, and Joyce Garcia at Clover Biopharmaceuticals, and our external service providers (contract research organisations, laboratories, clinical suppliers, and biostatistics) for their invaluable assistance in conducting the trial. We also thank Dynavax Technologies for providing the CpG 1018 adjuvant. We are particularly grateful to the Scientific Advisory Board members and The Coalition for Epidemic Preparedness Innovations (CEPI) team for their advice and guidance, and to the members of the data and safety monitoring board for their dedication to the trial and the endpoint adjudication committee for their expert analysis of the trial data. We also thank Keith Veitch (keithveitch communications, Amsterdam, the Netherlands) for assistance in writing and preparing the manuscript for submission (funded by Clover Biopharmaceuticals).
Contributors
All authors contributed to designing the study and preparing the protocol. Data analysis was done by an external service provider, who was supervised by PL. Interpretation and writing of the manuscript was done by all authors, led by RC. All authors had access to the analysed datasets and all authors made the decision to submit for publication. IS, HHH, PL, CB, CV, and RC had access to and verified the raw data.
Supplementary Material
References
- 1.Our World in Data Coronavirus (COVID-19) vaccinations. Dec 16, 2021. https://ourworldindata.org/covid-vaccinations
- 2.WHO Tracking SARS-CoV-2 variants. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/
- 3.WHO Update on Omicron. Nov 28, 2021. https://www.who.int/news/item/28-11-2021-update-on-omicron
- 4.WHO COVID-19 vaccine tracker and landscape. Nov 16, 2021. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
- 5.Huang Y, Yang C, Xu XF, Xu W, Liu SW. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin. 2020;41:1141–1149. doi: 10.1038/s41401-020-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richmond P, Hatchuel L, Dong M, et al. Safety and immunogenicity of S-Trimer (SCB-2019), a protein subunit vaccine candidate for COVID-19 in healthy adults: a phase 1, randomised, double-blind, placebo-controlled trial. Lancet. 2021;397:682–694. doi: 10.1016/S0140-6736(21)00241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richmond PC, Hatchuel L, Pacciarini F, et al. Persistence of the immune responses and cross-neutralizing activity with variants of concern following two doses of adjuvanted SCB-2019 COVID-19 vaccine. J Infect Dis. 2021 doi: 10.1093/infdis/jiab447. published online Sept 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ambrosino D, Han HH, Hu B, et al. Immunogenicity of SCB-2019 coronavirus disease 2019 vaccine compared with 4 approved vaccines. J Infect Dis. 2021;19 doi: 10.1093/infdis/jiab574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bravo L, Smolenov I, Han HH, et al. Efficacy of the adjuvanted subunit protein COVID-19 vaccine, SCB-2019: a phase 2 and 3 multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2022;399:461–472. doi: 10.1016/S0140-6736(22)00055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Food and Drug Administration Development and licensure of vaccines to prevent COVID-19: guidance for industry. https://www.fda.gov/media/139638/download
- 13.Laiton-Donato K, Franco-Muñoz C, Álvarez-Díaz DA, et al. Characterisation of the emerging B.1.621 variant of interest of SARS-CoV-2. Infect Genet Evol. 2021;95 doi: 10.1016/j.meegid.2021.105038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burki TK. Omicron variant and booster COVID-19 vaccines. Lancet Respir Med. 2021;10:e17. doi: 10.1016/S2213-2600(21)00559-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pulliam JRC, van Schalkwyk C, Govenfer N, et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of the Omicron variant in South Africa. MedRxiv. 2021 doi: 10.1101/2021.11.11.21266068. published online Dec 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolter N, Jassat W, Walaza S, et al. Early assessment of the clinical severity of the SARS-CoV-2 Omicron variant in South Africa. Lancet. 2022;399:437–446. doi: 10.1016/S0140-6736(22)00017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheizh A, Kerr S, Woolhouse M, McMenamin J, Robertson C. Severity of Omicron variant of concern and vaccine effectiveness against symptomatic disease: national cohort with nested test negative design study in Scotland. https://www.research.ed.ac.uk/en/publications/severity-of-omicron-variant-of-concern-and-vaccine-effectiveness- [DOI] [PMC free article] [PubMed]
- 18.Tartof SY, Slezak JM, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398:1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldberg Y, Mandel M, Bar-On YM, et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. 2021;385:e85. doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 COVID-19 vaccine over 6 months. N Engl J Med. 2021;385:e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naaber P, Tserel L, Kangro K, et al. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. Lancet Reg Health Eur. 2021;10 doi: 10.1016/j.lanepe.2021.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richmond PC, Hatchuel L, Pacciarini F, et al. Persistence of the immune responses and cross-neutralizing activity with Variants of Concern following two doses of adjuvanted SCB-2019 COVID-19 vaccine. J Infect Dis. 2021 doi: 10.1093/infdis/jiab447. published online Sept 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chemaitelly H, Bertollini R, Abu-Raddad LJ. Efficacy of natural immunity against SARS-CoV-2 reinfection with the Beta variant. N Engl J Med. 2021;385:2585–2586. doi: 10.1056/NEJMc2110300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Association of prior SARS-CoV-2 infection with risk of breakthrough infection following mRNA vaccination in Qatar. JAMA. 2021;326:1930–1939. doi: 10.1001/jama.2021.19623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gazit S, Shlezinger R, Perez G, et al. Comparing SARS-CoV-2 natural immunity to vaccine-induced immunity: reinfections versus breakthrough infections. MedRix. 2021 doi: 10.1101/2021.08.24.21262415. published online Aug 25. [DOI] [Google Scholar]
- 27.Spicer KB, Glick C, Cavanaugh AM, Thoroughman D. Protective immunity after natural infection with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) - Kentucky, USA, 2020. Int J Infect Dis. 2022;114:21–28. doi: 10.1016/j.ijid.2021.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kojima N, Shrestha NK, Klausner JD. A systematic review of the protective effect of prior SARS-CoV-2 infection on repeat infection. Eval Health Prof. 2021;44:327–332. doi: 10.1177/01632787211047932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mao Y, Wang W, Ma J, Wu S, Sun F. Reinfection rates among patients previously infected by SARS-CoV-2: systematic review and meta-analysis. Chin Med J (Engl) 2021;135:145–152. doi: 10.1097/CM9.0000000000001892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crotty S. Hybrid immunity. Science. 2021;372:1392–1393. [Google Scholar]
Uncited References
- 10.Sah P, Fitzpatrick MC, Zimmer CF, et al. Asymptomatic SARS-CoV-2 infection: a systematic review and meta-analysis. Proc Natl Acad Sci USA. 2021;118 doi: 10.1073/pnas.2109229118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Washington State Department of Health SARS-CoV-2 vaccine breakthrough surveillance and case information resource. Nov 24, 2021. https://www.doh.wa.gov/Portals/1/Documents/1600/coronavirus/data-tables/420-339-VaccineBreakthroughReport.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Once the study is completed, the datasets, including the redacted study protocol, redacted statistical analysis plan, and individual participants data supporting the results reported in this article, will be available 3 months from initial request to researchers who provide a methodologically sound proposal. All data sharing will be done at the discretion of the company governing body. The data will be provided after it is de-identified, in compliance with applicable privacy laws, data protection, and requirements for consent and anonymisation.