Abstract
To explore the role of atorvastatin in regulating intraocular pressure (IOP) in glaucoma in vivo, and to investigate its related molecular pathway in vitro, an ocular hypertension model was generated by intravitreal injection of an adenoviral vector encoding transforming growth factor (TGF)-β2 in the right eye of BALB/cJ mice, while the left was treated with an empty control adenovirus. To determine its anti-intraocular hypertension role, these induced hyper-IOP mice were gavaged with atorvastatin (20 mg/kg/day). Furthermore, extracellular matrix (ECM) factors were examined in the primary human trabecular meshwork (HTM) cells followed atorvastatin (0~200 µM) treatment in vitro. Whole genome microarray was employed to identify potential therapeutic target molecules associated with ECM regulation. Unilateral murine ocular hypertension was induced, via intravitreal injection of the adenoviral vector carrying the human TGF-β2 gene (Ad.hTGF-β2226/228), raising IOP from 12±1.6 to 32.3±0.7 mmHg (n=6, P<0.05) at day 15, which plateaued from day 15 to 30. Atorvastatin administration from day 15 to 30 decreased IOP from 32.3±0.7 to 15.4±1.1 mmHg (n=6, P<0.05) at day 30. Additionally, atorvastatin administration changed the morphology of cultured HTM cells from an elongated and adherent morphology into rounded, less elongated and less adherent cells, accompanied with suppressed expression of ECM. Gene Ontology and Genome analysis revealed that FGD4 (FYVE, RhoGEF and PH domain containing 4) might be a key factor contributing to these changes. Our data demonstrated that atorvastatin reduced TGF-β2-induced ocular hypertension in vivo, perhaps via modifying cellular structure and decreasing ECM, using the FGD4 signaling pathway, as demonstrated in HTM cells. Our findings provide some useful information for the management of glaucoma, with statin therapy revealing a potential novel therapeutic pathway for glaucoma treatment.
Keywords: atorvastatin, ocular hypertension mouse model, primary human trabecular meshwork cells, extracellular matrix, FGD4
Introduction
Primary open angle glaucoma (POAG) leads to irreversible blindness (1), which is characterized by the gradual apoptosis of retinal ganglion cells, resulting in visual field defects (2). Intraocular pressure (IOP) elevation is the major risk factor for the progression of POAG (3). However, the underlying pathogenesis of POAG remains inconclusive. Considering that ~75% of aqueous humor outflow occurs through the trabecular meshwork (TM) pathway (4), increased IOP-associated POAG is considered to be mainly due to elevated resistance to the TM outflow (5).
Most of the increased resistance to aqueous humor outflow is due to an increase in extracellular matrix (ECM) deposition (6-8). ECM contains elastin, collagens and fibronectin (FN). Considering the involvement of the ECM in increasing the resistance of the TM to aqueous humor outflow, pathological dysregulation of the ECM is strongly implicated in POAG, in part mediated via the matricellular proteins, which are important regulatory factors-extracellular proteins, including tenascin-C, secreted protein and rich in cysteine (SPARC) and connective tissue growth factor (CTGF), and communicate with each other and regulate their surrounding ECM (9,10). CTGF and SPARC may promote tissue fibrosis and abnormal tissue remodelling and disrupt ECM homeostasis, which eventually contributes to the pathogenesis of glaucoma (11,12). In addition, aging, actomyosin contractile systems (e.g. ciliary muscle contractions) and inflammation also contribute to the TM resistance to outflow (13,14)
FYVE, RhoGEF and PH domain containing 4 (FGD4) is a guanine nucleotide exchange factor specific for activation of cell division control protein 42 homolog (CDC42) (GTP bound) (15). The FGD4/CDC42 pathway is involved in the regulation of cell morphology, cytoskeleton organization, intercellular adhesion and ECM synthesis of non-ocular cells (16-18). Thus, the FGD4/CDC42 pathway may also contribute to the regulation of cell morphology, cytoskeletal organization, cell adhesion and ECM synthesis in human trabecular meshwork (HTM) cells.
There are several therapeutic agents available for reducing IOP in glaucoma (19). However, few of them are designed specifically for controlling the pathogenesis of POAG, e.g. targeting ECM accumulation in the TM. Moreover, the currently available anti-glaucoma agents can only decrease IOP, but not prevent the progression of, nor cure POAG (20,21).
Statins reduce serum cholesterol via selectively inhibiting HMG-CoA reductase for the biosynthesis of cholesterol (22-24). Statins are well known for their 'pleiotropic effects', e.g., anti-fibrotic, anti-inflammatory and immunomodulatory (25-29). Interestingly, statins also reduce the progression of POAG in specific populations (30-32), suggesting that statins minimize the risk of the development of glaucoma, as well as delay the progression of visual field defect (32). Because statins suppress ECM synthesis in non-ocular tissues, we hypothesized that statins can also decrease ECM dysregulation in human trabecular meshwork (HTM), constituting a potential anti-glaucoma mechanism (33,34). We demonstrated previously that atorvastatin improved aqueous humor outflow in a porcine whole-eye perfusion model ex vivo (35), but it is unclear whether atorvastatin is able to reduce IOP in POAG in vivo.
Transforming growth factor (TGF)-β2, expressed in TM cells, contributes to ECM generation (36). An adenoviral TGF-β2-induced ocular hypertension murine model was found to display a high glaucoma risk via decreasing aqueous outflow facility (36,37). Such an animal model is expected to be a useful model to investigate the pathogenesis of glaucoma (38).
Our present study aimed to investigate whether atorvastatin was able to reduce TGF-β2-induced intraocular hypertension in vivo, as well as to investigate the possible molecular expression in the dysregulation of the ECM in primary HTM cells following atorvastatin administration in vitro.
Materials and methods
Animals
All animals used in this research followed the ARVO statement for the Use of Animals (https://www.arvo.org/About/policies/statement-for-the-use-of-animals-in-ophthalmic-and-visual-research/). BALB/cJ mice (8 weeks; 3 females and 3 males per group, 4 groups and 24 mice in this study; 22~25 g), purchased from the Lingchang Animal Facility (Shanghai, China), were offered food and water ad libitum on a 12 h light/12 h dark cycle at 20°C room temperature. At the end of the experiment the animals were euthanized by cervical dislocation. The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Animal Care and Use Committee of the Huashan Hospital, Fudan University (Protocol #: JS-194, Approval Date: 19 February 2019).
Induction of ocular hypertension in vivo
The ocular hypertension model was induced by injecting an adenoviral vector of human TGF-β2 (Ad.hTGF-β2226/228) in 2 µl (6×107 pfu) intravitreally in the right eye, as described previously (36). The left eye was given carrier Ad with an empty vector (6×107 pfu in 2 µl), as a control. There was also a blank, no-injection control group. The Ad.hTGF-β2226/228-injected animals were further divided into groups with and without atorvastatin administration (20 mg/kg/day, gavage), starting at day 15. Thus, there were totally four groups of experimental mice: Ad.hTGF-β2226/228-induced (n=6); Ad.hTGF-β2226/228-induced with atorvastatin from day 15 (n=6); Ad. empty-injected (n=6); and non-injection (n=6). All IOP measurements were implemented using a Tonolab rebound tonometer (Icare), as described (39).
Trabecular meshwork cell culture
All experiments were carried out according to the guidelines of the Human Ethics Committee of Huashan Hospital, Fudan University (protocol #: 2020-611, approval date: 31 March 2020) (40). Primary HTM cells were isolated and characterized as described previously (41). There were 4 donors from Huashan Hospital, a Red Cross Hospital (Shanghai, China), aged 30, 35, 48 and 50 years, including 2 females and 2 males, respectively. Formal written consent was obtained from each of the donors in this study. All the donors had no previous diagnosis or history of eye disease. A more detailed description of the procedures for generating primary HTM cells were as follows. Human TM tissues were explanted from the corneal edge and were further minced in culture solution. The minced tissues were further cultured for 4 h at 37°C under 5% CO2 until the cells were adherent to the bottom of the culture flask. The culture flashes were washed with fresh culture medium to remove dead unadhered cells. The culture medium consisted of DMEM containing 15% fetal bovine serum (FBS) (Gibco; Thermo Fisher Scientific, Inc.). HTM cells were sub-cultured when the cells reached 80% confluence. The HTM cells were passaged at a ratio of 1:3 to maintain the same conditions. The third passage of HTM cells was identified and validated as HTM cells immunohistochemically for the specific proteins FN, vimentin and laminin (LN). The cultured HTM cells were stimulated with 250 and 500 nM dexamethasone (DEX) (Sigma-Aldrich; Merck KGaA) with fresh medium every 2 days until the 6th day. The level of myocilin (MYOC), which correlates with the trabecular meshwork glucocorticoid response, was assessed with western blot analysis. These HTM cells were passaged for 5 cultures in this study.
Atorvastatin treatment
The HTM cells were serum-deprived for 12 h until they reached 90% confluency. These cells were subsequently treated with atorvastatin (Sigma-Aldrich; Merck KGaA) at different dosages (0, 50, 100, 150 and 200 µM), and for different times (12, 24, 36 and 48 h). The morphological changes in the HTM cells were captured under phase contrast microscopy (ECLIPSE Ni-U; Nikon). The effect of atorvastatin on the viability of HTM cells was determined using a CCK-8 assay kit (Dojindo Molecular Technologies, Inc.) following atorvastatin treatment (0, 50, 100, 150, 200 µM), as previously described (35).
Real-time PCR
Whole cell RNA from HTM cells was extracted, using Trizol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Prime-Script RT reagent kit (RR036A; Takara Biotechnology Co.) was used for cDNA synthesis from 1 µg RNA. Real-time PCR was conducted using a qPCR kit (RR820A; Takara Biotechnology Co.), and the primers used are shown in Table I. The procedures have been described in detail previously (35).
Table I.
Sequences of the specific primers.
| Gene | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|
| Collagen I | ACGTCCTGGTGAAGTTGGT | CAGGGAAGCCTCTCTCTCCT |
| FN | AATATCTCGGTGCCATTTGC | AAAGGCAT GAAGCACTCAA |
| SPARC | ATGACGACGGCACCTACAG | TCGCGTTGGGGTAACTTTTCA |
| CTGF | GCAGGCTAGAGAAGCAGAGC | ATGTCTTCATGCTGGTGCAG |
| GAPDH | ACAGTCAGCCGCATCTTC | CTCCGACCTTCACCTTCC |
FN, fibronectin; SPARC, secreted protein and rich in cysteine; CTGF, connective tissue growth factor; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Western blot analysis
Western blot analysis was performed, as described previously (35,42). The concentration of each protein sample was determined using the Bradford protein-detection method (Bio-Rad, Laboratories, Inc.) and separated by SDS-polyacrylamide gel electrophoresis (PAGE) (10% acrylamide), then transferred onto a polyvinylidene fluoride (PVDF) membrane. The blots were incubated with the primary antibodies: rabbit anti-collagen I antibody (14695-1-AP, 1:1,000 dilution; Proteintech), rabbit anti-fibronectin antibody (15613-1-AP, 1:1,500 dilution; Proteintech), rabbit anti-CTGF antibody (ab6992, 1:1,000 dilution; Abcam), rabbit anti-SPARC antibody (15274-1-AP, 1:1,000 dilution, Proteintech), GAPDH (60004-1-Ig, 1:500 dilution, Proteintech) in blocking solution overnight at 4°C. Then the membranes were incubated with goat anti-rabbit secondary antibody (SE134, 1:1,000 dilution, Solarbio) at room temperature for 1 h, followed by washing with TBST for 3 times. Then, typically enhanced chemiluminescent (ECL) kit was used according to the manufacturer's instructions (POO18FS, Beyotime). A particular band of each sample was visualized by the Odyssey infrared imaging system (LI-COR, Inc.). The bands were analyzed using ImageJ software and normalized to each GAPDH band (version 1.48v; National Institutes of Health). The protein density of every band was analyzed with Image J software (http://imagej.nih.gov/ij/).
Immunofluorescence
Glass coverslips seeded with HTM cells were fixed with 4% paraformaldehyde. After 3 times washing with PBS, the primary antibody was bound at 4°C overnight: rabbit anti-collagen I antibody (14695-1-AP, 1:1,000 dilution; Proteintech), rabbit anti-fibronectin antibody (15613-1-AP, 1:1,500 dilution; Proteintech), rabbit anti-CTGF antibody (ab6992, 1:1,000 dilution; Abcam), rabbit anti-SPARC antibody (15274-1-AP, 1:1,000 dilution; Proteintech), rabbit anti-vimentin antibody (10366-1-AP, 1:1,000 dilution; Proteintech), rabbit anti-laminin (LN) antibody (23498-1-AP, 1:1,000 dilution; Proteintech). The procedures have been described in detail previously (35).
Array hybridization and data acquisition
Following 24 h atorvastatin or vehicle treatment (10, 25, 50, 100, 200 µM) in HTM cells, total RNA was extracted and purified using Trizol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Array hybridization was performed, using GeneChip® Hybridization, Wash and Stain kit (cat #900720, Affymetrix; Thermo Fisher Scientific, Inc.) in Hybridization Oven 645 (cat #00-0331-220V, Affymetrix; Thermo Fisher Scientific, Inc.) and Fluidics Station 450 (cat #00-0079, Affymetrix; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. The slide was scanned with the default settings of the GeneChip® Scanner 3000 (Cat #00-00212, Affymetrix) and Command Console® Software 4.0 (Affymetrix). Raw data were normalized using the MAS 5.0 algorithm (Affy packages in R).
GO and KEGG analysis
The differentially expressed genes were analyzed by Gene Oncology (GO) enrichment, and were implemented, using the top GO R packages based on the Fisher's exact test (43). Clusters of Orthologous Groups and KEGG databases were used to classify and analyze differentially expressed genes (44), and P<0.05 was used as the significance enrichment standard.
Statistical analysis
Data were analyzed using SPSS17.0 version 12.0 software (SPSS, Inc.). All data are represented as the mean ± standard deviation (SD). Analysis of variance (ANOVA) was used to compare results among two or more groups, followed by least significant difference (LSD) post hoc analysis; P<0.05 was deemed to be a statistically significant difference.
Results
Atorvastatin reduces intraocular pressure in a mouse model of ocular hypertension
IOP was increased 2-fold following intravitreal injection of Ad.hTGF-β2226/228 (12±1.6 vs. 24±1.7 mmHg, P<0.01) at day 5 (Fig. 1). A significantly elevated IOP of 32.3±0.7 mmHg was observed by day 15 following vector intravitreal injection, which plateaued until day 30, which was ~1.6-fold higher than the non-injection eyes (12.8±0.4 mmHg) or the vehicle (Ad. Empty) eyes (12.6±0.8 mmHg) (P<0.001). By day 20 (5 days after the commencement of the gavage of atorvastatin), IOP was significantly decreased in the hypertensive IOP group treated with atorvastatin compared to the non-atorvastatin-treated group (21.5±0.7 vs. 32.5±0.7 mmHg, P<0.01). By day 30, the IOP of the atorvastatin treatment subgroup of the Ad.hTGF-β2226/228-induced hypertensive IOP group continued to become significantly lower than that of the non-atorvastatin-treated group (15.4±1.1 vs. 32.3±0.7 mmHg, P<0.01). There were no adverse events during the period of the experiment.
Figure 1.

Mice intraocular pressure (IOP) following intravitreal injection of the Ad.hTGF-β2226/228 vector and gavage with atorvastatin. Four groups: Ad.hTGF-β2226/228 intravitreal injection (n=6); Ad.hTGF-β2226/228 intravitreal injection + daily gavage of atorvastatin (20 mg/kg) (n=6) from day 15; Ad.empty-injected treatment (n=6); no injection (n=6). *P<0.05; n represents the number of mice per group.
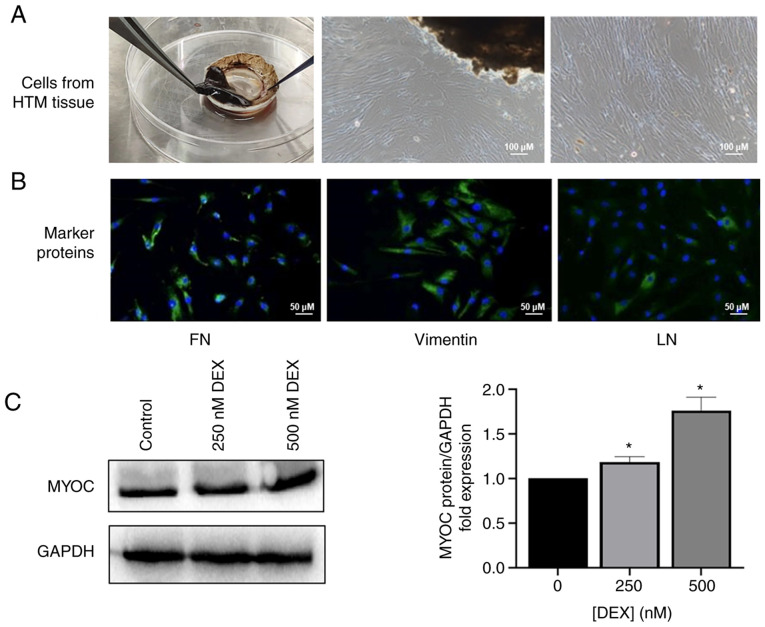
Atorvastatin toxicity and HTM cell morphology
The isolated primary HTM cells grew out from the HTM tissues (Fig. 2A) and expressed FN, vimentin and LN (Fig. 2B), and MYOC was increased after stimulation with DEX in a dose-dependent manner (Fig. 2C). There was no significant difference in viability of the HTM cells treated with and without atorvastatin (50-100 µM), except with atorvastatin at a dosage of 200 µM for 48 h (Fig. 3A). Thus, the time and the dose of atorvastatin were selected to be no more than 24 h nor over 200 µM in the subsequent experiments (Fig. 3A). There were morphological changes in the HTM cells following 24 h of statin treatment. The shape of the HTM cells was altered from an elongated and adherent cell morphology into rounded and detached cells with reduced elongation in a dose-dependent manner following statin treatment (Fig. 3B).
Figure 2.
Characterization of HTM cells. (A) HTM cells that grew out from the HTM tissues; (scale bar, 100 µm). (B) The expression of FN, vimentin, and LN of primary HTM cells was evaluated immunocytochemically; (scale bar, 50 µm). (C) The level of MYOC in HTM cells following treatment of DEX (250, 500 nM) for 6 days. *P<0.05. HTM, human trabecular meshwork; FN, fibronectin; LN, laminin; MYOC, myocilin; DEX, dexamethasone.
Figure 3.

(A) Effects of atorvastatin on the viability of HTM cells (n=4) (*P<0.05; n represents an independent experiment number using different major HTM cell lines. (B) Morphological changes in response to atorvastatin (50-200 µM) treatment in HTM cells (scale bar, 100 µm). HTM, human trabecular meshwork.
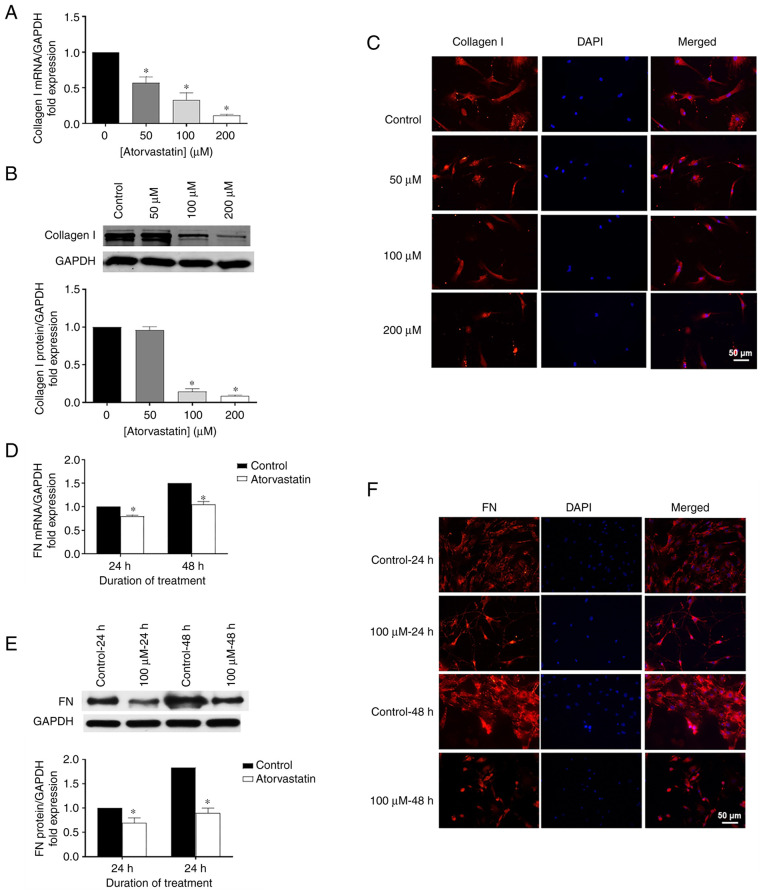
Effects of atorvastatin on expression levels of collagen I and FN
Using RT-qPCR, western blot analysis and immunohistochemistry, the role of atorvastatin on ECM expression was explored, particularly for collagen I and FN. Collagen I mRNA was significantly decreased in the HTM cells following atorvastatin treatment for 24 h (Fig. 4A) related to the change of dose, compared with control. Similar patterns were observed for FN mRNA in the HTM cells treated with 100 µM atorvastatin for 24 or 48 h, showing significantly decreased FN mRNA compared to the control (Fig. 4D). Consistent data were also obtained for protein expression using western blot analysis (Fig. 4B and E) and immunohistochemistry (Fig. 4C and F) in HTM cells following atorvastatin treatment.
Figure 4.
Effects of atorvastatin on decreasing (A) mRNA, (B) protein levels, and (C) nuclear localization of collagen I in HTM cells. Collagen I levels (n=4) were assessed. Effects of atorvastatin on decreasing (D) mRNA, (E) protein levels, and (F) nuclear localization of FN. FN levels (n=4) were assessed (*P<0.05; n represents an independent experiment number, using different major HTM cell lines; scale bar, 50 µm). HTM, human trabecular meshwork; FN, fibronectin.
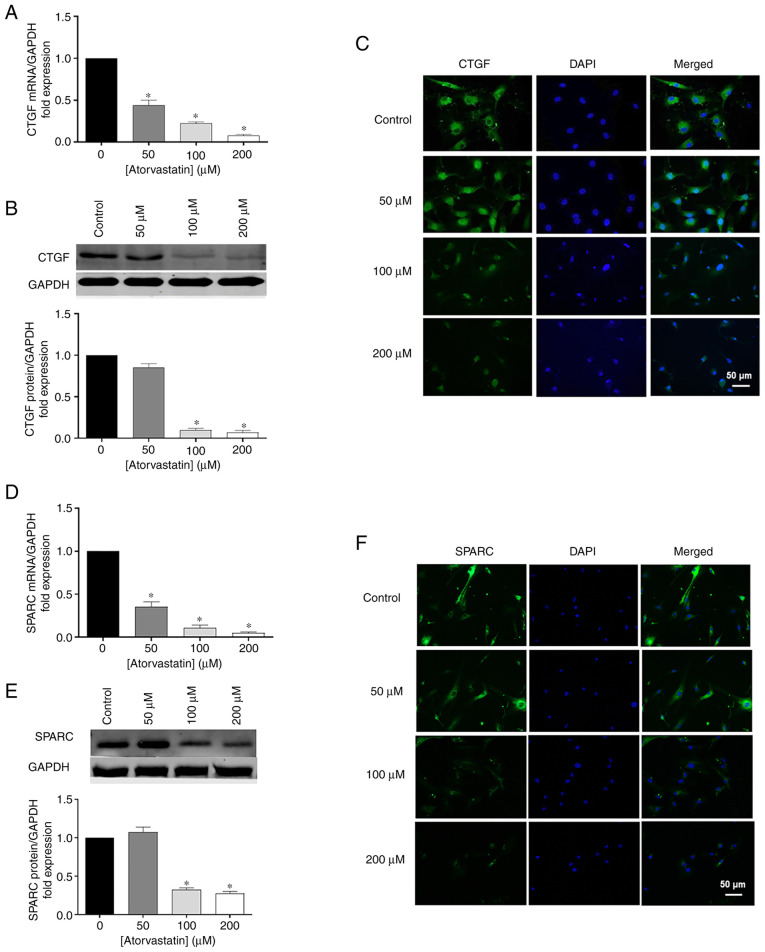
Effects of atorvastatin on CTGF and SPARC
In addition to ECM, atorvastatin inhibited CTGF and SPARC expression, which are two important matricellular proteins. To confirm the results obtained by RT-qPCR, Western blot analysis and immunohistochemistry were performed. CTGF and SPARC mRNA were significantly decreased in HTM cells followed atorvastatin treatment for 24 h in a dose-dependent manner, comparing with control (Fig. 5A and D). Similar trends were obtained by western blot analysis (Fig. 5B and E) and immunohistochemistry (Fig. 5C and F), showing that the protein products of CTGF and SPARC were reduced following atorvastatin treatment.
Figure 5.
Effects of atorvastatin on decreasing (A) mRNA, (B) protein levels, and (C) nuclear localization of CTGF in HTM cells. Effects of atorvastatin on decreasing (D) mRNA, (E) protein levels, and (F) nuclear localization of SPARC in HTM cells (*P<0.05; n=4, n represents an independent experiment number, using different major HTM cell lines; scale bar, 50 µm). HTM, human trabecular meshwork; CTGF, connective tissue growth factor; SPARC, secreted protein and rich in cysteine.
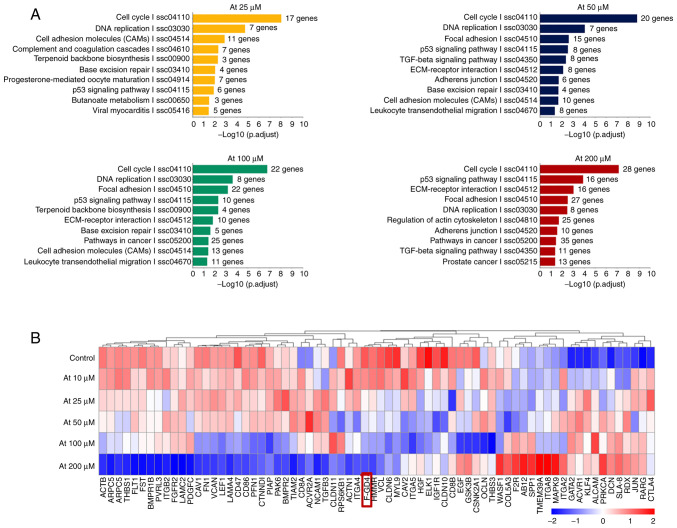
GO and KEGG analysis
Gene expression profile microarray was utilized for detection of a correlation of differential gene expression (Fig. 6). GO and KEGG gene enrichment analysis showed that in addition to lipid metabolism and cell cycle-related pathways (Fig. 6A), the differentially expressed genes mainly included 'cell adhesion molecules', 'focal adhesion', the 'TGF-β signaling pathway', 'ECM receptor interaction', 'adhesions junction', 'regulation of actin cytoskeleton and other extracellular matrix synthesis pathways', 'cell-cell adhesion pathways', and 'cytoskeleton related pathways'. These pathways are all related to POAG pathogenesis. Among these genes, FGD4 was downregulated most significantly in a concentration-dependent manner (Fig. 6B).
Figure 6.
(A) Expression and enrichment analysis of different genes in HTM cells treated with atorvastatin. (B) Expression levels of 'extracellular matrix synthesis pathway', 'cell-cell adhesion pathway', 'cytoskeletal pathway' related genes in HTM cells treated with atorvastatin. Among these genes, the FGD4 gene was downregulated the most in a drug concentration-dependent manner. HTM, human trabecular meshwork; FGD4, FYVE, RhoGEF and PH domain containing 4.
Verification of the expression of FGD protein isoforms in HTM and the inhibitory effect of atorvastatin on FGD4 gene expression in trabecular meshwork cells
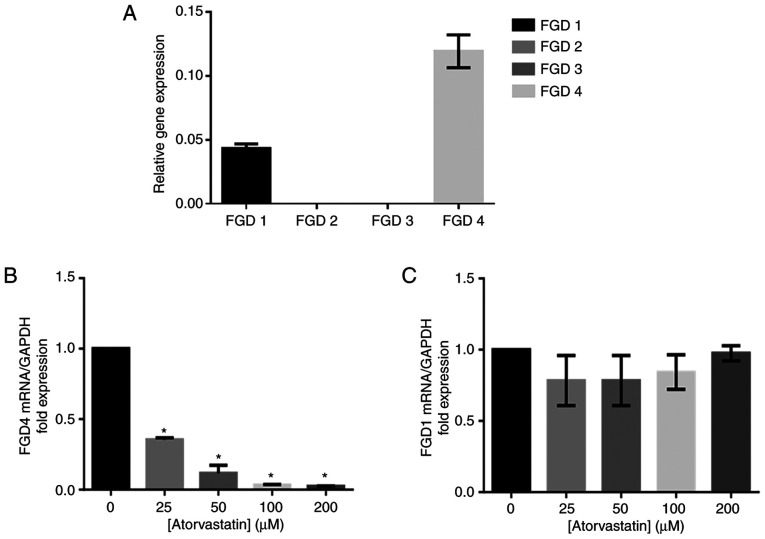
Our study found that the FGD4 gene was the most highly expressed isoform of FGD in HTM cells, and FGD1 was the second most highly expressed among the FGD genes (Fig. 7A). The expression of the FGD4 gene was decreased with increasing atorvastatin concentration (Fig. 7B). There was no significant change in FGD1 gene expression following atorvastatin treatment (Fig. 7C).
Figure 7.
(A) FGD4 was the most highly expressed isoform in HTM cells. (B) The expression level of FGD4 was decreased in response to the increased dosages of atorvastatin. *P<0.05 vs. the untreated control. (C) No significant difference in FGD1 expression was observed among the different atorvastatin concentrations. HTM, human trabecular meshwork; FGD4, FYVE, RhoGEF and PH domain containing 4.
Discussion
In the present study, we observed that atorvastatin reduced intraocular pressure (IOP) of ocular hypertension in vivo and in vitro, consistent with the observed morphologic change of isolated HTM cells. Atorvastatin also suppressed two key matricellular proteins [secreted protein and rich in cysteine (SPARC) and connective tissue growth factor (CTGF)] and decreased extracellular matrix (ECM) synthesis [collagen I and fibronectin (FN)] in human trabecular meshwork (HTM) cells. In addition, our data support the hypothesis that FYVE, RhoGEF and PH domain containing 4 (FGD4)/cell division control protein 42 homolog (CDC42) might be the pivotal factors to trigger these changes, which could be a novel potential therapeutic target for glaucoma pathogenesis and treatment. All the findings suggest that atorvastatin may be able to be a useful agent in the management of glaucoma.
Ocular hypertension induced by Ad.hTGF-β2226/228 can be reduced following atorvastatin treatment, suggesting that statins are effective in the management of ocular hypertension induced via the transforming growth factor (TGF)-β2 pathway, which is in line with others (37,38), showing hypertensive IOP is induced via the TGF-β2 route in vivo. However, the precise underlying mechanism of atorvastatin in reducing IOP is still unclear and will be clarified in the future, because elevated IOP is the most pivotal risk factor contributing to the progression of glaucoma (1,2).
Furthermore, there was no obvious cytotoxicity observed in the HTM cells in response to atorvastatin treatment, even at a rather high concentration, suggesting that the dosage of atorvastatin used in the present study is safe and reliable. Intercellular space between HTM cells was increased and HTM cell size was reduced following atorvastatin treatment in a dose-response manner, suggesting that there is a potential role for statins in reducing IOP, which may also be associated with a decrease in actin stress fibers and focal adhesion (45). The increased intercellular space and smaller size of HTM cells could improve permeability of the TM and reduce resistance of the TM to outflow. The permeability of the TM is acutely modulated by the combined action of contractile and volume-regulatory properties (46). Taken together, this could explain why atorvastatin reduced IOP via decreasing cell-cell adhesion and increasing intercellular space.
It has been reported that statins suppress ECM synthesis in various non-ocular tissues (33,34). Furthermore, there is a correlation between IOP and synthesis of ECM of the TM, where reducing ECM contributes to a decrease in outflow resistance (47), which is in line with our finding that atorvastatin decreased collagen I and FN expression in HTM cells. Thus, these data suggest that atorvastatin acts as a novel anti-glaucoma medicine, targeting the pathogenesis of primary open angle glaucoma (POAG). Moreover, we also found that atorvastatin inhibited matricellular protein expression (SPARC and CTGF), which enhance fibrosis and increase ECM deposition (10), providing a possible explanation for the involvement of a reduction in IOP at the molecular level.
FGD4 is closely related to the interaction among ECM receptors, adhesion junctions, and the regulation of the actin cytoskeleton, as demonstrated following GO and KEGG gene enrichment analysis. We found that FGD4 was significantly inhibited in response to atorvastatin treatment, suggesting the importance of FGD4 during the development of POAG. FGD4 activates CDC42 via GTP. CDC42 is one of the three members of the Rho protein guanosine triphosphatase (GTPase) family (48), which is closely related to the pharmacological effects of statins (49). The FGD4/CDC42 signaling pathway influences cytoskeleton remodelling and ECM generation (50,51), and thus might also play a pivotal role in glaucoma pathogenesis. Considering data from whole genome microarray, we believe that atorvastatin reduces IOP via the FGD4/CDC42 signaling pathway, which suppresses ECM expression and cytoskeleton remodelling, which will be verified in a future study.
In conclusion, the present study demonstrated that atorvastatin reduced IOP, accompanied by an anti-ECM synthesis effect, improved cellular spacing, and decreased focal adhesion proteins. Statins may be good candidates as a novel treatment for glaucoma. In future research, the precise underlying mechanisms of the FGD4/CDC42 signaling pathway involvement in the anti-glaucoma effect of atorvastatin will be investigated.
Acknowledgments
Not applicable.
Funding Statement
This study was supported by the Science and Technology Commission of Shanghai Municipality (Technology project 18441901400)
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
XYS and YYZ completed all experimental components and contributed to the interpretation of the results. YYC, WTL, LC, JLZ and YZ performed the statistical analysis and contributed to the interpretation of the results. YYC and WTL participated in the revision of this paper. YYZ contributed to the experimental design. XYS and YYZ wrote the paper. All authors read and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work (including all data) are appropriately investigated and resolved.
Ethics approval and consent to participate
The animal study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Animal Care and Use Committee of the Huashan Hospital, Fudan University (Protocol #: JS-194, Approval Date: 19 February 2019). The primary HTM cell experiments were carried out according to the guidelines of the Human Ethics Committee of Huashan Hospital, Fudan University (protocol #: 2020-611, approval date: 31 March 2020). Formal written consent was obtained from each of the donors in this study.
Patient consent for publication
Not applicable.
Competing interests
All the authors declare no competing interests. This research is original and has not been published elsewhere.
References
- 1.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: A review. JAMA. 2014;311:1901–1911. doi: 10.1001/jama.2014.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–1720. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- 3.De Moraes CG, Liebmann JM, Levin LA. Detection and measurement of clinically meaningful visual field progression in clinical trials for glaucoma. Prog Retin Eye Res. 2017;56:107–147. doi: 10.1016/j.preteyeres.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pederson JE, Gaasterland DE, MacLellan HM. Uveoscleral aqueous outflow in the rhesus monkey: Importance of uveal reabsorption. Invest Ophthalmol Vis Sci. 1977;16:1008–1017. [PubMed] [Google Scholar]
- 5.Larsson LI, Rettig ES, Brubaker RF. Aqueous flow in open-angle glaucoma. Arch Ophthalmol. 1995;113:283–286. doi: 10.1001/archopht.1995.01100030037018. [DOI] [PubMed] [Google Scholar]
- 6.Tam LC, Reina-Torres E, Sherwood JM, Cassidy PS, Crosbie DE, Lütjen-Drecoll E, Flügel-Koch C, Perkumas K, Humphries MM, Kiang AS, et al. Enhancement of outflow facility in the murine eye by targeting selected tight-junctions of Schlemm's canal endothelia. Sci Rep. 2017;7:40717. doi: 10.1038/srep40717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradley J, Vranka J, Colvis CM, Conger DM, Alexander JP, Fisk AS, Samples JR, Acott TS. Effect of matrix metalloproteinases activity on outflow in perfused human organ culture. Invest Ophthalmol Vis Sci. 1998;39:2649–2658. [PubMed] [Google Scholar]
- 8.Vranka JA, Kelley MJ, Acott TS, Keller KE. Extracellular matrix in the trabecular meshwork: Intraocular pressure regulation and dysregulation in glaucoma. Exp Eye Res. 2015;133:112–125. doi: 10.1016/j.exer.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wight TN, Potter-Perigo S. The extracellular matrix: An active or passive player in fibrosis? Am J Physiol Gastrointest Liver Physiol. 2011;301:G950–G955. doi: 10.1152/ajpgi.00132.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallace DM, Murphy-Ullrich JE, Downs JC, O'Brien CJ. The role of matricellular proteins in glaucoma. Matrix Biol. 2014;37:174–182. doi: 10.1016/j.matbio.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Brekken RA, Sage EH. SPARC, a matricellular protein: At the crossroads of cell-matrix. Matrix Biol. 2000;19:569–580. doi: 10.1016/S0945-053X(00)00105-0. [DOI] [PubMed] [Google Scholar]
- 12.Kuespert S, Junglas B, Braunger BM, Tamm ER, Fuchshofer R. The regulation of connective tissue growth factor expression influences the viability of human trabecular meshwork cells. J Cell Mol Med. 2015;19:1010–1020. doi: 10.1111/jcmm.12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamm ER. The trabecular meshwork outflow pathways: Structural and functional aspects. Exp Eye Res. 2009;88:648–655. doi: 10.1016/j.exer.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Cela D, Brignole-Baudouin F, Labbé A, Baudouin C. The trabecular meshwork in glaucoma: An inflammatory trabeculopathy? J Fr Ophtalmol. 2021;44:e497–e517. doi: 10.1016/j.jfo.2021.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Shahid M, George TB, Saller J, Haija M, Sayegh Z, Boulware D, Strosberg J, Chakrabarti R, Coppola D. FGD4 (Frabin) overexpression in pancreatic neuroendocrine neoplasms. Pancreas. 2019;48:1307–1311. doi: 10.1097/MPA.0000000000001422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sundararaman A, Mellor H. A functional antagonism between RhoJ and Cdc42 regulates fibronectin remodelling during angiogenesis. Small GTPases. 2021;12:241–245. doi: 10.1080/21541248.2020.1809927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ge J, Burnier L, Adamopoulou M, Kwa MQ, Schaks M, Rottner K, Brakebusch C. RhoA, Rac1, and Cdc42 differentially regulate αSMA and collagen I expression in mesenchymal stem cells. J Biol Chem. 2018;293:9358–9369. doi: 10.1074/jbc.RA117.001113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin J, Huang H, Lin L, Li W, Huang J. MiR-23a induced the activation of CDC42/PAK1 pathway and cell cycle arrest in human cov434 cells by targeting FGD4. J Ovarian Res. 2020;13:90. doi: 10.1186/s13048-020-00686-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheema A, Chang RT, Shrivastava A, Singh K. Update on the medical treatment of primary open-angle glaucoma. Asia Pac J Ophthalmol (Phila) 2016;5:51–58. doi: 10.1097/APO.0000000000000181. [DOI] [PubMed] [Google Scholar]
- 20.Rennie G, Wilkinson A, White A, Ruospo M, Teixeira-Pinto A, Strippoli G. Topical medical therapy and ocular perfusion pressure in open angle glaucoma: A systematic review and meta-analysis. Curr Med Res Opin. 2019;35:1421–1431. doi: 10.1080/03007995.2019.1595553. [DOI] [PubMed] [Google Scholar]
- 21.Adams CM, Stacy R, Rangaswamy N, Bigelow C, Grosskreutz CL, Prasanna G. Glaucoma-next generation therapeutics: Impossible to possible. Pharm Res. 2018;36:25. doi: 10.1007/s11095-018-2557-4. [DOI] [PubMed] [Google Scholar]
- 22.Vaughan CJ, Delanty N. Neuroprotective properties of statins in cerebral ischemia and stroke. Stroke. 1999;30:1969–1973. doi: 10.1161/01.STR.30.9.1969. [DOI] [PubMed] [Google Scholar]
- 23.Zacco A, Togo J, Spence K, Ellis A, Lloyd D, Furlong S, Piser T. 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors protect cortical neurons from excitotoxicity. J Neurosci. 2003;23:11104–11111. doi: 10.1523/JNEUROSCI.23-35-11104.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmeer C, Kretz A, Isenmann S. Statin-mediated protective effects in the central nervous system: General mechanisms and putative role of stress proteins. Restor Neurol Neurosci. 2006;24:79–95. [PubMed] [Google Scholar]
- 25.Song J, Deng PF, Stinnett SS, Epstein DL, Rao PV. Effects of cholesterol-lowering statins on the aqueous humor outflow pathway. Invest Ophthalmol Vis Sci. 2005;46:2424–2432. doi: 10.1167/iovs.04-0776. [DOI] [PubMed] [Google Scholar]
- 26.Nakagami H, Jensen KS, Liao JK. A novel pleiotropic effect of statins: Prevention of cardiac hypertrophy by cholesterol-independent mechanisms. Ann Med. 2003;35:398–403. doi: 10.1080/07853890310001294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson HL, Schwartz DM, Bhatt HR, McCulloch CE, Duncan JL. Statin and aspirin therapy are associated with decreased rates of choroidal neovascularization among patients with age-related macular degeneration. Am J Ophthalmol. 2004;137:615–624. doi: 10.1016/j.ajo.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 28.Polman CH, Killestein J. Statins for the treatment of multiple sclerosis: Cautious hope. Lancet. 2004;363:1570. doi: 10.1016/S0140-6736(04)16237-5. [DOI] [PubMed] [Google Scholar]
- 29.Endo A. The discovery and development of HMG-CoA reductase inhibitors. J Lipid Res. 1992;33:1569–1582. doi: 10.1016/S0022-2275(20)41379-3. [DOI] [PubMed] [Google Scholar]
- 30.Stein JD, Newman-Casey PA, Talwar N, Nan B, Richards JE, Musch DC. The relationship between statin use and open-angle glaucoma. Ophthalmology. 2012;119:2074–2081. doi: 10.1016/j.ophtha.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGwin G, McNeal S, Owsley C, Girkin C, Epstein D, Lee PP. Statins and other cholesterol-lowering medications and the presence of glaucoma. Arch Ophthalmol. 2004;122:822–826. doi: 10.1001/archopht.122.6.822. [DOI] [PubMed] [Google Scholar]
- 32.Talwar N, Musch DC, Stein JD. Association of daily dosage and type of statin agent with risk of open-angle glaucoma. JAMA Ophthalmol. 2017;135:263–267. doi: 10.1001/jamaophthalmol.2016.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu H, Zeng L, Peng H, Chen S, Jones J, Chew TL, Sadeghi MM, Kanwar YS, Danesh FR. HMG-CoA reductase inhibitor simvastatin mitigates VEGF-induced 'inside-out' signaling to extracellular matrix by preventing RhoA activation. Am J Physiol Physiol. 2006;291:F995–F1004. doi: 10.1152/ajprenal.00092.2006. [DOI] [PubMed] [Google Scholar]
- 34.Schaafsma D, Dueck G, Ghavami S, Kroeker A, Mutawe MM, Hauff K, Xu FY, McNeill KD, Unruh H, Hatch GM, Halayko AJ. The mevalonate cascade as a target to suppress extracellular matrix synthesis by human airway smooth muscle. Am J Respir Cell Mol Biol. 2011;44:394–403. doi: 10.1165/rcmb.2010-0052OC. [DOI] [PubMed] [Google Scholar]
- 35.Cong L, Fu S, Zhang J, Zhao J, Zhang Y. Effects of atorvastatin on porcine aqueous humour outflow and trabecular meshwork cells. Exp Ther Med. 2018;15:210–216. doi: 10.3892/etm.2017.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shepard AR, Millar JC, Pang IH, Jacobson N, Wang WH, Clark AF. Adenoviral gene transfer of active human transforming growth factor-β2 elevates intraocular pressure and reduces outflow facility in rodent eyes. Invest Ophthalmol Vis Sci. 2010;51:2067–2076. doi: 10.1167/iovs.09-4567. [DOI] [PubMed] [Google Scholar]
- 37.Fuchshofer R, Tamm ER. The role of TGF-β in the pathogenesis of primary open-angle glaucoma. Cell Tissue Res. 2012;347:279–290. doi: 10.1007/s00441-011-1274-7. [DOI] [PubMed] [Google Scholar]
- 38.Swaminathan SS, Oh DJ, Kang MH, Shepard AR, Pang IH, Rhee DJ. TGF-β2-mediated ocular hypertension is attenuated in SPARC-null mice. Invest Ophthalmol Vis Sci. 2014;55:4084–4097. doi: 10.1167/iovs.13-12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang WH, Millar JC, Pang IH, Wax MB, Clark AF. Noninvasive measurement of rodent intraocular pressure with a rebound tonometer. Invest Ophthalmol Vis Sci. 2005;46:4617–4621. doi: 10.1167/iovs.05-0781. [DOI] [PubMed] [Google Scholar]
- 40.Stamer WD, Roberts BC, Epstein DL, Allingham RR. Isolation of primary open-angle glaucomatous trabecular meshwork cells from whole eye tissue. Curr Eye Res. 2000;20:347–350. doi: 10.1076/0271-3683(200005)2051-1FT347. [DOI] [PubMed] [Google Scholar]
- 41.Stamer WD, Clark AF. The many faces of the trabecular meshwork cell. Exp Eye Res. 2017;158:112–123. doi: 10.1016/j.exer.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jurisic V, Srdic-Rajic T, Konjevic G, Bogdanovic G, Colic M. TNF-α induced apoptosis is accompanied with rapid CD30 and slower CD45 shedding from K-562 cells. J Membr Biol. 2011;239:115–122. doi: 10.1007/s00232-010-9309-7. [DOI] [PubMed] [Google Scholar]
- 43.Gracey AY, Fraser EJ, Li W, Fang Y, Taylor RR, Rogers J, Brass A, Cossins AR. Coping with cold: An integrative, multitissue analysis of the transcriptome of a poikilothermic vertebrate. Proc Natl Acad Sci. 2004;101:16970–16975. doi: 10.1073/pnas.0403627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kanehisa M, Goto S, Kawashima S, Okuno Y, Hattori M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004;32:D277–D280. doi: 10.1093/nar/gkh063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Brien ET, Kinch M, Harding TW, Epstein DL. A mechanism for trabecular meshwork cell retraction: ethacrynic acid initiates the dephosphorylation of focal adhesion proteins. Exp Eye Res. 1997;65:471–483. doi: 10.1006/exer.1997.0357. [DOI] [PubMed] [Google Scholar]
- 46.Honjo M, Tanihara H. Impact of the clinical use of ROCK inhibitor on the pathogenesis and treatment of glaucoma. Jpn J Ophthalmol. 2018;62:109–126. doi: 10.1007/s10384-018-0566-9. [DOI] [PubMed] [Google Scholar]
- 47.Faralli JA, Schwinn MK, Gonzalez JM, Jr, Filla MS, Peters DM. Functional properties of fibronectin in the trabecular meshwork. Exp Eye Res. 2009;88:689–693. doi: 10.1016/j.exer.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eliáš M, Klimeš V. Rho GTPases: Deciphering the evolutionary history of a complex protein family. Methods Mol Biol. 2012;827:13–34. doi: 10.1007/978-1-61779-442-1_2. [DOI] [PubMed] [Google Scholar]
- 49.Wang CY, Liu PY, Liao JK. Pleiotropic effects of statin therapy: Molecular mechanisms and clinical results. Trends Mol Med. 2008;14:37–44. doi: 10.1016/j.molmed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu HP, Chen CC, Wu CC, Huang YC, Liu SC, Liang Y, Chang KP, Chang YS. Epstein-Barr virus-encoded LMP1 interacts with FGD4 to activate Cdc42 and thereby promote migration of nasopharyngeal carcinoma cells. PLoS Pathog. 2012;8:e1002690. doi: 10.1371/journal.ppat.1002690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhoopathi P, Gondi CS, Gujrati M, Dinh DH, Lakka SS. SPARC mediates Src-induced disruption of actin cytoskeleton via inactivation of small GTPases Rho-Rac-Cdc42. Cell Signal. 2011;23:1978–1987. doi: 10.1016/j.cellsig.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.