Spondyloarthritis (SpA) is the most common extra-intestinal manifestation associated with active inflammatory bowel disease (IBD).1,2 The paucity of cohorts and trials using validated SpA diagnostic criteria and disease activity indices unfortunately limits the available data to define the efficacy of biologic therapy for IBD on joint symptoms. IBD-associated SpA can be classified into axial SpA or peripheral SpA (pSpA) (arthritis, enthesitis, or dactylitis) using diagnostic criteria established by Assessment of SpondyloArthritis International Society (ASAS).3 Validated clinical SpA disease activity indices are crucial to longitudinally track the response of SpA symptoms in a clinical setting4, 5. The aim of this study was to apply SpA diagnostic criteria and disease activity indices to assess intestinal and joint response to biologic therapy in IBD subjects with pSpA.
We analyzed 1032 IBD subjects (593 Crohn's disease, 439 ulcerative colitis) with prospective collection of clinical and endoscopic disease activity scores from the JRI Live Cell Biobank at Weill Cornell Medicine. Axial SpA or pSpA was defined by clinical and radiographic criteria established by the ASAS,3 and joint disease activity was assessed prospectively with the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI). Subjects with pSpA initiating biologic therapy for active intestinal disease were included in the longitudinal cohort.
Using ASAS diagnostic criteria, pSpA was the most prevalent extra-intestinal manifestation in IBD subjects and more prevalent in CD compared with UC (23.7% vs 12.4%, P < .0001, Table and Table A1). Subjects with pSpA were significantly more likely to be on steroids or biologic therapy and have a current or previous exposure to biologic therapy (63% vs 52%, P = .0094, Table). CD subjects with pSpA were less likely to be in clinical remission than those without SpA (43% vs 65%, P < .0001, Table), but no difference was noted in their Montreal classification (Table A2). Consistent with the overall concordance of pSpA with intestinal symptoms, subjects with active CD had a higher mean BASDAI than those in clinical remission (3.5 vs 2.6, P = .043, N = 106) (Table). No differences were observed in the Simple Endoscopic Score for Crohn's Disease or Mayo score between IBD and IBD-pSpA cohorts (CD: N = 258, UC: N = 434).
Table.
Cohort Demographics and Clinical Characteristics Stratified by pSpA
| Variable, n (%) | Total (n = 1032) | pSpA (n = 192) | No SpA (n = 840) | P-value |
|---|---|---|---|---|
| IBD type | <.0001 | |||
| Crohn’s disease | 593 (57) | 138 (72) | 455 (54) | |
| Ulcerative colitis | 439 (43) | 54 (28) | 385 (46) | |
| Age, y, mean (± SD) | 47.3 (16.4) | 45.9 (15.5) | 47.6 (16.6) | .27 |
| Sex | .0002 | |||
| Male | 447 (43) | 60 (31) | 387 (46) | |
| Female | 585 (57) | 132 (69) | 453 (54) | |
| Smoking status | .19 | |||
| Current | 29 (3) | 9 (5) | 20 (2) | |
| Former | 192 (19) | 38 (20) | 154 (19) | |
| Never | 776 (78) | 139 (75) | 637 (79) | |
| Previous surgery | .38 | |||
| Yes | 349 (34) | 69 (37) | 280 (34) | |
| No | 667 (66) | 117 (63) | 550 (66) | |
| Crohn’s disease | ||||
| HBI <5 | 350 (60) | 60 (43) | 290 (65) | < .0001 |
| Mean BASDAI (± SD) | 2.6 (2.2) | .043 | ||
| HBI ≥5 | 235 (40) | 78 (57) | 157 (35) | |
| Mean BASDAI (± SD) | 3.5 (2.2) | |||
| SES-CD | .59 | |||
| Total SES-CD, ≤3 | 56 (25) | 15 (27) | 41 (24) | |
| Total SES-CD, >3 | 172 (75) | 40 (73) | 132 (76) | |
| Ulcerative colitis | ||||
| Total Mayo score, ≤2 | 245 (56) | 28 (53) | 217 (57) | .57 |
| Mean BASDAI (± SD) | 2.6 (2.4) | 164 (43) | .51 | |
| Total Mayo score, >2 | 189 (44) | 25 (47) | ||
| Mean BASDAI (± SD) | 2.1 (2.1) | |||
| Current treatment | ||||
| 5-ASA | 423 (41) | 76 (40) | 347 (41) | .66 |
| Immunomodulator | 104 (10) | 22 (11) | 82 (10) | .48 |
| Steroids/biologics | 453 (44) | 100 (52) | 353 (42) | .011 |
| Steroids | 103 (10) | 23 (12) | 80 (10) | .31 |
| Biologics | 392 (38) | 84 (44) | 308 (37) | .068 |
| Anti-TNFα | 211 (20) | 41 (21) | 170 (20) | .73 |
| Vedolizumab | 87 (8) | 15 (8) | 72 (9) | .73 |
| Ustekinumab | 101 (10) | 28 (15) | 73 (9) | .0013 |
| Current/previous biologic | 558 (54) | 120 (63) | 438 (52) | .0094 |
| Infliximab | .36 | |||
| Responder | 123 (46) | 24 (40) | 99 (47) | |
| PNR | 67 (25) | 14 (23) | 53 (25) | |
| SNR | 79 (29) | 22 (37) | 57 (27) | |
| Adalimumab | < .0001 | |||
| Responder | 61 (30) | 8 (16) | 53 (35) | |
| PNR | 88 (44) | 17 (35) | 71 (46) | |
| SNR | 53 (26) | 24 (49) | 29 (19) | |
| Vedolizumab | .27 | |||
| Responder | 56 (46) | 9 (41) | 47 (47) | |
| PNR | 45 (37) | 11 (50) | 34 (34) | |
| SNR | 22 (18) | 2 (9) | 20 (20) | |
| Ustekinumab | .69 | |||
| Responder | 58 (59) | 17 (65) | 41 (57) | |
| PNR | 29 (30) | 6 (23) | 23 (32) | |
| SNR | 11 (11) | 3 (12) | 8 (11) |
Subject number and percentage are shown. Mann-Whitney test and Pearson’s chi-squared test were used to determine the P-value for differences between subjects with and without SpA.
PNR, primary nonresponse; SNR, secondary nonresponse.
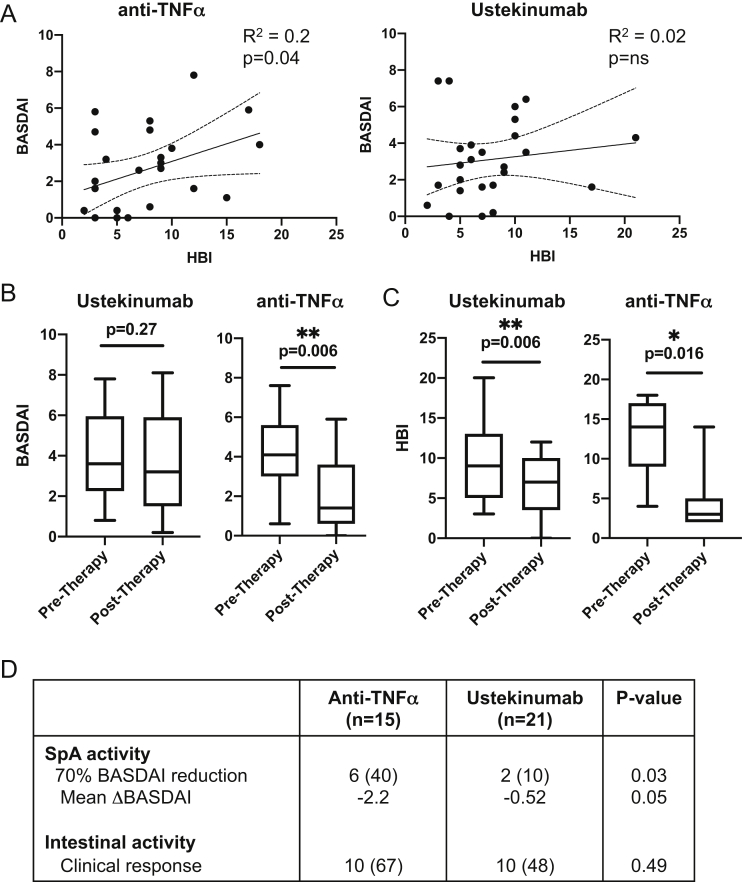
Although a higher proportion of subjects with pSpA were treated with ustekinumab (UST) than those without pSpA (15% vs 9%, P = .0013, Table), the impact of UST on pSpA in IBD is not clear. Linear regression analysis of intestinal disease activity (Harvey Bradshaw Index [HBI]) and joint disease activity (BASDAI) revealed a significant correlation for CD pSpA subjects treated with tumor necrosis factor-alpha inhibitors (anti-TNFα, N = 26, R2 = 0.2, P = .04), but not for CD pSpA subjects treated with UST (N = 28) (Figure A). To investigate this discordant response of joint symptoms in CD pSpA treated with UST, 36 sequential patients with IBD-pSpA initiating biologic therapy (21 with UST and 15 with anti-TNFα) for active intestinal disease were longitudinally assessed for intestinal and SpA symptoms before and after induction therapy (Table A3). Similar to previous reports,6 anti-TNFα therapy significantly reduced the BASDAI (4.3 vs 2.2, P = .006, Figure B) and HBI (12.4 vs 4.2, P = .016, Figure C). In contrast, induction therapy with UST resulted in no change in the BASDAI (4.0 vs 3.5, P = .27, Figure B) despite a significant reduction in the HBI (9.5 vs 6.4, P = .006, Figure C). Assessment of 70% reduction in the BASDAI revealed that a lower proportion of UST-treated patients achieved a joint clinical response in pSpA after induction therapy than anti-TNFα–treated patients (10% vs 40%, 0.03, Figure D). Furthermore, anti-TNFα therapy resulted in an average BASDAI reduction >1.1, whereas UST therapy did not, despite no significant difference between the two in intestinal clinical response.
Figure.
Intestinal but not joint activity is responsive to ustekinumab therapy in Crohn’s disease with peripheral spondyloarthritis. (A) Linear regression analysis of intestinal disease activity (Harvey Bradshaw Index) and joint disease activity (BASDAI) of CD pSpA subjects treated with anti-TNFα (n = 26) or ustekinumab (n = 28). (B) The BASDAI before and after ustekinumab or anti-TNFα induction therapy. Box plots indicate median and quartiles. P-values are indicated, 2-tailed paired T-tests. Ustekinumab, n = 21, anti-TNFα, n = 15. (C) The Harvey-Bradshaw Index before and after ustekinumab or anti-TNFα induction therapy. Box plots indicate median and quartiles. P-values are indicated, 2-tailed paired T-tests. Ustekinumab, n = 17, Anti-TNFα, n = 9. (D) Longitudinal assessment of intestinal disease activity (Harvey Bradshaw Index) and joint disease activity (BASDAI) before and after induction therapy. The Wilcoxon matched-pairs signed rank test was used to determine the P-value between subjects with and without SpA. Seventy percent reduction in the BASDAI and overall mean change in the ΔBASDAI after induction therapy are shown. Intestinal clinical response was defined by an HBI decrease of ≥3 (CD) or a partial Mayo decrease of ≥2 and ≥30% with a decrease in rectal bleeding subscore (RBS) of ≥1 point or an absolute RBS of 0 or 1 (UC).
SpA symptoms are common in IBD and frequently associated with intestinal disease activity, but the paucity of studies using validated diagnostic criteria and disease activity scoring limits our understanding of disease burden in this common entity. Using ASAS diagnostic criteria for SpA, our results confirm the high prevalence of pSpA in subjects with IBD along with the higher utilization of steroid and/or immunosuppressive therapy independent of intestinal disease activity. Consistent with the concordance of peripheral joint disease with intestinal disease, our data reveal that the validated BASDAI for SpA in subjects with pSpA correlates with intestinal disease and captures systemic symptoms not reflected solely in endoscopic severity. Collectively, this work highlights the value of using ASAS diagnostic criteria and SpA disease activity scores for future studies.
The higher prevalence of therapy in the pSpA cohort underscores the need for treatment algorithms based on the SpA phenotype and disease activity. Anti-TNFα therapy is an established first-line therapy for axial SpA7 and has been shown to be effective in CD-associated axial SpA and non-IBD pSpA.6 Although UST is effective for the treatment of intestinal CD symptoms and psoriatic arthritis, it is not effective therapy for axial SpA.8 Post hoc analysis of the UNITI-1/2 and IM-UNITI cohorts revealed no impact of UST on arthritis or arthralgia at week 6 or week 52 compared with placebo.9 Consistent with these findings, our data show that although UST and anti-TNFα induced equivalent rates of clinical response of intestinal disease, UST did not significantly improve systemic joint symptoms using validated SpA disease activity indexes.
Two possible explanations of these findings include timing of assessment and underlying biology of pSpA. Our assessment was limited to a single time point after induction and does not exclude the possibility of delayed effect of UST on the BASDAI at later time points. Alternatively, these findings may mechanistically indicate a restricted effect of IL-12/23 blockade on the intestinal symptoms of IBD. Supporting this point, recent data suggest that IL-23 selective blockade with guselkumab is effective in patients with active psoriatic SpA.10 Most subjects in our longitudinal cohort treated with anti-TNFα (8/15) and UST (12/21) were biologic experienced and may represent a cohort with refractory underlying disease; however, given the lack of recent previous exposure, it is unlikely that this discordance with UST reflects anti-TNFα withdrawal. With the emergence of additional therapies for IBD, our results highlight the need for clinical tracking of SpA disease activity in future studies to help define effective treatment algorithms for this unique clinical entity.
Footnotes
Conflict of Interest: Dana Lukin has served as a consultant for AbbVie, Boehringer Ingelheim, Palatin, and Pfizer and received grant support from AbbVie, Janssen, Takeda, and the Kenneth Rainin Foundation. Randy S. Longman has served as a consultant for Pfizer and Bristol Myers Squibb. Ellen Scherl has served as a consultant and on the advisory board for AbbVie, Entera Health, Evidera, GI Health Foundation, Janssen, Protagonist Therapeutics, Seres Health, Takeda Pharmaceuticals, and Bristol Myers Squibb, received grant support from Abbott (AbbVie), AstraZeneca, the CCFA, Janssen Research and Development, Pfizer, UCB, the UCSF–CCFA Clinical Research Alliance, Genentech, Seres Therapeutics, and Celgene Corporation, is a shareholder of Gilead, and has received nonbranded Speakers Bureau honoraria from GI Health Foundation and Janssen. The remaining authors disclose no conflicts.
Funding:NIH/NIDKK114252 (R.S.L.). Support for the Jill Roberts Institute (JRI) IBD Live Cell Bank is provided by the JRI, Jill Roberts Center for IBD, Cure for IBD, and the Rosanne H. Silbermann Foundation.
Ethical Statement: The corresponding author, on behalf of all authors, jointly and severally, certifies that their institution has approved the protocol for any investigation involving humans or animals and that all experimentation was conducted in conformity with ethical and humane principles of research.
Data Transparency Statement: All data, analytic methods, and study materials are available and provided in the manuscript or supplementary materials.
Material associated with this article can be found in the online version at https://doi.org/10.1016/j.gastha.2021.12.002.
Supplementary Materials
References
- 1.Vavricka S.R., et al. Am J Gastroenterol. 2011;106:110–119. doi: 10.1038/ajg.2010.343. [DOI] [PubMed] [Google Scholar]
- 2.Ott C., et al. Nat Rev Gastroenterol Hepatol. 2013;10:585–595. doi: 10.1038/nrgastro.2013.117. [DOI] [PubMed] [Google Scholar]
- 3.Rudwaleit M., et al. Ann Rheum Dis. 2011;70:25–31. doi: 10.1136/ard.2010.133645. [DOI] [PubMed] [Google Scholar]
- 4.Helliwell P.S., et al. RMD Open. 2020;6:e001149. doi: 10.1136/rmdopen-2019-001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.da Costa I.P., et al. Rev Bras Reumatol. 2015;55:48–54. doi: 10.1016/j.rbr.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Generini S., et al. Ann Rheum Dis. 2004;63:1664–1669. doi: 10.1136/ard.2003.012450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward M.M., et al. Arthritis Rheumatol. 2019;71:1599–1613. doi: 10.1002/art.41042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deodhar A., et al. Arthritis Rheumatol. 2019;71:258–270. doi: 10.1002/art.40728. [DOI] [PubMed] [Google Scholar]
- 9.Narula N., et al. United Eur Gastroenterol J. 2021;9:581–589. doi: 10.1002/ueg2.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mease P.J., et al. Lancet. 2020;395:1126–1136. doi: 10.1016/S0140-6736(20)30263-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.