Abstract
Objective
To assess the household secondary infection risk (SIR) of B.1.1.7 (Alpha) and non-Alpha lineages of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) among children.
Study design
During January to April 2021, we prospectively followed households with a SARS-CoV-2 infection. We collected questionnaires, serial nasopharyngeal swabs for reverse transcription polymerase chain reaction testing and whole genome sequencing, and serial blood samples for serology testing. We calculated SIRs by primary case age (pediatric vs adult), household contact age, and viral lineage. We evaluated risk factors associated with transmission and described symptom profiles among children.
Results
Among 36 households with pediatric primary cases, 21 (58%) had secondary infections. Among 91 households with adult primary cases, 51 (56%) had secondary infections. SIRs among pediatric and adult primary cases were 45% and 54%, respectively (OR, 0.79; 95% CI, 0.41-1.54). SIRs among pediatric primary cases with Alpha and non-Alpha lineage were 55% and 46%, respectively (OR, 1.52; 95% CI, 0.51-4.53). SIRs among pediatric and adult household contacts were 55% and 49%, respectively (OR, 1.01; 95% CI, 0.68-1.50). Among pediatric contacts, no significant differences in the odds of acquiring infection by demographic or household characteristics were observed.
Conclusions
Household transmission of SARS-CoV-2 from children and adult primary cases to household members was frequent. The risk of secondary infection was similar among child and adult household contacts. Among children, household transmission of SARS-CoV-2 and the risk of secondary infection was not influenced by lineage. Continued mitigation strategies (eg, masking, physical distancing, vaccination) are needed to protect at-risk groups regardless of virus lineage circulating in communities.
Keywords: alpha variant, children, COVID-19, household transmission
Abbreviations: CDC, US Centers for Disease Control and Prevention; COVID-19, Coronavirus disease 2019; GEE, Generalized estimating equation; NP, Nasopharyngeal; RT-PCR, Reverse transcription polymerase chain reaction; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; SIR, Secondary infection risk; VOC, Variant of concern
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), has affected more than 70 million people in the US, including children, as of January 2022.1 Compared with adults, children frequently exhibit SARS-CoV-2 infections that are less severe and are more often asymptomatic.2 , 3 Prior US household transmission investigations demonstrated that children acquired SARS-CoV-2 infection at similar rates as adults; however, less is known about transmission from children and how transmission might be affected by emerging variant lineages.4, 5, 6
Several variants of concern (VOCs) have emerged during the pandemic, which have affected SARS-CoV-2 transmission, susceptibility, and disease severity.7 , 8 The SARS-CoV-2 B.1.1.7 (Alpha) lineage was first identified in the UK in September 2020 and emerged in the US in December 2020, becoming the predominant variant within 4 months.7 , 9 From December 2020 to September 2021, the Alpha variant was classified as a VOC in the US. Prior studies have indicated that the Alpha variant was more efficiently transmitted compared with previous lineages.10 , 11
We conducted a household transmission investigation pre-B.1.617.2 (Delta) and pre-B.1.1.529 (Omicron) variant emergence, during the months of January to April 2021 when Alpha was the most prevalent lineage in the US. Using detailed epidemiological data and multiple testing modalities, we estimate household secondary infection risks (SIRs), describe characteristics associated with transmission and infection, and compare symptom profiles of children infected with Alpha and non-Alpha SARS-CoV-2 lineages in household settings.
Methods
Household Enrollment
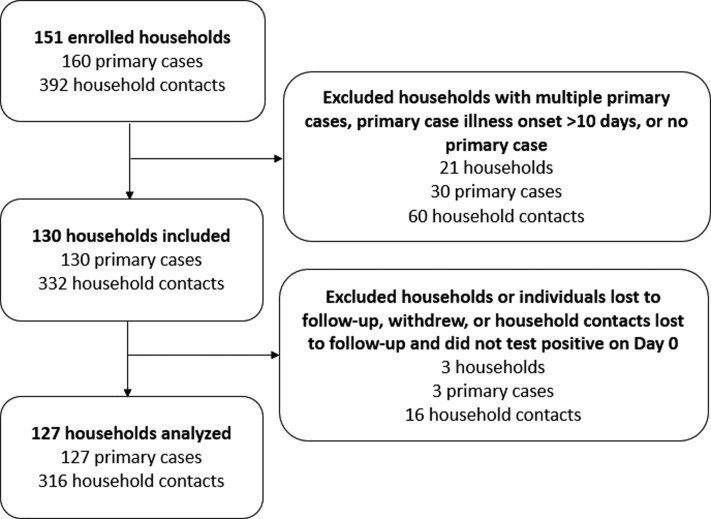
From January to April 2021, the US Centers for Disease Control and Prevention (CDC) was invited to collaborate with state and local health departments in San Diego County, California, and metropolitan Denver (Adam, Arapahoe, and Douglas counties), Colorado, to conduct household transmission investigations.12 Public health agencies reported persons in households with a positive SARS-CoV-2 reverse transcription polymerase chain reaction (RT-PCR) specimen to CDC’s investigation team to be screened for enrollment eligibility.12 Households were eligible if the primary case was not hospitalized at enrollment, lived with 1 or more persons, did not live in a congregate setting, and had illness onset 10 or fewer days before enrollment (Figure 1; available at www.jpeds.com).
Figure 1.
Enrollment of households in a COVID-19 household transmission investigation, California and Colorado, January to April 2021.
Household Case Classifications and Definitions
The primary case was defined as the person within the household with the earliest illness onset date. Illness onset date was defined as symptom onset date, or, if asymptomatic, collection date of the initial SARS-CoV-2 RT-PCR-positive specimen. Household contacts were household members spending 1 or more nights in the household during the infectious period of the primary case, defined as 2 days before through 10 days after the illness onset date.13 Households were excluded if all household members were lost to follow-up, withdrew during the 14-day follow-up, or if the primary case could not be determined because multiple persons in a household became symptomatic or tested positive on the same day (ie, co-primary infections).
Primary and secondary case classifications were retroactively assigned after the evaluation. Secondary cases were defined as household contacts who had a positive RT-PCR for SARS-CoV-2 during the investigation period, converted from SARS-CoV-2 immunoglobulin G (IgG) negative on enrollment to SARS-CoV-2 IgG positive on closeout without a history of vaccination, or were seropositive on enrollment with new-onset symptoms without a history of vaccination or a previous SARS-CoV-2 infection. Pediatric household members (aged <18 years) were classified as young children (≤11 years) or adolescents (12-17 years).
Household Visits
An in-person visit was conducted at enrollment (day 0) and closeout (day 14). Questionnaires capturing demographic characteristics, medical histories, recent symptoms, previous SARS-CoV-2 testing, and preventive behaviors were administered to all household members at enrollment and closeout. For young children, a parent or guardian assisted them in completing the questionnaire. A household-level questionnaire captured the physical characteristics of the residence (eg, square footage, number of bedrooms and bathrooms).
Blood for serology and nasopharyngeal (NP) swabs for RT-PCR were collected from household members on enrollment and closeout. Household members self-completed daily symptom diaries using a standardized form with yes/no responses. At enrollment, some households were offered daily home antigen testing by QuickVue At-Home OTC COVID-19 Test (Quidel). Household members were asked to perform an antigen test each day, regardless of symptom status. Antigen test results were submitted daily to the investigation team. During the 14-day follow-up period, if a household contact developed new or worsening symptoms, or newly tested positive by home antigen testing, an interim visit was conducted to collect an NP swab for RT-PCR from all household members. A parent or guardian used discretion to determine if children needed assistance with daily antigen testing and symptom diaries during the 14-day follow-up.
Vaccination Status and Symptom Categorization
Individuals were considered fully vaccinated 14 or more days after the completion of all recommended doses of an US Food and Drug Administration-authorized COVID-19 vaccine (the primary series), partially vaccinated if fewer than 14 days since completing the primary series or did not complete the series, and unvaccinated if no COVID-19 vaccine was received.14 The date and type of SARS-CoV-2 vaccination was verified through direct observation of vaccination cards when available.
Symptoms were categorized as constitutional (measured or subjective fever, chills, myalgia, and fatigue), upper respiratory (runny nose, nasal congestion, and sore throat), lower respiratory (cough, difficulty breathing, shortness of breath, wheezing, and chest pain), neurologic (headache, loss of taste, and loss of smell), and gastrointestinal (nausea and/or vomiting, diarrhea, and abdominal pain).
Laboratory Testing
The San Diego County Public Health Laboratories performed RT-PCR testing of NP swabs for SARS-CoV-2 using the Perkin Elmer New Coronavirus Nucleic Acid Detection Kit and Colorado Department of Public Health and Environment Laboratory used the TaqPath COVID-19 Combo Kit (ThermoFisher Scientific). Whole genome sequencing was conducted on at least one NP specimen from participants with a RT-PCR cycle threshold value of less than 35 (see Appendix for Supplemental Methods; available at www.jpeds.com). Serum was separated from whole blood samples and stored at –70°C until tested for presence of SARS-CoV-2-specific antibodies using the VITROS Immunodiagnostic Products Anti-SARS-CoV-2 IgG Reagent Pack (Ortho-Clinical Diagnostics), xMAP SARS-CoV-2 Multi-Antigen IgG Assay (Luminex), or Alinity i SARS-CoV-2 IgG assay (Abbott Laboratories).
The Phylogenetic Assignment of Named Global Outbreak Lineages was used to assign SARS-CoV-2 lineages to sequenced genomes.15 A lineage was assigned to each household as determined by the lineage of a representative sequence obtained from the primary case or sequenced household member if sequencing results were unavailable for the primary case. VOCs were defined based on the CDC variant classification scheme at the time the evaluation occured.8
Analyses
Demographic characteristics of pediatric household members were described by case classification (ie, primary case, secondary case, or uninfected household contact) and lineage (Alpha or non-Alpha lineages). Unadjusted ORs and 95% CIs were estimated using generalized estimating equations (GEEs) to compare the frequencies of symptoms between pediatric and adult cases, and among pediatric cases by lineage. GEEs with an exchangeable correlation matrix were used to account for household clustering.4 Wilcoxon rank-sum tests were used to compare symptom duration between pediatric and adult cases, and among pediatric cases by lineage. Those who remained symptomatic at closeout were treated as if their symptoms had resolved on day 14.
SIRs were calculated as the proportion of secondary cases among all nonexcluded household contacts. Contacts were excluded from SIR calculations and risk factor analyses if they were fully vaccinated or had a previously documented SARS-CoV-2 infection. SIRs were estimated (1) by age and lineage (Alpha vs non-Alpha) of the primary case and (2) by age and lineage of household contacts. ORs and 95% CIs were calculated using GEEs to compare SIRs in each primary and household contact age group and lineage group.
Among pediatric household contacts, unadjusted ORs and 95% CIs were estimated using GEEs to assess risk factors for infection. Within the GEE framework, type III tests were used to assess differences in percent positivity among age groups and lineage; P values were based on the limiting χ2 distributions.
Using Fisher exact tests, amino acid polymorphisms in the S-gene were screened for associations between the age of the primary case (pediatric or adult) and SIR (≥50% or <50%), evaluating each site at which 2 different amino acids were observed in 5 or more of the household representative sequences. P values of less than .05 were considered statistically significant. All analyses were performed using SAS 9.4 (SAS Institute Inc); figures were prepared using Microsoft Excel and R version 4.0.4 (R Foundation for Statistical Computing).
Ethical Considerations
This activity was reviewed by CDC and was conducted consistent with applicable federal law and CDC policy.16, 17, 18, 19, 20 All household members provided written consent, child assent with parental permission, or parent permission before participation.
Results
Individuals from 151 households were enrolled; 24 households were excluded during analysis (Figure 1). After exclusion, 127 households with a single primary case and 316 household contacts were available for analysis. The median time interval from primary case illness onset to enrollment was 6 days (range, 2-10). Pediatric household members represented 28% (36/127) of primary cases and 36% (115/316) of household contacts.
Of the 151 pediatric household members (including cases and household contacts), 73 (48%) were young children and 78 (52%) were adolescents (Table I ). The median age of pediatric household members was 12 years (IQR, 7-15). Underlying medical conditions were uncommon; the most frequently reported condition was chronic lung disease (13%). No pediatric cases were hospitalized, and none died. Most (147/151, 97%) pediatric household members were unvaccinated; 4 (3%) (2 primary and 2 secondary cases) were partially vaccinated. Alpha was the most common SARS-CoV-2 lineage identified in pediatric primary cases (n = 21 [58%]) and secondary cases (n = 39 [64%]). Demographic and clinical characteristics among pediatric cases with Alpha and non-Alpha lineages are displayed in Table II (available at www.jpeds.com).
Table I.
Demographic and clinical characteristics of pediatric household members, California and Colorado, January to April 2021 (n = 151)
| Characteristics | Pediatric primary cases (n = 36), n (%) | Pediatric secondary cases (n = 61), n (%) | Uninfected pediatric household contacts (n = 54), n (%) |
|---|---|---|---|
| Age, years | |||
| <5 | 6 (17) | 9 (15) | 8 (15) |
| 5-11 | 8 (22) | 25 (41) | 17 (31) |
| 12-17 | 22 (61) | 27 (44) | 29 (54) |
| Median (IQR) | 13 (7-15) | 11 (6-14) | 12 (7-15) |
| Sex | |||
| Male | 20 (56) | 33 (54) | 23 (43) |
| Female | 16 (44) | 28 (46) | 31 (57) |
| Race/ethnicity | |||
| Non-Hispanic White | 24 (67) | 26 (43) | 31 (57) |
| Non-Hispanic Black | 1 (3) | 2 (3) | 3 (6) |
| Non-Hispanic Asian | 0 (0) | 8 (13) | 3 (6) |
| Non-Hispanic American Indian/Alaska Native | 0 (0) | 2 (3) | 0 (0) |
| Non-Hispanic Native Hawaiian/Pacific Islander | 1 (3) | 0 (0) | 3 (6) |
| Non-Hispanic other∗ | 5 (14) | 3 (5) | 5 (9) |
| Hispanic (any race) | 5 (14) | 20 (33) | 9 (17) |
| Underlying medical conditions | |||
| Any medical condition | 6 (17) | 11 (18) | 15 (28) |
| Chronic lung disease (including asthma) | 2 (6) | 7 (11) | 10 (19) |
| Cardiovascular condition | 0 (0) | 0 (0) | 1 (2) |
| Immunocompromising condition | 0 (0) | 1 (2) | 2 (4) |
| Other chronic condition† | 4 (11) | 5 (8) | 7 (13) |
| Vaccination status‡ | |||
| Fully vaccinated | 0 (0) | 0 (0) | 0 (0) |
| Partially vaccinated | 2 (6) | 2 (3) | 0 (0) |
| Not vaccinated | 34 (94) | 59 (97) | 54 (100) |
These individuals either identified as Non-Hispanic Black, Asian, American Indian/Alaska Native, Native Hawaiian/Pacific Islander, belonging to a race/ethnicity not listed on the questionnaire or belonging to multiple races/ethnicities. Individuals were grouped together for analysis to increase power of analysis.
Other chronic conditions included hypothyroidism (1), neurological condition (3), anxiety/depression (3), attention deficit disorder (1), attention deficit hyperactivity disorder (6), allergies (1), juvenile idiopathic arthritis (1), and celiac disease (1).
Individuals were considered fully vaccinated ≥14 days after completion of all recommended doses of an US Food and Drug Administration-authorized COVID-19 vaccine, partially vaccinated if <14 days since completing the primary series or did not complete the series, and unvaccinated if no COVID-19 vaccine was received.
Four pediatric household contacts were seropositive at enrollment or reported prior SARS-CoV-2 infection and were excluded from the RT-PCR positivity, SIR, and risk factor analyses. Among the 36 pediatric primary cases and 111 nonexcluded pediatric household contacts, 96 (65%) had a SARS-CoV-2 RT-PCR-positive specimen collected during the evaluation (Table III; available at www.jpeds.com). One pediatric case with Alpha lineage never tested positive by RT-PCR but was classified as a case based on day 0 seropositivity and new onset of symptoms without a history of infection or vaccination.
RT-PCR positivity did not differ significantly by age; among pediatric cases, positivity did not differ significantly by lineage. Overall, 75% of pediatric (105/140) and 76% of adult (172/227) household members participated in daily antigen testing. Blood specimens for serological assessment were not available for 56 pediatric and 17 adult household members.
Symptoms among Pediatric Household Members
Among the 96 RT-PCR-positive pediatric cases, 26 (27%) were asymptomatic at the time of first positive test (data not shown). Ten of 96 (10%) pediatric cases remained asymptomatic throughout the 14-day follow-up period, whereas 7 of 164 (4%) adult cases remained asymptomatic.
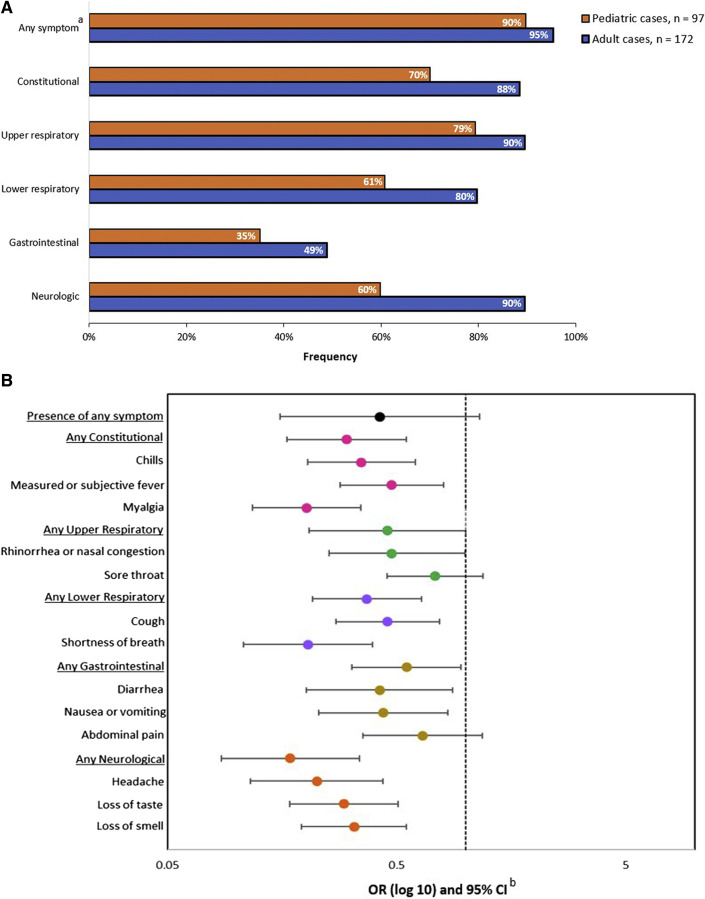
Among the 97 total pediatric cases, 87 (90%) reported at least 1 symptom during the evaluation (Figure 2 ). Upper respiratory (79%) and constitutional (70%) symptoms were the most common. The most reported individual symptoms were rhinorrhea or nasal congestion (72%), cough (61%), and headache (55%).
Figure 2.
Symptoms of COVID-19 among pediatric (n = 97) and adult (n = 172) household cases. A, Frequency of symptoms by age. B, ORs and 95% CIs for the presence of symptoms, comparing pediatric cases (n = 97) with adult household cases (n = 172). aSymptoms were categorized as constitutional (measured or subjective fever, chills, myalgia, and fatigue), upper respiratory (runny nose, nasal congestion, and sore throat), lower respiratory (cough, difficulty breathing, shortness of breath, wheezing, and chest pain), neurologic (headache, loss of taste, and loss of smell), and gastrointestinal (nausea and/or vomiting, diarrhea, and abdominal pain). bUnadjusted ORs and 95% CIs were estimated using generalized estimated equations with an exchangeable correlation matrix to account for household clustering. ORs and 95% CIs were graphed on a log scale.
When comparing pediatric and adult cases, pediatric cases had lower frequencies of reporting most symptoms (Figure 2). Pediatric cases were significantly less likely than adult cases to report constitutional (OR, 0.31; 95% CI, 0.15-0.65), lower respiratory (OR, 0.40; 95% CI, 0.22-0.75), gastrointestinal (OR, 0.54; 95% CI, 0.31-0.93), and neurological (OR, 0.10; 95% CI, 0.04-0.25) symptoms. The median symptom duration among pediatric cases was 3 days (IQR, 2-5 days), which did not differ significantly from the median among adults (4 days; IQR, 2-7 days) (P = .6).
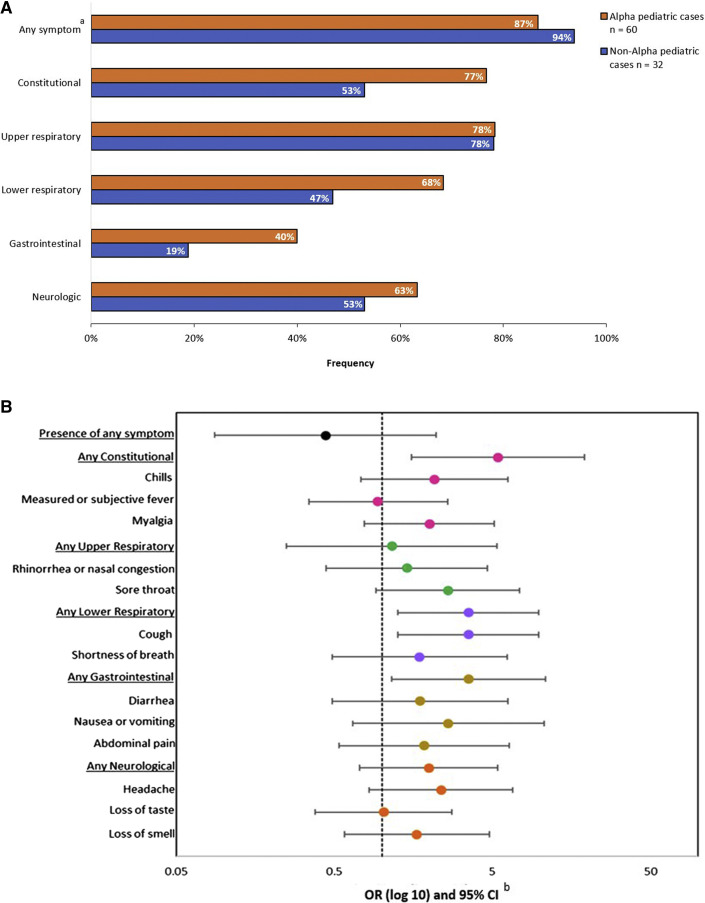
The frequencies of reporting any symptoms during this study were similar among pediatric cases with Alpha (87%) and non-Alpha (94%) lineages (OR, 0.44; 95% CI, 0.09-2.21) (Figure 3; available at www.jpeds.com). Pediatric cases with Alpha lineage were significantly more likely than cases with non-Alpha lineages to report constitutional (OR, 5.5; 95% CI, 1.55-19.20), lower respiratory (OR, 3.52; 95% CI, 1.26-9.86), and gastrointestinal (OR, 3.55; 95% CI, 1.16-10.89) symptoms. Pediatric cases with Alpha lineage experienced a similar duration of symptoms compared with non-Alpha lineages (median, 3 days vs 2 days, respectively; P = .64).
Figure 3.
Symptoms of COVID-19 among pediatric household cases by SARS-CoV-2 lineage (n = 92). A, Frequency of symptoms by lineage. B, ORs and 95% CIs for the presence of symptoms, comparing pediatric cases with B.1.1.7 (Alpha) lineage (n = 60) to pediatric cases with non-Alpha lineages (n = 32). aSymptoms were categorized as constitutional (measured or subjective fever, chills, myalgia, and fatigue), upper respiratory (runny nose, nasal congestion, and sore throat), lower respiratory (cough, difficulty breathing, shortness of breath, wheezing, and chest pain), neurologic (headache, loss of taste, and loss of smell), and gastrointestinal (nausea and/or vomiting, diarrhea, and abdominal pain). bUnadjusted ORs and 95% CIs were estimated using generalized estimated equations with an exchangeable correlation matrix to account for household clustering. ORs and 95% CIs were graphed on a log scale.
Secondary Infections Risks
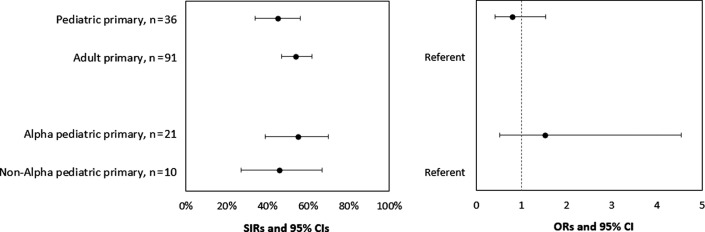
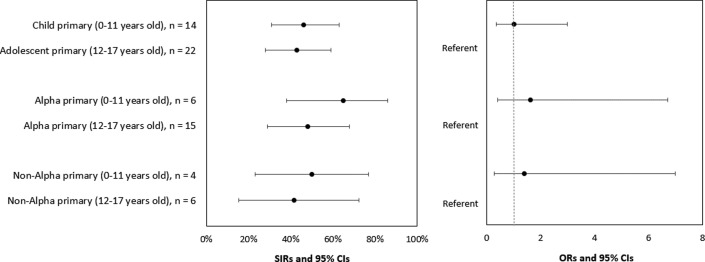
Among 36 households with pediatric primary cases, 21 (58%) had 1 or more secondary cases. Among 91 households with adult primary cases, 51 (56%) had 1 or more secondary cases. SIRs for household contacts of pediatric and adult primary cases were 45% and 54%, respectively (OR, 0.79; 95% CI, 0.41-1.54) (Figure 4 and Table IV; available at www.jpeds.com). The SIRs for household contacts of pediatric primary cases with Alpha and non-Alpha lineages were 55% and 46%, respectively (OR, 1.52; 95% CI, 0.51-4.53). The SIRs for the household contacts of young child and adolescent primary cases were 46% and 43%, respectively (OR, 1.02; 95% CI, 0.35-3.0) (Figure 5; available at www.jpeds.com).
Figure 4.
SIR of SARS-CoV-2 among household contacts, by age and SARS-CoV-2 lineage of primary cases, California and Colorado, January to April 2021. To account for household clustering, ORs and 95% CIs were calculated using GEEs with an exchangeable correlation matrix and used to compare the SIRs of the primary case to assess transmission risk (the probability of transmission from the primary case to household contacts) by age group (pediatric vs adult) and lineage group (B.1.1.7 [Alpha] vs non-Alpha). Four pediatric and 45 adult household contacts reported prior SARS-CoV-2 infection or seropositivity and were excluded from the SIR calculations. Specimens from 5 primary pediatric cases did not undergo sequencing; therefore, a lineage was not assigned to these individuals.
Figure 5.
SIR of SARS-CoV-2 among household contacts, by age and SARS-CoV-2 lineage of pediatric primary cases, California and Colorado, January to April 2021. To account for household clustering, ORs and 95% CIs were calculated by using GEEs with an exchangeable correlation matrix and used to compare SIRs of the primary case to assess transmission risk (the probability of transmission from the primary case to household contacts) by age group (pediatric vs adult) and lineage group (B.1.1.7 [Alpha] vs non-Alpha). Four pediatric and 45 adult household contacts reported prior SARS-CoV-2 infection or seropositivity and were excluded from the SIR calculations. Specimens from 5 primary pediatric cases did not undergo sequencing and therefore a lineage was not assigned to these individuals.
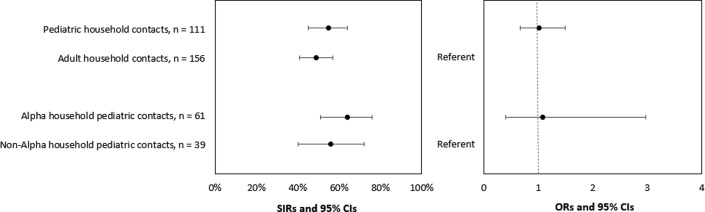
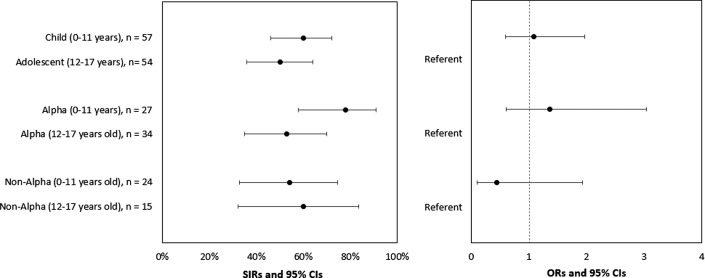
Among the 111 nonexcluded pediatric contacts, 61 (55%) tested positive by RT-PCR or seroconverted during the 14-day follow-up. The SIRs for pediatric and adult household contacts were 55% and 49%, respectively (OR, 1.01; 95% CI, 0.68-1.50) (Figure 6 and Table IV). The SIRs for pediatric contacts in households with Alpha and non-Alpha lineages were 64% and 56%, respectively (OR, 1.08; 95% CI, 0.40-2.98). The SIRs for young child and adolescent household contacts were 60% and 50%, respectively (OR, 1.09; 95% CI, 0.60-1.97) (Figure 7; available at www.jpeds.com). The inclusion of household contacts who were fully vaccinated or had a previously documented SARS-CoV-2 infection in SIR calculations did not result in significantly different SIRs than when these individuals were excluded (data not shown).
Figure 6.
SIR of SARS-CoV-2 among household contacts, by age and SARS-CoV-2 lineage, California and Colorado, January to April 2021. To account for household clustering, ORs and 95% CIs were estimated using GEEs with an exchangeable correlation matrix and used to compare SIRs of household contacts to assess infection risk (the probability of infection among household contacts) by age group (pediatric vs adult) and lineage group (B.1.1.7 [Alpha] vs non-Alpha). Four pediatric and 45 adult household contacts reported prior SARS-CoV-2 infection or seropositivity and were excluded from the SIR calculations. Lineages were assigned to uninfected pediatric household contacts based on the lineage of the household primary case. Eleven uninfected pediatric household contacts did not have a lineage assigned because specimens from primary cases did not undergo sequencing.
Figure 7.
SIR of SARS-CoV-2 among pediatric household contacts, by age and SARS-CoV-2 lineage, California and Colorado, January to April 2021. To account for household clustering, SIRs were estimated using GEEs with an exchangeable correlation matrix. ORs and 95% CIs were used to compare SIRs of household contacts to assess infection risk (the probability of infection among household contacts) by age group (pediatric vs adult) and lineage group (B.1.1.7 [Alpha] vs non-Alpha). Four pediatric household contacts reported prior SARS-CoV-2 infection or seropositivity and were excluded from the SIR calculations. Lineages were assigned to uninfected pediatric household contacts based on the lineage of the household primary case. Eleven uninfected pediatric household contacts did not have a lineage assigned because specimens from primary cases did not undergo sequencing.
Risk Factors for Infection Among Pediatric Contacts
There were no significant differences in the odds of acquiring infection among pediatric contacts by age, sex, race/ethnicity, underlying medical conditions, relationship to primary case, school or daycare attendance, number of household members, sharing of bedrooms and bathrooms, having direct or indirect contact with the primary case, maintaining physical distance (≥6 feet) with the primary case, or genomic lineage (Table V; available at www.jpeds.com).
Genomic Analysis
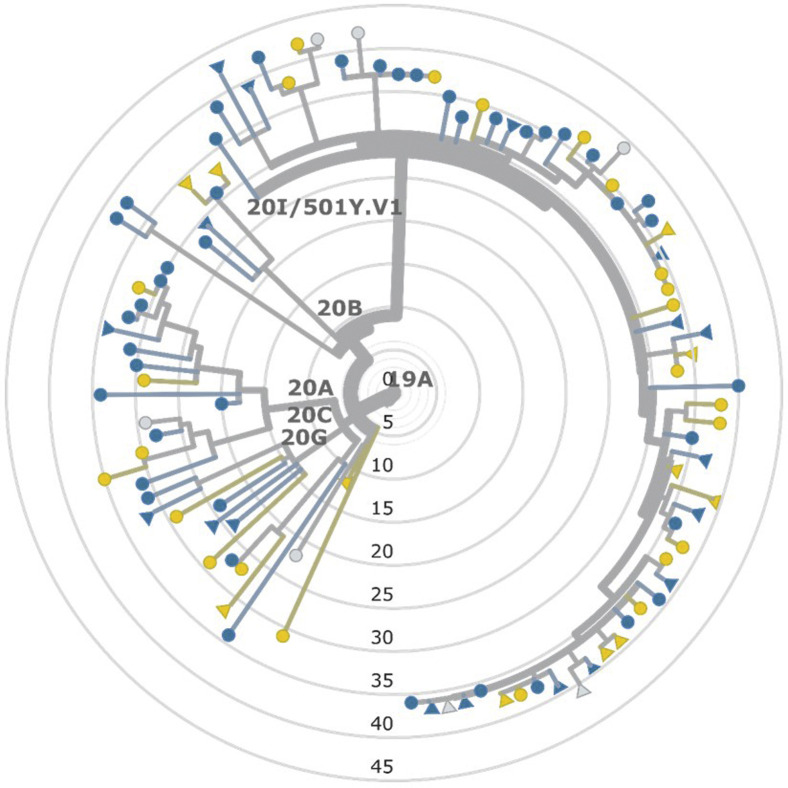
Sequence data were obtained for specimens from 212 household members from 104 households. Genetic analyses of RT-PCR-positive specimens showed no evidence of multiple introductions into households. Among the 59 households where SARS-CoV-2 sequences were recovered from multiple participants, lineages always matched, with sequences varying by 0-2 single nucleotide polymorphisms in 57 households and by 4 and 5 single nucleotide polymorphisms, respectively, in the remaining 2 households. No genetic association was detected with SIR differences between households or primary case age, based on the phylogeny of household representative sequences or with any of 13 S-gene amino acid polymorphisms from the same sequences (Figure 8; available at www.jpeds.com).
Figure 8.
Phylogenetic tree of representative SARS-CoV-2 genomes obtained from households in a COVID-19 household transmission investigation, by age and SIR, California and Colorado, January to April 2021. Sequence data were obtained for 212 household members from 104 households. Circles denote adult primary cases (n = 73); triangles denote pediatric primary cases (n = 31). A SIR of 50% or greater was binned as high and represented in blue (n = 62); a SIR of less than 50% was binned as low and represented in yellow (n = 35); gray denotes the absence of susceptible contacts within the household (n = 7).
Discussion
We present an analysis of SARS-CoV-2 household transmission among children immediately before the Delta wave of the pandemic when the Alpha variant was the most prominent lineage in the US. Household transmission of SARS-CoV-2 from child and adult primary cases to household members was frequent. We found similar SIRs among child and adult primary cases, contrary to prior household transmission investigations reporting SARS-CoV-2 transmission from adults at higher proportions than children.21, 22, 23, 24 Similarly, we found similar SIRs among young children and adolescent primary cases, despite prior studies reporting age-related differences in transmissibility.11 , 25 , 26 These findings could be explained by our universal testing independent of symptoms, allowing for comprehensive secondary case ascertainment among household contacts. Our ability to identify primary cases early in the acute phase of infection when they were likely most transmissible may have increased the robustness of our SIR estimates.27 , 28
We also found that pediatric household contacts were as likely as adult contacts to become infected with SARS-CoV-2. There is conflicting literature regarding how SARS-CoV-2 susceptibility among children compares with adults. Some studies have reported that children and adults acquired SARS-CoV-2 infection at similar proportions, and others have reported that children had lower susceptibility compared with adults.4, 5, 6 , 29, 30, 31 Elucidating pediatric susceptibility is complicated because transmission rates can be influenced by differences in behavior, exposure, and testing practices (ie, preferential testing of symptomatic individuals), which could lead to an underestimation of SARS-CoV-2 infections in children, who are less likely than adults to have symptoms when infected. Daily antigen testing likely facilitated the early identification of secondary cases and may have allowed us to identify more infections among asymptomatic household members compared with previous studies. The absolute proportion of symptomatic children in this investigation was higher than reported in previous studies.3 , 32 This finding could be attributed to differences in reporting symptoms daily, as was done in this investigation, compared with reporting symptoms retrospectively, which may underestimate the proportion of symptomatic children.
Although surveillance and modeling suggested that Alpha had increased transmissibility in community settings compared with non-Alpha lineages circulating at that time, in this investigation, household transmission of SARS-CoV-2 and risk of subsequent infection among children were not influenced by lineage.10 , 11 These findings suggest that substantial transmission can occur within households owing to shared living spaces, regardless of genomic lineage. At the time of investigation and analysis, many children were not eligible to receive COVID-19 vaccination, which underscores the importance of continued mitigation strategies (eg, masking, physical distancing) to protect children regardless of virus lineage circulating in the community.33, 34, 35, 36, 37, 38 Increases in pediatric cases, emergency department visits, and hospitalizations in the US, especially in situations where vaccinations among pediatric age groups are limited and in the context of emerging VOCs, such as Delta and Omicron, also highlight the importance of continued mitigation measures to protect this at-risk population.1 , 38 A further exploration of the relationship between household transmission and other emerging, highly transmissible lineages is warranted to aid in appropriate public health messaging and interventions to curb transmission.39
This analysis is subject to several limitations. First, the behavior of enrolled household members might not be reflective of behavior in the general population; those who enrolled might be more conscious of mitigating practices, which could have influenced transmission probability in this investigation. Similarly, exposures and transmission within households may differ from community transmission. Second, our sample size may have limited our ability to detect meaningful differences, especially regarding age strata within our pediatric population. We were unable to collect blood specimens from all pediatric household members, which may have influenced our ability to detect prior infection. This may have also affected our ability to identify pediatric household members who seroconverted during the investigation. Additionally, some pediatric infections may have been missed owing to seroconversion after the investigation concluded. Therefore, the true infection proportion among children may have been higher than reported. Although we attributed all illnesses to SARS-CoV-2 infection, it is possible that individuals who were only serologically positive with newly developed symptoms could have been ill from another respiratory pathogen. Finally, although our analysis attributes all transmission within households to primary cases, even with the viral genetic data, we cannot completely rule-out the possibility of tertiary transmission events (ie, subsequent contact-to-contact transmission events within a household). This factor could bias the SIR estimates and, therefore, limit the analysis of primary case and contact characteristics associated with transmission.
The transmission of SARS-CoV-2 from child and adult primary cases to other household members was frequent. Children were as likely as adults to acquire SARS-CoV-2 infection in household settings. Household transmission of SARS-CoV-2 and the risk of subsequent infection among children were not influenced by lineage, suggesting the substantial transmission that can occur within households owing to shared living spaces regardless of genomic lineage. Symptom profiles were different among children with Alpha and non-Alpha lineages, and between children and adults. These findings highlight the continued importance of mitigation measures to reduce SARS-CoV-2 transmission in households with at-risk groups. Although the Alpha variant is no longer a prominent circulating lineage, given the still limited information around SARS-CoV-2 transmission and susceptibility in children, this investigation adds to the growing literature providing understanding on the impact of current and emerging SARS-CoV-2 lineages among pediatric populations.
Data Statement
Data sharing statement available at www.jpeds.com.
Footnotes
The investigation was funded by the US CDC. The investigation was supported by the County of San Diego Health and Human Services Agency, California Department of Public Health, Colorado Department of Public Health and Environment, and Tri-County Health Department. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC. The authors declare no conficts of interest.
Contributor Information
the COVID-19 Laboratory & Testing Task Force:
Alexis Alford, Samuel Baird, Laura Bankers, Jazmin Bello, Shanna Bolcen, Peter Browning, Peter W. Cook, Ebenezer David, Jennifer L. Harcourt, Geir Hareland, Jr., Molly C. Hetherington-Rauth, Diana Ir, Shilpi Jain, Tao Lily Jia, Ralen Johnson, Anna Kelleher, Gimin Kim, Yan Li, Brian Lynch, Daniel Mallal, Panagiotis Maniatis, Rachel Marine, Magdalena Medrzycki, John M. Metz, Anna Maria Montmayeur, Kimberly M. Moss, Han Jia Justin Ng, Van Nyugen, Kristina Ortiz, Clinton R. Paden, So Hee Park, Krista Queen, Alexandria E.B. Rossheim, Vera Semenova, Samuel S. Shepard, Azaibi Tamin, Ying Tao, Alexandra Tejada-Strop, Phili Wong, Briana Zellner, and Jing Zhang
Appendix
Table II.
Demographic and clinical characteristics of pediatric cases and household contacts, by SARS-CoV-2 lineage, California and Colorado, January to April 2021 (n = 151)
| Characteristics | Pediatric SARS-CoV-2 cases (n = 97) |
Uninfected pediatric household contacts (n = 54), n (%) | ||
|---|---|---|---|---|
| Alpha lineage (n = 60), n (%) | Non-Alpha lineages∗ (n = 32), n (%) | Unable to sequence (n = 5), n (%) | ||
| Age, years | ||||
| <5 | 5 (8) | 7 (22) | 3 (60) | 8 (15) |
| 5-11 | 22 (37) | 10 (31) | 1 (20) | 17 (31) |
| 12-17 | 33 (55) | 15 (47) | 1 (20) | 29 (54) |
| Median age (IQR) | 13 (9-15) | 11 (6-15) | 9 (4-13) | 12 (7-15) |
| Sex | ||||
| Male | 36 (60) | 14 (44) | 3 (60) | 23 (43) |
| Female | 24 (40) | 18 (56) | 2 (40) | 31 (57) |
| Race/ethnicity | ||||
| Non-Hispanic White | 30 (50) | 17 (53) | 3 (60) | 31 (57) |
| Non-Hispanic Black | 3 (5) | 0 (0) | 0 (0) | 3 (6) |
| Non-Hispanic Asian | 8 (13) | 0 (0) | 0 (0) | 3 (6) |
| Non-Hispanic American Indian/Alaska Native | 2 (3) | 0 (0) | 0 (0) | 0 (0) |
| Non-Hispanic Native Hawaiian/Pacific Islander | 0 (0) | 0 (0) | 1 (20) | 3 (6) |
| Non-Hispanic other† | 6 (10) | 1 (3) | 1 (20) | 5 (9) |
| Hispanic (any race) | 11 (18) | 14 (44) | 0 (0) | 9 (17) |
| Underlying medical conditions | ||||
| Any medical condition | 11 (18) | 5 (16) | 1 (20) | 15 (28) |
| Chronic lung disease (any including asthma) | 5 (8) | 4 (13) | 0 (0) | 10 (19) |
| Cardiovascular condition | 0 (0) | 0 (0) | 0 (0) | 1 (2) |
| Immunocompromising condition | 1 (2) | 0 (0) | 0 (0) | 2 (4) |
| Other chronic condition‡ | 6 (10) | 2 (6) | 1 (20) | 7 (13) |
| Vaccination status§ | ||||
| Fully vaccinated | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Partially vaccinated | 3 (5) | 1 (3) | 0 (0) | 0 (0) |
| Not vaccinated | 57 (95) | 31 (97) | 5 (100) | 54 (100) |
Alpha is the B.1.1.7 SARS-CoV-2 lineage.
The following non-Alpha lineages were identified among pediatric cases: B.1.1.519, B.1.427 (Epsilon), P.1 (Gamma), B.1.2, B.1.526 (Iota), B.1, B.1.429 (Epsilon), and B.1.596.
These individuals either identified as Non-Hispanic Black, Asian, American Indian/Alaska Native, Native Hawaiian/Pacific Islander, belonging to a race/ethnicity not listed on the questionnaire or belonging to multiple races/ethnicities. Individuals were grouped together for analysis to increase power of analysis.
Other chronic conditions included hypothyroidism (1), neurological condition (3), anxiety/depression (3), attention deficit disorder (1), attention deficit hyperactivity disorder (6), allergies (1), juvenile idiopathic arthritis (1), and/or celiac disease (1).
Individuals were considered fully vaccinated ≥14 days after completion of all recommended doses of an US Food and Drug Administration-authorized COVID-19 vaccine, partially vaccinated if <14 days since completing the primary series or did not complete the series, and unvaccinated if no COVID-19 vaccine was received.
Table III.
SARS-CoV-2 RT-PCR positivity among pediatric and adult household members,∗ by age, symptom status, and lineage, California and Colorado, January to April 2021
| Household member age | <5 years (n = 23) | 5-11 years (n = 48) | 12-17 years (n = 76) | ≥18 years (n = 247) | P value† |
|---|---|---|---|---|---|
| RT-PCR-positive household members | 15 (65) | 32 (67) | 49 (64) | 164 (66) | .96 |
| Symptomatic cases‡ | 12/15 (80) | 30/32 (94) | 44/49 (90) | 157/164 (96) | .10 |
| Genomic lineage§ |
0-11 years |
12-17 years |
P value† | ||
|---|---|---|---|---|---|
| Alpha (n = 33) | Non-Alpha (n = 28) | Alpha (n = 49) | Non-Alpha (n = 21) | ||
| RT-PCR positive household members | 26 (79) | 17 (61) | 33 (67) | 15 (71) | .74 |
| Symptomatic cases‡ | 22/26 (85) | 16/17 (94) | 29/33 (88) | 14/15 (93) | .91 |
Four pediatric and 45 adult household contacts reported prior SARS-CoV-2 infection or seropositivity and were excluded from the RT-PCR positivity analysis. One pediatric case with B.1.1.7 (Alpha) lineage never tested positive by RT-PCR but was classified as a case based on serology testing and new-onset of symptoms.
Type III tests were used to assess differences in percent positivity among the 4 groups by age and genomic lineage; P values of less than .05 were considered statistically significant.
Percent of cases who were symptomatic. Individuals were classified as symptomatic if they reported symptoms at any time during the investigation.
Specimens from 5 primary pediatric cases did not undergo sequencing and therefore a lineage was not assigned to these individuals. Lineages were assigned to uninfected pediatric household contacts based on the lineage of the household primary case.
Table IV.
SIR of SARS-CoV-2 among household contacts, by age and SARS-CoV-2 lineage of primary cases and household contacts, California and Colorado, January to April 2021
| Characteristics | Primary cases, n | Secondary cases,∗ n | Total household contacts,† n | SIR (95% CI)‡ | OR (95% CI)§ |
|---|---|---|---|---|---|
| SIR by primary case characteristic¶ | |||||
| Primary case age | |||||
| Child | 36 | 37 | 83 | 0.45 (0.34-0.56) | 0.79 (0.41-1.54) |
| Adult | 91 | 100 | 184 | 0.54 (0.47-0.62) | Reference |
| Pediatric primary case age, y | |||||
| 0-11 | 14 | 19 | 41 | 0.46 (0.31-0.63) | 1.02 (0.35-3.00) |
| 12-17 | 22 | 18 | 42 | 0.43 (0.28-0.59) | Reference |
| Genomic lineage of pediatric primary case | |||||
| Alpha | 21 | 24 | 44 | 0.55 (0.39-0.70) | 1.52 (0.51-4.53) |
| Non-Alpha | 10 | 12 | 26 | 0.46 (0.27-0.67) | Reference |
| Alpha lineage and pediatric primary case age, y | |||||
| 0-11 | 6 | 11 | 17 | 0.65 (0.38-0.86) | 1.63 (0.40-6.70) |
| 12-17 | 15 | 13 | 27 | 0.48 (0.29-0.68) | Reference |
| Non-Alpha lineage and pediatric primary case age, y | |||||
| 0-11 | 4 | 7 | 14 | 0.50 (0.23-0.77) | 1.39 (0.28-6.99) |
| 12-17 | 6 | 5 | 12 | 0.42 (0.15-0.72) | Reference |
| SIR by household contact characteristic∗∗ | |||||
| Household contact age | |||||
| Child | – | 61 | 111 | 0.55 (0.45-0.64) | 1.01 (0.68-1.50) |
| Adult | – | 76 | 156 | 0.49 (0.41-0.57) | Reference |
| Pediatric household contact age, y | |||||
| 0-11 | – | 34 | 57 | 0.60 (0.46-0.72) | 1.09 (0.60-1.97) |
| 12-17 | – | 27 | 54 | 0.50 (0.36-0.64) | Reference |
| Genomic lineage of pediatric household contact | |||||
| Alpha | – | 39 | 61 | 0.64 (0.51-0.76) | 1.08 (0.40-2.98) |
| Non-Alpha | – | 22 | 39 | 0.56 (0.40-0.72) | Reference |
| Alpha lineage and pediatric household contact age, y | |||||
| 0-11 | – | 21 | 27 | 0.78 (0.58-0.91) | 1.37 (0.61-3.04) |
| 12-17 | – | 18 | 34 | 0.53 (0.35-0.70) | Reference |
| Non-Alpha lineage and pediatric household contact age, y | |||||
| 0-11 | – | 13 | 24 | 0.54 (0.32-0.74) | 0.45 (0.10-1.94) |
| 12-17 | – | 9 | 15 | 0.60 (0.32-0.84) | Reference |
–, not applicable.
Secondary cases were defined as household contacts who had a positive RT-PCR for SARS-CoV-2 during the investigation period, converted from SARS-CoV-2 IgG negative on enrollment to SARS-CoV-2 IgG positive on closeout without a history of vaccination, or were seropositive on enrollment with new-onset symptoms without a history of vaccination or a previous SARS-CoV-2 infection.
Four pediatric and 45 adult household contacts reported prior SARS-CoV-2 infection or seropositivity and were excluded from the SIR calculations.
SIRs were calculated as the proportion of secondary cases among all non-excluded household contacts.
Unadjusted ORs and 95% CIs were calculated using GEEs with an exchangeable correlation matrix and logit link to account for clustering within households. ORs were used to compare SIRs.
The probability of transmission among household contacts was compared by age of the primary case (pediatric vs adult). For pediatric primary cases, the probability of transmission among household contacts was compared by age of the pediatric primary case (young child [≤11 years] vs adolescent [12-17 years]) and by lineage group (B.1.1.7 [Alpha] vs non-Alpha). Specimens from 5 primary pediatric cases did not undergo sequencing and therefore a lineage was not assigned to these individuals.
The probability of infection among household contacts was compared by age of the household contact (pediatric vs adult). For pediatric household contacts, the probability of infection was compared by age of the pediatric household contact (young child [≤11 years] vs adolescent [12-17 years]) and by lineage group (B.1.1.7 [Alpha] vs non-Alpha). Lineages were assigned to uninfected pediatric household contacts based on the lineage of the household primary case.
Table V.
Risk factors associated with SARS-CoV-2 infection among pediatric household contacts,∗ California and Colorado, January to April 2021 (n = 111)
| Characteristics of pediatric household contacts | SARS-CoV-2-positive contacts (n = 61), n (%) | SARS-CoV-2-negative contacts (n = 50), n (%) | OR (95% CI)† |
|---|---|---|---|
| Age, years | |||
| <5 | 9 (15) | 8 (16) | 0.71 (0.30-1.66) |
| 5-11 | 25 (41) | 15 (30) | 0.95 (0.49-1.87) |
| 12-17 | 27 (44) | 27 (54) | Reference |
| Sex | |||
| Male | 33 (54) | 21 (42) | 1.01 (0.50-2.03) |
| Female | 28 (46) | 29 (58) | Reference |
| Race/ethnicity | |||
| Non-Hispanic White | 26 (43) | 29 (58) | Reference |
| Non-Hispanic other‡ | 15 (25) | 12 (24) | 0.85 (0.25-2.98) |
| Hispanic (any race) | 20 (33) | 9 (18) | 2.48 (0.80-7.62) |
| Underlying medical conditions | |||
| Any medical condition | 11 (18) | 14 (28) | 0.61 (0.27-1.40) |
| No underlying medical conditions | 50 (82) | 36 (72) | Reference |
| Asthma | 7 (11) | 9 (18) | 0.53 (0.19-1.47) |
| No asthma | 54 (89) | 41 (82) | Reference |
| Relationship to primary case | |||
| Child | 43 (70) | 28 (56) | 1.37 (0.54-3.47) |
| Sibling, extended family, or housemate | 18 (30) | 22 (44) | Reference |
| Attended school/daycare during the 2 weeks before exposure | |||
| Yes | 19 (31) | 21 (42) | 0.57 (0.28-1.15) |
| No | 42 (69) | 29 (58) | Reference |
| Number of household members | |||
| 2-4 | 20 (33) | 22 (44) | Reference |
| 5-6 | 34 (56) | 24 (48) | 1.65 (0.62-4.35) |
| ≥7 | 7 (11) | 4 (8) | 1.16 (0.15-9.32) |
| Shared bedroom or bathroom with the primary case | |||
| Yes | 35 (57) | 18 (36) | 1.44 (0.83-2.51) |
| No | 26 (43) | 32 (64) | Reference |
| Direct contact with primary case (eg, hand shaking, hugging) | |||
| Yes | 29 (48) | 18 (36) | 1.64 (0.35-7.68) |
| No | 32 (52) | 32 (64) | Reference |
| Indirect contact with primary case (eg, sharing utensils, plates, cups, other objects) | |||
| Yes | 12 (20) | 6 (12) | 1.30 (0.61-2.75) |
| No | 49 (80) | 44 (88) | Reference |
| Spent ≥10 minutes within 6 feet of primary case with or without mask | |||
| Yes | 55 (90) | 43 (86) | 1.31 (0.77-2.23) |
| No | 6 (10) | 7 (14) | Reference |
| Genomic lineage§ | |||
| Alpha | 39 (64) | 22 (44) | 2.11 (0.66-6.8) |
| Non-Alpha VOC | 15 (25) | 5 (10) | 3.91 (0.75-20.5) |
| Non-Alpha non-VOC | 7 (11) | 12 (24) | Reference |
| Duration of symptoms in days, median (IQR), n = 52 | 3 (2-5) | – | – |
–, not applicable.
Four of the 115 pediatric household contacts reported prior SARS-CoV-2 infection or seropositivity and were excluded from the risk factor analysis.
Unadjusted ORs and 95% CIs were calculated using GEEs with an exchangeable correlation matrix and logit link to account for clustering within households. ORs did not change when adjusting for household size.
These individuals either identified as Non-Hispanic Black, Asian, American Indian/Alaska Native, Native Hawaiian/Pacific Islander, belonging to a race/ethnicity not listed on the questionnaire or belonging to multiple races/ethnicities. Individuals were grouped together for analysis to increase power of analysis.
VOCs were defined based on the CDC variant classification scheme at the time the evaluation occurred. Alpha (B.1.1.7), Epsilon (B.1.427 and B.1.429), and Gamma (P.1) SARS-CoV-2 lineages were classified as VOCs; all other lineages identified were classified as non-VOCs. Eleven uninfected pediatric household contacts did not have a lineage assigned because specimens from primary cases did not undergo sequencing.
References
- 1.Centers for Disease Control and Prevention COVID data tracker 2021. https://covid.cdc.gov/covid-data-tracker/#datatracker-home
- 2.Ludvigsson J.F. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088–1095. doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong Y., Mo X., Hu Y., Qi X., Jiang F., Jiang Z., et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145:e20200702. doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 4.Laws R.L., Chancey R.J., Rabold E.M., Chu V.T., Lewis N.M., Fajans M., et al. Symptoms and transmission of SARS-CoV-2 among children—Utah and Wisconsin, March-May 2020. Pediatrics. 2021;147 doi: 10.1542/peds.2020-027268. :e2020027268. [DOI] [PubMed] [Google Scholar]
- 5.McLean H.Q., Grijalva C.G., Hanson K.E., Zhu Y.G., Deyoe J.E., Meece J.K., et al. Household transmission and clinical features of SARS-CoV-2 infections by age in 2 US communities. medRxiv. 2021 doi: 10.1542/peds.2021-054178. 2021;2021.08.16.21262121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yousaf A.R., Duca L.M., Chu V., Reses H.E., Fajans M., Rabold E.M., et al. A prospective cohort study in nonhospitalized household contacts with severe acute respiratory syndrome coronavirus 2 infection: symptom profiles and symptom change over time. Clin Infect Dis. 2021;73:e1841–e1849. doi: 10.1093/cid/ciaa1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paul P., France A.M., Aoki Y., Batra D., Biggerstaff M., Dugan V., et al. Genomic surveillance for SARS-CoV-2 variants circulating in the United States, December 2020-May 2021. MMWR Morb Mortal Wkly Rep. 2021;70:846–850. doi: 10.15585/mmwr.mm7023a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention SARS-CoV-2 variant classifications and definitions 2021. https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fcases-updates%2Fvariant-surveillance%2Fvariant-info.html
- 9.Galloway S.E., Paul P., MacCannell D.R., Johansson M.A., Brooks J.T., MacNeil A., et al. Emergence of SARS-CoV-2 B.1.1.7 lineage—United States, December 29, 2020-January 12, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:95–99. doi: 10.15585/mmwr.mm7003e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies N.G., Abbott S., Barnard R.C., Jarvis C.I., Kucharski A.J., Munday J.D., et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021;372:eabg3055. doi: 10.1126/science.abg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyngse F.P., Mølbak K., Skov R.L., Christiansen L.E., Mortensen L.H., Alertsen M., et al. Increased transmissibility of SARS-CoV-2 lineage B.1.1.7 by age and viral load. Nat Commun. 2021;12:7251. doi: 10.1038/s41467-021-27202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donnelly M.A.P., Chuey M.R., Soto R., Schwartz N.G., Chu V.T., Konkle S.T., et al. Household transmission of SARS-CoV-2 Alpha variant — United States, 2021. Clin Infect Dis. 2022;11:ciac125. doi: 10.1093/cid/ciac125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention COVID-19 contact tracing communication toolkit for health departments 2021. https://www.cdc.gov/coronavirus/2019-ncov/php/contact-tracing-comms.html
- 14.Scobie H.M., Johnson A.G., Suthar A.B., Severson R., Alden N.B., Balter S., et al. Monitoring incidence of COVID-19 cases, hospitalizations, and deaths, by vaccination status—13 U.S. jurisdictions, April 4-July 17, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1284–1290. doi: 10.15585/mmwr.mm7037e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rambaut A., Holmes E.C., O'Toole Á., Hill V., McCrone J.T., Ruis C., et al. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat Microbiol. 2020;5:1403–1407. doi: 10.1038/s41564-020-0770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.21 CFR §56.
- 17.42 USC §241(d).
- 18.5 USC §552a.
- 19.44 USC §3501 et seq.
- 20.45 CFR §46.102(l)(2).
- 21.Zhu Y., Bloxham C.J., Hulme K.D., Sinclair J.E., Tong Z.W.M., Steele L.E., et al. a meta-analysis on the role of children in severe acute respiratory syndrome coronavirus 2 in household transmission clusters. Clin Infect Dis. 2021;72:e1146–e1153. doi: 10.1093/cid/ciaa1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dattner I., Goldberg Y., Katriel G., Yaari R., Gal N., Miron Y., et al. The role of children in the spread of COVID-19: using household data from Bnei Brak, Israel, to estimate the relative susceptibility and infectivity of children. PLoS Comput Biol. 2021;17:e1008559. doi: 10.1371/journal.pcbi.1008559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soriano-Arandes A., Gatell A., Serrano P., Biosca M., Campillo F., Capdevila R., et al. Household SARS-CoV-2 transmission and children: a network prospective study. Clin Infect Dis. 2021;73:e1261–e1269. doi: 10.1093/cid/ciab228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyngse F.P., Kirkeby C.T., Halasa T., Andreasen V., Skov R.L., Moller F.T., et al. COVID-19 transmission within Danish households: a nationwide study from lockdown to reopening. medRxiv. 2020 doi: 10.2807/1560-7917.ES.2022.27.6.2001800. :2020.09.09.20191239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paul L.A., Daneman N., Schwartz K.L., Science M., Brown K.A., Whelan M., et al. Association of age and pediatric household transmission of SARS-CoV-2 infection. JAMA Pediatr. 2021;175:1151–1158. doi: 10.1001/jamapediatrics.2021.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grijalva C.G., Rolfes M.A., Zhu Y., McClean H.Q., Hanson K.E., Belongia E.A., et al. Transmission of SARS-COV-2 infections in households—Tennessee and Wisconsin, April-September 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1631–1634. doi: 10.15585/mmwr.mm6944e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ge Y., Martinez L., Sun S., Chen Z., Zhang F., Li F., et al. COVID-19 Transmission dynamics among close contacts of index patients with COVID-19: a population-based cohort study in Zhejiang Province, China. JAMA Intern Med. 2021;181:1343–1350. doi: 10.1001/jamainternmed.2021.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng H.Y., Jian S.W., Liu D.P., Ng T.C., Huang W.T., Lin H.H., et al. Contact tracing assessment of COVID-19 transmission dynamics in Taiwan and risk at different exposure periods before and after symptom onset. JAMA Intern Med. 2020;180:1156–1163. doi: 10.1001/jamainternmed.2020.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Viner R.M., Mytton O.T., Bonell C., Melendez-Torres G.J., Ward J., Hudson L., et al. Susceptibility to SARS-CoV-2 infection among children and adolescents compared with adults: a systematic review and meta-analysis. JAMA Pediatr. 2021;175:143–156. doi: 10.1001/jamapediatrics.2020.4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldstein E., Lipsitch M., Cevik M. On the effect of age on the transmission of SARS-CoV-2 in households, schools, and the community. J Infect Dis. 2021;223:362–369. doi: 10.1093/infdis/jiaa691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hua C.Z., Miao Z.P., Zheng J.S., Huang Q., Sun Q.F., Lu H.P., et al. Epidemiological features and viral shedding in children with SARS-CoV-2 infection. J Med Virol. 2020;92:2804–2812. doi: 10.1002/jmv.26180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J., Thoon K.C., Chong C.Y., Maiwald M., Kim K.Q., Nadua K., et al. Comparative analysis of symptomatic and asymptomatic SARS-CoV-2 infection in children. Ann Acad Med Singap. 2020;49:530–537. [PubMed] [Google Scholar]
- 33.Dawson P., Worrell M.C., Malone S., Tinker S.C., Fritz S., Maricque B., et al. Pilot investigation of SARS-CoV-2 secondary transmission in kindergarten through grade 12 schools implementing mitigation strategies—St. Louis County and City of Springfield, Missouri, December 2020. MMWR Morb Mortal Wkly Rep. 2021;70:449–455. doi: 10.15585/mmwr.mm7012e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rader B., White L.F., Burns M.R., Chen J., Brilliant J., Cohen J., et al. Mask-wearing and control of SARS-CoV-2 transmission in the USA: a cross-sectional study. Lancet Digit Health. 2021;3:e148–e157. doi: 10.1016/S2589-7500(20)30293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cowling B.J., Ali S.T., Ng T.W.Y., Tsang T.K., Li J.C.M., Fong M.W., et al. Impact assessment of non-pharmaceutical interventions against coronavirus disease 2019 and influenza in Hong Kong: an observational study. Lancet Public Health. 2020;5:e279–e288. doi: 10.1016/S2468-2667(20)30090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lam-Hine T., McCurdy S.A., Santora L., Duncan L., Corbett-Detig R., Kapusinszky B., et al. Outbreak associated with SARS-CoV-2 B.1.617.2 (Delta) variant in an elementary school—Marin County, California, May-June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1214–1219. doi: 10.15585/mmwr.mm7035e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yin S., Barnes K., Fisher R., Terashita D., Kim A.A. COVID-19 Case rates in transitional kindergarten through grade 12 schools and in the community—Los Angeles County, California, September 2020-March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1220–1222. doi: 10.15585/mmwr.mm7035e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siegel D.A., Reses H.E., Cool A.J., Shapiro C.N., Hsu J., Boehmer T.K., et al. Trends in COVID-19 cases, emergency department visits, and hospital admissions among children and adolescents aged 0-17 years—United States, August 2020-August 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1249–1254. doi: 10.15585/mmwr.mm7036e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dougherty K., Mannell M., Naqvi O., Matson D., Stone J. SARS-CoV-2 B.1.617.2 (Delta) Variant COVID-19 outbreak associated with a gymnastics facility—Oklahoma, April-May 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1004–1007. doi: 10.15585/mmwr.mm7028e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.