Abstract
Background
Heart failure self-management is essential to avoid decompensation and readmissions. Mobile apps seem promising in supporting heart failure self-management, and there has been a rapid growth in publications in this area. However, to date, systematic reviews have mostly focused on remote monitoring interventions using nonapp types of mobile technologies to transmit data to health care providers, rarely focusing on supporting patient self-management of heart failure.
Objective
This study aims to systematically review the evidence on the effect of heart failure self-management apps on health outcomes, patient-reported outcomes, and patient experience.
Methods
Four databases (PubMed, Embase, CINAHL, and PsycINFO) were searched for studies examining interventions that comprised a mobile app targeting heart failure self-management and reported any health-related outcomes or patient-reported outcomes or perspectives published from 2008 to December 2021. The studies were independently screened. The risk of bias was appraised using Cochrane tools. We performed a narrative synthesis of the results. The protocol was registered on PROSPERO (International Prospective Register of Systematic Reviews; CRD42020158041).
Results
A total of 28 articles (randomized controlled trials [RCTs]: n=10, 36%), assessing 23 apps, and a total of 1397 participants were included. The most common app features were weight monitoring (19/23, 83%), symptom monitoring (18/23, 78%), and vital sign monitoring (15/23, 65%). Only 26% (6/23) of the apps provided all guideline-defined core components of heart failure self-management programs: education, symptom monitoring, medication support, and physical activity support. RCTs were small, involving altogether 717 participants, had ≤6 months of follow-up, and outcomes were predominantly self-reported. Approximately 20% (2/10) of RCTs reported a significant improvement in their primary outcomes: heart failure knowledge (P=.002) and self-care (P=.004). One of the RCTs found a significant reduction in readmissions (P=.02), and 20% (2/10) of RCTs reported higher unplanned clinic visits. Other experimental studies also found significant improvements in knowledge, self-care, and readmissions, among others. Less than half of the studies involved patients and clinicians in the design of apps. Engagement with the intervention was poorly reported, with only 11% (3/28) of studies quantifying app engagement metrics such as frequency of use over the study duration. The most desirable app features were automated self-monitoring and feedback, personalization, communication with clinicians, and data sharing and integration.
Conclusions
Mobile apps may improve heart failure self-management; however, more robust evaluation studies are needed to analyze key end points for heart failure. On the basis of the results of this review, we provide a road map for future studies in this area.
Keywords: heart failure, self-management, mobile health, mobile app, secondary prevention, mobile phone
Introduction
Background
Heart failure affects approximately 40 million people worldwide [1]. A diagnosis of heart failure portends a poor prognosis, with a 12-month mortality rate of 17% for patients who are hospitalized and 7% for patients who are stable or ambulatory [2]. Hospitalization is associated with a 3-fold increased risk of death [3,4] and is preventable with good quality self-management [4-6], including symptom monitoring and taking prompt action when deterioration begins [4,7]. However, there are several barriers to achieving good quality self-management, such as lack of knowledge, symptom recognition, motivation, and confidence [8]. Addressing these can improve outcomes; yet, delivering such models for support at scale is challenging.
Mobile health (mHealth)—medical and public health practice supported by mobile devices, such as mobile phones, patient monitoring devices, personal digital assistants, and other wireless devices [9]—has excellent potential for cardiovascular disease prevention [10-14]. In particular, app interventions seem promising as they can automate the self-monitoring of physiological data, facilitate symptom and medication tracking, and provide reminders and personalized feedback to promote patient engagement [15-17]. To date, no systematic reviews have focused exclusively on mobile apps to support self-management of heart failure. Previous mHealth systematic reviews on heart failure have mostly reported remote monitoring interventions using older technologies such as phone calls and interactive voice response to transmit data to health care providers, rarely focusing on supporting patient self-management [18-27]. A total of 3 nonsystematic reviews evaluated the content and quality of existing commercial heart failure apps and mHealth interventions without assessing their impact or patient perspectives [28-30].
Aims
This systematic review aims to examine the role of mobile apps in heart failure self-management, specifically, their impact on improving (1) clinical outcomes, (2) patient-reported measures, and (3) self-management knowledge and behaviors and in addition, examine the acceptability and feasibility of these interventions, as well as patient perspectives, needs, and preferences for specific app features.
Methods
Database Search
A systematic search of the literature was performed in October 2019 and updated in December 2021 on PubMed, Embase, CINAHL, and PsycINFO, using several search terms such as mobile apps, heart failure, and self-management (Multimedia Appendix 1). The reference lists of relevant articles and gray literature such as dissertations, theses, and conference proceedings were also screened to ensure that all eligible studies were captured. The search was limited from 2008 onward as app stores were launched in that year [31]. No language limits were applied.
Eligibility Criteria
Studies were included if they (1) focused on adult patients with heart failure, (2) involved an intervention comprising a mobile app to support heart failure self-management (ie, provision of education and support to increase patients’ skills and confidence in managing their disease [32])—the mobile app could be a single component in the intervention or be combined with other intervention components (eg, wireless devices for remote monitoring)—(3) included any type or no comparison (eg, qualitative studies), (4) reported impact on any health outcome or patient-reported measure (eg, self-management and medication adherence) or focused on patients’ perspectives, and (5) were a primary research study involving the use or testing of the mobile app intervention. Studies were excluded if they (1) did not involve the use of the app by patients with heart failure and (2) assessed interventions without a clear component of heart failure self-management (eg, patients using the app only to input data to be analyzed by health care professionals).
Screening
The screening form was piloted by 2 investigators before beginning the screening process. The 2 investigators independently screened studies based on the information in their titles and abstracts and then performed the full-paper screening. Disagreements were resolved through discussion between the reviewers or by a third reviewer. Cohen κ statistic was used to measure intercoder agreement in the initial and full-text screening [33].
Data Extraction and Synthesis
One of the reviewers extracted the following information from the included studies: author, year of publication, country, study design, sample size, population characteristics, study duration or intervention use time, intervention characteristics (eg, technology components and others, mobile app features, and presence or absence of personalization), comparison, outcomes, and main results. The 2 investigators reviewed the data extraction form for consistency. The coding of behavior change techniques (BCTs) according to the BCT taxonomy [34] was conducted by 1 researcher and reviewed by another. Studies’ quality and risk of bias were appraised by 2 researchers using Cochrane’s risk of bias tool [35] for randomized controlled trials (RCTs) and the Risk Of Bias In Nonrandomized Studies of Interventions [36] tool for other experimental studies. Disagreements were resolved by a third reviewer. We performed a narrative synthesis of the studies. The PRISMA (Preferred Reporting Item for Systematic Reviews and Meta-Analyses) 2020 statement was followed (Multimedia Appendix 2) [37], and the protocol was registered on PROSPERO (International Prospective Register of Systematic Reviews; CRD42020158041).
Results
Search and Screening Results
The database search retrieved 1689 citations, from which 458 (27.1%) duplicates were removed (Figure 1). After title and abstract screening of the 1689 articles, 1189 (70.4%) were excluded. Full-text screening was conducted for 42 articles, and a further 26 (62%) papers were excluded (see Multimedia Appendix 3 for reasons for exclusion). A total of 12 additional papers were identified—1 (8%) from the reference list of the included studies and 11 (92%) from database alerts and search updates—leading to the inclusion of 28 articles [38-65] for final analysis (corresponding to 27 studies, as 1 study was published in 2 different articles [38,65]). The Cohen κ statistic was 0.81 (excellent agreement) for the title and abstract screening and 0.53 (fair agreement) for the full-text screening before the consensus agreement was reached [66].
Figure 1.
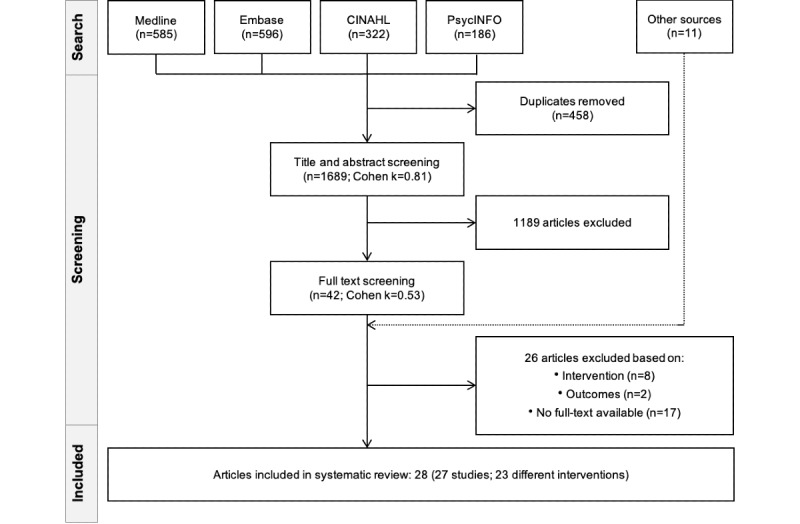
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram of the study selection process.
Study Characteristics
All 28 included articles [38-65] were published from 2012 onward and covered 27 studies and 23 interventions (n=4, 17% interventions were evaluated in ≥1 paper, using different study designs [39,44,47,52,56,59,63-65]). Of the 28 studies, there were 18 (64%) experimental studies [38-55], (n=10, 56% RCTs [38-47] and n=8, 44% quasi-experimental [48-55]; n=7, 39% with a qualitative component [38,44,46,49,51,52,54]; Table 1), 9 (32%) qualitative-only studies, 5 (18%) that included interviews [56-60], and 4 (14%) that involved a survey with open-ended questions (Table 2) [57-64]. Most studies were conducted in the United States (15/28, 54%) [39,40,44,45,48,50,52,54,56,58,60-64] and Canada (4/28, 14%) [47,51,55,59], and most were single-center, except for a few (5/28, 18%) [38,42,43,55,62]. There were 1397 participants (n=8-232 in experimental studies and 5-37 in qualitative studies), mean age was 63.4 years, 30% were women, 68% were White (from 15/28, 54% studies that reported on ethnicity), and the average education level was high (Multimedia Appendix 4 [38-65]). The study duration in the experimental studies ranged from 2 weeks to 12 months (average of 3.2 months). The 10 RCTs had a moderate risk of bias [35]; the quasi-experimental studies were of lower quality (Multimedia Appendix 5 [36,38-55]) [35].
Table 1.
Characteristics of experimental studies.
| First authora | Study design | Follow-up (months) | Sample size (intervention; control) | Age (years), mean | Women (%) | Intervention | Control | Main resultsb | |
| RCTsc | |||||||||
|
|
Clays et al [38,65] | RCT + interviews | 6 | 65 (38; 23) | 63 | 23 | App + devices (weight, BPd, pill organizer, and wrist band): monitoring weight, BP, physical activity, and HRe; psychological support; education | Standard care |
|
|
|
Schmaderer et al [39] | RCT (3 arms) | 3 | 74 (27; 26; 27) | 56.3 | 54 | App + wireless weight scale + Zoom visit with clinicians: monitoring medications and weight; automated feedback; graphical displays; education; clinician communication; reminders | App + wireless-weight scale: monitoring medications and weight |
|
|
|
Wei et al [40] | RCT + interviews | 1.5 | 28 (15; 13) | 63 | 25 | App + wireless weight scale: monitoring weight; manual input of diet sodium, and exercise, symptoms; automated feedback; graphical displays; education; clinician communication | Standard care + written education materials |
|
|
|
Yanicelli et al [41] | RCT | 3 | 40 (20; 20) | 52 | 20 | Telemonitoring via app: monitoring (manual input) weight, BP, HR, and symptoms | Standard care |
|
|
|
Rahimi et al [42] | RCT | 6 | 202 (101; 101) | 71.3 | 28 | Telemonitoring via tablet app + devices (weight, BP, and HR): monitoring weight, BP, HR, and symptoms; automated feedback; EMRo integration; graphical displays; education; clinician communication; reminders | Tablet app + devices; no clinician communication |
|
|
|
Wonggom et al [43] | RCT | 3 | 36 (17; 19) | 67.5 | 19 | App with avatar: education | Standard care |
|
|
|
Athilingam et al [44] | RCT + open-ended questionnaire | 1 | 18 (9; 9) | 53 | 56 | App + chest-worn sensor: monitoring HR and physical activity, weight, and BP, and symptoms; automated feedback; graphical displays; medication adherence; education | Standard care |
|
|
|
Goldstein et al [45] | RCT (2×2 factorial) + questionnaire | 1 | 60 (4 groups, 15 in each) | 69 | 35 | Arm 1: electronic pillbox; arm 2: arm 1 + medication reminder; arm 3: smartphone app; arm 4: arm 3 + medication reminder | Silent App or pillbox (no reminder) |
|
|
|
Vuorinen et al [46] | RCT + questionnaire and interview | 6 | 94 (47; 47) | 58 | 17 | Telemonitoring via app: monitoring (manual input) weight, BP, HR, and symptoms; automated feedback according to personal targets | Standard care |
|
|
|
Seto et al [47] | RCT | 6 | 100 (50; 50) | 54 | 21 | Telemonitoring via app + devices (weight and BP): monitoring symptoms; automated feedback; reminders for daily readings; graphical displays | Standard care |
|
| QEr studies | |||||||||
|
|
Heiney et al [48] | QE (1 arm)+questionnaire | 1 | 12 | NRs | 42 | App: monitoring (manual input) weight and symptoms; automated feedback; graphical displays; education | None |
|
|
|
Guo et al [49] | QE (1 arm) + intervein + questionnaire | 4 | 66 | 69 | 48 | Telemonitoring via app + devices (weight, BP, and HR): monitoring symptoms + medication; EMR viewing; graphical displays; remote consultations, clinician communication; visit reminders | None |
|
|
|
Park et al [50] | QE (1 arm) | 1 | 58 | 62 | 33 | Telemonitoring via 2 apps + devices (weight and BP): monitoring symptoms and patient-reported outcomes; education; reminders; alerts | None |
|
|
|
Ware et al [51] | QE (1 arm) + questionnaire + interview | 12 | 232; interview: 24 | 58; interview: 59 | 21; interview: 29 | Telemonitoring via app + devices (weight, BP, and HR): monitoring symptoms; automated feedback; graphical displays; reminders | None |
|
|
|
Foster [52] | QE (1 arm) + open-ended questionnaire | 0.5 | 10 | 65 | 40 | App: monitoring (manually) weight, BP, HR, and symptoms; automated feedback; medication reminders; education | None |
|
|
|
Suthipong [53] | QE (2 arms not randomized) | 3 | 120 (60; 60) | NR | 28 | App: monitoring (manually) weight, BP, symptoms, and liquid intake; automated feedback; medication adjustments; education; social support; clinician communication | Standard care |
|
|
|
Alnosayan et al [54] | QE (1 arm) + interview + questionnaire | 6 | 8 | 62 | 38 | Telemonitoring via app + devices (weight, BP, and glucose): monitoring symptoms; reminders; education; graphical displays | None |
|
|
|
Radhakrishna et al [55] | QE (1 arm) + questionnaire | 1 | 19 | NR | 11 | Game for tablet: education (quiz and rewards); reminders and tips on self-management | None |
|
aTable is presented in the following order: RCTs first, then quasi-experimental studies, in chronological order of year of publication;
bQualitative findings are included in the Results section.
cRCT: randomized controlled trial.
dBP: blood pressure.
eHR: heart rate.
fNS: nonstatistically significant.
gMeasured with the validated questionnaire Minnesota Living with Heart Failure Questionnaire [67].
hIndicates primary outcomes.
iMeasured with the validated questionnaire Self-Care of Heart Failure Index, which measures three subcomponents: self-management, self-confidence, and self-maintenance [68].
jMeasured with the validated questionnaire EuroQol–5 Dimensions.
kED: emergency department.
lMeasured with the validated questionnaire Atlanta Heart Failure Knowledge Test [69].
mMeasured with the validated questionnaire Kansas City Cardiomyopathy Questionnaire score.
nMeasured with the European Heart Failure Self-Care Behavior Scale.
oEMR: electronic medical record.
pNYHA: New York Heart Association functional classification.
qMeasured with the validated questionnaire Dutch Heart Failure Knowledge Scale.
rQE: quasi-experimental.
sNR: not reported.
tMeasured with the validated questionnaire Health-Related Quality of Life Scale 14.
Table 2.
Characteristics of qualitative studies.
| First author and country | Methods | Sample size | Age (years), mean | Women, n (%) | Length of app use | Intervention |
| Schmaderer, United States [56] | Interviews | 10 | 55.8 | 6 (60) | 12 weeks | Same as Schmaderer [39] (Table 1) |
| Woods, Australia [57] | Questionnaire + interview | 6 | 69 | 0 (0) | 14 days | Smartphone app: monitoring weight, BPa, HRb, fluid intake, exercise, diet, medication, well-being, and symptoms; graphical display of data; plan setting; reminders and alerts; medical documentation repository, appointments, and care team contacts |
| Foster, United States [63] | Questionnaires + open-ended questions | 10 | 65 | 4 (40) | 2 weeks | Same as Foster [52] (Table 1) |
| Portz, United States [62] | Questionnaire + open-ended questions | 30 | 66 | 18 (60) | NRc | Tablet app: monitoring weight and symptoms |
| Sebern, United States [61] | Focus group + open and closed ended questions | Patients: 4; caregivers: 4; clinicians: 7 | Patients: 74; caregivers: 72; clinicians: 34 | Patients: 1 (25); caregivers: 3 (75); clinicians: 6 (87) | NR | Tablet app: psychosocial intervention for partners (patients + their caregivers) based on share care, composed of communication (patients’ and caregivers’ preferences and values), decision-making and reciprocity; HFd education |
| Haynes, United States [60] | Interview (+ thinking aloud user observation) | Patients: 5; clinicians: 3 | NR | NR | 1 hour | Tablet app: monitoring weight, BP, and symptoms; medication tracking and reconciliation; care team contacts; appointment management |
| Srinivas, United States [58] | Interview + think-aloud user observation + questionnaire | 5 | 61 | 2 (40) | 60-90 minutes | Tablet app: monitoring weight, BP, HR, symptoms, physical activity, diet, and medication; HF education; daily behavior plan; motivational incentives and rewards |
| Athilingam, United States [64] | Questionnaires + open questions + user observation | Patients: 25; clinicians: 12 | Patients: 58; clinicians: NR | Patients: 10 (40); clinicians: NR | 1-2 hours | Same as Athilingam [44] (Table 1) |
| Seto, Canada [59] | Interview | Patients: 22; clinicians: 5 | Patients: 57; clinicians: NR | Patients: 4 (18); clinicians: NR | 6 months | Same as Seto [47] (Table 1) |
aBP: blood pressure.
bHR: heart rate.
cNR: not reported.
dHF: heart failure.
Intervention Characteristics
Across the 23 apps, the app was provided via a smartphone in 17 (74%) [38-54,57,59,63,64] and via a tablet in 6 (26%) interventions [42,55,58,60-62]. In addition to the app, 35% (8/23) of interventions included telemonitoring (ie, remote monitoring), with transfer of data to health care providers [41,42,46,47,49-51,54], and 65% (15/23) were solely focused on self-management support [38-40,43-45,48,52,53,55,57,58,60-62]. Approximately 9% (2/23) of apps provided patient access to electronic medical records [42,49], and 22% (5/23) of apps allowed direct clinician communication [39,40,42,49,53]. Approximately 48% (11/23) of apps involved patient or clinician co-design [38,40,42-44,47,51,55,57,58,60]. For 39% (9/23) of apps [38,40-42,48,51,53,57,58], the authors reported the use of personalization, mostly in the form of feedback to self-monitored measures (Multimedia Appendix 6 [38-65]).
The most frequent app features were weight monitoring (19/23, 83%), [38-42,44,46,47,49-54,57-60,62-64], symptom monitoring (18/23, 78%) [40-42,44,46-55,57-60,62-64], and vital signs monitoring (blood pressure and heart rate: 15/23, 65%; Figure 2; Multimedia Appendix 7 [38-55,57-65]) [38,41,42,44,46,47,49-54,57-60,63,64]. Automated monitoring through external wireless devices (eg, weight scale, blood pressure, and heart rate monitor) was present in 43% (10/23) of apps [38-40,42,44,47,49-51,54,59,64]. Of these 10 apps, 6 (60%) were part of a telemonitoring system (ie, the apps were connected to a health care service or clinical provider) [42,47,49-51,54,59]. None of the interventions included implantable cardiac devices. Most apps recommended daily monitoring of symptoms and vital signs, and reminders for monitoring were mentioned in 52% (12/23) of interventions [38-42,44,47,51,53-55,57]. Few studies detailed the format or specifics of symptom monitoring except for 22% (5/23) of interventions [41,46,54,55,62], which allowed for the recording of the presence or absence of specific symptoms, with 20% (1/5) of them based on a validated questionnaire [54] and 60% (3/5) of them also providing symptom severity scales [41,54,62].
Figure 2.
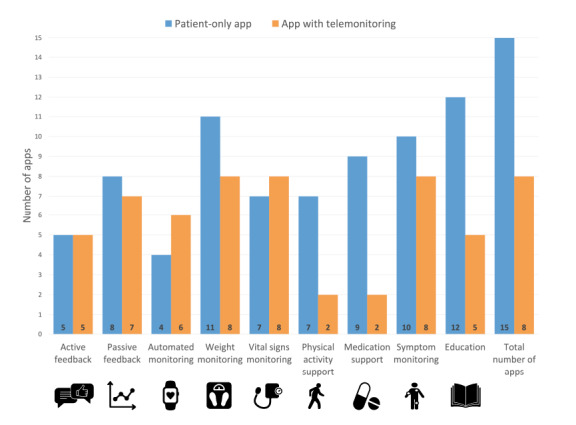
Features present in apps of included studies, grouped by type of app (patient-only app and app with telemonitoring, ie, with transfer of data to health care providers).
The most common BCTs presented in the studies were instructions on how to perform the behavior in 91% (21/23) of interventions [38-55,57-59,61,63,64], followed by self-monitoring of outcomes of behavior in 83% (19/23) [38-42,44,46-54,57-60,62-64], behavioral practice or rehearsal in 78% (18/23) [38-42,44,46-51,53-55,57,59,60,62,64], prompts or cues [38-42,44,45,47-51,53-55,57,59,60,64] and feedback on outcomes of behavior [38-42,44,46-51,53,54,57-59,62,64] in 74% (17/23) of interventions each. Feedback was active in 48% (11/23) of apps (ie, the app gave specific instructions to the patient in response to the individual information inputted by them) [40-42,44,46-48,51,53] and passive in 65% (15/23) of apps (ie, display of measurements in graphs) [38-40,42,44,47-51,54,57,58,62] (Multimedia Appendix 8 [38-55,57-65]).
Quantitative Results From Experimental Studies
The 10 included RCTs were small, often underpowered, with main outcomes self-reported, and the results were inconsistent. Approximately 20% (2/10) of RCTs found significant improvements in their primary outcomes: heart failure knowledge [43] and self-care [41]. One of the RCTs [47] reported several primary outcomes, showing improvements in self-care and quality of life. Approximately 40% (4/10) of RCTs did not show significant improvements in their primary outcomes (quality of life [38,39], self-care [38], achieving optimal medical therapy [42], medication adherence [45], and heart failure–related hospital days [46]). Approximately 20% (2/10) of RCTs indicated that their main aim was to assess feasibility [40,44].
Key clinical outcomes in heart failure were seldom reported (ie, mortality [46,47], emergency department visits [39,43,46,47], and hospital readmissions, [39,43,47,50,53]), with only 4% (1/28) of RCTs [39] and 7% (2/28) of quasi-experimental studies [50,53] showing a reduction in readmissions. Approximately 20% (2/10) of RCTs reported higher health care services use in the intervention groups than the control groups, including a higher number of unplanned clinic visits [46,47] and higher use of nurse resources (time and calls) and medication optimization [46].
Other significant improvements were inconsistently reported across experimental studies: heart failure–specific knowledge [40,44,55], self-care [38,52], hospital readmissions [50,53], depression and anxiety measures [38], quality of life [40], systolic blood pressure [42], diet [49], self-monitoring (blood pressure and weight) [49], medication adherence [49], 6-minute walking test [53], and pitting edema [53]. Engagement with the mobile app was reported in 11% (3/28) of studies, 67% (2/3) indicating that less than half of the participants accessed the app daily as recommended by the investigators [40,44] and another showing that 60% of participants used the app more than once a week, as recommended [49].
User Experience and Qualitative Results From Experimental and Qualitative Studies
Overview
User experience was assessed in 68% (19/28) of studies using questionnaires, interviews, and focus groups [40,43-46,48,49,51,52,54,57-65]. The most commonly used questionnaires, apart from those created specifically by study authors, were the System Usability Scale [54,58] and the Unified Theory of Acceptance and Use of Technology questionnaire [51,65].
Of the 28 studies, qualitative analysis to assess acceptability and user perspectives was conducted in 14 (50%) studies (n=8, 57% qualitative-only studies [57-64] and n=6, 21% as part of an experimental study [38,44,46,49,51,52,54]). Common themes were automated self-monitoring and feedback, personalization, communication with clinicians and data sharing and integration, and digital literacy and technical issues.
Automated Self-monitoring and Feedback
Most study participants appreciated and noted the importance of automated self-monitoring (particularly through wireless device integration [49,51,54,57,59,60]) and feedback mechanisms with easy-to-understand objective visual displays that could also be tracked by family and friends [46,49,51,54,57,59,64]. They also mentioned that comparing their tracked measures and symptoms with their targets increased goal motivation, symptom awareness, and understanding of the relationship between their lifestyle or behavioral choices and health status, encouraging them to better self-manage their condition [56,59,63].
Personalization
Participants in 18% (5/28) of studies noted the need for personalization of the intervention and content provided [51,57,60,62,65] and their preference for more personalization in the ability to report symptoms and needs, which ideally would also generate more relevant feedback [51,57,60]. Specifically, some participants suggested adding a free-writing field [60], additional symptoms [62], and flexibility to input and change information (eg, medication changes) [57]. Personalization of feedback and data displays was also raised, given that some patients found it difficult to interpret longitudinal graphs, and others suggested the ability to increase the size of buttons and text as a desirable feature [57,58]. In addition, the perceived usefulness of the educational content was associated with previous educational level and duration of heart failure, also indicating the importance of personalized educational content [52,55,57]. Reminders for tasks and medication were mentioned as very relevant by most participants in several studies [49,60,62,64].
Communication With Clinicians and Data Sharing and Integration
Participants in several studies considered that the app could be an excellent tool for communicating with clinicians and helping with care planning [49,54,56,57,60,61], particularly if it allowed for data sharing and integration with electronic medical records [49,57,60]. Sharing data easily with clinicians, family, and caregivers during emergencies was commonly considered advantageous [49,57,60].
Digital Literacy and Technical Issues
Low digital literacy and technical challenges were reported as barriers to using the app in 14% (4/28) of studies [44,49,51,54,57,58,60], and in 4% (1/28) of studies, they were reported as an impassable barrier for older patients without additional technical support [60]. Technical challenges were mentioned as affecting app use and intervention fidelity and were mainly related to difficulties in using the app, such as downloading it, setting reminders, and inputting data [49,57,58,63].
Discussion
Principal Findings
In this first systematic review targeting exclusively mobile apps for heart failure self-management, we identified 23 unique apps evaluated in quantitative and qualitative designs, with 8 (35%) being part of telemonitoring systems and connected to health care services. Common features of apps were weight, symptom, and vital signs monitoring and provision of education, medication reminders, and graphical visualization of data. Overall, few had robust efficacy evaluation frameworks—only 10 RCTs involving 717 participants, with ≤6 months of follow-up, substantial heterogeneity in interventions and outcomes, and hence little quantitative evidence to indicate efficacy. Few studies involved patients and clinicians in the design of apps, and few quantified app engagement metrics such as frequency of use during studies. Qualitative studies identified the automation of self-monitoring tasks and feedback, personalization of content and format, communication with clinicians, and data sharing and integration capabilities as key enablers.
Comparison With Existing Literature
Similar to previous systematic reviews of other digital technologies in heart failure (focused on nonapp mobile technologies, such as SMS text messaging, personal digital assistants, interactive voice response, and phone calls), our findings were mixed, with high heterogeneity and lack of detailed reporting of intervention characteristics [18-27] likely because of poor evaluation frameworks. In these reviews, the interventions did not commonly offer self-management support (eg, education and feedback), merely involving remote monitoring with regular digital transmission of physiological and other disease-related data from the patient’s home to a health care center. In addition, previous nonsystematic reviews seemingly with a focus on apps for heart failure self-management either only assessed the content and quality of commercially available apps [28-30] or broadened their inclusion criteria, including studies where the intervention was some type of mHealth technology but not an app (eg, SMS text messaging) [30]. In contrast, our systematic review is the first to focus exclusively on mobile apps for heart failure self-management (with or without clinician involvement via telemonitoring).
Despite the focus on heart failure self-management, the studies included in this review varied considerably in the types of self-management support features available in the apps. Core components of heart failure self-management programs, as defined in existing guidelines [2,5,70], include education, symptom monitoring, medication support, and physical activity support. Nevertheless, only 26% (6/23) of apps provided all these features [44,52,54,55,57,58], with more apps including features less supported by evidence in regard to their benefits in heart failure [5], such as daily weight monitoring. As a road map for future studies in this area, we encourage researchers and developers to follow the best available evidence [2,5,70] when designing and evaluating heart failure apps for self-management, focusing on features that have been systematically associated with improved outcomes. In addition, better reporting of intervention features is crucial to avoid what has been named as the black box of home telemonitoring [20], where the specific effective components of these interventions remain unknown.
Key outcomes in heart failure were seldom assessed in the included studies, hampering a complete evaluation of the impact of heart failure self-management apps. Overall, 1 RCT [39] and 2 quasi-experimental studies showed a significant reduction in readmissions [50,53], corresponding to the evaluation of 1 self-management app with telemonitoring and 2 without telemonitoring. Furthermore, 30% (3/10) of RCTs evaluated health care system use [43,46,47], with 67% (2/3) of them finding a higher number of unplanned clinic visits and medication optimization for participants in telemonitoring programs [46], although without significant changes in mortality, emergency department visits, or hospitalization [46,47]. Higher health care use may reflect earlier actions in the face of signs of worsening heart failure and provide opportunities for medication optimization. Such results may help explain the positive outcomes of telemonitoring interventions [26]. Longer and adequately powered studies measuring key clinical outcomes are needed to fully assess whether the potential benefits of self-management apps outweigh the costs of increased health care use.
Self-reported measures were commonly assessed in experimental studies, including validated questionnaires to measure heart failure knowledge, self-care, and quality of life [67-69]. Heart failure knowledge was significantly improved in 14% (4/28) of studies, all of which involved apps without telemonitoring [40,43,44,55]. Self-care was improved in 14% (4/28) of studies [38,41,47,52], 50% (2/4) of which involved apps with telemonitoring [41,47], and quality of life improved in 7% (2/28) of studies [40,47], 50% (1/2) of which involved telemonitoring [40]. There has been increasing recognition of the importance of including patient-reported outcomes as end points when evaluating interventions, as well as the benefits of collecting them routinely to improve care [71-73]. Digital technologies such as mobile apps can facilitate the capture of patient-reported outcomes, such as symptom status and severity [71], which can then be used by clinicians to guide care. Nevertheless, only one of the apps used a validated questionnaire for symptom monitoring [54]. The potential of mobile apps to collect patient-reported outcomes should be further explored in future studies, given their ability to promote patient-centered care and improve the quality of care for patients.
Overall, the evidence on the use of mobile apps for heart failure self-management is still lagging behind the large body of work supporting mHealth for remote monitoring, where significant reductions in all-cause mortality have been reported [19-22,26,27]. In our review, all included studies focused on supporting heart failure self-management, with 44% (8/18) of experimental studies including a telemonitoring component with clinician involvement [46,47,49-51,54]. Unfortunately, the small number, size, and quality of these studies do not enable us to draw conclusions regarding potential differences in efficacy between these 2 different types of mobile app interventions for heart failure self-management—with or without telemonitoring. Given the demonstrated benefits of self-management interventions more broadly [74] and remote monitoring [18-27], future research should explore the possibility that their combination may result in synergistic effects and higher efficacy in improving heart failure outcomes.
Personalization was valued in the studies included in this review, particularly personally relevant feedback and tailoring of the intervention to different levels of education and digital literacy. These findings are similar to those involving apps for other chronic diseases, showing that enabling customization (eg, editing information and choosing which aspects to track) is among the most appealing features and may enhance the usability, motivation, and engagement with the apps [17,75,76]. Future studies may explore the delivery of core BCTs (self-monitoring, feedback, and instruction on how to perform the behavior) and provide other techniques in a personalized manner, according to patient preferences and self-reported information [77] or based on machine learning algorithms using patient data collected over time (eg, from smartphone sensors or wireless monitoring devices) [78,79].
Limited experience in using technology can be a barrier to using mobile apps and may affect the utility and perceived benefit of mobile apps, as shown by our findings. The lack of confidence in using technology and perceived capability to benefit from it, as well as the workload required to learn how to use an app, are particularly challenging among older patients [80,81]. A study conducted to understand the main facilitators of and barriers to the use of mobile technology among older adults found that the most often mentioned barrier was the lack of knowledge on how to use it, whereas having previous experience of use was a facilitator [82]. However, older patients are willing to learn how to use mHealth technology and feel it may help them improve and maintain self-care behaviors [82,83]. Given that a large population of patients experiencing heart failure involves older adults, future app development needs to take into account specific characteristics of this population to design apps with simple navigation and ease of use [81].
Strengths and Limitations
This study presents several strengths. The PRISMA protocol was systematically followed. The screening process was pilot tested before its start, and there was good agreement between the independent reviewers. We also included both experimental and qualitative studies, enabling a better understanding of the impact, acceptance, and user preferences regarding mobile apps for heart failure self-management.
Some limitations should be considered in the interpretation of our results. First, given the heterogeneity between interventions and the small number of RCTs, a meta-analysis was not conducted. Second, the heterogeneity of study designs, sample sizes, follow-ups, interventions, and outcome measures among the experimental studies did not allow for consistent conclusions on the effectiveness of mobile apps in heart failure. Third, some studies in this review included analysis of adherence, acceptability, or usability of their interventions; however, although favorable trends were reported, the different measures and definitions used hindered reliable conclusions. Fourth, the socioeconomic and clinical characteristics of participants were rarely reported in the included studies; however, when reported, they suggested a high educational level and mild to moderate disease severity, potentially limiting the generalizability of the findings. Finally, the nature of this kind of research hampers the proper elucidation of the sociotechnical aspects of the interventions, which should be further evaluated in future studies (eg, using realist review methods).
Implications for Research, Clinical Practice, and Policy
Despite growing interest in the use of mobile apps for heart failure self-management, critical gaps remain in their design and evaluation, with lack of patient and clinician involvement and lack of robust evaluation to determine the populations that may benefit the most. Given the importance of patient preference and engagement in the successful delivery of heart failure interventions [26,27], co-design processes involving clinicians and patients and process evaluations assessing engagement and acceptability of the interventions are likely to improve intervention quality and consistency. Future studies should follow existing evidence in designing apps with features most likely to improve key patient-reported and clinical outcomes, adhering to recommendations derived from this study (Textbox 1). In addition, they should explore the efficacy and cost-effectiveness of mobile apps for heart failure self-management with and without a telemonitoring component. It is possible that self-management interventions without telemonitoring may be sufficient to improve outcomes in the early stages of disease in patients with a low risk of premature morbidity and mortality.
Recommendations for researchers and developers regarding apps for heart failure self-management.
Recommendations for researchers and developers
Researchers and developers, when designing and evaluating apps, should consider the following:
Follow the best available evidence
Align with clinical guidelines
Use co-design and pilot-testing to optimize products
Enable automated self-monitoring and feedback, personalization, communication with clinicians, and data sharing and integration
Report on specific functionalities and features of the apps
Evaluate effectiveness on relevant outcomes to heart failure patients; for example, clinical outcomes, health service use, and clinical measures
Report on adverse events or inadvertent effects; for example, increased health care use
Patient-reported outcomes, including self-care and experiences, are also important; however, consider the ability to compare such measures among studies
Research is needed to better understand how these interventions can be implemented in the real world and integrated into existing models of care, such as collaborative care models involving shared care between heart failure nurses, general practitioners, and cardiologists [84-86]. Integrating these interventions into such services may increase their benefits and leverage partnerships between patients and clinicians, possibly leading to a more seamless implementation in practice. Perhaps a future model of care for heart failure patients can involve using mobile technology to improve patients’ confidence and ability to manage their condition with greater autonomy, coupled with telemonitoring with clinician support for higher-risk patients.
Conclusions
This systematic review showed that research on the use of apps in heart failure self-management is still at an early stage, with limited evidence supporting its efficacy. RCTs are needed to fully ascertain the impact of these interventions. Future research should encompass greater involvement of end users and comprehensively measure patient engagement with the intervention.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. LBG was funded by the MQRES/RTPi Scholarship (20191550).
Abbreviations
- BCT
behavior change technique
- mHealth
mobile health
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROSPERO
International Prospective Register of Systematic Reviews
Search strategy.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist.
List of articles excluded after full-text review for not meeting inclusion criteria regarding the intervention, outcome, or unavailability of full-text.
Patients’ clinical and socioeconomic characteristics.
Quality assessment of included studies.
Presence of personalization in included studies.
Intervention features of included articles.
Behavior change techniques present in the interventions of included articles.
Footnotes
Authors' Contributions: LBG and LL were involved in the conceptualization and methodology, outcome data extraction, behavior change techniques coding, and risk of bias. LBG performed the database searching and writing of the original draft. Title, abstract, and full-text screening were performed by LBG, LL, and HLT. Validation, formal analysis, writing, review, and editing were performed by LBG, LL, HLT, RR, JC, CC, DK, JJA, and TS. LL, CC, RR, and JC provided supervision.
Conflicts of Interest: None declared.
References
- 1.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545–602. doi: 10.1016/S0140-6736(16)31678-6. https://linkinghub.elsevier.com/retrieve/pii/S0140-6736(16)31678-6 .S0140-6736(16)31678-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200. doi: 10.1093/eurheartj/ehw128.ehw128 [DOI] [PubMed] [Google Scholar]
- 3.Solomon SD, Dobson J, Pocock S, Skali H, McMurray JJ, Granger CB, Yusuf S, Swedberg K, Young JB, Michelson EL, Pfeffer MA, Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) Investigators Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation. 2007;116(13):1482–7. doi: 10.1161/CIRCULATIONAHA.107.696906.CIRCULATIONAHA.107.696906 [DOI] [PubMed] [Google Scholar]
- 4.Riegel B, Moser DK, Anker SD, Appel LJ, Dunbar SB, Grady KL, Gurvitz MZ, Havranek EP, Lee CS, Lindenfeld J, Peterson PN, Pressler SJ, Schocken DD, Whellan DJ, American Heart Association Council on Cardiovascular Nursing. American Heart Association Council on Cardiovascular Nursing. American Heart Association Council on Clinical Cardiology. American Heart Association Council on Nutrition‚ Physical Activity‚ and Metabolism. American Heart Association Interdisciplinary Council on Quality of Care and Outcomes Research State of the science: promoting self-care in persons with heart failure: a scientific statement from the American Heart Association. Circulation. 2009;120(12):1141–63. doi: 10.1161/CIRCULATIONAHA.109.192628.CIRCULATIONAHA.109.192628 [DOI] [PubMed] [Google Scholar]
- 5.NHFA CSANZ Heart Failure Guidelines Working Group. Atherton JJ, Sindone A, De Pasquale CG, Driscoll A, MacDonald PS, Hopper I, Kistler PM, Briffa T, Wong J, Abhayaratna W, Thomas L, Audehm R, Newton P, O'Loughlin J, Branagan M, Connell C. National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand: guidelines for the prevention, detection, and management of heart failure in Australia 2018. Heart Lung Circ. 2018;27(10):1123–208. doi: 10.1016/j.hlc.2018.06.1042. https://linkinghub.elsevier.com/retrieve/pii/S1443-9506(18)31777-3 .S1443-9506(18)31777-3 [DOI] [PubMed] [Google Scholar]
- 6.Ponikowski P, Anker SD, AlHabib KF, Cowie MR, Force TL, Hu S, Jaarsma T, Krum H, Rastogi V, Rohde LE, Samal UC, Shimokawa H, Budi Siswanto B, Sliwa K, Filippatos G. Heart failure: preventing disease and death worldwide. ESC Heart Fail. 2014;1(1):4–25. doi: 10.1002/ehf2.12005. doi: 10.1002/ehf2.12005. [DOI] [PubMed] [Google Scholar]
- 7.Clark AM, Spaling M, Harkness K, Spiers J, Strachan PH, Thompson DR, Currie K. Determinants of effective heart failure self-care: a systematic review of patients' and caregivers' perceptions. Heart. 2014;100(9):716–21. doi: 10.1136/heartjnl-2013-304852.heartjnl-2013-304852 [DOI] [PubMed] [Google Scholar]
- 8.Harkness K, Spaling MA, Currie K, Strachan PH, Clark AM. A systematic review of patient heart failure self-care strategies. J Cardiovasc Nurs. 2015;30(2):121–35. doi: 10.1097/JCN.0000000000000118. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization . mHealth: new horizons for health through mobile technologies: based on the findings of the second global survey on eHealth. Global observatory for eHealth - volume 3. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 10.Lu X, Yang H, Xia X, Lu X, Lin J, Liu F, Gu D. Interactive mobile health intervention and blood pressure management in adults. Hypertension. 2019;74(3):697–704. doi: 10.1161/HYPERTENSIONAHA.119.13273. [DOI] [PubMed] [Google Scholar]
- 11.Gandhi S, Chen S, Hong L, Sun K, Gong E, Li C, Yan LL, Schwalm JD. Effect of mobile health interventions on the secondary prevention of cardiovascular disease: systematic review and meta-analysis. Can J Cardiol. 2017;33(2):219–31. doi: 10.1016/j.cjca.2016.08.017.S0828-282X(16)30931-X [DOI] [PubMed] [Google Scholar]
- 12.Pfaeffli Dale L, Dobson R, Whittaker R, Maddison R. The effectiveness of mobile-health behaviour change interventions for cardiovascular disease self-management: a systematic review. Eur J Prev Cardiol. 2016;23(8):801–17. doi: 10.1177/2047487315613462.2047487315613462 [DOI] [PubMed] [Google Scholar]
- 13.Klimis H, Thakkar J, Chow CK. Breaking barriers: mobile health interventions for cardiovascular disease. Can J Cardiol. 2018;34(7):905–13. doi: 10.1016/j.cjca.2018.02.012.S0828-282X(18)30172-7 [DOI] [PubMed] [Google Scholar]
- 14.Piette JD, List J, Rana GK, Townsend W, Striplin D, Heisler M. Mobile health devices as tools for worldwide cardiovascular risk reduction and disease management. Circulation. 2015;132(21):2012–27. doi: 10.1161/CIRCULATIONAHA.114.008723. http://europepmc.org/abstract/MED/26596977 .CIRCULATIONAHA.114.008723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steinhubl SR, Muse ED, Topol EJ. Can mobile health technologies transform health care? JAMA. 2013;310(22):2395–6. doi: 10.1001/jama.2013.281078.1762473 [DOI] [PubMed] [Google Scholar]
- 16.Neubeck L, Lowres N, Benjamin EJ, Freedman SB, Coorey G, Redfern J. The mobile revolution--using smartphone apps to prevent cardiovascular disease. Nat Rev Cardiol. 2015;12(6):350–60. doi: 10.1038/nrcardio.2015.34.nrcardio.2015.34 [DOI] [PubMed] [Google Scholar]
- 17.Coorey GM, Neubeck L, Mulley J, Redfern J. Effectiveness, acceptability and usefulness of mobile applications for cardiovascular disease self-management: systematic review with meta-synthesis of quantitative and qualitative data. Eur J Prev Cardiol. 2018;25(5):505–21. doi: 10.1177/2047487317750913. [DOI] [PubMed] [Google Scholar]
- 18.Greenhalgh T, A'Court C, Shaw S. Understanding heart failure; explaining telehealth - a hermeneutic systematic review. BMC Cardiovasc Disord. 2017;17(1):156. doi: 10.1186/s12872-017-0594-2. https://bmccardiovascdisord.biomedcentral.com/articles/10.1186/s12872-017-0594-2 .10.1186/s12872-017-0594-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu Y, Gu X, Xu C. Effectiveness of telemedicine systems for adults with heart failure: a meta-analysis of randomized controlled trials. Heart Fail Rev. 2020;25(2):231–43. doi: 10.1007/s10741-019-09801-5. http://europepmc.org/abstract/MED/31197564 .10.1007/s10741-019-09801-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pekmezaris R, Tortez L, Williams M, Patel V, Makaryus A, Zeltser R, Sinvani L, Wolf-Klein G, Lester J, Sison C, Lesser M, Kozikowski A. Home telemonitoring in heart failure: a systematic review and meta-analysis. Health Aff (Millwood) 2018;37(12):1983–9. doi: 10.1377/hlthaff.2018.05087. [DOI] [PubMed] [Google Scholar]
- 21.Yun JE, Park JE, Park HY, Lee HY, Park DA. Comparative effectiveness of telemonitoring versus usual care for heart failure: a systematic review and meta-analysis. J Card Fail. 2018;24(1):19–28. doi: 10.1016/j.cardfail.2017.09.006.S1071-9164(17)31211-3 [DOI] [PubMed] [Google Scholar]
- 22.Kitsiou S, Paré G, Jaana M. Effects of home telemonitoring interventions on patients with chronic heart failure: an overview of systematic reviews. J Med Internet Res. 2015;17(3):e63. doi: 10.2196/jmir.4174. https://www.jmir.org/2015/3/e63/ v17i3e63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cajita MI, Gleason KT, Han HR. A systematic review of mHealth-based heart failure interventions. J Cardiovasc Nurs. 2016;31(3):E10–22. doi: 10.1097/JCN.0000000000000305. http://europepmc.org/abstract/MED/26544175 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carbo A, Gupta M, Tamariz L, Palacio A, Levis S, Nemeth Z, Dang S. Mobile technologies for managing heart failure: a systematic review and meta-analysis. Telemed J E Health. 2018;24(12):958–68. doi: 10.1089/tmj.2017.0269. [DOI] [PubMed] [Google Scholar]
- 25.Or CK, Tao D, Wang H. The effectiveness of the use of consumer health information technology in patients with heart failure: a meta-analysis and narrative review of randomized controlled trials. J Telemed Telecare. 2017;23(1):155–66. doi: 10.1177/1357633X15625540.1357633X15625540 [DOI] [PubMed] [Google Scholar]
- 26.Inglis SC, Clark RA, Dierckx R, Prieto-Merino D, Cleland JG. Structured telephone support or non-invasive telemonitoring for patients with heart failure. Cochrane Database Syst Rev. 2015;2015(10):CD007228. doi: 10.1002/14651858.CD007228.pub3. http://europepmc.org/abstract/MED/26517969 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inglis SC, Clark RA, Dierckx R, Prieto-Merino D, Cleland JG. Structured telephone support or non-invasive telemonitoring for patients with heart failure. Heart. 2017;103(4):255–7. doi: 10.1136/heartjnl-2015-309191.heartjnl-2015-309191 [DOI] [PubMed] [Google Scholar]
- 28.Wali S, Demers C, Shah H, Wali H, Lim D, Naik N, Ghany A, Vispute A, Wali M, Keshavjee K. Evaluation of heart failure apps to promote self-care: systematic app search. JMIR Mhealth Uhealth. 2019;7(11):e13173. doi: 10.2196/13173. https://mhealth.jmir.org/2019/11/e13173/ v7i11e13173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masterson Creber RM, Maurer MS, Reading M, Hiraldo G, Hickey KT, Iribarren S. Review and analysis of existing mobile phone apps to support heart failure symptom monitoring and self-care management using the mobile application rating scale (MARS) JMIR Mhealth Uhealth. 2016;4(2):e74. doi: 10.2196/mhealth.5882. https://mhealth.jmir.org/2016/2/e74/ v4i2e74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Athilingam P, Jenkins B. Mobile phone apps to support heart failure self-care management: integrative review. JMIR Cardio. 2018;2(1):e10057. doi: 10.2196/10057. https://cardio.jmir.org/2018/1/e10057/ v2i1e10057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin W, Sarro F, Jia Y, Zhang Y, Harman M. A survey of app store analysis for software engineering. IIEEE Trans Software Eng. 2017;43(9):817–47. doi: 10.1109/tse.2016.2630689. [DOI] [Google Scholar]
- 32.Institute of Medicine (US) Committee on the Crossing the Quality Chasm: Next Steps Toward a New Health Care System . In: The 1st annual crossing the quality chasm summit: a focus on communities. Adams KG, Corrigan J, editors. Washington, D.C: National Academies Press (US); 2004. [PubMed] [Google Scholar]
- 33.Orwin R. Evaluating coding decisions. In: Cooper H, Hedges LV, editors. The handbook of research synthesis. New York, NY: Russell Sage Foundation; 1993. pp. 139–62. [Google Scholar]
- 34.Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, Eccles MP, Cane J, Wood CE. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med. 2013;46(1):81–95. doi: 10.1007/s12160-013-9486-6. [DOI] [PubMed] [Google Scholar]
- 35.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions: Cochrane book series. Hoboken, NJ: Wiley; 2008. [Google Scholar]
- 36.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. http://www.bmj.com/lookup/pmidlookup?view=long&pmid=27733354 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. http://www.bmj.com/lookup/pmidlookup?view=long&pmid=33782057 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clays E, Puddu PE, Luštrek M, Pioggia G, Derboven J, Vrana M, De Sutter J, Le Donne R, Baert A, Bohanec M, Ciancarelli MC, Dawodu AA, De Pauw M, De Smedt D, Marino F, Pardaens S, Schiariti MS, Valič J, Vanderheyden M, Vodopija A, Tartarisco G. Proof-of-concept trial results of the HeartMan mobile personal health system for self-management in congestive heart failure. Sci Rep. 2021;11(1):5663. doi: 10.1038/s41598-021-84920-4. doi: 10.1038/s41598-021-84920-4.10.1038/s41598-021-84920-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmaderer MS, Struwe L, Loecker C, Lier L, Lundgren SW, Wichman C, Pozehl B, Zimmerman L. Mobile health self-management interventions for patients with heart failure: a pilot study. J Cardiovasc Nurs (forthcoming) 2021:-. doi: 10.1097/JCN.0000000000000846.00005082-900000000-99162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei KS, Ibrahim NE, Kumar AA, Jena S, Chew V, Depa M, Mayanil N, Kvedar JC, Gaggin HK. Habits heart app for patient engagement in heart failure management: pilot feasibility randomized trial. JMIR Mhealth Uhealth. 2021;9(1):e19465. doi: 10.2196/19465. https://mhealth.jmir.org/2021/1/e19465/ v9i1e19465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yanicelli LM, Goy CB, González VD, Palacios GN, Martínez EC, Herrera MC. Non-invasive home telemonitoring system for heart failure patients: a randomized clinical trial. J Telemed Telecare. 2021;27(9):553–61. doi: 10.1177/1357633X19899261. [DOI] [PubMed] [Google Scholar]
- 42.Rahimi K, Nazarzadeh M, Pinho-Gomes AC, Woodward M, Salimi-Khorshidi G, Ohkuma T, Fitzpatrick R, Tarassenko L, Denis M, Cleland J, SUPPORT-HF2 Study Group Home monitoring with technology-supported management in chronic heart failure: a randomised trial. Heart. 2020;106(20):1573–8. doi: 10.1136/heartjnl-2020-316773.heartjnl-2020-316773 [DOI] [PubMed] [Google Scholar]
- 43.Wonggom P, Nolan P, Clark RA, Barry T, Burdeniuk C, Nesbitt K, O'Toole K, Du H. Effectiveness of an avatar educational application for improving heart failure patients' knowledge and self-care behaviors: a pragmatic randomized controlled trial. J Adv Nurs. 2020;76(9):2401–15. doi: 10.1111/jan.14414. [DOI] [PubMed] [Google Scholar]
- 44.Athilingam P, Jenkins B, Johansson M, Labrador M. A mobile health intervention to improve self-care in patients with heart failure: pilot randomized control trial. JMIR Cardio. 2017;1(2):e3. doi: 10.2196/cardio.7848. https://cardio.jmir.org/2017/2/e3/ v1i2e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goldstein CM, Gathright EC, Dolansky MA, Gunstad J, Sterns A, Redle JD, Josephson R, Hughes JW. Randomized controlled feasibility trial of two telemedicine medication reminder systems for older adults with heart failure. J Telemed Telecare. 2014;20(6):293–9. doi: 10.1177/1357633X14541039. http://europepmc.org/abstract/MED/24958355 .1357633X14541039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vuorinen AL, Leppänen J, Kaijanranta H, Kulju M, Heliö T, van Gils M, Lähteenmäki J. Use of home telemonitoring to support multidisciplinary care of heart failure patients in Finland: randomized controlled trial. J Med Internet Res. 2014;16(12):e282. doi: 10.2196/jmir.3651. https://www.jmir.org/2014/12/e282/ v16i12e282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seto E, Leonard KJ, Cafazzo JA, Barnsley J, Masino C, Ross HJ. Mobile phone-based telemonitoring for heart failure management: a randomized controlled trial. J Med Internet Res. 2012;14(1):e31. doi: 10.2196/jmir.1909. https://www.jmir.org/2012/1/e31/ v14i1e31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heiney SP, Donevant SB, Arp Adams S, Parker PD, Chen H, Levkoff S. A smartphone app for self-management of heart failure in older African Americans: feasibility and usability study. JMIR Aging. 2020;3(1):e17142. doi: 10.2196/17142. https://aging.jmir.org/2020/1/e17142/ v3i1e17142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guo X, Gu X, Jiang J, Li H, Duan R, Zhang Y, Sun L, Bao Z, Shen J, Chen F. A hospital-community-family-based telehealth program for patients with chronic heart failure: single-arm, prospective feasibility study. JMIR Mhealth Uhealth. 2019;7(12):e13229. doi: 10.2196/13229. https://mhealth.jmir.org/2019/12/e13229/ v7i12e13229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park C, Otobo E, Ullman J, Rogers J, Fasihuddin F, Garg S, Kakkar S, Goldstein M, Chandrasekhar SV, Pinney S, Atreja A. Impact on readmission reduction among heart failure patients using digital health monitoring: feasibility and adoptability study. JMIR Med Inform. 2019;7(4):e13353. doi: 10.2196/13353. https://medinform.jmir.org/2019/4/e13353/ v7i4e13353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ware P, Dorai M, Ross HJ, Cafazzo JA, Laporte A, Boodoo C, Seto E. Patient adherence to a mobile phone-based heart failure telemonitoring program: a longitudinal mixed-methods study. JMIR Mhealth Uhealth. 2019;7(2):e13259. doi: 10.2196/13259. https://mhealth.jmir.org/2019/2/e13259/ v7i2e13259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Foster M. A mobile application for patients with heart failure: theory- and evidence-based design and testing. Comput Inform Nurs. 2018;36(11):540–9. doi: 10.1097/CIN.0000000000000465. [DOI] [PubMed] [Google Scholar]
- 53.Suthipong C. The feasibility of the heart failure mobile phone aide application (Android Apps.) on self-management measured by improved physical function and reduced hospital admissions related to heart failure in Thailand. Washington Research Library Consortium Digital Collections. 2018. [2022-02-10]. https://islandora.wrlc.org/islandora/object/cuislandora%3A213555 .
- 54.Alnosayan N, Chatterjee S, Alluhaidan A, Lee E, Houston Feenstra L. Design and usability of a heart failure mHealth system: a pilot study. JMIR Hum Factors. 2017;4(1):e9. doi: 10.2196/humanfactors.6481. https://humanfactors.jmir.org/2017/1/e9/ v4i1e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Radhakrishnan K, Toprac P, O'Hair M, Bias R, Kim MT, Bradley P, Mackert M. Interactive digital e-health game for heart failure self-management: a feasibility study. Games Health J. 2016;5(6):366–74. doi: 10.1089/g4h.2016.0038. http://europepmc.org/abstract/MED/27976955 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmaderer M, Miller JN, Mollard E. Experiences of using a self-management mobile app among individuals with heart failure: qualitative study. JMIR Nurs. 2021;4(3):e28139. doi: 10.2196/28139. http://europepmc.org/abstract/MED/34406966 .v4i3e28139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Woods LS, Duff J, Roehrer E, Walker K, Cummings E. Patients' experiences of using a consumer mHealth app for self-management of heart failure: mixed-methods study. JMIR Hum Factors. 2019;6(2):e13009. doi: 10.2196/13009. https://humanfactors.jmir.org/2019/2/e13009/ v6i2e13009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Srinivas P, Cornet V, Holden R. Human factors analysis, design, and evaluation of Engage, a consumer health IT application for geriatric heart failure self-care. Int J Hum Comput Interact. 2017;33(4):298–312. doi: 10.1080/10447318.2016.1265784. http://europepmc.org/abstract/MED/30429638 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seto E, Leonard KJ, Cafazzo JA, Barnsley J, Masino C, Ross HJ. Perceptions and experiences of heart failure patients and clinicians on the use of mobile phone-based telemonitoring. J Med Internet Res. 2012;14(1):e25. doi: 10.2196/jmir.1912. https://www.jmir.org/2012/1/e25/ v14i1e25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haynes SC, Kim KK. A mobile system for the improvement of heart failure management: evaluation of a prototype. AMIA Annu Symp Proc. 2017;2017:839–48. http://europepmc.org/abstract/MED/29854150 . [PMC free article] [PubMed] [Google Scholar]
- 61.Sebern MD, Sulemanjee N, Sebern MJ, Garnier-Villarreal M, Whitlatch CJ. Does an intervention designed to improve self-management, social support and awareness of palliative-care address needs of persons with heart failure, family caregivers and clinicians? J Clin Nurs. 2018;27(3-4):e643–57. doi: 10.1111/jocn.14115. [DOI] [PubMed] [Google Scholar]
- 62.Portz JD, Vehovec A, Dolansky MA, Levin JB, Bull S, Boxer R. The development and acceptability of a mobile application for tracking symptoms of heart failure among older adults. Telemed J E Health. 2018;24(2):161–5. doi: 10.1089/tmj.2017.0036. http://europepmc.org/abstract/MED/28696832 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Foster M. HF app to support self-care among community dwelling adults with HF: a feasibility study. Appl Nurs Res. 2018;44:93–6. doi: 10.1016/j.apnr.2018.10.007.S0897-1897(18)30577-9 [DOI] [PubMed] [Google Scholar]
- 64.Athilingam P, Labrador MA, Remo EF, Mack L, San Juan AB, Elliott AF. Features and usability assessment of a patient-centered mobile application (HeartMapp) for self-management of heart failure. Appl Nurs Res. 2016;32:156–63. doi: 10.1016/j.apnr.2016.07.001.S0897-1897(16)30052-0 [DOI] [PubMed] [Google Scholar]
- 65.Luštrek M, Bohanec M, Cavero Barca C, Ciancarelli MC, Clays E, Dawodu AA, Derboven J, De Smedt D, Dovgan E, Lampe J, Marino F, Mlakar M, Pioggia G, Puddu PE, Rodríguez JM, Schiariti M, Slapničar G, Slegers K, Tartarisco G, Valič J, Vodopija A. A personal health system for self-management of congestive heart failure (HeartMan): development, technical evaluation, and proof-of-concept randomized controlled trial. JMIR Med Inform. 2021;9(3):e24501. doi: 10.2196/24501. https://medinform.jmir.org/2021/3/e24501/ v9i3e24501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fleiss JL, Levin B, Paik MC. The measurement of interrater agreement. In: Fleiss JL, editor. Statistical methods for rates and proportions. 2nd edition. Hoboken, NJ: Wiley; 1981. pp. 212–36. [Google Scholar]
- 67.Middel B, Bouma J, de Jongste M, van Sonderen E, Niemeijer MG, Crijns H, van den Heuvel W. Psychometric properties of the Minnesota Living with Heart Failure Questionnaire (MLHF-Q) Clin Rehabil. 2001;15(5):489–500. doi: 10.1191/026921501680425216. [DOI] [PubMed] [Google Scholar]
- 68.Riegel B, Carlson B, Moser DK, Sebern M, Hicks FD, Roland V. Psychometric testing of the self-care of heart failure index. J Card Fail. 2004;10(4):350–60. doi: 10.1016/j.cardfail.2003.12.001.S1071916403008017 [DOI] [PubMed] [Google Scholar]
- 69.Reilly CM, Higgins M, Smith A, Gary RA, Robinson J, Clark PC, McCarty F, Dunbar SB. Development, psychometric testing, and revision of the Atlanta Heart Failure Knowledge Test. J Cardiovasc Nurs. 2009;24(6):500–9. doi: 10.1097/JCN.0b013e3181aff0b0. http://europepmc.org/abstract/MED/19858959 .00005082-200911000-00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey Jr DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL, American College of Cardiology Foundation. American Heart Association Task Force on Practice Guidelines 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147–239. doi: 10.1016/j.jacc.2013.05.019. https://linkinghub.elsevier.com/retrieve/pii/S0735-1097(13)02114-1 .S0735-1097(13)02114-1 [DOI] [PubMed] [Google Scholar]
- 71.Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GY, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL, ESC Scientific Document Group 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373–498. doi: 10.1093/eurheartj/ehaa612.5899003 [DOI] [PubMed] [Google Scholar]
- 72.Calvert M, Kyte D, Price G, Valderas JM, Hjollund NH. Maximising the impact of patient reported outcome assessment for patients and society. BMJ. 2019;364:k5267. doi: 10.1136/bmj.k5267. [DOI] [PubMed] [Google Scholar]
- 73.Rotenstein LS, Huckman RS, Wagle NW. Making patients and doctors happier - the potential of patient-reported outcomes. N Engl J Med. 2017;377(14):1309–12. doi: 10.1056/NEJMp1707537. [DOI] [PubMed] [Google Scholar]
- 74.Jonkman NH, Westland H, Groenwold RH, Ågren S, Atienza F, Blue L, Bruggink-André de la Porte PW, DeWalt DA, Hebert PL, Heisler M, Jaarsma T, Kempen GI, Leventhal ME, Lok DJ, Mårtensson J, Muñiz J, Otsu H, Peters-Klimm F, Rich MW, Riegel B, Strömberg A, Tsuyuki RT, van Veldhuisen DJ, Trappenburg JC, Schuurmans MJ, Hoes AW. Do self-management interventions work in patients with heart failure? An individual patient data meta-analysis. Circulation. 2016;133(12):1189–98. doi: 10.1161/CIRCULATIONAHA.115.018006. http://europepmc.org/abstract/MED/26873943 .CIRCULATIONAHA.115.018006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Birkhoff SD, Smeltzer SC. Perceptions of smartphone user-centered mobile health tracking apps across various chronic illness populations: an integrative review. J Nurs Scholarsh. 2017;49(4):371–8. doi: 10.1111/jnu.12298. [DOI] [PubMed] [Google Scholar]
- 76.Scott IA, Scuffham P, Gupta D, Harch TM, Borchi J, Richards B. Going digital: a narrative overview of the effects, quality and utility of mobile apps in chronic disease self-management. Aust Health Rev. 2020;44(1):62–82. doi: 10.1071/AH18064.AH18064 [DOI] [PubMed] [Google Scholar]
- 77.Dao KP, De Cocker K, Tong HL, Kocaballi AB, Chow C, Laranjo L. Smartphone-delivered ecological momentary interventions based on ecological momentary assessments to promote health behaviors: systematic review and adapted checklist for reporting ecological momentary assessment and intervention studies. JMIR Mhealth Uhealth. 2021;9(11):e22890. doi: 10.2196/22890. https://mhealth.jmir.org/2021/11/e22890/ v9i11e22890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tong HL, Quiroz JC, Kocaballi AB, Fat SC, Dao KP, Gehringer H, Chow CK, Laranjo L. Personalized mobile technologies for lifestyle behavior change: a systematic review, meta-analysis, and meta-regression. Prev Med. 2021;148:106532. doi: 10.1016/j.ypmed.2021.106532.S0091-7435(21)00116-X [DOI] [PubMed] [Google Scholar]
- 79.Castelyn G, Laranjo L, Schreier G, Gallego B. Predictive performance and impact of algorithms in remote monitoring of chronic conditions: a systematic review and meta-analysis. Int J Med Inform. 2021;156:104620. doi: 10.1016/j.ijmedinf.2021.104620. https://linkinghub.elsevier.com/retrieve/pii/S1386-5056(21)00246-X .S1386-5056(21)00246-X [DOI] [PubMed] [Google Scholar]
- 80.Masterson Creber RM, Hickey KT, Maurer MS. Gerontechnologies for older patients with heart failure: what is the role of smartphones, tablets, and remote monitoring devices in improving symptom monitoring and self-care management? Curr Cardiovasc Risk Rep. 2016;10(10):30. doi: 10.1007/s12170-016-0511-8. http://europepmc.org/abstract/MED/28713481 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cajita MI, Hodgson NA, Budhathoki C, Han HR. Intention to use mHealth in older adults with heart failure. J Cardiovasc Nurs. 2017;32(6):E1–7. doi: 10.1097/JCN.0000000000000401. http://europepmc.org/abstract/MED/28248747 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cajita MI, Hodgson NA, Lam KW, Yoo S, Han HR. Facilitators of and barriers to mHealth adoption in older adults with heart failure. Comput Inform Nurs. 2018;36(8):376–82. doi: 10.1097/CIN.0000000000000442. http://europepmc.org/abstract/MED/29742549 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hägglund E, Strömberg A, Hagerman I, Lyngå P. Theory testing of patient perspectives using a mobile health technology system in heart failure self-care. J Cardiovasc Nurs. 2019;34(6):448–53. doi: 10.1097/JCN.0000000000000595. [DOI] [PubMed] [Google Scholar]
- 84.Lee DS, Stukel TA, Austin PC, Alter DA, Schull MJ, You JJ, Chong A, Henry D, Tu JV. Improved outcomes with early collaborative care of ambulatory heart failure patients discharged from the emergency department. Circulation. 2010;122(18):1806–14. doi: 10.1161/CIRCULATIONAHA.110.940262.CIRCULATIONAHA.110.940262 [DOI] [PubMed] [Google Scholar]
- 85.Boom NK, Lee DS, Tu JV. Comparison of processes of care and clinical outcomes for patients newly hospitalized for heart failure attended by different physician specialists. Am Heart J. 2012;163(2):252–9. doi: 10.1016/j.ahj.2011.11.012.S0002-8703(11)00828-3 [DOI] [PubMed] [Google Scholar]
- 86.McAlister FA, Stewart S, Ferrua S, McMurray JJ. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: a systematic review of randomized trials. J Am Coll Cardiol. 2004;44(4):810–9. doi: 10.1016/j.jacc.2004.05.055. https://linkinghub.elsevier.com/retrieve/pii/S0735-1097(04)01123-4 .S0735-1097(04)01123-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search strategy.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist.
List of articles excluded after full-text review for not meeting inclusion criteria regarding the intervention, outcome, or unavailability of full-text.
Patients’ clinical and socioeconomic characteristics.
Quality assessment of included studies.
Presence of personalization in included studies.
Intervention features of included articles.
Behavior change techniques present in the interventions of included articles.


