Abstract
The in vitro potentiation of artemisinin by synthetic manganese porphyrin complexes has been recently reported (F. Benoit-Vical, A. Robert, and B. Meunier, Antimicrob. Agents Chemother. 43:2555–2558, 1999). Since the activity of artemisinin and synthetic antimalarial endoperoxides is related to their interaction with heme (S. R. Meshnick, A. Thomas, A. Ranz, C. M. Xu, and H. Z. Pan, Mol. Biochem. Parasitol. 49:181–190, 1991), an improvement of their efficiency may be expected in the presence of a synthetic metalloporphyrin having the same activating role as endogenous heme. With the aim to boost the activity of antimalarial endoperoxide drugs, we were thus led to evaluate the in vitro and in vivo potentiation of natural and synthetic drugs of this family by a nontoxic and cheap metalloporphyrin. The potentiation of artemisinin, β-artemether, and arteflene (Ro 42-1611) by synthetic heme models is reported. In vitro studies on the chloroquine-resistant Plasmodium falciparum FcB1-Columbia strain indicate a synergistic effect of the manganese complex of meso-tetrakis(4-sulfonatophenylporphyrin) (Mn-TPPS) on the activity of artemisinin or β-artemether, whereas this heme model has no influence on the activity of arteflene. A significant synergistic effect on rodent malaria was also observed in vivo between artemisinin and Mn-TPPS using Plasmodium vinckei petteri strain.
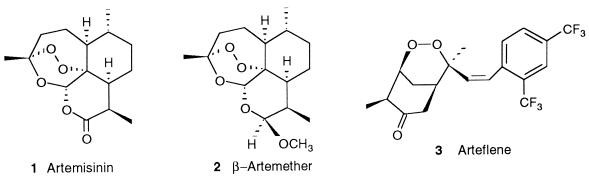
Artemisinin is a sesquiterpene lactone containing an endoperoxide that is critical for its pharmacological activity as an antimalarial drug. Artemisinin 1 and its hemisynthetic derivative artemether 2 (Fig. 1) are currently used to treat severe or multidrug-resistant Plasmodium falciparum malaria, including cerebral malaria. A synthetic compound with an endoperoxide function like arteflene, Ro 42-1611 (19), also exhibits strong antimalarial activity. Artemisinin derivatives are active during the intraerythrocytic stage of infection. The inhibition of hemozoin formation by artemisinin 1 has been proposed (25) but is still controversial (1). It has been proposed that the intraparasitic heme liberated during hemoglobin digestion might play an important role in the selective toxicity of artemisinin toward the parasite (24), and the reductive activation of artemisinin 1 or other endoperoxide-based antimalarial drugs by Fen heme is probably a key point in the mechanism of action of these drugs (8, 23, 33). In the presence of synthetic heme models such as the iron or manganese complexes of meso-tetraphenylporphyrin, it was recently found that artemisinin and many synthetic endoperoxides are able to generate alkylating intermediates via formation of either C-centered radicals (27, 32, 34) or other reactive species (5). For these compounds, the same mechanism of reductive activation by the synthetic metalloporphyrin or by heme itself gives rise to different drug-derived reactive species which can behave as alkylating agents with respect to heme or parasitic proteins. Such modifications of essential molecules of the parasite should be responsible for the antimalarial effect.
FIG. 1.
Structures of the endoperoxide antimalarial drugs 1 through 3.
We therefore looked for a possible potentiation of artemisinin, β-artemether, and arteflene activity by an added synthetic metalloporphyrin, which could act as enhancer of the drug-endoperoxide activation step as well as the endogenous iron(II) heme. This procedure was carried out on one hand in P. falciparum-infected human erythrocytes and on the other hand in murine malaria. In a previous study, the potentiation between artemisinin and the iron and manganese complexes of meso-tetrakis(4-sulfonatophenylporphyrin) (TPPS) and meso-tetrakis(3,5-disulfonatomesityl porphyrin) (TMPS) was evaluated in vitro (4). A synergistic effect was found between artemisinin and Mn-TPPS but not with the iron porphyrin analogue. In the present study, a similar behavior was observed with β-artemether in the presence of manganese porphyrin complexes in vitro. However, the metalloporphyrins used had no effect on the activity of the synthetic antimalarial drug arteflene. In vivo, on Plasmodium vinckei petteri, we observed a significant synergistic effect of Mn-TPPS on the activity of artemisinin but a decrease of the efficiency of β-artemether in the presence of the same metalloporphyrin. This apparent antagonistic effect can probably be attributed to an increased activation of β-artemether outside of its target in the presence of Mn-TPPS, this phenomenon leading to a lower concentration of “useful” drugs within the parasite.
MATERIALS AND METHODS
Materials.
The resin TEMEX, AG50W-X8, H+ form, was purchased from Touzart & Matignon (Vitry-sur-Seine, France). Artemisinin was purchased from Aldrich (Saint-Quentin Fallavier, France). Tetraphenylporphyrin and other commercially available chemicals were from Aldrich, Fluka (Saint-Quentin Fallavier, France) or Merck (Nogent-sur-Marne, France). Artemether and arteflene were gifts of Rhône-Poulenc-Rorer Doma (Antony, France) and Hoffmann-La Roche (Basel, Switzerland), respectively.
Porphyrin synthesis.
The hydrophobic ligand meso-tetramesitylporphyrin and the corresponding hydrosoluble octasulfonated derivative H2-TMPS were prepared as previously described by Hoffmann et al. (15) and Song et al. (36), respectively. The hydrosoluble tetrasulfonated derivative H2-TPPS was prepared by modification of a published synthesis (38) as follows. To recover the sulfonated porphyrin from an aqueous solution containing a huge amount of salt, n-tetrabutylammonium chloride was added (3 eq. per sulfonate group). The porphyrin containing four tetrabutylammonium counterions was then extracted with dichloromethane. The organic layer was washed with water, dried over magnesium sulfate, and evaporated to dryness. This desalted porphyrin was then dissolved in water and passed over a column of strongly acid resin AG50W-X8 pretreated with 1 M NaOH to regenerate the sodium sulfonate groups. The eluted aqueous solution was evaporated, and the pure tetrasodium salt of H2-TPPS was dried under vacuum.
The manganese complexes Mn-TPPS and Mn-TMPS were prepared by reaction of the corresponding ligands with MnII(OAc)2·4H2O (1.1 eq.) in refluxing water for 2 h. After cooling, the reaction mixture was treated with resin AG50W-X8, Na+ form, and filtered. The metalloporphyrins were dissolved in a minimum amount of methanol, precipitated by addition of diethyl ether, filtered, and dried under vacuum. For Mn-TPPS, the UV-vis (water) λ, in nanometers (ɛ, mM−1 × cm−1), is 378 (58), 400 (59), 420sh, 466 (100), 516 (7), 564 (12), 596 (9). For Mn-TMPS, the UV-vis (water) λ, in nanometers (ɛ, mM−1 × cm−1), is 378 (30), 400 (32), 420sh, 468 (80), 568 (6).
Parasites.
The chloroquine-resistant P. falciparum strain FcB1-Colombia was cultured in vitro using standard techniques (39) in a 5% CO2 atmosphere at 37°C (3). Cultures were synchronized by combination of gelatin (Plasmagel; Roger Bellon, Paris, France) and 5% d-sorbitol lysis (Merck, Darmstadt, Germany) (20, 21).
Antimalarial activity.
The in vitro antimalarial-drug-sensitivity microtest was adapted from the micromethod of Desjardins et al. (10). Drugs were tested three times in triplicate in 96-well plates with cultures at ring stage (synchronization interval, 16 h) at 0.5 to 1% parasitemia (hematocrit, 1%). For each test, the plates of parasite culture were incubated with drugs at decreasing concentrations for 32 h and 72 h because of the timing of the onset and cessation of DNA, RNA, and protein synthesis (14, 18). The first dilutions of artemisinin (Sigma, France) and metalloporphyrins were prepared in sterile dimethyl sulfoxide (Merck) and Milli-Q ultrapure water, respectively (1 mg/ml), and later dilutions were with RPMI 1640 (Life Technologies, Cergy Pontoise, France). Parasite growth was estimated by incorporation of [3H]hypoxanthine (Amersham-France, Les Ulis, France) (3). Concentrations inhibiting 50% of the parasite growth (IC50) were determined graphically in concentration versus percent inhibition curves for each endoperoxide drug and each metalloporphyrin after 32 h or 72 h of incubation, with [3H]hypoxanthine being added to the medium at 24 h and 56 h, respectively.
In vitro potentiation tests.
The effect of the manganese porphyrin complexes on the IC50 of endoperoxides was determined by potentiation experiments as described in reference 22. Isobolograms were constructed by plotting a pair of fractional IC50s for each combination of endoperoxide and metalloporphyrin. Endoperoxide fractional IC50s were calculated by dividing the IC50 of each endoperoxide combined with the metalloporphyrin by the IC50 of the endoperoxide alone, and these data were plotted on the horizontal axis. The corresponding metalloporphyrin fractional IC50 was calculated by dividing its fixed concentration by the IC50 of metalloporphyrin alone and was plotted on the vertical axis (13). An isobologram as a straight diagonal indicates an additive effect. Curves above or below the diagonal indicate antagonistic or synergistic effects, respectively. Results too close to the diagonal are considered as additive.
In vivo drug potentiation.
Cell line 106WH of P. vinckei petteri, kindly supplied by E. Deharo (Muséum National d'Histoire Naturelle, Paris, France), was maintained in mice by syringe passage. Female CD1 mice (6 weeks of age, 20 ± 2 g) were used in this study. The mice were kept at a temperature of 22 ± 3°C and provided with a standard diet and water. They were inoculated intraperitoneally with 2 × 107 infected erythrocytes on day 0. The 4-day test was carried out as previously described (26) and was repeated three times. For the determination of the 50% effective dose (ED50) values, groups of five mice were treated by intraperitoneal injection once daily for four consecutive days with a range of doses, beginning 3 h after infection on day 0. All experiments included a drug-free control group, three or four groups were treated with different doses of the endoperoxide, three groups were treated with different doses of Mn-TPPS administered alone, and three to five groups were treated with Mn-TPPS in combination with the endoperoxide (9, 31). For each endoperoxide drug, three independent experiments were made. Parasitemia was checked by Giemsa Diff-Quik (Dade Behring, Paris, France)-stained blood smears on day 4 (i.e., 24 h after the final treatment). The reduction of parasitemia in treated groups was calculated as a percentage of parasitemia of untreated control groups. The ED50 doses resulting in 50% decreases in parasitemia are expressed in milligrams per kilogram of body weight per day. The 95% confidence intervals were calculated by a statistical study. In order to evaluate the putative toxicity of Mn-TPPS, a group of healthy mice was treated with Mn-TPPS at 40 mg/kg of body weight/day for 4 days.
Statistical study.
The multiple comparison procedure of the Dunnett test (11) permitted us to examine the differences in vivo between all treated groups in comparison with the control group (PC-pcsm software, version 6.0, 1992; Delta-soft, Meylan, France).
RESULTS AND DISCUSSION
IC50 values and in vitro potentiation tests.
The IC50 values obtained for artemisinin, artemether, arteflene, Mn-TPPS, and Mn-TMPS on P. falciparum FcB1 strain are reported in Table 1. These results indicate that, on the chloroquine-resistant P. falciparum FcB1-Columbia strain, artemether is 3.5-fold as efficient as the parent drug artemisinin (IC50, 0.1 nM, compared to 0.35 nM after 32 h of incubation) and close to 500-fold as efficient as arteflene (IC50, 49 nM). The IC50 values of Mn-TPPS and Mn-TMPS were also determined and were found in the same range as previously reported (4). In fact, these metalloporphyrins are both devoid of significant antimalarial activity when used alone (IC50, 276 μM and 257 μM for Mn-TPPS and Mn-TMPS, respectively).
TABLE 1.
IC50 values of endoperoxides and manganese metalloporphyrins tested independently against the chloroquine-resistant P. falciparum strain FcB1-Columbia
| Drug | IC50 values for incubation time (h)
|
|||
|---|---|---|---|---|
| 32
|
72
|
|||
| ng/mla | nM | ng/mla | nM | |
| Artemisinin | 0.1 | 0.35 | 0.1 | 0.35 |
| Artemether | 0.03 (0.02) | 0.10 | 0.02 (0.01) | 0.07 |
| Arteflene | 20 (10) | 49 | 15 (10) | 37 |
| Mn-TMPS | 434 (135) | 257 | 196 (45) | 116 |
| Mn-TPPS | 306 (115) | 276 | 104 (36) | 94 |
Values in parentheses are standard deviations (n > 8).
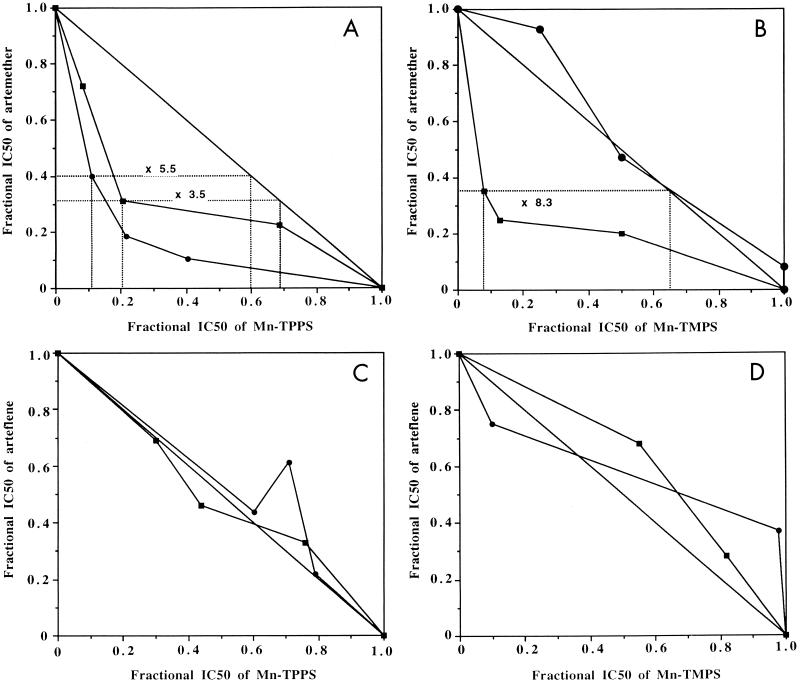
Artemether is a widely used derivative of artemisinin. It is effective against multidrug-resistant P. falciparum (29), and several studies in Asia have suggested that artemether is more effective than quinine in reducing the number of fatal issues in severe malaria (16). However, decreasing the efficient doses is a desirable goal in order to decrease the risk of resistance and also from an economic point of view. The potentiation of artemether by Mn-TPPS and Mn-TMPS is reported in Fig. 2A and B, respectively. A significant synergistic effect was observed in the presence of Mn-TPPS (Fig. 2A), the efficiency of artemether being multiplied by a factor of 3.5 or 5.5, after 32 or 72 h of incubation, respectively. This result is consistent with that obtained for the potentiation of artemisinin by Mn-TPPS on the same parasite strain (activity × 3.6 after 72 h) (4). On the other hand, Mn-TMPS also increased the efficiency of artemether after 32 h of incubation but was only additive after 72 h (Fig. 2B). An additive effect of Mn-TMPS associated with artemisinin after 72 h was also previously reported (4), but presently we have no rational explanation for the difference in effect of Mn-TMPS with respect to the incubation time.
FIG. 2.
Potentiation of artemether (A and B) and arteflene (C and D) by Mn-TPPS (A and C) and Mn-TMPS (B and D) on P. falciparum FcB1 strain after 32 h (■) or 72 h (●) of incubation.
Arteflene is a potent, long-lasting antimalarial drug, which is also active on chloroquine- and/or pyrimethamine-resistant strains (19). A clinical study of arteflene in the treatment of patients with mild malaria showed good efficacy and no adverse effects (35). However, other studies showed that the same dose was not sufficient to cure children with P. falciparum malaria (30). The potentiation of arteflene by simple and cheap molecules is of particular interest as is its combination with other antimalarial drugs.
The potentiation of arteflene by Mn-TPPS and Mn-TMPS is reported in Fig. 2C and D, respectively, after 32 and 72 h of incubation time. Both resulting isobolograms are close to the diagonal, therefore indicating that an additive effect was obtained, with the activity of arteflene mainly unchanged in the presence of a synthetic metalloporphyrin. When radiolabeled arteflene is incubated with P. falciparum-infected erythrocytes, this drug alkylates some parasite proteins (2). Furthermore, the in vitro activation of arteflene by a reduced-heme model produces drug-derived fragments which are able to react with nucleophilic residues of proteins to generate covalent adducts. It is therefore unlikely that arteflene did not react with Mn-TPPS or react to produce inert derivatives. The more reasonable hypothesis is that the in vivo activation of arteflene by sulfonated metalloporphyrin complexes probably occurs away from the parasite target of arteflene.
In vivo potentiation by Mn-TPPS.
The ED50 values of artemisinin and artemether on the P. vinckei petteri-infected mice were 3 ± 2 mg/kg of body weight and 0.3 ± 0.2 mg/kg of body weight, respectively, which is consistent with values previously reported by Jaquet et al. (19) and Posner et al. (28) (drugs injected subcutaneously on Plasmodium berghei).
In the 4-day test, the ED50 values of Mn-TPPS were found to be 25 ± 10 mg/kg of body weight per day. Since Mn-TPPS was shown to enhance in vitro the antimalarial activity of both artemisinin and artemether when incubated with the P. falciparum FcB1 strain and is devoid of toxicity in several cell lines (e.g., HeLa human fibroblast [4] and MT-4 lymphocyte [37]), we decided to treat a group of healthy mice with Mn-TPPS for 4 days. At a dose of 40 mg/kg of body weight per day, (close to twice as high as ED50), the treated mice were still alive, with normal behavior, after a 2-month period. No visible effect on organs was noticed during autopsy. These data confirmed the absence of toxicity of Mn-TPPS in mice.
The control-mouse group was inoculated with Plasmodium spp. and received no treatment but only physiological serum during the 4-day test. The mean parasitemia of untreated control mice on day 4 was found to vary from 32% to 63% (mean, 46%). No death occurred during the treatment. All but one (97%) of the nontreated control mice died between days 5 and 14.
The results obtained for the potentiation of the drug by Mn-TPPS on P. vinckei petteri were analyzed by using the Dunnett statistical test. For α = 0.05 and in bilateral situations, the groups of mice treated by the peroxide drug alone or by the peroxide associated with Mn-TPPS have ED50 values statistically different from those obtained with the untreated control group (11).
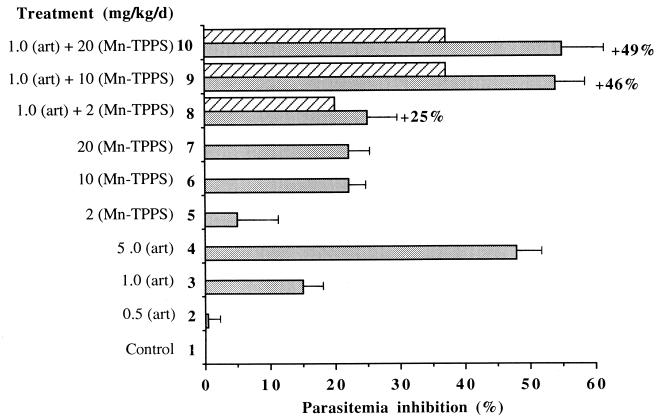
The results obtained for the potentiation of artemisinin and artemether by Mn-TPPS on P. vinckei petteri are reported in Fig. 3 and 4, respectively. They are expressed as percentages of parasitemia inhibition with respect to the control group. The inhibition of parasitemia of the control group (line 1) is therefore 0%. In Fig. 3, lines 2 to 4 and 5 to 7 report parasitemia inhibition in the presence of increasing doses of artemisinin alone and Mn-TPPS alone, respectively. Lines 8 to 10 report parasitemia inhibition in the presence of 1 mg of artemisinin per kg of body weight per day and increasing doses of Mn-TPPS (2, 10, and 20 mg/kg/day).
FIG. 3.
Parasitemia inhibition in mice treated with artemisinin (art) associated with Mn-TPPS. Hatched zones represent the expected inhibition if both drugs were additive.
FIG. 4.
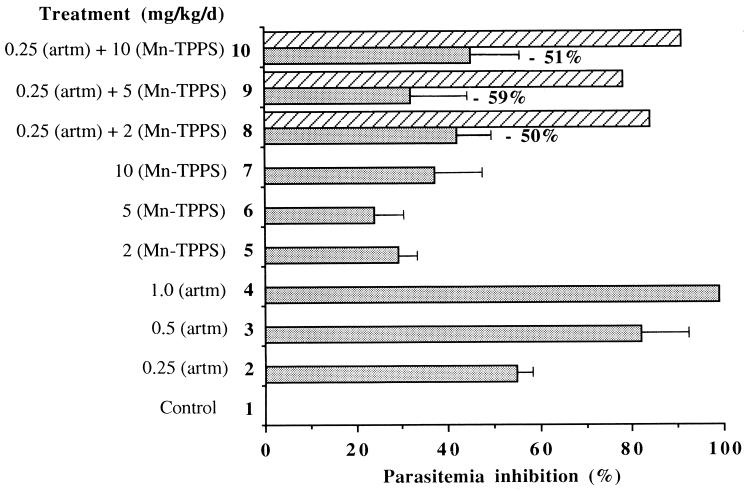
Parasitemia inhibition in mice treated with artemether (artm) associated with Mn-TPPS. Hatched zones represent the expected inhibition if both drugs were additive.
The potentiation was calculated as [inh (A + MnP) − (inh A + inh MnP)]/(inh A + inh MnP), where inh A is the parasitemia inhibition value due to the applied dose of artemisinin when used alone, inh MnP is the parasitemia inhibition value due to the applied dose of Mn-TPPS when used alone, and inh (A + MnP) is the parasitemia inhibition value due to artemisinin and Mn-TPPS used together.
When the subinhibitory dosage of 1 mg of artemisinin per kg of body weight per day was associated with 10 or 20 mg of Mn-TPPS per kg of body weight per day, the inhibition of parasitemia was 54% ± 7% and 55% ± 10%, respectively (lines 9 and 10 in Fig. 3), significantly higher than that of mice treated with artemisinin used alone (15% ± 3% [Fig. 3, line 3]) or the inhibition of parasitemia expected in the case of an additive effect (15% + 22% = 37%). The potentiations reached in these cases, 46 and 49% (Fig. 3, lines 9 and 10), were two similar values. With 1 mg of artemisinin per kg of body weight per day associated with 2 mg of Mn-TPPS per kg of body weight per day, the potentiation was 25% (line 8). The decreased parasitemia of mice treated with artemisinin associated with Mn-TPPS is consistent with the in vitro potentiation of artemisinin by the metalloporphyrin on the FcB1 strain.
The potentiation study of artemether reported in Fig. 4 is rather puzzling. When 0.25 mg of artemether per kg of body weight per day was associated with 2 or 10 mg of Mn-TPPS per kg body weight per day, in the hypothesis of an additive effect of these two drugs, 84 or 91%, respectively, of parasitemia inhibition was expected. In fact, only 42% ± 8% and 45% ± 10% of parasitemia inhibition was observed in these cases (Figure 4, lines 8 and 10).
This reduced efficiency of artemether in the presence of the metalloporphyrin did not correlate with the potentiation observed in vitro (Fig. 2B). The living mice are obviously more complex than the in vitro parasite culture medium, and artemether was found to be more fragile than artemisinin, especially in acidic conditions (34). It is also probable that artemether can be activated by the metalloporphyrin outside the parasite, far from the target heme or proteins, leading to a loss of “useful” artemether and then to a decrease of efficiency when artemether and Mn-TPPS are associated. In fact, different studies have shown that the results of antimalarial assays obtained in vitro with drug associations are difficult to extrapolate from one strain to another and from in vitro to in vivo (12). For example, on one hand, the association of artemisinin and mefloquine has been found to be additive in vitro on K1 and NF-54 strains (19). On the other hand, this association exhibits a synergistic effect in vivo on P. berghei (7) and is widely used against multidrug-resistant Plasmodium spp. in humans (17). Furthermore, it was recently reported that rats might be better animal models than mice for parasitical studies, due to the numerous differences between the immunity systems of the latter and that of humans (6).
The main result of the present in vivo potentiation study is that the artemisinin efficiency can be increased by ca. 50% in the presence of a nontoxic and cheap manganese porphyrin complex, which is by itself devoid of any antiplasmoidal activity. In addition, these results confirm that the activation of artemisinin by heme or a heme model in the present case is a key step in the mechanism of action of this antimalarial trioxane.
ACKNOWLEDGMENTS
This work was supported by the CNRS (Programme “Physique et Chimie du Vivant”) and by a grant from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (Director's Initiative Fund).
Rhône-Poulenc-Rorer Doma (Antony, France) and Hoffmann-La Roche (Basel, Switzerland) are gratefully acknowledged for a gift of β-artemether (Paluther) and arteflene (Ro 42-1611), respectively. We thank J. Bernadou (LCC-CNRS, Toulouse) for fruitful discussions and H. Maillols (Laboratoire de Technique Pharmaceutique Industrielle, UFR Sciences Pharmaceutiques, Université Montpellier I, Montpellier, France) for her help with statistical analyses of the results. We are grateful to J.-M. Bastide (Laboratoire d'Immunologie et Parasitologie, UFR Sciences Pharmaceutiques, Montpellier, France) for his constant interest throughout this work.
REFERENCES
- 1.Asawamahasakda W, Ittarat I, Chang C C, McElroy P, Meshnick S R. Effects of antimalarials and protease inhibitors on plasmodial hemozoin production. Mol Biochem Parasitol. 1994;67:183–191. doi: 10.1016/0166-6851(94)00128-6. [DOI] [PubMed] [Google Scholar]
- 2.Asawamahasakda W, Ittarat I, Pu Y-M, Ziffer H, Meshnick S R. Reaction of antimalarial endoperoxides with specific parasite proteins. Antimicrob Agents Chemother. 1994;38:1854–1858. doi: 10.1128/aac.38.8.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benoit F, Valentin A, Pélissier Y, Diafouka F, Marion C, Kone-Bamba D, Koné M, Mallié M, Yapo A, Bastide J M. In vitro antimalarial activity of vegetal extracts used in West African traditional medicine. Am J Trop Med Hyg. 1996;54:67–71. doi: 10.4269/ajtmh.1996.54.67. [DOI] [PubMed] [Google Scholar]
- 4.Benoit-Vical F, Robert A, Meunier B. Potentiation of artemisinin activity against chloroquine-resistant Plasmodium falciparum strains by using heme models. Antimicrob Agents Chemother. 1999;43:2555–2558. doi: 10.1128/aac.43.10.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cazelles J, Robert A, Meunier B. Characterization of the main radical and products resulting from a reductive activation of the antimalarial arteflene (Ro 42-1611) J Org Chem. 1999;64:6776–6781. doi: 10.1021/jo990744z. [DOI] [PubMed] [Google Scholar]
- 6.Cêtre C, Pierrot C, Cocude C, Lafitte S, Capron A, Capron M, Khalife J. Profiles of Th1 and Th2 cytokines after a primary infection by Schistosoma mansoni in the semipermissive rat host. Infect Immun. 1999;67:2713–2719. doi: 10.1128/iai.67.6.2713-2719.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chawira A N, Warhurst D C, Robinson B L, Peters W. The effect of combinations of qinghaosu (artemisinin) with standard antimalarial drugs in the suppressive treatment of malaria in mice. Trans R Soc Trop Med Hyg. 1987;81:554–558. doi: 10.1016/0035-9203(87)90404-4. [DOI] [PubMed] [Google Scholar]
- 8.Cumming J N, Ploypradith P, Posner G H. Antimalarial activity of artemisinin (qinghaosu) and related trioxanes: mechanism(s) of action. Adv Pharmacol. 1997;37:253–297. doi: 10.1016/s1054-3589(08)60952-7. [DOI] [PubMed] [Google Scholar]
- 9.Deloron P, Basc L K, Dubois B, Gaudin C, Clavier F, Le Bras J, Verdier F. In vitro and in vivo potentiation of chloroquine against malaria parasites by an enantiomer of amlodipine. Antimicrob Agents Chemother. 1991;35:1338–1342. doi: 10.1128/aac.35.7.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desjardins R E, Canfield C J, Haynes J D, Chulay J D. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother. 1979;16:710–718. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunnett C W. Biometrics. 1964;1964:482–491. [Google Scholar]
- 12.Fleck S L, Robinson B L, Peters W. The chemotherapy of rodent malaria. Ann Trop Med Parasitol. 1997;91:33–39. doi: 10.1080/00034983.1997.11813109. [DOI] [PubMed] [Google Scholar]
- 13.Frappier F, Jossang A, Soudon J, Calvo F, Rasoanaivo P, Ratsimamanga-Urveng S, Saez J, Schrével J, Grellier P. Bisbenzylisoquinolines as modulators of chloroquine resistance in Plasmodium falciparum and multidrug resistance in tumor cells. Antimicrob Agents Chemother. 1996;40:1476–1481. doi: 10.1128/aac.40.6.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graeser R, Wernli B, Franklin R M, Kappes B. Plasmodium falciparum protein kinase 5 and the antimalarial nuclear division cycles. Mol Biochem Parasitol. 1996;82:37–49. doi: 10.1016/0166-6851(96)02716-8. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann P, Robert A, Meunier B. Preparation and catalytic activities of the manganese and iron derivatives of Br8TMP and Cl12TMP, two robust porphyrin ligands obtained by halogenation of tetramesitylporphyrin. Bull Chem Soc Fr. 1992;129:85–97. [Google Scholar]
- 16.Hoffman S L. Artemether in severe malaria—still too many deaths. N Engl J Med. 1996;335:124–126. doi: 10.1056/NEJM199607113350209. [DOI] [PubMed] [Google Scholar]
- 17.Hung L N, de Vries P J, Thuy L T D, Lien B, Long H P, Hung T N, Nam N V, Anh T K, Kager P A. Single dose artemisinin-mefloquine versus mefloquine alone for uncomplicated falciparum malaria. Trans R Soc Trop Med Hyg. 1997;91:191–194. doi: 10.1016/s0035-9203(97)90221-2. [DOI] [PubMed] [Google Scholar]
- 18.Inselburg J, Banyal H S. Synthesis of DNA during the asexual cycle of Plasmodium falciparum in culture. Mol Biochem Parasitol. 1984;10:79–87. doi: 10.1016/0166-6851(84)90020-3. [DOI] [PubMed] [Google Scholar]
- 19.Jaquet C, Stohler H R, Chollet J, Peters W. Antimalarial activity of the bicyclic peroxide Ro 42-1611 (arteflene) in experimental models. Trop Med Parasitol. 1994;45:266–271. [PubMed] [Google Scholar]
- 20.Jensen J. Concentration from continuous culture of erythrocytes infected with trophozoites and schizonts of Plasmodium falciparum. Am J Trop Med Hyg. 1978;27:1274–1276. doi: 10.4269/ajtmh.1978.27.1274. [DOI] [PubMed] [Google Scholar]
- 21.Lambros C, Vanderberg J P. Synchronisation of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- 22.Martin S K, Oduola A M J, Milhous W K. Reversal of chloroquine resistance in Plasmodium falciparum by verapamil. Science. 1987;235:899–901. doi: 10.1126/science.3544220. [DOI] [PubMed] [Google Scholar]
- 23.Meshnick S R, Taylor T E, Kamchonwongpaisan S. Artemisinin and the antimalarial endoperoxides: from herbal remedy to targeted chemotherapy. Microbiol Rev. 1996;60:301–315. doi: 10.1128/mr.60.2.301-315.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meshnick S R, Thomas A, Ranz A, Xu C M, Pan H Z. Artemisinin (quinghaosu): the role of intracellular hemin in its mechanism of antimalarial action. Mol Biochem Parasitol. 1991;49:181–190. doi: 10.1016/0166-6851(91)90062-b. [DOI] [PubMed] [Google Scholar]
- 25.Orjih A U. Haemolysis of Plasmodium falciparum trophozoite-infected erythrocytes after artemisinin exposure. Br J Haematol. 1996;92:324–328. doi: 10.1046/j.1365-2141.1996.d01-1471.x. [DOI] [PubMed] [Google Scholar]
- 26.Peters W. Chemotherapy and drug resistance in malaria. 2nd ed. London, United Kingdom: Academic Press; 1987. [Google Scholar]
- 27.Posner G H, Oh C H, Wang D, Gerena L, Milhous W K, Meshnick S R, Asawamahasakda W. Mechanism-based design, synthesis, and in vitro antimalarial testing of new 4-methylated trioxanes structurally related to artemisinin: the importance of a carbon-centered radical for antimalarial activity. J Med Chem. 1994;37:1256–1258. doi: 10.1021/jm00035a003. [DOI] [PubMed] [Google Scholar]
- 28.Posner G H, Parker M H, Northrop J, Elias J S, Ploypradith P, Xie S, Shapiro T A. Orally active, hydrolytically stable, semisynthetic, antimalarial trioxanes in the artemisinin family. J Med Chem. 1999;42:300–304. doi: 10.1021/jm980529v. [DOI] [PubMed] [Google Scholar]
- 29.Price R N, Van Vught M, Nosten F, Luxemburger C, Brockman A, Phaipun L, Chongsuphajaisiddhi T, White N. Artesunate versus artemether for the treatment of recrudescent multidrug-resistant falciparum malaria. Am J Trop Med Hyg. 1998;59:883–888. doi: 10.4269/ajtmh.1998.59.883. [DOI] [PubMed] [Google Scholar]
- 30.Radloff P D, Phillips J, Nkeyi M, Sturchler D, Mitterlholzer M L, Kremsner P G. Arteflene compared with mefloquine for treating Plasmodium falciparum malaria in children. Am J Trop Med Hyg. 1996;55:259–262. doi: 10.4269/ajtmh.1996.55.259. [DOI] [PubMed] [Google Scholar]
- 31.Rasoanaivo P, Ratsimamanga-Urveg S, Rafatro H, Ramanitrahasimbola D, Palazzino G, Galeffi C, Nicoletti M. Alkaloids of Hernandia voyronii: chloroquine-potentiating activity and structure elucidation of herveline D. Planta Med. 1998;64:58–62. doi: 10.1055/s-2006-957367. [DOI] [PubMed] [Google Scholar]
- 32.Robert A, Meunier B. Characterization of the first covalent adduct between artemisinin and a heme model. J Am Chem Soc. 1997;119:5968–5969. [Google Scholar]
- 33.Robert A, Meunier B. Is alkylation the main mechanism of action of the antimalarial drug artemisinin? Chem Soc Rev. 1998;27:273–279. [Google Scholar]
- 34.Robert A, Meunier B. Alkylating properties of antimalarial artemisinin derivatives and synthetic trioxanes when activated by a reduced heme model. Chem Eur J. 1998;4:1287–1296. [Google Scholar]
- 35.Somo-Moyou R, Mittelholzer M L, Sorenson F, Haller L, Sturchler D. Efficacy of Ro 42-1611 (arteflene) in the treatment of patients with mild malaria. Trop Med Parasitol. 1994;45:288–291. [PubMed] [Google Scholar]
- 36.Song R, Robert A, Bernadou J, Meunier B. Sulfonated and acetamidosulfonylated tetraarylporphyrins as biomimetic oxidation catalysts under aqueous conditions. Inorg Chim Acta. 1998;272:228–234. [Google Scholar]
- 37.Song R, Witvrouw M, Schols D, Robert A, Balzarini J, De Clercq E, Bernadou J, Meunier B. Anti-HIV activities of anionic metalloporphyrins and related compounds. Antivir Chem Chemother. 1997;8:85–97. [Google Scholar]
- 38.Srivastava T S, Tsutsui M. Preparation and purification of tetrasodium meso-tetra(p-sulfophenyl)porphine. An easy procedure. J Org Chem. 1973;38:2103–2103. [Google Scholar]
- 39.Trager W, Jensen J. Human malaria parasite in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]