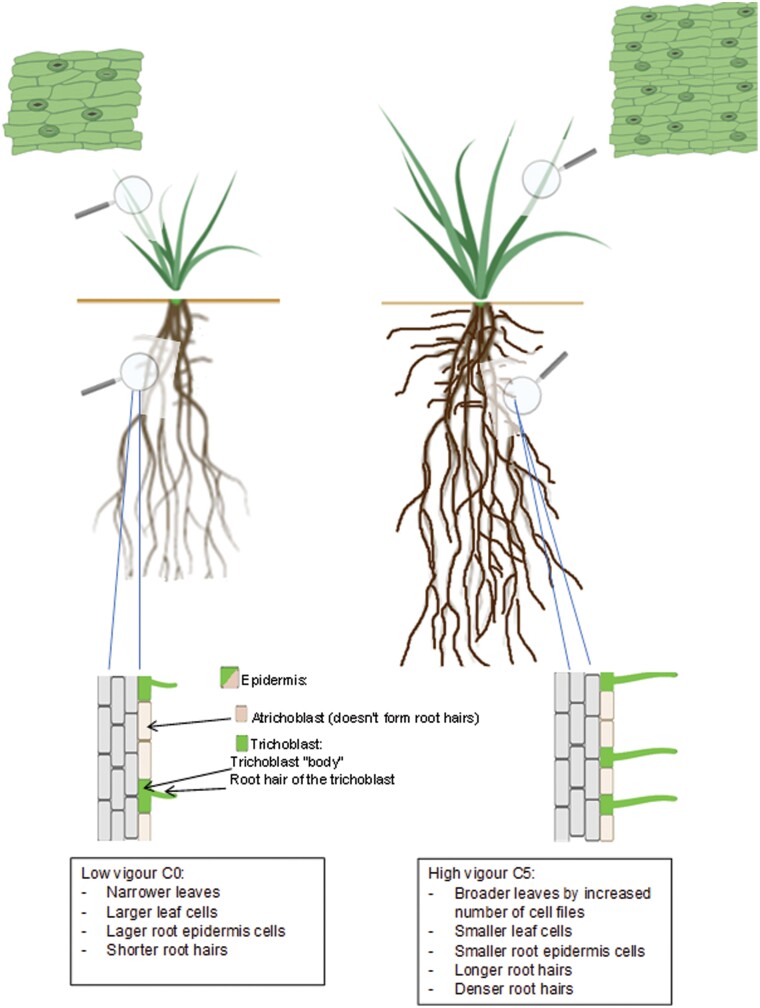
The selection for early shoot vigour in wheat is associated with increased root hair length and changes to cell size in roots and shoots.
Keywords: Cell body, cell size, competitiveness, early shoot vigour, rhizosheaths, root hairs, selection, trichoblast, wheat
Abstract
Six cycles of recurrent selection for early shoot vigour in wheat resulted in significant increases in leaf width and shoot biomass. Here, in replicated controlled-environment studies, the effect of early shoot vigour on root biomass, rhizosheath size, root hair length, and cell size in the roots and leaves was examined across different cycles of selection. Increased shoot vigour was associated with greater root biomass, larger rhizosheath size, and longer root hairs. Our findings demonstrate that rhizosheath size was a reliable surrogate for root hair length in this germplasm. Examination of the root epidermis revealed that the ‘cell body’ of the trichoblasts (hair-forming cells) and the atrichoblasts (non-hair-forming cells) decreased in size as shoot vigour increased. Therefore, in higher vigour germplasm, longer root hairs emerged from smaller trichoblasts so that total trichoblast volume (root hair plus cell body) was generally similar regardless of shoot vigour. Similarly, the sizes of the four main cell types on the leaf epidermis became progressively smaller as shoot vigour increased, which also increased stomatal density. The relationship between shoot vigour and root traits is considered, and the potential contribution of below-ground root traits to performance and competitiveness of high vigour germplasm is discussed.
Introduction
Early vigour in wheat (Triticum aestivum L.) is exemplified by rapid canopy growth, resulting in increased soil coverage (Richards, 1989), and is positively correlated with the length, width, and area of the first leaves (Rebetzke and Richards, 1999; Maydup et al., 2012). Leaf area and shoot biomass are factors that positively influence the ability of wheat to compete with weeds because they prevent competing species from accessing sunlight (Gaudet and Keddy, 1988). Plant height is also considered a beneficial trait for improving competitiveness as it is a major driver of above-ground biomass (Lemerle et al., 1996). However, during the Green Revolution of the 1960s, the average height of wheat crops was reduced by the introduction of dwarfing genes, Rht-B1b and Rht-D1b, to improve the harvest index and reduce lodging (Murphy et al., 2008). The resulting semi-dwarf cultivars exhibited reduced early vigour (Rebetzke et al., 2004) and lower shoot biomass, and were less competitive than their taller progenitors (Vandeleur and Gill, 2004). Considerable variation in early shoot vigour was identified among the semi-dwarf varieties, and additional selection for vigour generated modest increases in competitiveness without compromising the harvest index (Zerner et al., 2016). A focused breeding programme for early shoot vigour is thus expected to further enhance weed competitiveness (Rebetzke et al., 2018).
An intrapopulation breeding programme in wheat based on recurrent selection for early shoot vigour using a diverse set of globally sourced vigorous germplasm was described by Zhang et al. (2015). Progeny showing enhanced leaf width were selected and randomly intercrossed. This procedure was repeated over six cycles spanning 15 years, resulting in a near-linear increase in leaf width, leaf area index, and shoot biomass with each cycle of selection. While the positive impact of early shoot vigour on weed competitiveness is well established (Bertholdsson, 2005), the relationship between shoot vigour and other traits, including below-ground architecture and root development, is unclear. Further insight into whether the drivers of shoot vigour are restricted to the shoots or reflect a widespread change to plant development is required. It is unknown whether shoot vigour is linked with specific root traits that might also contribute to the enhanced competitiveness of that germplasm. After all, prior to canopy closure, the competition for below-ground resources is likely to be more intense than competition above-ground (Wilson, 1988; Kiær et al., 2013). Casper and Jackson (1997) also pointed out that ‘where’ and ‘when’ roots are deployed may be as important as average root density in explaining below-ground competitiveness.
Wheat cultivars with more vigorous shoots tend to possess larger root systems (Palta et al., 2011; Palta and Yang, 2014). One study demonstrated that the mature root systems of ‘vigorous’ wheats accumulated up to 66% more carbon than less vigorous commercial cultivars (Palta and Watt, 2009) and are more efficient at accessing nitrogen and phosphorus from the soil (Liao et al., 2004; Ryan et al., 2015). The effect of shoot vigour on other root traits, including root hair length and cell size, have not been investigated in detail, even though they could explain plant performance and reveal clues to the developmental mechanisms driving increased plant vigour.
Root hairs are likely to be associated with increased crop competitiveness as well. These protrusions from the trichoblasts on the root epidermis (Libault et al., 2010; Dolan, 2017) increase the volume of soil explored by the roots and enhance surface area required for absorption of water and nutrients (Passioura, 1988). Along with fine roots, root hairs enhance a plant’s ability to access critical soil resources for a relatively low carbon cost (Hutchings and de Kroon, 1994; Datta et al., 2011; Brown et al., 2012; Comas et al., 2013; Rongsawat et al., 2021). Root hair length, density, and distribution impact the plant’s ability to access resources (Gilroy and Jones, 2000; Ruiz et al., 2019), particularly in challenging environments (Carminati et al., 2017; Vincent et al., 2017). For example, a study with root hairless barley mutants demonstrated that under drought conditions the absence of root hairs reduced water and nutrient uptake by 3-fold (Marin et al., 2021). Indeed, some consider the volume of soil occupied by roots to be more important for competitiveness than the total size of the root system (Craine and Dybzinski, 2013). Interestingly, wheat exhibits variation in root hair length and density, and cultivars with long root hairs may also exhibit greater hair density (Gahoonia et al., 1997). Apart from increased soil exploration and nutrient absorption, root hairs may contribute to below-ground competitiveness through other rhizospheric interactions including the exudation of secondary metabolites that influence the growth of competing plants, or allelopathy (Holz et al., 2018). It has been suggested that plants with long, dense root hairs exhibit enhanced allelopathic potential (Bertin et al., 2003) by increased production and release of bioherbicides that suppress the growth of competing species (Weston, 2005; Weston et al., 2012).
Given the lack of information on the relationship between shoot vigour and below-ground development in wheat, we examined specific root traits in germplasm generated from the recurrent selection for increased shoot vigour. Specifically, we assessed root epidermal and leaf epidermal cell sizes to test the hypothesis that enhanced shoot vigour alters the number and size of cells in these organs. We also measured rhizosheath size as a proxy for root hair length (Delhaize et al., 2012, 2015) given that root hair assessments are particularly challenging (Watt et al., 1994; Pang et al., 2017). The hypothesis that trichoblast cell body size is associated with root hair length was also tested. We discuss how root traits could be contributing to the superior competitiveness of vigorous germplasm.
Materials and methods
Germplasm
Experiments were performed with wheat genotypes developed by recurrent selection for high shoot vigour in an intrapopulation wheat breeding programme (Zhang et al., 2015). Briefly, globally sourced wheat genotypes exhibiting high shoot vigour were randomly crossed to generate the first cycle. Progeny of these crosses showing the greatest vigour, as measured by leaf width, were selected and progeny-tested. Lines demonstrating greatest vigour were then intercrossed to generate the germplasm for the next cycle. Six cycles of selection were completed from Cycle 0 (C0, least vigorous) to Cycle 6 (C6, most vigorous), each of which included ~30 lines (Zhang et al., 2015). The lines examined in the present study were randomly sampled from Cycle 0 (C0), Cycle 1 (C1), Cycle 3 (C3), Cycle 4 (C4), Cycle 5 (C5), and Cycle 6 (C6). The numbering of these lines followed the nomenclature of Zhang et al. (2015), and the individual lines tested in this study from each cycle were as follows: C0 (lines 1, 6, 8, 10, and 17), C1 (lines 1, 2, 3, 11, 14, and 15), C3 (lines 1, 5, 11, 28, and 36), C4 (lines 17, 19, 21, 32, and 37), C5 (lines 4, 28, 31, 36, and 39), and C6 (lines 2, 26, 38, 39, and 54). Seed was obtained from plants that were grown together under the same optimal growing conditions in a glasshouse and stored at –18 °C until the start of the experiments.
Soils
As soil is a complex growth medium and its physical structure and composition interact with root growth (Bengough, 2003), two contrasting agricultural field soils were used in all rhizosheath growth experiments. Soil 1 was a Podzol obtained from the ‘Wallaroo’ site at the Ginninderra Experimental Station (35°17ʹS, 149°05ʹE; ACT Australia). Here, the topsoil was collected to a depth of 10 cm and was acidic (pH 4). The soil was limed with CaCO3 (2 g kg–1 of soil) to achieve a pH of 7. Soil 2 was a Red Kandosol collected from the Graham Centre Field in Wagga Wagga (35°03ʹS, 147°36ʹE; NSW Australia). The topsoil (0–10 cm) had a pH of 4.9 that had been previously limed with CaCO3 (1.5 g kg–1 of soil) to pH 7. Both soils were sieved through a 4 mm mesh and three random subsamples were oven-dried to determine soil moisture content. Deionized water was added to each soil to reach 50% field capacity at ~11% moisture content. For rhizosheath assays, the soils were placed in pots of volume 192 cm3 (40 × 40 × 120 mm) at a bulk density of 1.1 g cm–3. The rhizosheath experiment was replicated twice in the Podzol and once in the Red Kandosol.
Seedling growth
To minimize maternally induced variation among the genotypes examined (Evans and Bhatt, 1977; Peterson et al., 1989), all experimental comparisons were made on seedlings and plants grown from seeds of similar weight (47 ± 1 mg). Seed was treated with fungicide by soaking for 3 min in a Thiram® (1.4 g l–1) solution. Eight to 10 seeds of each genotype and replication were placed on wet filter paper in Petri dishes incubated at 4 °C for 3 d for germination at ambient temperature (~25 °C) for 24 h. Seedlings with roots between 3 mm and 7 mm long were selected to be sown. Transplanted seedlings were then placed in a growth cabinet with a 12/12 h day/night regime at 20/15 °C day/night and a daytime light intensity of 600 μmol m−2 s−1.
Shoot vigour assessment
For assessment of shoot vigour, six seedlings for each genotype from the cycles of recurrent selection were sown in opaque black pots (4 litres, 200 mm diameter, 190 mm height, ANOVApot®). Seedlings were sown vertically and spaced evenly in the pot filled with a soil mix (50% river sand/50% potting compost v/v). Pot position was randomized in growth cabinets (12/12 h day/night regime with temperatures set at 20/15 °C day/night, and daytime light intensity of 600 μmol m−2 s−1). The plants were harvested at leaf stage 4 (Z14) (Zadoks et al., 1974), and the widths of the first, second, and third leaves were assessed using a digital calliper. Roots and shoots were separated and dried for 72 h at 70 °C before weighing, and the root to shoot ratios of the various lines were calculated. The experiment, designed as a complete randomized block, was replicated four times.
Rhizosheath and root hair imaging and measurements
In each rhizosheath experiment, six seedlings per genotype were grown as described above under the heading ‘Seedling growth’. After 8 d, plants were gently removed from the pots so that the residual soil remained attached to the roots. The first three seminal roots were excised at the seed, and root length and fresh weight were measured (root plus soil) (Delhaize et al., 2012). Rhizosheath size is expressed as the ratio of the fresh weight to length of the three seminal roots. The roots were then washed to remove soil and stored in a 50% (v/v) ethanol solution. A 1 cm segment was excised from the middle of the first seminal root that had been removed from each plant, placed on a glass slide with 50% ethanol, and gently agitated to ensure root hairs were evenly spread from the epidermis. After covering with a glass coverslip, samples were observed using a Leica M205c® dissecting microscope equipped with a ×1.0 objective and light-emitting diode ring illumination, and imaged using a Leica IC90e® camera and Leica Application Suite V4.12 (LAS-X, Leica Microsystems; Fig. 1). Root hair length was estimated using the FIJI image processing package (version 1.53c) (Schindelin et al., 2012). The longest root hair was measured on both sides of the roots such that a total of 10 assessments were taken for each root sample as described previously (Delhaize et al., 2012).
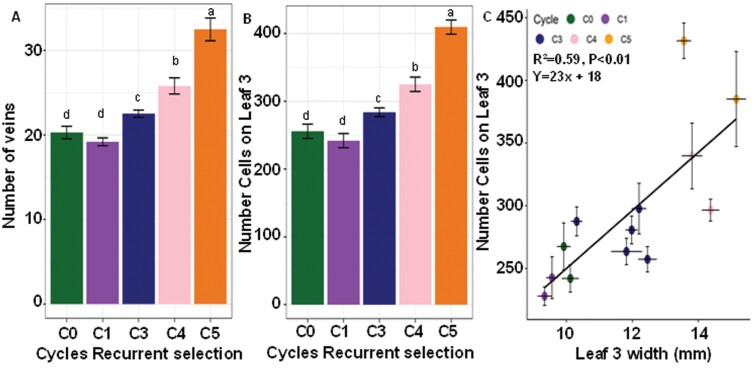
Fig. 1.
Number of veins and cell files across the leaves of C0 to C5 lines. Data show (A) the number of veins across the width of the third leaf, (B) the number of cell files over the width of the third leaf of the cycles for recurrent selection, and (C) the relationship between the width of leaf 3 and number of cell files. The error bars represent SEs (n=15 for vein and cell file numbers and n=24 for leaf width). Letters identify significant differences between means (P≤0.05).
Root cell measurements
Root epidermal cells were measured using tissues stored in 50% (v/v) ethanol that had been previously used to measure rhizosheath size and root hair length. Five 1 cm length root samples were excised from seedlings generated for all genotypes of the six recurrent selection cycles. Tissues were rehydrated by treatment with 25% ethanol (v/v) for 5 min followed by two deionized water rinses of 5 min using gentle agitation followed by staining in 0.1% (w/v) acridine orange (Sigma-Aldrich) in 5% (w/v) acetic acid for 2 min under a vacuum of ~0.07 MPa. Samples were rinsed twice in fresh distilled water for 2 min with gentle agitation, and mounted in water using glass slides and 0.17 mm thick coverslips.
Confocal images were obtained using a Leica SP8 confocal laser‐scanning microscope (Leica Microsystems, Australia) equipped with a ×20 (numeric aperture=0.5) water immersion objective and the Leica Application Suite V3.5 (LAS-X, Leica Microsystems). Images were collected with 488 nm excitation and 520–580 nm emission. Image z-stacks sufficient to capture the entire depth of the epidermal cells for each sample were collected using a 2.05 µm step size, this being the system-optimized value. Image z-stacks were deconvolved using the 3D deconvolution feature of LAS-X and its non-blind deblur algorithm with default system settings.
Measurements were collected on single plane images selected as being on the central line of each plane, and from several different planes in each stack. Surface areas of root epidermal cell planes were measured via multiple point polygons drawn to delineate the radial, transverse, and tangential planes using z-stack orthogonal projections (see Supplementary Fig. S1 for further explanation). Trichoblasts and atrichoblasts were identified by tracking the root hair through the z-stack to the epidermal cells of origin. Subsequently, trichoblasts and atrichoblasts were measured on tissues from the different recurrent selections for increased shoot vigour. Root hair density was assessed on genotypes from the two extreme cycles for vigour on these same images. A rectangle of 0.4 × 0.2 mm was placed over the centre of the root of each of the 10 samples per genotype. Trichoblasts in this rectangle were identified and counted and root hair density per mm2 was calculated. We define the ‘cell body’ of a trichoblast as the part of the root cell that is on the epidermal layer, excluding the protruding root hair (Supplementary Fig. S1A). Root hair diameter was measured at the base on 10 randomly chosen root hairs.
Leaf cell imaging and measurements
Measurements of leaf epidermal cells used 2 cm sections of the fully expanded third leaf excised 2 cm from the base of the leaf. These were fixed in 70% ethanol for 24 h and transferred to 1% w/v bleach (White King© sodium hypochlorite 42 g l–1) for clearing over several days (Botwright et al., 2005). Leaf pieces were mounted whole in water. Brightfield images were obtained using a Zeiss AxioImager Z1 microscope equipped with a Zeiss Axiocam 712 colour CCD camera (Carl Zeiss Micro-imaging GmbH, Jena, Germany) and plan-apochromat ×10 (numeric aperture=0.3) objective. Tiled images were captured to record epidermal cell position along with cell length and width measurements using Zeiss ZEN V2.6 (Carl Zeiss Micro-imaging GmbH).
Statistical analyses
A mixed linear model was fitted containing random components that identified the structure of the experimental design for each experiment: (i) pot position (row, column); and (ii) soil type. The ANOVA and estimation of least squares means was conducted considering genotypes as fixed effects using the function lm in ‘emmeans’ (Lenth et al., 2020) in the R (4.03) (R Core Team, 2020) software package. Pairwise comparisons between genotype means were obtained using the pairs function in the ‘emmeans’ package. The correlation between root hair length and rhizosheaths, and the relationship between the width of each of the three leaves and rhizosheath were tested using a Pearson’s correlation with the ‘cor.test’ in R.
Results
Shoot vigour, leaf width, and cell file number
Individual leaf traits were analysed to verify that the recurrent wheat lines varied significantly for vigour as previously described. Average widths of the first three fully emerged leaves increased significantly (P≤0.01) from the C0 lines, the least vigorous cycle, to the C5 lines, the most vigorous cycle (Supplementary Fig. S2). Average width of the first leaf in the C5 lines was 22% greater, 43% wider in the second leaf, and 39% greater in the third leaf compared with the C0 lines. There was a small but significant (P≤0.01) reduction in average leaf width for leaves 1, 2, and 3 between C5 and C6.
Closer examination indicated that the total number of veins across the leaves increased with increasing leaf width (Fig. 1A). The C5 lines had 65% more veins across the third leaf than the C0 and C1 lines. The number of cell files between adjacent veins (12.5 ± 0.2, SE) was not significantly different between the different selection cycles. Therefore, as the number of veins on the wider leaves increased, the total number of cell files across the leaves also increased (Fig. 1B). Leaves of C5 had an average of 410 ± 14 cells files across their width whereas C0 that had only 226 ± 14 cell files. Leaf width was strongly correlated with the number of cell files (r=0.77) (Fig. 1C).
Relationship between increased shoot vigour and root biomass
To investigate whether selection for shoot vigour affected root growth, shoot and root biomass of plants at growth stage Z14 were measured. Shoot biomass and root biomass increased between C0 and C6 of the recurrent selections (Supplementary Fig. S3A, B), and no clear relationship was noted between shoot vigour and root to shoot ratio (Supplementary Fig. S3C). Recurrent selection cycles C0 and C3 had root to shoot ratios that were not significantly different from one another. Similarly, the ratios for C1, C4, C5. and C6 were also not significantly different (P>0.05).
Relationship between increased shoot vigour, rhizosheath size, and root hair length
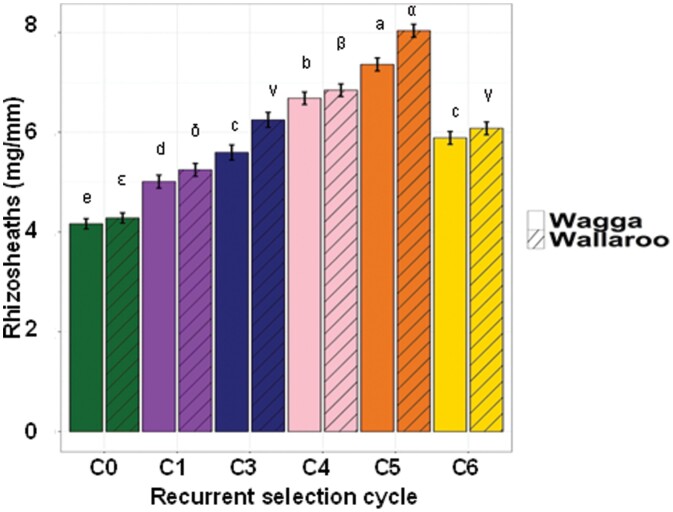
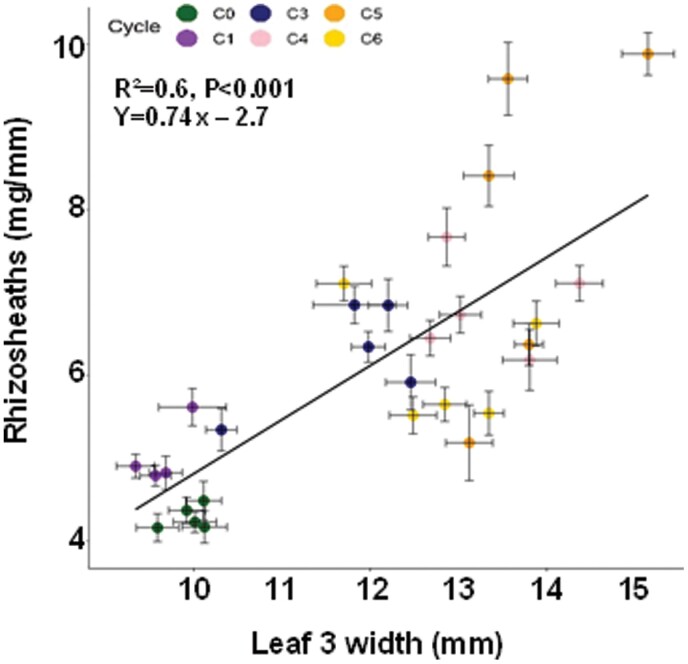
The seminal roots used to measure rhizosheath size had similar lengths across all cycles (Supplementary Fig. S). In contrast, rhizosheath size increased incrementally from the C0 to the C5 lines at ~17% per cycle, and then decreased (P<0.05) between C5 and C6 (Fig. 2). Average rhizosheath size in the C5 lines was 85% larger than in the C0 lines; however, C6 lines were 23% smaller than the C5 lines (P<0.05). Similar results were noted in experiments performed with both soils (Fig. 2), indicating that the differences in rhizosheath size were repeatable and robust. A significant correlation was detected between rhizosheath size and shoot vigour, with vigour assessed as width of the third leaf (Fig. 3).
Fig. 2.
Changes in rhizosheath size with recurrent selection for shoot vigour. Data show the rhizosheath size on lines from each cycle of the recurrent selections for shoot vigour. Rhizosheaths were assayed in Podzol and Red Kandosol soils. Error bars indicate the SE (n=60 per soil type and cycle number). Note, the analyses for the two soils were performed separately with a one-way ANOVA as experiments with the two soils were undertaken separately and at different times. The Roman letters indicate significant differences (P≤0.05) for the Red Kandosol, and the Greek letters indicate differences for the Podzol soil.
Fig. 3.
Relationship between shoot vigour and rhizosheath size. Rhizosheath size (mg mm–1 root length) was measured in the five lines from each of the recurrent selections and plotted against width of the third fully emerged leaf (shoot vigour). The error bars represent SEs for both leaf width and rhizosheaths (n=18 for rhizosheaths and n=24 for leaf width).
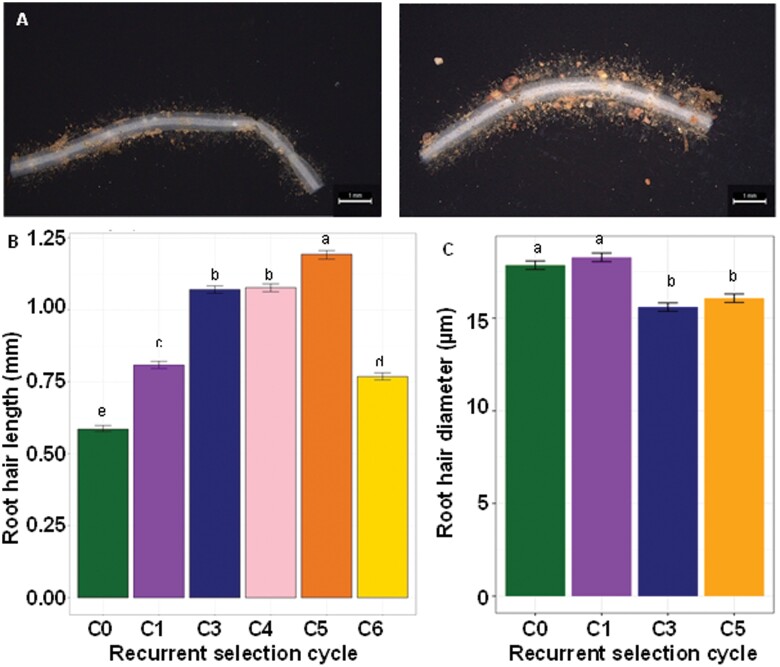
To further confirm that rhizosheath size was a reliable surrogate for root hair length in the vigour germplasm, direct measurements of root hair length were made on a subset of the roots previously used to measure rhizosheath size (Fig. 4A). Significant differences (P<0.01) in root hair length were detected between lines from the recurrent selection cycles. Root hair length doubled from the least vigorous C0 lines to the most vigorous C5 lines (Fig. 4B), and a strong linear correlation occurred between root hair length and rhizosheath size (r=0.87, P<0.001). This demonstrates that rhizosheath size was a useful proxy for root hair length in this germplasm (Supplementary Fig. S5). Root hair diameters were also measured at the point where root hairs emerged from the trichoblast cells. The average root hair diameter in the C5 lines was 14% smaller than in the C0 lines (Fig. 4C).
Fig. 4.
Influence of changes in shoot vigour on root hair length and diameter. The root tissues previously used for measuring rhizosheath size were rinsed of soil and preserved in 50% ethanol. The tissues were later photographed under the microscope. (A) Photographs of representative root segments taken from the seminal roots of C0 (left) and C5 (right) lines; scale bar=1 mm. (B) Root hair length of each of the recurrent selection cycles for increased shoot vigour (n=250 for each cycle). (C) Root hair diameter of a subsample of the recurrent selection cycles (n=250 for each cycle). The error bars represent SEs, and different letters identify significant differences in means at P=0.05.
Increased shoot vigour is associated with smaller cell sizes and increased root hair density
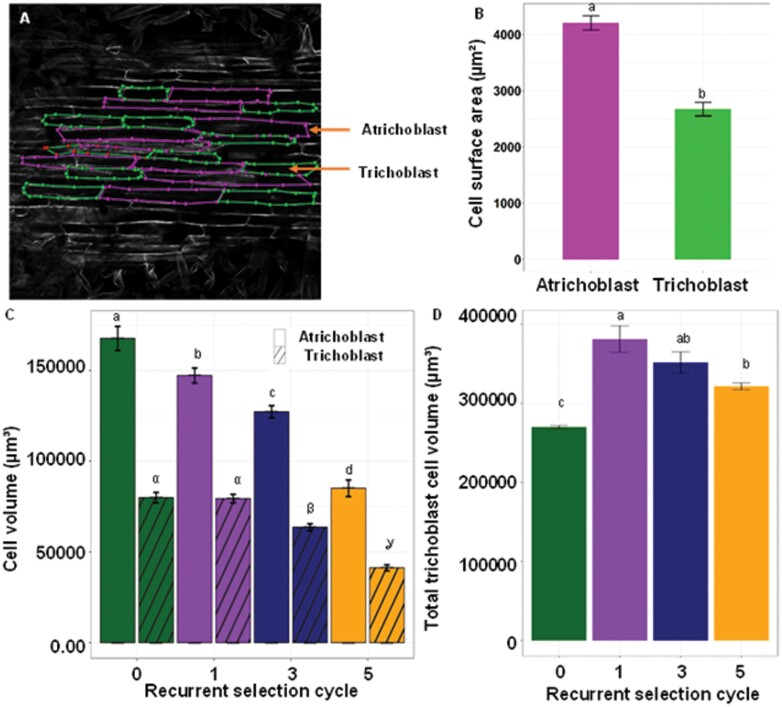
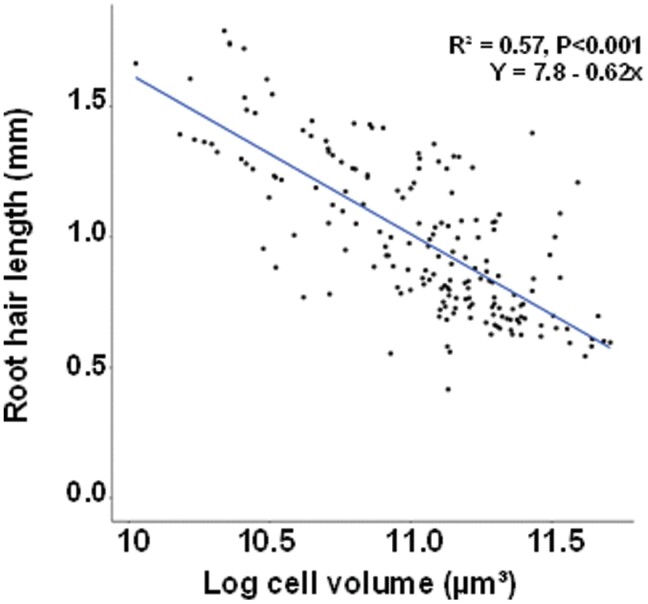
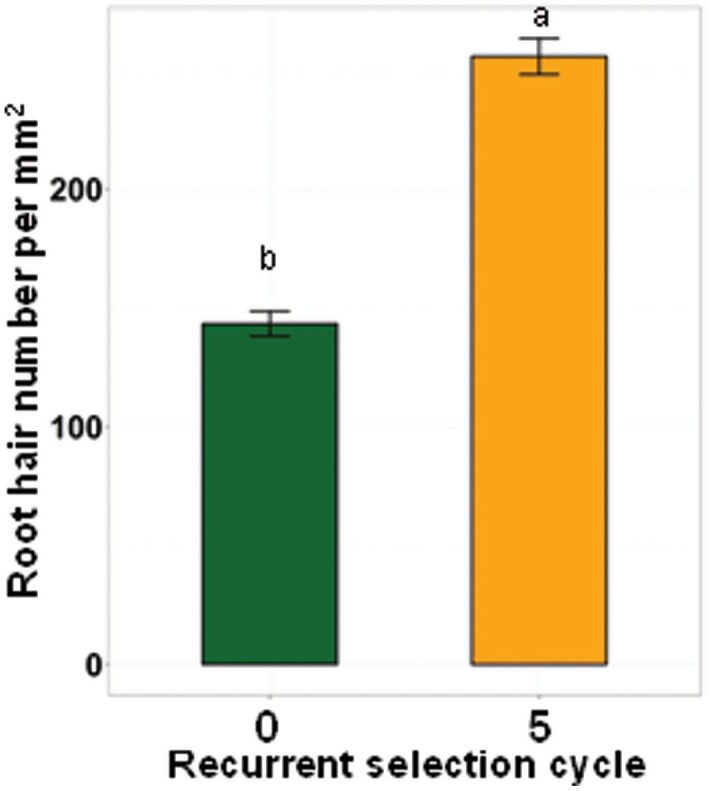
To investigate how increased vigour affected cell size, confocal microscopy was used to measure epidermal cell size on the roots, and widefield microscopy was used to measure the size of leaf epidermal cells (Supplementary Fig. S1B). Figure 5A highlights the cell bodies of the trichoblasts and atrichoblasts on the root epidermis. The area of these cells was measured in each plane (Fig. 5A). These measurements initially focused on the body of the cells and not the protruding root hairs. Figure 5B shows that the atrichoblasts were larger than the trichoblasts, and this was consistent for all the vigour selections. The cell bodies of both trichoblasts and atrichoblasts decreased from C0 to C5 as shoot vigour increased. Volumes of both cell types in C0 (least vigorous) lines were almost double those of C5 (most vigorous) (Fig. 5C). Root hair length was negatively correlated with the natural log of trichoblast body volume (Fig. 6). The reduced body sizes of the trichoblasts and atrichoblasts in the high vigour lines was associated with a significant increase of root hair density as shown in Fig. 7. Indeed, C5 had 34% more root hairs per mm2 than the less vigorous C0.
Fig. 5.
Influence of changes in shoot vigour on the size of root epidermal cells. (A) Single plane confocal microscopy image of root epidermal cells showing trichoblasts (green outline) and atrichoblasts (magenta outline). Trichoblasts were identified by changing the focus to identify those with root hairs. (B) Surface areas of the trichoblasts and atrichoblasts of C0 lines. The area measured was ‘surface 2’ of Supplementary Fig. S1 (n=64). (C) Cell body volumes of trichoblasts and atrichoblasts (excluding the root hair volume) for each cycle of the recurrent selection for shoot vigour (n=75 per cell type and cycle). (D) Total cell volumes of trichoblasts including root hair volume (n=75 per cell type and cycle). The error bars represent SEs, and different letters identify significant differences between means at P=0.05.
Fig. 6.
Relationship between trichoblast cell body volume and root hair length. Volumes of trichoblast bodies (transformed to the natural log) plotted against root hair length (n=180, samples issued from the rhizosheath experiment).
Fig. 7.
Influence of changes in shoot vigour on root hair density. Number of identified trichoblasts (cf Fig. 5A) per mm2 on roots of the two extreme cycles of the selection for increased shoot vigour. The error bars represent SEs, and different letters identify significant differences at P<0.05 (n=30).
Volumes of the protruding root hairs were then calculated from previous measurements of hair length and diameter (Fig. 4B, C). Total trichoblast volume was calculated by adding the hair volumes to the cell body volumes. Total trichoblast volumes were more similar across all recurrent selection cycles and did not show a consistent trend with shoot vigour (Fig. 5C). As shoot vigour increased, root hairs lengthened and largely compensated for the smaller volumes of the trichoblast body (Fig. 5D).
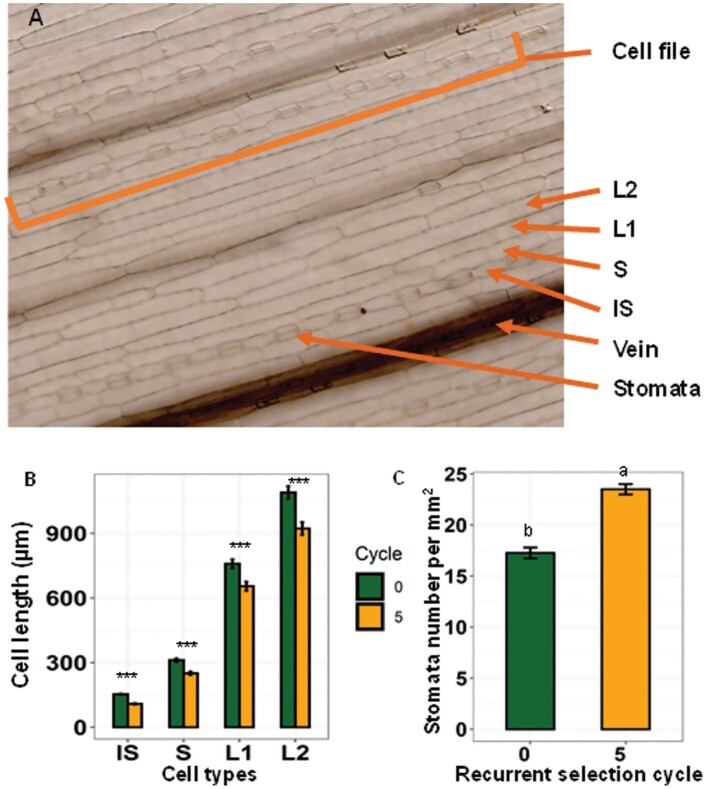
To establish if increased shoot vigour is only associated with changes to the size of root cells, we also measured the dimensions of four cell types on the leaves of lines from C0 and C5, which represent the extremes for shoot vigour in the germplasm examined (Fig. 8A). The length of all four cell types was significantly shorter (P<0.001) for the high vigour C5 lines than the less vigorous C0 lines. Compared with C0, the interstomatal cells of C5 were 27% shorter, the sister cells were 20% shorter, and the elongated cell files 1 and 2 were 22% and 16% shorter, respectively (Fig. 8B). In contrast, the widths of these cell types were not significantly different among the selection cycles (P>0.05), except for the interstomatal cells, which were 6% wider (P<0.01). The differences in interstomatal cell size were associated with changes in stomatal density on the leaves such that the average stomatal density for the C5 lines was 36% greater than that of the C0 lines (Fig. 8C).
Fig. 8.
Influence of changes in shoot vigour on the size of leaf epidermal cells. (A) Abaxial leaf surfaces of a wheat leaf showing the four cell types measured including the interstomatal cells (IS), sister cells (S), elongated cell 1 (L1), and elongated cell 2 (L2), the veins and cell files of stomata and IS cells. (B) Lengths of the four cell types. Significance levels represent comparisons between C0 and C5 for each cell type. ∗P< 0.05, ∗∗P<0.01, and ∗∗∗P<0.001 (n=100 per cell type and cycle). (C) Average number of stomata observed per square millimetre. The error bars represent SEs, and different letters identify significant differences at P<0.05 (n=75).
Discussion
This study used a novel wheat recurrent selection system expressing early vigour to examine whether changes to root and shoot cell properties were associated with early shoot vigour in progressive cycles of selection. Our results clearly demonstrate that recurrent selection for greater shoot biomass was strongly correlated with longer and denser root hairs and reduced cell sizes. Four different leaf cell types (interstomatal, sister, elongated 1, and elongated 2) (Fig. 8B) and two root epidermal cell types (trichoblasts and atrichoblasts) became progressively smaller as shoot vigour increased (Fig. 5C), indicating that the selection for early shoot vigour induced developmental changes throughout the plant. A summary of the developmental changes to shoots and roots associated with increased shoot vigour is provided in Fig. 9.
Fig. 9.
Diagram summarizing the impact of selection for shoot vigour on cells and organs.
Interestingly, while the cell bodies of trichoblasts on the roots of the high vigour lines were smaller than those on the less vigorous lines (Fig. 5C), the root hairs were significantly longer so the total trichoblast volume (combination of cell body and protruding root hair) showed no consistent relationship with shoot vigour (Fig. 5D). A similar inverse relationship between root hair length and trichoblast body size was recently reported in Arabidopsis (Sánchez-Fernández et al.,1997; Chen et al., 2015; Canales et al., 2017). Turgor pressure is an important driving force for hair elongation in trichoblasts (Mendrinna and Persson, 2015; Haas et al., 2020) along with cell wall composition and specific enzymes that contribute to wall loosening (Cosgrove, 2005; Winship et al., 2010; Kozlova et al., 2014; Zhang and Zhang, 2020). Turgor tends to be greater in smaller plant cells (Long et al., 2020), and higher turgor in the smaller trichoblasts could be the driving force for the development of longer root hairs in high vigour lines.
A corollary to the smaller epidermal cell size in high vigour lines is that the density of root hairs becomes significantly greater with high vigour, and we demonstrated this with direct measurements (Fig. 7). Therefore, not only do more vigorous lines have longer root hairs, they also exhibit greater density of root hairs than less vigorous lines. This is consistent with previous findings by Gahoonia et al. (1997) who demonstrated that wheat genotypes with longer root hairs also possessed more root hairs per unit area. In our study, the cell body sizes of the trichoblasts were consistently smaller than those of the atrichoblasts across all selection cycles (Fig. 5B). Asymmetry between trichoblasts and atrichoblasts was originally observed in Brassicae plants (Cormack, 1935). Our results are consistent with early observations in the Poacea by Row and Reeder (1957) and more recent reports in barley, Brachypodium distachyon, and rice (Oryza sativa L.) (Kim and Dolan, 2011; Marzec et al., 2013).
The size of the leaf epidermal cells also decreased as shoot vigour increased (Fig. 8A), and stomatal density was 36% greater in the high vigour lines compared with the less vigorous lines (Fig. 8B). Changes in stomatal density probably impact photosynthesis and transpiration, and may partly explain why early shoot vigour appears to stabilize yields in regions with variable rainfall (Wilson et al., 2015). Previous studies investigating the link between shoot vigour and leaf anatomy have reported contradictory findings. In agreement with the results presented here, Ter Steege et al. (2005) reported that early vigour in Aegilops tauschii was positively correlated with cell number and negatively correlated with maximum cell length. In contrast, increased vigour in ryegrass reportedly increased cell size and volume (Smith et al., 2003). Hybrid vigour in Arabidopsis was similarly associated with larger leaves and an increase in cell size (Greaves et al., 2015; Fujimoto et al., 2018). In wheat, Botwright et al. (2005) determined that one out of the four leaf cell types examined (‘long’ cells) was longer in a vigorous genotype while all other cell types did not differ in size. However, it should be emphasized that the vigorous germplasm examined by Botwright et al. (2005) was Vigour 18 which was one of the least vigorous lines included in C0 of the present study.
The wider leaves in the high vigour lines possessed significantly more veins and cell files across their width. Wider leaves have been linked to an increase in vein number in barley (Thirulogachandar et al., 2017) and with more cell files in wheat (Botwright et al., 2005; Spielmeyer et al., 2007). Vein number is an anatomical feature associated with plant performance because vein density can also impact photosynthetic efficiency. An increase in cell file number without a proportional increase in leaf vein number can negatively impact transport processes and photosynthesis (Feldman et al., 2017). We observed that leaf widths increased from C0 to C5 cycles, but significantly decreased from C5 to C6. Interestingly, using similar germplasm, Zhang et al. (2015) showed that leaf width remained stable in C5 and C6 lines. Our experiments used a subsample of the original genotypes assessed by Zhang et al. (2015) (five of 30 lines), which may account for these contrasting results. Nevertheless, an important finding to emerge from the germplasm examined in the present study was the strong positive associations between leaf width, leaf vein number, and leaf cell file number. In our study, root to shoot ratios remained relatively stable as shoot vigour increased, suggesting that carbon allocation between roots and shoots was generally conserved regardless of changes to vigour. This is consistent with previous conclusions using some of the progenitors of the germplasm examined in the present study (Palta et al., 2011; Palta and Yang, 2014).
This study confirmed that rhizosheath size is a reliable surrogate for root hair length in wheat (Delhaize et al., 2012, 2015). Rhizosheath size showed an average increase of 17% per selection cycle from C0 to C5 which is more than double the increase in leaf width of 7.3% per cycle described by Zhang et al. (2015). Indeed, as root hair length nearly doubled from C0 to C5, leaf width increased only by 35%. Nevertheless, a strong positive correlation was detected between shoot vigour (leaf width) and root hair length (rhizosheath size) across all lines.
Interestingly, both root hair length and shoot vigour in wheat are described as complex quantitative traits (Coleman et al., 2001; Horn et al., 2016). The surprising finding that these traits co-segregated with one another during the six cycles of recurrent selection for shoot vigour has two possible explanations. The first explanation is that genes controlling these two independent traits are closely linked and their co-selection was a consequence of their genetic linkage. A second explanation is that the genes controlling shoot vigour have pleotropic effects on root hair length. It is intriguing to consider the mechanisms that could link shoot vigour with root hair length, but the significant correlations observed between shoot vigour and cell size in the roots and the leaves clearly point to the fact that changes to whole-plant development have occurred.
Recent studies have shown that vigorous wheats are generally more competitive against weeds, and this is largely attributed to their ability to generate early biomass, intercept more light, and shade-out competitors (Mwendwa et al., 2020). We established here that high vigour lines also have larger root systems, longer and denser root hairs, and denser stomata, all of which could enhance soil exploration and increase the uptake of water and nutrients (Gilroy and Jones, 2000; Rongsawat et al., 2021). There appear to be surprising links between root hair growth and stomatal density, some of which have been reported previously. For instance, Hepworth et al. (2016) examined a range of transgenic Arabidopsis lines with altered stomatal patterning and reported a positive correlation between stomatal density and total root area, root hair density, and root hair length. While the mechanism for these relationships remains unclear, the authors suggested that increasing stomatal density increased transpiration and the demand for water which then stimulated root hair growth. However, in another Arabidopsis mutant carrying targeted knockouts in genes known to affect root hair growth, the authors found a negative relationship between root hair density and stomatal density. These results are intriguing, and the links between guard cell patterning and root hair growth deserve further attention.
The greater root hair length and density of the vigorous wheat lines is likely to improve competitiveness by facilitating the acquisition of essential resources (Craine and Dybzinski, 2013), particularly for plants grown under constrained environments as found for barley (Marin et al., 2021). For example, Haling et al. (2013) found that root hairs improved the ability of roots to penetrate soil, increased the root to soil contact, and enhanced P acquisition. Improved root penetration was evident in high strength soils (Haling et al., 2013) and in soils with variable densities (Bengough et al., 2016) where the hairs were thought to provide sufficient tensile strength to anchor root apices. These root traits might provide additional advantages to plants because they are also important for the release of both primary and secondary metabolites including allelochemicals (Yan et al., 2004; Weston et al., 2012). For example, in sorghum (Sorghum ssp.), root hairs have been identified as the sole site for the exudation of the allelochemical sorgoleone (Czarnota et al., 2003). In barley, hairless mutants were shown to exude three times less carbon into the soil compared with the wild type (Holz et al., 2018). In wheat, roots and rhizosphere processes are important for the release and transformation of benzoxazinones and phenoxazinones (Mwendwa et al., 2021). Since the more vigorous wheat lines had larger root systems with longer and denser root hairs, it is reasonable to speculate that they may also exude a greater amount of carbon into the rhizosphere, which may include allelochemicals useful for the suppression of competing species (Venturelli et al., 2015; Niculaes et al., 2018; Mwendwa et al. 2021).
Conclusion
This study demonstrated that recurrent selection for increased shoot vigour had pleotropic effects on other aspects of plant anatomy and growth. Greater shoot vigour was significantly correlated with larger root systems and with longer root hairs. Moreover, shoot vigour was linked with smaller epidermal cells on the roots and leaves which increased the density of root hairs and stomata. We conclude that the superior competitiveness of vigorous wheat lines could, in part, be explained by root traits that enhance their ability to acquire essential resources. Future work will investigate the involvement of hormones in early vigour and whether the exudation of allelopathic compounds could also contribute to the competitiveness of vigorous germplasm.
Supplementary data
The following supplementary data are available at JXB online.
Fig. S1. Influence of changes in shoot vigour on the size of root epidermal cell body.
Fig. S2. Leaf width of the cycles of the recurrent selection for shoot vigour.
Fig. S3. Influence of changes in shoot vigour on plant biomass and root to shoot ratio.
Fig. S4. Changes in root length with recurrent selection.
Fig. S5. Relationship between root hair length and rhizosheath size.
Acknowledgements
We thank Dr Washington Gapare (GRDC) for his assistance with statistical work in R, and Dr Brenton Brooks and Dave Smith (CSIRO) for their comments on the manuscript. Finally, we thank the CSIRO Black Mountain Micro Imaging Centre (BMIC), where the microimaging work was conducted.
Author contributions
P-WH, MD, VR, PH, SG, and PR: design and coordination; P-WH: conducting experiments; P-WH and PR: data collection; PH: collection of the confocal images; P-WH data analysis; LAW and GJR: supervision; P-WH, PR, and MD: writing with reviewing and/or comments by all authors.
Funding
This research is supported in part through the GRDC project, CSP00182, ‘Breeding weed competitive wheat’, and US00084, ‘New technologies for weed management in the Northern Region’. P-WH was supported by a CSU and CSIRO postgraduate research scholarship, and the AE Howard Memorial Trust through the Tim Healey Memorial Scholarship.
Conflict of interest
All authors have no conflict of interest to declare.
Data availability
The data supporting the findings of this study are available from the corresponding author, P.-W. Hendriks.
References
- Bengough AG, 2003. Root growth and function in relation to soil structure, composition, and strength. In: de Kroon H, Visser EJW,eds. Root ecology. Ecological studies (analysis and synthesis), Vol. 168. Berlin, Heidelberg: Springer, 151–171. [Google Scholar]
- Bengough AG, Loades K, McKenzie BM.. 2016. Root hairs aid soil penetration by anchoring the root surface to pore walls. Journal of Experimental Botany 67, 1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertholdsson NO. 2005. Early vigour and allelopathy—two useful traits for enhanced barley and wheat competitiveness against weeds. Weed Research 45, 94–102. [Google Scholar]
- Bertin C, Yang X, Weston LA.. 2003. The role of root exudates and allelochemicals in the rhizosphere. Plant and Soil 256, 67–83. [Google Scholar]
- Botwright TL, Rebetzke GJ, Condon AG, Richards RA.. 2005. Influence of the gibberellin-sensitive Rht8 dwarfing gene on leaf epidermal cell dimensions and early vigour in wheat (Triticum aestivum L.). Annals of Botany 95, 631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LK, George TS, Thompson JA, Wright G, Lyon J, Dupuy L, Hubbard SF, White PJ.. 2012. What are the implications of variation in root hair length on tolerance to phosphorus deficiency in combination with water stress in barley? Annals of Botany 110, 319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canales J, Contreras-Lopez O, M AJ, A. GR.. 2017. Nitrate induction of root hair density is mediated by TGA1/TGA4 and CPC transcription factors in Arabidopsis thaliana. The Plant Journal 92, 305–316. [DOI] [PubMed] [Google Scholar]
- Carminati A, Passioura JB, Zarebanadkouki M, Ahmed MA, Ryan PR, Watt M, Delhaize E.. 2017. Root hairs enable high transpiration rates in drying soils. New Phytologist 216, 771–781. [DOI] [PubMed] [Google Scholar]
- Casper BB, Jackson RB.. 1997. Plant competition underground. Annual Review of Ecology and Systematics 28, 545–570. [Google Scholar]
- Chen C-Y, Wu K, Schmidt W.. 2015. The histone deacetylase HDA19 controls root cell elongation and modulates a subset of phosphate starvation responses in Arabidopsis. Scientific Reports 5, 15708–15708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman RK, Gill GS, Rebetzke GJ.. 2001. Identification of quantitative trait loci for traits conferring weed competitiveness in wheat (Triticum aestivum L.). Australian Journal of Agricultural Research 52, 1235–1246. [Google Scholar]
- Comas LH, Becker SR, Cruz VMV, Byrne PF, Dierig DA.. 2013. Root traits contributing to plant productivity under drought. Frontiers in Plant Science 4, 442–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormack RGH. 1935. Investigations on the development of root hairs. New Phytologist 34, 30–54. [Google Scholar]
- Cosgrove DJ. 2005. Growth of the plant cell wall. Nature Reviews. Molecular Cell Biology 6, 850–861. [DOI] [PubMed] [Google Scholar]
- Craine JM, Dybzinski R.. 2013. Mechanisms of plant competition for nutrients, water and light. Functional Ecology 27, 833–840. [Google Scholar]
- Czarnota MA, Paul RN, Weston LA, Duke SO.. 2003. Anatomy of sorgoleone-secreting root hairs of sorghum species. International Journal of Plant Sciences 164, 861–866. [Google Scholar]
- Datta S, Kim C, Pernas M, Pires N, Proust H, Tam T, Vijayakumar P, Dolan L.. 2011. Root hairs: development, growth and evolution at the plant–soil interface. Plant and Soil 346, 1–14. [Google Scholar]
- Delhaize E, James RA, Ryan PR.. 2012. Aluminium tolerance of root hairs underlies genotypic differences in rhizosheath size of wheat (Triticum aestivum) grown on acid soil. New Phytologist 195, 609–619. [DOI] [PubMed] [Google Scholar]
- Delhaize E, Rathjen TM, Cavanagh CR.. 2015. The genetics of rhizosheath size in a multiparent mapping population of wheat. Journal of Experimental Botany 66, 4527–4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan L. 2017. Root hair development in grasses and cereals (Poaceae). Current Opinion in Genetics and Development 45, 76–81. [DOI] [PubMed] [Google Scholar]
- Evans LE, Bhatt GM.. 1977. Influence of seed size, protein content and cultivar on early seedling vigor in wheat. Canadian Journal of Plant Science 57, 929–935. [Google Scholar]
- Feldman AB, Leung H, Baraoidan M, Elmido-Mabilangan A, Canicosa I, Quick WP, Sheehy J, Murchie EH.. 2017. Increasing leaf vein density via mutagenesis in rice results in an enhanced rate of photosynthesis, smaller cell sizes and can reduce interveinal mesophyll cell number. Frontiers in Plant Science 8, 1883–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto R, Uezono K, Ishikura S, Osabe K, Peacock WJ, Dennis ES.. 2018. Recent research on the mechanism of heterosis is important for crop and vegetable breeding systems. Breeding Science 68, 145–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahoonia TS, Care D, Nielsen NE.. 1997. Root hairs and phosphorus acquisition of wheat and barley cultivars. Plant and Soil 191, 181–188. [Google Scholar]
- Gaudet CL, Keddy PA.. 1988. A comparative approach to predicting competitive ability from plant traits. Nature 334, 242–243 [Google Scholar]
- Gilroy S, Jones DL.. 2000. Through form to function: root hair development and nutrient uptake. Trends in Plant Science 5, 56–60. [DOI] [PubMed] [Google Scholar]
- Greaves IK, Gonzalez-Bayon R, Wang L, Zhu A, Liu P-C, Groszmann M, Peacock WJ, Dennis ES.. 2015. Epigenetic changes in hybrids. Plant Physiology 168, 1197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas KT, Wightman R, Meyerowitz EM, Peaucelle A.. 2020. Pectin homogalacturonan nanofilament expansion drives morphogenesis in plant epidermal cells. Science 367, 1003–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haling R, Brown L, Bengough AG, Young I, Hallett PD, White P, George T.. 2013. Root hairs improve root penetration, root–soil contact, and phosphorus acquisition in soils of different strength. Journal of Experimental Botany 64, 3711–3721. [DOI] [PubMed] [Google Scholar]
- Hepworth C, Turner C, Landim MG, Cameron D, Gray JE.. 2016. Balancing water uptake and loss through the coordinated regulation of stomatal and root development. PLoS One 11, e0156930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz M, Zarebanadkouki M, Kuzyakov Y, Pausch J, Carminati A.. 2018. Root hairs increase rhizosphere extension and carbon input to soil. Annals of Botany 121, 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R, Wingen LU, Snape JW, Dolan L.. 2016. Mapping of quantitative trait loci for root hair length in wheat identifies loci that co-locate with loci for yield components. Journal of Experimental Botany 67, 4535–4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings MJ, de Kroon H.. 1994. Foraging in plants: the role of morphological plasticity in resource acquisition. Advances in Ecological Research 25, 159–238. [Google Scholar]
- Kiær LP, Weisbach AN, Weiner J.. 2013. Root and shoot competition: a meta‐analysis. Journal of Ecology 101, 1298–1312. [Google Scholar]
- Kim CM, Dolan L.. 2011. Root hair development involves asymmetric cell division in Brachypodium distachyon and symmetric division in Oryza sativa. New Phytologist 192, 601–610. [DOI] [PubMed] [Google Scholar]
- Kozlova LV, Ageeva MV, Ibragimova NN, Gorshkova TA.. 2014. Arrangement of mixed-linkage glucan and glucuronoarabinoxylan in the cell walls of growing maize roots. Annals of Botany 114, 1135–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemerle D, Verbeek B, Cousens RD, Coombes NE.. 1996. The potential for selecting wheat varieties strongly competitive against weeds. Weed Research 36, 505–513. [Google Scholar]
- Lenth R, Singmann H, Love J, Buerkner P, Herve M.. 2020. Package ‘emmeans’: estimated marginal means, aka least-squares means. https://github.com/rvlenth/emmeans [Google Scholar]
- Liao MT, Fillery IRP, Palta JA.. 2004. Early vigorous growth is a major factor influencing nitrogen uptake in wheat. Functional Plant Biology 31, 121–129. [DOI] [PubMed] [Google Scholar]
- Libault M, Brechenmacher L, Cheng J, Xu D, Stacey G.. 2010. Root hair systems biology. Trends in Plant Science 15, 641–650. [DOI] [PubMed] [Google Scholar]
- Long Y, Cheddadi I, Mosca G, Mirabet V, Dumond M, Kiss A, Traas J, Godin C, Boudaoud A.. 2020. Cellular heterogeneity in pressure and growth emerges from tissue topology and geometry. Current Biology 30, 1504–1516. [DOI] [PubMed] [Google Scholar]
- Marin M, Feeney DS, Brown LK, et al. 2021. Significance of root hairs for plant performance under contrasting field conditions and water deficit. Annals of Botany 128, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzec M, Melzer M, Szarejko I.. 2013. Asymmetric growth of root epidermal cells is related to the differentiation of root hair cells in Hordeum vulgare (L.). Journal of Experimental Botany 64, 5145–5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maydup ML, Graciano C, Guiamet JJ, Tambussi EA.. 2012. Analysis of early vigour in twenty modern cultivars of bread wheat (Triticum aestivum L.). Crop and Pasture Science 63, 987–996. [Google Scholar]
- Mendrinna A, Persson S.. 2015. Root hair growth: it’s a one way street. F1000 Prime Reports 7, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KM, Dawson JC, Jones SS.. 2008. Relationship among phenotypic growth traits, yield and weed suppression in spring wheat landraces and modern cultivars. Field Crops Research 105, 107–115. [Google Scholar]
- Mwendwa JM, Brown WB, Weidenhamer JD, Weston PA, Quinn JC, Wu H, Weston LA.. 2020. Evaluation of commercial wheat cultivars for canopy architecture, early vigour, weed suppression, and yield. Agronomy 10, 983.– . [Google Scholar]
- Mwendwa JM, Weston PA, Weidenhamer JD, Fomsgaard IS, Wu H , Gurusinghe S, Weston LA.. 2021. Metabolic profiling of benzoxazinoids in the roots and rhizosphere of commercial winter wheat genotypes. Plant and Soil 466, 467–489. [Google Scholar]
- Niculaes C, Abramov A, Hannemann L, Frey M.. 2018. Plant protection by benzoxazinoids—recent insights into biosynthesis and function. Agronomy 8, 143. [Google Scholar]
- Palta J, Chen X, Milroy S, Rebetzke G, Dreccer M, Watt M.. 2011. Large root systems: are they useful in adapting wheat to dry environments? Functional Plant Biology 38, 347–354. [DOI] [PubMed] [Google Scholar]
- Palta J, Watt M.. 2009. Vigorous crop root systems. form and function for improving the capture of water and nutrients. In: Sadras V, Calderini D, eds. Crop physiology. Applications for genetic improvement and Agronomy. Amsterdam: Elsevier, 309–325. [Google Scholar]
- Palta J, Yang J.. 2014. Crop root system behaviour and yield. Field Crops Research. 165, 1–4. [Google Scholar]
- Pang J, Ryan MH, Siddique KHM, Simpson RJ.. 2017. Unwrapping the rhizosheath. Plant and Soil 418, 129–139. [Google Scholar]
- Passioura JB. 1988. Water transport in and to roots. Annual Review of Plant Physiology and Plant Molecular Biology 39, 245–265. [Google Scholar]
- Peterson CM, Klepper B, Rickman RW.. 1989. Seed reserves and seedling development in winter wheat. Agronomy Journal 81, 245–251. [Google Scholar]
- R Core Team . 2020. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Rebetzke GJ, Botwright TL, Moore CS, Richards RA, Condon AG.. 2004. Genotypic variation in specific leaf area for genetic improvement of early vigour in wheat. Field Crops Research 88, 179–189. [Google Scholar]
- Rebetzke G, Ingvordsen C, Newman P, Weston LA, French B, Gill G.. 2018. Delivering weed-competitive wheat breeding lines to growers. GRDC Grains Research & Development Corporation. [Google Scholar]
- Rebetzke GJ, Richards RA.. 1999. Genetic improvement of early vigour in wheat. Australian Journal of Agricultural Research 50, 291–301. [Google Scholar]
- Richards MC. 1989. Crop competitiveness as an aid to weed control. In: Proceedings of the Brighton Crop Protection Conference, Weeds. The British Crop Protection Council, 573–578. [Google Scholar]
- Rongsawat T, Peltier J-B, Boyer J-C, Véry A-A, Sentenac H.. 2021. Looking for root hairs to overcome poor soils. Trends in Plant Science 26, 83–94. [DOI] [PubMed] [Google Scholar]
- Row HC, Reeder JR.. 1957. Root-hair development as evidence of relationships among genera of Gramineae. American Journal of Botany 44, 596–601. [Google Scholar]
- Ruiz S, Koebernick N, Duncan S, et al. 2019. Significance of root hairs at the field scale—modelling root water and phosphorus uptake under different field conditions. Plant and Soil 447, 281–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PR, Liao M, Delhaize E, Rebetzke GJ, Weligama C, Spielmeyer W, James RA.. 2015. Early vigour improves phosphate uptake in wheat. Journal of Experimental Botany 66, 7089–7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Fernández R, Fricker M, Corben LB, S. WN, Shead N, J. LC, Van Montagu M, Inzé D, May MJ.. 1997. Cell proliferation and hair tip growth in the Arabidopsis root are under mechanistically different forms of redox control. Proceedings of the National Academy of Sciences, USA 94, 2745–2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, et al. 2012. Fiji: an open-source platform for biological-image analysis. Nature Methods 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KF, McFarlane NM, Croft VM, Trigg PJ, Kearney GA.. 2003. The effects of ploidy and seed mass on the emergence and early vigour of perennial ryegrass (Lolium perenne L.) cultivars. Animal Production Science 43, 481–486. [Google Scholar]
- Spielmeyer W, Hyles J, Joaquim P, Azanza F, Bonnett D, Ellis M, Moore C, Richards R.. 2007. A QTL on chromosome 6A in bread wheat (Triticum aestivum) is associated with longer coleoptiles, greater seedling vigour and final plant height. International Journal of Plant Breeding Research 115, 59–66. [DOI] [PubMed] [Google Scholar]
- Ter Steege M, Den Ouden F, Lambers H, Stam P, Peeters A.. 2005. Genetic and physiological architecture of early vigor in Aegilops tauschii, the D-genome donor of hexaploid wheat. A quantitative trait loci analysis. Plant Physiology 139, 1078–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirulogachandar V, Alqudah AM, Koppolu R, et al. 2017. Leaf primordium size specifies leaf width and vein number among row‐type classes in barley. The Plant Journal 91, 601–612. [DOI] [PubMed] [Google Scholar]
- Vandeleur RK, Gill GS.. 2004. The impact of plant breeding on the grain yield and competitive ability of wheat in Australia. Australian Journal of Agricultural Research 55, 855–861. [Google Scholar]
- Venturelli S, Belz RG, Kämper A, et al. 2015. Plants release precursors of histone deacetylase inhibitors to suppress growth of competitors. The Plant Cell 27, 3175–3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent C, Rowland D, Na C, Schaffer B.. 2017. A high-throughput method to quantify root hair area in digital images taken in situ. Plant and Soil 412, 61–80. [Google Scholar]
- Watt M, McCully ME, Canny MJ.. 1994. Formation and stabilization of rhizosheaths of Zea mays L. (effect of soil water content). Plant Physiology 106, 179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston L. 2005. History and current trends in the use of allelopathy for weed management. HortTechnology 15, 529–534. [Google Scholar]
- Weston LA, Ryan PR, Watt M.. 2012. Mechanisms for cellular transport and release of allelochemicals from plant roots into the rhizosphere. Journal of Experimental Botany 63, 3445–54. [DOI] [PubMed] [Google Scholar]
- Wilson JB. 1988. A review of evidence on the control of shoot: root ratio, in relation to models. Annals of Botany 61, 433–449. [Google Scholar]
- Wilson PB, Rebetzke GJ, Condon AG.. 2015. Of growing importance: combining greater early vigour and transpiration efficiency for wheat in variable rainfed environments. Functional Plant Biology 42, 1107–1115. [DOI] [PubMed] [Google Scholar]
- Winship LJ, Obermeyer G, Geitmann A, Hepler PK.. 2010. Under pressure, cell walls set the pace. Trends in Plant Science 15, 363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Liao H, Beebe SE, Blair MW, Lynch JP.. 2004. QTL mapping of root hair and acid exudation traits and their relationship to phosphorus uptake in common bean. Plant and Soil 265, 17–29. [Google Scholar]
- Zadoks JC, Chang TT, Konzak CF.. 1974. A decimal code for the growth stages of cereals. Weed Research 14, 415–421. [Google Scholar]
- Zerner MC, Rebetzke GJ, Gill GS.. 2016. Genotypic stability of weed competitive ability for bread wheat genotypes in multiple environments. Crop and Pasture Science 67, 695–702. [Google Scholar]
- Zhang D, Zhang B.. 2020. Pectin drives cell wall morphogenesis without turgor pressure. Trends in Plant Science 25, 719–722. [DOI] [PubMed] [Google Scholar]
- Zhang L, Richards RA, Condon AG, Liu DC, Rebetzke GJ.. 2015. Recurrent selection for wider seedling leaves increases early biomass and leaf area in wheat (Triticum aestivum L.). Journal of Experimental Botany 66, 1215–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author, P.-W. Hendriks.