Abstract
Stable isotopes at natural abundance are key tools to study physiological processes occurring outside the temporal scope of manipulation and monitoring experiments. Whole-molecule carbon isotope ratios (13C/12C) enable assessments of plant carbon uptake yet conceal information about carbon allocation. Here, we identify an intramolecular 13C/12C signal at tree-ring glucose C-5 and C-6 and develop experimentally testable theories on its origin. More specifically, we assess the potential of processes within C3 metabolism for signal introduction based (inter alia) on constraints on signal propagation posed by metabolic networks. We propose that the intramolecular signal reports carbon allocation into major metabolic pathways in actively photosynthesizing leaf cells including the anaplerotic, shikimate, and non-mevalonate pathway. We support our theoretical framework by linking it to previously reported whole-molecule 13C/12C increases in cellulose of ozone-treated Betula pendula and a highly significant relationship between the intramolecular signal and tropospheric ozone concentration. Our theory postulates a pronounced preference for leaf cytosolic triose-phosphate isomerase to catalyse the forward reaction in vivo (dihydroxyacetone phosphate to glyceraldehyde 3-phosphate). In conclusion, intramolecular 13C/12C analysis resolves information about carbon uptake and allocation enabling more comprehensive assessments of carbon metabolism than whole-molecule 13C/12C analysis.
Keywords: Carbon allocation, carbon stable isotopes, intramolecular isotope analysis, long time scales, ozone stress, primary carbon metabolism, triose-phosphate isomerase
Intramolecular 13C/12C analysis resolves information about carbon uptake and allocation (and associated environmental controls), enabling more comprehensive assessments of carbon metabolism, plant–environment interactions, and environmental variability than whole-molecule 13C/12C analysis.
Introduction
Plant carbon metabolism is a central component of the global carbon cycle. It both depends on and affects environmental properties. Improved understanding of long-term plant–environment interactions relies on information from plant archives (such as tree rings) because manipulation and monitoring experiments can only cover short to medium time scales. Stable carbon isotope (13C/12C) analysis is among the most advanced tools to extract physiological and environmental information from plant archives. Conventionally, average 13C/12C ratios of whole-plant metabolites are analysed. However, this approach neglects 13C/12C differences known to occur among individual carbon positions of plant metabolites (Abelson and Hoering, 1961). In contrast, we recently analysed intramolecular 13C/12C ratios in glucose extracted across an annually resolved Pinus nigra tree-ring time series (1961–1995) and reported intramolecular 13C signals (i.e. systematic 13C/12C variation confined to individual glucose carbon positions; Wieloch et al., 2018). Only after their ecophysiological origins have been elucidated can these archived signals become useful for applications within the plant and Earth sciences.
Based on our previous dataset (Wieloch et al., 2018), we have already pinpointed a 13C signal at tree-ring glucose C-4 and proposed that it informs about carbon flux around leaf cytosolic glyceraldehyde-3-phosphate dehydrogenases and associated energy metabolism (Wieloch, 2021; Wieloch et al., 2021). Here, we utilize the same dataset to isolate a 13C signal at tree-ring glucose C-5 and C-6. Since intramolecular 13C variation is governed (inter alia) by enzyme isotope effects and metabolite partitioning (Hayes, 2001), we hypothesize that the signal can be linked to shifts in carbon allocation and underlying environmental controls. Thus, we develop experimentally testable theories on ecophysiological mechanisms that can introduce the signal at glucose C-5 and C-6. To this end, we consider all enzyme reactions within central carbon metabolism of C3 plants. This includes the Calvin–Benson cycle (CBC), the photosynthetic carbon oxidation (PCO) cycle, starch and sucrose synthesis and degradation, cellulose synthesis, the pentose phosphate pathway, glycolysis, and carbon metabolism downstream of phosphoenolpyruvate (PEP). Carbon exchange between other biochemical pathways and the pathway leading to the formation of tree-ring glucose are presumably small, particularly when integrated over the course of growing seasons, the time frame of tree-ring formation. Thus, these processes cannot introduce 13C signals of substantial size into tree-ring archives. Furthermore, we only consider primary isotope effects (which occur at atoms with altered binding after chemical reactions). Sizes of secondary isotope effects (which occur at atoms with unaltered binding after chemical reactions due to indirect involvement in reaction mechanisms) are usually small and therefore unlikely to introduce detectable 13C signals into tree-ring archives. Finally, we present evidence supporting our theory. For this part, we reanalyse our own tree-ring dataset (Wieloch et al., 2018) in combination with publicly accessible climate data and 13C/12C data from an ozone treatment experiment published by Saurer et al. (1995).
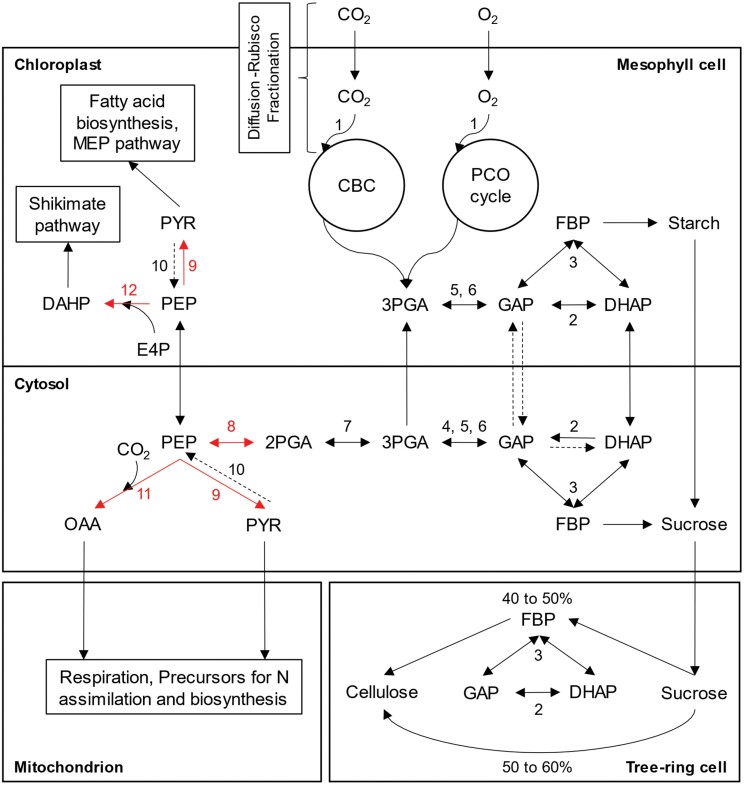
We distinguish two major types of 13C fractionation: diffusion–Rubisco fractionation, and post-Rubisco fractionation (Wieloch et al., 2018). Diffusion–Rubisco fractionation accompanies CO2 diffusion from ambient air into plant chloroplasts and subsequent carbon fixation by Rubisco (Figs 1, 2; Farquhar et al., 1982). It affects all carbon positions of plant glucose equally (Wieloch et al., 2018). In contrast, post-Rubisco fractionation results from metabolic processes downstream of Rubisco and is position specific (Figs 1–3). Deconvolution of the two fractionation types requires the intramolecular approach.
Fig. 1.
Central carbon metabolism in trees. Solid and dashed arrows represent substantial and negligible metabolite fluxes, respectively. Red arrows: reactions introducing the Δ5–6ʹ signal. CBC, Calvin–Benson cycle; PCO cycle, photosynthetic carbon oxidation cycle (photorespiration); MEP, non-mevalonate pathway. Metabolites: 2PGA, 2-phosphoglycerate; 3PGA, 3-phosphoglycerate; DAHP, 3-deoxy-d-arabino-heptulosonic acid 7-phosphate; DHAP, dihydroxyacetone phosphate; E4P, erythrose 4-phosphate; FBP, fructose 1,6-bisphosphate; GAP, glyceraldehyde 3-phosphate; OAA, oxaloacetate; PEP, phosphoenolpyruvate; PYR, pyruvate. Enzymes: (1) Rubisco; (2) TPI, triose-phosphate isomerase; (3) aldolase, fructose-bisphosphate aldolase; (4) np-GAPDH, irreversible non-phosphorylating glyceraldehyde-3-phosphate dehydrogenase; (5) p-GAPDH, reversible phosphorylating glyceraldehyde-3-phosphate dehydrogenase; (6) PGK, phosphoglycerate kinase; (7) PGM, phosphoglycerate mutase; (8) enolase, (9) PK, pyruvate kinase; (10) PPDK, pyruvate orthophosphate dikinase; (11) PEPC, phosphoenolpyruvate carboxylase; (12) DAHPS, 3-deoxy-d-arabino-heptulosonate 7-phosphate synthase. Figures 2 and 3 show the reactions in more detail. In contrast to its representation here, parts of the PCO cycle reside outside chloroplasts, in peroxisomes, and mitochondria. Localization of parts of the shikimate pathway in the cytosol is being debated (Maeda and Dudareva, 2012). To avoid clutter, not all metabolic intermediates are shown. For instance, conversion of 3PGA to GAP proceeds via 1,3-bisphosphoglycerate.
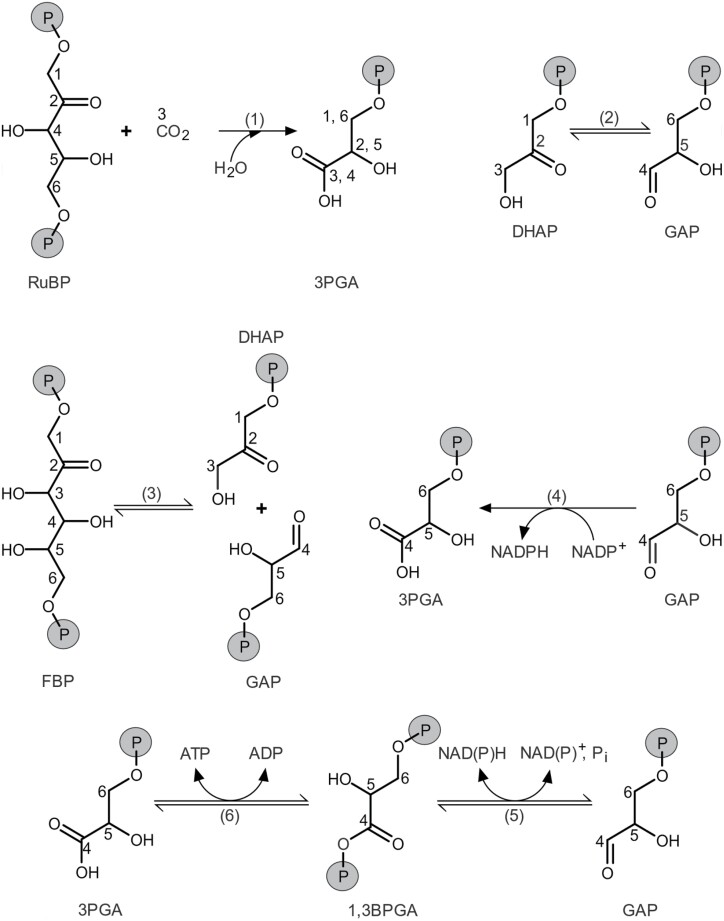
Fig. 2.
Formation and cleavage of bonds involving carbon atoms in central carbon metabolism. Carbon numbering is according to carbon positions in tree-ring glucose. Metabolites: 1,3BPGA, 1,3-bisphosphoglycerate; 3PGA, 3-phosphoglycerate; DHAP, dihydroxyacetone phosphate; FBP, fructose 1,6-bisphosphate; GAP, glyceraldehyde 3-phosphate; Pi, inorganic phosphate; RuBP, ribulose 1,5-bisphosphate. Numbers in parentheses denote enzymes: (1) Rubisco; (2) TPI, triose-phosphate isomerase; (3) aldolase, fructose-bisphosphate aldolase; (4) np-GAPDH, irreversible non-phosphorylating glyceraldehyde-3-phosphate dehydrogenase; (5) p-GAPDH, reversible phosphorylating glyceraldehyde-3-phosphate dehydrogenase; (6) PGK, phosphoglycerate kinase.
Our work makes several conceptual advances. (i) We show how constraints on signal propagation posed by metabolic networks can be used to narrow down signal origins. (ii) A conceptual model describes how the signal propagates from its origin to other glucose carbon positions and metabolite pools. Due to space restrictions, this model is presented in Supplementary Protocol S1. (iii) We revise current theory on plant isotope fractionation by ozone exposure. (iv) The present paper and a companion paper on the C-4 signal (Wieloch et al., 2021) develop theories that consider all relevant parts of metabolism and link intramolecular 13C signals with specific shifts in carbon allocation and their environmental causes. For isotope signals generated within complex metabolic networks, such comprehensive theories are required as a starting point for subsequent tailored experimental tests.
Materials and methods
Intramolecular 13C/12C ratios in tree-ring glucose of P. nigra from Vienna (Austria) were reported in Wieloch et al. (2018). They are expressed in terms of intramolecular 13C discrimination, Δiʹ, where i denotes individual carbon positions in tree-ring glucose (Wieloch et al., 2018; abbreviations and symbols are given in Table 1). In this notation, positive values denote discrimination against 13C. The prime denotes measurements subjected to a procedure that removes the 13C redistribution effect by triose phosphate cycling (Supplementary Protocol S2; Wieloch et al., 2018). This correction restores leaf-level 13C signals. The dataset comprises six annually resolved time series (one per glucose carbon) each covering the period 1961–1995 and containing 31 time points (n=6 × 31=186).
Table 1.
Abbreviations, terminology, and identifiers.
| Abbreviation | Metabolite | Identifier |
|---|---|---|
| 1,3BPGA | 1,3-Bisphosphoglycerate | CAS 1981-49-3 |
| 2PGA | 2-Phosphoglycerate | CAS 2553-59-5 |
| 3PGA | 3-Phosphoglycerate | CAS 820-11-1 |
| ADP | Adenosine diphosphate | CAS 58-64-0 |
| ATP | Adenosine triphosphate | CAS 56-65-5 |
| DAHP | 3-Deoxy-d-arabino-heptulosonic acid 7-phosphate | CAS 2627-73-8 |
| DHAP | Dihydroxyacetone phosphate | CAS 57-04-5 |
| E4P | Erythrose 4-phosphate | CAS 585-18-2 |
| FBP | Fructose 1,6-bisphosphate | CAS 488-69-7 |
| GAP | Glyceraldehyde 3-phosphate | CAS 591-59-3 |
| NAD+, NADH | Nicotinamide adenine dinucleotide | CAS 53-84-9 |
| OAA | Oxaloacetate | CAS 328-42-7 |
| PEP | Phosphoenolpyruvate | CAS 138-08-9 |
| Pi | Inorganic phosphate | CAS 14265-44-2 |
| PYR | Pyruvate | CAS 127-17-3 |
| Abbreviation | Enzyme | Identifier |
| Enolase | EC 4.2.1.11 | |
| Aldolase | Fructose-bisphosphate aldolase | EC 4.1.2.13 |
| DAHPS | 3-Deoxy-d-arabino-heptulosonate 7-phosphate synthase | EC 2.5.1.54 |
| np-GAPDH | Non-phosphorylating glyceraldehyde-3-phosphate dehydrogenase | EC 1.2.1.9 |
| p-GAPDH | Phosphorylating glyceraldehyde-3-phosphate dehydrogenase | EC 1.2.1.12/13 |
| PEPC | Phosphoenolpyruvate carboxylase | EC 4.1.1.31 |
| PEPCK | Phosphoenolpyruvate carboxykinase | EC 4.1.1.49 |
| PGK | Phosphoglycerate kinase | EC 2.7.2.3 |
| PGM | Phosphoglycerate mutase | EC 5.4.2.11 |
| PK | Pyruvate kinase | EC 2.7.1.40 |
| PPDK | Pyruvate orthophosphate dikinase | EC 2.7.9.1 |
| Rubisco | Ribulose-1,5-bisphosphate carboxylase/oxygenase | EC 4.1.1.39 |
| TPI | Triose-phosphate isomerase | EC 5.3.1.1 |
| Abbreviation | Other | |
| CBC | Calvin–Benson cycle | |
| MEP pathway | Non-mevalonate pathway | |
| [O 3 ] | Tropospheric ozone concentration | |
| PCO cycle | Photosynthetic carbon oxidation cycle | |
| rH | Relative humidity | |
| SD | Sunshine duration | |
| TPC | Triose phosphate cycling | |
| VPD | Air vapour pressure deficit | |
| ΔCi | Difference of intercellular CO2 concentrations | |
| δ 13Ca | 13C abundance of atmospheric CO2 | |
| Δδ13Cp | Difference of whole-molecule 13C abundances of plant matter | |
| Δ | Whole-molecule 13C discrimination | |
| Δ i | Intramolecular 13C discrimination | |
| Δ i ʹ | Intramolecular 13C discrimination corrected for TPC | |
| ΔΔ | Difference of 13C discrimination | |
| ΔΔDR | Difference of 13C discrimination due to diffusion–Rubisco fractionation |
Additionally, we reanalysed published differences in intercellular CO2 concentration, ΔCi, and whole-molecule 13C abundance, Δδ13Cp, between ozone-treated and control plants of Betula pendula grown in 1992 (Table 2; 90/40 nl O3 l–1 day/night versus <3 nl O3 l–1; Saurer et al., 1995). These authors used two different methods to determine ΔCi. Here, we calculated ΔCi averages. Corresponding differences in 13C discrimination by the diffusion–Rubisco interface were estimated as
Table 2.
Reanalysed data originally published by Saurer et al. (1995)
| C/LF | O3/LF | C/HF | O3/HF | |
|---|---|---|---|---|
| β±SD | 0.006 ± 0.002 | 0.013 ± 0.007∗∗∗ | 0.014 ± 0.005 | 0.017 ± 0.005∗ |
| ΔC i ±SD (ppm) | O 3 /LF–C/LF | O 3 /HF–C/HF | ||
| Steady state | 10 ± 16∗ | 34 ± 41∗∗ | ||
| Diurnal course | 5 ± 28 | 9 ± 35∗ | ||
| Δ δ 13 C p ±SD (‰) | O 3 /LF-C/LF | O 3 /HF–C/HF | ||
| Leaf cellulose | 1.1 ± 0.7∗ | 0.4 ± 0.9 | ||
| Stem cellulose | 1.3 ± 0.6∗∗ | 1.1 ± 0.6∗∗ | ||
Treatments: control group, C (<3 nl O3 l–1); ozone-treated group, O3 (90/40 nl O3 l–1 day/night); low fertilization, LF; high fertilization, HF. β denotes the carboxylation rate of phosphoenolpyruvate carboxylase relative to the total carboxylation rate of phosphoenolpyruvate carboxylase and Rubisco measured in vitro. ΔCi denotes differences in leaf intercellular CO2 concentrations between ozone-treated and control plants measured by two different methods (‘steady state’ and ‘diurnal course’). Δδ13Cp denotes differences in carbon isotope ratios between ozone-treated and control plants in leaf and stem cellulose. Significance levels of differences between ozone-treated and control plants: ∗P≤0.05; ∗∗P≤0.01; ∗∗∗P≤0.001.
| (1) |
where a and b denote discrimination factors of CO2 diffusion (4.4‰) and Rubisco carboxylation (29‰), respectively, and Ca denotes atmospheric CO2 concentration (356 ppm in 1992; Farquhar et al., 1982). Reported Δδ13Cp values (Table 2) were transformed to ΔΔ values as
| (2) |
where Ra denotes the 13C/12C ratio of atmospheric CO2, and ΔRp denotes the difference in 13C/12C ratios between ozone-treated and control plants. To utilize Δδ13Cp values reported in per mill, we transformed Equation 2 as
| (3) |
where δ13Ca denotes the 13C abundance of atmospheric CO2 (–8‰ in 1992; Leuenberger, 2007).
For the regression analysis and linear modelling, we used 13C/12C data from Wieloch et al. (2018) and publicly accessible climate data. Data of sunshine duration (SD) and relative humidity (rH) were acquired from the climate station Hohe Warte in Vienna (Klein Tank et al., 2002). Tropospheric ozone concentrations, [O3], were acquired from Stephansplatz, Laaer Berg, Hermannskogel, Hohe Warte, and Lobau (City of Vienna, Municipal Department 22).
Results and discussion
Tree-ring glucose exhibits a post-Rubisco signal at C-5 and C-6
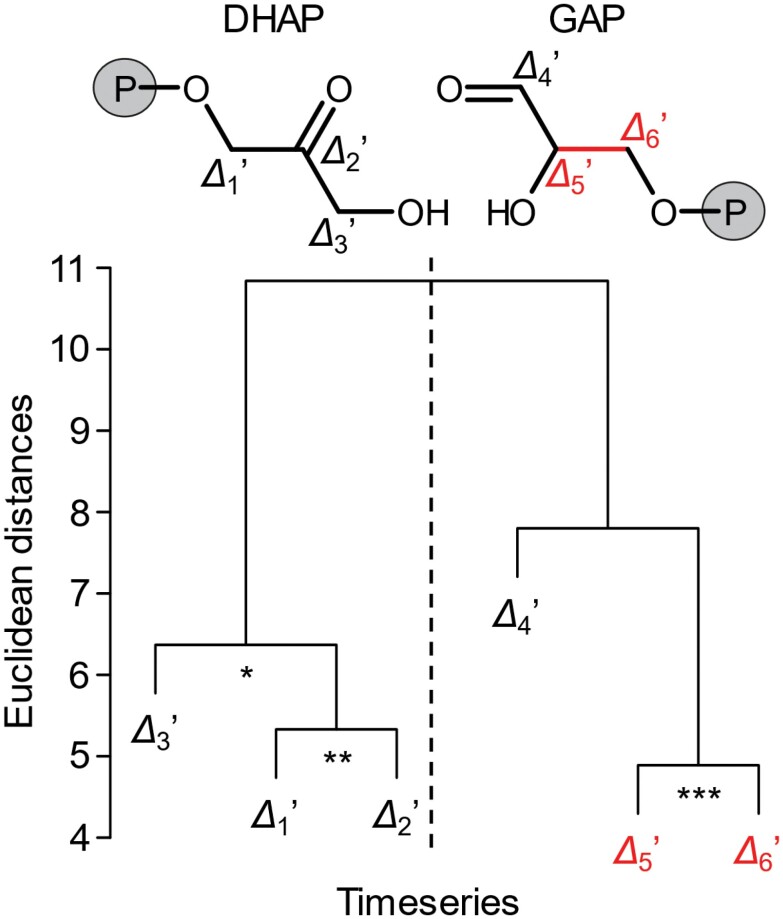
Figure 4 shows results of a hierarchical cluster analysis which groups Δiʹ time series according to co-variability (Wieloch et al., 2018); that is, Δiʹ time series carrying common 13C signals form clusters. Primary separation occurs between the Δ1ʹ to Δ3ʹ cluster and the Δ4ʹ to Δ6ʹ cluster. Average time series pertaining to these clusters are entirely uncorrelated (r=0.08, P>0.65, n=31). Thus, these clusters convey entirely different ecophysiological information. Wieloch et al. (2018) justified the use of air vapour pressure deficit (VPD) as a proxy of diffusion–Rubisco fractionation. While the average time series of the Δ1ʹ to Δ3ʹ cluster correlates highly significantly with VPD (r= –0.70, P=0.00001, n=31), the average time series of the Δ4ʹ to Δ6ʹ cluster is not significantly correlated (r= –0.30, P>0.05, n=31). This indicates that the diffusion–Rubisco signal is preserved at glucose C-1 to C-3 but not at C-4 to C-6. Among all Δiʹ, Δ5ʹ and Δ6ʹ exhibit the most significant correlation (r=0.61, P≤0.001, n=31). Since the diffusion–Rubisco signal is confined to glucose C-1 to C-3, we argue that C-5 and C-6 exhibit a strong post-Rubisco signal denoted the Δ5–6ʹ signal.
Fig. 4.
Clustering of Δiʹ time series due to co-variability. Δiʹ denotes time series of intramolecular 13C discrimination corrected for triose phosphate cycling (Wieloch et al., 2018). Red: time series discussed here. Data were measured on tree-ring glucose of Pinus nigra laid down from 1961 to 1995 at a dry site in the Vienna basin (n=6 × 31). Members of clusters marked by asterisks are correlated at the following significance levels: ∗P≤0.05; ∗∗ P≤0.01; and ∗∗∗P≤0.001. Precursors of tree-ring glucose, dihydroxyacetone phosphate (DHAP), and glyceraldehyde 3-phosphate (GAP) are shown as molecular structures. Modified figure from Wieloch et al. (2018).
Exclusion of metabolic locations as origin of the Δ5–6ʹ signal
Much is known about plant carbon metabolism. Based on this knowledge, we can exclude several metabolic locations as the origin of the Δ5–6ʹ signal as a first step in development of the theory. Note that the Δ5–6ʹ signal is introduced at the level of three-carbon compounds because reactions at other levels do not modify carbon bonds that become glucose C-5 and C-6.
Exclusion of the tree-ring cell as origin of the Δ5–6ʹ signal
13C labelling experiments provide compelling evidence for a complete or near-complete equilibration of triose phosphates in tree-ring cells of Quercus robur (Figs 1, 2; Hill et al., 1995). Numerous similar reports for other species and tissues suggest that triose phosphates in the cytosol of heterotrophic tissues are generally substantially equilibrated (Brown and Neish, 1954; Edelman et al., 1955; Neish, 1955, 1958; Shafizadeh and Wolfrom, 1955; Altermatt and Neish, 1956; Seegmiller et al., 1956; Shibko and Edelman, 1957; McConnell et al., 1958; Wolfrom et al., 1959; Keeling et al., 1988; Hatzfeld and Stitt, 1990; Viola et al., 1991). Only two 13C labelling studies report no evidence of substantial heterotrophic triose phosphate equilibration (Greathouse, 1953; Kikuta and Erickson, 1969). However, these authors analysed tissues during stages of exceptionally rapid fruit development with either high hexose phosphate flux into cotton-boll cellulose or high triose phosphate flux into avocado lipids (Greathouse, 1953; Brown and Neish, 1954; Kikuta and Erickson, 1969). Taken together, these reports suggest that heterotrophic triose phosphates are generally substantially equilibrated during normal growth.
The raw dataset of intramolecular 13C discrimination, Δi, in tree-ring glucose (Wieloch et al., 2018) exhibits significant correlations among all pairs of symmetry-related time series (Table 3); that is, significant correlations occur between Δ1 and Δ6, Δ2 and Δ5, and Δ3 and Δ4. These correlations probably result from carbon redistribution by triose phosphate cycling (TPC) which involves triose phosphate equilibration. Wieloch et al. (2018) describe this process mathematically and used the model to remove the TPC effect from Δi, yielding a TPC-free dataset, Δiʹ. In this latter dataset, significant correlations among pairs of symmetry-related time series are absent (Table 4). This provides strong evidence for the occurrence of substantial triose phosphate equilibration in tree-ring cells of the samples discussed here.
Table 3.
Correlation coefficients and significance levels obtained from cross-correlation analysis on Δi
| Δ 1 | Δ 2 | Δ 3 | Δ 4 | Δ 5 | Δ 6 | |
|---|---|---|---|---|---|---|
| Δ 1 | 1 | |||||
| Δ 2 | 0.60∗∗∗ | 1 | ||||
| Δ 3 | 0.31 | 0.52∗∗ | 1 | |||
| Δ 4 | 0.00 | 0.31 | 0.38∗ | 1 | ||
| Δ 5 | 0.37∗ | 0.42∗ | 0.24 | 0.39∗ | 1 | |
| Δ 6 | 0.55∗∗ | 0.48∗∗ | 0.31 | 0.11 | 0.69∗∗∗∗ | 1 |
∗P≤0.05; ∗∗P≤0.01; ∗∗∗P≤0.001; ∗∗∗∗P≤0.0001; n=6 × 31. Δi denotes time series of intramolecular 13C discrimination (Wieloch et al., 2018). Bold numbers refer to pairs of time series at symmetry-related glucose carbon positions. Data were measured on tree-ring glucose of Pinus nigra laid down from 1961 to 1995 at a dry site in the Vienna basin. This table was originally published as table 1 in Wieloch et al. (2018) and is provided here for convenience.
Table 4.
Correlation coefficients and significance levels obtained from cross-correlation analysis on Δiʹ
| Δ 1ʹ | Δ 2ʹ | Δ 3ʹ | Δ 4ʹ | Δ 5ʹ | Δ 6ʹ | |
|---|---|---|---|---|---|---|
| Δ 1 ʹ | 1 | |||||
| Δ 2 ʹ | 0.54∗∗ | 1 | ||||
| Δ 3 ʹ | 0.31 | 0.48∗∗ | 1 | |||
| Δ 4 ʹ | –0.12 | 0.10 | –0.12 | 1 | ||
| Δ 5 ʹ | 0.11 | –0.07 | 0.03 | 0.32 | 1 | |
| Δ 6 ʹ | 0.08 | 0.19 | 0.21 | 0.06 | 0.61∗∗∗ | 1 |
∗∗P≤0.01; ∗∗∗P≤0.001; n=6 × 31. Δiʹ denotes time series of intramolecular 13C discrimination corrected for triose phosphate cycling (Wieloch et al., 2018). Bold numbers refer to pairs of time series at symmetry-related glucose carbon positions. Data were measured on tree-ring glucose of Pinus nigra laid down from 1961 to 1995 at a dry site in the Vienna basin. This table was originally published as table 2 in Wieloch et al. (2018) and is provided here for convenience.
If a process in tree-ring cells had introduced a signal at carbon positions corresponding to glucose C-5 and C-6, triose phosphate equilibration would have transmitted it to carbon positions corresponding to glucose C-2 and C-1. The signal at C-5 would have had the same size as the signal at C-2, and the signal at C-6 would have had the same size as the signal at C-1. Please note that equally sized signals at symmetry-related glucose carbon positions are not removed by the method removing TPC effects (Wieloch et al., 2018). Since the Δ5–6ʹ signal is absent in Δ1ʹ and Δ2ʹ (Fig. 4), it must have been introduced at the leaf level.
Exclusion of the CBC and PCO cycle as origin of the Δ5–6ʹ signal
Introduction of the Δ5–6ʹ signal within the CBC or PCO cycle can be excluded because hexose phosphate synthesis includes conversion of photosynthetic/photorespiratory glyceraldehyde 3-phosphate (GAP; Δ4ʹ to Δ6ʹ) to dihydroxyacetone phosphate (DHAP; Δ3ʹ to Δ1ʹ) by triose-phosphate isomerase (TPI; Figs 1, 2). This would transmit any 13C signal present at GAP carbon positions corresponding to glucose C-5 and C-6 to DHAP carbon positions corresponding to glucose C-2 and C-1. More generally, metabolites feeding into the stromal GAP pool can be excluded as the origin of the Δ5–6ʹ signal based on the same reasoning.
Exclusion of reactions downstream of OAA, pyruvate, and DAHP as Δ5–6ʹ signal origin
Pyruvate kinase (PK) and pyruvate orthophosphate dikinase (PPDK) interconvert PEP and pyruvate (Figs 1, 3). The PK reaction is strongly on the side of pyruvate and considered nearly irreversible (Nageswara Rao et al., 1979; Tcherkez et al., 2011). In illuminated leaves of C3 plants, PPDK activity is either very low or undetectable, except for orchids and grasses (Hocking and Anderson, 1986). In illuminated leaves of Xanthium strumarium, flux from pyruvate to PEP is very small at ~0.05% of net CO2 assimilation (Tcherkez et al., 2011). In Arabidopsis thaliana, PPDK is up-regulated during leaf senescence which is believed to facilitate nitrogen remobilization (Taylor et al., 2010). This is of minor importance here because leaf senescence occurs during a short period relative to the multiyear life span of conifer needles. In Nicotiana tabacum, PPDK activity is increased up to 2.7-fold under strong drought (Doubnerová Hýsková et al., 2014). However, this should not result in significant flux in relation to fluxes in carbohydrate metabolism since basal PPDK activities in C3 plants are generally low (Hocking and Anderson, 1986; Tcherkez et al., 2011). Thus, flux from pyruvate to PEP should be small, and transmission of 13C signals in pyruvate to cytosolic carbohydrates by the PK/PPDK interface should be negligible.
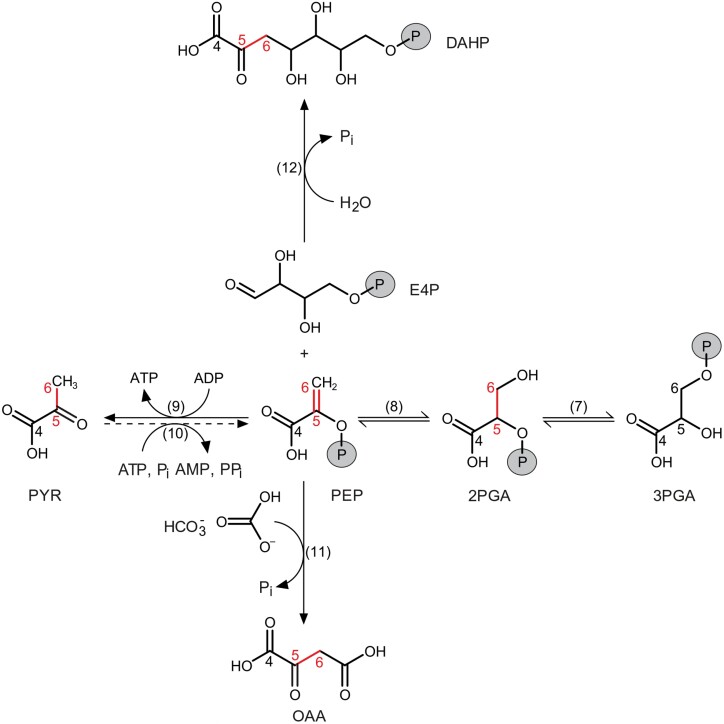
Fig. 3.
Formation and cleavage of bonds involving carbon atoms in central carbon metabolism. Solid and dashed arrows represent substantial and negligible metabolite fluxes, respectively. Red: carbon bond modifications possibly accompanied by primary isotope effects. Carbon numbering is according to carbon positions in tree-ring glucose. Metabolites: 2PGA, 2-phosphoglycerate; 3PGA, 3-phosphoglycerate; DAHP, 3-deoxy-d-arabino-heptulosonic acid 7-phosphate; E4P, erythrose 4-phosphate; HCO3–, bicarbonate; OAA, oxaloacetate; PEP, phosphoenolpyruvate; Pi, inorganic phosphate; PPi, pyrophosphate; PYR, pyruvate. Numbers in parentheses denote enzymes: (7) PGM, phosphoglycerate mutase; (8) enolase, (9) PK, pyruvate kinase; (10) PPDK, pyruvate orthophosphate dikinase; (11) PEPC, phosphoenolpyruvate carboxylase; (12) DAHPS, 3-deoxy-d-arabino-heptulosonate 7-phosphate synthase.
Phosphoenolpyruvate carboxylase (PEPC) and phosphoenolpyruvate carboxykinase (PEPCK) interconvert PEP and oxaloacetate (OAA). Conversion of PEP to OAA by PEPC is irreversible (Chollet et al., 1996). To our knowledge, there are no reports of PEPCK activity in mesophyll cells where bulk carbohydrate synthesis takes place (Pyke, 2001). PEPCK RNA and protein were not detected in leaves of Solanum lycopersicum irrespective of their developmental stage (Bahrami et al., 2001; Famiani et al., 2016). PEPCK protein or activity were not detected in leaves of Hordeum vulgare (Chen et al., 2000). In mature leaves of A. thaliana, PEPCK protein amount and activity are low and probably confined to specific cell types (Malone et al., 2007). In leaves of N. tabacum, PEPCK occurs in trichomes and stomata (Leegood et al., 1999; Malone et al., 2007). In leaves of Cucumis sativus, PEPCK occurs in trichomes and phloem cells (Leegood et al., 1999; Chen et al., 2004). In leaves of Oryza sativa, PEPCK occurs in hydathodes, stomata, and the vascular parenchyma (Bailey and Leegood, 2016). Thus, transmission of 13C signals in OAA to cytosolic carbohydrates by the PEPC/PEPCK interface should not occur.
3-Deoxy-d-arabino-heptulosonate 7-phosphate synthase (DAHPS) catalyses the irreversible reaction from PEP and erythrose 4-phosphate (E4P) to 3-deoxy-d-arabino-heptulosonic acid 7-phosphate (DAHP; Herrmann, 1995). To our knowledge, there are no reports of enzymes catalysing the back reaction. Thus, transmission of 13C signals in DAHP to cytosolic carbohydrates should not occur.
Taken together, leaf-level carbon fluxes from OAA, pyruvate, and DAHP to PEP are negligible or absent (Figs 1, 3). Therefore, reactions downstream of OAA, pyruvate, and DAHP cannot feed significant amounts of carbon and associated 13C signals into carbohydrate metabolism.
Exclusion of starch, sucrose, and cellulose metabolism, and the pentose phosphate pathway as origin of the Δ5–6ʹ signal
Reactions leading directly from stromal GAP to the formation of starch, sucrose, and cellulose, reactions remobilizing starch, and reactions of the pentose phosphate pathway do not simultaneously modify carbon bonds that become glucose C-5 and C-6. This excludes these pathways for Δ5–6ʹ signal introduction.
Origin of the Δ5–6ʹ signal
After excluding several metabolic locations as the origin of the Δ5–6ʹ signal (see ‘Exclusion of metabolic locations as origin of the Δ5–6ʹ signal’), the glycolytic pathway and PEP metabolism in leaves are left for consideration. Within this system, cytosolic GAP is used for PEP metabolism and sucrose synthesis (Fig. 1). Thus, GAP constitutes a central branch point in leaf carbon metabolism enabling isotope fractionation.
Leaf-level enolase, PEPC, PK, and/or DAHPS introduce the Δ5–6ʹ signal
Enolase, PEPC, PK, and DAHPS are the only enzymes which simultaneously modify carbon bonds that become glucose C-5 and C-6 (Figs 1–3). Reactions catalysed by these enzymes may be accompanied by 13C effects of substantial size and may thus introduce the Δ5–6ʹ signal. Enolase interconverts 2-phosphoglycerate (2PGA) and PEP (Figs 1, 3). In vivo, the reaction operates close to equilibrium (Kubota and Ashihara, 1990) and might thus be accompanied by an equilibrium isotope effect. Formation of the C=C double bond in PEP probably favours turnover of 13C isotopologues of 2PGA, leading to 13C enrichment in PEP. A 13C signal might then arise from varying allocation of PEP to downstream processes (Fig. 1). Increased downstream consumption would remove more 13C-enriched PEP and leave behind more 12C-enriched 2PGA for glucose synthesis.
Kinetic isotope effects may accompany the unidirectional conversions of PEP to OAA by PEPC, PEP to pyruvate by PK, and PEP and E4P to DAHP by DAHPS (Figs 1, 3). These reactions break the C=C double bond in PEP and can therefore be expected to favour turnover of 12C-isotopologues of PEP, leaving behind 13C-enriched PEP for glucose synthesis. Due to the usually larger size of kinetic isotope effects compared with equilibrium isotope effects, effects by PEPC, PK and DAHPS can be expected to outweigh any reciprocal effect by enolase. Thus, considering all four enzymes together, increasing turnover of PEP by PEPC, PK, and DAHPS can be expected to result in 13C-enriched tree-ring glucose (i.e. Δ5–6ʹ decreases).
Signal transmission to tree-ring glucose
Isotope signals generated at the level of leaf-cytosolic PEP or 2PGA need to be transmitted to GAP to then enter hexose phosphates and tree-ring glucose (Fig. 1). Transmission of a signal introduced by cytosolic enzymes is straightforward since the cytosolic glycolytic reactions between PEP and GAP are at equilibrium (Kubota and Ashihara, 1990). Transmission of a signal introduced by stromal enzymes is more intricate. First, it requires an incomplete or low-activity glycolytic pathway in leaf chloroplasts because signal equilibration with stromal triose phosphates would result in even signal distribution over all glucose carbon positions (Supplementary Protocol S1.6). An incomplete glycolytic pathway in leaf chloroplasts is supported by a reported lack of enolase in A. thaliana and O. sativa (van der Straeten et al., 1991; Prabhakar et al., 2009; Fukayama et al., 2015). Second, signal transmission from stromal PEP to C-5 and C-6 of cytosolic hexose phosphate requires chloroplast export of PEP. Transport of PEP across the chloroplasts’ inner membrane is mediated by the PEP/Pi translocator as counter-exchange with Pi, PEP, or 2PGA, and the putative in vivo preference for the transport of Pi, and PEP (Fischer et al., 1997; Flügge et al., 2011). Numerous stromal processes, such as the shikimate pathway and fatty acid biosynthesis, rely on PEP import from the cytosol (Streatfield et al., 1999; Flügge et al., 2011). Therefore, a net flux of PEP from the cytosol to chloroplasts can be expected. However, members of the phosphate translocator family are believed to be highly inefficient. For instance, merely 10% of the activity of the triose phosphate translocator is used for net export of triose phosphate from chloroplasts; 90% is wasted on futile counter-exchanges (Flügge, 1987, 1999). In addition, low stromal and high cytosolic Pi levels (Sharkey and Vanderveer, 1989) can be expected to promote chloroplast export of PEP. Consequently, efficient equilibration of cytosolic and stromal PEP pools, and 13C signals therein, can be expected. Thus, both cytosolic and stromal enzymes may contribute to the Δ5–6ʹ signal.
Signal introduction requires substantial carbon fluxes and flux variability
For the introduction of a 13C signal, a substantial share of the photosynthetically fixed carbon must be directed towards enolase, PEPC, PK, and/or DAHPS and their downstream derivatives. This share must vary substantially; in the present case, on the interannual time scale. Therefore, we will now discuss carbon fluxes through enolase, PEPC, PK, and DAHPS.
Commonly, PEPC is localized in the cytosol both in dispersion and bound to the outer mitochondrial membrane (Figs 1, 3; O’Leary et al., 2011). In leaf mesophyll cells of O. sativa, a putatively rare additional isoform occurs in chloroplasts (Masumoto et al., 2010; O’Leary et al., 2011). In C3 plants, PEPC provides OAA to replenish tricarboxylic acid cycle intermediates, and to support nitrogen assimilation and biosynthetic processes (O’Leary et al., 2011; O’Leary and Plaxton, 2017). On average, leaf carbon fixation by PEPC is believed to account for up to 5% of net CO2 assimilation (Melzer and O’Leary, 1987). Up-regulation of PEPC occurs (inter alia) with drought, salinity, ozone, nitrogen assimilation, and virus infections (see ‘Ecophysiological effects’; O’Leary et al., 2011). For instance, ozone triggers both an up-regulation of PEPC and a down-regulation of Rubisco (Dizengremel, 2001). In forest trees, the Rubisco/PEPC activity ratio can change from up to 25 in ozone-free air to ~2 under realistic levels of ambient ozone, redirecting carbon flux to maintenance and repair processes (Dizengremel, 2001).
Isoforms of PK are localized in both the cytosol and chloroplasts (Figs 1, 3; Ambasht and Kayastha, 2002). They provide pyruvate (inter alia) for mitochondrial respiration, fatty acid biosynthesis, and the non-mevalonate pathway (MEP). To our knowledge, estimates of the respiratory flux via PK in actively photosynthesizing leaves are unavailable. However, this flux may be substantial when photorespiration is low and thus co-vary with photorespiration and its environmental controls (Supplementary Protocol S3).
In illuminated photosynthetic tissue of A. thaliana, fatty acid biosynthesis can occur at a rate of 2.3 µmol C mg chlorophyll–1 h–1 (Bao et al., 2000). Based on this, we estimate an ~2% carbon flux relative to net CO2 assimilation into fatty acid biosynthesis (Supplementary Protocol S4). In leaves, this flux is predominantly controlled at the level of acetyl-CoA carboxylase (Page et al., 1994; Harwood, 2005; Ohlrogge et al., 2015). It responds to the stromal redox state (energy status) and associated environmental controls (Rawsthorne, 2002; Harwood, 2005; Geigenberger and Fernie, 2014).
The plastid-localized MEP pathway is yet another metabolic route carrying substantial flux. With isoprene as a major pathway product in some trees, it commonly consumes ~2% of net assimilated CO2 (Sharkey and Yeh, 2001). In forest trees, high temperature can enhance this fraction to up to 15% (Sharkey et al., 1996); a plant response believed to mitigate short-term high-temperature stress (Sharkey and Yeh, 2001).
DAHPS, located in both chloroplasts and the cytosol, is the first enzyme of the shikimate pathway (Figs 1, 3; Maeda and Dudareva, 2012). In vascular plants, 20–50% of the photosynthetically fixed carbon enters the pathway (Tohge et al., 2013). In trees, most of the flux can be expected to occur in heterotrophic tissues supporting lignin biosynthesis. To our knowledge, flux estimates for actively photosynthesizing leaves are unavailable. However, the shikimate pathway provides precursors for (inter alia) the aromatic amino acids phenylalanine, tryptophan, tyrosine, and their numerous derivatives. Thus, it should carry substantial flux in most tissues. In leaves of Prunus persica fed 13CO2, <6% of the label accumulated in a metabolite fraction comprising lipids, proteins, and residual compounds (Escobar-Gutiérrez and Gaudillère, 1997). Since the shikimate pathway contributes to the biosynthesis of this metabolite fraction among other pathways, its flux must be markedly below 6% of net assimilated CO2. In leaves of Helianthus annuus, Abadie et al. (2018) reported a flux of ~1% relative to net CO2 assimilation into the shikimate pathway product chlorogenate under normal growing conditions. Regulation of the shikimate pathway is primarily exerted by gene expression and post-translational modification in response to developmental and environmental cues (Entus et al., 2002; Mir et al., 2015). Relative carbon flux through the shikimate pathway can be expected to (inter alia) vary with light (Henstrand et al., 1992; Logemann et al., 2000; Entus et al., 2002), ozone (Janzik et al., 2005; Betz et al., 2009), physical wounding (Dyer et al., 1989; Keith et al., 1991), bacterial infection (Keith et al., 1991; Truman et al., 2006), fungal infestation (McCue and Conn, 1989; Henstrand et al., 1992; Görlach et al., 1995; Bischoff et al., 1996, 2001; Ferrari et al., 2007), and nitrogen availability (Scheible et al., 2004). For instance, in leaves of N. tabacum, induction of DAHPS increased up to 5-fold under ozone fumigation (160 nl l–1), and an increase in flux through the shikimate pathway was corroborated by increased levels of pathway products (Janzik et al., 2005). Performing an 83 d ozone fumigation experiment (160–190 nl l–1, 8 h d–1), Betz et al. (2009) reported evidence for increased carbon flux into the shikimate pathway in leaves of Fagus sylvatica.
Since PEPC, PK, and DAHPS are located downstream of enolase (Figs 1, 3), all four enzymes may contribute to the Δ5–6ʹ signal. Based on arguments given above, associated carbon fluxes and their variability can be expected to be substantial. Other leaf-level pathways consuming PEP, such as the cytosolic mevalonate pathway, may exert additional control over the Δ5–6ʹ signal.
Ecophysiological effects
The Δ5–6ʹ signal is independent of the diffusion–Rubisco signal at C-1 and C-2 (Fig. 4). Since diffusion–Rubisco fractionation initially affects all carbon entering glucose synthesis equally (see the Introduction), we propose that the Δ5–6ʹ signal exhibits two components of variance. The first component is inversely correlated with diffusion–Rubisco fractionation and removes the diffusion–Rubisco signal from glucose C-5 and C-6. The second component constitutes systematic variation independent of diffusion–Rubisco fractionation. In the following, we propose ecophysiological mechanisms for the introduction of each component starting with the independent component. Please note that, in the present case, the Δ5–6ʹ signal can be expected to be under environmental rather than developmental control (Supplementary Protocol S5).
Wieloch et al. (2018) studied effects of VPD, precipitation, soil moisture, temperature, and global radiation on the diffusion–Rubisco signal in their P. nigra samples. These authors found that VPD, a measure of environmental drought, exerts predominant control and pointed out that this agrees with expectations for the generally dry study site. Thus, the independent component of the Δ5–6ʹ signal is governed by environmental factors other than VPD.
The study site is ~10 km away from the city centre of Vienna and frequently exposed to substantial levels of tropospheric ozone (Oltmans et al., 1998; Ainsworth et al., 2012). Lefohn (1992) classified P. nigra as an ozone-sensitive tree species. Radiation stimulates the photochemical reactions of ozone formation (Ainsworth et al., 2012). Ozone triggers relative flux increases through the anaplerotic and shikimate pathways via PEPC and DAHPS, respectively (see ‘Signal introduction requires substantial carbon fluxes and flux variability’) and may thus cause 13C increases at PEP carbon positions that become glucose C-5 and C-6 (see ‘Leaf-level enolase, PEPC, PK, and/or DAHPS introduce the Δ5–6ʹ signal’). This may introduce an isotope signal independent of the diffusion–Rubisco signal due to independence at the level of environmental controls.
In contrast, a process mitigating ozone entry into plant leaves may explain the component of the Δ5–6ʹ signal which is inversely correlated with the diffusion–Rubisco signal. Dizengremel (2001) proposed that drought and ozone combined is a main recurring stress factor in forest ecosystems. Isohydric plant species, such as P. nigra, respond to drought by closing their stomata (Sade et al., 2012). Reduced stomatal conductance impedes ozone uptake (Tingey and Hogsett, 1985; Dobson et al., 1990; Dizengremel, 2001). In needles of Pinus halepensis, PEPC activities in control plants and plants exposed to mild drought stress were similar, strongly increased under ozone stress, but significantly less so under combined ozone and drought stress (Fontaine et al., 2003). Thus, anaplerotic flux rates can be expected to be highest under ozone stress but lower when ozone stress is accompanied by drought. While drought causes 13C enrichments at all glucose carbon positions due to diffusion–Rubisco fractionation (Wieloch et al., 2018), it can be expected to reduce ozone-induced 13C enrichments at glucose C-5 and C-6. This drought component of the ozone response may remove the diffusion–Rubisco signal from glucose C-5 and C-6. In Supplementary Protocol S3, we discuss how changes in substrate supply to mitochondrial oxidative phosphorylation (glycolytic pyruvate versus photorespiratory glycine) may additionally contribute to the component of the Δ5–6ʹ signal that is inversely correlated with diffusion–Rubisco fractionation.
Experimental evidence
Effects of tropospheric ozone on whole-molecule 13C/12C composition of plant cellulose
Growing B. pendula at increased ozone levels, several authors reported decreased 13C discrimination, Δ, in leaf and stem cellulose (Matyssek et al., 1992; Saurer et al., 1995). Intriguingly, these Δ decreases coincided with increased ratios of intercellular to ambient CO2 concentrations, Ci/Ca. As pointed out by Matyssek et al. (1992) and Saurer et al. (1995), this cannot be explained by the standard model of diffusion–Rubisco fractionation which predicts a positive correlation between Ci/Ca and Δ (Farquhar et al., 1982). Thus, post-Rubisco fractionation can be expected to cause these ozone-related isotope effects.
Matyssek et al. (1992) and Saurer et al. (1995) proposed that increased relative carbon fixation by PEPC due to ozone explains the Δ decreases because carbon fixed by PEPC is strongly 13C enriched compared with carbon fixed by Rubisco (Melzer and O’Leary, 1987). While this proposal is in line with significantly increased relative PEPC activities observed under ozone (Table 2), it conflicts with the set-up of carbon metabolism. PEPC-fixed carbon supplies downstream metabolism, yet no pathway carrying substantial flux exists that could transfer it into carbohydrate metabolism (see ‘Exclusion of reactions downstream of OAA, pyruvate, and DAHP as Δ5–6ʹ signal origin’). Above, we propose an ozone-dependent mechanism for the introduction of the Δ5–6ʹ signal which reconciles observations of Matyssek et al. (1992) and Saurer et al. (1995) with the set-up of carbon metabolism (see ‘Ecophysiological effects’).
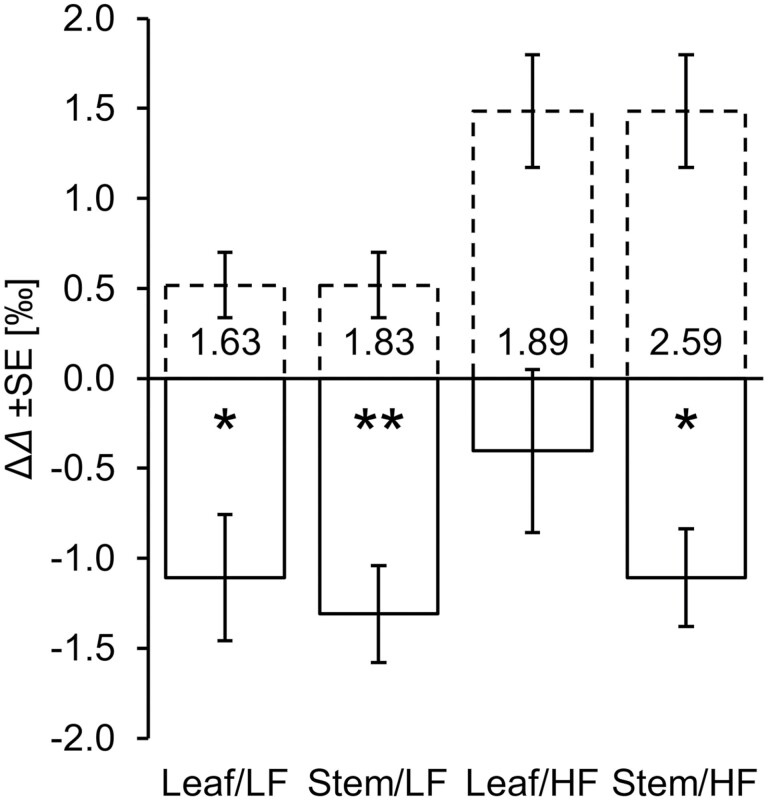
Saurer et al. (1995) reported differences in intercellular CO2 concentration, ΔCi, and whole-molecule 13C discrimination, ΔΔ, between ozone-treated and control plants. Plants grown with lower amounts of fertilizer (LF) exhibited ΔCi=7.5 ± 2.6 SE ppm, while plants grown with higher amounts of fertilizer (HF) exhibited ΔCi=21.5 ± 4.5 SE ppm. This corresponds to estimated increases in 13C discrimination by the diffusion–Rubisco interface of ΔΔDR=0.52 ± 0.18 SE ‰ and 1.49 ± 0.31 SE ‰, respectively (Fig. 5, dashed bars; Equation 1). However, Saurer et al. (1995) reported ΔΔ decreases in leaf and stem cellulose under both fertilization regimes (Fig. 5, solid bars; Equations 2, 3). With respect to ΔΔDR, these decreases are statistically significant (one-tailed t-test: P<0.05) except for leaf cellulose synthesized under HF conditions which comes, however, close to being statistically significant (P<0.08).
Fig. 5.
Differences in 13C discrimination between ozone-treated and control plants, ΔΔ (90/40 day/night versus <3 nl O3 l–1). ‘Leaf’ and ‘Stem’ refer to leaf and stem cellulose of Betula pendula, respectively. LF and HF refer to plants grown with low and high amounts of fertilizer, respectively. Solid bars: measured ΔΔ values. Dashed bars: expected ΔΔ values. Expected ΔΔ values were estimated using a model by Farquhar et al. (1982). This model describes 13C discrimination associated with plant carbon uptake including CO2 diffusion into plant leaves and assimilation by Rubisco. Numbers inside bars denote differences between measured and expected ΔΔ values. Statistically significant differences are marked by asterisks (one-tailed t-test: ∗P<0.05; ∗∗P<0.01). The difference of the Leaf/HF treatment is close to being statistically significant (P<0.08). This analysis is based on data published by Saurer et al. (1995).
In B. pendula, post-Rubisco fractionation causes average whole-molecule ΔΔ decreases of approximately –1.98 ± 0.58 SE ‰ (Fig. 5). Below, we propose that a fraction of the Δ5–6ʹ signal enters glucose C-1 to C-4 through indirect signal propagation via chloroplast metabolism (see ‘Signal propagation to all glucose carbons via chloroplast metabolism’). We estimate that the signal at C-5 and C-6 is 6.625-fold larger than at C-1 to C-4 (Supplementary Protocol S1.8). Thus, an approximately –1.98 ± 0.58 SE ‰ effect at the whole-molecule level scales to approximately –4.56 ± 1.34 SE ‰ effects at cellulose glucose C-5 and C-6 and to approximately –0.69 ± 0.20 SE ‰ effects at C-1 to C-4 (Supplementary Protocol S6). In P. nigra, measured Δ5–6ʹ values fall within a 5.80 ± 1.55 SE ‰ range (maximum=22.71 ± 0.99 SE ‰, minimum=16.91 ± 0.56 SE ‰). Wieloch et al. (2018) estimated that the Δ5–6ʹ time series contains 79% systematic and 21% error variance. Assuming the error is fully expressed in both the maximum and minimum value, we estimate a systematic time series range of ~4.58 ± 1.22 SE ‰ (5.80 ± 1.55 SE ‰×0.79). This largely equals the estimated effect at glucose C-5 and C-6 in ozone-treated B. pendula, corroborating the theory proposed above. Notably, occurrence of the post-Rubisco fractionation effect in leaf cellulose of B. pendula corroborates the proposed leaf-level origin of the Δ5–6ʹ signal.
Effect of tropospheric ozone on the Δ5–6ʹ signal in tree-ring glucose
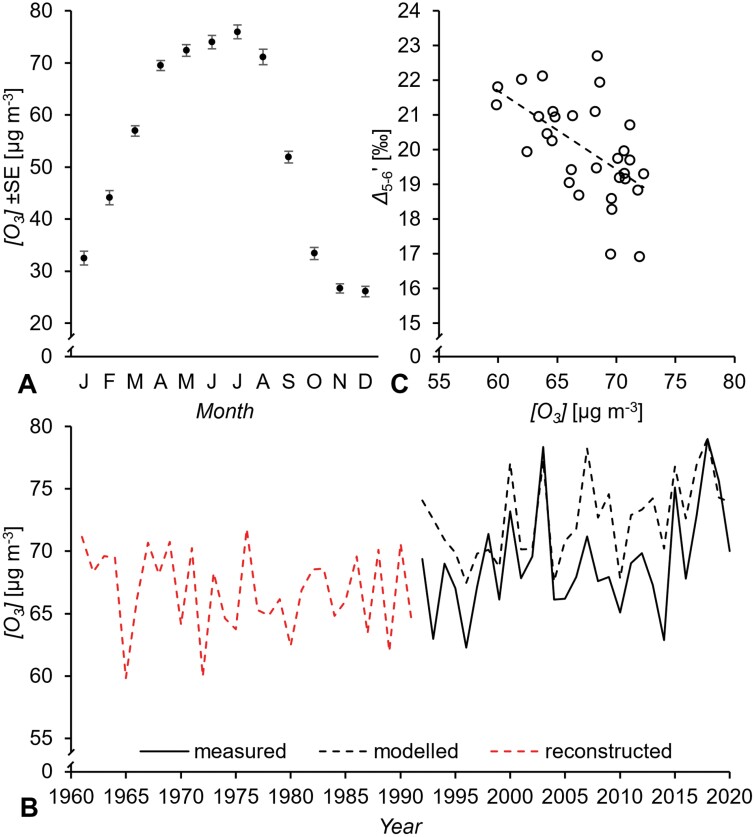
In Vienna, [O3] is measured at five sites. Complete time series for all sites are available since 1992. Intra-annually, the highest [O3] occurs during the period April to August (Fig. 6A) which can be expected to affect tree metabolism. Therefore, we calculated an April to August average time series for the Vienna region covering the period 1992–2020 (Fig. 6B, solid black line). We found that April to August SD and rH explain 59% of the time series variability (Fig. 6B, dashed black line, P<0.00001, n=29):
Fig. 6.
(A) Average monthly ozone concentrations, [O3], measured in Vienna over the period 1992–2020. (B) April to September [O3] in Vienna. Black solid line, measured [O3]; black and red dashed lines, modelled and reconstructed [O3], respectively, based on the relationship of [O3] with sunshine duration, SD, and relative humidity, rH: [O3]=58.28 + 0.02951SD–0.3697rH, R2=0.59, P<0.00001, n=29. (C) 13C discrimination at glucose C-5 and C-6, Δ5–6ʹ, as a function of [O3]: Δ5–6ʹ=35.24–0.2258[O3], R2=0.33, P<0.001, n=31. Isotope data were measured on tree-ring glucose of Pinus nigra laid down from 1961 to 1995 at a dry site in the Vienna basin. SD and rH were measured at the climate station Hohe Warte (Vienna). [O3] was measured at five stations in Vienna (Stephansplatz, Laaer Berg, Hermannskogel, Hohe Warte, and Lobau).
| (4) |
Other studies report similar relationships (Felipe-Sotelo et al., 2006; Kovač-Andrić et al., 2009). Based on Equation 4, we reconstructed [O3] for the Vienna region over the period 1961–1991 (Fig. 6B, dashed red line). We found that April to August [O3] (reconstructed for 1961–1991 and measured for 1992–1995) explains 33% of the Δ5–6ʹ time series variability (Fig. 6C, P<0.001, n=31):
| (5) |
Accounting for measurement error, 75% of the variance in Δ5–6ʹ is explainable by modelling (cf. Nilsson et al., 1996). Thus, [O3] explains 44% of the explainable variability in Δ5–6ʹ (33%/75%×100). This is substantial considering that measured and modelled [O3] time series merely exhibit 59% co-variability (Fig. 6B, solid and dashed black line). In summary, these results corroborate the proposed negative relationship between ozone stress and the Δ5–6ʹ signal (see ‘Ecophysiological effects’), and, by extension, the theoretical framework developed above.
Implications of the theory
Signal propagation at the level of TPI in the cytosol of leaves
The post-Rubisco signal at glucose C-5 and C-6 is independent of a signal at glucose C-1 and C-2 (Fig. 4; Wieloch et al., 2018); that is, substantial signal propagation from C-5 and C-6 to C-2 and C-1 is not supported by the data. This is surprising for the following reason: transmission of the Δ5–6ʹ signal from its origin, the lower end of the glycolytic pathway, into carbohydrate metabolism occurs via GAP (Fig. 1). Hence, signal independence requires negligible conversion of GAP (a precursor of glucose C-4 to C-6) to DHAP (a precursor of glucose C-1 to C-3) via leaf-cytosolic TPI (Figs 1, 2). Since TPI is often referred to as the prime example for the efficiency of enzyme catalysis, one would expect full equilibration of GAP and DHAP and inherent isotope signals. This view, however, is based on in vitro measurements of TPI kinetics. The following mechanisms may explain the apparent lack of equilibration and signal propagation in vivo.
In the light, chloroplast export of DHAP is favoured by the equilibrium position of stromal TPI, which is strongly on the side of DHAP (Walker, 1976; Knowles and Albery, 1977). Sharkey and Weise (2012) calculated that there should be >20 times more DHAP than GAP at equilibrium (Meyerhof and Junowicz-Kocholaty, 1943; Bassham and Krause, 1969). The substrate affinities of the triose phosphate translocator for DHAP and GAP are similar at Km=0.13 mM and Km=0.08 mM, respectively (Fliege et al., 1978). Thus, DHAP and GAP will be transported according to their concentrations; that is, 20 times more DHAP will be exported from chloroplasts to the cytosol. However, synthesis of fructose 1,6-bisphosphate uses DHAP and GAP at a 1:1 ratio. This may keep the concentration of leaf cytosolic GAP low. Flux of GAP into glycolysis and processes consuming glycolytic intermediates will additionally contribute to low cytosolic GAP concentrations. This may restrict the GAP to DHAP back-conversion. Furthermore, numerous common metabolites inhibit TPI competitively (Anderson, 1971; Grüning et al., 2014; Flügel et al., 2017; Li et al., 2019). In addition, the activity of cytosolic TPI decreases significantly upon treatment with reactive oxygen species, especially H2O2 (Lopez-Castillo et al., 2016). Thus, during active photosynthesis, a lack of isomeric and isotopic equilibrium between leaf cytosolic GAP and DHAP is conceivable. This would block the propagation of 13C signals in GAP to DHAP and enable independent 13C signals in Δ5–6ʹ and Δ1–2ʹ as observed.
Signal propagation to all glucose carbons via chloroplast metabolism
Cytosolic PEP, 2PGA, 3-phosphoglycerate, 1,3-bisphosphoglycerate, and GAP carry the Δ5–6ʹ signal from its putative origin (enolase, PEPC, PK, and DAHPS) directly into C-5 and C-6 of hexose phosphates (Figs 1–3). In addition, indirect signal propagation to C-1 to C-6 can be expected via import of 3-phosphoglycerate into chloroplasts (Supplementary Protocol S1.2). A model of signal propagation described in Supplementary Protocol S1 has several implications. First, observed Δ5–6ʹ signals at C-5 and C-6 are 6.625-fold larger than at C-1 to C-4 (Supplementary Protocol S1.8). Second, the original signal as introduced at the level of enolase, PEPC, PK, and DAHPS is 1.4-fold larger than the signal observed at glucose C-5 and C-6 (Supplementary Protocol S1.9). Third, clustering of Δ4ʹ with the Δ5ʹ to Δ6ʹ cluster (Fig. 4) can be explained by signal propagation via chloroplast metabolism (Supplementary Protocol S1.10).
Signal propagation to other plant metabolites
We propose that carbon flux changes around leaf cytosolic enolase, PEPC, PK, and DAHPS introduce the Δ5–6ʹ signal. Hence, upstream derivatives of 2PGA carbons corresponding to glucose C-5 and C-6 will inherit the signal (Fig. 1). Compared with plant cellulose, the signal will be distinctly smaller in chloroplast derivatives, such as starch, and distinctly larger in leaf sucrose synthesized during the photoperiod (Supplementary Protocol S1). This latter metabolite may be used to follow the Δ5–6ʹ signal on an hourly basis. Downstream derivatives of PEP carbons corresponding to glucose C-5 and C-6 will obtain an inverse Δ5–6ʹ signal. These differences may help to test the theory.
Turnover by PEPC, PK, and DAHPS can be expected to result in 13C-enriched PEP (see ‘Leaf-level enolase, PEPC, PK, and/or DAHPS introduce the Δ5–6ʹ signal’). Thus, our theory can help to explain the 13C/12C separation observed among plant compounds, specifically between 13C-enriched carbohydrates and 13C-depleted metabolites downstream of PK, PEPC, and DAHPS (Sharkey et al., 1991; Bathellier et al., 2017).
Implications for whole-molecule 13C/12C analysis
The Δ5–6ʹ signal has two components of variance (see ‘Ecophysiological effects’). One is inversely correlated with diffusion–Rubisco fractionation, and the other is independent. Both components have implications for studies of plant carbon uptake and associated properties by whole-molecule 13C/12C analysis. The inversely correlated component removes the diffusion–Rubisco signal from glucose C-5 and C-6. In addition, this signal is absent at glucose C-4 (Wieloch et al., 2018); that is, three out of six glucose carbon positions lack the diffusion–Rubisco signal. Thus, whole-molecule 13C/12C analysis captures an attenuated diffusion–Rubisco signal and underestimates the variability of the original signal and associated physiological properties, such as Ci/Ca and photosynthetic water use efficiency.
The independent component of the Δ5–6ʹ signal weakens signal extractions from whole-molecule 13C/12C measurements because it constitutes pseudorandom noise with respect to diffusion–Rubisco fractionation. This may explain why models of whole-molecule diffusion–Rubisco fractionation as functions of environmental properties often suffer from low explanatory powers, R2≤0.5 (Barbour and Song, 2014). In contrast, intramolecular 13C/12C analysis resolves information about distinct ecophysiological processes; a fundamental conceptual advancement enabling more adequate modelling of the variability of plant carbon uptake and associated environmental/developmental controls.
Tracking carbon allocation in other biological organisms
Whole-molecule 13C/12C analysis enables assessments of plant carbon uptake (Farquhar et al., 1982). According to theory reported here, intramolecular 13C/12C analysis enables additional assessment of downstream carbon allocation in actively photosynthesizing leaves. This includes carbon flux into the anaplerotic, shikimate, MEP, and fatty acid biosynthesis pathways, and mitochondrial respiration (Fig. 1). Intramolecular 13C signals are governed by a small set of physicochemical principles that apply generally (Schmidt et al., 2015). Thus, intramolecular 13C/12C analysis can be expected to enable retrospective assessment of carbon allocation in any biological organism including, for instance, disease-related shifts.
Utility of the Δ5–6ʹ signal
Laboratory experiments are limited to short time scales and in their capabilities to reproduce complex natural systems. Manipulation experiments on natural systems are limited to time scales of years and may suffer from spurious effects due to unnatural step changes in ambient conditions. In contrast, tree-ring analysis offers extensive temporal, spatial, species, and genotype coverage of natural systems that have not been subjected to unnatural step changes.
We propose that the Δ5–6ʹ signal reports flux into the anaplerotic pathway including CO2 uptake by PEPC (Figs 1, 3, 5, 6). In addition, it may report flux into mitochondrial respiration (Supplementary Protocol S3). Thus, signal analysis may enable a better understanding of plant and ecosystem carbon balances including the so-called CO2 fertilization effect.
Furthermore, intramolecular 13C/12C analysis enables analysis not only of carbon uptake–environment relationships but also of carbon allocation–environment relationships (Figs 5, 6) and thus more comprehensive assessments of flux-level plant performance. For instance, atmospheric CO2 and ozone concentrations have increased over recent years (Fig. 6B). Under business-as-usual scenarios, this will continue over the next decades (Turnock et al., 2018). While CO2 promotes leaf photosynthesis and net primary productivity, ozone has the very opposite effect (Ainsworth et al., 2012; IPCC, 2014). When this highly reactive chemical enters plant leaves through stomata, it causes harm to structure and function, and leads to major rearrangements in carbon metabolism. While ozone decreases carbon fixation, it increases carbon allocation to costly maintenance and repair processes (Dizengremel, 2001; Ainsworth et al., 2012). This includes increased carbon flux into the anaplerotic and shikimate pathway, and this resource investment is likely to be recorded in the Δ5–6ʹ signal. Ozone tolerance varies among species, with metabolic changes depending on the duration of ozone exposure (Fontaine et al., 2003; Ainsworth et al., 2012). Thus, the Δ5–6ʹ signal may support flux-level screenings for species/genotypes with the capacity to optimally adjust to prolonged ozone exposure (requires further investigation).
While glucose positions C-1 to C-3 preserve the VPD-dependent carbon uptake signal (Wieloch et al., 2018), this signal was removed from glucose C-5 and C-6 and replaced by an independent ozone-sensitive carbon allocation signal (Figs 5, 6). Thus, intramolecular 13C/12C analysis yields information about several environmental variables and may enable more powerful paleoenvironment reconstructions than whole-molecule analysis.
Lastly, sampling glucose at different developmental stages may enable the detection of shifts in carbon uptake and allocation over the life span of plants to better understand basic physiological processes such as plant senescence. In conclusion, intramolecular 13C/12C analysis opens up numerous new avenues of research within the plant and Earth sciences.
Supplementary data
The following supplementary data are available at JXB online.
Protocol S1. Signal propagation to all glucose carbons via chloroplast metabolism and implications.
Protocol S1.1. Signal propagation upon reimport of cytosolic metabolites into chloroplasts.
Protocol S1.2. Predominant import molecules.
Protocol S1.3. Predominant export molecules.
Protocol S1.4. Signal import into chloroplasts.
Protocol S1.5. Signal dilution and partial signal loss.
Protocol S1.6. Stromal signal redistribution
Protocol S1.7. Signal size in stromal triose phosphates.
Protocol S1.8. Signal size in cytosolic hexose phosphates.
Protocol S1.9. Original size of the Δ5–6ʹ signal.
Protocol S1.10. Signal propagation can explain the clustering of the Δ4ʹ to Δ6ʹ time series.
Protocol S1.11. The role of chloroplast starch in signal propagation.
Protocol S1.12. Signal import into chloroplasts via cytosolic 1,3BPGA, 2PGA, and PEP.
Protocol S1.13. Assumptions.
Protocol S2. Correction for 13C signal redistribution by triose phosphate cycling in tree-ring cells.
Protocol S3. Alternating substrate supply to oxidative phosphorylation may contribute to the Δ5–6ʹ signal.
Protocol S4. Carbon flux into fatty acid biosynthesis in illuminated photosynthetic tissue.
Protocol S5. Potential influence of environmental and developmental variables on the Δ5–6ʹ signal.
Protocol S6. Intramolecular isotope effects in response to ozone.
Acknowledgments
We thank Lisa Wingate (INRA, Ephyse Research Unit, Bordeaux, France), Richard Leegood (University of Sheffield, UK), and Nicole Linka (Heinrich Heine University Düsseldorf, Germany) for helpful comments.
Author contributions
TW: conceptualization, devising the physiological and isotope parts, and writing with input from TDS, JS, and RAW; TW: leading the research; TW: data analysis with input from RAW.
Conflict of interest
The authors declare no conflict of interest.
Funding
The research of JS was funded by the Swedish Research Council VR (2013-05219, 2018-04456), the Knut and Alice Wallenberg Foundation (‘NMR for Life’ facility and grant 2015.0047), and the Kempe foundations. The research of TDS was funded by the U.S. Department of Energy (DE-FG02-91ER2002). Partial salary support for TDS came from Michigan AgBioResearch.
Data availability
The data that support the findings of this study have been published previously by Wieloch et al. (2018) and Saurer et al. (1995).
References
- Abadie C, Bathellier C, Tcherkez G.. 2018. Carbon allocation to major metabolites in illuminated leaves is not just proportional to photosynthesis when gaseous conditions (CO2 and O2) vary. New Phytologist 218, 94–106. [DOI] [PubMed] [Google Scholar]
- Abelson PH, Hoering TC.. 1961. Carbon isotope fractionation in formation of amino acids by photosynthetic organisms. Proceedings of the National Academy of Sciences, USA 47, 623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth EA, Yendrek CR, Sitch S, Collins WJ, Emberson LD.. 2012. The effects of tropospheric ozone on net primary productivity and implications for climate change. Annual Review of Plant Biology 63, 637–661. [DOI] [PubMed] [Google Scholar]
- Altermatt HA, Neish AC.. 1956. The biosynthesis of cell wall carbohydrates. III. Further studies on formation of cellulose and xylan from labeled monosaccharides in wheat plants. Canadian Journal of Biochemistry and Physiology 34, 405–413. [PubMed] [Google Scholar]
- Ambasht PK, Kayastha AM.. 2002. Plant pyruvate kinase. Biologia Plantarum 45, 1–10. [Google Scholar]
- Anderson LE. 1971. Chloroplast and cytoplasmic enzymes II. Pea leaf triose phosphate isomerases. Biochimica et Biophysica Acta 235, 237–244. [DOI] [PubMed] [Google Scholar]
- Bahrami AR, Chen Z-H, Walker RP, Leegood RC, Gray JE.. 2001. Ripening-related occurrence of phosphoenolpyruvate carboxykinase in tomato fruit. Plant Molecular Biology 47, 499–506. [DOI] [PubMed] [Google Scholar]
- Bailey KJ, Leegood RC.. 2016. Nitrogen recycling from the xylem in rice leaves: dependence upon metabolism and associated changes in xylem hydraulics. Journal of Experimental Botany 67, 2901–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao X, Focke M, Pollard M, Ohlrogge J.. 2000. Understanding in vivo carbon precursor supply for fatty acid synthesis in leaf tissue. The Plant Journal 22, 39–50. [DOI] [PubMed] [Google Scholar]
- Barbour MM, Song X.. 2014. Do tree-ring stable isotope compositions faithfully record tree carbon/water dynamics? Tree Physiology 34, 792–795. [DOI] [PubMed] [Google Scholar]
- Bassham JA, Krause GH.. 1969. Free energy changes and metabolic regulation in steady-state photosynthetic carbon reduction. Biochimica et Biophysica Acta 189, 207–221. [DOI] [PubMed] [Google Scholar]
- Bathellier C, Badeck F-W, Ghashghaie J.. 2017. Carbon isotope fractionation in plant respiration. In: Tcherkez G, Ghashghaie J, eds. Advances in photosynthesis and respiration. Plant respiration: metabolic fluxes and carbon balance. Cham: Springer International Publishing AG, 43–68. [Google Scholar]
- Betz GA, Gerstner E, Stich S, Winkler B, Welzl G, Kremmer E, Langebartels C, Heller W, Sandermann H, Ernst D.. 2009. Ozone affects shikimate pathway genes and secondary metabolites in saplings of European beech (Fagus sylvatica L.) grown under greenhouse conditions. Trees 23, 539–553. [Google Scholar]
- Bischoff M, Rösler J, Raesecke HR, Görlach J, Amrhein N, Schmid J.. 1996. Cloning of a cDNA encoding a 3-dehydroquinate synthase from a higher plant, and analysis of the organ-specific and elicitor-induced expression of the corresponding gene. Plant Molecular Biology 31, 69–76. [DOI] [PubMed] [Google Scholar]
- Bischoff M, Schaller A, Bieri F, Kessler F, Amrhein N, Schmid J.. 2001. Molecular characterization of tomato 3-dehydroquinate dehydratase-shikimate:NADP oxidoreductase. Plant Physiology 125, 1891–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Neish AC.. 1954. The biosynthesis of cell wall carbohydrates—glucose-C14 as a cellulose precursor in wheat plants. Canadian Journal of Biochemistry and Physiology 32, 170–177. [PubMed] [Google Scholar]
- Chen ZH, Walker RP, Acheson RM, Tecsi LI, Wingler A, Lea PJ, Leegood RC.. 2000. Are isocitrate lyase and phosphoenolpyruvate carboxykinase involved in gluconeogenesis during senescence of barley leaves and cucumber cotyledons? Plant & Cell Physiology 41, 960–967. [DOI] [PubMed] [Google Scholar]
- Chen ZH, Walker RP, Tecsi LI, Lea PJ, Leegood RC.. 2004. Phosphoenolpyruvate carboxykinase in cucumber plants is increased both by ammonium and by acidification, and is present in the phloem. Planta 219, 48–58. [DOI] [PubMed] [Google Scholar]
- Chollet R, Vidal J, O’Leary MH.. 1996. Phosphoenolpyruvate carboxylase: a ubiquitous, highly regulated enzyme in plants. Annual Review of Plant Physiology and Plant Molecular Biology 47, 273–298. [DOI] [PubMed] [Google Scholar]
- Dizengremel P. 2001. Effects of ozone on the carbon metabolism of forest trees. Plant Physiology and Biochemistry 39, 729–742. [Google Scholar]
- Dobson MC, Taylor G, Freer-Smith PH.. 1990. The control of ozone uptake by Picea abies (L.) Karst. and P. sitchensis (Bong.) Carr. during drought and interacting effects on shoot water relations. New Phytologist 116, 465–474. [DOI] [PubMed] [Google Scholar]
- Doubnerová Hýsková V, Miedzińska L, Dobrá J, Vankova R, Ryšlavá H.. 2014. Phosphoenolpyruvate carboxylase, NADP-malic enzyme, and pyruvate, phosphate dikinase are involved in the acclimation of Nicotiana tabacum L. to drought stress. Journal of Plant Physiology 171, 19–25. [DOI] [PubMed] [Google Scholar]
- Dyer WE, Henstrand JM, Handa AK, Herrmann KM.. 1989. Wounding induces the first enzyme of the shikimate pathway in Solanaceae. Proceedings of the National Academy of Sciences, USA 86, 7370–7373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman J, Ginsburg V, Hassid WZ.. 1955. Conversion of monosaccharides to sucrose and cellulose in wheat seedlings. Journal of Biological Chemistry 213, 843–854. [PubMed] [Google Scholar]
- Entus R, Poling M, Herrmann KM.. 2002. Redox regulation of Arabidopsis 3-deoxy-d-arabino-heptulosonate 7-phosphate synthase. Plant Physiology 129, 1866–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar-Gutiérrez AJ, Gaudillère JP.. 1997. Carbon partitioning in source leaves of peach, a sorbitol-synthesizing species, is modified by photosynthetic rate. Physiologia Plantarum 100, 353–360. [Google Scholar]
- Famiani F, Paoletti A, Battistelli A, Moscatello S, Chen ZH, Leegood RC, Walker RP.. 2016. Phosphoenolpyruvate carboxykinase, pyruvate orthophosphate dikinase and isocitrate lyase in both tomato fruits and leaves, and in the flesh of peach and some other fruits. Journal of Plant Physiology 202, 34–44. [DOI] [PubMed] [Google Scholar]
- Farquhar GD, O’Leary MH, Berry JA.. 1982. On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Australian Journal of Plant Physiology 9, 121–137. [Google Scholar]
- Felipe-Sotelo M, Gustems L, Hernàndez I, Terrado M, Tauler R.. 2006. Investigation of geographical and temporal distribution of tropospheric ozone in Catalonia (North-East Spain) during the period 2000–2004 using multivariate data analysis methods. Atmospheric Environment 40, 7421–7436. [Google Scholar]
- Ferrari S, Galletti R, Denoux C, De Lorenzo G, Ausubel FM, Dewdney J.. 2007. Resistance to Botrytis cinerea induced in Arabidopsis by elicitors is independent of salicylic acid, ethylene, or jasmonate signaling but requires PHYTOALEXIN DEFICIENT3. Plant Physiology 144, 367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer K, Kammerer B, Gutensohn M, Arbinger B, Weber A, Hausler RE, Flügge UI.. 1997. A new class of plastidic phosphate translocators: a putative link between primary and secondary metabolism by the phosphoenolpyruvate/phosphate antiporter. The Plant Cell 9, 453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliege R, Flügge UI, Werdan K, Heldt HW.. 1978. Specific transport of inorganic phosphate, 3-phosphoglycerate and triosephosphates across the inner membrane of the envelope in spinach chloroplasts. Biochimica et Biophysica Acta 502, 232–247. [DOI] [PubMed] [Google Scholar]
- Flügel F, Timm S, Arrivault S, Florian A, Stitt M, Fernie AR, Bauwe H.. 2017. The photorespiratory metabolite 2-phosphoglycolate regulates photosynthesis and starch accumulation in Arabidopsis. The Plant Cell 29, 2537–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flügge U-I. 1987. Physiological function and physical characteristics of the chloroplast phosphate translocator. In: Biggins J, ed. Progress in Photosynthesis Research: Volume 3 Proceedings of the VIIth International Congress on Photosynthesis. Providence, RI, USA, 10–15 August 1986. Dordrecht: Springer Netherlands, 739–746. [Google Scholar]
- Flügge U-I. 1999. Phosphate translocators in plastids. Annual Review of Plant Physiology and Plant Molecular Biology 50, 27–45. [DOI] [PubMed] [Google Scholar]
- Flügge U-I, Häusler RE, Ludewig F, Gierth M.. 2011. The role of transporters in supplying energy to plant plastids. Journal of Experimental Botany 62, 2381–2392. [DOI] [PubMed] [Google Scholar]
- Fontaine V, Cabané M, Dizengremel P.. 2003. Regulation of phosphoenolpyruvate carboxylase in Pinus halepensis needles submitted to ozone and water stress. Physiologia Plantarum 117, 445–452. [DOI] [PubMed] [Google Scholar]
- Fukayama H, Masumoto C, Taniguchi Y, Baba-Kasai A, Katoh Y, Ohkawa H, Miyao M.. 2015. Characterization and expression analyses of two plastidic enolase genes in rice. Bioscience Biotechnology and Biochemistry 79, 402–409. [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Fernie AR.. 2014. Metabolic control of redox and redox control of metabolism in plants. Antioxidants & Redox Signaling 21, 1389–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görlach J, Raesecke HR, Rentsch D, Regenass M, Roy P, Zala M, Keel C, Boller T, Amrhein N, Schmid J.. 1995. Temporally distinct accumulation of transcripts encoding enzymes of the prechorismate pathway in elicitor-treated, cultured tomato cells. Proceedings of the National Academy of Sciences, USA 92, 3166–3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greathouse GA. 1953. Biosynthesis of C14-specifically labeled cotton cellulose. Science 117, 553–554. [DOI] [PubMed] [Google Scholar]
- Grüning NM, Du D, Keller MA, Luisi BF, Ralser M.. 2014. Inhibition of triosephosphate isomerase by phosphoenolpyruvate in the feedback-regulation of glycolysis. Open Biology 4, 130232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood JL. 2005. Fatty acid biosynthesis. In: Murphy DJ, ed. Plant lipids: biology, utilisation and manipulation. Oxford: Blackwell Publishing Ltd, 27–66. [Google Scholar]
- Hatzfeld W-D, Stitt M.. 1990. A study of the rate of recycling of triose phosphates in heterotrophic Chenopodium rubrum cells, potato tubers, and maize endosperm. Planta 180, 198–204. [DOI] [PubMed] [Google Scholar]
- Hayes JM. 2001. Fractionation of carbon and hydrogen isotopes in biosynthetic processes. Reviews in Mineralogy and Geochemistry 43, 225–277. [Google Scholar]
- Henstrand JM, McCue KF, Brink K, Handa AK, Herrmann KM, Conn EE.. 1992. Light and fungal elicitor induce 3-deoxy-d-arabino-heptulosonate 7-phosphate synthase mRNA in suspension cultured cells of parsley (Petroselinum crispum L.). Plant Physiology 98, 761–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann KM. 1995. The shikimate pathway: early steps in the biosynthesis of aromatic compounds. The Plant Cell 7, 907–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SA, Waterhouse JS, Field EM, Switsur VR, Ap Rees T.. 1995. Rapid recycling of triose phosphates in oak stem tissue. Plant, Cell & Environment 18, 931–936. [Google Scholar]
- Hocking CG, Anderson JW.. 1986. Survey of pyruvate, phosphate dikinase activity of plants in relation to the C3, C4 and CAM mechanisms of CO2 assimilation. Phytochemistry 25, 1537–1543. [Google Scholar]
- IPCC . 2014. Climate change 2014: Synthesis report. Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change. Geneva: IPCC. [Google Scholar]
- Janzik I, Preiskowski S, Kneifel H.. 2005. Ozone has dramatic effects on the regulation of the prechorismate pathway in tobacco (Nicotiana tabacum L. cv. Bel W3). Planta 223, 20–27. [DOI] [PubMed] [Google Scholar]
- Keeling PL, Wood JR, Tyson RH, Bridges IG.. 1988. Starch biosynthesis in developing wheat grain: evidence against the direct involvement of triose phosphates in the metabolic pathway. Plant Physiology 87, 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith B, Dong XN, Ausubel FM, Fink GR.. 1991. Differential induction of 3-deoxy-d-arabino-heptulosonate 7-phosphate synthase genes in Arabidopsis thaliana by wounding and pathogenic attack. Proceedings of the National Academy of Sciences, USA 88, 8821–8825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuta Y, Erickson LC.. 1969. Metabolism of glucose in relation to the lipid synthesis in the fruit of Persea americana MILL. Plant & Cell Physiology 10, 563–574. [Google Scholar]
- Klein Tank AMG, Wijngaard JB, Können GP, et al. 2002. Daily dataset of 20th-century surface air temperature and precipitation series for the European Climate Assessment. International Journal of Climatology 22, 1441–1453. [Google Scholar]
- Knowles JR, Albery WJ.. 1977. Perfection in enzyme catalysis: the energetics of triosephosphate isomerase. Accounts of Chemical Research 10, 105–111. [Google Scholar]
- Kovač-Andrić E, Brana J, Gvozdić V.. 2009. Impact of meteorological factors on ozone concentrations modelled by time series analysis and multivariate statistical methods. Ecological Informatics 4, 117–122. [Google Scholar]
- Kubota K, Ashihara H.. 1990. Identification of non-equilibrium glycolytic reactions in suspension-cultured plant cells. Biochimica et Biophysica Acta 1036, 138–142. [DOI] [PubMed] [Google Scholar]
- Leegood RC, Acheson RM, Técsi LI, Walker RP.. 1999. The many-faceted function of phosphoenolpyruvate carboxykinase in plants. In: Kruger NJ, Hill SA, Ratcliffe RG, eds. Regulation of primary metabolic pathways in plants. Dordrecht: Springer Netherlands, 37–51. [Google Scholar]
- Lefohn AS. 1992. Surface-level ozone exposures and their effects on vegetation. Chelsea: CRC Press. [Google Scholar]
- Leuenberger M. 2007. To what extent can ice core data contribute to the understanding of plant ecological developments of the past? In: Dawson TE, Siegwolf RTW, eds. Terrestrial ecology. Amsterdam: Elsevier, 211–233. [Google Scholar]
- Li J, Weraduwage SM, Preiser AL, Tietz S, Weise SE, Strand DD, Froehlich JE, Kramer DM, J H, Sharkey TD.. 2019. A cytosolic bypass and G6P shunt in plants lacking peroxisomal hydroxypyruvate reductase. Plant Physiology 180, 783–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logemann E, Tavernaro A, Schulz W, Somssich IE, Hahlbrock K.. 2000. UV light selectively coinduces supply pathways from primary metabolism and flavonoid secondary product formation in parsley. Proceedings of the National Academy of Sciences, USA 97, 1903–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Castillo LM, Jimenez-Sandoval P, Baruch-Torres N, Trasvina-Arenas CH, Diaz-Quezada C, Lara-Gonzalez S, Winkler R, Brieba LG.. 2016. Structural basis for redox regulation of cytoplasmic and chloroplastic triosephosphate isomerases from Arabidopsis thaliana. Frontiers in Plant Science 7, 1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H, Dudareva N.. 2012. The shikimate pathway and aromatic amino acid biosynthesis in plants. Annual Review of Plant Biology 63, 73–105. [DOI] [PubMed] [Google Scholar]
- Malone S, Chen ZH, Bahrami AR, Walker RP, Gray JE, Leegood RC.. 2007. Phosphoenolpyruvate carboxykinase in Arabidopsis: Changes in gene expression, protein and activity during vegetative and reproductive development. Plant & Cell Physiology 48, 441–450. [DOI] [PubMed] [Google Scholar]
- Masumoto C, Miyazawa S-I, Ohkawa H, Fukuda T, Taniguchi Y, Murayama S, Kusano M, Saito K, Fukayama H, Miyao M.. 2010. Phosphoenolpyruvate carboxylase intrinsically located in the chloroplast of rice plays a crucial role in ammonium assimilation. Proceedings of the National Academy of Sciences, USA 107, 5226–5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyssek R, Günthardt-Goerg MS, Saurer M, Keller T.. 1992. Seasonal growth, δ13C in leaves and stem, and phloem structure of birch (Betula pendula) under low ozone concentrations. Trees 6, 69–76. [Google Scholar]
- McConnell WB, Mitra AK, Perlin AS.. 1958. Studies on wheat plants using C14 compounds: VIII. Formation of amylose and amylopectin in the wheat kernel. Canadian Journal of Biochemistry and Physiology 36, 985–991. [PubMed] [Google Scholar]
- McCue KF, Conn EE.. 1989. Induction of 3-deoxy-d-arabino-heptulosonate-7-phosphate synthase activity by fungal elicitor in cultures of Petroselinum crispum. Proceedings of the National Academy of Sciences, USA 86, 7374–7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer E, O’Leary MH.. 1987. Anapleurotic CO2 fixation by phosphoenolpyruvate carboxylase in C3 plants. Plant Physiology 84, 58–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhof O, Junowicz-Kocholaty R.. 1943. The equilibria of isomerase and aldolase, and the problem of the phosphorylation of glyceraldehyde phosphate. Journal of Biological Chemistry 149, 71–92. [Google Scholar]
- Mir R, Jallu S, Singh TP.. 2015. The shikimate pathway: review of amino acid sequence, function and three-dimensional structures of the enzymes. Critical Reviews in Microbiology 41, 172–189. [DOI] [PubMed] [Google Scholar]
- Nageswara Rao BD, Kayne FJ, Cohn M.. 1979. 31P NMR studies of enzyme-bound substrates of rabbit muscle pyruvate kinase. Equilibrium constants, exchange rates, and NMR parameters. Journal of Biological Chemistry 254, 2689–2696. [PubMed] [Google Scholar]
- Neish AC. 1955. The biosynthesis of cell wall carbohydrates. II. Formation of cellulose and xylan from labeled monosaccharides in wheat plants. Canadian Journal of Biochemistry and Physiology 33, 658–666. [PubMed] [Google Scholar]
- Neish AC. 1958. The biosynthesis of cell wall carbohydrates. IV. Further studies on cellulose and xylan in wheat. Canadian Journal of Biochemistry and Physiology 36, 187–193. [PubMed] [Google Scholar]
- Nilsson MB, Dåbakk E, Korsman T, Renberg I.. 1996. Quantifying relationships between near-infrared reflectance spectra of lake sediments and water chemistry. Environmental Science & Technology 30, 2586–2590. [Google Scholar]
- Ohlrogge J, Browse J, Jaworski J, Somerville C.. 2015. Lipids. In: Buchanan BB, Gruissem W, Jones RL, eds. Biochemistry and Molecular Biology of Plants. Chichester, UK: John Wiley & Sons, Ltd, 337–400. [Google Scholar]
- O’Leary BM, Plaxton WC.. 2017. Mechanisms and functions of post-translational enzyme modifications in the organization and control of plant respiratory metabolism. In: Tcherkez G, Ghashghaie J, eds. Advances in Photosynthesis and Respiration. Plant respiration: metabolic fluxes and carbon balance. Cham: Springer International Publishing AG, 261–284. [Google Scholar]
- O’Leary B, Park J, Plaxton WC.. 2011. The remarkable diversity of plant PEPC (phosphoenolpyruvate carboxylase): recent insights into the physiological functions and post-translational controls of non-photosynthetic PEPCs. Biochemical Journal 436, 15–34. [DOI] [PubMed] [Google Scholar]
- Oltmans SJ, Lefohn AS, Scheel HE, et al. 1998. Trends of ozone in the troposphere. Geophysical Research Letters 25, 139–142. [Google Scholar]
- Page RA, Okada S, Harwood JL.. 1994. Acetyl-CoA carboxylase exerts strong flux control over lipid synthesis in plants. Biochimica et Biophysica Acta 1210, 369–372. [DOI] [PubMed] [Google Scholar]
- Prabhakar V, Löttgert T, Gigolashvili T, Bell K, Flügge UI, Häusler RE.. 2009. Molecular and functional characterization of the plastid-localized phosphoenolpyruvate enolase (ENO1) from Arabidopsis thaliana. FEBS Letters 583, 983–991. [DOI] [PubMed] [Google Scholar]
- Pyke K. 2001. Mesophyll . eLS. doi: 10.1002/9780470015902.a0002081.pub2 [DOI] [Google Scholar]
- Rawsthorne S. 2002. Carbon flux and fatty acid synthesis in plants. Progress in Lipid Research 41, 182–196. [DOI] [PubMed] [Google Scholar]
- Sade N, Gebremedhin A, Moshelion M.. 2012. Risk-taking plants: anisohydric behavior as a stress-resistance trait. Plant Signaling & Behavior 7, 767–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saurer M, Maurer S, Matyssek R, Landolt W, Günthardt-Goerg MS, Siegenthaler U.. 1995. The influence of ozone and nutrition on δ13C in Betula pendula. Oecologia 103, 397–406. [DOI] [PubMed] [Google Scholar]
- Scheible W-R, Morcuende R, Czechowski T, Fritz C, Osuna D, Palacios-Rojas N, Schindelasch D, Thimm O, Udvardi MK, Stitt M.. 2004. Genome-wide reprogramming of primary and secondary metabolism, protein synthesis, cellular growth processes, and the regulatory infrastructure of Arabidopsis in response to nitrogen. Plant Physiology 136, 2483–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HL, Robins RJ, Werner RA.. 2015. Multi-factorial in vivo stable isotope fractionation: causes, correlations, consequences and applications. Isotopes in Environmental and Health Studies 51, 155–199. [DOI] [PubMed] [Google Scholar]
- Seegmiller CG, Jang R, Mann W.. 1956. Conversion of radioactive hexoses to pectin in the strawberry. Archives of Biochemistry and Biophysics 61, 422–430. [DOI] [PubMed] [Google Scholar]
- Shafizadeh F, Wolfrom ML.. 1955. Biosynthesis of C14-labeled cotton cellulose from d-glucose-1-C14 and d-Glucose-6-C14. Journal of the American Chemical Society 77, 5182–5183. [Google Scholar]
- Sharkey TD, Loreto F, Delwiche CF, Treichel IW.. 1991. Fractionation of carbon isotopes during biogenesis of atmospheric isoprene. Plant Physiology 97, 463–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Singsaas EL, Vanderveer PJ, Geron C.. 1996. Field measurements of isoprene emission from trees in response to temperature and light. Tree Physiology 16, 649–654. [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Vanderveer PJ.. 1989. Stromal phosphate concentration is low during feedback limited photosynthesis. Plant Physiology 91, 679–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey T, Weise S.. 2012. Autotrophic carbon dioxide fixation. In: Eaton-Rye J, Tripathy B, Sharkey T, eds. Advances in Photosynthesis and Respiration. Photosynthesis: plastid biology, energy conversion and carbon assimilation. Dordrecht: Springer, 651–674. [Google Scholar]
- Sharkey TD, Yeh S.. 2001. Isoprene emission from plants. Annual Review of Plant Physiology and Plant Molecular Biology 52, 407–436. [DOI] [PubMed] [Google Scholar]
- Shibko S, Edelman J.. 1957. Randomization of the carbon atoms in glucose and fructose during their metabolism in barley seedlings. Biochimica et Biophysica Acta 25, 642–644. [DOI] [PubMed] [Google Scholar]
- Streatfield SJ, Weber A, Kinsman EA, Häusler RE, Li J, Post-Beittenmiller D, Kaiser WM, Pyke KA, Flügge UI, Chory J.. 1999. The phosphoenolpyruvate/phosphate translocator is required for phenolic metabolism, palisade cell development, and plastid-dependent nuclear gene expression. The Plant Cell 11, 1609–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor L, Nunes-Nesi A, Parsley K, Leiss A, Leach G, Coates S, Wingler A, Fernie AR, Hibberd JM.. 2010. Cytosolic pyruvate,orthophosphate dikinase functions in nitrogen remobilization during leaf senescence and limits individual seed growth and nitrogen content. The Plant Journal 62, 641–652. [DOI] [PubMed] [Google Scholar]
- Tcherkez G, Mahé A, Boex-Fontvieille E, Gout E, Guérard F, Bligny R.. 2011. Experimental evidence of phosphoenolpyruvate resynthesis from pyruvate in illuminated leaves. Plant Physiology 157, 86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingey DT, Hogsett WE.. 1985. Water stress reduces ozone injury via a stomatal mechanism. Plant Physiology 77, 944–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohge T, Watanabe M, Hoefgen R, Fernie AR.. 2013. Shikimate and phenylalanine biosynthesis in the green lineage. Frontiers in Plant Science 4, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truman W, de Zabala MT, Grant M.. 2006. Type III effectors orchestrate a complex interplay between transcriptional networks to modify basal defence responses during pathogenesis and resistance. The Plant Journal 46, 14–33. [DOI] [PubMed] [Google Scholar]
- Turnock ST, Wild O, Dentener FJ, et al. 2018. The impact of future emission policies on tropospheric ozone using a parameterised approach. Atmospheric Chemistry and Physics 18, 8953–8978. [Google Scholar]
- van der Straeten D, Rodrigues-Pousada RA, Goodman HM, van Montagu M.. 1991. Plant enolase: gene structure, expression, and evolution. The Plant Cell 3, 719–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola R, Davies HV, Chudeck AR.. 1991. Pathways of starch and sucrose biosynthesis in developing tubers of potato (Solanum tuberosum L.) and seeds of faba bean (Vicia faba L.)—elucidation by 13C-nuclear-magnetic-resonance spectroscopy. Planta 183, 202–208. [DOI] [PubMed] [Google Scholar]
- Walker DA. 1976. Plastids and intracellular transport. In: Stocking CR, Heber U, eds. Transport in plants III: intracellular interactions and transport processes. Berlin Heidelberg: Springer, 85–136. [Google Scholar]
- Wieloch T. 2021. A cytosolic oxidation–reduction cycle in plant leaves. Journal of Experimental Botany 72, 4186–4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieloch T, Ehlers I, Yu J, Frank D, Grabner M, Gessler A, Schleucher J.. 2018. Intramolecular 13C analysis of tree rings provides multiple plant ecophysiology signals covering decades. Scientific Reports 8, 5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieloch T, Werner RA, Schleucher J.. 2021. Carbon flux around leaf-cytosolic glyceraldehyde-3-phosphate dehydrogenase introduces a 13C signal in plant glucose. Journal of Experimental Botany 72, 7136–7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfrom ML, Webber JM, Shafizadeh F.. 1959. Studies on the biosynthesis and fragmentation of C14-labeled cotton cellulose and seed oil. Journal of the American Chemical Society 81, 1217–1221. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study have been published previously by Wieloch et al. (2018) and Saurer et al. (1995).