Abstract
Background:
A growing body of research has shown that underinsured patients are at increased risk of worse health outcomes compared to insured patients. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CRS-HIPEC) is performed largely at highly specialized cancer centers and may pose challenges for the underinsured. This study investigates surgical outcomes following CRS-HIPEC for insured and underinsured patients with peritoneal carcinomatosis.
Methods:
We performed a retrospective cohort study of 125 patients undergoing CRS-HIPEC between 2013–2019. Patients were categorized into two groups. The insured group was comprised of patients with private insurance at the time of CRS-HIPEC or who obtained it during the follow-up period, while the underinsured group consisted of patients with Medicare, Medicaid, or self-pay. Perioperative and oncologic outcomes were compared between the two groups.
Results:
A total of 102 (82.3%) patients were insured and 22 (17.7%) patients were underinsured. There were no significant differences in age, medical morbidities, primary tumor characteristics, peritoneal carcinomatosis index, or completion of cytoreduction score between the two groups. The median overall survival (OS) for insured patients was 64.8 months and was 52.9 months for underinsured patients (p=0.01). Additionally, insured patients had a significantly longer follow-up time. Underinsurance status was also associated with increased hospital and intensive care unit length of stay, and higher rate of Clavien-Dindo classification III-IV complications.
Conclusion:
In this retrospective study conducted at a large urban specialized cancer center, private insurance status was associated with increased overall survival and longer follow-up period. Furthermore, underinsurance status was associated with increased perioperative morbidity.
Introduction
Peritoneal carcinomatosis (PC) is a late presentation of gastrointestinal, gynecological, or primary peritoneal malignancies that results in the dissemination of cancer throughout the peritoneum1. The foundation of treatment of PC consists of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CRS-HIPEC)2. As there is increasing data to support the use of CRS-HIPEC, the operation has become more popular and available2. However, these surgeries are typically available in specialized cancer centers with surgical personnel and support staff with expertise in managing these patients. Disparities in the timing of cancer diagnosis, treatment, and survival exist across different ethnic and socioeconomic groups within the United States3. Several studies have demonstrated that privately insured patients with cancer, including gastrointestinal malignancies, have improved survival compared to patients with no insurance or government insurance3–8. Insurance status influences a patient’s ability to obtain prompt high-quality surgical care and may also act as a surrogate for other sociodemographic factors that may influence oncologic and perioperative outcomes. In a large retrospective study utilizing the National Cancer Database, multivariate analyses demonstrated that private insurance was associated with increased overall survival at one year. Additionally, underinsured patients had significantly more medical comorbidities4. A similar study of over 3 million patients showed that underinsured patients were more likely to present with advanced-stage cancer compared to their privately insured counterparts9.
Due to the highly specialized nature of CRS-HIPEC and the medical complexity of patients with PC, it is imperative to investigate the disparities in outcomes in patients with PC. These investigations would deepen the ongoing conversation regarding socioeconomic health disparities in the United States and allow us to mitigate factors related to such disparities. The impact of insurance status on outcomes in patients with PC is largely unknown. A recent study found no difference in overall survival between insured and underinsured patients who underwent CRS-HIPEC, but only 31 patients were included and only 6 patients were alive at the end of the study period10. The aim of this study is to examine the impact of insurance status on oncologic and perioperative outcomes in patients undergoing CRS-HIPEC for peritoneal carcinomatosis. To date, this is the largest study comparing outcomes in patients undergoing CRS-HIPEC based on insurance status.
Materials and Methods
Data Sources and Definitions
The present study was approved by Institutional Review Board of Vanderbilt University (IRB # 200638) and utilizes a retrospectively created database of all patients with PC who underwent CRS-HIPEC with curative intent from 2013–2019 at Vanderbilt University Medical Center (Nashville, TN). We utilized this prospectively maintained database to collect patient demographic information, such as gender, age, race, BMI, state residence, American Society of Anesthesiologists (ASA) class, and Eastern Cooperative Oncology Group (ECOG) performance status as well at treatment information. Oncologic data, such as tumor origin and grade, lymphovascular invasion, and perineural invasion were based on final pathology report from the surgical specimen at the time of CRS-HIPEC. Additionally, the peritoneal surface disease severity score (PSDSS), peritoneal carcinomatosis index (PCI), and complete cytoreduction score (CCR) were obtained from the surgeon’s CRS-HIPEC operative note.
Patients were categorized into two groups based on their insurance status. Patients with solely commercial private insurance at the time of CRS-HIPEC were included within in the insured group (PI). Patients who maintained their previously obtained private insurance after receiving government benefits or who supplemented their Medicare benefits with private insurance were included in the insured group. The underinsured group (UI) consisted of patients with documentation of no insurance, or proof of Medicaid with or without Medicare at the time of CRS-HIPEC. Since about half of Medicare beneficiaries quality for Medicaid, which serves low-income patients, this cohort is comprised of patients with socioeconomic characteristics that prevent them from purchasing supplemental or sole private insurance11. In addition, patients who had evidence of loss of insurance during the follow-up period or had no documentation of insurance status were excluded from this study. To further understand each group’s geographic access to care, distance traveled was derived by using the estimated driving distance from the centroid of the zip code listed in the patient’s primary residence to the centroid of the zip code of this institution. The primary outcome was overall survival (OS) defined as time (months) from CRS-HIPEC to death. The secondary outcomes included follow-up time, defined as time (months) from CRS-HIPEC to last known follow-up with a surgeon or oncologist, or death, as well as intensive care unit length of stay (ICU LOS), hospital length of stay, and rate of Clavien-Dindo classification III/IV complication.
Statistical Analysis
Demographic and oncologic factors were compared based on insurance status as previously defined. Categorical variables are recorded as percentages compared using Chi-squared test, and continuous variables are recorded as means and compared using Kruskal-Wallis test. OS was calculated using the Kaplan-Meier method and groups were compared using the log-rank test. Multivariate regression analysis of factors associated with overall survival, including insurance status, ASA class, tumor grade, PCI, and CCR score was performed using Cox regression analysis. All analyses were performed using IBM Statistical Product and Service Solutions for Mac, Version 27 (IBM Corp., Armonk, N.Y., USA) software package and statistical significance was set at p=0.05.
Results
We identified 125 patients in the database who underwent CRS-HIPEC from 2013–2019, all of whom had documented insurance status. Overall, 51% were male and median age was 50.5 years. The median overall survival was 50.2 months and median follow-up time was 36.9 months for the entire patient population. Of the patients included in our analysis, 82.3% (102) were in the privately insured group (PI) and 17.7% (22) were in the underinsured group (UI). The median age of patients in the PI and UI cohorts were 52.6 years and 59.7 years, respectively. When comparing demographic and oncologic characteristics between the two groups, no significant differences were seen in gender, age, race, BMI, ASA class, ECOG performance status, state residence, PSDSS, Charlson comorbidity index, primary tumor location, or tumor grade. However, patients in the UI group were more likely to have primary tumors with lymphovascular invasion than their counterparts in the PI group (52.2% vs 19.6%, p = 0.003). Data on patient demographics and pathologic features are outlined in Table 1.
Table 1:
Demographics and Oncologic Characteristics among Insured and Underinsured Patients
| Variables | Insured % (n) | Underinsured % (n) | p Value |
|---|---|---|---|
| Total | 82.3% (102) | 17.7% (22) | |
| Gender | |||
| Male | 51% (52) | 50.0% (11) | 0.91 |
| Female | 49% (50) | 50.0% (11) | |
| Age (median) | 52.6 years | 57.7 years | 0.29 |
| Race | 0.49 | ||
| White | 86.2% (88) | 90.9% (20) | |
| Black | 12.7% (13) | 4.3% (1) | |
| Other | 1% (1) | 4.3% (1) | |
| BMI (mean ± std) | 28.9 ± 3.2 | 29.2 ± 5.3 | 0.93 |
| In-State Residence | 67.6% (69) | 69.1% (15) | 0.83 |
| Miles Travelled (mean ± std) | 127.4 ± 95.5 | 112.6 ± 89.1 | 0.5 |
| ASA Class | 0.43 | ||
| 1 | 1.0% (1) | 0% (0) | |
| 2 | 15.7% (16) | 22.7% (5) | |
| 3 | 80.4% (82) | 68.2% (15) | |
| 4 | 2.9% (3) | 9.1% (2) | |
| ECOG Performance Status | 0.20 | ||
| 0 | 29.4% (30) | 36.4% (8) | |
| 1 | 32.4% (33) | 22.7% (5) | |
| 2 | 2% (2) | 0% (0) | |
| 3 | 0% (0) | 4.5% (1) | |
| Unknown | 36.2% (37) | 36.4% (8) | |
| Peritoneal Surface Disease Severity Score (mean ± std) |
7.0 ± 2.1 | 7.8 ± 4.2 | 0.10 |
| Charlson Comorbidity Index (mean ± std) | 7.3 ± 1.4 | 8.6 ± 20 | 0.12 |
| Synchronous Disease | 79.2% (80) | 81.8% (18) | 0.80 |
| Primary Colorectal Tumor | 25.5% (26) | 40.9% (9) | 0.15 |
| Primary Appendiceal Tumor | 58.8% (60) | 54.5% (12) | 0.82 |
| Primary Peritoneal Mesothelioma | 7.8% (8) | 0% (0) | 0.19 |
| Primary Ovarian Tumor | 3.8% (4) | 4.5% (1) | 0.61 |
| Primary Other Tumor | 4.9% (5) | 0% (0) | 0.66 |
| Tumor Grade | 0.68 | ||
| High-grade | 27.7% (24) | 27.3% (6) | |
| Intermediate Grade | 19.1% (16) | 27.3% (6) | |
| Low Grade | 53.2% (46) | 45.4% (10) | |
| Lymphovascular Invasion | 19.6% (20) | 50% (12) | 0.001 |
| Perineural Invasion | 10.9% (11) | 22.7% (5) | 0.04 |
When compared to PI patients, patients in the UI group had increased estimated blood loss (760mL vs 550mL; p=0.04) and had slightly increased PCI score, but did not reach statistical significance (19.5 vs 14.8, p=0.07). Patients in the UI group had a significantly higher rate of postoperative admission to the intensive care unit (26.1% vs 13%; p=0.03), longer intensive care unit length of stay (7.5 days vs 4.25 days; p=0.04) and hospital length of stay (16.0 days vs 12.0 days; p=0.002). These patients were more likely to be discharged to a skilled nursing facility or inpatient rehab when compared to PI patients (26.1% vs 2.9%; p <0.001) and had an increased rate of Clavien-Dindo classification III or IV complications within 30 postoperative days (56.5% vs 19.6%; p = 0.002). Median follow-up duration after index CRS-HIPEC was significantly shortened in the UI group [25.1 months interquartile range (IQR) 16.5 – 33.7 months)] compared to the PI group ( 51.6 months, IQR 40.2 – 63.0 months)(p <0.001). Patients in both groups had similar rates of neoadjuvant and adjuvant chemotherapy, number of cycles received, and rate of chemotherapy regimen completion. UI patients had significantly shortened overall survival (48.9 months, IQR 44.5 – 53.3 months) and disease-free survival (12.2 months, IQR 8.7 – 15.7 months) compared to patients in the PI group (OS 64.8 months, IQR 48.5 – 80.1 months ; p=0.007) (DFS 24.9 months, IQR 21.2 – 28.6 months; p=0.02). Perioperative and follow-up data for patients in each group are summarized in Table 2.
Table 2:
Perioperative Characteristics Among Insured and Underinsured patients
| Variables | Insured % (n) | Underinsured % (n) | p Value |
|---|---|---|---|
| Total | 82.2% (102) | 17.8% (22) | |
| Peritoneal Carcinomatosis Index (mean ± std) | 14.8 ± 9.3 | 18.9 ± 11.2 | 0.09 |
| Completion of Cytoreduction Score | 0.44 | ||
| 0 | 87% (89) | 77.3% (17) | |
| 1 | 13% (13) | 22.7% (5) | |
| Operative Time (mean ± std) | 588.5 ± 134.2 minutes | 639.5 ± 16.1 minutes | 0.14 |
| Estimated Blood Loss (mean ± std) | 550 ± 125 mL | 718 ± 161 mL | 0.04 |
| Intensive Care Unit Admission | 9.8% (10) | 27.3% (6) | 0.02 |
| ICU Length of Stay (mean ± std) | 4.25 ± 6.7 days | 7.7 ± 3.57days | 0.03 |
| Hospital Length of Stay (mean ± std) | 12.0 ± 5.9 days | 16.3 ± 7.2 days | 0.002 |
| Discharge to home | 97.1% (99) | 72.7% (16) | <0.001 |
| Clavien-dindo III/IV Complication | 19.6% (20) | 59.1% (13) | 0.001 |
| Neoadjuvant Chemotherapy | 52.9% (54) | 50% (11) | 0.88 |
| Number of Cycles (mean ± std) | 9.5 ± 2.4 | 9.6 ± 2.5 | 0.9 |
| Adjuvant Chemotherapy | 19.6% (20) | 27.3% (6) | 0.37 |
| Number of Cycles (mean ± std) | 6.8 ± 0.7 | 7.1 ± 0.4 | 0.9 |
| Completion of Chemotherapy Regimen | 90.5% (67) | 63.6% (14) | 0.45 |
| Follow-up time (median) | 51.6 months | 28.1 months | <0.001 |
| Overall Survival (median) | 64.8 months | 52.9 months | 0.01 |
| Disease-free Survival (median) | 24.9 months | 13.2 months | 0.03 |
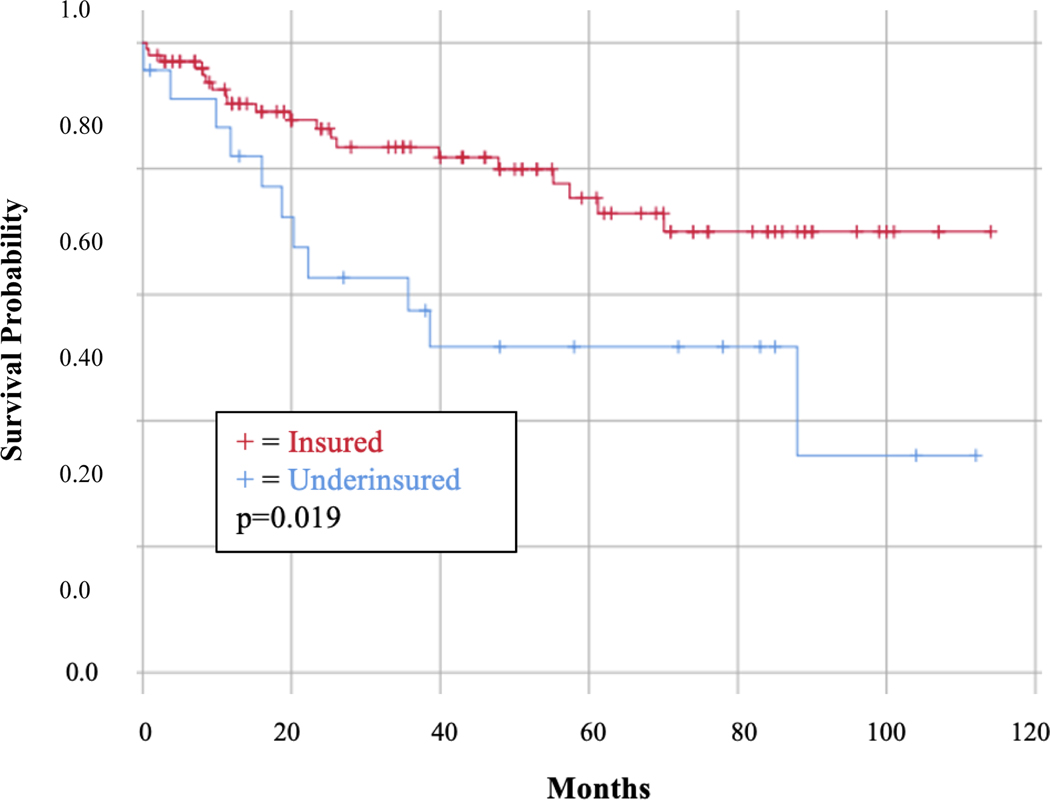
Additionally, the 5-year OS was significantly lower for UI patients than PI patients (52.4% vs 67.2%; p=0.015) as displayed in Figure 1. In a Cox multivariate analysis controlling for PCI, CCR >0, high tumor grade, and ASA class ≥ 3, being underinsured was independently associated with worsened OS in patients with peritoneal carcinomatous undergoing CRS-HIPEC [HR 1.42, 95% CI 0.17–0.82; p=0.03), which is summarized in Table 3.
Figure 1:
Overall Survival by Insurance Status
Table 3:
Cox Multivariate regression analysis of factors associated with overall survival
| Variables | HR | 95% CI | p Value |
|---|---|---|---|
| Underinsured | 1.49 | 0.20 – 0.85 | 0.02 |
| Peritoneal Carcinomatosis Index | 0.04 | 0.97 – 1.09 | 0.64 |
| Completion of Cytoreduction Score > 0 | 0.14 | 0.19 – 3.82 | 0.85 |
| High Tumor Grade | 1.1 | 0.19 – 0.70 | 0.002 |
| ASA Class ≥ 3 | 0.23 | 0.22 – 2.85 | 0.72 |
Discussion
tTo date, this is the largest examination of the impact of insurance status on outcomes for patients undergoing CRS-HIPEC. The disparities identified are almost exclusively related to perioperative and oncologic outcomes, rather than disease severity at presentation. This is in contrast to previous studies demonstrating that uninsured patients and patients with low socioeconomic status have increased medical comorbidities and present with advanced disease3–5,7,9,12,13. In our single-institution cohort, there were no significant differences in age, BMI, ASA class, ECOG performance status, Charlson comorbidity index, or prevalence of synchronous disease at presentation. UI patients did have a slightly higher PSDSS score , which is comprised of PCI, tumor grade, and patient symptoms, which suggest more symptomatic disease or delayed presentation. Pathologic tumor assessment showed similar tumor grades between the two groups, but UI patients were more likely to have lymphovascular invasion, suggesting a more aggressive tumor biology. In this study where all patients underwent surgery for peritoneal carcinomatosis, which is a late-stage form of cancer, such baseline disparities may not be apparent. It is likely that patients who are referred to specialized centers that perform CRS-HIPECs have similar medical comorbidities that do not pose prohibitive operative risk regardless of insurance status.
In the United States, it is estimated that 14.7% of the adult population is uninsured and 20.4% have public insurance, yielding approximately 73 million people who self-pay for medical expenses or receive insurance coverage through a state of federal government-subsidized policy14. Several studies have demonstrated that uninsured patients have inadequate access to medical care across several medical specialties and thus have disparate outcomes when compared to insured patients6,15–19. Such disparities have been demonstrated in chronic diseases as well as cancer, including breast, colorectal, head, neck, and gynecologic malignancies6,13,20–23. Specifically, among uninsured cancer patients, significant delays in access to care and completion of recommended therapy have detrimental effects on prognosis and cancer-related survival. While health insurance coverage is an important factor to health access in this country, coverage does not necessarily translate to access. Healthcare providers are less likely to accept public insurance compared to private insurance, particularly in rural areas and can pose increasing challenges for those seeking specialty care24,25. While Medicaid provides health care coverage for many low-income families, sequala of low socioeconomic status including lack of transportation, food insecurity, and competing priorities pose further barriers. It is important to note that significantly disparate oncologic outcomes were observed between the two groups despite no estimated difference in distance travelled, suggesting that geographic access to care is not necessarily a limited factor for underinsured patients. Strikingly, while patients in the underinsured group had significantly shorter follow-up time, they underwent similar number of rounds of adjuvant chemotherapy and completed their course at a similar rate. In this context, these findings suggest that competing priorities that are associated with specific socioeconomic factors are also associated with lack of insurance or government subsidized insurance regardless of geographic location.
Additionally, Medicare beneficiaries, who are older and have chronic medical conditions, typically have incomes less than twice the federal poverty level and almost 25% of beneficiaries live below the poverty line26. Previous studies have demonstrated different outcomes based on primary payer status across a variety of clinical settings including cancer and surgery, with patients on Medicaid and/or Medicare performing worse than privately insured patients5,13,20,21. For these reasons, the population of focus for this study was patients who relied on self-pay, Medicaid, or Medicare for their healthcare expenses, which we classified as underinsured.
There are several limitations in this study. While this is the largest study examining the impact of insurance status on outcomes after CRS-HIPEC, this is a retrospective analysis from a single institution in Tennessee. Thus, there may be several unidentified factors that are unique to patients in Tennessee, particularly regarding income, housing costs, social support, and transportation. We lack data regarding socioeconomic status, which may be the underlying common denominator associated with underinsurance status and worse outcomes and shortened follow-up time. However, given that Medicaid beneficiaries are typically low-income and more than 20% of Medicare beneficiaries are low-income, our methods have likely identified patients with low-income as part of the UI group. Additionally, since CRS-HIPECs are performed at specialized centers, there is likely a referral bias from providers in the community. Underinsured patients are known to have more difficult access to specialized care and have more medical comorbidities. Thus, they may never be referred to a center familiar with CRS-HIPEC by their community provider, which may explain the fairly homogenous PI and UI groups seen in this cohort.
Specific targeted interventions taken by our institution and others can help broaden access to the specialized care that patients with peritoneal carcinomatosis require. Educational outreach initiatives aimed at informing non-surgical and surgical providers, particularly in rural or community settings, may expand a referral base prevent patients from being denied a potential CRS-HIPEC before entering our institution. Furthermore, forming and acquiring satellite clinics and medical centers that are aligned with a centralized specialized or academic center, sometimes referred to as a “hub-and-spoke” model, facilities the consolidation of resources and streamlines care that increases access to multidisciplinary and specialized treatment.
Underinsurance status, as defined as the use of self-pay, Medicaid, or Medicare, is associated with worse overall survival in patients with peritoneal carcinomatosis undergoing CRS-HIPEC, in a multivariate analysis. Additionally, underinsurance status was associated with worse perioperative outcomes. As the landscape of health insurance continues to evolve in the United States, disparate outcomes should continue to be identified with the goal of improving outcomes for vulnerable patient populations.
Synopsis.
Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy is performed at specialized cancer centers, which pose challenges for underinsured patients. This retrospective study demonstrates that underinsured patients have worse overall survival, increased postoperative morbidity, and shorter follow-up times compared to privately insured patients.
Acknowledgements
We would like to acknowledge our funding source, the National Cancer Institute (T32CA106183).
Footnotes
Disclosures: None
References
- 1.Dehal A, Smith JJ, Nash GM. Cytoreductive surgery and intraperitoneal chemotherapy: an evidence-based review-past, present and future. J Gastrointest Oncol 2016;7:143–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auer RC, Sivajohanathan D, Biagi J, Conner J, Kennedy E, May T. Indications for hyperthermic intraperitoneal chemotherapy with cytoreductive surgery: a clinical practice guideline. Curr Oncol 2020;27:146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niu X, Roche LM, Pawlish KS, Henry KA. Cancer survival disparities by health insurance status. Cancer Med 2013;2:403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robbins AS, Pavluck AL, Fedewa SA, Chen AY, Ward EM. Insurance status, comorbidity level, and survival among colorectal cancer patients age 18 to 64 years in the National Cancer Data Base from 2003 to 2005. J Clin Oncol 2009;27:3627–33. [DOI] [PubMed] [Google Scholar]
- 5.Walker GV, Grant SR, Guadagnolo BA, et al. Disparities in stage at diagnosis, treatment, and survival in nonelderly adult patients with cancer according to insurance status. J Clin Oncol 2014;32:3118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fedewa SA, Lerro C, Chase D, Ward EM. Insurance status and racial differences in uterine cancer survival: a study of patients in the National Cancer Database. Gynecol Oncol 2011;122:63–8. [DOI] [PubMed] [Google Scholar]
- 7.Kwok J, Langevin SM, Argiris A, Grandis JR, Gooding WE, Taioli E. The impact of health insurance status on the survival of patients with head and neck cancer. Cancer 2010;116:476–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slatore CG, Au DH, Gould MK, American Thoracic Society Disparities in Healthcare G. An official American Thoracic Society systematic review: insurance status and disparities in lung cancer practices and outcomes. Am J Respir Crit Care Med 2010;182:1195–205. [DOI] [PubMed] [Google Scholar]
- 9.Halpern MT, Ward EM, Pavluck AL, Schrag NM, Bian J, Chen AY. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncol 2008;9:222–31. [DOI] [PubMed] [Google Scholar]
- 10.Chokshi RJ, Kim JK, Patel J, Oliver JB, Mahmoud O. Impact of insurance status on overall survival after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (CRS-HIPEC). Pleura Peritoneum 2020;5:20200105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Data Anaysis Brief: Medicare-Medicaid Dual Enrollment 2006 – 2018. 2019. (Accessed 1, at chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/viewer.html?pdfurl=https%3A%2F%2Fwww.cms.gov%2FMedicare-Medicaid-Coordination%2FMedicare-and-Medicaid-Coordination%2FMedicare-Medicaid-Coordination-Office%2FDataStatisticalResources%2FDownloads%2FMedicareMedicaidDualEnrollmentEverEnrolledTrendsDataBrief2006–2018.pdf&clen=676901&chunk=true.)
- 12.Esnaola NF, Gebregziabher M, Finney C, Ford ME. Underuse of surgical resection in black patients with nonmetastatic colorectal cancer: location, location, location. Ann Surg 2009;250:549–57. [DOI] [PubMed] [Google Scholar]
- 13.Parikh AA, Robinson J, Zaydfudim VM, Penson D, Whiteside MA. The effect of health insurance status on the treatment and outcomes of patients with colorectal cancer. J Surg Oncol 2014;110:227–32. [DOI] [PubMed] [Google Scholar]
- 14.Cha AE, Cohen RA. Reasons for Being Uninsured Among Adults Aged 18–64 in the United States, 2019. NCHS Data Brief 2020:1–8. [PubMed] [Google Scholar]
- 15.Ahmad TR, Chen LM, Chapman JS, Chen LL. Medicaid and Medicare payer status are associated with worse surgical outcomes in gynecologic oncology. Gynecol Oncol 2019;155:93–7. [DOI] [PubMed] [Google Scholar]
- 16.Bleicher RJ, Ruth K, Sigurdson ER, et al. Preoperative delays in the US Medicare population with breast cancer. J Clin Oncol 2012;30:4485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bradley CJ, Given CW, Dahman B, Fitzgerald TL. Adjuvant chemotherapy after resection in elderly Medicare and Medicaid patients with colon cancer. Arch Intern Med 2008;168:521–9. [DOI] [PubMed] [Google Scholar]
- 18.Dobie SA, Baldwin LM, Dominitz JA, Matthews B, Billingsley K, Barlow W. Completion of therapy by Medicare patients with stage III colon cancer. J Natl Cancer Inst 2006;98:610–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farkas DT, Greenbaum A, Singhal V, Cosgrove JM. Effect of insurance status on the stage of breast and colorectal cancers in a safety-net hospital. J Oncol Pract 2012;8:16s–21s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LaPar DJ, Bhamidipati CM, Mery CM, et al. Primary payer status affects mortality for major surgical operations. Ann Surg 2010;252:544–50; discussion 50–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livingston EH, Langert J. The impact of age and Medicare status on bariatric surgical outcomes. Arch Surg 2006;141:1115–20; discussion 21. [DOI] [PubMed] [Google Scholar]
- 22.Ward EM, Fedewa SA, Cokkinides V, Virgo K. The association of insurance and stage at diagnosis among patients aged 55 to 74 years in the national cancer database. Cancer J 2010;16:614–21. [DOI] [PubMed] [Google Scholar]
- 23.Martin S, Ulrich C, Munsell M, Taylor S, Lange G, Bleyer A. Delays in cancer diagnosis in underinsured young adults and older adolescents. Oncologist 2007;12:816–24. [DOI] [PubMed] [Google Scholar]
- 24.Decker SL. Two-thirds of primary care physicians accepted new Medicaid patients in 2011–12: a baseline to measure future acceptance rates. Health Aff (Millwood) 2013;32:1183–7. [DOI] [PubMed] [Google Scholar]
- 25.Ward E, Halpern M, Schrag N, et al. Association of insurance with cancer care utilization and outcomes. CA Cancer J Clin 2008;58:9–31. [DOI] [PubMed] [Google Scholar]
- 26.Schoen C, Davis K, Willink A. Medicare Beneficiaries’ High Out-of-Pocket Costs: Cost Burdens by Income and Health Status. Issue Brief (Commonw Fund) 2017;11:1–14. [PubMed] [Google Scholar]