Abstract
Autoimmune hepatitis (AIH) is a chronic, autoimmune disease of the liver that occurs when the body’s immune system attacks liver cells, causing the liver to be inflamed. AIH is one of the manifestations of a coronavirus disease 2019 (COVID-19), as well as an adverse event occurring after vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Few cases of AIH have been described after vaccination with two messenger RNA (mRNA)-based vaccines—BTN162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna)—against SARS-CoV-2. Herein, we report a case of AIH occurring after Pfizer-BioNTech COVID-19 vaccine. A 27-year-old female presented with jaundice and hepatomegaly, appearing 14 days after receiving the second dose of Pfizer-BioNTech vaccine. Her laboratory results showed abnormal liver function with high total immunoglobulin G level. She was diagnosed with AIH with histologic finding and successfully treated with oral prednisolone. We report an AIH case after COVID-19 vaccination in Korea.
Keywords: Coronavirus Disease 2019, Vaccine, Autoimmune Hepatitis
Graphical Abstract
INTRODUCTION
The coronavirus disease 2019 (COVID-19) pandemic, putatively caused by the widespread transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has resulted in 257,469,528 laboratory-confirmed cases of infection and 5,158,211 deaths globally as of November 28, 2021.1 Rapid vaccine development, however, has significantly mitigated severe COVID-19 illness. Two messenger RNA (mRNA) COVID-19 vaccines, BNT162b2 (Pfizer-BioNTech, New York, NY, USA/Mainz, Germany) and mRNA-1273 (Moderna, Cambridge, MA, USA), were granted emergency use authorization by the United States Food and Drug Administration in December 2020. SARS-CoV-2 infection has been associated with the development of autoimmune processes.2 Because SARS-CoV-2 harbors the same protein motif the mRNA vaccine codes for, it is plausible that these vaccines could trigger autoimmune diseases in predisposed patients.3,4 Autoimmune hepatitis (AIH) is a polygenic multifactorial disease that may be triggered by specific environmental factors, such as viral infections, resulting in the loss of self-tolerance to autoantigens in genetically susceptible individuals.5
CASE DESCRIPTION
We treated a 27-year-old female nurse who developed AIH after COVID-19 vaccination. She had no known history of liver disease and did not use herbal remedies or alcohol. She received a second dose of the Pfizer-BioNTech COVID-19 vaccine on March 30, 2021, and, since April 6, 2021, symptoms of nausea, vomiting, headache, fever, and dark urine continued. Accordingly, she was hospitalized via the emergency room 14 days after COVID-19 vaccination. A COVID-19 polymerase chain reaction test, performed at the local hospital on April 7 and 12, 2021, was negative. The physical examination was unremarkable, except for scleral icterus, jaundice, and palpable hepatomegaly. In the emergency room, laboratory investigations were significant for the following: bilirubin, 8.6 mg/dL; aspartate aminotransferase (AST), 1,004 U/L; alanine aminotransferase (ALT), 1,478 U/L; alkaline phosphatase, 182 U/L, white blood cell count, 6,720/μL (neutrophils, 46.8%); hemoglobin, 13.0 g/dL; platelet count 373,000/μL; blood urea nitrogen/creatinine, < 5.0/0.54 mg/dL (estimated glomerular filtration rate, 145.0 mL/min/1.73 m2); and prothrombin international normalized ratio, 1.1. Laboratory results were negative for hepatitis A, B, C, and E, Epstein-Barr virus, cytomegalovirus, herpes simplex virus types 1 and 2, and human immunodeficiency virus. Antinuclear antibody (ANA) was positive (1:80; mixed pattern). Other antibodies (including anti-mitochondrial, anti-smooth muscle, liver-kidney microsomal, and antineutrophil cytoplasmic antibodies) were negative. Total immunoglobulin G (IgG) level was 1,641 mg/dL (normal range, 549–1,584 mg/dL). Ceruloplasmin, transferrin saturation, thyroid function test, and serum protein electrophoresis were all normal. Abdominal ultrasound revealed splenomegaly (12.5 cm) without cirrhosis and gallbladder wall thickening.
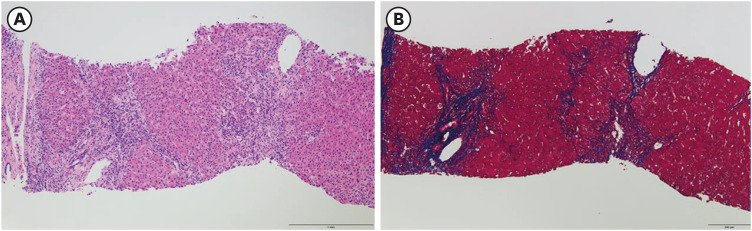
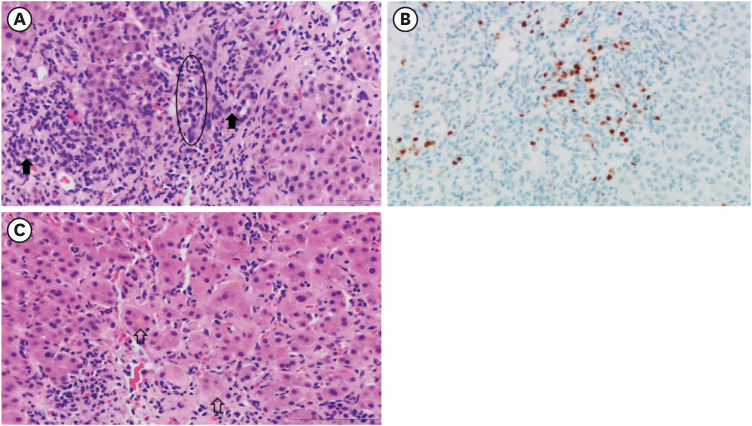
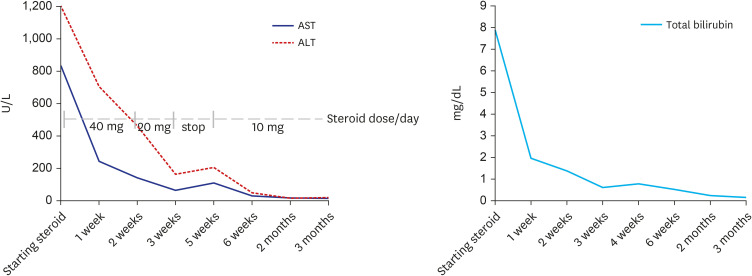
Liver dynamic computed tomography revealed no evidence of biliary lithiasis or biliary dilation, and ultrasound-guided transabdominal liver biopsies were obtained. In microscopic examination, 17 portal tracts were identified. Although there was a focal bridging, the overall lobular architecture was preserved in the low magnification. Some portal tracts were widened by moderate inflammation with periportal fibrosis (Fig. 1). The portal inflammation was composed of mainly lymphocytes, clusters of plasma cells and few eosinophils, extending into proto-lobular interface (interface hepatitis) (Fig. 2A). The immunohistochemical staining for plasma cell markers, MUM1 and CD138 confirmed significant plasma cell infiltration in portal tracts as well as in lobules (Fig. 2B). Diffuse moderate necroinflammatory damage in lobules, associated with perivenular hepatocytes degeneration, mild cholestasis with hepatocytic rosettes (Fig. 2C) and sinusoidal inflammation were found. Other than COVID-19 vaccination, no other drug, herbal supplement, or toxin use were reported by the patient. The revised original score for AIH pretreatment was 18 (results > 15 suggest definite AIH). Treatment with oral prednisolone (40 mg daily) was initiated. Plasma ALT, AST, and total bilirubin levels over time, and before and after treatment, are summarized in Fig. 3. After three weeks of treatment, diarrhea and fever developed, and she was transferred to the hospital’s emergency room. Treatment with prednisolone (20 mg daily) was discontinued with the diagnosis of enteritis. Four days after admission, symptoms were relieved, and she was discharged from hospital with steroid discontinuation. Two weeks after stopping therapy, there were biochemical signs of an AIH relapse; therefore, treatment with oral prednisolone (10 mg daily) was restarted. Liver enzyme levels were completely normalized and the patient’s symptoms significantly improved.
Fig. 1. The microphotograph of low magnification of liver biopsy shows portal widening with periportal fibrosis (A) hematoxylin and eosin ×100, (B) Masson trichrome ×100.
Fig. 2. Histological finding. (A) The porto-lobular interface shows severe inflammation composed of lymphocytes, clausters of plasma cells (circle) and a few eosinophils. Bile ducts (closed arrow) are not damaged (H&E, ×400). (B) The photomicrography of MUM1 immunohistochemical stain demonstrates numerous plasma cell infiltration (×400). (C) The lobules show diffuse degeneration of hepatocytes, mild cholestasis in hepatocytic rosettes (opened arrow) and sinusoidal lymphoplasma cells infiltration (H&E ×400).
H&E = hematoxylin and eosin.
Fig. 3. Trends of serum ALT, AST and total bilirubin over time.
ALT = alanine aminotransferase, AST = aspartate aminotransferase.
Ethics statement
This report was approved by the Institutional Review Board of Wonju Severance Christian Hospital, and the requirement for informed consent was waived (subject number: CR320172).
DISCUSSION
We described a case of AIH that developed in a patient after vaccination with the Pfizer-BioNTech COVID-19 vaccine, which was resolved with steroid treatment. To date, four cases of AIH have been reported after Pfizer-BioNTech COVID-19 vaccination in the literature (Table 1)6,7,8, the first of which was reported by Bril et al.3 The patient was a 35-year-old woman in her third month postpartum who developed AIH after COVID-19 vaccination. In this case, AIH exhibited some atypical features: autoantibodies other than ANA were negative and eosinophils were present on liver histology. Similarly, Lodato et al.6 reported a case of AIH occurring after vaccination, with no development of autoantibodies and eosinophil infiltrate in liver histology. Thereafter, two patients had a history of Hashimoto’s disease, high IgG levels, and typical findings on biopsy, unlike the above cases.7,8 Although our patient had no autoimmune disease, autoantibodies were positive, and IgG level was high. In addition, our case had typical findings on biopsy and responded well to steroid therapy. It is thought to be a new-onset AIH triggered by COVID-19 vaccination, but periportal fibrosis was observed in histological examination. However, in case with acute onset, there may be minimal fibrosis. Moreover, symptoms developed after the second vaccination in this patient, but there is a possibility that inflammation may have occurred even though there were no symptoms after the first vaccination.
Table 1. Characteristics of patients with autoimmune hepatitis after Pfizer-BioNTech COVID-19 vaccine.
| Study | Sex | Age | Autoimmune history | Onset time | Antibodies | IgG | Biopsy | Steroid response |
|---|---|---|---|---|---|---|---|---|
| Bril et al.3 | Female | 35 | None | 1 week after 1st vaccination | ANA | Normal | Compatible | Yes |
| Lodato et al.6 | Female | 43 | None | 2 days after completing vaccination | Negative | Normal | Compatible | Yes |
| Rocco et al.7 | Female | 80 | Hashimoto disease | 1 week after completing vaccination | ANA | High | Compatible | Yes |
| Avci et al.8 | Female | 61 | Hashimoto disease | 1 month after 1st vaccination | ANA | High | Compatible | Yes |
COVID-19 = coronavirus disease 2019, IgG = immunoglobulin G, ANA = antinuclear antibody.
Because causality cannot be definitively confirmed, it is possible that this association was coincidental. However, severe cases of SARS-CoV-2 infection are characterized by autoinflammatory dysregulation.9 Because the viral spike protein appears to be responsible, it is plausible that spike-directed antibodies induced by vaccination may also trigger autoimmune conditions in predisposed individuals.10 In support of this, several cases of immune thrombocytopenia have been reported days after COVID-19 vaccination. Vaccines protect the host from the virus by inducing antibody generation against viral peptides.11 Autoimmunity can develop due to cross-reactivity to the generated antibodies. The epitopes used for induction of the host immune system may mimic the structure of self-peptides, and antibodies that develop after vaccination may cause cross-reactivity directed to the self.12
Given the close temporal relationship between vaccination and onset of symptoms, we hypothesized that vaccination against COVID-19 could have triggered the development of AIH in our patient. To the best of our knowledge, this is the first reported episode of AIH that developed post-COVID-19 vaccination in Korea. Whether a causal relationship exists between COVID-19 vaccination and the development of AIH remains to be determined. Nevertheless, it is necessary to raise awareness about potential side effects that will likely emerge as more individuals are vaccinated.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Kang SH.
- Data curation: Kang SH, Cho MY.
- Formal analysis: Kang SH, Cho MY.
- Investigation: Kang SH.
- Visualization: Kang SH.
- Writing - original draft: Kang SH, Cho MY, Kim MY.
- Writing - review & editing: Kang SH, Kim MY, Cho MY, Baik SK.
References
- 1.World Health Organization. Global surveillance for COVID-19 caused by human infection with COVID-19 virus: interim guidance 2020. [Updated 2020]. [Accessed November 28, 2021]. https://apps.who.int/iris/handle/10665/331506 .
- 2.Liu Y, Sawalha AH, Lu Q. COVID-19 and autoimmune diseases. Curr Opin Rheumatol. 2021;33(2):155–162. doi: 10.1097/BOR.0000000000000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bril F, Al Diffalha S, Dean M, Fettig DM. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: causality or casualty? J Hepatol. 2021;75(1):222–224. doi: 10.1016/j.jhep.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee EJ, Cines DB, Gernsheimer T, Kessler C, Michel M, Tarantino MD, et al. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am J Hematol. 2021;96(5):534–537. doi: 10.1002/ajh.26132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Czaja AJ. Autoimmune liver disease. Curr Opin Gastroenterol. 2004;20(3):231–240. doi: 10.1097/00001574-200405000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Lodato F, Larocca A, D’Errico A, Cennamo V. An unusual case of acute cholestatic hepatitis after m-RNABNT162b2 (Comirnaty) SARS-CoV-2 vaccine: coincidence, autoimmunity or drug-related liver injury. J Hepatol. 2021;75(5):1254–1256. doi: 10.1016/j.jhep.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rocco A, Sgamato C, Compare D, Nardone G. Autoimmune hepatitis following SARS-CoV-2 vaccine: may not be a casuality. J Hepatol. 2021;75(3):728–729. doi: 10.1016/j.jhep.2021.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avci E, Abasiyanik F. Autoimmune hepatitis after SARS-CoV-2 vaccine: new-onset or flare-up? J Autoimmun. 2021;125:102745. doi: 10.1016/j.jaut.2021.102745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehrenfeld M, Tincani A, Andreoli L, Cattalini M, Greenbaum A, Kanduc D, et al. COVID-19 and autoimmunity. Autoimmun Rev. 2020;19(8):102597. doi: 10.1016/j.autrev.2020.102597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vojdani A, Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020;217:108480. doi: 10.1016/j.clim.2020.108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sadarangani M, Marchant A, Kollmann TR. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat Rev Immunol. 2021;21(8):475–484. doi: 10.1038/s41577-021-00578-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malonis RJ, Lai JR, Vergnolle O. Peptide-based vaccines: current progress and future challenges. Chem Rev. 2020;120(6):3210–3229. doi: 10.1021/acs.chemrev.9b00472. [DOI] [PMC free article] [PubMed] [Google Scholar]