Abstract
From 2019, life in the world has mainly been determined by successive waves of the COVID-19 epidemic. During this time, the virus structure, action, short- and long-term effects of the infection were discovered, and treatments were developed. This epidemic undoubtedly affected people's lives, but increasing attention is also being paid to the effects of the epidemic on the environment. Following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines, a global scoping review of peer-reviewed information has been conducted on the use of over-the-counter non-steroidal anti-inflammatory drugs in the treatment of symptoms of SARS-CoV-2 infections and their positive and negative effects on the human body, the effects of non-steroidal anti-inflammatory drugs (NSAIDs) on aquatic organisms, and their adverse effects on non-target organisms. The literature from 1998 to 2021 was analysed using the Scopus®, Web of Science™ (WoS) and Google Scholar databases. As non-steroidal anti-inflammatory drugs place a heavy burden on the environment, all reports of the presence of these drugs in the environment during the pandemic period have been thoroughly analysed. Of the 70 peer-reviewed records within the scope, only 14% (n = 10) focussed on the analysis of non-steroidal anti-inflammatory drugs concentrations in wastewater and surface waters during the pandemic period. The percentage of these works indicates that it is still an open topic, and this issue should be supplemented with further reports in which the results obtained during the pandemic, which has been going on for several years, will be published. The authors hope this review will inspire scientists to investigate the problem of non-steroidal anti-inflammatory drugs in the environment to protect them for the next generation.
Keywords: NSAIDs, SARS-CoV-2, Toxicity, Organisms, Pollution
Graphical abstract
1. Introduction
In December 2019, the first cases of the disease caused by severed acute respiratory syndrome coronavirus 2 (SARS-CoV-2) appeared in Wuhan, China. In the first months of 2020, over 200 people had already been affected by this disease in countries around the world. Initially, the pandemic hit the United States, Spain and Italy the most (Yunus et al., 2020). However, the pandemic effects were reflected not only in the economy and social relationships but also in the environment. The latter is widely commented on in the scientific literature, both in the positive aspect (reduction of greenhouse gas emissions, suspended particulate matter) and in the negative aspect (pollution of wastewater with anti-inflammatory and anti-viral drugs, personal protective equipment, etc.) (Rupani et al., 2020; Shakil et al., 2020; Usman et al., 2020; Yunus et al., 2020). For example, in China, carbon dioxide emissions decreased by 25% during the lockdown, which is about 1 million tonnes of carbon dioxide less than the same period in the previous year. Simultaneously, hospitals generated 240 tonnes of medical waste per day in this city compared to the 50 tonnes per day in the pre-COVID-19 period (Shakil et al., 2020). In COVID-19 therapy, apart from dedicated antiviral drugs, auxiliary drugs, including NSAIDs, have been used. The latter are widely used in outpatient treatment. The results of clinical trials indicate that the use of NSAIDs in therapies is relatively safe, and that is how they function in the social consciousness. However, many reports in the literature indicate that exposure to these drugs by non-target organisms can lead to a wide range of side effects, ranging from behavioural changes through damage to internal organs and developmental disorders (Madikizela and Ncube, 2021; Wojcieszyńska and Guzik, 2020). During epidemics an increasing burden of bioactive substances in wastewater treatment plants is observed (Nason et al., 2021; Praveenkumarreddy et al., 2021). These substances usually are not completely degraded, and along with sewage treatment plant outflows, they get into the environment, affecting the organisms that exist in it. Taking this into account, the paper characterized NSAIDs as one of the most frequently used drug groups in the home treatment of Covid-19 and the risks of using them during an epidemic, both for humans and the environment. Considering this, the article characterized NSAIDs as one of the most commonly used drug groups in the home treatment of Covid-19 and the risks associated with their use during an epidemic, both for humans and the environment, based on a literature review data analysis conducted following the PRISMA guidelines. This analysis should identify the main causes of the release of NSAIDs into the environment. In addition, it should allow the verification of the thesis whether the COVID-19 epidemic has had a significant impact on increasing environmental concentrations of NSAIDs and, therefore, whether the risk of exposure to non-target organisms to these drugs has increased. The review, pointing to the risks associated with the increased use of NSAIDs during epidemics, will change the scientific community's position on the need to monitor these pharmaceuticals in the outflow of sewage treatment plants. The emphasis on the problem by opinion-forming circles may, as a result, contribute to legal changes related to the monitoring of NSAIDs and the establishment of limit values for concentrations of these drugs in the outflows of sewage treatment plants by legislative bodies around the world. In addition, increased public awareness will contribute to the responsible use and disposal of NSAIDs.
2. Materials and methods
According to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines (Moher et al., 2009), the following databases were searched, the Scopus®, Web of Science ™ (WoS) and Google Scholar, in order to create a collection of scientific articles only in English. A time limit has been applied, from 1998 when the first report of the presence of NSAIDs in the aquatic environment appeared (Ternes, 1998). When searching the databases, the phrases “COVID-19 and NSAIDs” OR “NSAIDs toxicity human” OR “NSAIDs toxicity non-target organisms” OR “NSAIDs in Covid-19 therapy” OR “COVID-19 NSAIDs in environment” OR “COVID-19 NSAIDs in water” OR “NSAIDs sources” were used. Both original experimental works, as well as reviews and communications, were used for the analysis. The first selection factors used in the analysis were the publication date. Any paper about the presence of NSAIDs in the environment published before the beginning of the COVID-19 epidemic and related to the toxicity of NSAIDs from the past twenty years were rejected. The second selection excluded papers because of duplicates and titles that are not informative enough. After reviewing the abstracts of works selected in the second stage, the third selection rejected papers unrelated to the topic. The final selection was made after reading the full texts of the papers and works concerning the presence of NSAIDs in the environment and was rejected if they were published during the pandemic, but the presented data were from before this period. Finally, 70 items were used in the present review (Fig. 1 ).
Fig. 1.

Article selection flow chart.
3. NSAIDs in Covid-19 therapy - facts and myths
Coronavirus disease 2019 (COVID-19) is caused by the SARS-CoV-2 virus belonging to the beta-coronavirus family (Babaei et al., 2020; Lam et al., 2020). SARS-CoV-2 is a single-stranded, positive-sense RNA-enveloped virus. Structural proteins, as well as non-structural and accessory proteins, are encoded in the virus genome. In the pathogenesis of this disease, non-structural proteins such as 3-chymotrypsin-like protease (3CLpro), papain-like protease (PLpro), helicase, RNA-dependent RNA polymerase (RdRp) and structural protein (spike protein) play a significant role. The spike (S) protein consists of two subunits. The attachment of the coronavirus to the ACE2 receptor, which acts as a transmembrane serine protease 2 on the surface of the host cells, cleaves the spike (s) protein into S1 and S2 subunits. The S1 subunit is responsible for the cellular tropism and attachment of the virus with the host cell, while the S2 subunit plays a role in viral fusion with the cell membrane (Pujari et al., 2020). COVID-19 is characterized by a vast spectrum of symptoms, including runny nose, sore throat, fever, dry cough, headache, general weakness, hypogeusia and hyposmia, muscle pain, shortness of breath, diarrhoea, or acute respiratory distress syndrome and multiorgan failure. The variety of symptoms result from the attachment of the SARS-CoV-2 virus to the angiotensin-converting enzyme 2 (ACE2) receptor, which is present on the surface of cells in various organs, including the lungs, heart, kidneys, intestines, and arteries (Babaei et al., 2020; Pujari et al., 2020).
In the course of the disease requiring hospitalization, the most commonly used groups of drugs are anti-viral drugs to treat inflammatory diseases, immunomodulating agents, and angiotensin-converting enzyme inhibitors. Most of the targeted anti-viral therapies were aimed to reduce the essential virus proteins (3CLpro and Plpro) responsible for its replication and packaging. Hence, previously approved drugs for treating SARS and MERS virus infections - disulfiram, lopinavir, ritonavir - were widely used. However, patients are most often treated symptomatically using primary nonsteroidal anti-inflammatory and antitussive drugs during home treatment. This type of treatment affects the majority, i.e. nearly 80% of patients with COVID-19, and is most often out of control (Boregowda et al., 2020; Lam et al., 2020; Pujari et al., 2020).
The recent months have been full of reports related to the use of NSAIDs in COVID therapy. These reports contain radically different information (De Girolamo et al., 2020; Giollo et al., 2021; Javid et al., 2020; Lund et al., 2020; Paprocki, 2020). Moreover, many authors indicate that the therapeutic effect of NSAIDs in SARS-CoV-2 infection is not only related to their anti-inflammatory effect but also antithrombotic, cardioprotective and even anti-viral properties (Babaei et al., 2020). The undeniable fact is that the use of NSAIDs reduces the cytokine storm that also occurs in COVID-19. Among other things, popular ibuprofen minimizes the level of interleukin-6 in human tissues and sputum (De Girolamo et al., 2020; Giollo et al., 2021; Javid et al., 2020). However, attention is also often paid to the risks associated with NSAIDs in COVID therapy. Many authors emphasize that this risk should be analysed from two different perspectives. The first, with the risk of long-term use of NSAIDs by patients before infection with SARS-CoV-2, and the second, with the risk of using NSAIDs in COVID-19 therapy (De Girolamo et al., 2020; Giollo et al., 2021). De Girolamo et al. (2020) do not recommend using NSAIDs in COVID-19 treatment due to the risk of complications from the respiratory and vascular systems. At the same time, emphasizing that the therapy of patients treated with NSAIDs in whom the SARS-CoV-2 virus has not been detected should not be modified. Moreover, Crighton et al. (2020) recommended using NSAIDs for toothache in dental treatment for both COVID-19 positive and negative patients. In turn, Giollo et al. (2021) suggest that people taking long-term NSAIDs in the case of infection with coronavirus should not be treated with these drugs. Moreover, a higher level of ACE2 secretion was observed after the use of ibuprofen, resulting in a more severe course of COVID-19. A report prepared in France based only on four patients showed a worsening of the symptoms in COVID-19 after ibuprofen usage (Kawthalkar, 2020). However, no unequivocal reports link the increase in COVID-19 mortality or the severity of COVID-19 after ibuprofen use during therapy (Lund et al., 2020; Paprocki, 2020). It only indicates that diabetics with higher ACE2 levels are more likely to develop acute disease (Paprocki, 2020; Pergolizzi et al., 2020). Zolk et al. (2020) indicate that undoubtedly indirectly, the mechanism of action of NSAIDs may contribute to the complicated course of pneumonia. This is because NSAIDs inhibit significant inflammation symptoms, such as fever and pain, which leads to a delayed diagnosis of pneumonia and a delay in initiating the appropriate therapy.
A review performed by the World Health Organization (WHO) of 73 studies revealed no influence of NSAIDs on severe adverse events, effect on long-term survival or quality of life in patients with COVID-19 (Kawthalkar, 2020). In turn, the Expert Working Group on the Commission of Human Medicines in the U.K. has looked into an attempt to link ibuprofen therapy to acute COVID-19 cases. There was no unequivocal evidence linking a cytokine storm to NSAID use. At the same time, this commission, due to inconclusive results, recommends the use of paracetamol under the condition of restrictive dosing (Pergolizzi et al., 2020).
So far, the results and conclusions related to the safety of NSAIDs are incredibly different and even mutually exclusive. One of the reasons is the size of the analysed groups. Reports based on the analysis of small, even several-person groups have appeared in prestigious literature. This precludes a correct statistical analysis. This is all the more surprising that after more than two years of the pandemic, the group of infected people, on the day of writing the article, has reached almost 490 million. This would allow for a broad and comprehensive analysis of the results, but it requires the cooperation of scientific and therapeutic units worldwide.
4. NSAIDs characterization and their toxicity to human and non-target organisms
Nonsteroidal anti-inflammatory drugs (NSAIDs) belong to a broad group of drugs with diverse structural profiles but similar action modes, anti-inflammatory, antipyretic and analgesic properties. They are among the most commonly consumed medications either by prescription or over-the-counter (Osafo et al., 2017). Today, more than 50 different NSAIDs are available on the global pharmaceutical market, and almost 35 million people use them daily (Fokunang et al., 2018). Among the most popular NSAIDs today, apart from acetylsalicylic acid, are ibuprofen, diclofenac, naproxen, ketoprofen, piroxicam, mefenamic acid, celecoxib or rofecoxib (Lonappan et al., 2016; Osafo et al., 2017). Although not directly classified as NSAIDs in pharmacology textbooks, paracetamol without an anti-inflammatory component is also discussed along with these drugs (Jóźwiak-Bębenista and Nowak, 2014).
NSAIDs are non-selective inhibitors of cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2) isoenzymes. Both isoforms are responsible for converting arachidonic acid into prostaglandins, prostacyclins and thromboxanes (Fokunang et al., 2018; Lucas et al., 2019).
NSAIDs inhibit COX enzymes by blocking the entrance of arachidonic acid to a hydrophobic channel, leading to the enzyme's active site (Bjarnason et al., 2018). COX-1 is expressed constitutively in most mammalian cells. Prostaglandins produced by this isoform play a role in gastro and renal protection, macrophage differentiation, platelet aggregation and mucus production. It was confirmed that COX-1 has a limited role in the inflammatory process. However, its non-selective inhibition by NSAIDs during treatment may have adverse effects (Osafo et al., 2017). COX-2 is an inducible enzyme expressed during tissue injury and is active in various tissues such as the vascular endothelium and rheumatoid synovial endothelial cells mediating inflammation, pain and fever. An increase in COX-2 levels results in enhanced synthesis of pro-inflammatory prostaglandins in the arachidonic acid pathway (Fig. 2 ). The degree of influence of NSAIDs on COX became the basis for the classification of these drugs into non-target NSAIDs, including diclofenac, ketoprofen, aspirin, naproxen, flunixin, ibuprofen, flurbiprofen, indomethacin and meglumine, COX-2 preferential inhibitors including meloxicam, etodolac, nimesulide, carprofen, and highly selective COX-2 inhibitors including celecoxib, robenacoxib, rofecoxib, lumiracoxib and etoricoxib (Lucas et al., 2019; Rigas et al., 2020). The differences in the selectivity of COXs result from differences in the structure of the hydrophobic channel of enzymes. Conventional NSAIDs have access to both types of channels and bind to the enzyme via their carboxyl or enolic groups. The COX-1 channel is smaller and does not allow access to COX-2 inhibitors. In turn, the substrate specificity of COX-2 is enhanced by the presence of a polar binding site for the aryl sulfonamide and sulfone groups of COX-2 selective inhibitors (Bjarnason et al., 2018).
Fig. 2.
Arachidonic acid pathway with the participation of cyclooxygenases and the sites of transformation inhibition. * - acylation site Ser530 by group transfer from arginine (Al-Turki et al., 2010; Bjarnason et al., 2018; Fokunang et al., 2018).
Despite the unquestionable effectiveness in treating pain and inflammation, NSAIDs may have harmful effects, especially as NSAIDs are one of the most commonly overdosed drugs (Hunter et al., 2011). In the kidneys, prostacyclins act as vasodilators in the afferent arteriole and, in this way, increase renal perfusion. This mechanism is a negative feedback on the renin-angiotensin-aldosterone system and the sympathetic nervous system. Its inhibition by NSAIDs may result in acute vasoconstriction and, consequently, acute renal injury (Hunter et al., 2011; Lucas et al., 2019). It has also been shown that topical use of NSAIDs in ophthalmology can lead to superficial punctuate keratitis, corneal infiltrates, epithelial defects, and even corneal melt (Rigas et al., 2020). Weng et al. (2011) indicate that the use of NSAIDs in the course of soft tissue infections by Streptococcus pyogenes may lead to an increase in pro-inflammatory cytokines and the risk of septic shock, and consequently to higher mortality. The negative effect of COX-1 inhibitors on the gastrointestinal tract has been demonstrated in many studies (Bjarnason et al., 2018). Damage to the stomach and small intestine results from a reduction in the prostaglandins level in the mucosa due to the inhibition of COX-1. Hence, it seemed that selective COX-2 inhibitors should not cause such effects (Bjarnason et al., 2018). However, Szweda et al. (2013) showed that even selective COX-2 inhibitor such as robenacoxib might induce some damage to the canine colon. It is probably because COX-2 inhibition activates the lipooxygenase pathway with leukotrienes production, damaging the gastrointestinal mucosa.
The action of paracetamol is also related to the inhibition of COX enzymes. However, it does not induce the adverse effects of NSAIDs on the gastrointestinal tract. Whereas the inhibition of COX by NSAIDs relies on the competition with arachidonic acid for the active site of the enzyme, paracetamol is a factor reducing an iron cation in protoporphyrin IX in the peroxidase part of cyclooxygenases (Guzik and Wojcieszyńska, 2019; Jóźwiak-Bębenista and Nowak, 2014; Tittarelli et al., 2017). This radical generates tyrosine radicals in the cyclooxygenase part of the enzyme, which are necessary to catalyse the oxidation reaction of arachidonic acid into 15-hydroxyperoxide (the latter is reduced to prostaglandin H in the peroxidase part of the enzyme) (Fig. 3 ). The inflamed tissue is characterized by high levels of peroxides, which are antagonists of paracetamol and thus reduce the inhibition of prostaglandin production (Guzik and Wojcieszyńska, 2019; Jóźwiak-Bębenista and Nowak, 2014).
Fig. 3.
Mechanism of COX action and its inhibition by paracetamol (Guzik and Wojcieszyńska, 2019; Jóźwiak-Bębenista and Nowak, 2014; Tittarelli et al., 2017).
It is also known that NSAIDs usage, probably except for naproxen, is connected with an increased cardiovascular risk (Li et al., 2020). It is hypothesized that differential inhibition of COX isoenzymes influences the cardiovascular safety of NSAIDs. COX-1 produces thromboxane A2, which causes platelet aggregation, vasoconstriction and intensify vascular and cardiac remodelling (Li et al., 2020). However, most typical NSAIDS do not inhibit COX-1 sufficient to inhibit platelet activation. Only naproxen has a sufficiently long half-life for effective platelet COX-1 inhibition and platelet aggregation prevention. More COX-2 is selectivity correlated with a higher cardiovascular risk because COX-2 produces anti-inflammatory prostacyclin, a cardioprotective molecule in the circulatory system (Angiolillo and Weisman, 2017; Hunter et al., 2011). Probably, similar adverse cardiovascular reactions may occur after long-term use of paracetamol. In contrast to inflammatory tissues, endothelial cells are characterized by a low level of peroxides, which is insufficient to abolish paracetamol activity against COX-2 (Jóźwiak-Bębenista and Nowak, 2014, Tittarelli et al., 2017).
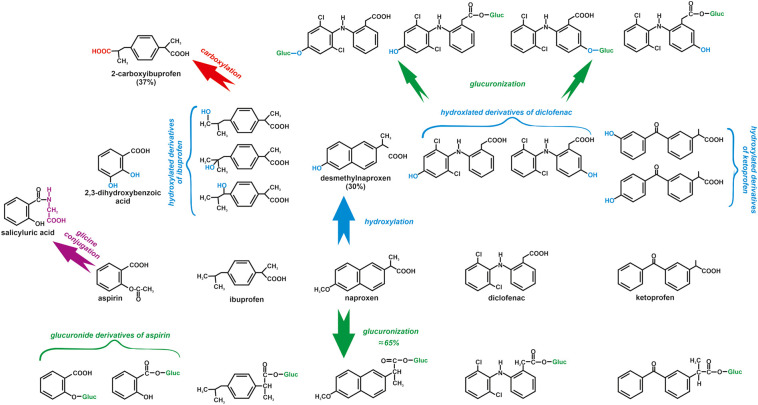
NSAIDs are metabolized in the liver of humans and other mammals during oxidation and conjugation reactions (Fig. 4 ). Most NSAIDs are metabolized by cytochrome P-450, which plays a crucial role in the oxidation of drugs in humans, plants, fish, and aquatic invertebrates. The glucuronic acid conjugation with carboxylated NSAIDs is catalysed by the uridine diphosphoglucuronosyl transferase and results in acyl glucuronides (Parolini, 2020). The reactive intermediates formed during the metabolism of NSAIDs may result in covalent binding to DNA, protein or other macromolecules and cause adverse effects (Niu et al., 2015). Ultimately metabolites of NSAIDs are usually excreted with the urine together with 10–20% of parent NSAID (Fokunang et al., 2018; Hunter et al., 2011). 90% of paracetamol undergoes metabolism in the liver through conjugation with glucuronic acid, sulfuric acid and cysteine. 5% of paracetamol is eliminated by the kidney in an unchanged form, and 5% of the drug is hydroxylated by cytochrome P-450 in the liver to N-acetyl-p-benzoquinone imine (NAPQI). This toxic metabolite is inactivated by glutathione sulphydryl groups to mercapturic acid and excreted via urine. In the case of low glutathione levels or over-dosage of paracetamol, when NAPQI overwhelms the level of glutathione, NAPQI covalently binds to hepatocyte macromolecules leading to structural and metabolic damage to the liver (Fig. 5 ) (Jeong et al., 2019; Lee, 2017; Jóźwiak-Bębenista and Nowak, 2014; Mossanen and Tacke, 2015; Tittarelli et al., 2017). Moreover, NAPQI causes Fe(II) oxidation to Fe(III) and methaemoglobin formation. Cats, whose erythrocytes are characterized by a low level of methaemoglobin reductase, are susceptible to this process (Siroka and Svobodova, 2013; Steenbergen, 2003).
Fig. 4.
Transformation of NSAIDs in the microsomal liver fraction (Fokunang et al., 2018; Hunter et al., 2011; Parolini, 2020).
Fig. 5.
Metabolic biotransformation of paracetamol (Jeong et al., 2019; Jóźwiak-Bębenista and Nowak, 2014; Lee, 2017; Mossanen and Tacke, 2015; Siroka and Svobodova, 2013; Steenbergen, 2003).
Due to the high consumption of NSAIDs, improper disposal and the low efficiency of sewage treatment plants, these drugs are currently among the most frequently detected environmental micro-pollutants (Parolini, 2020; Lonappan et al., 2016). Moreover, the accumulation of NSAIDs in water organisms has been noted. NSAIDs were detected in the tissue of organisms such as Gammarus fossarum, Potamopyrgus antipodarum, Chironomus riparius, Hyalella azteca, Utterbackia imbecillis, Corbicula fluminea, Exopalaemon modestus, Erpobdella octoculata, Phagocata vitta, Unio tumidus, Anadonta anatine or Dreissena polymorpha (Parolini, 2020). Therefore, in recent years, more and more attention has been paid to the acute and chronic toxicity of NSAIDs to non-target organisms. Many years of research have shown the toxic effects of acetylsalicylic acid, paracetamol, diclofenac, ibuprofen and naproxen on freshwater invertebrates. Detailed data on this subject can be found in the paper of Parolini (2020). It has also been shown that in Gammarus pulex and H. azteca, diclofenac is transformed into several oxidation products and conjugates, including two untypical types – diclofenac taurine conjugate and diclofenac methyl ester. An S-adenosylmethionine-dependent carboxylic acid methyltransferase catalyses the transformation of diclofenac to its methyl ester derivative in H. azteca. The LC50 of diclofenac estimated for H. azteca was 216 mg/L, while the LC50 of diclofenac methyl ester was reduced to 0.53 mg/L. These results indicate that acute toxicity of the methylated derivative of diclofenac increases 430-fold compared to the parent compound. Because O-methyltransferases have also been identified in plants, bacteria, yeast and humans, it cannot be ruled out that such a toxic metabolite of diclofenac may also be formed in these organisms (Fu et al., 2020).
Binukumari et al. (2016) observed a decrease in blood parameters in Cirrhinus mrigala, such as red blood cells (RBC), haemoglobin (Hb), mean corpuscular volume (MCV), and mean corpuscular haemoglobin (MCH) after both short- and long-term exposure to diclofenac. A particular example of the toxicity of NSAIDs to non-target organisms is the nephrotoxicity of NSAIDs in birds (Cuthbert et al., 2016; Ramzan et al., 2015). Flunixin has been reported to cause this damage in cranes and flamingos. This same effect was also observed for broiler chicken after diclofenac treatment (Ramzan et al., 2015). Between 1990 and 2007, the population of the three species of vultures in South Asia (Gyps bengalensis, Gyps indicus and Gyps tenuirostris) decreased by as much as 97%. The reason was the veterinary use of diclofenac in cattle treatment, the carcasses of which became food for vultures (Cuthbert et al., 2016; Swan et al., 2006). Renal damage in birds caused impairment of the normal excretion of uric acid from the body, such that it started crystallizing on the surface and inside the visceral organs resulting in visceral gout and ultimately death (Lonappan et al., 2016; Ramzan et al., 2015). For this reason, diclofenac appears to be dangerous to all species of vultures, not only Asian but also other scavenging birds (Camina et al., 2018; Swan et al., 2006). Several studies have also revealed the toxic effect of NSAIDs even at low concentrations toward aquatic organisms such as Cyprinus carpio, Salmo trutta fario, Oncorhynchus mykiss, Gasterosteus aculeatus and Tinca tinca (Fu et al., 2020; Lonappan et al., 2016; Stancova et al., 2017).
A strong effect of the subchronic exposure to naproxen on the early life stages of common carp had been observed by Sehonova et al. (2017). Hatching delay, developmental retardation, and gill lamella's deformations were noticed (Sehonova et al., 2017). Diclofenac has very similar effects in fish as naproxen, but at much lower concentrations and thus posed a much higher risk to fish than naproxen. It was shown that fish exposed for 21 days to the concentration of 1232 μg/L of naproxen showed a 25% mortality and numerous histopathological changes in the kidneys. A similar response with diclofenac was already observed at a concentration of 4.6 μg/L (lowest concentration tested) (Näslund et al., 2020). In turn, after 28 days of exposure to 3.38 nM diclofenac cytological alterations were observed in the liver, kidney and gills of Oncorhynchus mykiss, (Chen et al., 2014). It has been proven that diclofenac even at an environmentally observed concentration may lead to tissue damage in Salmo trutta (Lonappan et al., 2016). In embryo zebrafish (Danio rerio), after four-days of diclofenac exposure, Chen et al. (2014) observed shorter body length, smaller eye, pericardial and body oedema, lack of liver, intestine and circulation, muscle degeneration, abnormal pigmentation, and the alteration of some genes expression, which may cause defects in the cardiovascular and nervous systems. In Oryzias latipes, diclofenac affected the growth in the egg phase, reduced hatchability and delayed hatching (Lonappan et al., 2016). Stancova et al. (2017) observed a decrease in glutathione peroxidase and catalase activities after the exposure of Tinca tinca to ibuprofen and diclofenac, which may lead to increased oxidative stress. Moreover, the mixture of these pharmaceuticals induced oxidative stress at environmentally relevant concentrations (Stancova et al., 2017). The cell response to the formation of free radicals is the induction of antioxidant enzymes and changes in the transcription of genes associated with the detoxifying pathway. These processes are associated with the high energy expenditure of the organism, which in turn is reflected in changes in many physiological processes, including reproduction. In addition, oxidative stress can also lead to DNA damage and changes at the cellular level. Moreover, NSAIDs can cause teratogenic effects (Świacka et al., 2020).
As a result of the literature analysis, a surprisingly small number of literature reports related to the study of chronic toxicity, which in the case of NSAIDs is crucial due to the low concentrations found in the environment of these relatively resistant structures degradation, were found. Therefore, non-target organisms are exposed to these drugs in the long term, often over generations, which may eventually lead to the degeneration of the population. It is all the more surprising that most of the literature reports concern acute toxicity to non-target organisms that do not occur in the environment. In conclusion, although NSAIDs function as entirely safe in public awareness, the above review clearly indicated that they negatively influence biocenoses at environmental concentrations.
5. The impact of Covid-19 therapy with NSAIDs on the environment
Research into water pollution with pharmaceuticals has been ongoing since the 1990s when Ternes published his famous work on water pollution with prescriptions (Ternes, 1998). Particular attention is given to monitoring the drugs most commonly used in the population, such as hormones, antibiotics, antihypertensive drugs, and over-the-counter NSAIDs (Godoy et al., 2015; Kraemer et al., 2019; Wojcieszyńska and Guzik, 2020; Wojcieszyńska et al., 2020). Despite the great interest of the world of science in this problem, there is still no legal regulation mandating systematic testing of the aquatic environment's condition in terms of drug content. The emergence of the SARS-CoV-2 pandemic made it even more difficult to conduct systematic research due to the introduction of a complete lockdown in many countries. The obtained and published results of NSAID concentrations in the environment are often not representative, because they come from research centres using various methodologies, often not validated, and analyses are conducted inconsistently, at irregular intervals, and samples are collected from unrepresentative areas. On the other hand, there are more and more alarming reports that the raging epidemic substantially impacts the environment. Large amounts of drugs, including antibiotics, anti-viral and anti-inflammatory drugs, and personal protective equipment, are sent to sewage and landfills (Usman et al., 2020). During the pandemic, there has been an increase in the consumption of disposable plastic equipment, used both by healthcare professionals and the rest of the population, such as personal protective devices, gloves and masks for medical workers, disposable plastic items for life support tools, respirators, and common plastic items containing medical needles (Rume and Islam, 2020; Rupani et al., 2020). It is estimated that during the first lockdown, Wuhan in China produced 190 million tonnes of medical waste per day, more than during normal time. The use of a huge amount of disinfectants has led to the killing of non-target beneficial species, which can cause an ecological imbalance (Rume and Islam, 2020). On the other hand, a reduction in environmental pollution has been observed, which is associated with reduced vehicle traffic and the exclusion of many industries from operating for a month or longer due to the pandemic (Rupani et al., 2020; Shakil et al., 2020). Among other things, it has been shown to improve the quality of surface waters in terms of suspended particulate matter (SPM) in Lake Vembanad, India. Based on the established turbidity algorithm from the Landsat-8 OLI images estimated that the concentration of SPM during the blockage period decreased on average by 16% compared to with pre-closing period. Compared to the previous year, a 34% decrease in SPM was observed in April 2020 (Yunus et al., 2020). However, we cannot ignore the overwhelming impact of the pandemic on water pollution from treatment and disinfection agents. In the absence of targeted therapy, various substances have been used in the treatment of Covid-19 throughout the year. In the initial period of the pandemic, known anti-viral, anti-malarial and anti-inflammatory drugs were introduced into treatment. Also, in reports from China and India, recommendations for the use of traditional medicinal plants to treat COVID-19 can be found. However, the most commonly used drugs were chloroquine and hydroxychloroquine, lopinavir, remdesivir, favipiravir and azithromycin. Despite divergent data, NSAIDs have also been widely used in the treatment of Covid-19, especially in patients treated at home. With over-the-counter availability, they are often used without medical supervision to treat the basic symptoms of Covid, and NSAIDs can be found in the sewage treatment plant along with domestic and hospital sewage (Barcelo, 2020). After reaching the sewage plant, these drugs undergo a partial biological or chemical transformation, often into intermediates with a higher toxicity than the parent drug (Marchlewicz et al., 2017; Wojcieszyńska and Guzik, 2020). Increasing contamination with these drugs and their degradation products was already observed in the period before the pandemic in the waters of all continents (Godoy et al., 2015; Lacina et al., 2012; Lonappan et al., 2016; Madikizela and Chimuka, 2017; Shanmugam et al., 2014; Ternes, 1998; Wojcieszyńska and Guzik, 2020). However, it seems that SARS-CoV-2 infections contributed to the escalation of this problem. From 2019 to 2021, a lot of drugs belong to NSAIDs were detected in the world's water systems at concentrations ranging from a few nanograms to hundreds of micrograms per litre. The most frequently detected drugs were diclofenac, ibuprofen, naproxen, acetaminophen and ketoprofen (Jurado et al., 2021; Madikizela and Ncube, 2021; Nason et al., 2021; Omotola and Olatunji, 2020; Praveenkumarreddy et al., 2021; Reinstadler et al., 2021; Thalla and Vannarath, 2020).
Ajibola et al. (2021) conducted a drug concentration analysis on the influent and effluent of treatment plants located near hospitals in the cities of Ijaiye and Alausa and in the Dandaru River in Nigeria. The highest concentration of ibuprofen was observed in the influent to the treatment plant in Alausa (45 μg/L), and the highest concentration of diclofenac in the influent to the treatment plant in Ijaiye (166 μg/L). It was surprising that the high concentration of ibuprofen appeared in the Dandaru river (60 μg/L). These authors demonstrated a high environmental risk of ibuprofen to fish and diclofenac to bacteria (Ajibola et al., 2021). Omotola and Olatunji (2020) studied the pollution of the Msunduzi and Umgeni rivers in KwaZulu-Natal Province of South Africa by two popular nonsteroidal anti-inflammatory drugs - diclofenac and paracetamol and showed that the level of the observed concentrations ranged from 40 ng/l to 51.94 μg/L and from 96.70 to almost 152 μg/L, respectively. In the effluent of the wastewater treatment plant in South Africa ketoprofen at 91 μg/L was also detected (Madikizela and Ncube, 2021). Meanwhile, before 2018, naproxen, ibuprofen and diclofenac concentrations were 6.84, 19.2 and 9.69 μg/L, respectively in the Mbokodweni River, also in KwaZulu-Natal Province of South Africa (Madikizela and Chimuka, 2017).
In India, a country particularly hit by the coronavirus pandemic, a large share of NSAIDs has been observed in both rivers and sewage treatment plant inflows. Particularly high concentrations of ketoprofen (2747.29 μg/L), aspirin (125–2213.36 μg/L) and naproxen (3–2132.48 μg/L) were observed in the inflow to the treatment plant (Praveenkumarreddy et al., 2021; Thalla and Vannarath, 2020). On the other hand, in the Gurupura River in south-west India, the following concentrations were found, naproxen 8.8 μg/L, ketoprofen 1.5 μg/L, diclofenac 1.6 μg/L, ibuprofen 0.17 μg/L and aspirin 0.02 μg/L (Praveenkumarreddy et al., 2021). Studies of Praveenkumarreddy et al. (2021) on the ecotoxicological risk of NSAIDs in this river have shown, among other things, a medium risk related to the appearance of ibuprofen and naproxen concerning the Hydra attenuata polyp. In addition, ibuprofen was also toxic to Vibrio fischeri, Daphnia magna and algae (Praveenkumarreddy et al., 2021). In contrast, diclofenac has shown an average ecological risk to Ceriodaphnia dubia. In turn, Thalla and Vannarath (2020) have shown a high ecotoxicological risk of ibuprofen for fish and algae and a medium risk in the case of ketoprofen, aspirin, naproxen and diclofenac for fish, crustacean and algae.
High concentrations of NSAIDs in the environment are also observed on the European continent. For example, in the Besós river flowing through the urbanized areas of the metropolitan district of Barcelona (Spain), the following NSAIDs were detected, ketoprofen (42–133 ng/L), ibuprofen (73–126 ng/L), diclofenac (199–469 ng/L), mefenamic acid (8–13 ng/L), salicylic acid (54–109 ng/L), propyphenazone (14–85 ng/L) and phenazone (5–37 ng/L). The high concentration of diclofenac corresponded to the identified 4-hydroxy derivative metabolite. In addition, the appearance of the studied drugs in this region was also shown in the aquifer (Jurado et al., 2021). Also on the South American continent, the occurrence of high concentrations of paracetamol and diclofenac and their high environmental risk have been observed. Paracetamol concentrations even above 500 μg/L were found in the sewage treatment plants in Juliaca (Peru) (Nieto-Juarez et al., 2021). In turn, Reinstadler et al. (2021) monitored the concentration of paracetamol in the wastewater of a sewage treatment plant in Innsbruck during the lockdown in Austria between 12 March 2020 and 15 April 2020. The results were compared to those between March 2019 and January 2020. The authors found a decrease in paracetamol concentration in the wastewater, probably due to the changing lifestyle, limiting social contacts, and thus the spread of diseases such as colds and flu, in the treatment of which this drug is commonly used (Reinstadler et al., 2021). However, analysis of samples from the East Shore Water Pollution Abatement Facility wastewater treatment plant, which supplies the population of 200,000 New Haven residents in the USA, showed that during the period of full lockdown from March 19 to June 30, 2020, the content of paracetamol in the wastewater increased significantly (Nason et al., 2021). The authors suggested that this was because acetaminophen may be used to treat symptoms of COVID-19 such as headache and fever (Nason et al., 2021).
According to the PRISMA guidelines, an analysis of the literature indicated that only a tiny percentage of the available literature actually relates to studies of NSAID concentrations in the environment during the pandemic period. Most of the literature that appeared in this period publishes data from before the pandemic period. These modest data probably result from the introduced lockdowns in virtually all regions of the world, which significantly hindered the implementation of projects and sampling for analysis. In addition, they may indicate that the problem of increased NSAID consumption during a pandemic was not identified. This is supported by reports mainly on the increase amount of personal protective equipment, antibiotics or antiviral drugs in the environment. Without constant and regular monitoring of NSAIDs, this approach can lead to uncontrolled growth in these drugs' surface waters, especially given that the pandemic is becoming endemic. The review indicates a need for more extensive research by the scientific community in monitoring and studying the fate of NSAIDs in the environment.
6. Conclusion
The literature analysis indicated that the frequent use of NSAIDs as drugs supporting COVID-19 therapy and slight changes in the structure of these drugs during changes in the body is the reason for the appearance of NSAIDs in the environment in an unchanged or only slightly altered form. NSAIDs are observed in rivers and aquifers and in the inflows and outflows of wastewater treatment plants worldwide. A literature review also indicated a high ecotoxicological risk of NSAIDs to aquatic organisms. Data analysis from two years of the pandemic allowed for the systematization of information on the role of NSAIDs in COVID-19 therapy. At the same time, analysis of the papers shows a knowledge gap regarding the effects of the pandemic on the content of drugs in waters, which clearly indicates the need for constant monitoring of the environment regarding the presence of NSAIDs both during and after the pandemic.
Funding
This research was funded by the National Science Centre, Poland (grant number 2018/29/B/NZ9/00424).
CRediT authorship contribution statement
Conceptualization: DW, HG, UG.
Visualization: UG.
Funding acquisition: UG.
Project administration: UG.
Supervision: DW, UG.
Writing – original draft: DW, HG, UG.
Writing – review & editing: DW, HG, UG.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Urszula Guzik reports financial support was provided by National Science Centre Poland.
Editor: Damià Barceló
References
- Ajibola A.S., Adebiyi A.O., Nwaeke D.O., Ajibola F.O., Adewuyi G.O. Analysis, occurrence and ecological risk assessment of diclofenac and ibuprofen residues in wastewater from three wastewater treatment plants in south-western Nigeria. J. Appl. Sci. Environ. Manag. 2021;25:333–340. [Google Scholar]
- Al-Turki D.A., Abou-Zeid L.A., Shehata I.A., Al-Omar M.A. Therapeutic and toxic effects of new NSAIDs and related compounds: a review and prospective study. Int. J. Pharmacol. 2010;6:813–825. [Google Scholar]
- Angiolillo D.J., Weisman S.M. Clinical pharmacology and cardiovascular safety of naproxen. Am. J. Cardiovasc. Drugs. 2017;17:97–107. doi: 10.1007/s40256-016-0200-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babaei F., Mirzababaei M., Nassiri-Asl M., Hosseinzadeh H. Review of registered clinical trials for the treatment of COVID-19. Drug Dev. Res. 2020;1–20 doi: 10.1002/ddr.21762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcelo D. An environmental and health perspective for COVID-19 outbreak: meteorology and air quality influence, sewage epidemiology indicator, hospitals disinfection, drug therapies and recommendations. J. Environ. Chem. Eng. 2020;8 doi: 10.1016/j.jece.2020.104006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binukumari S., Priyadarsini V., Vasanthi J. Impact of diclofenac drug on the biochemical composition of the fresh water fish, Cirrhinus mrigala. Int. J. Adv. Res. Biol. Sci. 2016;3:153–159. [Google Scholar]
- Bjarnason I., Scarpignato C., Holmgren E., Olszewski M., Rainsford K.D., Lanas A. Mechanisms of damage to the gastrointestinal tract from nonsteroidal anti-inflammatory drugs. Gastroenterology. 2018;154:500–514. doi: 10.1053/j.gastro.2017.10.049. [DOI] [PubMed] [Google Scholar]
- Boregowda U., Gandhi D., Jain N., Khanna K., Gupta N. Comprehensive literature review and evidence evaluation of experimental treatment in COVID 19 contagion. Clin. Med. Insights Circ. Respir. Pulm. Med. 2020;14:1–7. doi: 10.1177/1179548420964140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camina A., Aguilera J., Sarrazini F., Duriez O. Potential exposure to diclofenac in Spain of European vultures. Vulture News. 2018;75:1–20. [Google Scholar]
- Chen J.B., Gao H.W., Zhang Y.L., Zhang Y., Zhou X.F., Li Ch.Q., Gao H.P. Developmental toxicity of diclofenac and elucidation of gene regulation in zebrafish (Danio rerio) Sci. Rep. 2014;4:4841. doi: 10.1038/srep04841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crighton A.J., McCann C.T., Todd E.J., Brown A.J. Safe use of paracetamol and high-dose NSAID analgesia in dentistry during the COVID-19 pandemic. Br. Dent. J. 2020;229:15–18. doi: 10.1038/s41415-020-1784-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert R.J., Taggart M.A., Saini M., Sharma A., Das A., Kulkurni M.D., Deori P., Ranade S., Shringarpure R.N., Galligan T.H., Green R.E. Continuing mortality of vultures in India associated with illegal veterinary use of diclofenac and potential threat from nimesulide. Oryx. 2016;51:104–112. [Google Scholar]
- De Girolamo L., Peretti G.M., Maffulli N., Brini A.T. Covid-19 – the real role of NSAIDs in Italy. J. Orthop. Surg. Res. 2020;15:165. doi: 10.1186/s13018-020-01682-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fokunang Ch.N., Fokunang E.T., Frederick K., Ngameni B., Ngadjui B. Overview of nonsteroidal anti-inflammatory drugs (nsaids) in resource limited countries. MOJ Toxicol. 2018;4:5–13. [Google Scholar]
- Fu Q., Fedrizzi D., Kosfeld V., Schlechriem Ch., Ganz V., Derrer S., Rentsch D., Hollender J. Biotransformation chenges bioaccumulation and toxicity of diclofenac in aquatic organisms. Environ. Sci. Technol. 2020;54:4400–4408. doi: 10.1021/acs.est.9b07127. [DOI] [PubMed] [Google Scholar]
- Giollo A., Adami G., Gatti D., Idolazzi L., Rossini M. Coronavirus disease (Covid-19) and nonsteroidal anti-inflammatory drugs (NSAID) Ann. Rheum. Dis. 2021;80(2) doi: 10.1136/annrheumdis-2020-217598. [DOI] [PubMed] [Google Scholar]
- Godoy A.A., Kummrow F., Pamplin P.A.Z. Occurrence, ecotoxicological effects and risk assessment of antihypertensive pharmaceutical residues in the aquatic environment - a review. Chemosphere. 2015;138:281–291. doi: 10.1016/j.chemosphere.2015.06.024. [DOI] [PubMed] [Google Scholar]
- Guzik U., Wojcieszyńska D. In: Microbes and Enzymes in Soil Health and Bioremediation. Microorganisms for Sustainability. Kumar A., Sharma S., editors. Vol. 16. Springer Nature Singapore Pte Ltd; 2019. Biodegradation of nonsteroidal anti-inflammatory drugs and their influence on soil microorganisms. Chapter 16; pp. 379–401. [Google Scholar]
- Hunter L.J., Wood D.M., Dargan P.I. The patterns of toxicity and management of acute nonsteroidal anti-inflammatory drug (NSAID) overdose. Open Access Emerg. Med. 2011;3:39–48. doi: 10.2147/OAEM.S22795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javid M.J., Javid D.J., Zebardast J. Rescue therapy in Covid-19: NSAIDs and mineralocorticoid receptor antagonists (MRBs) Austin J. Surg. 2020;7:1248. [Google Scholar]
- Jeong T.B., Kim J.H., Kim S.H., Lee S., Son S.W., Lim Y., Cho J.Y., Hwang D.Y., Kim K.S., Kwak J.H., Jung Y.S. Comparison of toxic responses to acetaminophen challenge in ICR mice originating from different sources. Lab. Anim. Res. 2019;35(16):1–7. doi: 10.1186/s42826-019-0017-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jóźwiak-Bębenista M., Nowak J.Z. Paracetamol: mechanism of action, applications and safety koncern. Acta Pol. Pharm. 2014;71:11–23. [PubMed] [Google Scholar]
- Jurado A., Vazquez-Sune E., Pujades E. Urban groundwater contamination by non-steroidal anti-inflammatory drugs. Water. 2021;13:720. [Google Scholar]
- Kawthalkar A.S. Musculoskeletal interventions in the era of COVID-19: current scenario and review of literature regarding procedures, practices, and precautions. Indian J. Musculoskelet. Radiol. 2020;2:20–25. [Google Scholar]
- Kraemer S.A., Ramachandran A., Perron G.G. Antibiotic pollution in the environment: from microbial ecology to public policy. Microorganisms. 2019;7:180. doi: 10.3390/microorganisms7060180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacina P., Zenatova P., Vavrova M. The assessment of contamination of selected river streams in the Czech Republic by human and veterinary drug residues with liquid and gas chromatography. Fresenius Environ. Bull. 2012;21:3318–3324. [Google Scholar]
- Lam S., Lombardi A., Ouanounou A. COVID-19: a review of the proposed pharmacological treatments. Eur. J. Pharmacol. 2020;886 doi: 10.1016/j.ejphar.2020.173451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W.M. Acetaminophen (APAP) hepatotoxicity – isn't it time for APAP to go away? J. Hepatol. 2017;67:1324–1331. doi: 10.1016/j.jhep.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Yu Ch., Zeng X. Comparative efficacy of traditional non-selective NSAIDs and selective cyclooxygenase-2 inhibitors in patients with acute gout: a systematic review and meta-analysis. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2019-036748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonappan L., Brar S.K., Das R.K., Verma M., Surampalli R.Y. Diclofenac and its transformation products: environmental occurrence and toxicity – a review. Environ. Int. 2016;96:127–138. doi: 10.1016/j.envint.2016.09.014. [DOI] [PubMed] [Google Scholar]
- Lucas G.N.C., Leitao A.C.C., Xavier R.M.F., De Francesco Daher E., da Silva Junior G.B. Pathophysiological aspects of nephropathy caused by nonsteroidal anti-inflammatory drugs. Braz. J. Nephrol. 2019;41:124–130. doi: 10.1590/2175-8239-JBN-2018-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund L.Ch., Reilev M., Hallas J., Kristensen K.B., Thomsen R.W., Christiansen Ch.F., Sorensen H.T., Johansen N.B., Brun N.C., Voldstedlung M., Stovring H., Thomasen M.K., Christensen S., Pottegard A. Association of nonsteroidal anti-inflammatory drug use and adverse outcomes among patients hospitalized with influenza. JAMA Netw. Open. 2020;3(7) doi: 10.1001/jamanetworkopen.2020.13880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madikizela L.M., Chimuka L. Occurrence of naproxen, ibuprofen, and diclofenac residues in wastewater and river water of KwaZulu-Natal Province in South Africa. Environ. Monit. Assess. 2017;189:348. doi: 10.1007/s10661-017-6069-1. [DOI] [PubMed] [Google Scholar]
- Madikizela L.M., Ncube S. Occurrence and ecotoxicological risk assessment of non-steroidal anti-inflammatory drugs in South African aquatic environment: what is known and the missing information? Chemosphere. 2021;280 doi: 10.1016/j.chemosphere.2021.130688. [DOI] [PubMed] [Google Scholar]
- Marchlewicz A., Guzik U., Hupert-Kocurek K., Nowak A., Wilczyńska S., Wojcieszyńska D. Toxicity and biodegradation of ibuprofen by Bacillus thuringiensis B1(2015b) Environ. Sci. Pollut. Res. 2017;24:7572–7584. doi: 10.1007/s11356-017-8372-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- Mossanen J.C., Tacke F. Acetaminophen-induced acute liver injury in mice. Lab. Anim. 2015;49:30–36. doi: 10.1177/0023677215570992. [DOI] [PubMed] [Google Scholar]
- Näslund J., Asker N., Fick J., Larsson D.G.J., Norrgren L. Naproxen affects multiple organs in fish but is still an environmentally better alternative to diclofenac. Aquat. Toxicol. 2020;227 doi: 10.1016/j.aquatox.2020.105583. [DOI] [PubMed] [Google Scholar]
- Nason S.L., Lin E., Eitzer B., Koelmel J., Peccia J. Changes in sewage sludge chemical signatures during a COVID-19 community lockdown, Part 1: traffic, drugs, mental health, and disinfectants. Environ. Toxicol. Chem. 2021 doi: 10.1002/etc.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto-Juarez J.I., Torres-Palma R.A., Botero-Coy A.M., Hernandez F. Pharmaceuticals and environmental risk assessment in municipal wastewater treatment plants and rivers from Peru. Environ. Int. 2021;155 doi: 10.1016/j.envint.2021.106674. [DOI] [PubMed] [Google Scholar]
- Niu X., de Graaf I.A.M., Langeaar-Makkinje M., Horvatovich P., Groothuis G.M.M. Diclofenac toxicity in human intestine ex vivo is not related to the formation of intestinal metabolites. Arch. Toxicol. 2015;89:107–119. doi: 10.1007/s00204-014-1242-6. [DOI] [PubMed] [Google Scholar]
- Omotola E.O., Olatunji O.S. Quantification of selected pharmaceutical compounds in water using liquid chromatography-electrospray ionization mass spectrometry (LC-ESI-MS) Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e05787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osafo N., Agyare Ch., Obiri D.D., Antwi A.O. In: Nonsteroidal Anti-Inflammatory Drugs. Al-kaf A.G.A., editor. IntechOpen; Rijeka: 2017. Mechanism of action of nonsteroidal anti-inflammatory drugs. Chapter 2, 5-15. [DOI] [Google Scholar]
- Paprocki M. Nonsteroidal anti-inflammatory drugs (NSAIDS) in Covid-19 patient. Disaster Emerg. Med. J. 2020;5:1–2. [Google Scholar]
- Parolini M. Toxicity of the nonsteroidal anti-inflammatory drugs (NSAIDs) acetylsalicylic acid, paracetamol, diclofenac, ibuprofen and naproxen towards freshwater invertebrates: a review. Sci. Total Environ. 2020;740 doi: 10.1016/j.scitotenv.2020.140043. [DOI] [PubMed] [Google Scholar]
- Pergolizzi J.V., Varrassi G., Magnusson P., LeQuang J.A., Paladini A., Taylor R., Wollmuth Ch., Breve F., Christo P. COVID-19 and NSAIDS: a narrative review of knowns and unknowns. Pain Ther. 2020;9:353–358. doi: 10.1007/s40122-020-00173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praveenkumarreddy Y., Vimalkumar K., Ramaswamy B.R., Kumar V., Singhal R.K., Basu H., Gopal Ch.M., Vandana K.E., Bhat K., Udayashankar H.N., Balakrishna K. Assessment of non-steroidal anti-inflammatory drugs from selected wastewater treatment plants of Southwestern India. Emerg. Contam. 2021;7:43–51. [Google Scholar]
- Pujari R., Thommana M.V., Mercedes B.R., Serwat A. Therapeutic options for COVID-10: a review. Cureus. 2020;12(9) doi: 10.7759/cereus.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramzan M., Ashraf M., Hashmi H.A., Iqbal Z., Anjum A.A. Evaluation of diclofenac sodium toxicity at different concentrations in relation to time using broiler chicken model. J. Anim. Plant Sci. 2015;25:357–365. [Google Scholar]
- Reinstadler V., Ausweger V., Grabher A.L., Kreidl M., Huber S., Grander J., Haslacher S., Singer K., Schlapp-Hackl M., Sorg M., Erber H., Oberacher H. Montoring drug consumption in Innsbruck during coronavirus disease 2019 (COVID-19) lockdown by wastewater analysis. Sci. Total Environ. 2021;757 doi: 10.1016/j.scitotenv.2020.144006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigas B., Huang W., Honkanen R. NSAID-induced corneal melt: clinical importance, pathogenesis, and risk mitigation. Surv. Ophthalmol. 2020;65:1–11. doi: 10.1016/j.survophthal.2019.07.001. [DOI] [PubMed] [Google Scholar]
- Rume T., Islam S.M.D.-U. Environmental effects of COVID-19 pandemic and potential strategies of sustainability. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e04965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupani P.F., Nilashi M., Abumalloh R.A., Asadi S., Samad S., Wang S. Coronavirus pandemic (COVID-19) and its natural environmental impacts. Int. J. Environ. Sci. Technol. 2020;1:1–12. doi: 10.1007/s13762-020-02910-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehonova P., Plhalova L., Blahova J., Doubkova V., Prokes M., Tichy F., Fiorino E., Faggio C., Svobodova Z. Toxicity of naproxen sodium and its mixture with tramadol hydrochloride on fish early life stages. Chemosphere. 2017;188:414–423. doi: 10.1016/j.chemosphere.2017.08.151. [DOI] [PubMed] [Google Scholar]
- Shakil M.H., Munim Z.H., Tasnia M., Sarowar S. COVID-19 and the environment: a critical review and research agenda. Sci. Total Environ. 2020;745 doi: 10.1016/j.scitotenv.2020.141022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam G., Sampath S., Selvaraj K.K., Larsson D.G.J., Ramaswamy B.R. Nonsteroidal anti-inflammatory drugs in Indian rivers. Environ. Sci. Pollut. Res. 2014;21:921–931. doi: 10.1007/s11356-013-1957-6. [DOI] [PubMed] [Google Scholar]
- Siroka Z., Svobodova Z. The toxicity and adverse effects of selected drugs in animals – overview. Pol. J. Vet. Sci. 2013;16:181–191. doi: 10.2478/pjvs-2013-0027. [DOI] [PubMed] [Google Scholar]
- Stancova V., Plhalova L., Blahova J., Zivna D., Bartoskova M., Siroka Z., Marsalek P., Svobodova Z. Effects of the pharmaceutical contaminants ibuprofen, diclofenac, and carbamazepine alone, and in combination, on oxidative stress parameters in early life stages of tench (Tinca tinca) Vet. Med. 2017;62:90–97. [Google Scholar]
- Steenbergen V. Acetaminophen and cats.A dangerous combination. Vet. Tech. 2003;24:43–45. [Google Scholar]
- Swan G.E., Cuthbert R., Quevedo M., Green R.E., Pain D.J., Bartels P., Cunningham A.A., Duncan N., Meharg A.A., Oaks J.L., Parry-Jones J., Shultz S., Taggart M.A., Verdoorn G., Wolter K. Toxicity of diclofenac to Gyps vultures. Biol. Lett. 2006;2:279–282. doi: 10.1098/rsbl.2005.0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Świacka K., Michnowska A., Maculewicz J., Caban M., Smolarz K. Toxic effects of NSAIDs in non-target species: a review from the perspective of the aquatic environment. Environ. Pollut. 2020;273 doi: 10.1016/j.envpol.2020.115891. [DOI] [PubMed] [Google Scholar]
- Szweda M., Szarek J., Dublan K., Gesek M., Mecik-Kronenberg T. Effect of selected nonsteroidal anti-inflammatory drugs on the pathomorphology of the mucous membrane of the canine colon. Vet. Med. 2013;58:430–436. [Google Scholar]
- Ternes T.A. Occurrence of drugs in German sewage treatment plants and rivers. Water Res. 1998;12:3245–3260. [Google Scholar]
- Thalla A.K., Vannarath A.S. Occurence and environemntal risks of nonsteroidal anti-inflammatory drugs in urban wastewater in the southwest monsson region of India. Environ. Monit. Assess. 2020;192:193. doi: 10.1007/s10661-020-8161-1. [DOI] [PubMed] [Google Scholar]
- Tittarelli R., Pellegrini M., Scarpellini M.G., Marinelli E., Bruti V., Di Luca N.M., Busardo F.P., Zaami S. Hepatotoxicity of paracetamol and related fatalities. Eur. Rev. Med. Pharmacol. Sci. 2017;32:95–101. [PubMed] [Google Scholar]
- Usman M., Farooq M., Hanna K. Environmental side effects of the indjudicious use of antimicrobials in the era of COVID-19. Sci. Total Environ. 2020;745 doi: 10.1016/j.scitotenv.2020.141053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng T.Ch., Chen Ch.Ch., Toh H.S., Tang H.J. Ibuprofen worsens streptococcus pyogenes soft tissue infections in mice. J. Microbiol. Immunol. Infect. 2011;44:418–423. doi: 10.1016/j.jmii.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Wojcieszyńska D., Guzik U. Naproxen in the environment: its occurrence, toxicity to non-target organisms and biodegradation. Appl. Microbiol. Biotechnol. 2020;104:1849–1857. doi: 10.1007/s00253-019-10343-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcieszyńska D., Marchlewicz A., Guzik U. Suitability of immobilized systems for microbiological degradation of endocrine disrupting compound. Molecules. 2020;25(19):4473. doi: 10.3390/molecules25194473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunus A.P., Masago Y., Hijioka Y. COVID-19 and surface water quality: improved lake water quality during the lockdown. Sci. Total Environ. 2020;731 doi: 10.1016/j.scitotenv.2020.139012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolk O., Hafner S., Schmidt Ch.Q. COVID-19 pandemic and therapy with ibuprofen or rennin-angiotensin system blockers: no need for interruptions or changes in ongoing chronic treatments. Naunyn-Schiedeberg's Arch. Pharmacol. 2020;393:1131–1135. doi: 10.1007/s00210-020-01890-6. [DOI] [PMC free article] [PubMed] [Google Scholar]