Abstract
Objective
The objective of this study was to evaluate how COVID-19 affects patients with acute ischemic or hemorrhagic stroke outcome.
Materials and methods
This retrospective study was performed on adult patients (> 18 years old) with stroke (ischemic or hemorrhagic) who were admitted to hospital with or without COVID-19. The primary outcome was stroke-related disability, which was measured by mRS at baseline and discharge. Hospital duration, intensive care unit (ICU) admission, and mortality were considered the secondary outcomes.
Results
From February 2019 until August 2020, we recruited and analyzed 151 patients, 42 of whom had COVID-19 based on RT-PCR tests or lung CT scan findings. COVID-19 positive patients had higher baseline and final mRS scores than the control group (4.46 ± 0.67 vs 4.79 ± 0.61, P: 0.001, 3.83 ± 1.22 vs 4.46 ± 0.67, P: 0.001). Moreover, stroke patients with COVID-19 experienced a more severe disease and required a higher rate of ICU admission (17 vs 0, P:0.001) and longer hospitalization compared to those without COVID-19 (8.50 ± 7.86 vs 7.5 ± 11.20, P: 0.021). Also, mortality was higher in the COVID-19 group (19 vs 13, P:0.001). There was not any significant differences between the two groups in terms of the involvement of cerebral arteries and type of stroke. Male sex, COVID-19, and ICU admission were the main independent risk factors for death.
Conclusion
The results of the study showed stroke patients (ischemic or hemorrhagic) with COVID-19 can have more disabilities and incur more hospital complications and mortality than non-COVID-19 patients.
Keywords: Ischemic stroke, Hemorrhagic stroke, COVID-19, Outcome
Introduction
On December 2019, the first patients with coronavirus disease 2019 (COVID-19) infection were diagnosed in China.1 Approximately 228 million people have been infected with COVID-19 until September 2021, and 4700,000 of them have died due to the disease.
The initial symptoms reported for the disease focused on the respiratory system, and included fever, shortness of breath, productive and nonproductive cough, and chest pain.2 , 3 However, with the worldwide spread of the disease, many symptoms, including neurological ones, were observed in COVID-19 patients. The most critical neurological complications recognized so far are cerebrovascular disorders involving intracranial hemorrhage (ICH), ischemic stroke, and cerebral venous thrombosis.4 , 5 Stroke appears to be a rare COVID-19 complication, but it can cause significant morbidity and mortality.6 Patients with vascular risk factors associated with stroke, such as aging, diabetes, hypertension, obesity, and previous cardiac or cerebrovascular disease, are also at increased risk of mortality and morbidity by COVID-19.7
COVID-19-induced hypoxia and inflammation may play a role in the onset, progression, and prognosis of ischemic stroke. Therefore, they should be on the lookout for COVID-19 (especially in patients with neurological symptoms) and initial precautions should be taken for them.8
Due to the physiopathology of COVID-19 and the increased reports of neurological symptoms, we decided to evaluate the possible effects of COVID-19 on patients with stroke (ischemic or hemorrhagic) and to compare the results with those of stroke patients who were not infected with COVID-19.
Materials and methods
Setting and study registration
This retrospective study was performed on hospitalized patients with stroke who were admitted at Ibne Sina hospital. This is a 320-bed teaching hospital and provides one of the main referral neurology centers in Sari, Iran, that annually admits around 250 stroke patients. The Ethics Committee of Mazandaran University of Medical Sciences approved the study (IR.MAZUMS.REC.1399.549).
Setting and patients
All adult patients (Age ≥ 18 years old) who were admitted to hospital due to stroke with or without COVID-19 were included. Besides relevant clinical symptoms, COVID-19 diagnosis was confirmed by the reverse transcription polymerase chain reaction (RT-PCR) or chest computed tomography (CT) scan in all the patients. Stroke diagnosis was based on clinical symptoms and brain CT/MRI findings recorded during patients’ hospital stay. Any patient about whom there was no clinical information in the case files was excluded from the study.
Data collection and measurement
The collected data were related to the period between from February 2019 to August 2020. Initially, we extracted all information related to patients with COVID-19 which had been recorded during the mentioned period. Patients whose throat swab sample or tracheal secretion test were positive for SARS-CoV-2 in the real-time RT-PCR test or whose pulmonary CT scan showed COVID-19 infection were considered as cases of COVID-19. Brain CT scans of patients with signs of stroke (ischemic or hemorrhagic) were recorded and re-evaluated by neurologists. Demographics including age, sex, drug history, underlying disease, laboratory tests, intensive care unit (ICU) admission, mortality, hospital stay duration, drug history, and medication during hospitalization were recorded and evaluated.
As the primary outcome of the study, stroke disability was measured using mRS at hospital admission time and on the last day of hospitalization. Regarding the mRS, it should be stated that it is a clinically based scoring system for stroke disability. This scale is scored as follows: 0: No symptoms at all; 1: No significant disability despite symptoms, able to carry out all usual duties and activities; 2: Slight disability, unable to carry out all previous activities, but able to look after one's own affairs without assistance; 3: Moderate disability, requiring some help, but able to walk without assistance; 4: Moderately severe disability, unable to walk without assistance and unable to attend to one's own bodily needs without assistance; 5: Severe disability, bedridden, incontinent and requiring constant nursing care and attention; and 6: Dead.9 Hospital duration, ICU admission, and mortality were regarded as secondary outcomes. The ICU admission criterion was defined as the need for invasive interventions such as mechanical ventilation, vasopressors, and organ failure support.
Statistical analysis
The collected data were analyzed using SPSS v16. Baseline information and mean and standard deviation were used to evaluate data with normal distribution, and median and interquartile range (IQR) were used to analyze data with no normal distribution. Continuous variables were compared using the Wilcoxon rank-sum test. Also, classified variables were expressed as number and percentage and compared using Chi-squared test. P < 0.05 was considered statistically significant.
Results
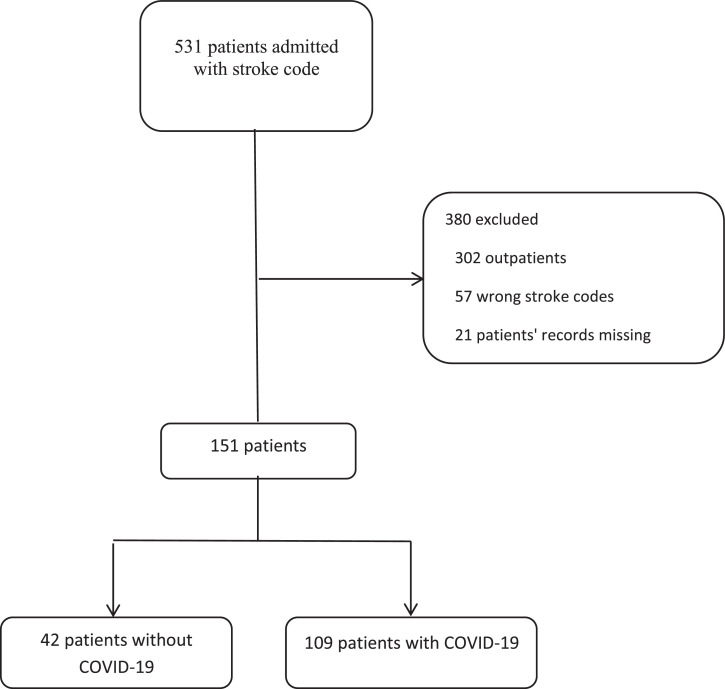
This analysis was performed on 531 patients hospitalized with neurological symptoms during the study period. Of this population, 380 patients were excluded due to lack of enough clinical data for a definite stroke diagnosis. Accordingly, clinical characteristics of 151 patients with stroke were evaluated. Forty two patients had coincidence of stroke and COVID-19, and their data were compared with other stroke patients who were not infected with COVID-19. The mean age of patients was 68.93 years, which was not significantly different between the two study groups. In addition, men accounted for 53.6% of patients; also, 23.5% of men developed COVID-19, while this rate was 32.9% in women. Also, in terms of height and weight (obtained from the patients' files and through calculating their BMI), the subjects were divided into three categories: <25, 25-30, and> 30, whose frequencies of a positive test for COVID-19 were 36.4%, 19.6%, and 31%, respectively. The mean white blood cell (WBC) count had significantly increased in COVID-19 group (11719 vs 9174.9, P: 0.001). The most common underlying disease was hypertension (72.2%), followed by diabetes mellitus (34.4%), ischemic heart disease (31.8%), and thyroid disease (7.3%). Concerning drug history, the results showed that angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers (ACEi/ARBs) were used significantly higher in non-COVID-19 stroke patients. The clinical characteristics and demographic data of the study subjects are shown in Table 1 .
Table 1.
Demographic characteristics and baseline clinical characteristics.
|
n (%) |
COVID-19, n(%) |
||||
|---|---|---|---|---|---|
| Variable | Negative | Positive | P-value | ||
| Age (mean ±SD) | 68.93±13.85 | 68.20 ± 13.72 | 70.79 ± 14.18 | 0.307 | |
| Gender | Male | 81 (53.6) | 62 (76.5) | 19 (23.5) | 0.199 |
| Female | 70 (46.4) | 47 (67.1) | 23 (32.9) | ||
| BMI (kg/m2) | <25 | 44 (34.1) | 28 (63.6) | 16 (36.4) | 0.165 |
| 25-30 | 56 (43.4) | 45 (80.4) | 11 (19.6) | ||
| >30 | 29 (22.5) | 20 (69.0) | 9 (31.0) | ||
| Cigarette smoking | 17 (11.3) | 14 (82.4) | 3 (17.6) | 0.321 | |
| Baseline laboratory test, mean ± SD | |||||
| White blood cells, (μ/L) | 9860.8 ± 3864.6 | 9174.9 ± 3413.7 | 11719 ± 4419.0 | 0.001 | |
| Hemoglobin, g/dl | 12.24 ± 2.6 | 12.25 ± 2.7 | 12.22 ± 2.3 | 0.596 | |
| LDH, mg/dl | 497.1 ± 400.0 | 424.1 ± 155.2 | 605.8 ± 626.8 | 0.586 | |
| Platelet count (/mm3) | 235128 ± 70.963 | 236762 ± 72.259 | 230897 ± 68.222 | 0.574 | |
| Underlying disease, | |||||
| Diabetes mellitus, | 52 (34.4) | 41 (78.8) | 11 (21.2) | 0.186 | |
| Hypertension | 109 (72.2) | 79 (72.5) | 30 (27.5) | 0.898 | |
| Ischemic heart disease | 48 (31.8) | 36 (75.0) | 12 (25.0) | 0.598 | |
| Thyroid disease | 11 (7.3) | 10 (90.9) | 1 (9.1) | 0.150 | |
| Drug history, | |||||
| ACEI/ARBS | 137 (90.7) | 104 (75.9) | 33 (24.1) | 0.001 | |
| Statins | 111 (73.5) | 84 (75.7) | 27 (24.3) | 0.111 | |
| Beta blockers | 116 (76.8) | 86 (74.1) | 30 (25.9) | 0.330 | |
| Cerebral arteries involvement | |||||
| Artery of Heubner | 3 (2.0) | 1 (33.3) | 2 (66.7) | 0.187 | |
| ICA | 4 (2.6) | 2 (50.0) | 2 (50.0) | 0.309 | |
| Lateral lenticulostriate | 11 (7.3) | 10 (90.9) | 1 (9.1) | 0.292 | |
| PCA | 7 (4.6) | 4 (57.1) | 3 (42.9) | 0.398 | |
| M2 inferior division | 7 (4.6) | 6 (85.7) | 1 (14.3) | 0.674 | |
| M2 superior division | 15 (9.9) | 12 (80.0) | 3 (20.0) | 0.561 | |
| M1 artery | 8 (5.3) | 4 (50.0) | 4 (50.0) | 0.219 | |
| ICH | 24 (15.9) | 15 (62.5) | 9 (37.5) | 0.248 | |
LDH: Lactate dehydrogenase, ACEI: Angiotensin-converting enzyme inhibitor, ARB: Angiotensin II receptor blocker, SD: Standard deviation, ICA: Internal carotid artery, PCA: Posterior cortical atrophy, ICH: Intra-cerebral hemorrhage, COVID-19: Coronavirus disease 2019.
All patients who were admitted to the ICU were infected with COVID-19 and the difference was significant between the two groups (P: <0.001). Also, mortality and hospital stay duration were significantly higher in the COVID-19 group (13 vs 19, P: <0.001, 7.52 vs 8.5, P:0.021). In terms of stroke disability, COVID-19 patients had a higher mean mRS at hospital admission and at the end of hospitalization, meaning that patients with coincidence of stroke and COVID-19 experienced more disability (4.46 vs 4.79, P: 0.001, 3.83 vs 4.93, P: <0.001), Table 2 ).
Table 2.
Clinical outcomes of the study.
| Variable | N (%) | COVID-19, n(%) |
P-value | ||
|---|---|---|---|---|---|
| Negative | Positive | ||||
| Admission to ICU | Yes | 17 (11.3) | 0 (0.0) | 17 (100.0) | <0.001 |
| No | 134 (88.7) | 109 (81.3) | 25 (18.7) | ||
| Death | Yes | 32 (21.2) | 13 (40.6) | 19 (59.4) | <0.001 |
| No | 119 (78.8) | 96 (80.7) | 23 (19.3) | ||
| Duration of hospitalization*, (days) | 7.78±10.42 | 7.52±11.20 | 8.50±7.86 | 0.021 | |
| Baseline MRS* | 4.55±0.67 | 4.46±0.67 | 4.79±0.61 | 0.001 | |
| Final MRS* | 4.13±1.30 | 3.83±1.22 | 4.93±1.18 | <0.001 | |
Values shown by mean ± SD, ICU: intensive care unit, MRS: modified Rankin Scale, COVID-19: Coronavirus disease 2019, SD: Standard deviation.
We also evaluated the findings of brain CT scans during hospitalization and compared the cerebral arteries, but no significant differences were observed between the two studies (Table 1).
To identify the factors contributing to the mortality of stroke patients, we performed multiple logistic regression analysis. According to the results, men were 2.68 times more likely to die than women. The chance of death in patients with coincidence of stroke and COVID-19 was 4.41 times higher than in cases without COVID-19. Finally, individuals admitted to ICU were 3.5 times more likely to die than others, although this relationship was not significant (Table 3 ).
Table 3.
Independent risk factors of mortality in patients with stroke.
| Variables | OR | P-value |
| Male | 2.68 | 0.045 |
| ICU admission | 3.50 | 0.069 |
| COVID-19 | 4.41 | 0.005 |
ICU: intensive care unit, COVID-19: Coronavirus disease 2019, OR: Odd Ratio
Discussion
The results of our study showed that patients with stroke and COVID-19 coincidence had a higher mRS at onset and discharge. This means COVID-19 could exacerbate stroke-related disability. It was found that mortality, ICU admission, and hospital stay duration were higher in the COVID-19 group. Furthermore, male sex, COVID-19, and the need for ICU admission were correlated with higher rate of mortality in patients with stroke. Meanwhile, it should be mentioned that the main cause of death in COVID-19 patients was related to respiratory and multi-organ failure.
In a retrospective study on COVID-19 patients with or without acute cerebrovascular disease (CVD) in Wuhan, China, Li et al. found that 10 (4.6%) of the 219 patients with COVID-19 had an acute ischemic stroke, and one (0.5%) had a cerebral hemorrhage. In this case series, older age, hypertension, diabetes, and history of CVD were major identified risk factors among COVID-19 patients with new onset CVD. In addition, they mentioned CVD could be a negative prognostic factor in COVID-19 patients.10 It should be considered that the authors studied only symptomatic COVID-19 patients, which leads to overlooking other non-symptomatic patients who might develop stroke.
In a multi-center cohort study by Mart-Fabregas et al., stroke severity was measured using the National Institute of Health Stroke Scale (NIHSS), and it was revealed that patients with COVID-19 experienced a more severe stroke than non-COVID-19 patients, indicating extensive vascular involvement in the former group.11 This result is also consistent with our study, which measured stroke severity with mRS. Specifically, in individuals who had COVID-19 and stroke simultaneously, mRS was higher at both admission and discharge.
Other research findings highlight the significant incidence of stroke in young people with COVID-19 (aged 33–49) as well as a low prevalence of common stroke risk factors and increased markers of inflammation (ferritin) and coagulation (D-dimer and fibrinogen).12
High rates of ischemic stroke in young patients with COVID-19 and no vascular risk factors illuminate the specific mechanisms of COVID-19. Thus, for example, direct viral invasion and inflammation cause abnormalities in the vascular endothelium.13
In the study of Naval-Baudin et al., 100 patients with confirmed ischemic stroke were recruited; and the demographics and the main imaging findings were compared between the two groups of patients according to SARS-CoV-2 PCR results. Nineteen patients had PCR positive, and the final analysis demonstrated that the COVID-19 group had a lower baseline mRS and more hospital mortality.14 The results of this study are similar to ours. However, unlike our study, their findings did not include hemorrhagic stroke; additionally, the possibility of false negative of SARS-CoV-2 PCR at admission time makes the initial diagnosis of COVID-19 uncertain.
Hess et al. suggested that ACE2 depletion by SARS2 might impair the brain endothelial function and induce stroke. Therefore, the use of ACEi and ARBs might raise some concerns regarding COVID-19 deterioration.15 However, the results of our study showed patients who took ACEI and ARBs were less likely to develop COVID-19. While there is no conclusive evidence confirming this effect, it is in line with the recommendation of the American Heart Association that the use of ACEi and ARBs should be continued in COVID-19 patients as they may be beneficial.16
A retrospective case series of 86 patients with acute stroke during SARS-CoV-2 outbreak showed that COVID-19 is an independent risk factor for in-hospital stroke and mortality. The authors also reported that older age and ICH were associated with higher mortality.17 Despite the similarity of these findings to our results, it should be noted the authors in that research included patients with imaging-confirmed stroke at the time of admission, which underestimates the incidence of COVID-19-induced stroke. In addition, the groups of that study were not compared at the same time, which might have affected the patients’ outcome.
Our study has some limitations. The large number of our patients with mild symptoms of stroke were not included. Due to the critical situation of COVID-19 pandemic, most of these patients were not hospitalized. Therefore, the number of patients with stroke might have been underestimated. Due to limited resources, diagnosis of COVID-19 patients was mainly based on lung CT scan findings, whose specificity is questionable—their suggestiveness and utility notwithstanding.
Conclusion
In conclusion, according to our results, COVID-19 is associated with higher hospital duration, ICU admission, and mortality in patients with stroke. Although the rate of ischemic or hemorrhagic stroke in patients with COVID-19 was not significantly different from the control group, the former group had a more severe disability (higher mRS) at admission and discharge. Further studies need to be undertaken demonstrate that how COVID-19 alters the physiopathology and nature of stroke (Fig. 1 ).
Fig 1.
Study diagram.
Declaration of Competing Interest
None.
References
- 1.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Qureshi A.I., Abd-Allah F., Al-Senani F., et al. Management of acute ischemic stroke in patients with COVID-19 infection: report of an international panel. Int J Stroke. 2020;15(5):540–554. doi: 10.1177/1747493020923234. [DOI] [PubMed] [Google Scholar]
- 3.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wölfel R., Corman V.M., Guggemos W., et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 5.Mao L., Jin H., Wang M., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galán J.T.G. Stroke as a complication and prognostic factor of COVID-19. Neurología. 2020;35(5):318–322. doi: 10.1016/j.nrl.2020.04.015. (English Edition) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cummings M.J., Baldwin M.R., Abrams D., et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Q., Guan X., Wu P., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson J.T., Hareendran A., Hendry A., Potter J., Bone I., Muir K.W. Reliability of the modified Rankin Scale across multiple raters: benefits of a structured interview. Stroke. 2005;36(4):777–781. doi: 10.1161/01.STR.0000157596.13234.95. Apr. [DOI] [PubMed] [Google Scholar]
- 10.Li Y., Li M., Wang M., et al. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol. 2020;5(3):279–284. doi: 10.1136/svn-2020-000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martí-Fàbregas J., Guisado-Alonso D., Delgado-Mederos R., et al. Impact of COVID-19 infection on the outcome of patients with ischemic stroke. Stroke. 2021;52(12):3908–3917. doi: 10.1161/STROKEAHA.121.034883. STROKEAHA. 121.034883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z., Yang Y., Liang X., et al. COVID-19 associated ischemic stroke and hemorrhagic stroke: incidence, potential pathological mechanism, and management. Front Neurol. 2020;11:1152. doi: 10.3389/fneur.2020.571996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spence J.D., De Freitas G.R., Pettigrew L.C., et al. Mechanisms of stroke in COVID-19. Cerebrovasc Dis. 2020;49(4):451–458. doi: 10.1159/000509581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naval-Baudin P., Rodriguez Caamaño I., Rubio-Maicas C., et al. COVID-19 and ischemic stroke: clinical and neuroimaging findings. J Neuroimaging. 2021;31(1):62–66. doi: 10.1111/jon.12790. [DOI] [PubMed] [Google Scholar]
- 15.Hess D.C., Eldahshan W., Rutkowski E. COVID-19-related stroke. Transl Stroke Res. 2020;11(3):322–325. doi: 10.1007/s12975-020-00818-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bozkurt B., Kovacs R., Harrington B. Joint HFSA/ACC/AHA statement addresses concerns re: using RAAS antagonists in COVID-19. J Card Fail. 2020;26(5):370. doi: 10.1016/j.cardfail.2020.04.013. May 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katz J.M., Libman R.B., Wang J.J., et al. Cerebrovascular complications of COVID-19. Stroke. 2020;51(9):e227–ee31. doi: 10.1161/STROKEAHA.120.031265. [DOI] [PMC free article] [PubMed] [Google Scholar]