Abstract
MYL-1402O (Abevmy®, Lextemy®) is a biosimilar of the reference anti-vascular endothelial growth factor antibody bevacizumab. Abevmy® is approved for use in all indications for which reference bevacizumab is approved, including the treatment of non-small cell lung cancer (NSCLC) and other solid cancers. Lextemy® is approved for all indications as reference bevacizumab, except in recurrent ovarian cancer. MYL-1402O has similar physicochemical and pharmacodynamic properties to those of reference bevacizumab, and the pharmacokinetic similarity of the agents has been shown in healthy male subjects. MYL-1402O demonstrated clinical efficacy equivalent to that of reference bevacizumab in patients with non-squamous NSCLC. The tolerability, safety and immunogenicity profiles of MYL-1402O were consistent with those of reference bevacizumab. The role of reference bevacizumab in the management of solid cancers is well established and MYL-1402O provides an effective biosimilar alternative for patients requiring bevacizumab therapy.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11523-021-00858-7.
| Digital Features for this Adis Biosimilar Brief can be found at 10.6084/m9.figshare.17074784. |
MYL-1402O: Key Points
| Biosimilar to reference bevacizumab. |
| Equivalent efficacy and tolerability to reference bevacizumab in patients with stage IV non-squamous NSCLC. |
| Similar pharmacokinetic and pharmacodynamic properties to those of reference bevacizumab. |
| MYL-1402O (as Abevmy®) is approved for all indications for which reference bevacizumab is approved. |
Introduction
MYL-1402O (Abevmy®, Lextemy®) is a biosimilar of the reference monoclonal anti-vascular endothelial growth factor antibody bevacizumab. Abevmy® is approved for the same indications as the reference drug in the EU (Table 1) [1]. Lextemy is approved for the same indications as bevacizumab, apart from recurrent ovarian cancer [2]. The pharmacokinetic similarity of MYL-1402O to EU- and US-sourced reference bevacizumab has been demonstrated [3]. This article summarizes, from an EU perspective, the key features of MYL-1402O and its clinical use in the treatment of solid cancers, focusing on non-squamous non-small cell lung cancer (NSCLC).
Table 1.
MYL-1402O (Abevmy®) prescribing summary in the EUa,b [1]
| Approved indications | |
|---|---|
| Colorectal cancer | Treatment of adult patients with metastatic carcinoma of the colon or rectum |
| Breast cancer | In combination with paclitaxel as first-line treatment of adult patients with metastatic breast cancerc |
| In combination with capecitabine as first-line treatment of adult patients with metastatic breast cancer in whom treatment with other chemotherapy options including taxanes or anthracyclines is not considered appropriate. Patients who have received taxane and anthracycline containing regimens in the adjuvant setting within the last 12 months should be excluded from treatment with bevacizumab in combination with capecitabinec | |
| Lung cancer | In addition to platinum-based chemotherapy as first-line treatment of adult patients with unresectable advanced, metastatic or recurrent non-small cell lung cancer other than predominantly squamous cell histology |
| In combination with erlotinib as first-line treatment of adult patients with unresectable advanced, metastatic or recurrent non-squamous non-small cell lung cancer with EGFR activating mutations | |
| Kidney cancer | In combination with interferon alfa-2a as first-line treatment of adult patients with advanced and/or metastatic renal cell cancer |
| Ovarian cancer | In combination with carboplatin and paclitaxel as front-line treatment of adult patients with advanced (FIGO stages III B, III C and IV) epithelial ovarian, fallopian tube, or primary peritoneal cancer |
| In combination with carboplatin and gemcitabine or in combination with carboplatin and paclitaxel in adults with first recurrence of platinum-sensitive epithelial ovarian, fallopian tube or primary peritoneal cancer who have not received prior therapy with bevacizumab or other VEGF inhibitors or VEGF receptor–targeted agents | |
| In combination with paclitaxel, topotecan, or pegylated liposomal doxorubicin in adults with platinum-resistant recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who received no more than two prior chemotherapy regimens and who have not received prior therapy with bevacizumab or other VEGF inhibitors or VEGF receptor-targeted agents | |
| Cervical cancer | In combination with paclitaxel and cisplatin or paclitaxel and topotecan in adults with persistent, recurrent, or metastatic carcinoma of the cervix |
EGFR epidermal growth factor receptor, FIGO International Federation of Gynecology and Obstetrics, VEGF vascular endothelial growth factor
aMYL-1402O is available as a 25 mg/ml concentrate for solution for intravenous infusion in 100 mg and 400 mg vials. Consult local prescribing information for details including pre- and post-medications, contraindications, warning and precautions
bAbevmy® is approved for all indications that are approved for reference bevacizumab [1], whereas Lextemy® is not approved for the treatment of recurrent ovarian cancer [2]
cRefer to local prescribing information for details regarding EGFR2 status
Clinical Pharmacology
Pharmacokinetic equivalence of MYL-1402O to EU- and US-sourced bevacizumab was demonstrated in a pharmacokinetic study in healthy male subjects (Table 2). A parallel study design was selected as the half-life of bevacizumab is approximately 20 days. Although a subtherapeutic dose (1 mg/kg) of bevacizumab was administered to limit exposure in healthy subjects, this dose was within the range where the pharmacokinetics of bevacizumab are expected to be linear [3].
Table 2.
Biosimilarity summary of MYL-1402O
| Mechanism of action | Anti-VEGF antibody that inhibits the binding of VEGF to VEGF receptors on the surface of endothelial cells; inhibits tumour angiogenesis and subsequently inhibits tumour growth [1, 2] |
| Physicochemical characterisation | Similar to EU-sourced reference bevacizumab with respect to primary, secondary and higher order structure. Differences in purity, charge variants, oxidation and post-translational modifications did not appear to have a clinically significant effect [4] |
| Differences in post-translational modifications included a decrease in non-glycosylated heavy chains, an increase in total sialic acid and increases in high mannose, total galactose and total afucosylated species [4] | |
| Pharmacodynamic similarity | The Fab region demonstrated similar binding kinetics and potency as reference bevacizumab against VEGF165, VEGF121 and VEGF189 [4] |
| Fc receptor binding kinetics were generally consistent with reference bevacizumab; minor differences were within method variability [4] | |
| Pharmacokinetic similarity | Pharmacokinetic similarity of MYL-1402O to EU- and US-sourced bevacizumab was established in a parallel three-arm study in healthy male subjects; the ratios and the 90% CIs of natural log-transformed parameters (AUC∞, AUCt and Cmax) were within the prespecified equivalence criteria of 0.80–1.25 [3] |
| Immunogenicity | In patients with stage IV non-squamous NSCLC, the incidence of treatment-emergent ADAs was 6.5% in 337 MYL-1402O recipients and 4.8% in 334 reference bevacizumab recipients [5] |
| In healthy male subjects, the incidence of ADA-positive subjects in the MYL-1402O arm was comparable to the EU- and US-sourced bevacizumab arms at all measured time points (days 15–99) [3] | |
| Subjects with higher levels of ADAs compared with lower levels of ADAs did not demonstrate clinically relevant differences in bevacizumab AUC∞, AUCt and Cmax [3] | |
| Efficacy and tolerability | Comparable efficacy between MYL-1402O and reference bevacizumab in patients with stage IV non-squamous NSCLC; the RD and the 95% CI of the RD, in addition to the 90% CI of the ratios of objective response rates, were within their equivalence margins [5] |
| The safety and tolerability profile of MYL-1402O was similar to that of reference bevacizumab in patients with stage IV non-squamous NSCLC [5] |
ADA(s) anti-drug antibody(antibodies), AUC∞ area under the serum concentration-time curve from time 0 extrapolated to infinity, AUCt AUC to last quantifiable concentration, CI confidence interval, Cmax maximum serum concentration, F(ab/c) fragment (antigen-binding/crystallisable), NSCLC non-small cell lung cancer, RD risk difference, VEGF vascular endothelial growth factor
MYL-1402O demonstrated pharmacodynamic equivalence and similarity in physicochemical properties to EU-sourced bevacizumab in pre-clinical studies (Table 2). Differences in post-translational modifications were detected in MYL-1402O (Table 2), which have the potential to affect the efficacy of biological products via changes in antibody-dependent cellular cytotoxicity (ADCC) or complement-dependent cytotoxicity (CDC). However, as bevacizumab does not exhibit ADCC nor CDC, these differences in post-translational modifications are unlikely to be clinically relevant [4].
Clinical Efficacy
The efficacy of MYL-1402O and reference bevacizumab was investigated in a multicentre, randomised, double-blind phase III equivalence trial in 671 patients with stage IV non-squamous NSCLC (Table 2, Fig. 1). Patients aged ≥ 18 years were treated intravenously with MYL-1402O or reference bevacizumab 15 mg/kg in 3-week cycles for 18 weeks, in addition to carboplatin to a target area under the concentration-time curve of 6 mg/mL·min and paclitaxel 175 or 200 mg/m2. Treatment was continued until disease progression or unacceptable toxicity, whichever occurred earlier. Treatment response was evaluated by independent review using Response Evaluation Criteria in Solid Tumours 1.1 criteria at any time point within the first 18 weeks of treatment. After the initial 18-week combination treatment period, monotherapy with MYL-1402O or reference bevacizumab 15 mg/kg once every 3 weeks was available in eligible patients [i.e. those with stable disease or who had achieved a complete (CR) or partial (PR) response] for a further 24 weeks. Patients who at week 42 had maintained stable disease or who had achieved a CR or PR continued to receive MYL-1402O or reference bevacizumab until disease progression or discontinuation of treatment or termination of study. Baseline characteristics were generally well balanced between treatment arms [5].
Fig. 1.
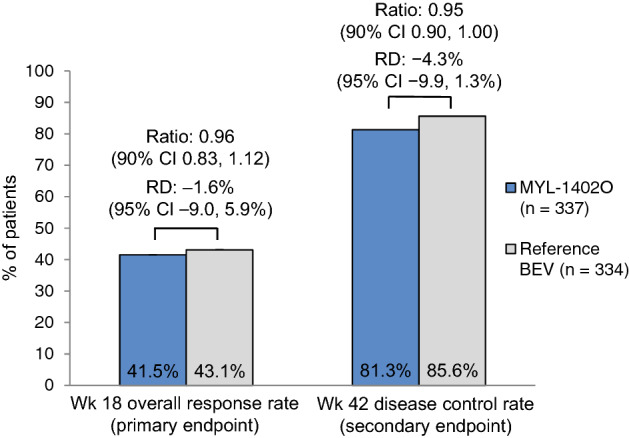
Response rates in the intent-to-treat population following treatment with MYL-1402O or reference bevacizumab in combination with carboplatin and paclitaxel in patients with stage IV non-squamous non-small cell lung cancer. Bevacizumab (as MYL-1402O or reference bevacizumab) 15 mg/kg was administered intravenously in 3-week cycles [5]. BEV bevacizumab, CI confidence interval, RD risk difference
Therapeutic equivalence of MYL-1402O to reference bevacizumab was demonstrated at week 18 analysis in the intent-to-treat population (primary efficacy analysis; Fig. 1). Overall response rates were similar between the MYL-1402O and reference bevacizumab treatment arms, and both EMA and FDA requirements for equivalence were met. The 95% confidence intervals of the risk difference were contained within the predefined equivalence margin of ± 12.5% (EMA requirement), and the 90% confidence intervals of the ratio of the overall response rate fell within the predefined equivalence margin of 0.73–1.36 (FDA requirement) [5].
Analyses at week 42 supported the therapeutic equivalence of MYL-1402O to reference bevacizumab [5]. The disease control rate in the MYL-1402O treatment arm was congruent with the rate in the bevacizumab treatment arm (Fig. 1). No significant differences between the MYL-1402O and reference bevacizumab arms were reported in median progression-free survival (7.6 months vs 9.0 months) and overall survival [OS] (70.0% vs 75.4%) rates; median OS was not reached in both treatment arms. The duration of response was consistent between treatment arms (7.7 vs 6.9 months) [5]. Data beyond week 42 indicated the OS in the MYL-1402O and reference bevacizumab arms was 71.9 and 77.3 weeks [4].
Tolerability and Safety
The safety and tolerability profile of MYL-1402O was similar to reference bevacizumab during a phase III trial in patients with stage IV non-squamous NSCLC. In total, 664 patients were analysed in the safety set, including 335 patients in the MYL-1402O treatment arm. In the MYL-1402O and reference bevacizumab treatment arms, treatment-related adverse events (TRAEs) were reported in 35.8% vs 35.0% of patients, serious TRAEs were reported in 5.1% vs 6.7% of patients, TRAEs leading to treatment discontinuation in 3.9% vs 4.0% of patients and TRAEs leading to death in 2.1% and 1.2% of patients. The most commonly reported TRAEs with an incidence ≥ 5% of patients were anaemia (6.6% vs 6.7%), thrombocytopenia (6.3% vs 5.5%) and diarrhoea (6.0% vs 3.6%) [4].
The incidence of adverse events of special interest (AESI) were generally similar between the MYL-1402O and reference bevacizumab treatment arms. The incidence of any-grade AESI at week 18 (i.e. when patients received combination therapy with MYL-1402O or reference bevacizumab and chemotherapy) in the MYL-1402O and reference bevacizumab treatment arms was 16.1% vs 20.1%; hypertension (3.6% vs 3.3%) and epistaxis (1.5% vs 5.2%) were the only AESI with an incidence ≥ 3% in any treatment arm. Grade ≥ 3 AESI were reported in 6.9% vs 7.0% of patients. At week 42 (i.e. when patients received monotherapy with MYL-1402O or reference bevacizumab), the incidence of any-grade (10.5% vs 8.0%) and grade ≥ 3 AESI (2.0% vs 2.5%) were consistent between treatment arms [4].
Immunogenicity
The immunogenicity of MYL-1402O was comparable to reference bevacizumab (Table 2). During a phase III trial in patients with stage IV non-squamous NSCLC, the incidence of treatment-emergent anti-drug antibodies (ADAs) was similar between the MYL-1402O and reference bevacizumab treatment arms (Table 2) [5]. In healthy male subjects, the majority (≥ 89%) of patients in the MYL-1402O, EU- and US-sourced reference bevacizumab treatment arms tested positive for ADAs on day 15. A similar decrease in ADA-positive subjects over time was observed across the trial arms [3]. Furthermore, in an analysis of the total trial population, higher levels of ADAs did not appear to have clinically significant effects on the pharmacokinetics of bevacizumab in healthy male subjects (Table 2) [3].
Conclusion
MYL-1402O is a bevacizumab biosimilar with similar physicochemical and functional properties to the reference product (Table 2). Based on the efficacy, tolerability and safety characteristics in clinical data available from patients with non-squamous NSCLC (Table 2), MYL-1402O (as Abevmy®) has been approved in the EU for all indications for which reference bevacizumab is approved (Table 1).
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
During the peer review process the manufacturer of MYL-1402O was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
Arnold Lee is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Footnotes
The manuscript was reviewed by: K. Araki, Department of Medical Oncology, Gunma Prefectural Cancer Center, Ota, Japan; P. Gascon, Department of Hematology-Oncology, University of Barcelona, Barcelona, Spain
The original version of this article was revised due to a retrospective Open Access order.
Change history
4/18/2022
A Correction to this paper has been published: 10.1007/s11523-022-00882-1
References
- 1.European Medicines Agency. Abevmy (bevacizumab): EU summary of product characteristics. 2021. https://www.ema.europa.eu/. Accessed 24 Nov 2021.
- 2.European Medicines Agency. Lextemy (bevacizumab): EU summary of product characteristics. 2021. https://www.ema.europa.eu/. Accessed 24 Nov 2021.
- 3.Hummel M, Bosje T, Shaw A, et al. A pharmacokinetics study of proposed bevacizumab biosimilar MYL-1402O vs EU-bevacizumab and US-bevacizumab. J Cancer Res Clin Oncol. 2021 doi: 10.1007/s00432-021-03628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Medicines Agency. Abevmy (bevacizumab): EU public assessment report. 2021. https://www.ema.europa.eu/. Accessed 24 Nov 2021.
- 5.Socinski MA, Waller CF, Idris T, et al. Phase III double-blind study comparing the efficacy and safety of proposed biosimilar MYL-1402O and reference bevacizumab in stage IV non-small-cell lung cancer. Ther Adv Med Oncol. 2021 doi: 10.1177/17588359211045845. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


