Abstract
Recently, Staphylococcus aureus strains with intermediate resistance to vancomycin, the antibiotic of last resort, have been described. Multiple changes in peptidoglycan turnover and structure contribute to the resistance phenotype. Here, we describe that structural changes of teichoic acids in the cell envelope have a considerable influence on the susceptibility to vancomycin and other glycopeptides. S. aureus cells lacking d-alanine esters in teichoic acids exhibited an at least threefold-increased sensitivity to glycopeptide antibiotics. Furthermore, the autolytic activity of the d-alanine mutant was reduced compared to the wild-type, and the mutant was more susceptible to the staphylolytic enzyme lysostaphin. Vancomycin inhibited autolysis at very high concentrations but neither in the wild-type nor in the mutant was the autolytic activity influenced in the range of the MIC. Mutant cells had a considerably higher capacity to bind vancomycin.
Many important antimicrobial agents such as β-lactam and glycopeptide antibiotics attack the components of bacterial cell wall turnover and synthesis. However, the increasing prevalence of strains of the major human pathogen Staphylococcus aureus with resistance to almost all available antibiotics has become a threatening problem (10). Recently, reduced susceptibilities to the glycopeptides vancomycin and teicoplanin, which represent the last options for the treatment of multidrug-resistant staphylococci have been reported among clinical S. aureus isolates (8, 17). While the glycopeptide resistance systems of enterococci are well characterized (1, 22), the mechanisms influencing the susceptibility to vancomycin and teicoplanin in staphylococci are less understood. Multiple changes in the cell wall involving the rate of peptidoglycan precursor synthesis, cell wall thickness, peptidoglycan cross-linking, and amount of muropeptides with deamidated glutamine residues have been reported to influence the sensitivity of S. aureus to glycopeptide antibiotics (6, 7). In vancomycin- and teicoplanin-resistant laboratory mutants, a dramatic increase of monomeric muropeptides containing the d-alanyl-d-alanine dipeptide, the binding motive of glycopeptide antibiotics, has been observed (18, 19). The binding sites in mature peptidoglycan compete with the d-alanyl-d-alanine dipeptides of lipid-bound murein precursors, the vancomycin target sites, for binding of vancomycin (18). Not only does vancomycin block peptidoglycan synthesis, but it also has the capacity to inhibit autolysins of the cell wall (18).
Besides peptidoglycan, the S. aureus cell wall contains polymers of alternating phosphate and alditol groups called teichoic acids (3). These polymer chains are either covalently connected to the peptidoglycan (wall teichoic acids) (15) or to membrane glycolipids (lipoteichoic acids) (4). The highly charged teichoic acids are involved in the control of autolysin activity (3). We have recently described an S. aureus mutant lacking d-alanine in the teichoic acids since the dltABCD operon responsible for the d-alanine transfer into teichoic acids was disrupted (14). This pathway is not involved in the synthesis of murein precursors. The mutant was sensitive to cationic pore-forming antimicrobial peptides such as nisin from Lactococcus lactis, α-defensins from the human immune system, and related peptides. Here, we report that the absence of d-alanine esters in teichoic acids has a profound influence on the susceptibility to glycopeptide antibiotics and on autolysin activity.
Activity of antibiotics against the dlt mutant and the parental strain.
We compared the MICs of various antibiotics for S. aureus Sa113 wild-type, dlt mutant, and dlt mutant complemented with plasmid pRBdlt1. Construction of the mutant and plasmid has been described recently (14). Serial threefold dilutions of the various antibiotics in LB broth (1% tryptone, 0.5% yeast extract, 0.5% NaCl [pH 7.0]) were inoculated with 1/100 volume of precultures which had been adjusted to the same cell density. Tubes with 400-μl aliquots were shaken at 37°C, and after 8 h the A600 was determined. The values in Table 1 represent the antibiotic concentrations causing a 75% reduction of growth as calculated by interpolation. The assay was carried out at least two times with several identical samples, yielding very reproducible results. While the mutant was only slightly more sensitive (less than twofold) than the wild type to methicillin, cefazolin, erythromycin, and tetracycline, a considerable increase of the susceptibility (more than threefold) to the glycopeptide antibiotics vancomycin, teicoplanin, and balhimycin was observed. Complementation of the mutant with plasmid pRBdlt1 bearing the intact dltABCD operon restored the tolerance to glycopeptide antibiotics to wild-type levels. Balhimycin belongs to the vancomycin class of glycopeptides. It is not approved for medical use yet (11, 13).
TABLE 1.
Susceptibility of S. aureus Sa113 to various antibiotics
| Antibiotic | MIC (μg/ml) for:
|
||
|---|---|---|---|
| Wild type | dltA::spc | dltA::spc(pRBdlt1) | |
| Vancomycin | 1.4 | 0.39 | 1.4 |
| Teicoplanin | 1.3 | 0.40 | 1.1 |
| Balhimycin | 1.4 | 0.37 | 1.3 |
| Methicillin | 0.72 | 0.66 | 0.67 |
| Cefazolin | 0.11 | 0.062 | 0.10 |
| Erythromycin | 0.30 | 0.17 | 0.20 |
| Tetracycline | 0.16 | 0.097 | 0.10 |
Autolytic properties and lysostaphin-induced lysis.
The d-alanine esters introduce positively charged amino groups into the otherwise negatively charged teichoic acids. In Bacillus subtilis, the absence of d-alanine from the polymers has been reported to cause an alteration of the activity of autolytic enzymes, which are considered to bind to teichoic acids by ionic interactions (21). Since vancomycin has been shown to inhibit the autolytic activity of S. aureus strains at high concentrations (18), we compared the autolytic properties of the parental and the mutant strain and a possible impact of any changes on the sensitivity to vancomycin.
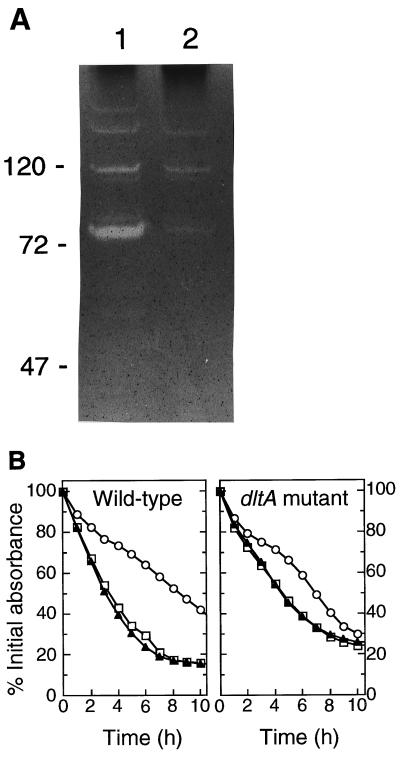
Cell wall-lytic enzymes were isolated from wild-type and dlt mutant bacteria and visualized by zymographic analysis as described elsewhere (12, 16). Briefly, autolysins were isolated from bacteria grown for 12 h in BM broth (LB broth supplemented with 0.1% glucose and 0.1% K2HPO4) by boiling the cells with 1% sodium dodecyl sulfate (SDS) and separated by Tricine-SDS-polyacrylamide gel electrophoresis with gels containing heat-inactivated S. aureus dlt mutant cells (2 mg/ml). Autolytic enzyme activities (clearing zones) were visualized by staining the gels with methylene blue. The autolysin patterns of the two strains were very similar (Fig. 1A). However, the clearing zones from the mutant were much smaller than those of the wild type, suggesting that autolysins were less efficiently detached from the mutant cell walls. No differences were observed in the autolysin patterns of the supernatants (data not shown).
FIG. 1.
Zymographic analysis (A) and spontaneous autolysis with or without vancomycin (B) of S. aureus Sa113 wild type and dltA mutant. (A) Equal amounts of cell wall-associated proteins from S. aureus Sa113 wild type (lane 1) and from the isogenic dltA mutant (lane 2) were applied to an SDS-polyacrylamide gel containing heat-inactivated staphylococcal cells. Molecular mass standards are indicated in kilodaltons. (B) Equal amounts of cells were incubated without vancomycin (triangles), with 1 μg of vancomycin per ml (squares), or with 10 μg of vancomycin per ml (circles). The values are given as percentages of the initial A600.
In order to compare the spontaneous autolysis of wild-type S. aureus and the dlt mutant, the strains were grown until late logarithmic phase in BM broth and washed twice with ice-cold water. Equal amounts of bacteria were resuspended in sodium phosphate buffer (10 mM, pH 7.0), and the decrease of the A600 was monitored during incubation at 30°C. When no vancomycin was added, the dltA mutant exhibited a reduced rate of autolysis (decrease of the cell density of 9.6%/h in the mutant vs. 16%/h in the wild type in the linear parts of the curves). Binding of the cationic autolysins to anionic teichoic acids is regarded as a control mechanism that reduces their activity (4); the stronger negative charge of the teichoic acids in the mutant is thus likely to further increase the inhibition of autolysins. Addition of 10 μg of vancomycin per ml caused a profound inhibition of the spontaneous autolysis of both strains. However, a vancomycin concentration of 1 μg/ml, which is close to the MIC had no influence on the autolytic behavior of both the parental and mutant strain. Therefore, it seems unlikely that vancomycin exerts its higher activity against the mutant via increased inhibition of autolysis.
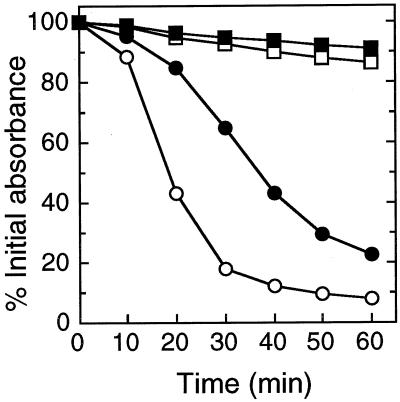
While the activity of endogenous cell wall-lytic enzymes seemed to be reduced, the mutant was considerably more sensitive to the glycyl-glycyl endopeptidase lysostaphin, a bacteriocin-like enzyme from Staphylococcus simulans bv. staphylolyticus (20) (Fig. 2). Its activity was analyzed as described above for spontaneous autolysis except that bacteria were washed and resuspended in phosphate-buffered saline (10 mM sodium phosphate buffer containing 130 mM NaCl; pH 7.4). This observation suggests a role of anionic teichoic acids in the binding of lysostaphin, which has previously been shown to bind to the cell wall via its cationic C-terminal domain (2). Whether the polymers represent the receptor molecules or have an indirect influence on lysostaphin binding or activity remains to be analyzed.
FIG. 2.
Lysostaphin-mediated lysis of wild type (solid symbols) and dltA mutant (empty symbols). Equal amounts of cells were incubated without (squares) or with (circles) lysostaphin (1 μg/ml). The values are given as percentages of the initial A600.
Vancomycin binding capacity.
Since the altered surface charge of the mutant might influence the affinity for vancomycin, the capacities of wild-type and mutant cells to bind vancomycin were compared. Bacteria were grown in BM broth until late logarithmic phase, washed twice in sodium phosphate buffer (100 mM, pH 7.0), and resuspended in the same buffer to a final cell density of 2.5 × 1010/ml. After addition of vancomycin (1 μg/ml), the cells were incubated for 20 min at ambient temperature and subsequently removed by centrifugation. The amounts of unbound antibiotic were determined by reversed-phase high-performance liquid chromatography using a linear gradient of 0 to 100% acetonitrile in 0.1% trifluoroacetic acid over 20 column volumes on a Spherisorb ODS2 column (Grom Analytik, Herrenberg, Germany). While in the supernatant of the wild type (means ± standard deviations of three independent determinations each) 54% ± 3% of the applied vancomycin was detected, the supernatant of the mutant contained only 39% ± 7%, which indicates that the binding capacity for vancomycin was increased in the dlt mutant cells.
The phosphate contents of the cell walls, which provide a measure for the teichoic acid content, were very similar in the wild type and dlt mutant (means ± standard deviations of three independent determinations each: 0.69 ± 0.05 μmol/mg cell wall dry mass in the wild-type versus 0.65 ± 0.05 μmol/mg in the dlt mutant). Cell walls were isolated and analyzed as described recently (14) except that cells and cell walls were washed and resuspended in water instead of sodium acetate buffer and that extraction with SDS was repeated twice.
Concluding comments.
Our studies demonstrate that teichoic acids have a considerable influence on the susceptibility to vancomycin and related substances. This is consistent with two recently published studies. (i) Sieradzki and Tomasz demonstrated that treatment of cell walls with hydrofluoric acid, which removes the cell wall teichoic acids, reduces the binding capacity of a resistant S. aureus strain for vancomycin (18). (ii) Gutmann and coworkers described that almost twice the normal amount of d-alanine was attached to lipoteichoic acids of vancomycin-resistant Enterococcus faecium strains (5). Since glycopeptide antibiotics bind to d-alanyl-d-alanine dipeptides, it is tempting to speculate that the d-alanine substituents of teichoic acids constitute alternative binding sites for the antibiotics. However, the dlt mutant bound more vancomycin than the wild-type strain. Moreover, the teichoic acid d-alanine esters contain positively charged amino groups while the terminal d-alanine residues of murein peptides contain negatively charged carboxyl groups. Therefore, a direct binding of vancomycin to the d-alanine esters of teichoic acids is unlikely.
We found no indications for an autolysin-mediated inhibition of the dlt mutant by vancomycin but an increased binding capacity of mutant cells for vancomycin, which may at least in part be responsible for the sensitivity of the mutant to glycopeptide antibiotics. Since the teichoic acid content and the degree of d-alanylation vary among S. aureus strains (9), an increased amount of d-alanine esters may contribute to vancomycin resistance in S. aureus.
Acknowledgments
We thank Vera Augsburger for excellent technical assistance and Stefan Pelzer for providing purified balhimycin.
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (GO 371/3-1).
REFERENCES
- 1.Arthur M, Reynolds P, Courvalin P. Glycopeptide resistance in enterococci. Trends Microbiol. 1996;4:401–407. doi: 10.1016/0966-842X(96)10063-9. [DOI] [PubMed] [Google Scholar]
- 2.Baba T, Schneewind O. Target cell specificity of a bacteriocin molecule: a C-terminal signal directs lysostaphin to the cell wall of Staphylococcus aureus. EMBO J. 1996;15:4789–4797. [PMC free article] [PubMed] [Google Scholar]
- 3.Fischer W. Lipoteichoic acid and teichoic acid biosynthesis. Targets of new antibiotics? In: Hakenbeck R, editor. New targets for new antimicrobial agents. Heidelberg, Germany: Spektrum Akademischer Verlag; 1997. pp. 47–50. [Google Scholar]
- 4.Fischer W. Physiology of lipoteichoic acids in bacteria. Adv Microb Physiol. 1988;29:233–302. doi: 10.1016/s0065-2911(08)60349-5. [DOI] [PubMed] [Google Scholar]
- 5.Gutmann L, Al-Obeid S, Billot-Klein D, Ebnet E, Fischer W. Penicillin tolerance and modification of lipoteichoic acid associated with expression of vancomycin resistance in VanB-type Enterococcus faecium D366. Antimicrob Agents Chemother. 1996;40:257–259. doi: 10.1128/aac.40.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanaki H, Kuwahara-Arai K, Boyle-Vavra S, Daum R S, Labischinski H, Hiramatsu K. Activated cell wall synthesis is associated with vancomycin resistance in methicillin-resistant Staphylococcus aureus clinical strains Mu3 and Mu50. J Antimicrob Chemother. 1998;42:199–209. doi: 10.1093/jac/42.2.199. [DOI] [PubMed] [Google Scholar]
- 7.Hanaki H, Labischinski H, Inaba Y, Kondo N, Murakami H, Hiramatsu K. Increase in glutamine-non-amidated muropeptides in the peptidoglycan of vancomycin-resistant Staphylococcus aureus strain Mu50. J Antimicrob Chemother. 1998;42:315–320. doi: 10.1093/jac/42.3.315. [DOI] [PubMed] [Google Scholar]
- 8.Hiramatsu K, Aritaka N, Hanaki H, Kawasaki S, Hosoda Y, Hori S, Fukuchi Y, Kobayashi I. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet. 1997;350:1670–1673. doi: 10.1016/S0140-6736(97)07324-8. [DOI] [PubMed] [Google Scholar]
- 9.Jenni R, Berger-Bächi B. Teichoic acid content in different lineages of Staphylococcus aureus NCTC8325. Arch Microbiol. 1998;170:171–178. doi: 10.1007/s002030050630. [DOI] [PubMed] [Google Scholar]
- 10.Lowy F D. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 11.Nadkarni S R, Patel M V, Chatterjee S, Vijayakumar E K, Desikan K R, Blumbach J, Ganguli B N, Limbert M. Balhimycin, a new glycopeptide antibiotic produced by Amycolatopsin sp. Y-86,21022. Taxonomy, production, isolation and biological activity. J Antibiot (Tokyo) 1994;47:334–341. doi: 10.7164/antibiotics.47.334. [DOI] [PubMed] [Google Scholar]
- 12.Oshida T, Tomasz A. Isolation and characterization of a Tn551 autolysis mutant of Staphylococcus aureus. J Bacteriol. 1992;174:4952–4959. doi: 10.1128/jb.174.15.4952-4959.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pelzer S, Süssmuth R, Heckmann D, Recktenwald J, Huber P, Jung G, Wohlleben W. Identification and analysis of the balhimycin biosynthetic gene cluster and its use for manipulating glycopeptide biosynthesis in Amycolatopsis mediterranei DSM5908. Antimicrob Agents Chemother. 1999;43:1565–1573. doi: 10.1128/aac.43.7.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peschel A, Otto M, Jack R W, Kalbacher H, Jung G, Götz F. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins and other antimicrobial peptides. J Biol Chem. 1999;274:8405–8410. doi: 10.1074/jbc.274.13.8405. [DOI] [PubMed] [Google Scholar]
- 15.Pooley H M, Karamata D. Teichoic acid synthesis in Bacillus subtilis: genetic organization and biological roles. In: Ghuysen J-M, Hakenbeck R, editors. Bacterial cell wall. Amsterdam, The Netherlands: Elsevier Science B.V.; 1994. pp. 187–197. [Google Scholar]
- 16.Schneewind O, Model P, Fischetti V A. Sorting of protein A to the staphylococcal cell wall. Cell. 1992;70:267–281. doi: 10.1016/0092-8674(92)90101-h. [DOI] [PubMed] [Google Scholar]
- 17.Sieradzki K, Roberts R B, Haber S W, Tomasz A. The development of vancomycin resistance in a patient with methicillin-resistant Staphylococcus aureus infection. N Engl J Med. 1999;340:517–523. doi: 10.1056/NEJM199902183400704. [DOI] [PubMed] [Google Scholar]
- 18.Sieradzki K, Tomasz A. Inhibition of cell wall turnover and autolysis by vancomycin in a highly vancomycin-resistant mutant of Staphylococcus aureus. J Bacteriol. 1997;179:2557–2566. doi: 10.1128/jb.179.8.2557-2566.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sieradzki K, Tomasz A. Suppression of glycopeptide resistance in a highly teicoplanin-resistant mutant of Staphylococcus aureus by transposon inactivation of genes involved in cell wall synthesis. Microb Drug Resist. 1998;4:159–168. doi: 10.1089/mdr.1998.4.159. [DOI] [PubMed] [Google Scholar]
- 20.Thumm G, Götz F. Studies on prolysostaphin processing and characterization of the lysostaphin immunity factor (Lif) of Staphylococcus simulans biovar staphylolyticus. Mol Microbiol. 1997;23:1251–1265. doi: 10.1046/j.1365-2958.1997.2911657.x. [DOI] [PubMed] [Google Scholar]
- 21.Wecke J, Madela K, Fischer W. The absence of d-alanine from lipoteichoic acid and wall teichoic acid alters surface charge, enhances autolysis and increases susceptibility to methicillin in Bacillus subtilis. Microbiology. 1997;143:2953–2960. doi: 10.1099/00221287-143-9-2953. [DOI] [PubMed] [Google Scholar]
- 22.Woodford N. Glycopeptide-resistant enterococci: a decade of experience. J Med Microbiol. 1998;47:849–862. doi: 10.1099/00222615-47-10-849. [DOI] [PubMed] [Google Scholar]