Abstract
Introduction.
Given that the US Food and Drug Administration (FDA) authorized reduced exposure messaging to be used in IQOS marketing, we examined: 1) IQOS ad content; 2) advertising media channels; and 3) changes in advertising efforts over time.
Methods.
We conducted a mixed-methods study regarding IQOS ad content (headlines, themes, images), ad occurrence characteristics (including content, media channels, and adspend) in the US from August 2019 to April 2021 using Numerator advertising/marketing data.
Results.
Across 24 unique ads and 591 occurrences (84.6% online, 8.0% print, 7.4% mobile), there was $4,902,110 total adspend (98.9% allocated to print). Across unique ads, occurrences, and adspend, prominent themes included “real tobacco” (45.8%, 47.4%, 30.1%, respectively), less odor/ash (29.2%, 21.0%, 29.9%), and switching from cigarettes (25.0%, 19.5%, 69.4%), and images mainly featured the product alone (58.2%, 61.4%, 99.5%) or with women (25.0%, 19.1%, 0.3%). Per occurrences and adspend, the most prominent media channel themes (e.g., magazine/website topics) were technology (19.3%, 10.6%), women’s fashion (18.1%, 26.2%), weather/news (9.0%, 15.3%), and entertainment/pop culture/gaming (8.5%, 23.1%). Ad themes appearing only post-FDA authorization included switching from traditional cigarettes, same-day/home-delivery, convenience (e.g., use indoors), reduced exposure to some dangerous substances, science/research, and distinction from e-cigarettes. Overall adspend per occurrence increased post-authorization (p=.016); the highest adspend per unique ad (69.3% of total) focused on ads featuring reduced exposure.
Conclusions.
Regulatory efforts must be informed by ongoing surveillance of IQOS marketing efforts and its impacts, particularly how specific consumer subgroups (e.g., tobacco non-users, women, young people) are impacted by marketing exposure.
Keywords: Tobacco industry, tobacco control, marketing, heated tobacco products
INTRODUCTION
Heated tobacco products (HTPs)1,2 represent a growing segment of the global tobacco market.3–7 The largest market share of HTPs is held by Philip Morris International’s (PMI) product, IQOS, and their accompanying disposable tobacco sticks (HEETS).8 IQOS, first released in Japan in 2014, is now sold in over 60 countries and has over 17 million users.9
After considering the premarket tobacco application (PMTA) for IQOS, FDA issued an order in April 2019 authorizing its marketing in the US.10 Since Philip Morris USA (PM – under parent company Altria) began to expand IQOS markets in the US in Fall 2019,11 IQOS has penetrated markets in Georgia, Virginia, North Carolina, and South Carolina. Even prior to its entry into the US, adult awareness of HTPs increased from 9.3% to 12.4% from 2016 to 2017, with ever and current use roughly doubling to 2.2% and 1.1%, respectively.3,12
In the US, specific FDA authorization is required to advertise a tobacco product as “reduced harm” or “reduced exposure”, which is pursued via the modified risk tobacco product (MRTP) application pathway.13 Requirements for obtaining authorization to use reduced exposure messaging is less rigorous compared to reduced harm. On July 7, 2020, FDA authorized IQOS to use reduced exposure messaging in its marketing14 but did not authorize the use of reduced risk or harm messaging due to insufficient evidence of reduced harm or risk of tobacco-related diseases.15 Among the authorized reduced exposure claims are: “IQOS heats tobacco but does not burn it”, “[it] significantly reduces the production of harmful and potentially harmful chemicals”, and “scientific studies have shown that switching completely from cigarettes to the IQOS system significantly reduces your body’s exposure to harmful or potentially harmful chemicals”.16 Unfortunately, reduced exposure messages may be interpreted as reduced risk,17 messages regarding “switching completely” may be misperceived by consumers,17,18 and IQOS users are unlikely to “switch completely”.18
Given the HTP’s potential harm to public health,7,19–21 ongoing surveillance is needed to estimate the impact of HTPs and related marketing;22,23 in fact, FDA requires PM to conduct post-marketing surveillance to monitor public health impact.24 Marketing strategies of HTPs are important to examine, as these strategies impact how HTPs are perceived and used. Advertising attracts new users,25–29 promotes continued use,30–32 builds brand loyalty,33 and expands tobacco markets.27,28,30,33 For newly introduced products such as HTPs, advertising is fundamental to their success,34 as the first exposure is the most influential for short-term sales or gains.35
Marketing campaigns, such as those developed by the tobacco industry,36,37 are based on market research, which segments populations based on some type of similarity, such as sociodemographics, behaviors, reactions to marketing messages, or psychographics (e.g., attitudes, needs, wants, goals, lifestyles).37–40 These strategies inform both: 1) the channels through which advertising campaigns are disseminated; and 2) the messages used to attract consumers. In terms of media channels, outside of point-of-sale advertising,41,42 PMI has used various channels to market IQOS, including traditional, new, and social media,43–49 as well as more novel approaches such as brand ambassadors.50,51
Regarding messaging strategies, HTPs have been marketed globally as an alternative to combustible cigarettes, often using claims such as “cleaner” and “reduced-risk” products,50 despite limited evidence of such claims.2,52 Other messaging strategies have focused on values and lifestyle characteristics, such as being a high-end, fashionable alternative to smoking; a device for tech-savvy consumers;53 or as a clean, chic, and pure product, which seemingly aligns with cultural values of order, cleanliness, quality, and respect for others in Japan.54 These are just a few examples of messaging strategies previously used by PMI.
With FDA authorization to market IQOS using reduced exposure messaging, it is critical to understand the extent to which this authorization has shifted both PM’s messaging strategies and the channels through which PM is disseminating its marketing, as these changes likely impact targeted consumers. Thus, this study examined: 1) IQOS ad content; 2) prominent media channels through which IQOS is advertised (per number of occurrences, adspend, and digital impressions); and 3) changes in advertising efforts (i.e., content, adspend, media channels) over time, particularly before versus after FDA authorized IQOS to use modified exposure messaging.
METHODS
Data Source
Data for this study were purchased from Numerator (formerly Competitrack), a media spend tracking company that tracks over 2,600 English language media outlets in the US, which has been used in previous public health and tobacco-related research.55–57 Numerator systematically tracks a broad range of marketing channels, including print ads (in newspapers, magazines, and inserts), TV, radio, cinema, mobile, online displays/videos, outdoor, and social media, as well as direct marketing on a contractual basis.58 A defined classification system is used to code the advertiser, unique ad content (i.e., visual content/imagery, headlines/other prominent text), if the ad was “recut” (i.e., revision of a prior/existing ad), station/channel, market (e.g., national or designated market area), number of occurrences of the ads (i.e., ad campaigns, which could use: [a] existing or recut ad content or unique ad content and [b] similar or distinct channels and markets), run dates, cost estimates (i.e., adspend), and number of digital impressions (i.e., digital views or engagements with the ads as derived by Web-indexing technology, audience measurement data from comScore, and estimated cost per thousand impressions for each monitored site), among other variables.58
Procedures
We conducted a mixed-methods study regarding IQOS adspend (i.e., amount of money spent on advertising), adspend per media channel, and messaging strategies. The sampling frame included all data regarding IQOS advertising since its launch in the US in August 2019 (first IQOS ad) to April 2021.
Ads during this timeframe included only print, online display, and mobile. Print ads are determined via manual review of hard copy and digital publications within top national newspapers and magazines sourced from multiple markets to identify regional and demographic buys.58 Online display ads are ad occurrences for display/banner advertising for Interactive Advertising Bureau standard ad sizes (e.g. medium rectangle, leaderboard)59 running on websites in desktop/laptop environments, recorded using proprietary Web indexing technology involving daily page visits across thousands of sites, including top ad-supported websites (e.g., Amazon.com).58 Mobile ads include ad occurrences running in mobile environments (phones/tablets), including display/banner advertising on websites and apps and video advertising served against video content on websites, captured by software monitoring of top ad-supported mobile-enabled websites/apps, as determined by high-traffic sites/apps.58
Data Management & Analysis
Ad occurrences were attached to metadata regarding whether it featured unique ad content versus recut ad content, run date of the occurrence, media type (i.e., print, online display, mobile), media channel (e.g., magazine, website), ad size, adspend, and number of digital impressions (for online display and mobile; see Appendix A for sample ad descriptions).
To describe ad content, recut ads were categorized alongside the respective original ad. For each unique individual ad, we recorded the headline (see Table 1 for headlines) and then assigned headline themes (see Table 2 for headline themes). Two members of the authorship team (CJB and KFR) developed a list of themes (which were not discrete categories) and applied them independently to all headlines (intercoder reliability Kappa=100%). We also confirmed image content (which were designed as discrete codes) with the data provided by Numerator.
Table 1.
Ad Headlines and Example Content Pre- and Post-MRTP, August 2019 to April, 2021*
| Ad Headline | Occurrences (of 591 total) N (%) |
|---|---|
| Both Pre- and Post-MRTP | |
| Get IQOS | 128 (21.7) |
| Pre-MRTP | |
| The Future of Tobacco is Here | 89 (15.1) |
| Real Tobacco Meets Innovative Technology | 42 (7.3) |
| Get IQOS Real Tobacco Less Odor | 17 (2.9) |
| Meet IQOS Real Tobacco No Ash Less Odor a | 11 (1.9) |
| Meet IQOS The Future of Real Tobacco Has Arrived Real Tobacco No Ash Less Odor | 13 (2.2) |
| The Future of Real Tobacco Has Arrived | 6 (1.0) |
| Post-MRTP | |
| Get IQOS Real Heated Tobacco is Here with Less Odor | 62 (10.5) |
| IQOS Uses Heatsticks Made with Real Tobacco§ | 61 (10.3) |
| With Heat Control Technology Real Tobacco is Heated Never Burned | 39 (6.6) |
| Reduce your Body’s Exposure to Harmful Chemicals§ b | 35 (5.9) |
| Why Make the Switch from Cigarettes to IQOS? | 16 (2.7) |
| We Built a New Way to Enjoy Tobacco with Heat Control Technology | 15 (2.5) |
| Real Heated Tobacco is Here with Less Odor | 14 (2.4) |
| Purchase an IQOS Bundle with Same-Day Delivery in Select Cities§ | 11 (1.9) |
| IQOS Is the Only Tobacco Product of Its Kind | 10 (1.7) |
| Too Busy to Make an Appointment? Experience IQOS at Home | 10 (1.7) |
| Order an IQOS Bundle with Same-Day Delivery in Select Cities | 6 (1.0) |
| Half Off! Use Promo Code Device50 At Getiqos.Com | 3 (0.51) |
| Experience Heated Tobacco Sooner than Ever | 2 (0.34) |
| It’s Not a Vape It’s Not an E-Vape It’s Real Tobacco with Less Odor and No Ash c | 1 (0.17) |
|
a 2nd highest adspend: $1,294,900 (26.4% overall; 97.8% pre-MRTP) ƚ Description: Black background with white text: “Meet IQOS® REAL TOBACCO | NO ASH | LESS ODOR” and image of black device and holder. Warnings included: Top of ad: “WARNING: This product contains nicotine. Nicotine is an addictive chemical.” Bottom of ad: “SURGEON GENERAL’S WARNING: Smoking by Pregnant Women May Result in Fetal Injury, Premature Birth, and Low Birth Weight.” |
b Highest adspend: $3,395,900 (69.3% overall; 94.9% post-MRTP) ƚ Description: White background with black text: “Reduce your body’s exposure to harmful chemicals by switching completely from cigarettes to IQOS®. The IQOS® system heats tobacco but does not burn it. This significantly reduces the production of harmful and potentially harmful chemicals. Scientific studies have shown that switching completely from conventional cigarettes to the IQOS® system significantly reduces your body’s exposure to harmful or potentially harmful chemicals. Learn more at getIQOS.com” and image of white device and three boxes of HeatSticks® (one grey, two turquoise – one labeled “Smooth Menthol”) Warnings included: Top of ad: “WARNING: This product contains nicotine. Nicotine is an addictive chemical.” Bottom of ad: “SURGEON GENERAL’S WARNING: Smoking by Pregnant Women May Result in Fetal Injury, Premature Birth, and Low Birth Weight.” |
c 3rd highest adpsend: $155,800 (3.2% overall; 4.4% post-MRTP) ƚ Description: White background with black text: “It’s not a vape. It’s not an e-cig. It’s real tobacco with less odor and no ash. IQOS® IS THE ONLY PRODUCT OF ITS KIND THAT CAN MAKE THIS CLAIM. The IQOS® system heats tobacco but does not burn it. This significantly reduces the production of harmful and potentially harmful chemicals. Scientific studies have shown that switching completely from conventional cigarettes to the IQOS® system significantly reduces your body’s exposure to harmful or potentially harmful chemicals. Want to learn more? Visit getIQOS.com” and with image of white holder and device and green and white Marlboro box. Warnings included: Top of ad: “WARNING: This product contains nicotine. Nicotine is an addictive chemical.” Bottom of ad: “Smoking Causes Lung Cancer, Heart Disease, Emphysema, And May Complicate Pregnancy.” |
Run date beginning before July 7, 2020 (pre-MRTP) vs. after (post-MRTP).
Ad headline represents 2 unique ads.
Contact corresponding author for ad images.
Table 2.
Characteristics of Unique Ads (N=24), August 2019 to April 2021
| Total N=24 | Pre-MRTP* N=9 | Post-MRTP N=15 | ||
|---|---|---|---|---|
| Variable | N (%) or M (SD) | N (%) or M (SD) | N (%) or M (SD) | p |
| Ad Headline Theme, N (%) § | ||||
| Real tobacco | 11 (45.8) | 6 (66.7) | 5 (33.3) | .113 |
| Less odor/ash | 7 (29.2) | 3 (33.3) | 4 (26.7) | .728 |
| Switch | 6 (25.0) | -- | 5 (33.3) | -- |
| Same-day/home delivery | 5 (20.8) | -- | 5 (33.3) | -- |
| Convenience (e.g., use indoors) | 4 (16.7) | -- | 4 (26.7) | -- |
| Not burned/heat control | 4 (16.7) | 1 (11.1) | 3 (20.0) | .572 |
| Includes tag: Get IQOS | 3 (12.5) | 2 (22.2) | 1 (6.7) | .265 |
| Future | 3 (12.5) | 3 (33.3) | -- | -- |
| Innovation/Technology | 3 (12.5) | 1 (11.1) | 2 (13.3) | .873 |
| Reduced exposure | 3 (12.5) | -- | 3 (20.0) | -- |
| Enjoyment/satisfaction | 1 (4.2) | 1 (11.1) | -- | -- |
| Promotions (e.g., half off) | 1 (4.2) | -- | 1 (6.7) | -- |
| Science/research | 1 (4.2) | -- | 1 (6.7) | -- |
| Distinct from e-cigarettes | 1 (4.2) | -- | 1 (6.7) | -- |
| Ad Visual/Image Theme, N (%) | .451 | |||
| Product | 14 (58.3) | 6 (66.7) | 8 (53.3) | |
| Woman/Product | 6 (25.0) | 1 (11.1) | 5 (33.3) | |
| Text | 2 (8.3) | 1 (11.1) | 1 (6.7) | |
| Man/Product | 1 (4.2) | -- | 1 (6.7) | |
| Woman/Man/Product | 1 (4.2) | 1 (11.1) | -- | |
| Spend per Ad ($ in thousands), M (SD) | 204.3 (596.7) | 147.9 (143.4) | 238.1 (689.9) | .729 |
| Impressions per Ad (in thousands), M (SD) | 1051.5 (1350.8) | 1546.1 (1798.1) | 747.1 (944.3) | .218 |
Run date beginning before July 7, 2020 (pre-MRTP) vs. after (post-MRTP).
Not discrete categories.
Across ad occurrences, we also categorized media channels by theme (e.g., news, weather, fashion, technology; see Appendix B for media channel themes). Two members of the authorship team (CJB and CNW) developed a list of themes (which were discrete categories) and applied them independently to all media channels (intercoder reliability Kappa=91%); discordantly coded channels were discussed and recoded with consensus. Parent companies of the media channels were also coded to show the major companies distributing IQOS marketing.
Adspend estimates are based on the following metrics: for print, open rates from multiple media monitoring sources (e.g., Nielsen, SQAD);58 for online display, top-line digital spend from an industry source to estimate aggregate spend for the trackable digital web-based universe for the given run week, projected spend per site, and media value (per ad frequency and other proprietary inputs);58 and for mobile, top-line digital spend from an industry source to estimate aggregate spend for the trackable app-spend universe for the given run week, projected spend per app, and media value.58
The run date of each occurrence was categorized by their begin date as either before or after July 7, 2020, reflecting the pre- versus post-MRTP authorization periods. Adspend and digital impressions were also characterized by quarter, starting in 2019 Quarter 3 (July-Sept) to 2021 Quarter 2 (Apr-incomplete).
Quantitative data were analyzed using SPSS 26.0,60 with alpha set at .05. To explore ad content, descriptive statistics were used to characterize ad headline themes and visual themes, as well as average adspend per ad and digital impressions per ad. We then analyzed these themes using bivariate analyses to characterize differences pre- versus post-MRTP. To examine prominent media channels and themes of media channels through which IQOS is advertised, we conducted descriptive analyses, characterizing the occurrences, which included metrics regarding media type, ad headlines used, ad headline and visual themes, media channels, ad size, average adspend per occurrence, and average number of digital impressions per occurrence. Bivariate analyses were then used to examine occurrences launched pre-versus post-MRTP. Subsequently, we conducted analyses, characterizing adspend by media type, ad headlines used, ad headline and visual themes, and media channels. We also explored digital impressions by media channel.
RESULTS
Across the study timeframe, there were 24 unique ads (i.e., unique content) and 591 ad occurrences, distributed across print, online, and mobile. These ads were distributed to the entire general US market (rather than geographically restricted markets). During this period, there was $4,902,110 total adspend and 22,029,900 impressions.
Ad Content Characteristics
Table 1 provides the list of unique ad headlines, as well as the number of occurrences they represent (see Appendix A for example ad content). Most headlines were used only once across ads (albeit with considerable overlap in content), and all but one of the headlines (i.e., “Get IQOS”) were exclusive to either the pre- or post-MRTP period. Table 2 shows that references to “real tobacco” occurred in 45.8% (11/24) of unique ads, with other prominent references including less odor/ash (29.2%; 7/24), switching from cigarettes (25.0%; 6/24), same-day/home delivery (20.8%; 5/24), convenience (16.7%; 4/24), and not burned/heat control (16.7%; 4/24). Themes only appearing post-MRTP included switching from traditional cigarettes, same-day/home delivery, convenience (e.g., use indoors), reduced exposure, science/research, and distinction from e-cigarettes. The majority of ads featured only the product itself (58.3%; 14/24), with the product being featured with a woman in 25.0% (6/24), with a man in 4.2% (1/24), and with both a man and woman in 4.2% of the ads (1/24). Average adspend per unique ad was $19,530 (SD=$45,530), and the average number of digital impressions per unique ad was 48,400 (SD=54,550), with no differences pre- versus post-MRTP. The highest adspend per unique ad was $3,395,900 ($69.3%), with the headline “Reduce your Body’s Exposure to Harmful Chemicals” (Table 1).
Ad Occurrence Characteristics
Displayed in Table 3, most occurrences (84.6%) were via online displays, with print and mobile representing ~8% of occurrences, respectively. The number of occurrences per ad headline theme varied, with prominent messages including “real tobacco” and “get IQOS” (47.4% and 35.0% of occurrences, respectively) – both pre- and post-MRTP – followed by less odor/ash (21.0%), switching (19.5%), future (18.3%), innovation/technology (17.8%), convenience (15.6%), and not burned/heat control (13.5%). Noteworthy is that headlines with themes emphasizing real tobacco, switching, convenience, not burned, same-day/home delivery, enjoyment/satisfaction, reduced exposure, and science/research base represented a greater proportion of ads post- versus pre-MRTP (p’s<.05). Also noteworthy is that, while ad visual themes were largely product-centric for both periods, more ads depicted people, particularly women, post- versus pre-MRTP (p<.001). The majority of occurrences depicted new ads (59.2%), especially in the post-MRTP period (68.0%). Across occurrences, ad sizes were quite large, being at least medium rectangle (i.e., 300 × 250 pixels59).
Table 3.
Characteristics of Ad Occurrences (N=591), August 2019 to April 2021
| Total N=591 | Pre-MRTP* N=269 | Post-MRTP N=322 | ||
|---|---|---|---|---|
| Variable | N (%) or M (SD) | N (%) or M (SD) | N (%) or M (SD) | p |
| Media Type, N (%) | <.001 | |||
| Online Display | 500 (84.6) | 253 (94.1) | 247 (76.7) | |
| 47 (8.0) | 11 (4.1) | 36 (11.2) | ||
| Mobile | 44 (7.4) | 5 (1.9) | 39 (12.1) | |
| Ad Headline Theme, N (%) § | ||||
| Real tobacco | 281 (47.5) | 107 (39.8) | 174 (54.0) | .001 |
| Includes tag: Get IQOS | 207 (35.0) | 96 (35.7) | 111 (34.5) | .758 |
| Less odor/ash | 124 (21.0) | 48 (17.8) | 76 (23.6) | .087 |
| Switch | 115 (19.5) | -- | 115 (35.7) | -- |
| Future | 108 (18.3) | 108 (40.1) | -- | -- |
| Innovation/Technology | 106 (17.9) | 47 (17.5) | 59 (18.3) | .789 |
| Convenience (e.g., use indoors) | 92 (15.6) | 7 (2.6) | 85 (26.4) | <.001 |
| Not burned/heat control | 80 (13.5) | 5 (1.9) | 75 (23.3) | <.001 |
| Reduced exposure | 45 (7.6) | -- | 45 (14.0) | -- |
| Same-day/home delivery | 29 (4.9) | -- | 29 (9.0) | -- |
| Enjoyment/satisfaction | 15 (2.5) | 2 (0.7) | 13 (4.0) | .011 |
| Science/research | 10 (1.7) | -- | 10 (3.1) | -- |
| Promotions (e.g., half off) | 3 (0.5) | -- | 3 (0.9) | -- |
| Distinct from e-cigarettes | 1 (0.2) | -- | 1 (0.3) | -- |
| Ad Visual/Image Theme, N (%) a | <.001 | |||
| Product | 363 (61.4) | 195 (72.5) | 168 (52.2) | |
| Woman/Product | 113 (19.1) | 23 (8.6) | 90 (28.0) | |
| Text | 96 (16.2) | 38 (14.1) | 58 (18.0) | |
| Woman/Man/Product | 13 (2.2) | 13 (4.8) | -- | |
| Man/Product | 6 (1.0) | -- | 6 (1.9) | |
| New (vs. Recut/Same as), N (%) | 350 (59.2) | 131 (48.7) | 219 (68.0) | <.001 |
| Ad Size, N (%)*** b | <.001 | |||
| Medium Rectangle | 203 (34.4) | 78 (29.0) | 125 (38.8) | |
| Half Page Ad | 153 (25.9) | 115 (42.8) | 38 (11.8) | |
| Custom Large | 145 (24.5) | 60 (22.3) | 85 (26.4) | |
| Full | 28 (4.7) | 10 (3.7) | 18 (5.6) | |
| Inside Front or Back Cover | 18 (3.0) | 1 (0.4) | 17 (5.3) | |
| Media Channel Theme, N (%) ƚ c | <.001 | |||
| Technology | 114 (19.3) | 53 (19.7) | 61 (18.9) | |
| Women’s fashion | 107 (18.1) | 31 (11.5) | 76 (23.6) | |
| Home | 56 (9.5) | 17 (6.3) | 39 (12.1) | |
| Weather/news | 53 (9.0) | 36 (13.4) | 17 (5.3) | |
| Entertainment/pop culture/gaming | 50 (8.5) | 4 (1.5) | 46 (14.3) | |
| Sports | 34 (5.8) | 29 (10.8) | 5 (1.6) | |
| Finance/business | 28 (4.7) | 24 (8.9) | 4 (1.2) | |
| Men’s fashion | 24 (4.1) | 10 (3.7) | 14 (4.3) | |
| Travel | 22 (3.7) | 4 (1.5) | 18 (5.6) | |
| Yahoo | 20 (3.4) | 19 (7.1) | 1 (0.3) | |
| Other | 83 (14.0) | 42 (15.6) | 41 (12.7) | |
| Spend per Occurrence ($ in thousands), M (SD) | 8.3 (31.1) | 4.9 (23.9) | 11.1 (35.8) | .016 |
| Impressions per Occurrence (in thousands), M (SD) | 40.5 (109.6) | 39.6 (120.2) | 41.3 (99.4) | .852 |
Run date began before July 7, 2020 (pre-MRTP) vs. after (post-MRTP).
Not discrete categories.
Not applicable for mobile.
All significant in pairwise tests except: atext; bcustom large and full; and cmen’s fashion.
The most prominent media channel themes were technology (19.3%) and women’s fashion (18.1%), followed by home (9.5%), weather/news (9.0%), and entertainment/pop culture/gaming (8.5%; Table 3 & Figure 1a; see Appendix B for specific channels). Post-MRTP, there were more occurrences across marketing channels thematically oriented to women’s fashion, home, entertainment/pop culture/gaming, and travel (p’s<.05).
Figure 1.
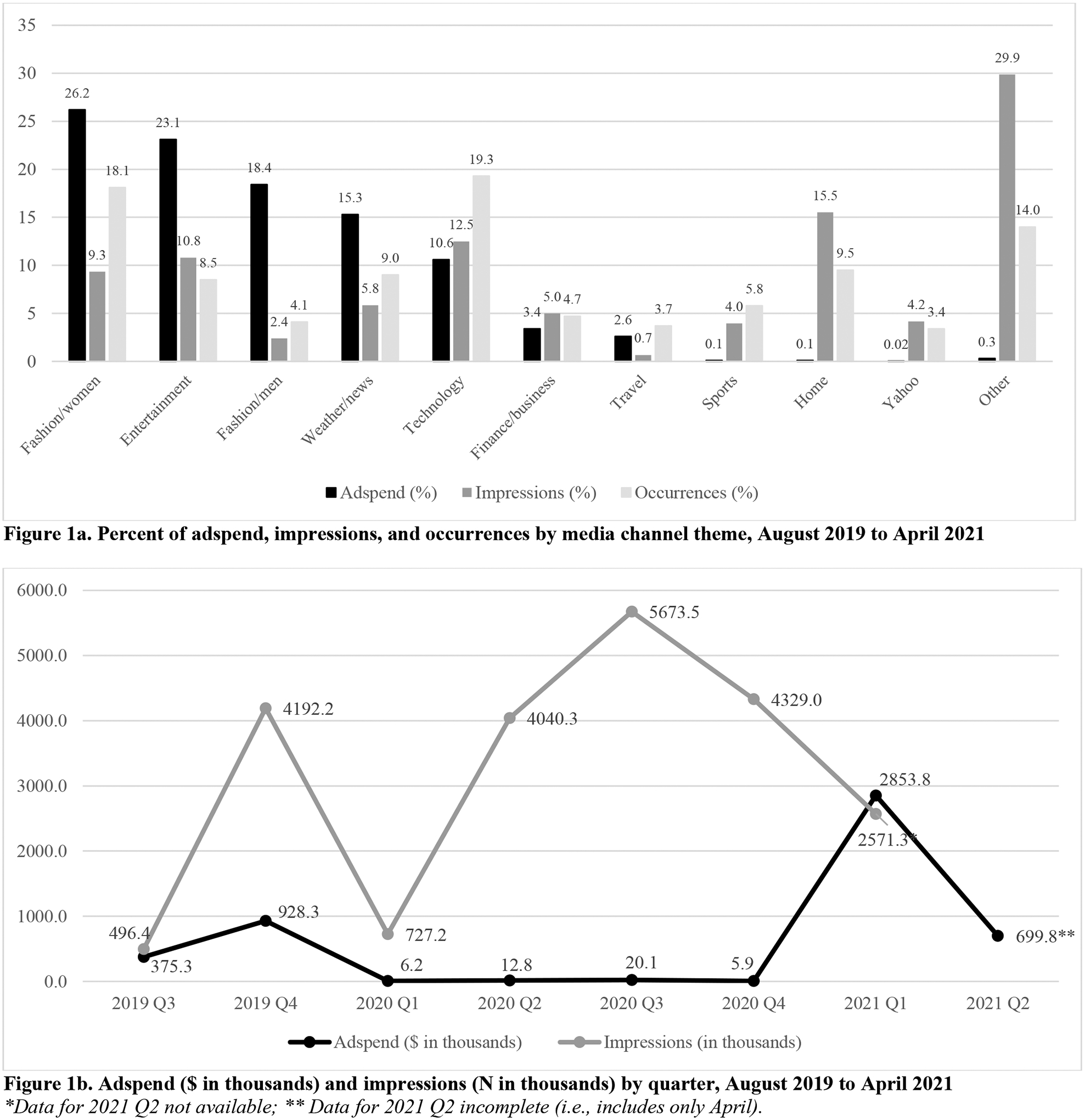
Advertising characteristics across media channels and time
Note: Ad occurrences defined as ad campaigns, which could use: [a] existing or recut ad content or unique ad content and [b] similar or distinct channels and markets. Digital impressions defined as digital views or engagements with the ads as derived by Web-indexing technology, audience measurement data from comScore, and estimated cost per thousand impressions for each monitored site.
Average adspend per occurrence was $8,290 (SD=$31,070), which increased post-MRTP (p=.016). The average number of digital impressions per occurrence was 40,500 (SD=109,640), which did not significantly change pre- to post-MRTP.
Adspend and Impressions
Table 4 highlights adspend across different dimensions of occurrences. Overall, total adspend across the study timeframe was $4,902,110, with the majority being spent after MRTP authorization ($3,578,030; 73.0%; Figure 1b). Almost all adspend (~99%) went toward print advertising and three main ads (Table 1). Adspend was particularly allocated to ad themes related to switching from cigarettes, reduced exposure, real tobacco, and less odor/ash. Almost all adspend (>99%) was allocated to ads depicting the product only. The greatest amount of adspend was allocated to media channels representing women’s fashion (26.2%), entertainment/pop culture/gaming (23.1%), men’s fashion (18.4%), weather/news (15.3%), and technology (10.6%; Figure 1a).
Table 4.
Adspend Across Dimensions of $4.9 Million Total Adspend, 2019–2021
| Total $4,902,110 (100.0%) | Pre-MRTP* $1,324,080 (27.0%) | Post-MRTP $3,578,030 (73.0%) | ||
|---|---|---|---|---|
| Variable | N (%) Cost to the $000’s | N (%) Cost to the $000’s | N (%) Cost to the $000’s | p |
| Media Type | ||||
| 4846.5 (98.9) | 1294.9 (97.8) | 3551.7 (99.3) | .263 | |
| Online Display | 43.2 (0.9) | 29.0 (2.2) | 14.2 (0.4) | .034 |
| Mobile | 12.4 (0.3) | 0.2 (0.02) | 12.2 (0.3) | .157 |
| Ad Headline Theme | ||||
| Switch | 3404.2 (69.4) | -- | 3404.2 (95.1) | -- |
| Reduced exposure | 3398.7 (69.3) | -- | 3398.7 (95.0) | -- |
| Real tobacco | 1472.9 (30.1) | 1303.8 (98.5) | 169.1 (4.7) | <.001 |
| Less odor/ash | 1464.5 (29.9) | 1299.1 (98.1) | 165.4 (4.6) | <.001 |
| Distinct from e-cigarettes | 155.8 (3.2) | -- | 155.8 (4.4) | -- |
| Includes tag: Get IQOS | 24.2 (0.5) | 10.0 (0.8) | 14.2 (0.4) | .489 |
| Future | 14.6 (0.3) | 14.6 (1.1) | -- | -- |
| Convenience (e.g., use indoors) | 10.2 (0.2) | 0.3 (0.02) | 10.0 (0.3) | .452 |
| Innovation/Technology | 9.0 (0.2) | 4.5 (0.3) | 4.5 (0.1) | .363 |
| Not burned/heat control | 8.4 (0.2) | 0.1 (0.01) | 8.2 (0.2) | .368 |
| Science/research | 2.8 (0.1) | -- | 2.8 (0.1) | -- |
| Same-day/home delivery | 1.0 (0.02) | -- | 1.0 (0.03) | -- |
| Enjoyment/satisfaction | 0.5 (0.01) | 0.01 (0.01) | 0.5 (0.01) | .423 |
| Promotions (e.g., half off) | 0.3 (0.01) | -- | 0.3 (0.01) | -- |
| Ad Visual/Image Theme § | ||||
| Product | 4877.2 (99.5) | 1317.4 (99.5) | 3559.8 (99.5) | <.001 |
| Woman/Product | 14.1 (0.3) | 1.6 (0.1) | 12.5 (0.4) | .269 |
| Text | 9.0 (0.2) | 3.4 (0.3) | 5.5 (0.2) | .886 |
| Woman/Man/Product | 1.6 (0.03) | 1.6 (0.1) | -- | -- |
| Man/Product | 0.3 (0.01) | -- | 0.3 (0.01) | -- |
| Media Channel Theme | ||||
| Women’s fashion | 1284.6 (26.2) | 414.0 (31.3) | 870.7 (24.3) | .808 |
| Entertainment/pop culture/gaming | 1132.6 (23.1) | 0.2 (0.02) | 1132.4 (31.7) | .252 |
| Men’s fashion | 900.9 (18.4) | 499.8 (37.7) | 401.1 (11.2) | .403 |
| Weather/news | 750.9 (15.3) | 17.2 (1.3) | 733.8 (20.5) | .003 |
| Technology | 519.3 (10.6) | 383.3 (28.9) | 136.0 (3.8) | .215 |
| Finance/business | 164.8 (3.4) | 1.3 (0.1) | 163.5 (4.6) | <.001 |
| Travel | 128.7 (2.6) | 0.1 (0.01) | 128.6 (3.6) | .646 |
| Sports | 3.3 (0.1) | 3.1 (0.2) | 0.2 (0.01) | .236 |
| Home | 2.6 (0.1) | 0.5 (0.04) | 2.1 (0.1) | .434 |
| Yahoo | 0.9 (0.02) | 0.8 (0.1) | 0.1 (0.01) | .239 |
| Other | 13.6 (0.3) | 3.9 (0.3) | 9.7 (0.3) | <.001 |
Run date began before July 7, 2020 (pre-MRTP) vs. after (post-MRTP).
Not discrete categories.
Regarding digital impressions (not shown in tables), there were a total of 22,029,900 impressions, with 63.2% earned via online display and 26.8% earned via mobile channels. The number and proportions of digital impressions earned across media channel themes largely reflected occurrences across media channels (Figure 1a), but did not necessarily reflect adspend across media channels (Figure 1a) or adspend across quarters (Figure 1b).
DISCUSSION
This study is the first to examine IQOS marketing in the US across different media platforms and the extent to which it changed pre- to post MRTP authorization. Notably, adspend was highest during the initial emergence in the US market and then in the later part of 2020 and into 2021, after MRTP authorization. The modest adspend in 2020 may be related to COVID-19, which led to state shelter-in-place orders beginning in March 2020 and impacted US population behavior throughout 2020 and into 2021.61 In addition, ads were circulated mainly in print, online, and via mobile channels. Print ads accounted for the vast majority (~99%) of adspend, although online displays accounted for the majority (~85%) of occurrences, as well as digital impressions.
Regarding ad content, most headlines were used only once; however, certain themes (e.g., real tobacco) were featured across ads. Moreover, certain themes (i.e., real tobacco, less odor/ash, innovation/technology) were consistently used pre- to post-MRTP, which has been noted in other countries as well.53 Messages regarding reduced exposure, promoting switching from traditional cigarettes, and eluding to research to support claims of reduced exposure emerged post-MRTP, as anticipated. Some of these messages (e.g., technology, switching, health claims) have also been used by various e-cigarette brands.55,62 Other prominent messages also emerged post-MRTP that may be reflections of other societal events: 1) same-day/home delivery and convenience, potentially related to COVID-19 state shelter-in-place orders and their impacts on usual in-person consumerism;63,64 and 2) distinguishing IQOS from e-cigarettes, potentially related to increased media coverage of e-cigarette and vaping associated lung injury (EVALI).65
Noteworthy, most ad content and occurrences featured the IQOS product itself; however, 29.2% of ad content and 21.3% of ad occurrences included women in the visual content (vs. 8.4% of ad content and 3.2% of ad occurrences including men). Along these lines, media channels thematically oriented toward women’s fashion accounted for the largest share of adspend and among the most ad occurrences. Moreover, many of these women’s fashion media channels target younger women; for example, Self targets ages 18–30,66 21% of Glamour’s readership is ages 18–24 (with another 23% being 25–34),67 and per Conde Nast’s website, Teen Vogue is “the destination for the next generation of influencers” and “educates, enlightens, and empowers young people, arming them with all they need to lead stylish and informed lives”.68 Additionally, media channels targeting entertainment/pop culture/gaming were also prominent in terms of both occurrences and adspend. In short, these findings raise concerns about targeting women, as well as young people, which contradicts PM’s stated target consumers of 24 and older.11 Furthermore, research in countries with established IQOS markets, specifically in Italy69 and South Korea,70 indicate comparable numbers of never and current smokers who have tried or intend to try IQOS69 and that current IQOS users are more likely to also smoke conventional cigarettes and/or e-cigarettes.70 These findings also contradict the industry’s claims that the target market is smokers and that conventional cigarette smokers will switch to IQOS.
It is also important to note how these strategies have been used by PM for prior products. For example, one study comparing MarkTen advertising strategies relative to other e-cigarette brands (i.e., Vuse, NJoy, Blu) indicated that – like the other e-cigarette brands – MarkTen ads largely focused its imagery on the product itself.55 However, MarkTen was unique in its featuring of people in its ads, particularly women and young people,55 as current findings show in relation to IQOS. MarkTen was also unique, relative to the other brands, in its minimal focus on flavors,55 which is also notable with regard to IQOS advertising. Adspend for MarkTen was distinct from the others in its allocation of all adspend on print ads, while the others also prominently leveraged TV and online advertising.55 A more recent product that is noteworthy and relevant is JUUL, an e-cigarette of which Altria, the parent company of PM, has 35% stake.71 JUUL’s marketing strategies have involved youthful looking men and women using JUUL, how discrete the device and its use are,72 and messaging that misleads about the risk of addiction.48,73 JUUL is also highly discussed on social media platforms such as Twitter, Instagram, YouTube, and Reddit.48 While JUUL more recently invested in campaigns to reduce underage access, much of these efforts have been in reaction to scrutiny by FDA, public health, and media regarding underage use – and have focused on media used by health and legislative organizations.74 Collectively, these experiences of PM-affiliated products in the US highlight concerns about targeting young people and women, as well as leveraging online, mobile, and social media, which may provide opportunities to circumvent regulatory efforts that prohibit targeting young people and use of certain language (e.g., “reduced risk”) in ad content.48
These findings have implications for research and practice. Ongoing surveillance is needed to monitor the content and distribution channels of IQOS advertising, as well as its impact on consumers. In particular, current findings indicate the need to monitor the impact on young people and women and to monitor the online, mobile, and social media environments. Notably, current findings must be included in PM’s post-market surveillance studies, which “must include procedures for monitoring and assessing previously unreported (new) findings both in published or unpublished studies conducted by you or on your behalf and in published or otherwise available studies regarding the MRTPs and consumer perception, behavior, or health” and include studies of impact on youth and non-users of tobacco, which could include women who are seemingly targeted consumers.24 In addition, international research is also needed to monitor the impact of FDA’s MRTP authorization, as PMI has used it to promote IQOS around the world, including mischaracterizing FDA’s MRTP order as evidence that IQOS is a reduced harm product and leveraging it in efforts to minimize government regulation of IQOS.21,75–85 This is highly controversial, as this is occurring in countries that have ratified the WHO Framework Convention on Tobacco Control,21,75–85 which in fact calls for a comprehensive ban on tobacco product advertising, promotion, and sponsorship.86 Finally, another concern is how consumers perceive ad headlines such as “Why Make the Switch from Cigarettes to IQOS?” when situated on ads that have warning labels indicating “Quitting Smoking Now Greatly Reduces Serious Risks to Your Health”, as there is evidence from other countries that PMI has strategized to pitch its products against such health warning labels/campaigns.87
Limitations
This study was limited by a relatively small number of ads available, which precluded using advanced analyses. However, descriptive analyses indicated notable themes and changes from pre- to post-MRTP authorization. In addition, COVID-19 and other societal events likely impacted IQOS marketing.63,64 In addition, this study focused on explicitly-paid advertising and did not include point-of-sale marketing (which has been the focus of limited research in the US88 and elsewhere41,42,89,90), promotion via social media,53,91 or other ways in which industry influences media (e.g., “unpaid” articles,92 communication with policymakers and retailers44,89). These other strategies also require ongoing surveillance.
Conclusions
As IQOS markets in the US expand, ongoing marketing and population impact surveillance – by regulatory agencies and independent researchers – is needed to inform regulatory efforts. Language and imagery used in advertising IQOS, particularly post-MRTP, must be scrutinized to determine how consumers are interpreting the potential harms of IQOS and their perceptions of how and why IQOS should be used (e.g., to facilitate smoking cessation, to project positive self-image) and who should use IQOS (e.g., young people, cigarette users) based on IQOS’ marketing content and channels. This is critical as FDA continues to review MRTP applications and authorize tobacco products to use reduced exposure or harm claims. Furthermore, strategic international collaborations are needed to advance and inform regulatory efforts, especially given PMI’s leveraging of FDA’s MRTP authorization in its marketing globally and to influence policy in other countries.85,93
Supplementary Material
What This Paper Adds.
What is already known on this subject?
Worldwide, there is increasing use of heated tobacco products (HTPs), generally marketed as an alternative to combustible cigarettes, often using claims that use of HTPs is less harmful than cigarettes and other combustible tobacco products.
For newly introduced products such as HTPs, advertising is fundamental to product success.
IQOS was the first HTP to receive US FDA authorization to use “reduced exposure” claims (i.e., that use reduces exposure to harmful or potentially harmful substances relative to cigarettes), which does not allow language regarding reduced harm or risk.
What important gaps in knowledge exist on this topic?
The impacts of such FDA authorization – in terms of PM’s subsequent marketing strategies and related consumer perceptions and behaviors – are unknown.
Thus, it is critical to examine the marketing of IQOS in the USA, particularly before and after authorization to use reduced exposure claims.
What this study adds?
There was a surge in adpsend after IQOS received FDA modified exposure authorization.
Certain themes (i.e., real tobacco, less odor/ash, innovation/technology) were consistently used before and after modified exposure authorization, while messages regarding reduced exposure and switching from traditional cigarettes emerged post-authorization.
Ad content, occurrences, and adspend indicated targeting of women (i.e., featured in ads, relevant media channels) as well as those under age 24.
Acknowledgements
The authors acknowledge and thank other members of our research team, including Orly Manor, PhD and our staff, as well as the members of the External Advisory Board for their input to the study’s design, methodology, and interpretation of results (by alphabetical order): Joanna Cohen PhD, Nadav Davidovitch MD, PhD, Milka Dunchin MD, Anat Gesser-Edelsburg PhD, Michael Eriksen ScD, Haim Geva Haspil, Lisa Henriksen PhD, Elad Godinger, Shira Kislev, James Thrasher PhD.
Funding
This research was supported by the National Cancer Institute (R01CA239178-01A1; MPIs: Berg, Levine) and by the Israel Lung and Tuberculosis Association (MPIs: Bar-Zeev, Levine). Dr. Berg is also supported by other NIH funding, specifically the US National Cancer Institute (R01CA179422-01; PI: Berg; R01CA215155-01A1; PI: Berg), the US Fogarty International Center/National Institutes of Health (NIH) (1R01TW010664-01; MPIs: Berg, Kegler), and the National Institute of Environmental Health Sciences/Fogarty (D43ES030927-01; MPIs: Berg, Marsit, Sturua).
Footnotes
Human Subjects Statement
This study was deemed exempt from Institutional Review Board approvals, per George Washington University’s Institutional Review Board.
Declaration of Interests
Yael Bar-Zeev has received fees for lectures from Pfizer Ltd, Novartis NCH, and GSK Consumer Health (distributors of smoking cessation pharmacotherapy in Israel) in the past (2012- July2019). Hagai Levine had received fees for lectures from Pfizer Israel Ltd (distributor of a smoking cessation pharmacotherapy in Israel) in 2017. Lorien Abroms receives royalties for the sale of Text2Quit and is a shareholder in Welltok, Inc.
References
- 1.Robertson L, Cameron C, McGee R, Marsh L, Hoek J. Point-of-sale tobacco promotion and youth smoking: a meta-analysis. Tob Control. 2016;25(e2):e83–e89. [DOI] [PubMed] [Google Scholar]
- 2.St.Helen G, Jacob Iii P, Nardone N, Benowitz NL. IQOS: examination of Philip Morris International’s claim of reduced exposure. Tob Control. 2018;27(Suppl 1):s30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nyman AL, Weaver SR, Popova L, et al. Awareness and use of heated tobacco products among US adults, 2016–2017. Tob Control. 2018;27(Suppl 1):s55–s61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sutanto E, Miller C, Smith DM, et al. Prevalence, Use Behaviors, and Preferences among Users of Heated Tobacco Products: Findings from the 2018 ITC Japan Survey. Int J Environ Res Public Health. 2019;16(23). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu X, Lugo A, Spizzichino L, Tabuchi T, Pacifici R, Gallus S. Heat-not-burn tobacco products: concerns from the Italian experience. Tobacco Control. 2019;28(1):113. [DOI] [PubMed] [Google Scholar]
- 6.Czoli CD, White CM, Reid JL, RJ OC, Hammond D. Awareness and interest in IQOS heated tobacco products among youth in Canada, England and the USA. Tob Control. 2020;29(1):89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ratajczak A, Jankowski P, Strus P, Feleszko W. Heat Not Burn Tobacco Product—A New Global Trend: Impact of Heat-Not-Burn Tobacco Products on Public Health, a Systematic Review. International Journal of Environmental Research and Public Health. 2020;17(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berg CJ, Bar-Zeev Y, Levine H. Informing iQOS Regulations in the United States: A Synthesis of What We Know. SAGE Open. 2020;10(1):2158244019898823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Philip Morris International. In how many countries do you sell your cigarette brands? https://www.pmi.com/faq-section/faq/in-how-many-countries-do-you-sell-your-cigarette-brands#. Published 2020. Accessed July 1, 2021.
- 10.US Food and Drug Administration. Marketing Order, FDA Submission Tracking Numbers (STNs): PM0000424- PM0000426, PM0000479, IQOS System Holder and Charger and Marlboro Heatsticks, 2019. Available: https://www.fda.gov/tobacco-products/premarket-tobacco-product-applications/premarket-tobacco-product-marketing-orders [Accessed 6 Aug 2020]. 2019.
- 11.Food and Drug Administration. FDA permits sale of IQOS Tobacco Heating System through premarket tobacco product application pathway. Food and Drug Administration. https://www.fda.gov/news-events/press-announcements/fda-permits-sale-iqos-tobacco-heating-system-through-premarket-tobacco-product-application-pathway. Published 2019. Accessed July 1, 2021. [Google Scholar]
- 12.Berg CJ, Romm KF, Patterson B, Wysota CN. Heated tobacco product awareness, use, and perceptions in a sample of young adults in the U.S. Nicotine Tob Res. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Family Smoking Prevention and Tobacco Control Act. PUB L. No. 111–31, 123 STAT. 1776 2009;1776.
- 14.Administration USFaD. Scientific Review of Modified Risk Tobacco Product Application (MRTPA) Under Section 911(d) of the FD&C Act. 07/07/2020 2020.
- 15.US Food and Drug Administration. Exposure Modification Modified Risk Granted Orders, FDA submission tracking numbers (STNs): MR0000059-MR0000061, MR0000133, 2020. Available: https://www.fda.gov/media/139797/download [Accessed 6 Aug 2020]. 2020.
- 16.Food and Drug Administration. Philip Morris Products S.A. Modified Risk Tobacco Product (MRTP) Applications. Food and Drug Administration. https://www.fda.gov/tobaccoproducts/labeling/marketingandadvertising/ucm546281.htm. Published 2018. Accessed December 29, 2018.
- 17.El-Toukhy S, Baig SA, Jeong M, Byron MJ, Ribisl KM, Brewer NT. Impact of modified risk tobacco product claims on beliefs of US adults and adolescents. Tob Control. 2018;27(Suppl 1):s62–s69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKelvey K, Popova L, Kim M, et al. IQOS labelling will mislead consumers. Tob Control. 2018;27(Suppl 1):s48–s54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hatsukami DK, Carroll DM. Tobacco harm reduction: Past history, current controversies and a proposed approach for the future. Prev Med. 2020;140:106099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Enserink M Tobacco giant’s research largesse ignites controversy. Science. 2018;359(6376):622–623. [DOI] [PubMed] [Google Scholar]
- 21.Gilmore AB, Braznell S. US regulator adds to confusion around heated tobacco products. BMJ. 2020;370:m3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashley DL. Premarket Tobacco Application (PMTA) Technical Project Lead (TPL) Review: Swedish Match North America, Inc. In: Center for Tobacco Products FaDA, Department of Health and Human Services, ed 2015. [Google Scholar]
- 23.Ashley DL. Modified Risk Tobacco Product (MRTP) ApplicationTechnical Proj ect Lead (TPL) Review: Swedich Match North America, Inc. In: Center for Tobacco Products FaDA, Department of Health and Human Services, ed 2014. [Google Scholar]
- 24.Food and Drug Administration. IQOS Modified Risk Granted Orders - Exposure Modification. Food and Drug ADministration. https://www.fda.gov/media/139797/download. Published 2020. Accessed August 15, 2021. [Google Scholar]
- 25.Chen X, Cruz TB, Schuster DV, Unger JB, Johnson CA. Receptivity to protobacco media and its impact on cigarette smoking among ethnic minority youth in California. J Health Commun. 2002;7(2):95–111. [DOI] [PubMed] [Google Scholar]
- 26.Evans N, Farkas A, Gilpin E, Berry C, Pierce JP. Influence of tobacco marketing and exposure to smokers on adolescent susceptibility to smoking. J Natl Cancer Inst. 1995;87(20):1538–1545. [DOI] [PubMed] [Google Scholar]
- 27.Hanewinkel R, Isensee B, Sargent JD, Morgenstern M. Cigarette advertising and adolescent smoking. Am J Prev Med. 2010;38(4):359–366. [DOI] [PubMed] [Google Scholar]
- 28.Lovato C, Linn G, Stead LF, Best A. Impact of tobacco advertising and promotion on increasing adolescent smoking behaviours. Cochrane Database Syst Rev. 2003(4):CD003439. [DOI] [PubMed] [Google Scholar]
- 29.Paynter J, Edwards R. The impact of tobacco promotion at the point of sale: a systematic review. Nicotine Tob Res. 2009;11(1):25–35. [DOI] [PubMed] [Google Scholar]
- 30.Gilpin EA, White MM, Messer K, Pierce JP. Receptivity to tobacco advertising and promotions among young adolescents as a predictor of established smoking in young adulthood Am J Public Health. 2007;97(8):1489–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi WS, Ahluwalia JS, Harris KJ, Okuyemi K. Progression to established smoking: the influence of tobacco marketing. Am J Prev Med. 2002;22(4):228–233. [DOI] [PubMed] [Google Scholar]
- 32.Burton S, Clark L, Jackson K. The association between seeing retail displays of tobacco and tobacco smoking and purchase: findings from a diary-style survey. Addiction. 2012;107(1):169–175. [DOI] [PubMed] [Google Scholar]
- 33.Pucci LG, Siegel M. Exposure to brand-specific cigarette advertising in magazines and its impact on youth smoking. Prev Med. 1999;29(5):313–320. [DOI] [PubMed] [Google Scholar]
- 34.Sethuraman R, Tellis GJ, Briesch R. How well does advertising work? Generalizations from a meta-analysis of brand advertising elasticity. Journal of Marketing Research. 2011;48:457–471. [Google Scholar]
- 35.Vakratsas D, Ambler T. How advertising works: What do we really know? Journal of Marketing Research. 1999;63:26–43. [Google Scholar]
- 36.Anderson SJ, Glantz SA, Ling PM. Emotions for sale: cigarette advertising and women’s psychosocial needs. Tob Control. 2005;14(2):127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ling PM, An LC, Lein EB. Using tobacco industry psychographic measures to describe college smokers and nonsmokers. Society of Nicotine and Tobacco Research; February 20, 2004, 2004; Scottsdale, AZ. [Google Scholar]
- 38.Ling PM, Glantz SA. Why and how the tobacco industry sells cigarettes to young adults: evidence from industry documents. Am J Public Health. 2002;92(6):908–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katz SK, Lavack AM. Tobacco related bar promotions: insights from tobacco industry documents. Tob Control. 2002;11 Suppl 1:I92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sepe E, Ling PM, Glantz SA. Smooth moves: bar and nightclub tobacco promotions that target young adults. Am J Public Health. 2002;92(3):414–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mathers A, Schwartz R, O’Connor S, Fung M, Diemert L. Marketing IQOS in a dark market. Tob Control. 2018. [DOI] [PubMed] [Google Scholar]
- 42.Bar-Zeev Y, Levine H, Rubinstein G, Khateb I, Berg CJ. IQOS point-of-sale marketing strategies in Israel: a pilot study. Isr J Health Policy Res. 2019;8(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kopel E, Davidovitch N, Levine H. Using All Means to Protect Public Health in Israel From Emerging Tobacco Products. Am J Public Health. 2017;107(10):1599–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosen L, Kislev S. The IQOS Campaign in Israel. Tob Induced Dis. 2018;16(Suppl1):A732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Auer R, Concha-Lozano N, Jacot-Sadowski I, Cornuz J, Berthet A. Heat-Not-Burn Tobacco Cigarettes: Smoke by Any Other Name. JAMA Intern Med. 2017;177(7):1050–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Linder-Ganz R In the newspapers, in digital and in the field: Philip Morris poured millions of shekels on the publication of IQOS in Israel. The Marker Web site. https://www.themarker.com/news/health/1.6035501. Published 2018. Accessed July 1, 2021.
- 47.Huang J, Kornfield R, Szczypka G, Emery S. A Cross-sectional Examination of Marketing and Promotion of Electronic Cigarettes on Twitter. Tobacco Control. 2014;23(3):iii26–iii30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang J, Duan Z, Kwok J, et al. Vaping versus JUULing: how the extraordinary growth and marketing of JUUL transformed the US retail e-cigarette market. Tob Control. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kavuluru R, Han S, Hahn EJ. On the popularity of the USB flash drive-shaped electronic cigarette Juul. Tob Control. 2019;28(1):110–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.World Health Organization. Heated tobacco products (HTPs) market monitoring information sheet file:///https://apps.who.int/iris/bitstream/handle/10665/273459/WHO-NMH-PND-18.7-eng.pdf. Published 2018, July. Accessed July 1, 2021.
- 51.PMI Digital Lab. iQOS brand voice guidelines. https://www.documentcloud.org/documents/4331963-IQOS-Brand-Voice%20Guidelines.html. Published 2018. Accessed July 1, 2021.
- 52.Jackler RK, Ramamurthi D, Axelrod A, et al. Global Marketing of IQOS The Philip Morris Campaign to Popularize “Heat Not Burn” Tobacco.: Stanford University School of Medicine; February 21, 2020. 2020. [Google Scholar]
- 53.Jackler RK, Ramamurthi D, Axelrod AK. Global Marketing of IQOS: The Philip Morris Campaign to Popularize “Heat Not Burn” Tobacco (White Paper), 2020. Available: http://tobacco.stanford.edu/iqosanalysis/. 2020.
- 54.Hair EC, Bennett M, Sheen E, et al. Examining perceptions about IQOS heated tobacco product: consumer studies in Japan and Switzerland. Tob Control. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haardorfer R, Cahn Z, Lewis M, et al. The Advertising Strategies of Early E-cigarette Brand Leaders in the United States. Tob Regul Sci. 2017;3(2):222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moran MB, Heley K, Czaplicki L, Weiger C, Strong D, Pierce J. Tobacco advertising features that may contribute to product appeal among US adolescents and young adults. Nicotine Tob Res. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cantrell J, Ganz O, Emelle B, et al. Mobile marketing: an emerging strategy to promote electronic nicotine delivery systems. Tob Control. 2017;26(e2):e1–e3. [DOI] [PubMed] [Google Scholar]
- 58.Numerator. US Media Types, Methodology & Tracking Universe. Numerator. https://help.www2.numerator.com/en/collections/1734170-us-media-types-methodology-tracking-universe. Published 2021. Accessed July 1, 2021. [Google Scholar]
- 59.Interactive Advertising Bureau. Fixed Size Ad Specifications. Interactive Advertising Bureau. https://www.iab.com/wp-content/uploads/2019/04/IABNewAdPortfolio_LW_FixedSizeSpec.pdf. Published 2021. Accessed August 15, 2021. [Google Scholar]
- 60.IBM Corp. IBM SPSS Statistics for Windows, Version 26.0. IBM Corp. Published 2019. Accessed July 1, 2021. [Google Scholar]
- 61.National Bureau of Economic Research. Tracking Public and Private Responses to the COVID-19 Epidemic: Evidence from State and Local Government Actions. National Bureau of Economic Research. http://www.nber.org/papers/w27027. Published 2020. Accessed July 1, 2021. [Google Scholar]
- 62.Dutra LM, Grana R, Glantz SA. Philip Morris research on precursors to the modern e-cigarette since 1990. Tob Control. 2017;26(e2):e97–e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zangerl C How has COVID-19 impacted consumer behaviors & organizational responses. Boston, MA: Northeastern University;2020. [Google Scholar]
- 64.Zwanka RJ, Buff C. COVID-19 Generation: A Conceptual Framework of the Consumer Behavioral Shifts to Be Caused by the COVID-19 Pandemic. Journal of International Consumer Marketing 2020;33(1):58–67. [Google Scholar]
- 65.Navon L, Jones CM, Ghinai I, et al. Risk Factors for E-Cigarette, or Vaping, Product Use-Associated Lung Injury (EVALI) Among Adults Who Use E-Cigarette, or Vaping, Products -Illinois, July-October 2019. MMWR Morb Mortal Wkly Rep. 2019;68(45):1034–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vega T Self Magazine Refocuses for a Younger Audience. The New York Times Web site. https://www.nytimes.com/2013/02/11/business/media/self-magazine-widens-its-focus-for-a-younger-audience.html#:~:text=%E2%80%9CBeing%20fit%20and%20fashionable%20are,are%20obsessed%20with%20social%20media. Published 2013. Accessed July 1, 2021.
- 67.Conde Nast. Glamour Circulation. Conde Nast Web; site. https://www.condenast.ru/en/portfolio/magazines/glamour/circulation/. Published 2020. Accessed July 1, 2021. [Google Scholar]
- 68.Conde Nast. Teen Vogue. Conde Nast. https://www.condenast.com/brands/teen-vogue/. Published 2021. Accessed August 14, 2021. [Google Scholar]
- 69.Liu X, Lugo A, Spizzichino L, Tabuchi T, Pacifici R, Gallus S. Heat-not-burn tobacco products: concerns from the Italian experience. Tob Control. 2019;28(1):113–114. [DOI] [PubMed] [Google Scholar]
- 70.Kim J, Yu H, Lee S, Paek YJ. Awareness, experience and prevalence of heated tobacco product, IQOS, among young Korean adults. Tob Control. 2018;27(Suppl 1):s74–s77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lavito A In high-stakes votes, FDA advisors say evidence doesn’t back Philip Morris’ claims. CNBC 2018. [Google Scholar]
- 72.Ramamurthi D, Chau C, Jackler RK. JUUL and other stealth vaporisers: hiding the habit from parents and teachers. Tob Control. 2018. [DOI] [PubMed] [Google Scholar]
- 73.Harty D JUUL hopes to reinvent e-cigarette ads with “Vaporized” campaign Ad Age. 2015.
- 74.Kostygina G, Szczypka G, Czaplicki L, et al. Promoting corporate image or preventing underage use? Analysis of the advertising strategy and expenditures of the JUUL parent education for youth vaping prevention campaign. Tob Control. 2021. [DOI] [PubMed] [Google Scholar]
- 75.Malta. To. Philip Morris receives FDA authorisation to market IQOS in US, 2020. Available: https://timesofmalta.com/articles/view/philip-morris-receives-fda-authorisation-to-market-iqosin-us.811917 [Accessed 10 Oct 2020]. 2020.
- 76.Leyco C, Bulletin M. Philip Morris urges PH to adopt US FDA finding, 2020. Available: https://mb.com.ph/2020/09/07/philip-morris-urges-ph-to-adopt-us-fda-finding/ [Accessed 10 Oct 2020]. 2020.
- 77.Nyasa Times. Malawi: Agriculture Minister Says Smokeless Cigarette Technologies to Boost Malawi Tobacco Industry, 2020. Available: https://allafrica.com/stories/202009100285.html [Accessed 10 Oct 2020]. 2020. [Google Scholar]
- 78.All Africa. Zimbabwe: Heating System to Rescue Tobacco Industry, 2020. Available: https://allafrica.com/stories/202009070903.html [Accessed 10 Oct 2020]. 2020. [Google Scholar]
- 79.Mushava E NewsDay Zimbabwe. Boost for Zim’s tobacco industry, 2020. Available: https://www.newsday.co.zw/2020/07/boost-for-zims-tobacco-industry/ [Accessed 31 Aug 2020]. 2020. [Google Scholar]
- 80.Satigui S All Africa. Africa: Modified Risk Tobacco Product - Historic FDA Decision and Lessons for Africa, 2020. Available: https://allafrica.com/stories/202007090930.html [Accessed 31 Aug 2020]. 2020. [Google Scholar]
- 81.Han-soo L Korea Biomedical Review. FDA designation of IQOS as modified risk should change regulatory regime, 2020. Available: http://www.koreabiomed.com/news/articleView.html?idxno=9180 [Accessed 10 Oct 2020]. 2020. [Google Scholar]
- 82.Byung-yeul B The Korea Times. Philip Morris’ IQOS gets FDA approval to market as modified risk product, 2020. Available: https://www.koreatimes.co.kr/www/tech/2020/07/694_292616.html [Accessed 31 Aug 2020]. 2020. [Google Scholar]
- 83.Chow T Harbour Times. FDA statement on IQOS may prevent ban on alternative smoking products in Hong Kong, 2020. Available: https://harbourtimes.com/?s=FDA+statement+on+IQOS+may+prevent+ban+on+alternative+smoking+products+in+Hong+Kong [Accessed 31 Aug 2020]. 2020. [Google Scholar]
- 84.Tobacco Tactics. University of Bath. PMI promotion of IQOS using FDA MRTP order, 2020. Available: https://tobaccotactics.org/wiki/pmi-iqos-fda-mrtp-order/ [Accessed 10 Oct 2020]. 2020.
- 85.Lempert LK, Bialous S, Glantz S. FDA’s reduced exposure marketing order for IQOS: why it is not a reliable global model. Tob Control. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.World Health Organization. WHO Framework Convention on Tobacco Control. Available at https://www.who.int/fctc/text_download/en/. Accessed January 1, 2021. 2003.
- 87.Bar-Zeev Y, Berg CJ, Kislev S, et al. Tobacco legislation reform and industry response in Israel. Tob Control. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Churchill V, Weaver SR, Spears CA, et al. IQOS debut in the USA: Philip Morris International’s heated tobacco device introduced in Atlanta, Georgia. Tob Control. 2020;29(e1):e152–e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Berg CJ, Bar-Zeev Y, Levine H. Informing IQOS regulations in the United States: A synthesis of what we know. Sage Open. 2020:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bar-Zeev Y, Berg CJ, L.C. A, et al. Assessment of IQOS Marketing Strategies at Points-of-Sale in Israel at a Time of Regulatory Transition. Nicotine Tob Res. 2021. doi: 10.1093/ntr/ntab142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jackler RK, Li VY, Cardiff RAL, Ramamurthi D. Promotion of tobacco products on Facebook: policy versus practice. Tob Control. 2019;28(1):67–73. [DOI] [PubMed] [Google Scholar]
- 92.Gilchrist M Lost Amid Misinformation: Real People, Real Science, Real Progress. Washington Post. 2021;May 25. [Google Scholar]
- 93.Conference of the Parties to the WHO Framework Convention on Tobacco Control. Decision: FCTC/COP8(22) - Novel and emerging tobacco products, 2018. Available: https://www.who.int/fctc/cop/sessions/cop8/FCTC__COP8(22).pdf?ua=1 [Accessed 10 Oct 2020]. 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


