Abstract
Background
Evidence of the consequences of different prehospital pathways before mechanical thrombectomy (MT) in large vessel occlusion stroke is inconclusive. The aim of this study was to investigate the infarct extent and progression before and after MT in directly admitted (mothership) versus transferred (drip and ship) patients using the Alberta Stroke Program Early CT Score (ASPECTS).
Methods
ASPECTS of 535 consecutive large vessel occlusion stroke patients eligible for MT between 2015 to 2019 were retrospectively analyzed for differences in the extent of baseline, post-referral, and post-recanalization infarction between the mothership and drip and ship pathways. Time intervals and transport distances of both pathways were analyzed. Multiple linear regression was used to examine the association between infarct progression (baseline to post-recanalization ASPECTS decline), patient characteristics, and logistic key figures.
Results
ASPECTS declined during transfer (9 (8–10) vs 7 (6-9), p<0.0001), resulting in lower ASPECTS at stroke center presentation (mothership 9 (7–10) vs drip and ship 7 (6–9), p<0.0001) and on follow-up imaging (mothership 7 (4–8) vs drip and ship 6 (3–7), p=0.001) compared with mothership patients. Infarct progression was significantly higher in transferred patients (points lost, mothership 2 (0–3) vs drip and ship 3 (2–6), p<0.0001). After multivariable adjustment, only interfacility transfer, preinterventional clinical stroke severity, the degree of angiographic recanalization, and the duration of the thrombectomy procedure remained predictors of infarct progression (R 2=0.209, p<0.0001).
Conclusions
Infarct progression and postinterventional infarct extent, as assessed by ASPECTS, varied between the drip and ship and mothership pathway, leading to more pronounced infarction in transferred patients. ASPECTS may serve as a radiological measure to monitor the benefit or harm of different prehospital pathways for MT.
Keywords: stroke, CT, intervention
Introduction
In many regions of the world, the two most frequent treatment pathways in the management of acute large vessel occlusion strokes are intravenous thrombolysis in the nearest thrombolysis facility (drip and ship concept) prior to mechanical thrombectomy in endovascular capable comprehensive stroke centers or, as the main alternative, direct transfer to a comprehensive stroke center for mechanical thrombectomy (mothership concept).1 The population based benefit or harm of either scenario is region specific and not externally generalizable.2 Although certain stroke networks have evaluated prehospital triage systems based on stroke severity scales, it will likely remain elusive, from a methodological perspective, to study optimal prehospital routing strategies by performing trials where the treatment pathway is randomized in the field and which at the same time will account for region specific factors or by performing such trials separately by region.2 3
Functional clinical measures are the standard of reference as primary endpoints in randomized stroke trials. However, observational investigation using standardized and widely used radiological measures, such as the Alberta Stroke Program Early CT Score (ASPECTS), may be valuable to indicate benefit or harm in a setting where randomization of different treatment pathways for acute large vessel occlusion stroke remains a serious obstacle or is not feasible across multiple centers, each with distinct region specific features.1 2 4 Recently, ASPECTS has been proposed as an imaging tool to assess the dynamics of infarction during interfacility transfer.5 6 We add to previous studies on ASPECTS by investigating whether the infarct extent before and after mechanical thrombectomy differs between the mothership and drip and ship pathways, and aim to quantify the imaging defined effect size of pre- to postinterventional worsening of stroke over time for both pathways.
Methods
This retrospective observational study was approved by the local ethics committee. The period of observation was from May 2015 to July 2019. A total of 535 consecutive anterior circulation ischemic stroke patients intended to be treated by mechanical thrombectomy at an academic comprehensive stroke center in our regiopolitan area were analyzed to investigate whether preinterventional and early postinterventional follow-up ASPECTS at 24–48 hour after symptom onset differed between the mothership and drip and ship treatment pathways for mechanical thrombectomy (online supplemental figure 1). In addition, we compared the course of decline in ASPECTS over time (difference in follow-up ASPECTS to baseline) between the two treatment pathways, and assessed the association of key patient, interventional, and logistic variables with ASPECTS-defined infarct progression.
neurintsurg-2020-017155supp001.pdf (186.5KB, pdf)
The stroke network of the regiopolitan area surveyed covers a population of ~1.4 million inhabitants and is composed of one academic comprehensive stroke center and 11 associated hospitals within a maximum ground transfer distance of 149 km.7 To ensure guideline compliant neurointerventional and pharmacological stroke treatment in a 24/7 setting,1 the academic comprehensive stroke center provides teleradiological and teleneurological support on request (ie, the assessment of thrombectomy eligibility), coordination of patient transfer from associated hospitals (individual decision making), and, following transfer, subsequent mechanical thrombectomy if indicated.7
All symptomatic acute ischemic stroke patients with proven occlusion of the distal internal carotid artery, middle cerebral artery M1 segment, and proximal M2 segment were eligible for study entry. Of those, we included patients either directly admitted or transferred from associated referring hospitals for which initial non-invasive imaging by means of non-contrast CT and CT angiography were available for both the comparison with non-contrast CT (Somatom Definition AS, Siemens Healthcare, Erlangen, Germany) at stroke center presentation and at 24–48 hours (postinterventional imaging, cut-off for contrast agent resorption).8 Thrombectomy eligibility was guided using consensus recommendations or current guidelines if available.1
The following demographic, clinical, radiological, interventional, and logistical data were collected according to the available medical records: age, sex, baseline medication and medical history, time of symptom onset, heart rate and blood pressure at presentation, National Institutes of Health Stroke Scale (NIHSS) score at presentation and at 24 hours, time of non-invasive and angiographic image acquisition, occlusion location, baseline ASPECTS (referring hospital/comprehensive stroke center), ASPECTS after patient transfer and at 24–48 hour, and alteplase administration. Imaging data were stored in the respective picture archiving and communication system and were available via teleradiological access. ASPECTS values were independently evaluated by two of the authors (FC/JH) trained in ASPECTS scoring (http://www.aspectsinstroke.com), both blinded to clinical information. Interobserver agreement was assessed by the intraclass correlation coefficient (ICC). Recanalization success was assessed by the modified Treatment in Cerebral Ischemia Scale (mTICI) and determined by finding a consensus between two examiners with longstanding expertise in vascular neurointervention (AMK/MP). A mTICI score of ≥2 b was defined as successful recanalization.
Logistic key figures were obtained as described previously.7 Briefly, the overall treatment chain ranging from symptom onset to stroke center admission and initiation of mechanical thrombectomy was divided into chronological intervals. For each patient, we reconstructed and geocoded the actual transport routes by time and distance between symptom onset, initial hospital, and the comprehensive stroke center, applying Google’s Distance Matrix Application Programming Interface (Mountain View, California, USA). All calculations were carried out under the assumption that the documented time of image acquisition (referring hospital/comprehensive stroke center) approximates the time of clinical presentation. The time interval between non-invasive imaging (non-contrast CT) at the referring facility and the subsequent CT at the comprehensive stroke center was defined as time surrogate for the duration of the overall transfer process.
The manuscript was prepared according to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement for observational studies.9
Statistical analysis
All data were stored and processed in Microsoft Office 365 ProPlus Excel (V.2102, Microsoft Corporation, Redmond, Washington, USA). Statistical analyses were performed using GraphPad Prism (GraphPad Prism 9.1, GraphPad Software, San Diego, California, USA) and MedCalc 19.7.2 (MedCalc Software, Ostend, Belgium). Gaussian distribution was tested by the D'Agostino and Pearson omnibus normality test. Standard descriptive statistical measures were used, including median (IQR) for numerical data or absolute and relative frequency distribution for categorial variables. ICC was used as a statistical measure of interobserver agreement for radiological scoring of ASPECTS and is given with 95% CI. Single comparison between two groups was done by χ2 test for categorial variables and by unpaired t test or Mann–Whitney U test for parametric and non-parametric data. The Kruskal–Wallis test was used for non-parametric repeated measures and followed by Dunn’s multiple comparison test. Variables of univariate association (p<0.1) were chosen for entry into multiple linear regression to evaluate the association between ASPECTS decline over time, patient characteristics, and logistic key figures. Stepwise backwards selection procedures were used to fit a model to these variables. A two sided p value <0.05 was predetermined as the threshold for statistical significance.
Results
Between May 2015 and July 2019, 535 consecutive patients with large vessel occlusion of the anterior circulation were primarily admitted (mothership) or secondarily referred (drip and ship) to our comprehensive stroke center with the intention to be treated by mechanical thrombectomy. The demographic, clinical, and radiological characteristics according to the prehospital pathway (mothership vs drip and ship) are given in table 1.
Table 1.
Patient characteristics
| Characteristics | MS (n=221) | DS (n=314) | P value |
| Age (years) (median (IQR)) | 77 (66–83) | 77 (66–82) | 0.912 |
| Males (n (%)) | 104 (47) | 137 (42) | 0.433 |
| Medical history (n (%)) | |||
| Hypertension | 179 (81) | 259 (82) | 0.659 |
| Diabetes mellitus | 53 (24) | 61 (19) | 0.205 |
| Hyperlipidemia | 72 (33) | 91 (29) | 0.373 |
| Atrial fibrillation | 69 (31) | 131 (42) | 0.014 |
| Smoking history | 79 (36) | 96 (31) | 0.209 |
| Baseline medication (n (%)) | |||
| Antithrombotic medication | 110 (50) | 161 (51) | 0.733 |
| Antihypertensive drugs | 156 (71) | 227 (72) | 0.667 |
| Clinical presentation | |||
| Unknown time of symptom onset (n (%)) | 38 (17) | 33 (11) | 0.025 |
| NIHSS at CSC presentation (median (IQR)) | 16 (11–20) | 14 (10–14) | 0.016 |
| Systolic blood pressure mm Hg) (median (IQR)) | 158 (137–179) | 160 (144–225) | 0.125 |
| Diastolic blood pressure (mm Hg) (median (IQR)) | 80 (70–95) | 85 (72–99) | 0.35 |
| Heart rate (beats/min) (median (IQR)) | 78 (67–90) | 79 (67–90) | 0.982 |
| Treatment | |||
| IV alteplase (n (%)) | 109 (49) | 164 (52) | 0.508 |
| Intervention (n (%)) | |||
| No MT after interfacility transfer | 60 (19) | ||
| Recanalization after remote IV alteplase | 10 (3) | ||
| No large vessel occlusion after transfer | 5 (2) | ||
| Far distal occlusion not suitable for MT | 6 (2) | ||
| Far advanced infarct progression not suitable for MT | 34 (11) | ||
| No consent for treatment | 2 (1) | ||
| No endovascular access route to the occlusion location | 3 (1) | ||
| Onset to puncture (min) (median (IQR)) | 136 (110–164) | 266 (230–326) | <0.0001 |
| Occlusion location (n (%))* | |||
| M1 | 131 (59) | 195 (62) | 0.509 |
| M2 | 33 (15) | 60 (19) | 0.209 |
| ICA | 90 (41) | 121 (39) | 0.61 |
| Stent retrieval maneuvers (median (IQR)) | 2 (1–3) | 2 (1–3) | 0.779 |
| Successful recanalization (mTICI ≥2 b) (n (%)) | 181 (82) | 219 (87) | 0.001 |
| Duration of MT procedure (min) (median (IQR)) | 70 (45–111) | 75 (49–99) | 0.947 |
| Onset to recanalization (min) (median (IQR)) | 210 (168–271) | 346 (296–416) | <0.0001 |
| Cervical ICA stenting (n (%)) | 36 (16) | 52 (17) | 0.934 |
| Clinical outcome | |||
| NIHSS score 24 hours postintervention (median (IQR)) | 6 (1–15) | 5 (2–13) | 0.854 |
| Intracranial hemorrhage (n (%)) | 35 (16) | 42 (13) | 0.425 |
| Inhouse mortality (n (%)) | 41 (19) | 51 (16) | 0.486 |
Values are number (%) for categorial variables and median (IQR) for continuous variables.
*Including combined vessel occlusions.
CSC, comprehensive stroke center; DS, drip and ship; ICA, internal carotid artery; IV, intravenous; M1/M2, middle cerebral artery segment; MS, mothership; MT, mechanical thrombectomy; mTICI, modified Treatment in Cerebral Ischemia Scale; NIHSS, National Institutes of Health Stroke Scale.
There were no differences in patient age (mothership 77 (66-83) vs drip and ship 77 (66-82) years, p=0.912) or gender distribution (mothership 47% men vs 42% for drip and ship, p=0.433). Hypertension was the most prevalent comorbid disease and similarly distributed across both groups (mothership 81% vs drip and ship 82%, p=0.659). The rate of atrial fibrillation was significantly higher in transferred patients (mothership 31% vs drip and ship 42%, p=0.014). No differences were observed for the use of antithrombotic (mothership 50% vs drip and ship 50%, p=0.733) or antihypertensive drugs (mothership 71% vs drip and ship 72%, p=0.667). We found significantly more patients with unknown time of symptom onset (mothership 17% vs drip and ship 11%, p=0.025) and higher degrees of stroke severity in the mothership group (NIHSS, mothership 16 (11-20) vs drip and ship 14 (10-14), p=0.016). Both groups showed elevated systolic blood pressure levels at the time of stroke center presentation (mothership 158 (137-179) vs drip and ship 160 (144-225) mm Hg, p=0.125). The rate of intravenous alteplase administration was almost 50% in both groups (mothership 49% vs drip and ship 52%, p=0.508). In 34 patients (11%) after interfacility transfer, eventual mechanical thrombectomy was not performed because of significant infarct progression on arrival.
Transport distances and quantitative time metrics are given in table 2. In the drip and ship group, interfacility transfer delayed the initiation of mechanical thrombectomy by 319% (picture to puncture time mothership 57 (47-75) vs drip and ship 182 (144-217) min, p<0.0001).
Table 2.
Logistic key figures
| MS (n=221) | DS (n=314) | P value | |
| Ground bound transport distances (km) (median (IQR)) | |||
| Onset to RH | 12 (4–21) | ||
| RH to CSC | 48 (23–50) | ||
| Onset to CSC* | 9 (3–23) | 60 (36–82) | <0.0001 |
| Time metrics (min) (median (IQR)) | |||
| Onset to RH imaging | 83 (63–118) | ||
| RH to CSC imaging | 141 (112–181) | ||
| Onset to CSC imaging | 72 (56–102) | 232 (191–291) | <0.0001 |
| Picture to puncture time† | 57 (47–75) | 182 (144–217) | <0.0001 |
*Including all transfer routes.
†Picture to puncture time=time interval between initial CT scan and groin puncture at the CSC.
CSC, comprehensive stroke center; DS, drip and ship; MS, mothership; RH, referring hospital.
The middle cerebral artery M1 segment (mothership 59% vs drip and ship 62%, p=0.509) and the distal internal carotid artery (mothership 41% vs drip and ship 39%, p=0.61) were the most common sites of vascular occlusion in both groups (table 1). In both groups, a median of two retrieval maneuvers (mothership 2 (1–3) vs drip and ship 2 (1–3), p=0.779) were necessary to achieve successful recanalization (mTICI ≥2 b). Although statistically significant, the rate of successful recanalization of the infarct related artery was only slightly higher in transferred patients (mothership 82% vs drip and ship 87%, p=0.001). Interfacility transfer extended the time interval from symptom onset to recanalization by 136 min (onset to recanalization time mothership 210 (168-271) vs drip and ship 346 (296-416) min, p<0.0001), while the overall duration of thrombectomy procedures (puncture to final recanalization result) did not differ between the two groups (mothership 70 (45-111) vs drip and ship 75 (49-99), p=0.947). No differences were observed regarding the occurrence of tandem lesions represented by cervical internal carotid artery stenosis of ≥50% requiring angioplasty with or without stenting (mothership 16% vs drip and ship 17%, p=0.934). Short term clinical improvement in the mothership group did not significantly differ from the drip and ship group (NIHSS 24 hours post intervention mothership 6 (1–15) vs drip and ship 5 (2-13), p=0.854). There were no intergroup differences with regard to intracranial hemorrhage (mothership 16% vs drip and ship 13%, p=0.425) or death during hospital stay (mothership 19% vs drip and ship 16%, p=0.486).
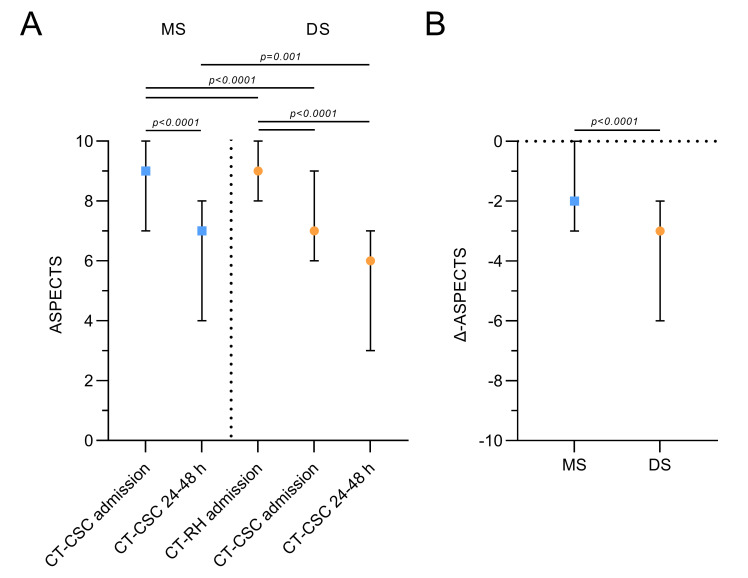
Serial ASPECTS are given in figure 1A and online supplemental table 1. The ICC for interobserver agreement of ASPECTS was good to excellent in both groups (mothership 0.876 (95% CI 0.788 to 0.924); drip and ship 0.958 (0.9381 to 0.971)).10 Interfacility transfer in the drip and ship pathway was associated with a significant drop in median ASPECTS on stroke center admission by 2 points (preinterventional ASPECTS, comprehensive stroke center 7 (6–9) vs external ASPECTS 9 (8–10), p<0.0001). Preinterventional ASPECTS on stroke center admission was significantly lower in drip and ship (7 (6–9)) versus mothership (9 (7–10)) patients (p<0.0001). Infarct extension on follow-up imaging was significantly more favorable in the mothership pathway (mothership 7 (4–8) vs drip and ship 6 (3–7), p=0.001). Consistently, the decline in ASPECTS between baseline (CT at remote hospitals for drip and ship patients and at stroke center presentation for mothership patients) and postinterventional follow-up imaging (figure 1B) was significantly steeper in transferred patients (points lost, mothership 2 (0–3) vs drip and ship 3 (2–6), p<0.0001).
Figure 1.
(A) Serial Alberta Stroke Program Early CT Score (ASPECTS) of directly admitted (mothership (MS)) and referred patients (drip and ship (DS)) at the time of initial imaging, at admission to the comprehensive stroke center (CSC), and at 24–48 hours after symptom onset. (B) Decline in ASPECTS between baseline and follow-up imaging according to treatment pathway. Data are median (IQR). RH, referring hospital.
Multiple linear regression was performed to identify relevant recorded patient characteristics (ie, age, sex, unknown time of symptom onset, baseline ASPECTS, NIHSS at presentation (comprehensive stroke center), blood pressure, heart rate, hypertension, diabetes mellitus, hyperlipidemia, atrial fibrillation, smoking, antithrombotic medication and use of antihypertensive drugs, intravenous thrombolysis, time interval from symptom onset to groin puncture, site of occlusion, stent retrieval maneuvers, angiographic degree of recanalization, duration of thrombectomy, time interval from symptom onset to recanalization, and stenting) and logistic key figures (ie, interfacility transfer, air transport, transfer time, and ground bound transport distance) associated with ASPECTS decline (online supplemental table 2). A model covering all variables of univariate association (p<0.1) is given in table 3, revealing that only interfacility transfer (drip and ship), NIHSS at presentation, degree of angiographic recanalization (mTICI), and duration of the thrombectomy procedure were significant predictors of infarct progression (adjusted R 2=0.209, p<0.0001).
Table 3.
Multiple regression model of ASPECTS decline between baseline and postinterventional follow-up imaging
| Variable | B | SE | T | P value |
| Interfacility transfer (drip and ship) | 3.898 | 1.031 | 3.782 | 0.0002 |
| NIHSS at CSC presentation | 0.13 | 0.029 | 4.517 | <0.0001 |
| mTICI | −0.543 | 0.236 | −2.303 | 0.023 |
| Duration of MT procedure | 0.015 | 0.004 | 3.535 | 0.0006 |
R 2=0.231, adjusted R 2=0.209, F=10.501, p<0.0001
ASPECTS, Alberta Stroke Program Early CT Score; CSC, comprehensive stroke center; MT, mechanical thrombectomy; mTICI, modified Treatment in Cerebral Ischemia Scale; NIHSS, National Institutes of Health Stroke Scale.
Discussion
Prehospital triage and interfacility transfer are critical factors that potentially worsen clinical outcome in acute large vessel occlusion stroke.11 12 So far, there are no data on the extent to which different prehospital pathways affect radiological measures of structural damage and the dynamic progression of cerebral infarction before and after mechanical thrombectomy, which can be semiquantitatively assessed through radiological scoring of ASPECTS.4–6 13–15 Our study addressed this issue with the following main results. Preinterventional ASPECTS declined significantly during interfacility transfer, resulting in significantly lower ASPECTS at stroke center presentation and on follow-up imaging in the drip and ship group compared with the mothership group. Infarct progression from baseline to postinterventional imaging was significantly higher in transferred patients. In the multivariable model, only interfacility transfer, NIHSS at presentation, degree of angiographic recanalization, and duration of the thrombectomy procedure proved to be independent predictors of structural infarct progression.
ASPECTS is a 10 point non-contrast CT scoring system of early ischemic changes within the middle cerebral artery territory.4 For large vessel occlusion stroke, ASPECTS has been increasingly exposed to internal validation with good estimates of internal consistency (Crohnbach’s alpha=0.859) and varying interobserver reliability (ICC=0.672–0.834).1 16 17 In our study, good to excellent levels of inter-rater agreement could be achieved, further supporting that ASPECTS is a reliable tool to assess the extent of cerebral infarction across different CT scanners, scan acquisition protocols, and time points.5 10
Recently, ASPECTS has been used to investigate the dynamics of infarct progression during interfacility transfer, revealing that one of three patients becomes ineligible for mechanical thrombectomy based on ASPECTS imaging criteria,6 13 and that every 1 point increase in ASPECTS decline per hour correlates with a 23 fold lower probability of good functional outcome (modified Rankin Scale score of 0–2) at 90 days.5 In our study, the ASPECTS decline of 2 points that occurred during interfacility transfer was more severe than observed previously by others (1 point), although transfer times were nearly identical.5 14 This discrepancy in early ASPECTS decline may be best explained by the rate of distal internal carotid artery occlusions which was three times higher in our observation. This highlights that not rigid absolute time windows but rather local cerebral pathophysiology and individual collateral recruitment, which critically depend on precise occlusion location, likely affects the dynamics of how the penumbra is transformed into infarction.15 18–20 Prospective registry data revealed that significant treatment delays in the drip and ship treatment pathway (onset to recanalization time, mothership 202 (160-265) vs drip and ship 312 (255-286) min, p<0.0001) lead to worse clinical outcome (modified Rankin Scale score of 0–1 at 90 days, mothership 47,4% vs drip and ship 38%, p=0.005).21 This is in line with the fact that a significant proportion of large vessel occlusion stroke patients (≥70%) can be assigned to the categories of rapid and intermediate infarct progressors,18 22 in whom the frequency of good outcome (modified Rankin Scale score of 0–2) is highly time dependent and declines from 64.1% for those recanalized within 180 min to 46.1% for those recanalized within 480 min.23 In our study, multimodal imaging selection was applied to identify recanalization responders in the extended or unknown time window,24 25 which consequently dissolved the association of time and imaging defined pre- to postinterventional worsening of stroke. Importantly, interfacility transfer remained the most powerful predictor of structural infarct progression.
Although ASPECTS values following interfacility transfer were significantly lower in our cohort, short term functional outcomes at 24 hours were not different between the two prehospital pathways. Each ASPECTS value, by definition, is associated with a spectrum of different infarct volumes for which a strong correlation with NIHSS has been reported (r=0.79, p<0.0001).4 26 However, for small infarct volumes, this correlation does not hold.27 There are various explanations as to why a greater infarct extent may remain neutral with regard to functional outcome, as was observed in our cohort. The clinical endpoint of NIHSS may be biased towards good functional outcome as it does not reflect more subtle neuropsychological deficits.28 Alternatively, or in addition, NIHSS may have underestimated the degree of favorable outcome due to the short follow-up period. Also, the beta error of statistical decision making may have occurred for which this retrospective study may have been at risk because statistical power was not adjustable a priori.
There are certain limitations to this study. First, all referring hospitals were heterogeneously located in peri-urban, urban, and rural areas. This reflects specific geographical and organizational characteristics of our region but limits external generalizability. Second, ASPECTS is an established marker of baseline infarct extent but is not established for the quantification of infarct extent on postinterventional follow-up imaging. Third, ASPECTS is unable to detect volumetric infarct progression due to edema in already affected ASPECTS regions, which may have reached substantial levels at the time of follow-up imaging.29
Conclusion
Infarct progression and postinterventional infarct extent, as assessed by ASPECTS, varied between the drip and ship and mothership pathways for mechanical thrombectomy, leading to more pronounced infarction in transferred patients. ASPECTS may serve as a radiological measure to monitor benefit or harm of different prehospital pathways and to advocate for the direct transfer to a comprehensive stroke center in regions with similar landscapes and infrastructure.
Acknowledgments
We thank T Guenthner-Lengsfeld, Y Xiong, J Kunz, and F Essig for their support in data acquisition and patient handling.
Footnotes
Contributors: AMK, MP, and MS designed the study. AMK, FC, JH, and WM were responsible for patient inclusion. AMK, FC, JH, JF, AGM, and FW acquired the data. AMK, FC, JH, and MS analyzed the data. AMK, FC, JH, MKS, GS, MP, and MS interpreted the data. AMK supervised the study. AMK, MP, and MS wrote the manuscript. All authors read and approved the final manuscript.
Funding: Funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation), Projektnummer 374031971-TRR 240, Projektnummer 413657723-Clinician Scientist programme UNION CVD.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The study was approved by the ethics committee of the University Hospital of Würzburg.
References
- 1. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019;50:E344–418. 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 2. Lima FO, Mont'Alverne FJA, Bandeira D, et al. Pre-hospital assessment of large vessel occlusion strokes: implications for modeling and planning stroke systems of care. Front Neurol 2019;10:1–8. 10.3389/fneur.2019.00955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jadhav AP, Campbell BCV. Ongoing advances in medical and interventional treatments of large vessel occlusion stroke. Stroke 2021;52:1115–7. 10.1161/STROKEAHA.121.033448 [DOI] [PubMed] [Google Scholar]
- 4. Barber PA, Demchuk AM, Zhang J, et al. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. Lancet 2000;355:1670–4. 10.1016/S0140-6736(00)02237-6 [DOI] [PubMed] [Google Scholar]
- 5. Sun C-HJ, Connelly K, Nogueira RG, et al. ASPECTS decay during inter-facility transfer predicts patient outcomes in endovascular reperfusion for ischemic stroke: a unique assessment of dynamic physiologic change over time. J Neurointerv Surg 2015;7:22–6. 10.1136/neurintsurg-2013-011048 [DOI] [PubMed] [Google Scholar]
- 6. Mokin M, Gupta R, Guerrero WR, et al. ASPECTS decay during inter-facility transfer in patients with large vessel occlusion strokes. J Neurointerv Surg 2017;9:442–4. 10.1136/neurintsurg-2016-012331 [DOI] [PubMed] [Google Scholar]
- 7. Kollikowski AM, Amaya F, Stoll G, et al. Impact of landmark endovascular stroke trials on logistical performance measures: a before-and-after evaluation of real-world data from a regional stroke system of care. J Neurointerv Surg 2019;11:563–8. 10.1136/neurintsurg-2018-014286 [DOI] [PubMed] [Google Scholar]
- 8. Dekeyzer S, Nikoubashman O, Lutin B, et al. Distinction between contrast staining and hemorrhage after endovascular stroke treatment: one CT is not enough. J Neurointerv Surg 2017;9:394–8. 10.1136/neurintsurg-2016-012290 [DOI] [PubMed] [Google Scholar]
- 9. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495–9. 10.1016/j.ijsu.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 10. Cicchetti DV. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assess 1994;6:284–90. 10.1037/1040-3590.6.4.284 [DOI] [Google Scholar]
- 11. Ismail M, Armoiry X, Tau N, et al. Mothership versus drip and ship for thrombectomy in patients who had an acute stroke: a systematic review and meta-analysis. J Neurointerv Surg 2019;11:14–19. 10.1136/neurintsurg-2018-014249 [DOI] [PubMed] [Google Scholar]
- 12. Zhou MH, Kansagra AP. Effect of routing paradigm on patient-centered outcomes in acute ischemic stroke. J Neurointerv Surg 2019;11:251–6. 10.1136/neurintsurg-2018-013994 [DOI] [PubMed] [Google Scholar]
- 13. Fuentes B, Alonso de Leciñana M, Ximénez-Carrillo A, et al. Futile interhospital transfer for endovascular treatment in acute ischemic stroke: The Madrid Stroke Network Experience. Stroke 2015;46:2156–61. 10.1161/STROKEAHA.115.009282 [DOI] [PubMed] [Google Scholar]
- 14. Purrucker JC, Mattern N, Herweh C, et al. Electronic Alberta Stroke Program Early CT score change and functional outcome in a drip-and-ship stroke service. J Neurointerv Surg 2020;12:252–5. 10.1136/neurintsurg-2019-015134 [DOI] [PubMed] [Google Scholar]
- 15. Boulouis G, Lauer A, Siddiqui AK, et al. Clinical imaging factors associated with infarct progression in patients with ischemic stroke during transfer for mechanical thrombectomy. JAMA Neurol 2017;74:1361–7. 10.1001/jamaneurol.2017.2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Finlayson O, John V, Yeung R, et al. Interobserver agreement of ASPECT score distribution for noncontrast CT, CT angiography, and CT perfusion in acute stroke. Stroke 2013;44:234–6. 10.1161/STROKEAHA.112.665208 [DOI] [PubMed] [Google Scholar]
- 17. Farzin B, Fahed R, Guilbert F, et al. Early CT changes in patients admitted for thrombectomy: intrarater and interrater agreement. Neurology 2016;87:249–56. 10.1212/WNL.0000000000002860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rocha M, Jovin TG. Fast versus slow progressors of infarct growth in large vessel occlusion stroke: clinical and research implications. Stroke 2017;48:2621–7. 10.1161/STROKEAHA.117.017673 [DOI] [PubMed] [Google Scholar]
- 19. Kollikowski AM, Schuhmann MK, Nieswandt B, et al. Local leukocyte invasion during hyperacute human ischemic stroke. Ann Neurol 2020;87:466–79. 10.1002/ana.25665 [DOI] [PubMed] [Google Scholar]
- 20. Brozici M, van der Zwan A, Hillen B. Anatomy and functionality of leptomeningeal anastomoses: a review. Stroke 2003;34:2750–62. 10.1161/01.STR.0000095791.85737.65 [DOI] [PubMed] [Google Scholar]
- 21. Froehler MT, Saver JL, Zaidat OO, et al. Interhospital transfer before thrombectomy is associated with delayed treatment and worse outcome in the STRATIS registry (Systematic Evaluation of Patients Treated with Neurothrombectomy Devices for Acute Ischemic Stroke). Circulation 2017;136:2311–21. 10.1161/CIRCULATIONAHA.117.028920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Broocks G, Rajput F, Hanning U, et al. Highest lesion growth rates in patients with hyperacute stroke. Stroke 2018;50:STROKEAHA118023457. 10.1161/STROKEAHA.118.023457 [DOI] [PubMed] [Google Scholar]
- 23. Saver JL, Goyal M, van der Lugt A, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA 2016;316:1279–88. 10.1001/jama.2016.13647 [DOI] [PubMed] [Google Scholar]
- 24. Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018;378:NEJMoa1706442. 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
- 25. Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018;378:708–18. 10.1056/NEJMoa1713973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brott T, Marler JR, Olinger CP, et al. Measurements of acute cerebral infarction: lesion size by computed tomography. Stroke 1989;20:871–5. 10.1161/01.STR.20.7.871 [DOI] [PubMed] [Google Scholar]
- 27. Schiemanck SK, Post MWM, Witkamp TD, et al. Relationship between ischemic lesion volume and functional status in the 2nd week after middle cerebral artery stroke. Neurorehabil Neural Repair 2005;19:133–8. 10.1177/154596830501900207 [DOI] [PubMed] [Google Scholar]
- 28. Brott T, Adams HP, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989;20:864–70. 10.1161/01.STR.20.7.864 [DOI] [PubMed] [Google Scholar]
- 29. Tipirneni-Sajja A, Christensen S, Straka M, et al. Prediction of final infarct volume on subacute MRI by quantifying cerebral edema in ischemic stroke. J Cereb Blood Flow Metab 2017;37:3077–84. 10.1177/0271678X16683960 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
neurintsurg-2020-017155supp001.pdf (186.5KB, pdf)
Data Availability Statement
Data are available upon reasonable request.