Abstract
Objective
To examine associations between Mycoplasma genitalium infection during pregnancy and adverse outcomes.
Methods
We did a systematic review of observational studies. We searched Medline, EMBASE, the Cochrane Library and CINAHL up to 11 August 2021. Studies were included if they compared preterm birth, spontaneous abortion, premature rupture of membranes, low birth weight or perinatal death between women with and without M. genitalium. Two reviewers independently assessed articles for inclusion and extracted data. We used random-effects meta-analysis to estimate summary ORs and adjusted ORs, with 95% CIs, where appropriate. Risk of bias was assessed using established checklists.
Results
We identified 116 records and included 10 studies. Women with M. genitalium were more likely to experience preterm birth in univariable analyses (summary unadjusted OR 1.91, 95% CI 1.29 to 2.81, I2=0%, 7 studies). The combined adjusted OR was 2.34 (95% CI 1.17 to 4.71, I2=0%, 2 studies). For spontaneous abortion, the summary unadjusted OR was 1.00 (95% CI 0.53 to 1.89, I2=0%, 6 studies). The adjusted OR in one case–control study was 0.9 (95% CI 0.2 to 3.8). Unadjusted ORs for premature rupture of membranes were 7.62 (95% CI 0.40 to 145.86, 1 study) and for low birth weight 1.07 (95% CI 0.02 to 10.39, 1 study). For perinatal death, the unadjusted OR was 1.07 (95% CI 0.49 to 2.36) in one case–control and 38.42 (95% CI 1.45 to 1021.43) in one cohort study. These two ORs were not combined, owing to heterogeneity. The greatest risk of bias was the failure in most studies to control for confounding.
Conclusion
M. genitalium might be associated with an increased risk of preterm birth. Further prospective studies, with adequate control for confounding, are needed to understand the role of M. genitalium in adverse pregnancy outcomes. There is insufficient evidence to indicate routine testing and treatment of asymptomatic M. genitalium in pregnancy.
PROSPERO registration number
CRD42016050962.
Keywords: mycoplasma, pregnancy, systematic review, meta-analysis
Introduction
Bacterial STIs during pregnancy, such as Chlamydia trachomatis and Neisseria gonorrhoeae have been reported to be associated with one or more of the following adverse pregnancy and perinatal outcomes: spontaneous abortion, preterm birth (PTB), premature rupture of membranes (PROM), low birth weight (LBW) and perinatal death.1–6 In pregnancy, the inflammatory response resulting from infections that ascend to the upper genital tract provides a plausible biological mechanism for the association between STIs and preterm birth.7 It is hypothesised that preterm labour is a common pathway of a cascade of proinflammatory cytokine production, for which endocervical pathogens are one of the triggers.7 If associations observed in epidemiological studies reflect a causal pathway, early detection and treatment of STIs in pregnancy is a potential intervention. In observational epidemiological studies, it is essential to understand whether there are confounding factors that are known to be associated with both an exposure (eg, an STI) and an outcome (eg, preterm birth) and to control for them in multivariable statistical analyses. Systematic reviews show that potential confounders, such as young age, lower socioeconomic position and smoking are often not controlled for, however.5 6
Mycoplasma genitalium is the most recently identified bacterial STI. The prevalence of M. genitalium in high-income countries is around 1% in studies among the general population and is similar among pregnant women,8 but M. genitalium has been found in 12% or more of pregnant women in studies in South Africa and Papua New Guinea.9 10 The strength of association between M. genitalium during pregnancy and poor pregnancy outcomes is still unclear.11 In a systematic review of observational studies published up to 2014, Lis et al found associations with preterm birth and spontaneous abortion, but not with stillbirth.11 That review included studies with self-reported outcomes and the potential effects of confounding factors could not be examined because the estimates in the meta-analyses combined both unadjusted and adjusted estimates. Other outcomes, such as PROM, LBW and perinatal death, were not considered. As nucleic acid amplification tests (NAATs) for M. genitalium detection are increasingly used for widespread testing in populations including pregnant women, an updated review of the evidence about associations between M. genitalium and objectively documented adverse pregnancy and perinatal outcomes is warranted.
The primary objective of this study was to assess the association between M. genitalium infection in pregnancy and PTB. Secondary outcomes were spontaneous abortion, PROM, LBW and perinatal death.
Methods
This systematic review and meta-analysis is registered in the PROSPERO database (CRD42016050962) and follows a published protocol, which also addresses N. gonorrhoeae and other genital mycoplasmas.12 We report our findings using the Preferred Reporting Items for Systematic reviews and Meta-Analyses 2020 (online supplemental table S1).13
sextrans-2021-055352supp001.pdf (304.2KB, pdf)
Eligibility criteria
Studies reporting on M. genitalium during pregnancy, labour or the immediate postpartum period were eligible for inclusion if they reported on any of the following outcomes (in order of occurrence during pregnancy): spontaneous abortion, PROM (preterm and term), PTB, LBW, and perinatal or neonatal death. We included clinical trials, cohort, case–control and cross-sectional studies but excluded individual case reports, case series, opinion articles and studies without a comparison group.
Information sources and search strategy
We searched Medline, Excerpta Medica database (EMBASE), the Cumulative Index to Nursing and Allied Health Literature (CINAHL) and the Cochrane library databases from 1948 to 11 August 2021. Search terms combined thesaurus and free-text terms for pregnancy and M. genitalium and the outcomes of interest. The search strategy is published12 and listed in online supplemental text S1. We examined reference lists of included studies for additional articles. The searches did not apply language restrictions, but we included only articles published in English or German (languages spoken fluently by review team members).
Study selection and data extraction
One reviewer (LV) screened titles and abstracts (online supplemental text S2). Two reviewers (LV, DE-G) independently screened the full text of potentially relevant articles and extracted data independently into a standardised, piloted form in a Research Electronic Data Capture database (REDCap, Vanderbilt University, Tennessee, USA) recording study design, participant characteristics, presence or absence of M. genitalium, pregnancy, perinatal or neonatal outcomes, and other STI and genital infections. Standard definitions for outcomes were used,12 or if necessary, we used the definitions used by the authors. If results were described for more than one anatomical site, we used the following order of preference: vaginal or cervical swabs, urine, amniotic fluid, placenta because the original site of infection is the genital tract, with other sites reflecting increasingly distant sites of potential ascending infection. All diagnoses were made by NAATs. Discrepancies were resolved by discussion or by the decision of a third reviewer (NL, CF).
Risk of bias in individual studies
Two reviewers assessed the risk of bias in each study independently (LV, DE-G or CF), using checklists published by the UK National Institute for Health and Care Excellence for case–control and cohort studies.14 A third reviewer resolved discrepancies (NL). Each study was assessed for internal and external validity overall as having all or most of checklist criteria fulfilled (++), some checklist criteria fulfilled (+) or few or no checklist criteria fulfilled (−), and the main sources of bias were recorded.
Data synthesis and analysis
We used the ‘metan’ command in Stata (V.15.1; StataCorp, College Station, Texas, USA) for analyses. We used the OR as the measure of association for all study designs, on the assumption that the risk ratio and OR would be similar, as the outcomes of interest are usually rare events. We calculated the crude OR and its 95% CI based on raw data from the paper, or we extracted the published values if raw data were not available. If there were no events in one group, we applied a continuity correction, adding 0.5 to each cell. Where authors reported a multivariable analysis, we extracted the adjusted OR (aOR, with its 95% CI) and recorded the variables included in the model. We examined forest plots for each outcome, by study design, and used the I2 statistic to examine the level of variability in effect estimates due to heterogeneity between studies other than that due to chance.15
For outcomes reported by two or more studies, we used random-effects models for meta-analyses,15 based on an assessment of statistical and clinical heterogeneity. The random-effects model is appropriate for meta-analysis of observational studies because it assumes that there are differences between studies in the underlying effects because of heterogeneity in study populations and measurement of exposures and outcomes.16 We first examined estimates for cohort and case–control studies separately. Where appropriate, we estimated a summary OR (and 95% CI) and a prediction interval, which displays the expected range of effect estimates in future studies.15 For adjusted estimates, we used the same approach as for the unadjusted analyses. For outcomes for which there were at least two studies of the same design, we categorised study locations as high income and non-high income (combining low-income and middle-income countries), based on the 2019 World Bank list.17
Risk of bias across studies and certainty of the body of evidence
We planned to examine publication bias by generating a funnel plot for outcomes reported by 10 or more studies. We did not conduct any subgroup analyses. We used the Grading of Recommendations Assessment, Development and Evaluation approach, adapted to assess the certainty of the evidence about the possible causal association18 between M. genitalium and each outcome.
Results
The searches of electronic databases identified 116 records and we screened 104 records after exclusion of duplicates. Of 26 full-text articles assessed for eligibility (online supplemental figure S1), we included 10 studies, which reported on 18 outcomes (table 1, online supplemental table S2).3 19–27
Table 1.
Summary of study characteristics of included studies
| First author, publication year, reference number | Study design | Timing of specimen collection | Specimen type | Total enrolled, N | Sample size for outcome, events in women with Mycoplasma genitalium/total with the outcome, n/n (%) |
||||
| PTB | PROM | LBW | SAB | PND | |||||
| Agger, 201421 | Cohort | 1st or 2nd trimester | Endocervical swab | 783 | 676, 0/54 (0) |
NR | NR | NR | NR |
| Averbach, 201319 | Cohort* | 1st or 2nd trimester | Endocervical swab | 100 | 66, 1/11 (9) |
NR | 81, 1/11 (9) |
81, 1/9 (11) |
NR |
| Choi, 201222 | Case–control | NR | Vaginal swab | 217 | 217, 0/100 (0) |
NR | NR | NR | NR |
| Edwards, 200623 | Cohort | NR | Not clear | 137 | 134† | NR | NR | NR | NR |
| Hitti, 20103 | Case–control* | <48 hours post partum | Endocervical swab | 1338 | 1328, 29/661 (4) |
NR | NR | NR | NR |
| Kataoka, 200624 | Cohort | 1st trimester | Vaginal swab | 1040 | 871, 0/15 (0) |
871, 0/7 (0) |
NR | 877, 0/5 (0) |
872, 0/1 (0) |
| Labbe, 200220 | Case–control | <24 hours post partum | Endocervical swab | 1014 | 799, 16/119 (13) |
NR | NR | 653, 2/53 (4) |
725, 8/125 (6) |
| Oakeshott, 200425 | Cohort | 1st trimester | Urine | 1216 | 699, 0/39 (0) |
NR | NR | 894, 1/92 (1) |
NR |
| Rahimkhani, 201827 | Cohort | 1st or 2nd trimester | Urine | 119 | NR | NR | NR | 119, 6/31 (19) |
NR |
| Short, 201026 | Case–control* | NR | Urine | 216 | NR | NR | NR | 213, 3/82 (4) |
NR |
*Authors reported both univariable and multivariable analyses.
†Numerator and denominator not reported in text. OR and 95% CI, as reported by authors, used in meta-analysis.
LBW, low birth weight; NR, not reported; PROM, premature rupture of membranes; PND, perinatal death; PTB, preterm birth; SAB, spontaneous abortion.
Study locations and sociodemographic information are reported in online supplemental table S3. Briefly, seven studies took place in high-income countries,19 21–26 and three in low/middle-income countries.3 20 27 Seven studies took place in urban locations.3 19 22–26 Age was reported in three studies,19 25 27 eight reported on ethnicity,19–26 four included smokers3 19 23 26 and two included women with multiple pregnancies.3 21 In three studies, authors reported adjusted ORs from multivariable analyses.3 19 26
The authors of seven studies reported timing of specimen collection: specimens were obtained during the first trimester in two studies,24 25 during the first or second trimester in three,19 21 27 and in the early postpartum period in two studies3 20 (table 1, online supplemental table S2). The sample types were endocervical swabs in four studies,3 19–21 urine in three25–27 and vaginal swabs in two studies.22 24 In one study, specimen type was unclear.23 In three studies, women who tested positive for an STI were given antibiotic treatment.3 22 24 In one study in Japan, the authors reported that they gave antibiotics if C. trachomatis and/or N. gonorrhoeae were detected but not for any Mycoplasma spp alone, that is, in the absence of C. trachomatis or N. gonorrhoeae.24 This was also the only study that reported the timing of antibiotic treatment (first or second trimester) (online supplemental table S4).
In all included studies, the authors tested for one or more other STI or genital infections (online supplemental tables S5–S8 report on coinfections with M. genitalium in included studies). C. trachomatis was tested for in all but one study20 and was detected in 2.2%–7.5% of women. N. gonorrhoeae was tested for in seven studies3 19–24 and detected in 0.0%–7.9% of women. In four studies, 0.0%–4.8% of women had positive serological tests for syphilis19 20 22 23 and in four studies, bacterial vaginosis was diagnosed in 0.8%–56.0% of women.19 22 23 25 In six studies, one or more of M. hominis, Ureaplasma urealyticum, U. parvum, Trichomonas vaginalis, herpes simplex virus type 2 or HIV were also reported3 20–24 (online supplemental table S9).
Risk of bias
In case–control studies, the potential for selection bias could not be assessed because response rates for cases and controls were only reported in one study.3 In both case–control and cohort studies, the risk of confounding was high because potential confounding factors were often not reported and multivariable analyses were conducted in very few studies. Among the four case–control studies, all or most of checklist criteria (++) were completed for internal validity for two studies.3 26 For external validity, three of these studies had some checklist criteria (+) completed3 20 22 (online supplemental table S10). Five of the six cohort studies had some checklist criteria (+) completed for internal validity19 21 23–25 and two for external validity21 23 (online supplemental table S11). There were too few studies to assess publication bias using funnel plots for any outcome.
Preterm birth
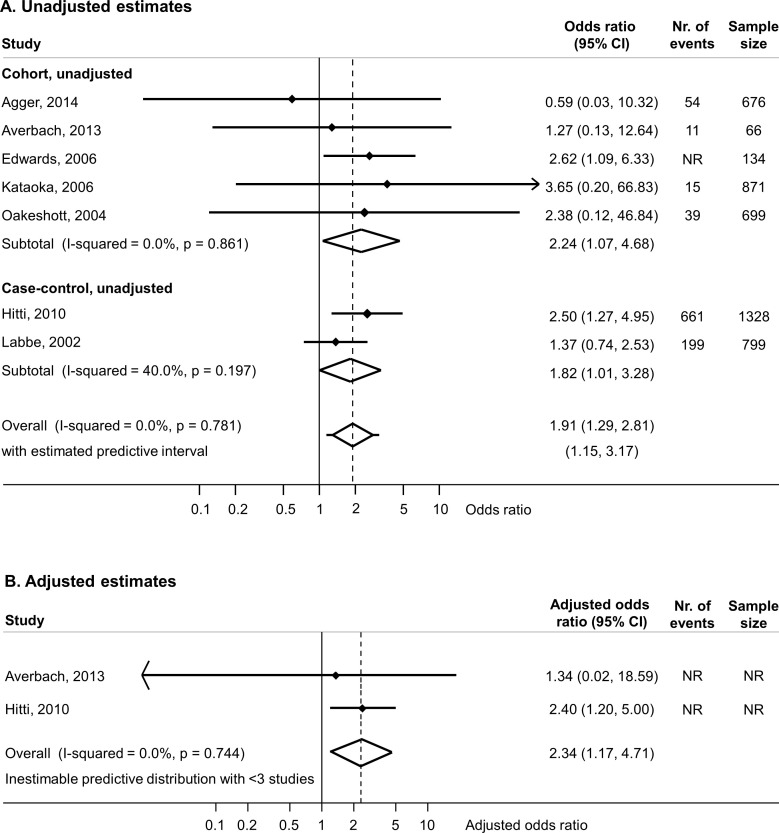
Eight of the ten included studies reported on M. genitalium and the primary outcome, PTB.3 19–25 One study was not included in meta-analysis because the authors reported that no woman tested positive for M. genitalium infection.22 Of the seven studies included in the meta-analysis of univariable results, five cohort studies reported on 2446 women,19 21 23–25 and two case–control studies reported on 2127 women.3 20 The meta-analysis of all seven studies found an OR 1.91 (95% CI 1.29 to 2.81, I2=0%) and increased odds of PTB in both cohort studies and case–control studies (figure 1A). Two studies reported the results of multivariable analyses. In the case–control study in Peru, age, cigarette smoking, second trimester bleeding, twin gestation and prior PTB were controlled for.3 In a cohort study in the USA, maternal age and history of preterm delivery were controlled for.19 The aOR in each study was similar to the unadjusted OR in the same study (figure 1A, B).3 19 The summary aOR was 2.34 (95% CI 1.17 to 4.71, I2=0%, 2 studies, table 2, figure 1B).
Figure 1.
Random-effects meta-analysis of studies reporting on the association between Mycoplasma genitalium during pregnancy and preterm birth. Forest plots show effect estimates for each study for unadjusted estimates (A) and adjusted estimates (B). In studies reporting multivariable analyses, the numbers of events or total number of observations included were not reported (NR). For each study, the solid diamond is the point estimate, the lines either side are the 95% CIs. A line ending in an arrow means that the confidence limit lies beyond the values of the x-axis. The open diamond is the summary estimate. The lines either side of the open diamond show the prediction interval if there are three or more studies in the meta-analysis. The x-axis is on the log scale.
Table 2.
Summary estimates for associations between Mycoplasma genitalium in pregnancy and adverse pregnancy and perinatal outcomes
| Outcome, study design | Analysis | Number of studies, design | Sample size for outcome | I2 % | Summary OR (95% CI) |
| Preterm birth | Unadjusted, meta-analysis | 7 5 cohort, 2 case–control |
4573 | 0 | 1.91 (1.29 to 2.81) |
| Adjusted, meta-analysis | 2 1 cohort, 1 case–control |
NR | 0 | 2.34 (1.17 to 4.71) | |
| Spontaneous abortion | Unadjusted, meta-analysis | 6 4 cohort, 2 case–control |
2837 | 0 | 1.00 (0.53 to 1.89) |
| Adjusted | 1 case–control | 216 | NA | 0.9 (0.2 to 3.8)* | |
| Premature rupture of membranes | Unadjusted | 1 cohort | 871 | NA | 7.62 (0.40 to 145.86) |
| Low birth weight | Unadjusted | 1 cohort | 81 | NA | 1.07 (0.02 to 10.39) |
| Perinatal death | Unadjusted | 1 cohort | 872 | NA | 38.42 (1.45 to 1021.43) |
| Unadjusted | 1 case–control | 725 | NA | 1.07 (0.49 to 2.36) |
*Adjusted OR reported to one decimal place, as in the publication, reference 25.
NA, not applicable; NR, not reported.
Spontaneous abortion
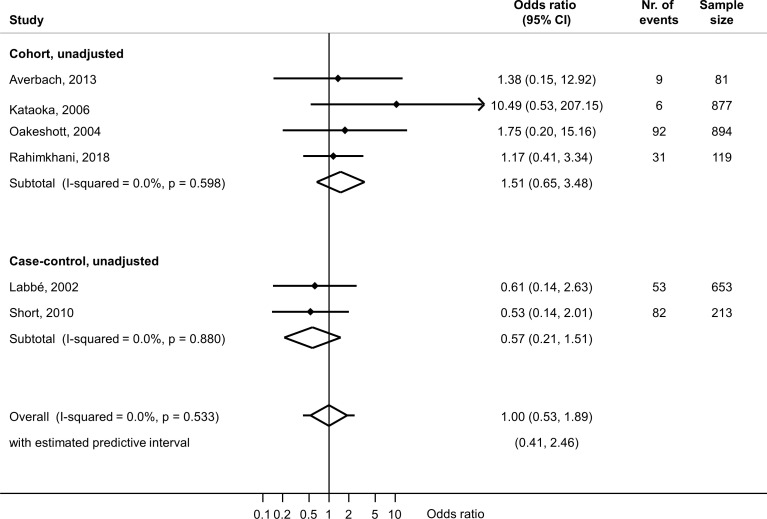
Six studies reported on associations with spontaneous abortion: four cohort studies including 1971 women19 24 25 27 and two case--control studies including 866 women.20 26 The summary unadjusted OR from all six studies was 1.00 (95% CI 0.53 to 1.89, I2=0%) (figure 2). Only one case–control study reported an aOR 0.9 (95% CI 0.2 to 3.8), adjusting for age, history of spontaneous abortion, smoking and gestational age26 (table 2).
Figure 2.
Random-effects meta-analysis of studies reporting an unadjusted association between Mycoplasma genitalium during pregnancy and spontaneous abortion. Forest plots show effect estimates for each study. For each study, the solid diamond is the point estimate, the lines either side are the 95% CIs. A line ending in an arrow means that the confidence limit lies beyond the values of the x-axis. The open diamond is the summary estimate. The lines either side of the open diamond show the prediction interval. The x-axis is on the log scale. Only one study reported a multivariable analysis (adjusted OR 0.90, 95% CI 0.2 to 3.8) (ref 26).
Premature rupture of membranes
One cohort study from Japan provided data about the univariable association between M. genitalium and PROM (OR 7.62, 95% CI 0.40 to 145.86, n=871) (table 2).24
Low birth weight
One cohort study from the USA reported on the univariable association between M. genitalium and LBW (OR 1.07; 95% CI 0.02 to 10.39, n=81) (table 2).19
Perinatal death
Two studies provided data about univariable associations between M. genitalium and perinatal death (online supplemental figure S2).20 24 In a case–control study in Guinea-Bissau, the OR was 1.07 (95% CI 0.49 to 2.36, n=725).20 In one cohort study in Japan, the OR was 38.42 (95% CI 1.45 to 1021.43, n=872).26 Owing to heterogeneity (I2=77%), we did not combine these estimates (table 2, online supplemental figure S2).
Certainty of evidence
The certainty of evidence of causality (online supplemental table S12) was low for the outcomes preterm birth and spontaneous abortion, and very low for all other outcomes, based on assessment of study design and analysis.
Discussion
This systematic review included 10 studies that reported on associations between M. genitalium and adverse pregnancy outcomes. For PTB, the summary unadjusted OR was 1.91 (95% CI 1.29 to 2.81, I2=0%, 7 studies) and summary aOR 2.34 (95% CI 1.17 to 4.71, I2=0%, 2 studies) with low between-study heterogeneity. For spontaneous abortion, the summary estimate of the unadjusted OR was 1.00 (95% CI 0.53 to 1.89, I2=0%, 6 studies). Only one study reported on the outcomes PROM, LBW and two reported on perinatal death.
Strengths and weaknesses
Strengths of this systematic review are that we followed a protocol with a priori methods and we tried to reduce subjectivity by having two independent reviewers select studies for inclusion, extract data and assess the risk of bias. We examined adjusted effect estimates, where reported. It is important to report the confounder-adjusted estimate prominently, even when the data are sparse, because this is the most relevant measure for systematic reviews of observational studies that examine potential causal associations.16 We also examined findings from case–control and cohort studies separately, because the different study designs are subject to different biases.28 In this review, it made sense to combine the estimates for the outcomes PTB and spontaneous abortion because the strength of association in both was compatible. The main weakness of the review methods was that, despite a broad search strategy, we may have missed relevant studies in languages other than English or German. Given the small number of included studies, we could not assess the possibility of publication bias statistically.
Comparison with other studies and interpretation
Our findings update and add to those of the systematic review by Lis et al.11 Despite a large increase in the availability of testing for M. genitalium, the number of published studies investigating associations with adverse pregnancy outcomes has not increased substantially since 2014, when the search of Lis et al ended. In contrast with Lis et al, we only included studies in which outcomes were directly observed, so there was no potential for recall bias in studies that rely on self-reported outcomes, and we examined the results of unadjusted and confounder-adjusted analyses separately. No new studies about the association between M. genitalium and PTB have been published since 2014. We found an association in meta-analysis of unadjusted estimates, and in the two studies with a multivariable analysis, the increased risk of preterm birth in women with M. genitalium, compared with those without, persisted.3 19 The potential for confounding cannot be assessed in detail; however, both studies adjusted for age, but not all potentially relevant confounders were considered. For spontaneous abortion, we included six studies and did not find evidence of an association with M. genitalium in univariable analyses, or in the only study reporting a multivariable analysis (aOR 0.9, 95% CI 0.2 to 3.8).26 In contrast, Lis et al reported an unadjusted summary OR of 1.82 (95% CI 1.10 to 3.03), but only one of the included studies did not use self-reported outcomes.11 The certainty of evidence for these outcomes is low because reliance on unadjusted findings means that the estimate from fully confounder-adjusted analyses might be substantially different. For all other outcomes, the evidence is very uncertain because of the paucity of studies.
The summary point estimates for the association between M. genitalium and PTB (case–control studies, OR 1.82; 95% CI 1. 01 to 3.28 and cohort studies, OR 2.24; 95% CI 1.07 to 4.68) appear higher than those obtained from systematic reviews of studies of associations with other bacterial STIs, although the overlap in CIs for all estimates means that the finding might be due to chance. For C. trachomatis, the reported summary unadjusted OR for preterm labour in case–control studies was 1.29 (95% CI 1.11 to 1.50, I2=82%, 10 studies) and for cohort studies 1.54 (95% CI 1.48 to 1.60, I2=98%, 13 studies).5 Tang et al 5 identified many more studies about C. trachomatis overall and found that summary confounder-adjusted ORs were lower than the unadjusted estimates. For T. vaginalis, the summary unadjusted risk ratio was 1.42 (95% CI 1.15 to 1.75, I2=63%, 9 studies)29 and for N. gonorrhoeae, the summary unadjusted OR was 1.55 (95% CI 1.21 to 1.99, 18 studies).6 Interpretation of the evidence overall is limited by poor reporting, partly because M. genitalium was not a primary study objective in many studies, with analyses done retrospectively using stored samples. Additionally, reporting of information about antibiotic treatment, the trimester in which treatment was given and coinfections was poor. Only 3 out of the 10 studies reported information on antibiotic treatment, and only 1 study specified the trimester in which treatment was given. We documented if studies tested for other STIs and if coinfections with STI were reported. Unfortunately, coinfections with STIs were often not reported, which made it difficult to assess if they were confounding variables.
Implications for practice and research
This systematic review found some evidence that M. genitalium might increase the risk of PTB, but not spontaneous abortion. The limitations of the evidence available from published studies mean that there is a low level of certainty about these estimated effect sizes and there is insufficient evidence to determine whether M. genitalium is causally associated with PTB or other adverse pregnancy and perinatal outcomes. Future studies examining the association between M. genitalium infection and adverse pregnancy and birth outcomes are needed. These studies should be designed prospectively, with adequate statistical power to conduct multivariable analyses that control for potential confounding and should report on coinfections and provision and timing of antibiotic treatment during pregnancy. There are ongoing trials of the effectiveness of testing for STIs in pregnancy,30 31 in which the association between M. genitalium and the prespecified outcomes could be examined. Randomised controlled trials will be needed to determine whether an intervention to offer screening and treatment for M. genitalium in pregnancy reduces PTB or other adverse pregnancy outcomes. In view of the propensity for, and increasing levels of, antimicrobial resistance to azithromycin,32 testing and treatment for asymptomatic M. genitalium in pregnancy is not indicated at present.
Key messages.
Few studies have examined associations between Mycoplasma genitalium and adverse pregnancy and perinatal outcomes.
There is some evidence of an association between M. genitalium in pregnancy and preterm birth, but no evidence of an association with spontaneous abortion.
There is insufficient evidence to recommend screening and treatment for asymptomatic M. genitalium infection in pregnancy.
Footnotes
Handling editor: Bea Vuylsteke
Twitter: @nicolamlow
Contributors: LV, DE-G, AV and NL conceived the study and wrote the protocol. LV and DE-G led the study, including screening and data extraction. CF and DE-G undertook all statistical analyses, with support from NL. CF, DE-G and LV wrote the first draft of the manuscript. AV and NL reviewed the first draft and made important intellectual contributions to the revised version. All authors read, provided feedback and approved the final manuscript. NL is the guarantor of the study.
Funding: LV receives salary support from the Australian National Health and Medical Research Council (NHMRC), through an early career fellowship. DE-G received salary support from r4d programme (Swiss Programme for Research on Global Issues for Development) (grant number IZ07Z0-160909). AV receives salary support from the Australian NHMRC, through a Career Development Fellowship. NL obtained funding from the Swiss National Science Foundation (grant numbers IZ07Z0-160909 and 197831).
Disclaimer: The funders played no role in the development of the protocol or the analyses conducted.
Competing interests: NL is an associate editor of Sexually Transmitted Infections.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data are available in the manuscript or the online supplemental materials.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study does not involve human participants.
References
- 1. Mullick S, Watson-Jones D, Beksinska M, et al. Sexually transmitted infections in pregnancy: prevalence, impact on pregnancy outcomes, and approach to treatment in developing countries. Sex Transm Infect 2005;81:294–302. 10.1136/sti.2002.004077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Donders GG, Desmyter J, De Wet DH, et al. The association of gonorrhoea and syphilis with premature birth and low birthweight. Genitourin Med 1993;69:98–101. 10.1136/sti.69.2.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hitti J, Garcia P, Totten P, et al. Correlates of cervical Mycoplasma genitalium and risk of preterm birth among Peruvian women. Sex Transm Dis 2010;37:81–5. 10.1097/OLQ.0b013e3181bf5441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cotch MF, Pastorek JG, Nugent RP, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. The vaginal infections and Prematurity Study Group. Sex Transm Dis 1997;24:353–60. 10.1097/00007435-199707000-00008 [DOI] [PubMed] [Google Scholar]
- 5. Tang W, Mao J, Li KT, et al. Pregnancy and fertility-related adverse outcomes associated with Chlamydia trachomatis infection: a global systematic review and meta-analysis. Sex Transm Infect 2020;96:322–9. 10.1136/sextrans-2019-053999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vallely LM, Egli-Gany D, Wand H. Adverse pregnancy and neonatal outcomes associated with Neisseria gonorrhoeae: systematic review and meta-analysis. Sex Transm Infect 2021;97:104–11. 10.1136/sextrans-2020-054653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. BJOG 2006;113 Suppl 3:17–42. 10.1111/j.1471-0528.2006.01120.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baumann L, Cina M, Egli-Gany D, et al. Prevalence of Mycoplasma genitalium in different population groups: systematic review andmeta-analysis. Sex Transm Infect 2018;94:255–62. 10.1136/sextrans-2017-053384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Scoullar MJL, Boeuf P, Peach E, et al. Mycoplasma genitalium and other reproductive tract infections in pregnant women, Papua New Guinea, 2015-2017. Emerg Infect Dis 2021;27:894–904. 10.3201/eid2703.201783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smullin CP, Green H, Peters R, et al. Prevalence and incidence of Mycoplasma genitalium in a cohort of HIV-infected and HIV-uninfected pregnant women in Cape Town, South Africa. Sex Transm Infect 2020;96:501–8. 10.1136/sextrans-2019-054255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma genitalium infection and female reproductive tract disease: a meta-analysis. Clin Infect Dis 2015;61:418–26. 10.1093/cid/civ312 [DOI] [PubMed] [Google Scholar]
- 12. Vallely LM, Egli-Gany D, Pomat W, et al. Adverse pregnancy and neonatal outcomes associated with Neisseria gonorrhoeae, Mycoplasma genitalium, M. hominis, Ureaplasma urealyticum and U. parvum: a systematic review and meta-analysis protocol. BMJ Open 2018;8:e024175. 10.1136/bmjopen-2018-024175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. NICE . The social care guidance manual: process and methods. UK: National Institute for Health and care Excellence, 2013. [PubMed] [Google Scholar]
- 15. Riley RD, Higgins JPT, Deeks JJ. Interpretation of random effects meta-analyses. BMJ 2011;342:d549. 10.1136/bmj.d549 [DOI] [PubMed] [Google Scholar]
- 16. Dekkers OM, Vandenbroucke JP, Cevallos M, et al. COSMOS-E: guidance on conducting systematic reviews and meta-analyses of observational studies of etiology. PLoS Med 2019;16:e1002742. 10.1371/journal.pmed.1002742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Worldbank . List of economies 2019. Available: https://databank.worldbank.org/data/download/site-content/CLASS.xls [Accessed 13th February 2022].
- 18. Schünemann H, Hill S, Guyatt G, et al. The grade approach and Bradford Hill's criteria for causation. J Epidemiol Community Health 2011;65:392–5. 10.1136/jech.2010.119933 [DOI] [PubMed] [Google Scholar]
- 19. Averbach SH, Hacker MR, Yiu T, et al. Mycoplasma genitalium and preterm delivery at an urban community health center. Int J Gynaecol Obstet 2013;123:54–7. 10.1016/j.ijgo.2013.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Labbé A-C, Frost E, Deslandes S, et al. Mycoplasma genitalium is not associated with adverse outcomes of pregnancy in Guinea-Bissau. Sex Transm Infect 2002;78:289–91. 10.1136/sti.78.4.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Agger WA, Siddiqui D, Lovrich SD, et al. Epidemiologic factors and urogenital infections associated with preterm birth in a midwestern U.S. population. Obstet Gynecol 2014;124:969–77. 10.1097/AOG.0000000000000470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Choi SJ, Park SD, Jang IH, et al. The prevalence of vaginal microorganisms in pregnant women with preterm labor and preterm birth. Ann Lab Med 2012;32:194–200. 10.3343/alm.2012.32.3.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Edwards RK, Ferguson RJ, Reyes L, et al. Assessing the relationship between preterm delivery and various microorganisms recovered from the lower genital tract. J Matern Fetal Neonatal Med 2006;19:357–63. 10.1080/00207170600712071 [DOI] [PubMed] [Google Scholar]
- 24. Kataoka S, Yamada T, Chou K, et al. Association between preterm birth and vaginal colonization by mycoplasmas in early pregnancy. J Clin Microbiol 2006;44:51–5. 10.1128/JCM.44.1.51-55.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oakeshott P, Hay P, Taylor-Robinson D, et al. Prevalence of Mycoplasma genitalium in early pregnancy and relationship between its presence and pregnancy outcome. BJOG 2004;111:1464–7. 10.1111/j.1471-0528.2004.00276.x [DOI] [PubMed] [Google Scholar]
- 26. Short VL, Jensen JS, Nelson DB. Mycoplasma genitalium among young, urban pregnant women. Infect Dis Obstet Gynecol 2010;2010:984760–8. 10.1155/2010/984760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rahimkhani M, Mordadi A, Gilanpour M. Detection of urinary Chlamydia trachomatis, Mycoplasma genitalium and human papilloma virus in the first trimester of pregnancy by PCR method. Ann Clin Microbiol Antimicrob 2018;17:25. 10.1186/s12941-018-0276-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Low N. Chlamydia trachomatis and reproductive health: what can we learn from systematic reviews of observational studies? Sex Transm Infect 2020;96:315–7. 10.1136/sextrans-2019-054279 [DOI] [PubMed] [Google Scholar]
- 29. Silver BJ, Guy RJ, Kaldor JM, et al. Trichomonas vaginalis as a cause of perinatal morbidity: a systematic review and meta-analysis. Sex Transm Dis 2014;41:369–76. 10.1097/OLQ.0000000000000134 [DOI] [PubMed] [Google Scholar]
- 30. Grant JS, Chico RM, Lee AC, et al. Sexually transmitted infections in pregnancy: a narrative review of the global research gaps, challenges, and opportunities. Sex Transm Dis 2020;47:779–89. 10.1097/OLQ.0000000000001258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vallely AJ, Pomat WS, Homer C, et al. Point-of-care testing and treatment of sexually transmitted infections to improve birth outcomes in high-burden, low-income settings: study protocol for a cluster randomized crossover trial (the wantaim trial, Papua New Guinea). Wellcome Open Res 2019;4:53. 10.12688/wellcomeopenres.15173.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Machalek DA, Tao Y, Shilling H, et al. Prevalence of mutations associated with resistance to macrolides and fluoroquinolones in Mycoplasma genitalium: a systematic review and meta-analysis. Lancet Infect Dis 2020;20:1302–14. 10.1016/S1473-3099(20)30154-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
sextrans-2021-055352supp001.pdf (304.2KB, pdf)
Data Availability Statement
All data are available in the manuscript or the online supplemental materials.