Abstract
Introduction
Cystic fibrosis (CF) is a severe monogenic disorder caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. Several types of CFTR modulators (correctors/potentiators) have been developed to overcome protein dysfunction associated with these mutations. CFTR modulator therapy is now available for the major CF-causing mutations; however, 10% of people with CF remain without causal treatments. By combining investigational and market-approved CFTR modulators, we aimed to maximise functional rescue of iva-, luma- and tezacaftor refractory mutants G85E and N1303K.
Methods
We used the well-established forskolin-induced swelling (FIS) in primary rectal organoids to assess responses to different CFTR corrector and potentiator types. The FIS analysis was performed with brightfield microscopy, allowing both 1-h and 24-h follow-up. Corrector and potentiator activity of elexacaftor was investigated.
Results
For G85E, maximal rescue was observed by a combination of elexacaftor and corr4a. For N1303K, the quadruple combination teza-elexa-ivacaftor with apigenin was required to obtain a rescue similar to that of luma-ivacaftor rescued F508del. Elexacaftor rescued G85E and N1303K by different mechanisms, with chronic corrector effects on G85E and acute potentiation of N1303K only in the presence of ivacaftor. Synergy in N1303K rescue for iva-elexacaftor and apigenin suggests at least three potentiator mechanisms for this mutant. 24-h FIS identified ivacaftor as the main CFTR modulator for N1303K and elexacaftor and apigenin as co-potentiators.
Conclusions
Novel combinations of CFTR modulators can further improve functional rescue of G85E and N1303K in rectal organoids, although for N1303K, more effective CFTR modulators are still needed.
Short abstract
Organoids can guide personalised medicine in cystic fibrosis. Novel modulator combinations rescue G85E and N1303K beyond Trikafta. Label-free, long-term organoid monitoring unravels major contributors to the functional rescue of these rare CFTR mutants. https://bit.ly/3AKYJnz
Introduction
Cystic fibrosis (CF) affects over 85 000 people worldwide and is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene [1–3]. CFTR regulates chloride, bicarbonate and fluid transport across secretory epithelia in many organs. Currently, over 2100 mutations have been described (http://www.genet.sickkids.on.ca/Home.html) of which at least 382 are confirmed disease-causing (https://cftr2.org/). These mutations disturb the protein synthesis (Class I/II/V/VII), function (Class III/IV) or stability (Class VI) at the plasma membrane (PM) of CFTR, or a combination of those [4, 5].
Small molecules have been developed to improve CFTR processing and trafficking (“correctors”) or its gating (“potentiators”). Corrector and potentiator types have been proposed to reflect the distinct mechanisms of action of different CFTR modulators (supplementary table S1 and [6–9]). To date, four molecules have received market approval either alone or in combination: 1) potentiator ivacaftor (VX-770; type I potentiator), 2) correctors lumacaftor (VX-809; type I corrector) and its derivative 3) tezacaftor (VX-661; type I corrector), and 4) corrector elexacaftor (VX-445; type III corrector). Highly effective CFTR modulator treatment is now approved for the majority of people with CF (PwCF): either ivacaftor monotherapy (Kalydeco™) [10] or teza-elexa-ivacaftor triple-combination therapy (Trikafta™, Kaftrio™ in Europe) [11, 12]. Prior to the approval of Trikafta™, two other modulator combinations had been approved for PwCF homozygous for the most common mutation, F508del (c.1521_1523delCTT): Orkambi™ (luma-ivacaftor) and Symdeko™ (Symkevi™ in Europe, teza-ivacaftor) [13, 14]. In December 2020, the Food and Drug Administration (FDA) announced a label extension for Kalydeco™, Symdeko™ and Trikafta™ to qualify over 100 additional rare mutations for these therapies [15].
CF cell models have been instrumental in guiding treatment decisions in PwCF, a strategy called theratyping [16]. While the main goal is to identify PwCF who could benefit from a specific treatment by assessing the responsiveness of a given CFTR genotype to modulator therapy, it also allows to gain insights into the molecular defects of the mutation(s) studied [5]. Importantly, theratyping allows comparison of modulator responses, in either a mutation- or patient-specific approach within a given preclinical model. In particular the many different, rare mutations do not support standard clinical trial design and therefore depend on theratyping. In that light, the development of organoids as a biomarker of CFTR function and the possibility to quantify drug responsiveness was a major breakthrough [17, 18]. CFTR modulator responses, quantified by forskolin-induced swelling (FIS), correlate well with non-gastrointestinal clinical end-points in PwCF, including lung function (forced expiratory volume in 1 s) and sweat chloride concentration [17, 19]. To date, evidence supports that organoids can be used to guide personalised medicine in CF [20]. Their most prominent advantages include the ease and minimal discomfort of obtaining rectum biopsies, which have led to the rapid growth of different biobanks around the world, including the UZ Leuven biobank used here. In particular, many rare genotypes have been included. Further, the rapid in vitro expansion of stem-cell derived organoids speeds up preclinical assessment, allowing to obtain functional data within a month after biopsy collection. Based on this favourable profile, we opted to use primary rectal organoids for our study.
One mutation for which Trikafta™ was recently approved by FDA label extension is G85E (c.254G>A; 0.4% of alleles, CFTR2 database). G85E, located in the first membrane-spanning domain (MSD1), causes severely impaired processing and trafficking, completely abolishing CFTR-mediated ion transport [21]. We and others have previously shown that this mutant is drug-refractory to potentiators and type I correctors alone or in combination [8, 21]. Recently, elexa-teza-ivacaftor has been shown to partially rescue G85E function [8, 22]. The exact mode of action of elexacaftor has not been fully uncovered, but recent reports show that besides its known corrector function, it also acts as a potentiator, with mutation-specific activity [9, 22]. Therefore, we aimed to study elexacaftor both as corrector (24-h incubation) and potentiator (added acutely). We further sought to investigate whether the severe trafficking defect of G85E was maximally corrected by the corrector activity of elexacaftor, which has been shown to stabilise nucleotide binding domain 1 (NBD1) [8], or whether correctors with different mechanisms could further improve correction. We thus tested tezacaftor (correcting the MSD–NBD interface) and a type II corrector (stabilising NBD2/MSD2), i.e. the investigational corrector 4a (corr4a; C4) [7, 23].
Many studies have tried to rescue the severe NBD2 mutant N1303K (c.3909C>G), the 4th most common mutation (1.6% of alleles in CFTR2). N1303K has been shown to be an atypical CFTR mutant in many ways, by both its trafficking and functional defects [24]. In fact, to date there is no consensus on which of these two defects is the major cause of N1303K dysfunction and, hence, should be the main focus of therapeutic targeting [25, 26]. N1303K is completely refractory to iva-, luma- and tezacaftor in patient-derived organoids and airway cultures, but recent reports show a modest functional rescue in cell lines and primary human bronchial epithelial cells (HBE) with elexa-teza-ivacaftor [8, 22], which precluded N1303K from inclusion in the Trikafta™ FDA label extension [27]. We therefore investigated whether a rational choice of modulators, i.e. NBD2/MSD2 corrector corr4a and co-potentiator apigenin (type II potentiator), would augment the limited but hopeful functional correction induced by Trikafta™ [28].
Methods
Human rectal organoid generation and biobanking
Informed consent was obtained prior to biopsy collection, in accordance with the ethical committee of UZ Leuven (S56329). Human rectal organoids were generated from rectum biopsies of PwCF as previously described, and biobanked after successful culture [19, 29]. Organoids from the biobank were thawed and cultured for evaluation in this study.
Forskolin-induced swelling assay in human rectal organoids
Responses to CFTR modulator combinations were determined in the FIS assay, which was performed as described previously [18, 21, 29], with modifications. Briefly, organoids with F508del, G85E or N1303K genotypes were mechanically disrupted and plated in Matrigel® Matrix (#356231; Corning, New York, NY, USA) in 96-well plates. Corrector compounds (lumacaftor/tezacaftor/elexacaftor (S1565/S7059/S8851; all 3 µM; all Selleckchem, Houston, TX, USA)) and corr4a (5 µM; 219673; Merck, Kenilworth, NJ, USA) or DMSO controls were added for 24 h prior to FIS. At the time of analysis 0.8 µM of forskolin (Selleckchem; S2449) was added together with either potentiator compound (ivacaftor (Selleckchem; S1144; 3 µM) and/or apigenin (Selleckchem; S2262; 50 µM)) or DMSO controls. In experiments where the potentiator activity of elexacaftor was investigated, there was no pre-incubation with this compound. Rather, it was added together with the other potentiators. In selected experiments, calcein green AM live dye (5 µM; C34852; Invitrogen, Waltham, MA, USA) was added to fluorescently label organoids. In most experiments, however, organoids were imaged label-free using brightfield microscopy. Fluorescent and brightfield images were taken every 10 min for 1 h or every 30 min for 24 h (for brightfield only) on the ZEISS LSM880 system at 5X magnification. Tiff files were analysed in ImageJ. Customised macros were developed to determine total organoid area in each image, by removing the background and enhancing the contrast of each image. Colour thresholds recognising organoid structures were set and holes in the mask were filled, after which it was despeckled to remove noise. The area of the resulting mask was determined for each timepoint, and organoid swelling was calculated as the relative increase in this area over time, which was analysed in Microsoft Excel. Compound responses between t=0 and t=60 min or t=24 h were calculated as the area under the curve (AUC) of the organoid swelling in GraphPad Prism 9.
Flow cytometry plasma membrane density in CFTR overexpressing HEK293T cells
HEK293T cells overexpressing CFTR variants with an extracellular triple haemagglutinin (3HA) tag [30] were plated in 96-well plates, and 24 h prior to analysis cells were treated either with corrector combinations of tezacaftor (3 µM), elexacaftor (3 µM) and corr4a (5 µM), or DMSO controls. PM density was then determined as described previously [21] and corrected for differences in mRNA expression levels measured by reverse transcriptase PCR (RT-PCR). For RT-PCR, RNA was isolated using Aurum Total RNA mini kit (#7326820; Bio-Rad, Hercules, CA, USA) and converted to cDNA with the High-Capacity cDNA Reverse Transcription Kit (4368813; Thermo Fischer Scientific, Waltham, MA, USA). Samples contained LightCycler 480 SYBR Green I Mastermix (04707516001; Roche, Basel, Switzerland) and CFTR (Fw:GCAGTTGATGTGCTTGGCTA; Rev:ACTGCCGCACTTTGTTCTCT) or β-actin (Fw:TCACCCACACTGTGCCCATCTACGA; Rev: CAGCGGAACCGCTCATTGCCAATGG) primers and were run and analysed in LightCycler 480 (Roche). CFTR mRNA expression levels were normalised to β-actin levels.
Statistics
At least three independent repeats of each experiment were performed and are shown as dots (mean±sem) in the diagrams in figures 1–4 and supplementary figures S2 and S4. Each experiment was performed in triplicate/quadruplicate. All statistical analysis was performed in GraphPad Prism 9. Pearson correlation r2 was determined between fluorescent and brightfield FIS analysis. Different treatments were analysed with ANOVA with multiple comparisons, unless specified otherwise in the text.
Results
Label-free analysis of forskolin-induced swelling using brightfield microscopy
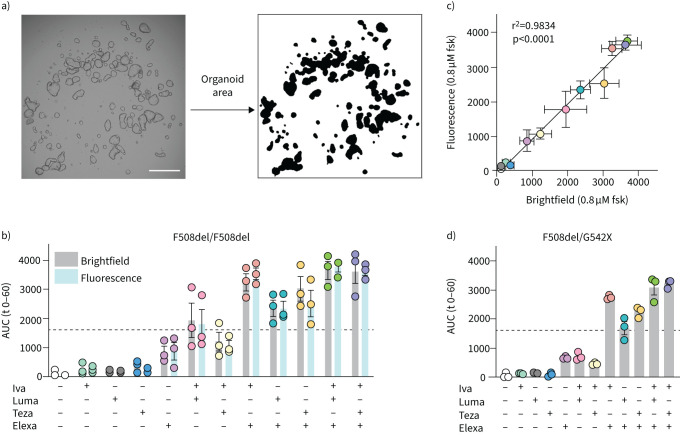
We set out to optimise a label-free version of the FIS assay which would allow long-term monitoring of organoid swelling. Calcein, the most commonly used fluorescent dye to label organoids in FIS, diffuses out of cells over time (supplementary figure S1A). Re-application of calcein at later timepoints was not feasible due to further loss of the signal-to-noise ratio and toxicity (supplementary figure S1B,C). In brightfield microscopy by contrast, no labels are needed for organoid detection. An example of a brightfield image converted to a binary mask representing the total organoid surface area is shown in figure 1a. In order to validate our optimised analysis, market-approved CFTR modulators were tested alone and in existing and novel combinations on F508del/F508del organoids (figure 1b). The two analysis methods, brightfield and fluorescence microscopy (calcein labelling), were performed in parallel and showed an excellent correlation (r2=0.98, p<0.0001) (figure 1c). Therefore, all subsequent FIS assays were analysed using brightfield microscopy.
FIGURE 1.
Label-free analysis of forskolin (fsk)-induced swelling (FIS) by brightfield microscopy allows evaluation of cystic fibrosis transmembrane conductance regulator (CFTR) modulator responses. Brightfield and fluorescence microscopy readouts for FIS of rectal organoids were compared for several market-approved CFTR modulators. a) Detection of organoid area from brightfield images. Left: brightfield image of rectal organoids at 5× magnification. Right: mask of total organoid area after analysis. Scale bar: 500 μm. b) F508del/F508del organoids were treated with combinations of CFTR correctors (lumacaftor (Luma), tezacaftor (Teza), elexacaftor (Elexa); all at 3 μM) or DMSO 24 h before analysis. At the start of the assay, fsk (0.8 μM) with or without the CFTR potentiator ivacaftor (Iva) (3 μM) was added, and organoid swelling was assessed over the next 60 min using either brightfield or fluorescence microscopy. Area under the curve (AUC) of the relative increase in organoid area over time is shown. c) Correlation between brightfield and fluorescence readouts for each modulator combination in b. d) FIS analysis as described in b of F508del/G542X organoids treated with the same modulator combinations. Each circle in b and d represents the result of an independent experiment, each performed in quadruplicate. Bars show mean+sem. Dotted line represents the arbitrary threshold for relevant functional rescue (average response of F508del/F508del organoids to Luma-Iva).
Limited effect of type I corrector on top of elexa-ivacaftor in F508del rectal organoids
We first quantified rescue of F508del function by combinations of the currently approved CFTR modulators (figure 1b) as a reference for further comparison with G85E and N1303K responses. As a proxy for a relevant response to modulator treatment, the published average response of F508del/F508del organoids to luma-ivacaftor (Orkambi™; 1600 AUC after 60 min, figure 1b, dotted line [19]) was set as the arbitrary threshold in all experiments. A clear additive effect was observed when elexacaftor was added to luma-ivacaftor or teza-ivacaftor in line with published data for elexa-teza-ivacaftor [8, 31]. Intriguingly, adding a second corrector (luma- or tezacaftor) to elexa-ivacaftor did not seem to further improve CFTR function in human rectal organoids with F508del (p=0.99 and p=0.15 for homozygous F508del and F508del/G542X(c.1624G>T) (figure 1d) organoids, respectively; CFTR modulator responses of G85E/F508del and N1303K/F508del organoids are shown in supplementary figure S2).
Functional rescue of G85E by elexacaftor alone
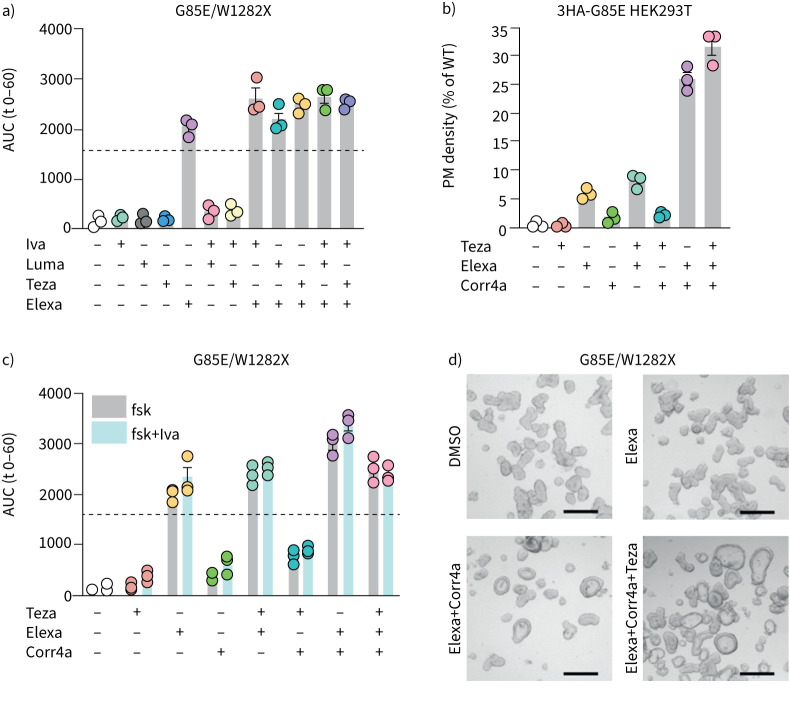
We next evaluated drug responsivity in two G85E genotypes, both compound heterozygous, one with F508del on the other allele and the other with the W1282X (c.3846G>A) mutation for which Trikafta/Kaftrio™ is not approved. Indeed, in contrast to the previously market approved CFTR modulator combinations, elexa-teza-ivacaftor could functionally rescue G85E in both organoid samples well above the threshold considered relevant (figure 2a; supplementary figure S2). Interestingly, we observed that elexacaftor by itself resulted in a similar response compared to elexa-teza-ivacaftor in G85E/W1282X organoids (ANOVA, multiple comparisons, p=0.17).
FIGURE 2.
Elexacaftor (Elexa) alone restores G85E function. Cystic fibrosis transmembrane conductance regulator (CFTR) modulators were added at a concentration of 3 μM, except for corrector 4a (Corr4a) (5 μM), and organoids were analysed by brightfield microscopy. a) Forskolin (fsk)-induced swelling (FIS) analysis of G85E/W1282X organoids treated with CFTR modulators or DMSO controls after stimulation with 0.8 μM fsk for 1 h. b) Plasma membrane (PM) density of HEK293T cells overexpressing 3HA-G85E-CFTR treated with CFTR correctors or DMSO controls, measured by flow cytometry. PM density is determined as the percentage of positive cells multiplied with the median fluorescence intensity of positive cells, relative to 3HA-WT-CFTR PM density after correction for mRNA expression, determined by reverse transcriptase PCR. 10 000 events were analysed per well. c) FIS analysis of G85E/W1282X organoids treated with CFTR modulators or DMSO controls after stimulation with 0.8 μM fsk for 1 h. d) Brightfield images of G85E/W1282X organoids after 24 h incubation with mentioned CFTR modulators or DMSO controls. Scale bars: 200 μm. Each circle represents the result of an independent experiment, each performed in triplicate or quadruplicate. Bars show mean+sem. Dotted line represents the arbitrary threshold for relevant functional rescue (average response of F508del/F508del organoids to lumacaftor (Luma)-ivacaftor (Iva)). AUC: area under the curve; WT: wild-type; Teza: tezacaftor.
Corrector combinations synergistically improve G85E PM density and function
We previously reported that G85E suffers from a severe trafficking defect, which reduces PM expression below 1% of wild-type CFTR (WT-CFTR) [21] (figure 2b). To address if the elexacaftor-induced functional rescue we observed in organoids originated from a rescue in trafficking, in line with elexacaftor's corrector activity, we determined the PM density of G85E (figure 2b). As hypothesised, elexacaftor increased PM density to ∼6% of WT-CFTR (13-fold increase over DMSO; p=0.0032), with no additive benefit of tezacaftor and elexacaftor (p=0.56). We set out to further enhance G85E trafficking by evaluating different corrector combinations. Adding corr4a onto elexacaftor led to a synergistic increase in PM density to ∼26% of WT-CFTR (62-fold increase over DMSO; 4-fold increase over elexacaftor alone, p<0.001). Rescue by corr4a in the absence of elexacaftor did not reach significance. We next evaluated corr4a-mediated rescue in G85E/W1282X organoids, to verify whether it would enhance function of (elexacaftor-corrected) G85E (figure 2c). Combined elexacaftor and corr4a synergistically rescued G85E function compared to elexacaftor only (p<0.0001). In elexacaftor-corr4a and in particular the triple corrector (teza-elexacaftor-corr4a)-treated organoids, CFTR activity was observed even in the absence of forskolin stimulation. These organoids showed a visible lumen (figure 2d), the phenotype of rectal organoids from non-CF subjects [18] and F508del/F508del organoids rescued with teza-elexacaftor (supplementary figure S3), this in contrast to the dense lumen-less phenotype of CF organoids. The effect of ivacaftor on top of corrector-mediated rescue was minor (figure 2c).
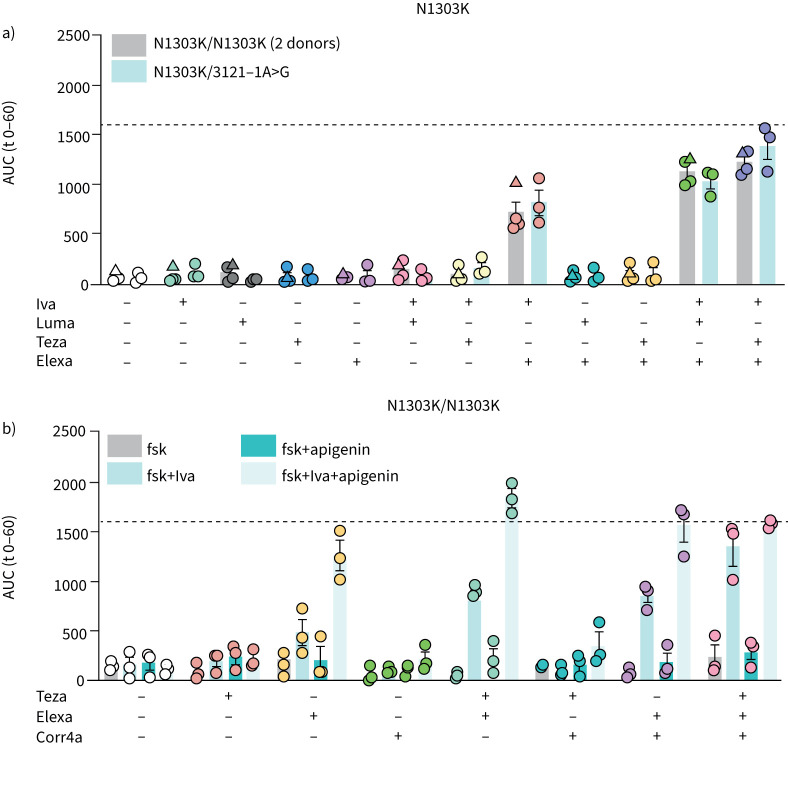
Functional rescue of N1303K minimally requires elexa-ivacaftor combinations
We used a rational choice of CFTR modulators to investigate whether NBD2 corrector corr4a and co-potentiator apigenin would further augment the modest functional improvement by elexa-teza-ivacaftor [8, 22, 28, 32]. First, we reproduced this functional correction, now in the rectal organoid model (figure 3a). Although significantly higher than previously approved modulator therapies (ivacaftor, luma-ivacaftor, teza-lumacaftor), CFTR function did not surpass the threshold for proposed relevance (dotted line, figure 3a) in either homozygous N1303K or N1303K/3121-1A>G compound heterozygous organoids, the latter carrying a class I mutation on the second allele. The minimally effective combination necessary to rescue N1303K-CFTR function above baseline was ivacaftor plus elexacaftor (p<0.0001, both genotypes), although adding tezacaftor provided significant benefit (p<0.0001, both genotypes). To next investigate whether we could further increase N1303K function, we combined the above correctors and potentiators with apigenin or corr4a. Apigenin synergistically enhanced elexa-ivacaftor rescued N1303K (figure 3b). Compared to elexa-teza-ivacaftor, adding apigenin doubled CFTR function, thereby reaching levels with predicted clinical benefit. Corr4a, however, could not functionally rescue N1303K alone or in combination with ivacaftor or ivacaftor plus apigenin (figure 3b).
FIGURE 3.
Elexacaftor (Elexa)-ivacaftor (Iva) combinations restore some cystic fibrosis transmembrane conductance regulator (CFTR) function in N1303K rectal organoids. CFTR modulators were added at a concentration of 3 μM, except for apigenin (50 μM) and corrector 4a (Corr4a) (5 μM) and rectal organoids were analysed by brightfield microscopy. a) Forskolin (fsk)-induced swelling (FIS) analysis of N1303K/N1303K and N1303K/3121-1A>G organoids treated with approved CFTR modulators or DMSO controls after stimulation with 0.8 μM fsk for 1 h. Circles/triangles represent data from organoids from two different donors with the same genotype. b) FIS analysis of N1303K/N1303K organoids treated with CFTR modulators or DMSO controls after stimulation with 0.8 μM fsk for 1 h. Each circle/triangle represents the result of an independent experiment, each performed in triplicate/quadruplicate. Bars show mean+sem. Dotted line represents the arbitrary threshold for relevant functional rescue (average response of F508del/F508del organoids to lumacaftor (Luma)-Iva). AUC: area under the curve; Teza: tezacaftor.
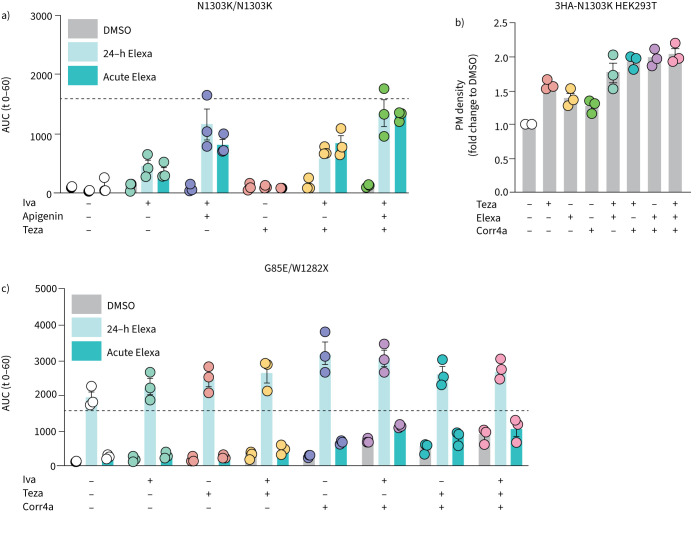
Elexacaftor rescues N1303K and G85E by different mechanisms
Based on recent reports stating that elexacaftor acts both as a corrector and potentiator [8, 9, 22, 33], we took a closer look at the mechanism(s) by which elexacaftor restores function of G85E and N1303K. 24-h incubation and acute addition of elexacaftor resulted in similar levels of N1303K functional rescue for all compound combinations tested (figure 4a; 2-way ANOVA, p=0.52). Here, acute addition represents the potentiator activity of elexacaftor, while 24-h incubation shows the combined effect of potentiator and corrector activities. Since the FIS assay does not allow discrimination of corrector and potentiator activities with 24-h incubation, we investigated the corrector activity via PM density in HEK293T cells (figure 4b). Elexacaftor improved PM expression of N1303K a modest but significant ∼1.3-fold over DMSO (p=0.03), compared to ∼1.5-fold for tezacaftor (p=0.001). Corr4a alone could not significantly augment N1303K PM expression (p=0.21). The triple corrector teza-elexacaftor-corr4a yielded a ∼2-fold increase in PM N1303K. Comparing 24-h incubation and acute elexacaftor treatment in the FIS assay confirmed that, in contrast to N1303K, pre-incubation with elexacaftor was necessary to achieve relevant levels of G85E rescue (figure 4c).
FIGURE 4.
Elexacaftor (Elexa) mediates rescue of N1303K and G85E by different mechanisms. Cystic fibrosis transmembrane conductance regulator (CFTR) modulators were added at 3 μM except for corrector 4a (Corr4a) (5 μM) and apigenin (50 μM). Forskolin (fsk)-induced swelling (FIS) analysis of a) N1303K/N1303K and c) G85E/W1282X organoids treated with CFTR modulators and activated with 0.8 μM fsk. Elexa was added 24 h before analysis together with the CFTR correctors (24-h) or at the start of the assay together with CFTR potentiators (acute). Organoid swelling was evaluated by brightfield microscopy for the next hour. b) Plasma membrane (PM) density of HEK293T cells overexpressing 3HA-N1303K-CFTR treated with different CFTR correctors/corrector combinations or DMSO controls, measured by flow cytometry. PM density is determined as the percentage of positive cells multiplied with the median fluorescence of positive cells, put relative to 3HA-WT-CFTR PM density after correction for mRNA expression, determined by reverse transcriptase PCR. 10 000 events were analysed per well. Each circle represents the result of an independent experiment, each based on a triplicate. Bars shows mean+sem. AUC: area under the curve; Iva: ivacaftor; Teza: tezacaftor.
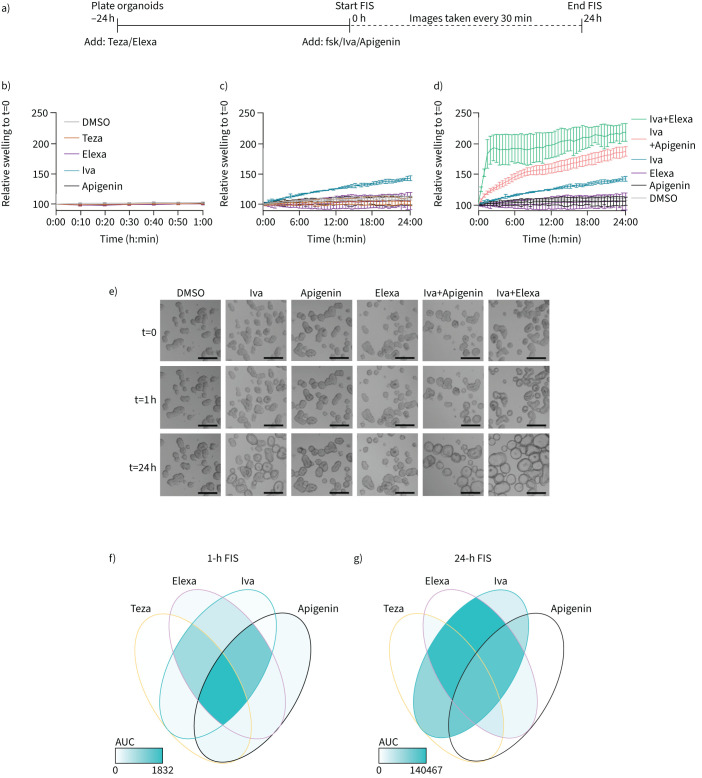
Long-term FIS reveals ivacaftor as the major potentiator for N1303K
Finally, we aimed to unravel which CFTR modulator is the major contributor to the functional rescue of N1303K. To this end, we optimised an extended version of FIS more sensitive for detecting small improvements in CFTR function as accumulated swelling is analysed over a 24-h rather than 1-h period (figure 5a). This allowed detection of near-linear swelling for CFTR modulators and combinations undetectable at 1 h (figure 5b–d). Since the combination of ivacaftor and elexacaftor was the minimal treatment needed to moderately rescue N1303K function in the 1-h FIS (figure 3), we now investigated within the 24-h detection window, how each modulator on its own affected N1303K function. We identified that only ivacaftor mono-treatment resulted in a functional correction, in contrast to mono-treatment with tezacaftor, elexacaftor or apigenin, suggesting a major role of ivacaftor to improve the gating defect of N1303K (figure 5c). Ivacaftor was effectively co-potentiated though by apigenin and elexacaftor (figure 5d and e). The Venn diagrams summarise the contribution of each modulator tested for N1303K in the 1-h and 24-h detection window, respectively (figure 5f and g).
FIGURE 5.
Elexacaftor (Elexa) and apigenin co-potentatiate ivacaftor (Iva) rescue of N1303K in rectal organoids. Cystic fibrosis transmembrane conductance regulator (CFTR) modulators were added at a concentration of 3 μM, except for apigenin (50 μM) to N1303K/N1303K organoids. a) Scheme of 24-h forskolin (fsk)-induced swelling (FIS). Tezacaftor (Teza) and Elexa were added 24 h before the start of analysis. Fsk (0.8 μM), Iva and apigenin were added and organoid swelling was analysed every 30 min for 24 h. b–d) Time course of 1-h (b) and 24-h FIS (c and d) in N1303K/N1303K organoids with mentioned compounds. Time course shows mean±sd of representative experiments. e) Brightfield images of organoids in d at time points 0, 1 and 24 h. Scale bars: 200 μm. f and g) Venn diagram summarising responses of CFTR modulators or combinations in N1303K/N1303K organoids in 1-h FIS (f), based on data from figure 3d, or 24-h FIS (g), based on data in supplementary figure S4.
Discussion
Over the past decade, four CFTR modulator therapies have reached the market and have provided a causal therapy for the majority of PwCF. For the remaining 10% of PwCF, however, no causal treatment is available. The remaining untreatable mutations are mainly non-sense, frameshift and deletion mutations, but also several missense mutations. N1303K is the most common missense mutation without approved modulators. With over 2100 PwCF registered in the CFTR2 database, an effective treatment for this mutation remains an important need for the CF community. G85E is the 13th most common CFTR mutation, also refractory to iva-, luma- and tezacaftor. Elexa-teza-ivacaftor was approved for this mutation through label extension of Trikafta™ by the FDA but not the European Medicines Agency (EMA). Nevertheless, in light of its previous modulator refractoriness, questions about G85E's underlying molecular defects remain. The FIS assay performed in patient-derived rectal organoids is a robust and well-validated preclinical model for phenotyping and drug testing [17, 19, 34], a procedure called theratyping [16]. We have used patient-derived rectal organoids to test the latest approved CFTR modulator, elexacaftor, as well as combinations of known types of correctors (I/II/III) and potentiators (I/II) with distinct mechanisms of action for previously drug-refractory mutants G85E and N1303K. By combining approved and investigational types of CFTR modulators we found novel combinations that enhanced G85E and N1303K function beyond that of the Trikafta/Kaftrio™ triple (teza-elexa-ivacaftor), which is currently the best combination with market approval. Our study also showed for both F508del and G85E that not all compounds of the triple combination contributed to CFTR functional rescue in rectal organoids, suggesting a remaining need to optimise combination therapies for in-patient use. Finally, we identified a distinct mode of action of CFTR modulator elexacaftor for both CFTR mutants studied. For G85E, it predominantly restored its folding defect and coupled to that, CFTR function, whereas for N1303K, it largely improved its gating defect when combined minimally with ivacaftor.
Indeed, as elexacaftor is the latest CFTR modulator to reach market approval, the full scale of its effects is still under investigation, especially for the many rare mutations that cause CF. To date, several studies have investigated the effect of elexacaftor in cell lines and primary HBE both for the most common mutation F508del as well as for several gating and processing mutations, including G85E and N1303K [8, 9, 22, 35]. However, in rectal organoids, only the added effect of elexacaftor on top of teza-ivacaftor for F508del was recently reported [31]. In our study, we set out to investigate novel combinations of CFTR modulators beyond the market-approved combinations, aiming to achieve maximised functional rescue for these specific mutations, as well as better understand the underlying molecular defects. For determining the minimal level of organoid responsivity correlating with reported clinical benefit, we set the published average response of F508del/F508del organoids to luma-ivacaftor treatment (Orkambi™) as cut-off [19]. Interestingly, we observed that adding tezacaftor did not further enhance elexa-ivacaftor rescue of F508del much. The same limited benefit of tezacaftor on top of elexacaftor was observed for G85E. For N1303K, a clear benefit of tezacaftor on top of elexa-ivacaftor was observed in the 1-h FIS. Our study thus suggests that the contribution of each of the three individual components of Trikafta/Kaftrio™ should be investigated case by case.
For G85E, mono-treatment with elexacaftor was sufficient to restore CFTR function to clinically relevant levels, and adding extra CFTR modulators (iva-, luma- or tezacaftor) provided only limited benefit (figure 2a). Currently, only the triple teza-elexa-ivacaftor (Trikafta™) is FDA-approved for PwCF carrying the G85E mutation, but an elexacaftor monotherapy might be considered in the future. A synergistic effect on top of elexacaftor was observed for type II corrector corr4a (figure 2c). This investigational compound was previously described as a corrector of F508del by stabilising MSD2/NBD2 [7, 23, 36]. For G85E it appears to act as a co-corrector, dependent on simultaneous elexacaftor rescue. Notably, G85E organoids pre-incubated with elexacaftor-corr4a and even more so, teza-elexacaftor-corr4a showed CFTR functionality even in the absence of forskolin stimulation. This is indicative of potent rescue, which is sufficient to rescue both trafficking and ion channel function of G85E.
On the other hand, effective rescue of N1303K remains more elusive. In the 1-h FIS, we identified elexa-ivacaftor as the minimal treatment necessary to restore detectable CFTR function in organoids, although below the arbitrary threshold for effectivity (figure 3a). Interestingly, co-potentiator apigenin approximately doubled CFTR function of elexa-ivacaftor and teza-elexa-ivacaftor rescued N1303K organoids (figure 3b). The exact mechanism of apigenin and other co-potentiators has not yet been elucidated, but in line with previous cell line data [6], our study shows that ivacaftor is necessary for apigenin-mediated rescue of N1303K function. Pre-incubation with elexacaftor was not necessary to restore N1303K function in the presence of ivacaftor, suggesting N1303K rescue is mainly achieved by (co-)potentiation of its gating defect (figure 4a). The potentiator activity of elexacaftor has previously been described for other CFTR mutants, in particular gating mutants in need of a potentiator [9, 22, 33], but we report for the first time that elexacaftor's potentiator activity also is the main mode of action for N1303K. Indeed, elexacaftor only moderately increased N1303K PM density (figure 4b), in contrast to G85E (figure 2b). Laselva and colleagues [22] investigated the potentiator effect of elexacaftor and ivacaftor on N1303K in cell lines, reporting that both showed some minimal potentiator activity by itself. Our study by contrast shows that even when CFTR functional responses were magnified by long-term FIS (24 h), no potentiator or corrector activity of elexacaftor was detectable in the absence of ivacaftor in organoids, which is a primary cell model expressing physiological levels of CFTR. Only ivacaftor alone allowed some functional rescue of N1303K in the 24-h FIS, in contrast to mono-treatment of tezacaftor, elexacaftor or apigenin, suggesting a major role of ivacaftor to improve N1303K gating (figure 5b). Our study shows that at least for N1303K, elexacaftor acts as a co-potentiator of ivacaftor (figure 5c). The synergy of apigenin on top of elexa-ivacaftor (figure 3b) suggests there are at least three different mechanisms for CFTR potentiation, some of which are dependent on each other (summarised in figure 5f and g; supplementary table S1). In follow-up studies, the co-potentiators elexacaftor and apigenin should be evaluated in more mechanistic assays, such as by patch clamp, to allow precise elucidation of the different co-potentiator mechanisms. Our study thus underscores that N1303K requires more effective CFTR modulators, as even the six proposed modulator types (i.e. three corrector and three potentiator types, supplementary table S1) only moderately restore CFTR function in organoids. In particular, CFTR potentiation, with or without supporting correction, appears necessary to fully restore N1303K function.
In conclusion, while some of the tested modulators used in this study are investigational, these data may serve to further optimise drug discovery initiatives for these mutants, in particular N1303K, to reach further functional rescue, and coupled to that, clinical benefit in PwCF. In light of this, the label-free 24-h FIS assay we developed may accelerate identifying drug responses for difficult to correct mutations, due to its increased sensitivity and potential to be implemented in labs without the need for large, expensive confocal microscopy equipment.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary figures 00716-2021.SUPPL_FIGS (240.4KB, pdf)
Table S1 00716-2021.TABLES1 (148.4KB, pdf)
Acknowledgements
We acknowledge Kris De Boeck, Marijke Proesmans and Mieke Boon (UZ Leuven, Dept of Pediatrics, Leuven, Belgium) for valuable scientific discussion of the data presented within this manuscript. We thank Abida Bibi (KU Leuven, Development and Regeneration, Leuven, Belgium) for technical support with organoid biobanking and subculturing.
Provenance: Submitted article, peer reviewed.
Author contributions: M.M. Ensinck, M.S. Carlon, A.S. Ramalho and F. Vermeulen conceptualised the research questions. M.M. Ensinck and L. De Keersmaecker performed all experiments. A.S. Ramalho, S. Cuyx, S. Van Biervliet and F. Vermeulen assisted with or collected organoid samples. M.M. Ensinck and M.S. Carlon analysed the data and prepared figures. Data were discussed with all authors. M.M. Ensinck and M.S. Carlon wrote the original draft, which was revised and edited by all.
Conflict of interest: M.M. Ensinck has nothing to disclose.
Conflict of interest: L. De Keersmaecker has nothing to disclose.
Conflict of interest: A.S. Ramalho has nothing to disclose.
Conflict of interest: S. Cuyx reports receiving research grants (funds paid to institution) from Belgische Vereniging Kindergeneeskunde, Mucovereniging/Association Muco and Fonds Forton, outside the submitted work.
Conflict of interest: S. Van Biervliet has nothing to disclose.
Conflict of interest: L. Dupont has nothing to disclose.
Conflict of interest: F. Christ reports receiving grants or contracts (funds paid to institution) from FWO Flanders (SBO: S001221N) outside the submitted work.
Conflict of interest: Z. Debyser reports receiving grants or contracts (funds paid to institution) from FWO Flanders (SBO: S001221N) outside the submitted work.
Conflict of interest: F. Vermeulen reports receiving grants or contracts (funds paid to institution) from the HIT-CF consortium, Vertex Pharmaceuticals, Fonds Wetenschappelijk Onderzoek, Association Muco and the Cystic Fibrosis Foundation; and participation on a data safety monitoring or advisory board for Vertex Pharmaceuticals. All disclosures made outside the submitted work.
Conflict of interest: M.S. Carlon reports receiving grants or contracts (funds paid to institution) from Koning Boudewijnstichting and the Belgian CF patient association (2017-J1810150-207746), and FWO Flanders (SBO: S001221N), outside the submitted work.
Support statement: This study was supported by Koning Boudewijnstichting grant 2017-J1810150-207746, KU Leuven Internal Funds – C3 grant C32/15/027, and Fonds Wetenschappelijk Onderzoek grants 12Z5920N, 1S29917N and S001221N. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Kerem B, Rommens JM, Buchanan JA, et al. Identification of the cystic fibrosis gene: genetic analysis. Science 1989; 245: 1073–1080. doi: 10.1126/science.2570460 [DOI] [PubMed] [Google Scholar]
- 2.Riordan JR, Rommens JM, Kerem B, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 1989; 245: 1066–1073. doi: 10.1126/science.2475911 [DOI] [PubMed] [Google Scholar]
- 3.Rommens JM, Iannuzzi MC, Kerem B, et al. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science 1989; 245: 1059–1065. doi: 10.1126/science.2772657 [DOI] [PubMed] [Google Scholar]
- 4.De Boeck K, Amaral MD. Progress in therapies for cystic fibrosis. Lancet Respir Med 2016; 4: 662–674. doi: 10.1016/S2213-2600(16)00023-0 [DOI] [PubMed] [Google Scholar]
- 5.Veit G, Avramescu RG, Chiang AN, et al. From CFTR biology toward combinatorial pharmacotherapy: expanded classification of cystic fibrosis mutations. Mol Biol Cell 2016; 27: 424–433. doi: 10.1091/mbc.e14-04-0935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phuan P-W, Tan J-A, Rivera AA, et al. Nanomolar-potency ‘co-potentiator’ therapy for cystic fibrosis caused by a defined subset of minimal function CFTR mutants. Sci Rep 2019; 9: 17640. doi: 10.1038/s41598-019-54158-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okiyoneda T, Veit G, Dekkers JF, et al. Mechanism-based corrector combination restores ΔF508-CFTR folding and function. Nat Chem Biol 2013; 9: 444–454. doi: 10.1038/nchembio.1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veit G, Roldan A, Hancock MA, et al. Allosteric folding correction of F508del and rare CFTR mutants by elexacaftor-tezacaftor-ivacaftor (Trikafta) combination. JCI Insight 2020; 5: e139983. doi: 10.1172/jci.insight.139983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Veit G, Vaccarin C, Lukacs GL. Elexacaftor co-potentiates the activity of F508del and gating mutants of CFTR. J Cyst Fibros 2021; 20: 895–898. doi: 10.1016/j.jcf.2021.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramsey BW, Davies J, McElvaney NG, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 2011; 365: 1663–1672. doi: 10.1056/NEJMoa1105185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heijerman HGM, McKone EF, Downey DG, et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet 2019; 394: 1940–1948. doi: 10.1016/S0140-6736(19)32597-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Middleton PG, Mall MA, Drevinek P, et al. Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med 2019; 381: 1809–1819. doi: 10.1056/NEJMoa1908639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wainwright CE, Elborn JS, Ramsey BW, et al. Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med 2015; 373: 220–231. doi: 10.1056/NEJMoa1409547 [DOI] [PubMed] [Google Scholar]
- 14.Taylor-Cousar JL, Munck A, McKone EF, et al. Tezacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del. N Engl J Med 2017; 377: 2013–2023. doi: 10.1056/NEJMoa1709846 [DOI] [PubMed] [Google Scholar]
- 15.V ertex . Vertex Announces FDA Approvals of TRIKAFTA® (elexacaftor/tezacaftor/ivacaftor and ivacaftor), SYMDEKO® (tezacaftor/ivacaftor and ivacaftor) and KALYDECO® (ivacaftor) for use in people with CF with certain rare mutations. 2020. https://investors.vrtx.com/news-releases/news-release-details/vertex-announces-fda-approvals-trikaftar
- 16.Clancy JP, Cotton CU, Donaldson SH, et al. CFTR modulator theratyping: current status, gaps and future directions. J Cyst Fibros 2019; 18: 22–34. doi: 10.1016/j.jcf.2018.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dekkers JF, Berkers G, Kruisselbrink E, et al. Characterizing responses to CFTR-modulating drugs using rectal organoids derived from subjects with cystic fibrosis. Sci Transl Med 2016; 8: 344ra384. doi: 10.1126/scitranslmed.aad8278 [DOI] [PubMed] [Google Scholar]
- 18.Dekkers JF, Wiegerinck CL, De Jonge HR, et al. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med 2013; 19: 939–945. doi: 10.1038/nm.3201 [DOI] [PubMed] [Google Scholar]
- 19.Ramalho AS, Fürstová E, Vonk AM, et al. Correction of CFTR function in intestinal organoids to guide treatment of cystic fibrosis. Eur Respir J 2021; 57: 1902426. doi: 10.1183/13993003.02426-2019 [DOI] [PubMed] [Google Scholar]
- 20.Noordhoek J, Gulmans V, van der Ent K, et al. Intestinal organoids and personalized medicine in cystic fibrosis: a successful patient-oriented research collaboration. Curr Opin Pulm Med 2016; 22: 610–616. doi: 10.1097/MCP.0000000000000315 [DOI] [PubMed] [Google Scholar]
- 21.Ensinck M, De Keersmaecker L, Heylen L, et al. Phenotyping of rare CFTR mutations reveals distinct trafficking and functional defects. Cells 2020; 9: 754. doi: 10.3390/cells9030754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laselva O, Bartlett C, Gunawardena TNA, et al. Rescue of multiple class II CFTR mutations by elexacaftor+tezacaftor+ivacaftor mediated in part by the dual activities of elexacaftor as both corrector and potentiator. Eur Respir J 2021; 57: 2002774. doi: 10.1183/13993003.02774-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pedemonte N. Small-molecule correctors of defective F508-CFTR cellular processing identified by high-throughput screening. J Clin Invest 2005; 115: 2564–2571. doi: 10.1172/JCI24898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noel S, Sermet-Gaudelus I, Sheppard DN. N1303K: leaving no stone unturned in the search for transformational therapeutics. J Cyst Fibros 2018; 17: 555–557. doi: 10.1016/j.jcf.2018.07.009 [DOI] [PubMed] [Google Scholar]
- 25.Liu Q, Sabirzhanova I, Yanda MK, et al. Rescue of CFTR NBD2 mutants N1303K and S1235R is influenced by the functioning of the autophagosome. J Cyst Fibros 2018; 17: 582–594. doi: 10.1016/j.jcf.2018.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Destefano S, Gees M, Hwang T-C. Physiological and pharmacological characterization of the N1303K mutant CFTR. J Cyst Fibros 2018; 17: 573–581. doi: 10.1016/j.jcf.2018.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ve rtex . Am I eligible for Trikafta®. www.trikafta.com/who-trikafta-is-for Date last accessed: 13 September 2021.
- 28.Phuan P-W, Son J-H, Tan J-A, et al. Combination potentiator (‘co-potentiator’) therapy for CF caused by CFTR mutants, including N1303K, that are poorly responsive to single potentiators. J Cyst Fibros 2018; 17: 595–606. doi: 10.1016/j.jcf.2018.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vonk AM, van Mourik P, Ramalho AS, et al. Protocol for application, standardization and validation of the forskolin-induced swelling assay in cystic fibrosis human colon organoids. STAR Protoc 2020; 1: 100019. doi: 10.1016/j.xpro.2020.100019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma M, Pampinella F, Nemes C, et al. Misfolding diverts CFTR from recycling to degradation. J Cell Biol 2004; 164: 923–933. doi: 10.1083/jcb.200312018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furstova E, Dousova T, Beranek J, et al. Response to elexacaftor/tezacaftor/ivacaftor in intestinal organoids derived from people with cystic fibrosis. J Cyst Fibros 2022; 21: 243–245. [DOI] [PubMed] [Google Scholar]
- 32.Huang Y, Paul G, Lee J, et al. Elexacaftor/tezacaftor/ivacaftor improved clinical outcomes in a patient with N1303K-CFTR based on in vitro experimental evidence. Am J Respir Crit Care Med 2021; 204: 1231–1235. doi: 10.1164/rccm.202101-0090LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaughnessy CA, Zeitlin PL, Bratcher PE. Elexacaftor is a CFTR potentiator and acts synergistically with ivacaftor during acute and chronic treatment. Sci Rep 2021; 11: 19810. doi: 10.1038/s41598-021-99184-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cuyx S, Ramalho AS, Corthout N, et al. Rectal organoid morphology analysis (ROMA) as a promising diagnostic tool in cystic fibrosis. Thorax 2021; 76: 1146–1149. doi: 10.1136/thoraxjnl-2020-216368 [DOI] [PubMed] [Google Scholar]
- 35.Becq F, Mirval S, Carrez T, et al. The rescue of F508del-CFTR by elexacaftor/tezacaftor/ivacaftor (Trikafta) in human airway epithelial cells is underestimated due to the presence of ivacaftor. Eur Respir J 2022; 59: 2100671. doi: 10.1183/13993003.00671-2021 [DOI] [PubMed] [Google Scholar]
- 36.Laselva O, Molinski S, Casavola V, et al. Correctors of the major cystic fibrosis mutant interact through membrane-spanning domains. Mol Pharmacol 2018; 93: 612–618. doi: 10.1124/mol.118.111799 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary figures 00716-2021.SUPPL_FIGS (240.4KB, pdf)
Table S1 00716-2021.TABLES1 (148.4KB, pdf)