Abstract
Craniofacial tissue injuries, diseases, and defects, including those within bone, dental, and periodontal tissues and salivary glands, impact an estimated 1 billion patients globally. Craniofacial tissue dysfunction significantly reduces quality of life, and successful repair of damaged tissues remains a significant challenge. Blood vessels and nerves are colocalized within craniofacial tissues and act synergistically during tissue regeneration. Therefore, the success of craniofacial regenerative approaches is predicated on successful recruitment, regeneration, or integration of both vascularization and innervation. Tissue engineering strategies have been widely used to encourage vascularization and, more recently, to improve innervation through host tissue recruitment or prevascularization/innervation of engineered tissues. However, current scaffold designs and cell or growth factor delivery approaches often fail to synergistically coordinate both vascularization and innervation to orchestrate successful tissue regeneration. Additionally, tissue engineering approaches are typically investigated separately for vascularization and innervation. Since both tissues act in concert to improve craniofacial tissue regeneration outcomes, a revised approach for development of engineered materials is required. This review aims to provide an overview of neurovascularization in craniofacial tissues and strategies to target either process thus far. Finally, key design principles are described for engineering approaches that will support both vascularization and innervation for successful craniofacial tissue regeneration.
Keywords: engineered tissue regeneration, craniofacial tissue, neurovascularization, biomaterial design, growth factor, cell therapy, hydrogel
Graphical Abstract

1. INTRODUCTION
The craniofacial complex is composed of various tissues including cranial, maxillary, and mandibular bones, dental and periodontal tissues, and salivary glands.1,2 These structures provide critical functions including protection of the brain and oral cavity, mastication, defense against bacteria, and assisting in digestion.3 Destruction or loss of craniofacial tissues has many underlying causes. Craniofacial bone defects can occur following injury, tumor resection, or as a result of dysfunctional development.4 Dental pulp and periodontal tissues can be damaged by dental caries and periodontitis.5,6 Xerostomia, or chronic dry mouth, occurs due to bystander damage of salivary glands during radiation treatments or destruction due to Sjögren’s Syndrome.7-9 Craniofacial tissue dysfunction significantly reduces quality of life, and successful repair of damaged tissues remains a significant challenge.10 The current lack of approaches to restore function to cranial and oral tissues demands innovative tissue engineering and regenerative medicine approaches.
While the field of tissue engineering has made remarkable progress, vascularization remains a significant and long-standing hurdle for engineered scaffolds including those for craniofacial tissues.11 Additionally, craniofacial tissues are highly innervated. Nerves are found in close proximity to blood vessels and are increasingly recognized as critical coregulators of tissue regeneration.12-14 While vascularization has been targeted with tissue engineering approaches in cranial and alveolar bone defect healing15,16 and dental tissue regeneration,17,18 innervation has received much less attention, and coordinated innervation and vascularization has not been directly investigated for craniofacial tissue regeneration.
Here, we review the biological processes underpinning both vascularization and innervation and their crosstalk in craniofacial tissues. We then describe methods employed to date to promote angiogenesis and innervation, either directly in the context of craniofacial tissue regeneration or in general for tissue engineering. Finally, we discuss the microenvironmental cues that may pave the way for successful neurovascular tissue engineering strategies for craniofacial tissues.
2. INNERVATION AND ANGIOGENESIS IN CRANIOFACIAL TISSUE REGENERATION
2.1. Innervation and Angiogenesis after Injury.
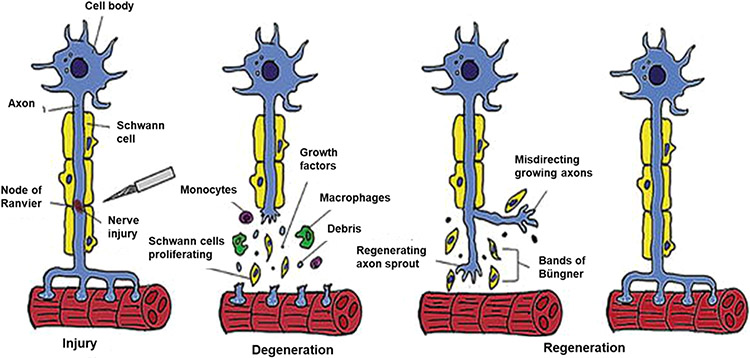
Peripheral nerves can regenerate after injury. Over the first 24 h following axotomy, nerve stumps retract due to dissolution of the cell membranes adjacent to the injury, and macrophages are recruited to the injury site. Distal axons undergo Wallerian degeneration (Figure 1), which is the coordinated removal of the axon and tissue debris by Schwann cells and macrophages. Schwann cells (SCs) proliferate in response to myelin debris and macrophage-secreted cytokines, then align in tubes known as Bungner bands, which express chemotactic cues including nerve growth factor (NGF) to attract and guide proximal axon reinnervation, resulting in functional recovery of their tissue targets (Figure 1).19-22 Schwann cell proliferation can also be stimulated by target organ-derived trophic factors such as glial derived growth factor (GDGF) and brain-derived neurotrophic factor (BDNF).22
Figure 1.
Depiction of Wallerian degeneration [Reproduced with permission from ref 21. Copyright 2017 IntechOpen].
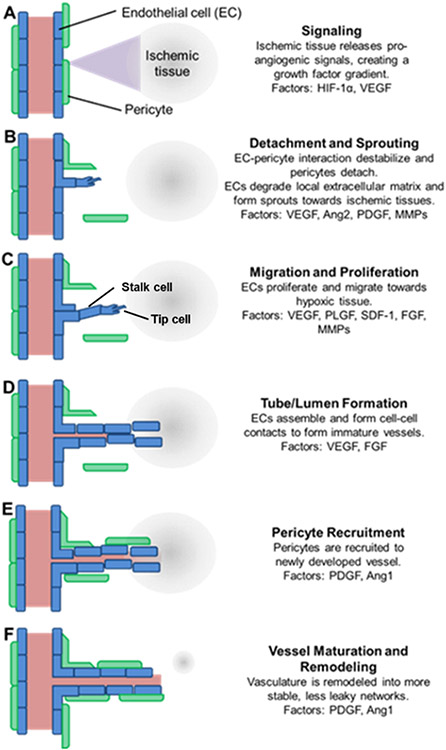
Angiogenesis occurs in response to tissue hypoxia, or low oxygen tension, following injury-mediated disruption of vascularization. A representation of this process is shown in Figure 2, which includes a summary of key cells and growth factors. First, cells in ischemic tissue increase production of hypoxia inducible factor 1α (HIF-1α), which induces production of vascular endothelial growth factor (VEGF) that diffuses into the nearby tissue. These factors signal to pericytes and endothelial cells (ECs) of nearby vasculature, causing detachment of pericytes and sprouting of endothelial tip cells toward the signal gradient. Tip cells then migrate in the direction of the gradient, degrading the local extracellular matrix to enable stalk cells to proliferate and form vessels. Stalk cells align in tube-like luminal structures, forming an immature vascular network. Pericytes are then recruited to the newly formed vasculature, stabilizing the immature vessels, and participating in vessel remodeling.
Figure 2.
Angiogenesis is a tightly controlled process involving numerous cells and factors. (A) Ischemic tissue increases production of hypoxia inducible factor 1α (HIF-1α), which induces production of vascular endothelial growth factor (VEGF) that (B) signals to pericytes (green) and endothelial cells (ECs, blue), resulting in detachment of pericytes and sprouting of endothelial tip cells toward the VEGF gradient. (C) Tip cells (blue) then migrate, degrade the matrix, and (D) enable stalk cells (blue) to proliferate and form vessels via alignment into tube-like luminal structures, followed by (E) pericyte recruitment and (F) further remodeling and maturation. HIF-1α, hypoxia inducible factor 1α; VEGF, vascular endothelial growth factor; Ang2, angiopoietin 2; PDGF, platelet derived growth factor; MMPs, matrix metalloproteinases; PLGF, placenta growth factor; SDF-1, stromal cell-derived factor 1; FGF, fibroblast growth factor; Ang1, angiopoietin 1 [Reproduced with permission from ref 32. Copyright 2015 Frontiers].
Vascularization and innervation share many developmental and anatomical similarities including reciprocal signals that influence their development. To establish proper connections within the vasculature and nervous system, chemotaxis of axons or blood vessels is orchestrated by similar structures: the growth cone in axons or tip cells in vessels.19,23,24 Endothelial and nerve cells can both secrete angiogenic and neurogenic factors during vessels and nerve regeneration. Recent studies suggest that endothelial cell paracrine factors provide critical cues for recruiting and controlling innervation.25,26 Specifically, endothelial cells secrete artemin and neurotrophin 3 that recruit axons to track alongside vessels.27,28 In turn, nerves secrete VEGF in a spatiotemporally controlled manner to coordinate vessel growth.29 Further, stimulation of neuronal activity can also trigger angiogenesis.30,31
2.2. Coupled Innervation and Angiogenesis in Craniofacial Tissue Regeneration.
2.2.1. Craniofacial Tissues Are Vascularized and Innervated.
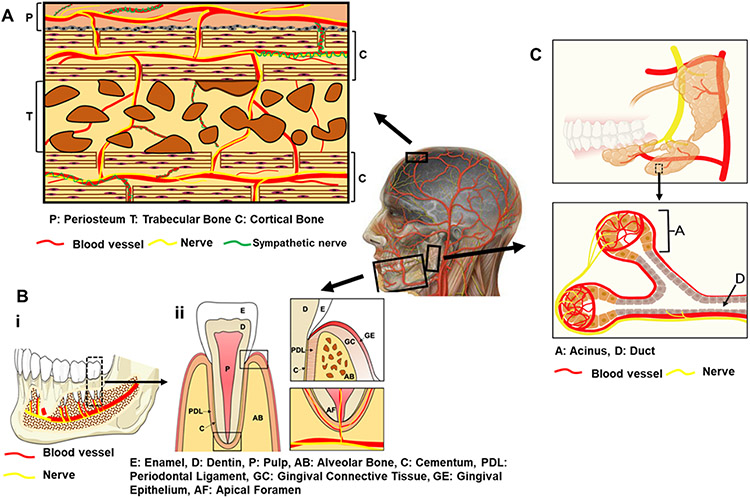
Craniofacial tissues, including cranial and mandibular bone, dental tissue, and salivary glands, are highly vascularized and innervated with blood vessels and nerves in close juxtaposition (Figure 3). For example, the calvarial periosteum and diploë, the cancellous bone separating the inner and outer layers of the cortical bone of the skull, are innervated by peripheral nerve fibers, including both sensory and sympathetic fibers.33,34 Some small sensory nerve fibers depart blood vessels and wind through the periosteum, while the main sensory nerves follow blood vessels, terminating in the lining layer of periosteum or continuing along vessels into bone via Volkmann’s canals.35 Sympathetic fibers often wrap around blood vessels penetrating from the periosteum into the diploë.35
Figure 3.
Anatomy of vascularized and innervated craniofacial tissues (A) cranial bone, (B) (i) mandibular bone (ii) and tooth, and (C) salivary glands. Part C was created with BioRender.com, agreement number: MB22XPLPSX.
Dental vessels and nerves course from the inferior alveolar nerve and vessels in the mandible (Figure 3Bi) and from branches of the maxillary artery and nerve in the maxilla, entering the dental pulp primarily through the apical foramen at the tooth root end (apex) (Figure 3Bii). Arterioles in the central pulp region supply a peripheral capillary network adjacent to dentin walls.36 Autonomic nerve fibers are found primarily in association with the vasculature, while sensory nerve fibers form a network around the pulp periphery and can enter dentin tubules in close relationship to odontoblasts.37 Additionally, the periodontal ligament (PDL) surrounding the cementum lining the tooth root and connecting alveolar bone, is vascularized and innervated.38 Conversely, cementum is avascular and contains no nerves.39 Progenitor and stem cell populations in both dental pulp and PDL are closely associated with the neurovasculature40,41 and sensory denervation can inhibit stem cell proliferation and differentiation.42
Vessels and nerves are also colocalized within the salivary gland (Figure 3C). Vessels enter the salivary gland from the external carotid artery and closely associate with the duct network of the salivary tissue together with nerves43 (Figure 3C). These vessels form dense capillary networks around the terminal ends of the secretory acinar units, which are also enveloped by nerves.44 Salivary gland innervation, similar to the pulp and PDL, is required for gland function and repair, controlling salivary secretion, and possibly maintaining salivary epithelial progenitor cell populations.45-47 Thus, vessels and nerves colocalize and play important roles in tissue homeostasis in craniofacial tissues.
2.2.2. Innervation and Angiogenesis Coordinate Craniofacial Tissue Regeneration.
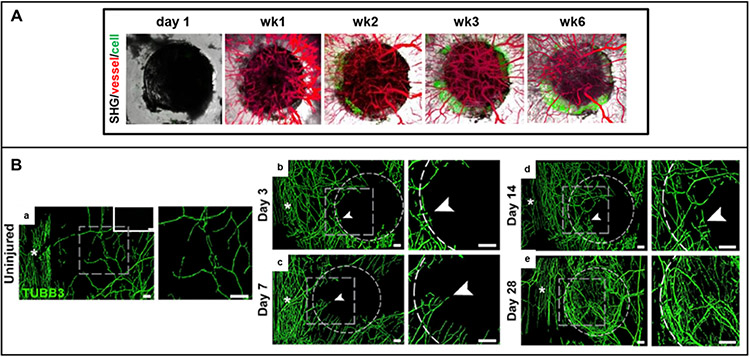
Blood vessels and nerves synergistically coordinate tissue regeneration.48-54 In bone, healing is initiated by hematoma and inflammation, which leads to cytokine release from inflammatory cells, followed by increased blood flow and cell migration to the injured site.55 Mesenchymal stem cells (MSCs) and osteoprogenitors migrate to the defect area and differentiate into osteoblasts to form the ossification center.56 Within the hypoxic wound, osteoblasts synthesize and secrete VEGF in response to elevated levels of HIF-1α, which then stimulates angiogenesis. Blood vessels sprouting from preexisting vessels to the defect center can be observed within the first week after injury in mouse cranial defect models57,58 (Figure 4A). Inflammatory cytokines, such as interleukin-1 (IL-1) and tumor necrosis factor (TNF)-α, directly induce NGF expression and downstream nuclear factor kappa B (NF-kB) signaling in calvarial osteoblasts to stimulate nerve recruitment.59 Reinnervation, which is associated with blood vessels, can be observed as early as 3 days postinjury in mouse cranial defects (Figure 4B).60 Nerves play diverse roles during osteogenesis, regulating the canonical/ß-catenin Wnt signaling pathway and expression of Connexin 43 (Cx43) and N-cadherin in MSCs61 as well as secreting vasculogenic neuropeptides, including neuropeptide Y (NPY), calcitonin gene-related peptide (CGRP), and substance P (SP), to stimulate angiogenesis.62,63 Increased angiogenesis recruits additional osteoprogenitors to the repair site, along with nutrients, oxygen, and minerals necessary for mineralization. Concomitantly, endothelial cells produce osteogenic factors, such as transforming growth factor beta (TGF-β) and bone morphogenetic protein-2 (BMP2), to further promote osteogenic differentiation and bone mineralization.64,65
Figure 4.
(A) Longitudinal tracking of cranial bone defect healing in Col2.3GFP transgenic mice over a 6-week period illustrates osteogenesis and angiogenesis. Vessel, red; osteoblasts, green [Reproduced with permission from ref 57. Copyright 2018 Springer eBook]. (B) Reinnervation during cranial bone defect repair: representative whole-mount tile scans (left) and high-magnification images (right) of TUBB3 (beta III tubulin)-stained cranial defects (b–e) from days 3 to 28 postinjury compared to uninjured controls (a); TUBB3+ nerve fibers appear green; dashed white circles indicates margins of defect; white asterisks indicate midline suture and white scale bar, 200 mm [Reproduced with permission from ref 60. Copyright 2020 Elsevier].
Dental and periodontal tissue repair also requires the orchestrated effort of vessels and nerves. Dental pulp has an inherent, albeit limited, regenerative capacity, which requires mesenchymal cell infiltration, innervation, and angiogenesis from adjacent healthy tissues into the pulp space.66 Dental pulp injuries result in hypoxia where pulp cells respond by secreting HIF-1α to drive downstream expression of VEGF, platelet derived growth factor (PDGF), fibroblast growth factor (FGF)-2, and angiopoietins to promote angiogenesis.67 Additionally, pulpal nerves respond to injury with sympathetic nerves releasing NPY and norepinephrine leading to vasoconstriction, while sensory neurons release neuropeptides such as SP and CGRP, which promote vasodilation and recruit immune cells.68 Neuropeptides induce nerve sprouting adjacent to the pulpal injury, with further neuropeptides released from these sprouting fibers leading to additional immune cell recruitment, vasodilation, and continued healing.37 The pulp chamber of mature teeth presents a particularly challenging space for tissue regeneration: new nerves and vessels must sprout from existing vessels and nerve trunks up to 15 mm away and enter through a narrow apical foramen 0.2 to 0.4 mm in diameter.69,70
Similar to bone, initial healing of the periodontium is characterized by the release of cytokines such as PDGF, TGF-β, VEGF, and bFGF from platelets and infiltrating immune cells, which then stimulates angiogenesis and recruitment of host cells from adjacent periodontal tissues.71 Recombinant PDGF-BB and autologous platelet-derived factors promote periodontal wound healing and tissue regeneration72,73 and likely stimulate angiogenesis during this process. PDL nerves produce low levels of neuropeptides during tissue homeostasis74 yet retain the ability to remodel and regenerate in response to external stimuli.75,76 The PDL responds to orthodontic force with nerve fiber sprouting, increased production of both sensory (SP, CGRP) and sympathetic (NPY, tyrosine hydroxylase) neuropeptides, and increased capillary density together with transient local bone resorption.77-79 PDL nerves also express the high affinity neurotrophin receptor tropomyosin receptor kinase B (TrkB)80 and high levels of NGF receptor p75-NGFR and growth associated protein-43 (GAP-43), factors associated with neuroplasticity.81 Neurotrophins and neurotrophin receptors are also expressed by periodontal ligament cells,82 and cells within healing periodontal defects, including infiltrating endothelial cells, express TrkB.83 The epithelial rests of Malassez (ERM) and associated nerves may also play a key role in periodontal wound healing,84 as sensory denervation results in a reduction in the size and number of ERM85 and ankylosis of tooth to bone.86
There are few studies examining the role of vessels and nerves in salivary gland regeneration, despite studies indicating their importance in secretory acinar cells regeneration. During gland development, signaling from CD31+ endothelial cells via vascular endothelial growth factor receptor 2 (VEGFR2) is required for proper tissue patterning by helping maintain Kit+ progenitor cells and promoting branching morphogenesis.43 Additionally, secretion of vasoactive intestinal peptide (VIP) by innervating ganglia is necessary for duct growth and lumen formation during branching morphogenesis,87 and parasympathetic innervation promotes SRY-Box transcription factor 2 (SOX2) expression by progenitor cells in the developing gland.88 Expression of SOX2 is essential for the production of secretory acinar cells, but not duct cells, and is maintained in a subset of cells in adult gland,89 which has been shown to replace acinar cells under homeostatic conditions.89,90
2.3. Failures in Angiogenesis or Innervation Result in Deficient Craniofacial Tissue Regeneration.
Innervation and vascularization are both necessary for successful craniofacial tissue regeneration. For example, a significant decrease in ossification of calvaria has been observed in conditional VEGF knockout mice or those with impaired FGF signaling due to inadequate angiogenesis.91,92 Inhibition of VEGFR2 or depletion of endothelial cells embryonically leads to loss of secretory acinar cells.43 Additionally, deletion of NGF leads to dramatic reductions in nerve sprouts, angiogenesis, and delayed regeneration of cranial defects.60 Sensory denervation also leads to alterations in osteoclasts, which subsequently delay cranial bone defect healing,93 bone formation during mandibular repair,94 orthodontic tooth movement,95 and mineralization of new bone within the periodontium.96,97 Denervation also leads to decreases in pulp cell proliferation98 and increased susceptibility to pulpal necrosis after bacterial exposure.99 Sympathectomy or treatment with sympathetic antagonists during tooth movement suppresses osteoclast activity but not bone formation79 and also leads to periodontal bone loss via increased production of inflammatory cytokines in the periodontal tissues and subsequent osteoclast activity.100 Moreover, denervation90 or disruption of innervation prevents salivary gland regeneration in ductal ligation models, despite progenitor cell maintenance,101-103 suggesting a critical role for innervation.
Altogether, these studies emphasize the necessity of both angiogenesis and innervation for successful craniofacial tissue regeneration and point to the possibility of superior integration with the host tissue and minimum long-term complications for regenerative approaches that target both processes.
3. CURRENT CRANIOFACIAL TISSUE REGENERATIVE APPROACHES THAT TARGET INNERVATION OR VASCULARIZATION
3.1. Growth Factor Delivery.
3.1.1. Growth Factor Delivery to Stimulate Reinnervation.
The growing appreciation of the role of reinnervation in tissue regeneration has resulted in several studies exploiting delivery of neuronal growth factors and neuropeptides to promote craniofacial tissue regeneration. These growth factors include NGF, BDNF, SP, galanin, and neurturin (NTRN). For example, NGF has been incorporated into bone scaffolds to treat rat cranial defects, showing an increase in innervation but limited new bone formation.104,105 NGF delivery via alginate hydrogels has also been used to increase bone and cementum formation in periodontal defects.106 Local injection of SP promoted bone formation during mandibular distraction osteogenesis in rats,94 and BDNF delivery from collagen sponges107,108 or using hyaluronic acid hydrogels83,109-111 led to increased bone, PDL, and cementum formation compared to scaffold controls. Galanin-loaded collagen sponges promoted bone formation in inflammatory periodontal defects.112 Overexpression of NTRN prior to irradiation was protective of salivary gland innervation and function,113 and laminin matrices with entrapped NTRN promoted neurite outgrowth from isolated parasympathetic submandibular ganglia (PSG), reduced neuronal apoptosis, and increased the number of end buds, progenitor cells, and innervation in mouse submandibular glands exposed to irradiation.101 Additionally, salivary gland cell sphere cultures combined with mesenchyme and PSG in matrices containing NRTN became innervated and underwent branching similar to development.114
3.1.2. Growth Factor Delivery to Promote Vascularization.
A common approach to achieve vascularization is delivery of angiogenic proteins including VEGF, FGFs, and PDGF.115,116 For example, VEGF,117,118 FGF-2,15 and PDGF16 delivery enhanced local angiogenesis and osteogenesis in some cranial defect models. However, another study showed vascularization and new bone regeneration after scaffold-mediated VEGF led to similar results as a control group, with only 26% of the defect covered by newly formed bone 12 weeks after injury,117 suggesting that VEGF-mediated vascularization alone is insufficient to support a robust regenerative response. Previous studies have also demonstrated the ability of VEGF and FGF-2 to promote dental pulp angiogenesis.119-121 The delivery of VEGF (together with dental pulp stem cells (DPSCs)) from heparin-conjugated gelatin microspheres in tooth roots also supported formation of vascularized pulp tissue.122
3.1.3. Delivery of Multiple Growth Factors to Stimulate Innervation or Vascularization.
Combinatorial approaches of neurogenic and angiogenic and, in some cases, osteogenic growth factors have also shown promise. Recent studies suggest delivery of multiple pro-angiogenic proteins can result in therapeutically relevant and long-lasting vascularization, which is necessary for regeneration.123-126 For example, combined delivery of VEGF and BMP-2 promoted osteogenesis and angiogenesis in cranial defects.127,128 Additionally, the combined delivery of VEGF or FGF2 with NGF and BMP-7 to dental pulp chambers led to angiogenesis, recellularization, and new dentin formation.129 NGF has also been delivered by alginate hydrogels together with BMP-2, resulting in synergistic effects on periodontal bone and cementum formation.106 Additionally, fibrin hydrogel-mediated delivery of pro-angiogenic VEGF and FGF9, which recruits vessel stabilizing mural cells and maintains neuronal cell viability and function,130,131 enhanced salivary gland regeneration and saliva production in a gland injury model.132
3.1.4. Peptides as Alternatives to Growth Factors for Vascularization.
Protein delivery approaches can suffer from poor long-term outcomes due to insufficient protein stability and rapid clearance133-135 as well as challenges associated with precise control over growth factor release kinetics.11,136 Therefore, therapeutic peptides have recently gained interest as an alternative to proteins. Their smaller size137 gives them more versatile synthetic schemes than proteins and allows peptides to be delivered at higher concentrations to target tissues when applied locally. Additionally, peptides often do not require complex tertiary structures for bioactivity, increasing in vivo stability.138 For example, VIP-conjugated hydrogels have been reported to enhance cranial bone defect repair, 139 where VIP not only stimulated tube formation and VEGF expression but also promoted bone marrow stem cell (BMSC) differentiation by activating the Wnt-β-catenin signaling pathway. Self-assembling peptide hydrogels for pulpal regeneration were synthesized to contain a pro-angiogenic peptide sequence, QK, derived from VEGF or dentonin, an odontogenic peptide.140 Roots treated with hydrogels without bioactive peptides or containing only the odontogenic peptide formed disorganized connective tissue, while hydrogels containing QK showed formation of pulpal tissues with new odontoblast-like cells, blood vessels, and nerves. A recent study delivered dimethyloxalylglycine, a glycine derivative that inhibits prolyl hydroxylase to activate HIF-1α, via nanosilicates entrapped within poly(L-lactide-co-glycolide) (PLGA) scaffolds, to promote angiogenesis and tissue regeneration in periodontal defects.141
3.2. Implantation of Prevascularized or Innervated Scaffolds.
Several techniques have been employed to develop prevascularized constructs.142-150 These approaches exploit both fully differentiated as well as progenitor cell types. For example, DPSCs were precultured in endothelial differentiation media to form Von Willebrand factor (vWF) and CD31-positive reticular structures, which supported formation of vascularized pulp tissue and differentiated odontoblasts.151 Additionally, a preformed endothelial network was incorporated into bone scaffolds to promote vascularization and osteogenesis in a rat cranial defect model.152 However, only 25% of increased bone volume was observed despite increased blood vessel volume after 8 weeks, a result that may be due to no or poor innervation.152 Prevascularization strategies face challenges to ensure stable tissue formation and anastomosis within host tissues. EC-only derived vasculature is commonly unstable, lasting only a few days in vitro. Cocultures of ECs and vessel-stabilizing MSCs or other pericyte-like cells can extend vascular network stability for >60 days in vitro in engineered extracellular matrices and facilitate tissue integration in vivo.153,154 As an example, spheroids composed of BMSCs and human umbilical vein endothelial cells (HUVECs) were encapsulated in fibrin scaffolds to form vascular networks prior to implanting the scaffolds in rat cranial defects.155 The preformed vascular networks survived and anastomosed with host tissue within 1 week of implantation but ultimately did not significantly enhance bone regeneration compared to controls.155
Innervated tissue engineering approaches have been utilized in a few applications to recapitulate Wallerian degeneration to guide nerve integration and promote healing. Though not specific to craniofacial tissues, preinnervated tissue engineered muscle was formed using a coculture of myocytes and motor neurons on aligned nanofibrous scaffolds. The presence of neurons enhanced the differentiation and maturation of skeletal myocytes in vitro and significantly increased satellite cell migration, microvessel formation and revascularization, and neuromuscular junction density in vivo.156 Though not a direct preinnervation approach, incorporating sensory nerve tracts in bioceramic-based scaffolds has been reported to enhance both neurogenesis and bone formation.157
4. DEVELOPMENT OF TISSUE ENGINEERING APPROACHES FOR NEUROVASCULARIZATION
Tissue engineering approaches that address both innervation and vascularization hold great potential to promote craniofacial tissue regeneration158 as opposed to targeting only vascularization159-161 or innervation.162-165 These approaches can be adopted from traditional tissue engineering approaches and include cells,166,167 synthetic168-170 and natural166,171,172 scaffolds, growth factors,173,174 and combinations therein,127,175 which are detailed in the following sections.
4.1. Cells for Neurovascular Tissue Regeneration.
A variety of cell types have been used to promote neurovascularization for craniofacial tissue regeneration. These include stem cells, such as MSCs, DPSCs, and induced pluripotent stem cells (iPSCs), SCs, and ECs. Cells participate in regeneration via many mechanisms, including tissue-specific matrix deposition as well as secretion of growth factors, to facilitate tissue recruitment and neurovascular tissue development. For example, SCs can direct axon migration and regulate their proliferation via neurotrophic factor expression (Figure 4);176 ECs can mediate angiogenesis and osteogenesis by producing paracrine factors177 and proliferation, migration, and assembly into tubular structures178 (Figure 2). Therefore, SCs166,179-181 and ECs167,182-184 have been incorporated in tissue engineering constructs to promote peripheral nerve regeneration and angiogenesis during bone repair, respectively. Other systems have utilized cocultured stem cells/SCs175,185,186 and stem cells/ECs.187-192 MSCs have been shown to differentiate into osteoprogenitors as well as Schwann-like cells193,194 and may give rise to neurons186 and enhance axonal regeneration.185 Though controversial, some studies have also reported that MSCs can differentiate into endothelial cells.195,196 Additionally, MSCs cocultured with ECs have been reported to act as pericytes during angiogenesis in vitro197 and in vivo,198 where MSCs localize along the endothelial tubes and encourage vascular network formation by secreting angiogenic growth factors such as VEGF and bFGF.199,200 Human DPSCs have been reported to enhance HUVEC migration and VEGF secretion as well as promote ECM deposition and mineralization during dental pulp regeneration.201 Hence, the integration of cocultured SCs/ECs or SCs/ECs/stem cells into cellular approaches may improve neurovascularization for craniofacial tissue regeneration. However, the clinical application of many cell types has been limited by source of human cells and lack of in vitro expansion capacity.202,203 In this regard, iPSCs, which can be reproducibly differentiated into myriad cell types including ECs204 and neurons,205 are promising alternatives. Previous studies have demonstrated that iPSC-derived ECs significantly increase vascularization in vivo.206,207 Moreover, iPSC-derived neural crest stem cells (NCSCs) and NCSC-SCs have been reported to encourage nerve regeneration in vivo, where they interacted with host axons and produce neurogenic factors (NGF and BDNF).208 Though the application of iPSCs for craniofacial neurovascularization has not been reported, its potential has been discussed.209-211
4.2. Design of Biomaterials for Neurovascularization.
Biomaterials used in craniofacial tissue engineering must be designed to support peripheral nerves and vessels. Biomaterial design, therefore, must take into account many complex design requirements including biophysical and biochemical cues.212,213 Moreover, biomaterials should be biodegradable and may also need to provide control over release of therapeutic factors to encourage cell migration, axonal growth, and vessel infiltration.214-216 In the following sections, we summarize various biomaterials classes and design criteria enabled by new chemistries and fabrication techniques for the regeneration of neurovascularized tissues.
4.2.1. Biomaterial Classes for Neurovascularization.
Because of excellent biocompatibility, natural polymers, such as collagen,171,217-219 chitosan,172,220-222 and silk,166 have been utilized to promote neurovascularization in vivo. For example, chitosan has been reported to support ~50% regeneration and tissue reinnervation in a rat critical nerve gap model.222 Neovascularization has also been observed in chitosan scaffolds after 12-weeks of implantation in vivo.223 Additionally, cell/growth factor-loaded collagen-based scaffolds have been reported to promote nerve regeneration224 and vascularization225 in vivo. However, the use of natural polymers is hindered by their complex chemical structures, uncontrolled degradation, and thermal sensitivity.226 Alternatively, synthetic polymers can be tailored to match biophysical and biochemical properties demanded by vascularization and innervation and enable delivery of cells and bioactive factors. Previous studies have demonstrated that polymers such as poly(glycolic acid) (PGA),227,228 poly(lactic acid) (PLA),229-231 PLGA,232-235 and poly(caprolactone) (PCL),236-238 combined with cells168 and growth factors,169 can encourage peripheral nerve regeneration in vivo. However, application of synthetic polymers in nerve repair still remains a clinical challenge.239,240 Even biomaterials approved by the FDA for specific applications, such as NeuraGen,241 Neuromaix,242 and NEUROLAC,243 provide only limited repair for nerve defects longer than 3 cm. Synthetic polymers, such as PGA,244,245 PLA, and PLGA,161,246,247 have also been developed as delivery vehicles of angiogenic growth factors to promote neovascularization in vivo. Nevertheless, the effects of these scaffolds on angiogenesis can be hindered because release profiles of the incorporated growth factors are difficult to control and often do not match what is required to achieve regeneration.248
Various hydrogel biomaterials have also been explored in neural and vascular tissue engineering. Hydrogels have great utility in tissue engineering in general due to high water content, ability to be tailored via functionalization by exogenous biochemical cues, incorporation of cross-linkers degradable by various modes and rates, and incorporation and control over growth factor release.32,249-254 Poly(ethylene glycol) (PEG)255 and gelatin-based hydrogels256 promoted the regeneration of rat sciatic nerves, as evidenced by increased axon number and diameter. Gelatin hydrogels have been used in multiple studies to promote angiogenesis during bone repair through the delivery of various growth factors.248,257-259 PEG-based hydrogels have also been used to deliver growth factors159 and cells260,261 to improve angiogenesis during bone regeneration in vivo. Despite promise, hydrogel-based materials have not yet been effectively used to promote both angiogenesis and innervation in the regeneration of craniofacial tissues.262,263
4.2.2. Biomaterial Physical Cues for Neurovascularization.
Biomaterials designed for neurovascularization should provide a 3D microenvironment with proper biophysical cues, including pore size/porosity, stiffness, and degradation dynamics, to support endothelial and nerve cell growth and migration. Previous studies have demonstrated that a pore size of 35–100 μm with at least 50% porosity is optimal for blood vessel formation.264,265 Cells may rapidly migrate from scaffolds with pore sizes too large, and smaller pore size can hinder oxygen and nutrition diffusion and cell migration, resulting in unstable vessels.266 The optimal pore size for innervation was reported to be in the ~5–30 μm range with >80% porosity to enable nutrient and waste exchange and neuron infiltration.267-269
The morphology, organization, and function of endothelial270 and nerve tissues271 have also been reported to be influenced by substrate stiffness. In 3D culture systems, which have greater relevance for tissue engineering than 2D studies, more robust neurite outgrowth was observed in softer matrices (0.5 kPa) than stiffer matrices (1.4 kPa,272 2.1 kPa273). Encapsulated nerve cells also showed higher gene expression level of neural differentiation and neuron maturation markers including Synapsin1, βIII-tubulin (TUBB3), and Neuronal nuclear antigen (NeuN) in softer matrices.272 Similarly, greater spreading and branching of microvessels formed by ECs were observed with decreased matrix stiffness: from 17 to 7 kPa274 and from 0.5 Pa to 0.2 kPa.275 Additionally, stiffer matrices (~0.5 kPa) encourage EC spreading and branching versus softer matrices (~0.1 kPa).275,276 Though absolute stiffness ranges may vary due to divergent design parameters, generally more compliant matrices favor vascularization and innervation.
Cell migration and sprouting not only depend on the original matrix stiffness but also its degradation mode and rate.277-279 For example, our previous study found that matrix metalloproteinase (MMP)-degradable hydrogels better support EC sprouting than hydrolytically degradable hydrogels with a similar initial modulus (~10 kPa).280 Additionally, endothelial281,282 and nerve 283,284 tissues can sense their surrounding mechanical environment and secrete enzymes, such as MMPs, to degrade and remodel ECM dynamically. Indeed, softer matrices (~2 kPa) upregulated nerve cell expression of MMP-2 and MMP-3 compared to stiffer matrices (~3.5 kPa).285
An additional challenge for some craniofacial tissues, including cranial and alveolar bones, is the requirement for structural stability during tissue regeneration. Many current approaches include a stiff scaffold component that slowly resorbs as new bone is deposited and matures.286 For instance, design criteria for periodontal tissue scaffolds often include a “bone” component with a distinct design from PDL and cementum portions of the scaffold.287 Even if each component of such multiphasic scaffolds is porous and interconnected, ingrowth of host neurovasculature may be hindered by complex, slowly degrading structures. Thus, composite scaffolds that combine sufficiently porous or soft, degradable structures required for optimal neurovascularization together with stiffer, dense components that can direct bone growth and stability may be required in certain craniofacial locations.
4.2.3. Biomaterial Biochemical Cues for Neurovascularization.
Given the critical role of cell-secreted growth factors (GFs) in driving transplanted cell bioactivity, therapeutic factors can be used to promote neurovascularization. For example, delivery of NGF,288-290 GDNF,291-293 and BDNF294,295 from synthetic nerve conduits improves axon regeneration in vivo. Previous studies have also demonstrated enhanced vascularization and bone regeneration by singular and dual delivery of VEGF,160,161 PDGF,296 and VEGF/BMP2.127,297 Furthermore, FGF delivery has been reported to improve neurogenesis,298 angiogenesis,299 and bone regeneration.300,301 Consequently, therapeutic cues for tissue engineered neurovascular approaches will likely include combinations of proangiogenic and neural growth factors, which necessitates further investigation to identify the critical growth factors and define the appropriate doses and temporal availabilities to achieve regenerative success.
While GFs can be physically encapsulated/immobilized into or absorbed on the surface of biomaterials, these approaches are susceptible to a fast or burst release of factors immediately after contact with physiological fluids.302 For example, burst release of VEGF and BMP-2 from poly(L-lactic acid) (PLLA) scaffolds was observed only after 24 h postimplantation in vivo, and >80% of these growth factors were released after 14 days (Figure 6A).303 This temporal profile is unlikely to match any native GF release patterns, and GF bioactivity may be compromised by unstable release profiles.248 For example, in cranial defects, angiogenesis and reinnervation do not occur until 3–7 days after injury and then continue for up to 6 weeks (Figure 4). Covalent immobilization of GFs to biomaterials through carbodiimide, 304,305 thiol–ene, 250,252,253,306,307 and enzymatic307,308 conjugations can eliminate the possibility of initial burst release and has emerged as a promising approach for improved GF delivery kinetics. In this case, GF release depends on biomaterial degradation or hydrolytic or enzymatic cleavage of the bond between GFs and the biomaterial.32,250 Additionally, inspired by the natural interactions between ECM and GFs, bioaffinity tethering approaches have been applied for more controlled delivery systems. For example, due to the interaction between GFs (e.g., BMP2, VEGF, and FGF-2) and heparin sulfate of the natural ECM, biomaterials have been decorated with heparin or heparin sulfate-mimetic molecules to bind GFs.290,309,310 The higher affinity of heparin-GF binding not only contributes to more extended and stable release profiles but also facilitates localized and spatially regulated signaling.309,311
Figure 6.
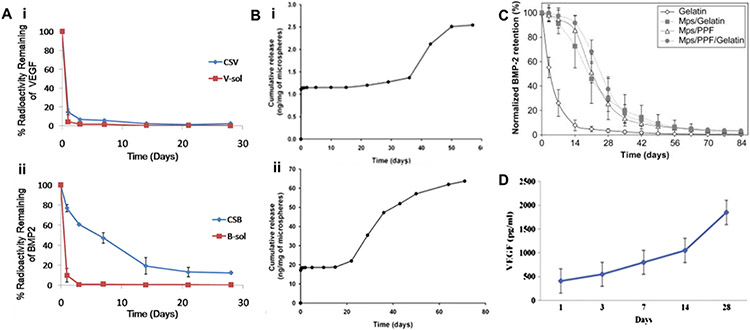
(A) In vivo release profiles of (i) VEGF and (ii) BMP-2 were obtained after implantation of scaffold into the subcutaneous tissue mice for 28 days,303 where CSB and CSV represent nanocomposite fibrous scaffold (CS) loaded with BMP2 (B) and VEGF (V), respectively [Reproduced with permission from ref 303. Copyright 2018 Elsevier]. (B) Release kinetics of PLGA microspheres steady release of neurotrophic growth factors was detected for at least 60 days (i, BDNF) and 80 days (ii, GDNF) [Reproduced with the permission from ref 358. Copyright 2010 Springer Nature]. (C) Normalized in vivo release profile of BMP-2 from the four different implants in a rat subcutaneous implantation model, where Mps = PLGA microparticles loaded with BMP-2, and PPF = poly(propylene fumarate) [Reproduced with the permission from ref 359. Copyright 2008 Elsevier]. (D) Cumulative release of VEGF from the biohybrid scaffold with PLGA nanofibers, which displays a sustained release of VEGF over 28 days [Reproduced with the permission from ref 355. Copyright 2014 Elsevier].
During regeneration, integrin transmembrane receptors mediate cell–substrate binding, which is critical for adhesion and migration.312,313 Apart from forming focal adhesions, binding between integrin subunits (α and β) and ECM ligands alters actin cytoskeleton structures and actively transduces biochemical and signaling cascades.314 Therefore, biomaterials have been functionalized by ECM protein mimetic peptides specifically to encourage neurogenesis and angiogenesis. Laminin mimetic peptides (CDPGYIGSR or CSRARKQAA-SIKVAVSAD,315 and YIGSR316) immobilized within chitosan hydrogels have been reported to promote nerve regeneration in vivo. Additionally, the peptide, RGD, which is ubiquitously found within myriad extracellular matrix proteins, has been incorporated into poly(caprolactone)-based materials to promote SCs outgrowth in vitro317 and axonal regeneration and functional recovery in vivo.318 Laminin (YIGSR,319 SIKVAV320) and collagen (e.g., GFOGER159 and P15 (PQGIAGQRGVV)321) mimetic peptides, along with RGD322 have also been applied to stimulate angiogenesis in vitro and in vivo. Additionally, fibronectin-derived peptide REDV (GREDVY),323 osteopontin-derived peptide SVVYGLR,324 and VEGF-binding domain-derived peptide PR1P (DRVQRQ-TTTVVA)325 have been used to functionalize biomaterials to enhance angiogenesis in vivo.
4.2.4. New Approaches to Meet Biomaterials Design Requirements for Neurovascularization.
Recently, various techniques have been applied to fabricate complex, multicomponent biomaterials to better meet the demands for neurovascularization. For example, synthetic polymers, such as poly(caprolactone) (PCL), poly(dimethylsiloxane) (PDMS), and hydrogels can be fabricated into vascular326,327 and neuronal328,329 scaffolds using three-dimensional (3D) printing techniques. Perfusable 3D-printed vascular networks and porous neural scaffolds have been reported to support angiogenesis330-332 and neurogenesis,328,333 respectively, in vitro. A 3D printed PCL scaffold doped with tricalcium phosphate (TCP) supported vascularized new bone formation in a rat calvarial defect model,334 where the microchannels in the scaffold facilitated diffusion of nutrients to printed cells, overcoming the diffusion limit of 100–200 μm for cell survival in engineered tissues.335 3D printing has also been used to fabricate scaffolds for alveolar bone and periodontal tissue regeneration, primarily to localize growth factors and transplanted cells and guide cell orientation during healing.336 3D printing technology is also being explored for salivary gland regeneration, focusing on secretory and duct cell patterning and organoid printing.337-340 The integration of nerves and vasculature is likely to be a key step in the full integration and successful regeneration of salivary gland tissue using these methods. Therefore, 3D bioprinting has promise for fabricating scaffolds for bone and dental tissues regeneration, which provide features targeting vascularization and innervation.
A combination of favorable biochemical and biomechanical cues can be achieved by designing materials to support and respond to tissue infiltration as required during neurovascularization and tissue regeneration. In particular, MMP-degradable hydrogels are promising materials from which to develop neurovascularization strategies.341 Both endothelial cell migration and vessel extension during angiogenesis and axonal outgrowth and pathfinding during innervation occur via degradation of the surrounding ECM by MMPs.342,343 MMP-degradable hydrogels can be optimized to support host tissue recruitment and integration by modifying the degradation kinetics of the MMP-susceptible linkages.167,240,344-346 Moreover, the potential of MMP-degradable hydrogels to improve both angiogenesis and neurogenesis has been investigated in vitro347,348 and in vivo.349,350 Indeed, our recent study demonstrated that a tissue engineered periosteum composed of MMP-degradable hydrogels improved allograft healing by promoting rapid innervation and vascularization (Figure 5).280 We have also found that MMP-degradable hydrogels improve the organization, apicobasal polarization, and retention of salivary gland acinar cell phenotype in tissue-mimetic culture systems.351,352
Figure 5.
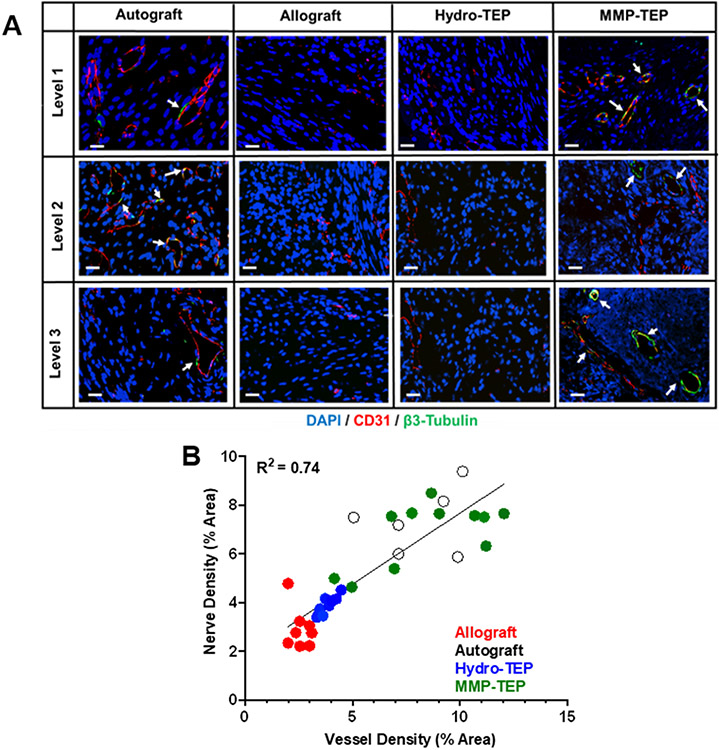
(A) Representative confocal images of costained of CD31 (blood vessels, red) and β3-tubulin (nerves, green) on the cross sections of autografts, allografts, allografts modified by hydrolytically or MMP degradable tissue engineered periosteum (Hydro-TEP, MMP-TEP) at levels proximal (1), medial (2), and distal (3) in relation to the femoral head (scale bar = 20 μm) at 3-week postsurgery. (B) Blood vessel density-dependent effects on nerve density, where regression analysis demonstrates a linear relationship (R2 = 0.74) between revascularization and reinnervation, indicating their synergistic coordination during bone defect healing [Reproduced with permission from ref 280. Copyright 2021 Elsevier].
As control over growth factor delivery from polymeric scaffolds is still challenging,303 various fabrication techniques, such as microsphere formation353 and electrospinning,354,355 have been investigated. The GF release kinetics from microspheres can be adjusted by altering the molecular weight and composition of the copolymer.356 A sustained 4-week release of NGF357 and up to 80-days of steady release of BDNF and GDNF358 (Figure 6B) from PLGA microspheres have been reported, where the release of NGF significantly enhanced neurite outgrowth in vitro. The in vivo release profile of BMP-2 from PLGA microspheres has been shown to be an S-shaped curve over 84 days without any observed burst release at early time points (Figure 6C).359 PLGA microspheres were also developed to exhibit minimal initial in vitro release of VEGF in the first 7 days after loading and then reaching a plateau at day 20.355
Another fabrication method to prolong the release of entrapped GFs is via electrospinning. Electrospinning techniques can manufacture scaffolds with ECM-like structure including variable degrees of porosity and adjustable mechanical properties.360 Growth factor release can be controlled by the extruded materials’ orientation and geometries.361 Electrospun PLGA nanofibers have been reported to slow the release of FGF-2354 and VEGF355 in vitro, showing a 28-day sustained in vitro release profile of VEGF (Figure 6D).
5. PERSPECTIVES ON NEUROVASCULARIZATION STRATEGIES FOR CRANIOFACIAL TISSUE REGENERATION
Despite great progress in the fundamental understanding of neurovascularization during development and regeneration and biomaterials design and fabrication, progress in implementing these innovations for the repair of craniofacial tissue defects is still in its infancy. Given the complex nature of craniofacial tissues, the integration of multiple tissues remains a critical barrier. For example, during craniofacial bone regeneration, osteogenic, neurogenic, and endothelial cells may require different microenvironments to holistically integrate to coordinate healing.158,362 Challenges in manipulating multiple cell types, particularly those originating from different tissues, as well as spatial regulation of complex cell-cell interactions, remain unaddressed.363 Additionally, the clinical translation of cell-based therapies can be hindered by complicated cell isolation processes, long-term loss of viability, regulatory hurdles, and adverse host responses,364 some which may be overcome through the use of iPSCs and their offer of nearly infinite patient-specific cells and cell types.365 Successful neurovascularization strategies are also likely to require spatially and temporally regulated growth factor/bioactive peptide release. Considering the multifactorial and differential effects of growth factors on various cell types, a significant challenge is determining and delivering optimal combinations and concentrations of growth factors with appropriate temporal availabilities to coordinate successful craniofacial tissue regeneration.366 Nevertheless, recruitment of host neurovascular tissues can be modulated within tissue engineering approaches through careful design of biomaterial mechanics, degradation rate and mechanism (hydrolytic or enzymatic), and adhesive ligand concentration.148-150,367-370 MMP-degradable hydrogels,341 incorporation of critical growth factors or iPSCs-derived target cells, prevascularization, and preinnervation strategies, as well as new tissue engineering techniques such as 3D printing, have promise for the development of biomaterials that can improve the regeneration of craniofacial tissues by promoting neurovascularization.
ACKNOWLEDGMENTS
This work was supported by the National Science Foundation (CAREER Award Nos. CBET1450987 and DMR2103553 (to D.S.W.B.), and DGE1419118 (to M.R.N.)), National Institutes of Health (NIH) P30 AR069655, R01 AR064200, R01 AR056696, R01 AR071363, UG3DE027695, and UH3 DE027695 (to D.S.W.B.), T32 ES007026 (to J.A.M.), and UL1 TR002001 (University of Rochester Clinical and Translational Science Institute Pilot Award to D.F.). D.F. is supported by the UR Joan Wright Goodman Dissertation Fellowship.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors declare no competing financial interest.
Contributor Information
Yiming Li, Department of Biomedical Engineering, University of Rochester, Rochester, New York 14627, United States; Department of Orthopaedics and Center for Musculoskeletal Research, University of Rochester Medical Center, Rochester, New York 14642, United States.
David Fraser, Department of Orthopaedics and Center for Musculoskeletal Research, University of Rochester Medical Center, Rochester, New York 14642, United States; Eastman Institute for Oral Health, University of Rochester Medical Center, Rochester, New York 14620, United States; Translational Biomedical Sciences Program, University of Rochester Medical Center, Rochester, New York 14642, United States.
Jared Mereness, Department of Biomedical Engineering, University of Rochester, Rochester, New York 14627, United States; Department of Orthopaedics and Center for Musculoskeletal Research, University of Rochester Medical Center, Rochester, New York 14642, United States; Department of Environmental Medicine, University of Rochester Medical Center, Rochester, New York 14642, United States.
Amy Van Hove, Department of Biomedical Engineering, University of Rochester, Rochester, New York 14627, United States; Department of Orthopaedics and Center for Musculoskeletal Research, University of Rochester Medical Center, Rochester, New York 14642, United States.
Sayantani Basu, Department of Biomedical Engineering, University of Rochester, Rochester, New York 14627, United States; Department of Orthopaedics and Center for Musculoskeletal Research, University of Rochester Medical Center, Rochester, New York 14642, United States.
Maureen Newman, Department of Biomedical Engineering, University of Rochester, Rochester, New York 14627, United States; Department of Orthopaedics and Center for Musculoskeletal Research, University of Rochester Medical Center, Rochester, New York 14642, United States.
Danielle S. W. Benoit, Department of Biomedical Engineering, University of Rochester, Rochester, New York 14627, United States; Department of Orthopaedics and Center for Musculoskeletal Research, University of Rochester Medical Center, Rochester, New York 14642, United States; Eastman Institute for Oral Health, University of Rochester Medical Center, Rochester, New York 14620, United States; Translational Biomedical Sciences Program, Department of Environmental Medicine, and Department of Biomedical Genetics and Center for Oral Biology, University of Rochester Medical Center, Rochester, New York 14642, United States; Materials Science Program and Department of Chemical Engineering, University of Rochester, Rochester, New York 14627, United States.
REFERENCES
- (1).Warren SM; Fong KD; Chen CM; Loboa EG; Cowan CM; Lorenz HP; Longaker MT Tools and techniques for craniofacial tissue engineering. Tissue Eng. 2003, 9 (2), 187–200. [DOI] [PubMed] [Google Scholar]
- (2).Petrovic V; Zivkovic P; Petrovic D; Stefanovic V Craniofacial bone tissue engineering. Oral surgery, oral medicine, oral pathology and oral radiology 2012, 114 (3), No. e1. [DOI] [PubMed] [Google Scholar]
- (3).Zhang W; Yelick PC Craniofacial tissue engineering. Cold Spring Harbor Perspect. Med 2018, 8 (1), No. a025775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Li J; Gsaxner C; Pepe A; Morais A; Alves V; von Campe G; Wallner J; Egger J Synthetic skull bone defects for automatic patient-specific craniofacial implant design. Sci. Data 2021, 8 (1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Ozdemir D Dental caries: the most common disease worldwide and preventive strategies. Int. J. Biol 2013, 5 (4), 55. [Google Scholar]
- (6).Kinane DF; Stathopoulou PG; Papapanou PN Periodontal diseases. Nat. Rev. Dis Primers 2017, 3, 17038. [DOI] [PubMed] [Google Scholar]
- (7).Jasmer KJ; Gilman KE; Muñoz Forti K; Weisman GA; Limesand KH Radiation-Induced Salivary Gland Dysfunction: Mechanisms, Therapeutics and Future Directions. J. Clin. Med 2020, 9 (12), 4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Limesand KH; Said S; Anderson SM Suppression of Radiation-Induced Salivary Gland Dysfunction by IGF-1. PLoS One 2009, 4 (3), No. e4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Konings AWT; Coppes RP; Vissink A On the mechanism of salivary gland radiosensitivity. Int. J. Radiat. Oncol., Biol., Phys 2005, 62 (4), 1187–1194. [DOI] [PubMed] [Google Scholar]
- (10).Szpalski C; Barr J; Wetterau M; Saadeh PB; Warren SM Cranial bone defects: current and future strategies. Neurosurgical focus 2010, 29 (6), E8. [DOI] [PubMed] [Google Scholar]
- (11).Lovett M; Lee K; Edwards A; Kaplan DL Vascularization strategies for tissue engineering. Tissue Eng., Part B 2009, 15 (3), 353–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Kannan RY; Salacinski HJ; Sales K; Butler P; Seifalian AM The roles of tissue engineering and vascularisation in the development of micro-vascular networks: a review. Biomaterials 2005, 26 (14), 1857–75. [DOI] [PubMed] [Google Scholar]
- (13).Liu Y; Chan JK; Teoh SH Review of vascularised bone tissue-engineering strategies with a focus on co-culture systems. J. Tissue Eng. Regener. Med 2015, 9 (2), 85–105. [DOI] [PubMed] [Google Scholar]
- (14).Roux BM; Cheng MH; Brey EM Engineering clinically relevant volumes of vascularized bone. J. Cell. Mol. Med 2015, 19 (5), 903–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Kigami R; Sato S; Tsuchiya N; Sato N; Suzuki D; Arai Y; Ito K; Ogiso B Effect of basic fibroblast growth factor on angiogenesis and bone regeneration in non-critical-size bone defects in rat calvaria. J. Oral Sci 2014, 56 (1), 17–22. [DOI] [PubMed] [Google Scholar]
- (16).Shah NJ; Hyder MN; Quadir MA; Courchesne N-MD; Seeherman HJ; Nevins M; Spector M; Hammond PT Adaptive growth factor delivery from a polyelectrolyte coating promotes synergistic bone tissue repair and reconstruction. Proc. Natl. Acad. Sci. U. S. A 2014, 111 (35), 12847–12852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Huang GTJ; Yamaza T; Shea LD; Djouad F; Kuhn NZ; Tuan RS; Shi S Stem/Progenitor Cell–Mediated De Novo Regeneration of Dental Pulp with Newly Deposited Continuous Layer of Dentin in an In Vivo Model. Tissue Eng., Part A 2010, 16 (2), 605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Dissanayaka WL; Zhang C The Role of Vasculature Engineering in Dental Pulp Regeneration. Journal of Endodontics 2017, 43 (9), S102–S106. [DOI] [PubMed] [Google Scholar]
- (19).Huber AB; Kolodkin AL; Ginty DD; Cloutier JF Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annu. Rev. Neurosci 2003, 26, 509–63. [DOI] [PubMed] [Google Scholar]
- (20).Scheib J; Hoke A Advances in peripheral nerve regeneration. Nat. Rev. Neurol 2013, 9 (12), 668–76. [DOI] [PubMed] [Google Scholar]
- (21).Alvites RD; Santos ARC; Varejóo ASP; Maurício A Olfactory Mucosa Mesenchymal Stem Cells and Biomaterials: A New Combination to Regenerative Therapies after Peripheral Nerve Injury. Mesenchymal Stem Cells–Isolation, Characterization and Applications 2017, 55235. [Google Scholar]
- (22).Terenghi G Peripheral nerve regeneration and neurotrophic factors. J. Anat 1999, 194 (1), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Dickson BJ Molecular mechanisms of axon guidance. Science 2002, 298 (5600), 1959–64. [DOI] [PubMed] [Google Scholar]
- (24).Tessier-Lavigne M; Goodman CS The molecular biology of axon guidance. Science 1996, 274 (5290), 1123–33. [DOI] [PubMed] [Google Scholar]
- (25).Kannan S; Lee M; Muthusamy S; Blasiak A; Sriram G; Cao T Peripheral sensory neurons promote angiogenesis in neurovascular models derived from hESCs. Stem Cell Res. 2021, 52, 102231. [DOI] [PubMed] [Google Scholar]
- (26).Grasman JM; Kaplan DL Human endothelial cells secrete neurotropic factors to direct axonal growth of peripheral nerves. Sci. Rep 2017, 7 (1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Honma Y; Araki T; Gianino S; Bruce A; Heuckeroth R; Johnson E; Milbrandt J Artemin is a vascular-derived neurotropic factor for developing sympathetic neurons. Neuron 2002, 35 (2), 267–82. [DOI] [PubMed] [Google Scholar]
- (28).Kuruvilla R; Zweifel LS; Glebova NO; Lonze BE; Valdez G; Ye H; Ginty DD A neurotrophin signaling cascade coordinates sympathetic neuron development through differential control of TrkA trafficking and retrograde signaling. Cell 2004, 118 (2), 243–55. [DOI] [PubMed] [Google Scholar]
- (29).Mukouyama YS; Shin D; Britsch S; Taniguchi M; Anderson DJ Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell 2002, 109 (6), 693–705. [DOI] [PubMed] [Google Scholar]
- (30).Black JE; Isaacs KR; Anderson BJ; Alcantara AA; Greenough WT Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc. Natl. Acad. Sci. U. S. A 1990, 87 (14), 5568–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Kokaia Z; Lindvall O Neurogenesis after ischaemic brain insults. Curr. Opin. Neurobiol 2003, 13 (1), 127–32. [DOI] [PubMed] [Google Scholar]
- (32).Van Hove AH; Benoit DS Depot-based delivery systems for pro-angiogenic peptides: a review. Front. Bioeng. Biotechnol 2015, 3, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Herskovits M; Hallas B; Singh I Study of sympathetic innervation of cranial bones by axonal transport of horseradish peroxidase in the rat: preliminary findings. Cells Tissues Organs 1993, 147 (3), 178–183. [DOI] [PubMed] [Google Scholar]
- (34).Kosaras B; Jakubowski M; Kainz V; Burstein R Sensory innervation of the calvarial bones of the mouse. J. Comp. Neurol 2009, 515 (3), 331–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Hill EL; Elde R Distribution of CGRP-, VIP-, DβH-, SP-, and NPY-immunoreactive nerves in the periosteum of the rat. Cell Tissue Res. 1991, 264 (3), 469–480. [DOI] [PubMed] [Google Scholar]
- (36).Hilkens P; Lambrichts I; Bronckaers A Current and Future Views on Pulpal Angiogenesis. In Clinical Approaches in Endodontic Regeneration: Current and Emerging Therapeutic Perspectives; Duncan HF, Cooper PR, Eds.; Springer International Publishing: Cham, 2019; pp 37–53. [Google Scholar]
- (37).Yu C; Abbott PV An overview of the dental pulp: its functions and responses to injury. Aust. Dent. J 2007, 52 (1 Suppl), S4–16. [DOI] [PubMed] [Google Scholar]
- (38).Beertsen W; McCulloch CA; Sodek J The periodontal ligament: a unique, multifunctional connective tissue. Periodontology 2000 1997, 13, 20–40. [DOI] [PubMed] [Google Scholar]
- (39).Foster B; Somerman M Cementum. In Mineralized Tissues in Oral and Craniofacial Science; McCauley LK, Somerman MJ, Eds.; Wiley, 2013. [Google Scholar]
- (40).Kaukua N; Shahidi MK; Konstantinidou C; Dyachuk V; Kaucka M; Furlan A; An Z; Wang L; Hultman I; Ahrlund-Richter L; Blom H; Brismar H; Lopes NA; Pachnis V; Suter U; Clevers H; Thesleff I; Sharpe P; Ernfors P; Fried K; Adameyko I Glial origin of mesenchymal stem cells in a tooth model system. Nature 2014, 513 (7519), 551–4. [DOI] [PubMed] [Google Scholar]
- (41).Men Y; Wang Y; Yi Y; Jing D; Luo W; Shen B; Stenberg W; Chai Y; Ge W-P; Feng JQ; Zhao H Gli1+ Periodontium Stem Cells Are Regulated by Osteocytes and Occlusal Force. Dev. Cell 2020, 54 (5), 639–654. [DOI] [PubMed] [Google Scholar]
- (42).Zhao H; Feng J; Seidel K; Shi S; Klein O; Sharpe P; Chai Y Secretion of Shh by a Neurovascular Bundle Niche Supports Mesenchymal Stem Cell Homeostasis in the Adult Mouse Incisor. Cell Stem Cell 2014, 14 (2), 160–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Kwon HR; Nelson DA; DeSantis KA; Morrissey JM; Larsen M Endothelial cell regulation of salivary gland epithelial patterning. Development 2017, 144 (2), 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).de Paula F; Teshima THN; Hsieh R; Souza MM; Nico MMS; Lourenco SV Overview of Human Salivary Glands: Highlights of Morphology and Developing Processes. Anat. Rec 2017, 300 (7), 1180–1188. [DOI] [PubMed] [Google Scholar]
- (45).Knox SM; Lombaert IM; Reed X; Vitale-Cross L; Gutkind JS; Hoffman MP Parasympathetic innervation maintains epithelial progenitor cells during salivary organogenesis. Science 2010, 329 (5999), 1645–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Holmberg KV; Hoffman MP Anatomy, biogenesis and regeneration of salivary glands. Monogr Oral Sci. 2014, 24, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Porcheri C; Mitsiadis TA Physiology, Pathology and Regeneration of Salivary Glands. Cells 2019, 8 (9), 976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Carmeliet P Blood vessels and nerves: common signals, pathways and diseases. Nat. Rev. Genet 2003, 4 (9), 710–20. [DOI] [PubMed] [Google Scholar]
- (49).Carmeliet P; Tessier-Lavigne M Common mechanisms of nerve and blood vessel wiring. Nature 2005, 436 (7048), 193–200. [DOI] [PubMed] [Google Scholar]
- (50).Stegen S; van Gastel N; Carmeliet G Bringing new life to damaged bone: the importance of angiogenesis in bone repair and regeneration. Bone 2015, 70, 19–27. [DOI] [PubMed] [Google Scholar]
- (51).Marsell R; Einhorn TA The biology of fracture healing. Injury 2011, 42 (6), 551–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Schindeler A; McDonald MM; Bokko P; Little DG Bone remodeling during fracture repair: The cellular picture. Semin. Cell Dev. Biol 2008, 19 (5), 459–66. [DOI] [PubMed] [Google Scholar]
- (53).Claes L; Recknagel S; Ignatius A Fracture healing under healthy and inflammatory conditions. Nat. Rev. Rheumatol 2012, 8 (3), 133–43. [DOI] [PubMed] [Google Scholar]
- (54).Ratko TA; Belinson SE; Samson DJ; Bonnell C; Ziegler KM; Aronson N Bone Morphogenetic Protein:The State of the Evidence of On-Label and Off-Label Use; Agency for Healthcare Research and Quality: Rockville, MD, 2010. [PubMed] [Google Scholar]
- (55).Kolar P; Gaber T; Perka C; Duda GN; Buttgereit F Human early fracture hematoma is characterized by inflammation and hypoxia. Clin. Orthop. Relat. Res 2011, 469 (11), 3118–3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Wang J; Glimcher M Characterization of matrix-induced osteogenesis in rat calvarial bone defects: II. Origins of bone-forming cells. Calcif. Tissue Int 1999, 65 (6), 486–493. [DOI] [PubMed] [Google Scholar]
- (57).Zhang X Intravital imaging to understand spatiotemporal regulation of osteogenesis and angiogenesis in cranial defect repair and regeneration. In Somatic Stem Cells; Springer, 2018; pp 229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Holstein J; Becker S; Fiedler M; Garcia P; Histing T; Klein M; Laschke M; Corsten M; Pohlemann T; Menger M Intravital microscopic studies of angiogenesis during bone defect healing in mice calvaria. Injury 2011, 42 (8), 765–771. [DOI] [PubMed] [Google Scholar]
- (59).Takano S; Uchida K; Miyagi M; Inoue G; Fujimaki H; Aikawa J; Iwase D; Minatani A; Iwabuchi K; Takaso M Nerve growth factor regulation by TNF-α and IL-1β in synovial macrophages and fibroblasts in osteoarthritic mice. J. Immunol. Res 2016, 2016, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Meyers CA; Lee S; Sono T; Xu J; Negri S; Tian Y; Wang Y; Li Z; Miller S; Chang L; et al. A neurotrophic mechanism directs sensory nerve transit in cranial bone. Cell Rep. 2020, 31 (8), 107696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Silva D The role of sensory nervous system in the regulation of bone formation, remodeling, and repair. Human Health and Pathology; Université de Bordeaux, 2017. [Google Scholar]
- (62).El Karim IA; Linden GJ; Irwin CR; Lundy FT Neuropeptides regulate expression of angiogenic growth factors in human dental pulp fibroblasts. Journal of endodontics 2009, 35 (6), 829–833. [DOI] [PubMed] [Google Scholar]
- (63).Aoki M; Tamai K; Saotome K Substance P-and calcitonin gene-related peptide-immunofluorescent nerves in the repair of experimental bone defects. International orthopaedics 1994, 18 (5), 317–324. [DOI] [PubMed] [Google Scholar]
- (64).Matsubara H; Hogan DE; Morgan EF; Mortlock DP; Einhorn TA; Gerstenfeld LC Vascular tissues are a primary source of BMP2 expression during bone formation induced by distraction osteogenesis. Bone 2012, 51 (1), 168–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Hu K; Olsen BR Vascular endothelial growth factor control mechanisms in skeletal growth and repair. Dev. Dyn 2017, 246 (4), 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Murray PE; Garcia-Godoy F; Hargreaves KM Regenerative Endodontics: A Review of Current Status and a Call for Action. Journal of Endodontics 2007, 33 (4), 377–390. [DOI] [PubMed] [Google Scholar]
- (67).Rombouts C; Giraud T; Jeanneau C; About I Pulp Vascularization during Tooth Development, Regeneration, and Therapy. J. Dent. Res 2017, 96 (2), 137–144. [DOI] [PubMed] [Google Scholar]
- (68).Caviedes-Bucheli J; Muñoz HR; Azuero-Holguín MM; Ulate E Neuropeptides in dental pulp: the silent protagonists. J. Endod 2008, 34 (7), 773–88. [DOI] [PubMed] [Google Scholar]
- (69).Diogenes A Trigeminal Sensory Neurons and Pulp Regeneration. Journal of Endodontics 2020, 46 (9), S71–S80. [DOI] [PubMed] [Google Scholar]
- (70).Morfis A; Sylaras SN; Georgopoulou M; Kernani M; Prountzos F Study of the apices of human permanent teeth with the use of a scanning electron microscope. Oral Surg., Oral Med., Oral Pathol 1994, 77 (2), 172–6. [DOI] [PubMed] [Google Scholar]
- (71).Aukhil I Biology of wound healing. Periodontology 2000 2000, 22 (1), 44–50. [DOI] [PubMed] [Google Scholar]
- (72).Tavelli L; Ravidá A; Barootchi S; Chambrone L; Giannobile WV Recombinant Human Platelet–Derived Growth Factor: A Systematic Review of Clinical Findings in Oral Regenerative Procedures. JDR Clinical & Translational Research 2021, 6 (2), 161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Del Fabbro M; Karanxha L; Panda S; Bucchi C; Nadathur Doraiswamy J; Sankari M; Ramamoorthi S; Varghese S; Taschieri S Autologous platelet concentrates for treating periodontal infrabony defects. Cochrane Database Syst. Rev 2018, 11, Cd011423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Youn SH; Sakuda M; Kurisu K; Wakisaka S Regeneration of periodontal primary afferents of the rat incisor following injury of the inferior alveolar nerve with special reference to neuropeptide Y-like immunoreactive primary afferents. Brain Res. 1997, 752 (1–2), 161–9. [PubMed] [Google Scholar]
- (75).Kvinnsland I; Heyeraas KJ Effect of traumatic occlusion on CGRP and SP immunoreactive nerve fibre morphology in rat molar pulp and periodontium. Histochemistry 1992, 97 (2), 111–20. [DOI] [PubMed] [Google Scholar]
- (76).Kato J; Wakisaka S; Kurisu K Immunohistochemical changes in the distribution of nerve fibers in the periodontal ligament during an experimental tooth movement of the rat molar. Cells Tissues Organs 1996, 157 (1), 53–62. [DOI] [PubMed] [Google Scholar]
- (77).Vandevska-Radunovic V; Kvinnsland S; Kvinnsland IH Effect of experimental tooth movement on nerve fibres immunoreactive to calcitonin gene-related peptide, protein gene product 9.5, and blood vessel density and distribution in rats. Eur. J. Orthod 1997, 19 (5), 517–29. [DOI] [PubMed] [Google Scholar]
- (78).Yamaguchi M; Nakajima R; Kasai K Mechanoreceptors, Nociceptors, and Orthodontic Tooth Movement. Seminars in Orthodontics 2012, 18 (4), 249–256. [Google Scholar]
- (79).Kondo M; Kondo H; Miyazawa K; Goto S; Togari A Experimental tooth movement-induced osteoclast activation is regulated by sympathetic signaling. Bone 2013, 52 (1), 39–47. [DOI] [PubMed] [Google Scholar]
- (80).Atsumi Y; Hayashi S; Nakakura-Ohshima K; Maeda T; Kurisu K; Wakisaka S Heterogeneous localizations of Trk B among individual periodontal Ruffini endings in the rat incisor. Arch. Histol. Cytol 1999, 62 (5), 435–40. [DOI] [PubMed] [Google Scholar]
- (81).Maeda T; Ochi K; Nakakura-Ohshima K; Youn SH; Wakisaka S The Ruffini ending as the primary mechanoreceptor in the periodontal ligament: its morphology, cytochemical features, regeneration, and development. Crit. Rev. Oral Biol. Med 1999, 10 (3), 307–27. [DOI] [PubMed] [Google Scholar]
- (82).Kurihara H; Shinohara H; Yoshino H; Takeda K; Shiba H Neurotrophins in cultured cells from periodontal tissues. J. Periodontol 2003, 74 (1), 76–84. [DOI] [PubMed] [Google Scholar]
- (83).Konishi A; Takeda K; Fujita T; Kajiya M; Matsuda S; Kittaka M; Shiba H; Kurihara H Sequential process in brain-derived neurotrophic factor-induced functional periodontal tissue regeneration. Eur. J. Oral Sci 2016, 124 (2), 141–150. [DOI] [PubMed] [Google Scholar]
- (84).Xiong J; Gronthos S; Bartold PM Role of the epithelial cell rests of Malassez in the development, maintenance and regeneration of periodontal ligament tissues. Periodontology 2000 2013, 63 (1), 217–233. [DOI] [PubMed] [Google Scholar]
- (85).Yamashiro T; Fujiyama K; Fukunaga T; Wang Y; Takano-Yamamoto T Epithelial rests of Malassez express immunoreactivity of TrkA and its distribution is regulated by sensory nerve innervation. J. Histochem. Cytochem 2000, 48 (7), 979–984. [DOI] [PubMed] [Google Scholar]
- (86).Fujiyama K; Yamashiro T; Fukunaga T; Balam TA; Zheng L; Takano-Yamamoto T Denervation Resulting in Dento-Alveolar Ankylosis Associated with Decreased Malassez Epithelium. J. Dent. Res 2004, 83 (8), 625–629. [DOI] [PubMed] [Google Scholar]
- (87).Nedvetsky PI; Emmerson E; Finley JK; Ettinger A; Cruz-Pacheco N; Prochazka J; Haddox CL; Northrup E; Hodges C; Mostov KE; Hoffman MP; Knox SM Parasympathetic Innervation Regulates Tubulogenesis in the Developing Salivary Gland. Dev. Cell 2014, 30 (4), 449–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Chatzeli L; Gaete M; Tucker AS Fgf10 and Sox9 are essential for the establishment of distal progenitor cells during mouse salivary gland development. Development 2017, 144 (12), 2294–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Emmerson E; May AJ; Nathan S; Cruz-Pacheco N; Lizama CO; Maliskova L; Zovein AC; Shen Y; Muench MO; Knox SM SOX2 regulates acinar cell development in the salivary gland. eLife 2017, 6, No. e26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Emmerson E; May AJ; Berthoin L; Cruz-Pacheco N; Nathan S; Mattingly AJ; Chang JL; Ryan WR; Tward AD; Knox SM Salivary glands regenerate after radiation injury through SOX2-mediated secretory cell replacement. EMBO Mol. Med 2018, 10 (3), e8051 DOI: 10.15252/emmm.201708051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Duan X; Bradbury SR; Olsen BR; Berendsen AD VEGF stimulates intramembranous bone formation during craniofacial skeletal development. Matrix Biol. 2016, 52, 127–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Kim J-M; Shin H-I; Cha S-S; Lee CS; Hong BS; Lim S; Jang H-J; Kim J; Yang YR; Kim Y-H; et al. DJ-1 promotes angiogenesis and osteogenesis by activating FGF receptor-1 signaling. Nat. Commun 2012, 3 (1), 1–11. [DOI] [PubMed] [Google Scholar]
- (93).Henmi A; Nakamura M; Echigo S; Sasano Y Involvement of sensory neurons in bone defect repair in rats. J. Electron Microsc 2011, 60 (6), 393–400. [DOI] [PubMed] [Google Scholar]
- (94).Jones RE; Salhotra A; Robertson KS; Ransom RC; Foster DS; Shah HN; Quarto N; Wan DC; Longaker MT Skeletal stem cell-schwann cell circuitry in mandibular repair. Cell Rep. 2019, 28 (11), 2757–2766 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Yamashiro T; Fujiyama K; Fujiyoshi Y; Inaguma N; Takano-Yamamoto T Inferior alveolar nerve transection inhibits increase in osteoclast appearance during experimental tooth movement. Bone 2000, 26 (6), 663–669. [DOI] [PubMed] [Google Scholar]
- (96).Lv L; Wang Y; Zhang J; Zhang T; Li S Healing of periodontal defects and calcitonin gene related peptide expression following inferior alveolar nerve transection in rats. J. Mol. Histol 2014, 45 (3), 311–20. [DOI] [PubMed] [Google Scholar]
- (97).Yu X; Lv L; Zhang J; Zhang T; Xiao C; Li S Expression of neuropeptides and bone remodeling-related factors during periodontal tissue regeneration in denervated rats. J. Mol. Histol 2015, 46 (2), 195–203. [DOI] [PubMed] [Google Scholar]
- (98).Chiego DJ Jr.; Klein RM; Avery JK; Gruhl IM Denervation-induced changes in cell proliferation in the rat molar after wounding. Anat. Rec 1986, 214 (4), 348–352. [DOI] [PubMed] [Google Scholar]
- (99).Byers MR; Taylor PE Effect of Sensory Denervation on the Response of Rat Molar Pulp to Exposure Injury. J. Dent. Res 1993, 72 (3), 613–618. [DOI] [PubMed] [Google Scholar]
- (100).Kim Y; Hamada N; Takahashi Y; Sasaguri K; Tsukinoki K; Onozuka M; Sato S Cervical sympathectomy causes alveolar bone loss in an experimental rat model. J. Periodontal Res 2009, 44 (6), 695–703. [DOI] [PubMed] [Google Scholar]
- (101).Knox SM; Lombaert IM; Haddox CL; Abrams SR; Cotrim A; Wilson AJ; Hoffman MP Parasympathetic stimulation improves epithelial organ regeneration. Nat. Commun 2013, 4, 1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Proctor GB; Carpenter GH Regulation of salivary gland function by autonomic nerves. Auton. Neurosci 2007, 133 (1), 3–18. [DOI] [PubMed] [Google Scholar]
- (103).Wang X; Li Z; Shao Q; Zhang C; Wang J; Han Z; Wang S; Qin L The intact parasympathetic nerve promotes submandibular gland regeneration through ductal cell proliferation. Cell Proliferation 2021, 54 (7), No. e13078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (104).Chen W-H; Mao C.-q.; Zhuo L.-l.; Ong JL Beta-nerve growth factor promotes neurogenesis and angiogenesis during the repair of bone defects. Neural Regener. Res 2015, 10 (7), 1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (105).Jin P; Yin F; Huang L; Zheng L; Zhao J; Zhang X Guangxi cobra venom-derived NGF promotes the osteogenic and therapeutic effects of porous BCP ceramic. Exp. Mol. Med 2017, 49 (4), No. e312. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- (106).Yan XZ; Ge SH; Sun QF; Guo HM; Yang PS A pilot study evaluating the effect of recombinant human bone morphogenetic protein-2 and recombinant human beta-nerve growth factor on the healing of Class III furcation defects in dogs. J. Periodontol 2010, 81 (9), 1289–98. [DOI] [PubMed] [Google Scholar]
- (107).Takeda K; Shiba H; Mizuno N; Hasegawa N; Mouri Y; Hirachi A; Yoshino H; Kawaguchi H; Kurihara H Brain-derived neurotrophic factor enhances periodontal tissue regeneration. Tissue Eng. 2005, 11 (9–10), 1618–29. [DOI] [PubMed] [Google Scholar]
- (108).Ramalho I; Bergamo E; Lopes A; Medina-Cintrón C; Neiva R; Witek L; Coelho P Periodontal Tissue Regeneration Using Brain-Derived Neurotrophic Factor Delivered by Collagen Sponge. Tissue Eng., Part A 2019, 25 (15–16), 1072–1083. [DOI] [PubMed] [Google Scholar]
- (109).Jimbo R; Tovar N; Janal MN; Mousa R; Marin C; Yoo D; Teixeira HS; Anchieta RB; Bonfante EA; Konishi A; Takeda K; Kurihara H; Coelho PG The Effect of Brain-Derived Neurotrophic Factor on Periodontal Furcation Defects. PLoS One 2014, 9 (1), No. e84845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (110).Jimbo R; Singer J; Tovar N; Marin C; Neiva R; Bonfante EA; Janal MN; Contamin H; Coelho PG Regeneration of the cementum and periodontal ligament using local BDNF delivery in class II furcation defects. J. Biomed. Mater. Res., Part B 2018, 106 (4), 1611–1617. [DOI] [PubMed] [Google Scholar]
- (111).Takeda K; Sakai N; Shiba H; Nagahara T; Fujita T; Kajiya M; Iwata T; Matsuda S; Kawahara K; Kawaguchi H; Kurihara H Characteristics of high-molecular-weight hyaluronic acid as a brain-derived neurotrophic factor scaffold in periodontal tissue regeneration. Tissue Eng., Part A 2011, 17 (7–8), 955–67. [DOI] [PubMed] [Google Scholar]
- (112).Ma W; Lyu H; Pandya M; Gopinathan G; Luan X; Diekwisch TGH Successful Application of a Galanin-Coated Scaffold for Periodontal Regeneration. J. Dent. Res 2021, 100 (10), 1144–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (113).Ferreira JNA; Zheng C; Lombaert IMA; Goldsmith CM; Cotrim AP; Symonds JM; Patel VN; Hoffman MP Neurturin Gene Therapy Protects Parasympathetic Function to Prevent Irradiation-Induced Murine Salivary Gland Hypofunction. Mol. Ther.–Methods Clin. Dev 2018, 9, 172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (114).Vining KH; Lombaert IMA; Patel VN; Kibbey SE; Pradhan-Bhatt S; Witt RL; Hoffman MP Neurturin-containing laminin matrices support innervated branching epithelium from adult epithelial salispheres. Biomaterials 2019, 216, 119245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (115).Losordo DW; Dimmeler S Therapeutic angiogenesis and vasculogenesis for ischemic disease. Part I: angiogenic cytokines. Circulation 2004, 109 (21), 2487–91. [DOI] [PubMed] [Google Scholar]
- (116).Papanas N; Maltezos E Growth factors in the treatment of diabetic foot ulcers: new technologies, any promises? Int. J. Lower Extremity Wounds 2007, 6 (1), 37–53. [DOI] [PubMed] [Google Scholar]
- (117).Yonamine Y; Matsuyama T; Sonomura T; Takeuchi H; Furuichi Y; Uemura M; Izumi Y; Noguchi K Effectable application of vascular endothelial growth factor to critical sized rat calvaria defects. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology 2010, 109 (2), 225–231. [DOI] [PubMed] [Google Scholar]
- (118).Peng H; Usas A; Olshanski A; Ho AM; Gearhart B; Cooper GM; Huard J VEGF improves, whereas sFlt1 inhibits, BMP2-induced bone formation and bone healing through modulation of angiogenesis. J. Bone Miner. Res 2005, 20 (11), 2017–2027. [DOI] [PubMed] [Google Scholar]
- (119).Mullane EM; Dong Z; Sedgley CM; Hu JCC; Botero TM; Holland GR; Nör JE Effects of VEGF and FGF2 on the Revascularization of Severed Human Dental Pulps. J. Dent. Res 2008, 87 (12), 1144–1148. [DOI] [PubMed] [Google Scholar]
- (120).Yadlapati M; Biguetti C; Cavalla F; Nieves F; Bessey C; Bohluli P; Garlet GP; Letra A; Fakhouri WD; Silva RM Characterization of a Vascular Endothelial Growth Factor–loaded Bioresorbable Delivery System for Pulp Regeneration. Journal of Endodontics 2017, 43 (1), 77–83. [DOI] [PubMed] [Google Scholar]
- (121).Nabeshima CK; Valdivia JE; Caballero-Flores H; Arana-Chavez VE; Machado MEDL Immunohistological study of the effect of vascular Endothelial Growth Factor on the angiogenesis of mature root canals in rat molars. J. Appl. Oral Sci 2018, 26, e20170437 DOI: 10.1590/1678-7757-2017-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (122).Li X; Ma C; Xie X; Sun H; Liu X Pulp regeneration in a full-length human tooth root using a hierarchical nanofibrous microsphere system. Acta Biomater. 2016, 35, 57–67. [DOI] [PubMed] [Google Scholar]
- (123).Sylven C; Hao XJ; Silva EA; Mansson-Broberg A; Grinnemo KH; Siddiqui AJ; Dellgren G; Wardell E; Brodin LA; Mooney DJ Angiogenic effects of sequential release of VEGF-A(165) and PDGF-BB with alginate hydrogels after myocardial infarction. Cardiovasc. Res 2007, 75 (1), 178–185. [DOI] [PubMed] [Google Scholar]
- (124).Mooney DJ; Chen RR; Silva EA; Yuen WW Spatiotemporal VEGF and PDGF delivery patterns blood vessel formation and maturation. Pharm. Res 2007, 24 (2), 258–264. [DOI] [PubMed] [Google Scholar]
- (125).Layman H; Sacasa M; Murphy AE; Murphy AM; Pham SM; Andreopoulos FM Co-delivery of FGF-2 and G-CSF from gelatin-based hydrogels as angiogenic therapy in a murine critical limb ischemic model. Acta Biomater. 2009, 5 (1), 230–239. [DOI] [PubMed] [Google Scholar]
- (126).Brudno Y; Ennett-Shepard AB; Chen RR; Aizenberg M; Mooney DJ Enhancing microvascular formation and vessel maturation through temporal control over multiple pro-angiogenic and pro-maturation factors. Biomaterials 2013, 34 (36), 9201–9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (127).Patel ZS; Young S; Tabata Y; Jansen JA; Wong ME; Mikos AG Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone 2008, 43 (5), 931–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (128).Liu K; Meng C-X; Lv Z-Y; Zhang Y-J; Li J; Li K-Y; Liu F-Z; Zhang B; Cui F-Z Enhancement of BMP-2 and VEGF carried by mineralized collagen for mandibular bone regeneration. Regenerative Biomaterials 2020, 7 (4), 435–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (129).Kim JY; Xin X; Moioli EK; Chung J; Lee CH; Chen M; Fu SY; Koch PD; Mao JJ Regeneration of dental-pulp-like tissue by chemotaxis-induced cell homing. Tissue Eng., Part A 2010, 16 (10), 3023–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (130).Behr B; Leucht P; Longaker MT; Quarto N Fgf-9 is required for angiogenesis and osteogenesis in long bone repair. Proc. Natl. Acad. Sci. U. S. A 2010, 107 (26), 11853–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (131).Huang JY; Lynn Miskus M; Lu HC FGF-FGFR Mediates the Activity-Dependent Dendritogenesis of Layer IV Neurons during Barrel Formation. J. Neurosci 2017, 37 (50), 12094–12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (132).Nam K; Dean SM; Brown CT; Smith RJ Jr.; Lei P; Andreadis ST; Baker OJ Synergistic effects of laminin-1 peptides, VEGF and FGF9 on salivary gland regeneration. Acta Biomater. 2019, 91, 186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (133).Laham RJ; Rezaee M; Post M; Sellke FW; Braeckman RA; Hung D; Simons M Intracoronary and intravenous administration of basic fibroblast growth factor: Myocardial and tissue distribution. Drug Metab. Dispos 1999, 27 (7), 821–826. [PubMed] [Google Scholar]
- (134).Ozawa CR; Banfi A; Glazer NL; Thurston G; Springer ML; Kraft PE; McDonald DM; Blau HM Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J. Clin. Invest 2004, 113 (4), 516–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (135).Silva EA; Mooney DJ Spatiotemporal control of vascular endothelial growth factor delivery from injectable hydrogels enhances angiogenesis. J. Thromb. Haemostasis 2007, 5 (3), 590–598. [DOI] [PubMed] [Google Scholar]
- (136).Liu Y; Chan JK; Teoh SH Review of vascularised bone tissue-engineering strategies with a focus on co-culture systems. J. Tissue Eng. Regener. Med 2015, 9 (2), 85–105. [DOI] [PubMed] [Google Scholar]
- (137).Lehninger AL; Nelson DL; Cox MM Lehninger principles of biochemistry, 3rd ed.; Worth Publishers: New York, 2000. [Google Scholar]
- (138).Finetti F; Basile A; Capasso D; Di Gaetano S; Di Stasi R; Pascale M; Turco CM; Ziche M; Morbidelli L; D’Andrea LD Functional and pharmacological characterization of a VEGF mimetic peptide on reparative angiogenesis. Biochem. Pharmacol 2012, 84 (3), 303–311. [DOI] [PubMed] [Google Scholar]
- (139).Shi L; Feng L; Zhu M-L; Yang Z-M; Wu T-Y; Xu J; Liu Y; Lin W-P; Lo JHT; Zhang J-F; et al. Vasoactive intestinal peptide stimulates bone marrow-mesenchymal stem cells osteogenesis differentiation by activating Wnt/β-catenin signaling pathway and promotes rat skull defect repair. Stem Cells Dev. 2020, 29 (10), 655–666. [DOI] [PubMed] [Google Scholar]
- (140).Siddiqui Z; Sarkar B; Kim KK; Kadincesme N; Paul R; Kumar A; Kobayashi Y; Roy A; Choudhury M; Yang J; Shimizu E; Kumar VA Angiogenic hydrogels for dental pulp revascularization. Acta Biomater. 2021, 126, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (141).Shang L; Liu Z; Ma B; Shao J; Wang B; Ma C; Ge S Dimethyloxallyl glycine/nanosilicates-loaded osteogenic/angiogenic difunctional fibrous structure for functional periodontal tissue regeneration. Bioactive Materials 2021, 6 (4), 1175–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (142).Chen YC; Lin RZ; Qi H; Yang Y; Bae H; Melero-Martin JM; Khademhosseini A Functional Human Vascular Network Generated in Photocrosslinkable Gelatin Methacrylate Hydrogels. Adv. Funct. Mater 2012, 22 (10), 2027–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (143).Chwalek K; Tsurkan MV; Freudenberg U; Werner C Glycosaminoglycan-based hydrogels to modulate heterocellular communication in in vitro angiogenesis models. Sci. Rep 2015, 4, 4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (144).Culver JC; Hoffmann JC; Poche RA; Slater JH; West JL; Dickinson ME Three-dimensional biomimetic patterning in hydrogels to guide cellular organization. Adv. Mater 2012, 24 (17), 2344–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (145).Kusuma S; Shen YI; Hanjaya-Putra D; Mali P; Cheng L; Gerecht S Self-organized vascular networks from human pluripotent stem cells in a synthetic matrix. Proc. Natl. Acad. Sci. U. S. A 2013, 110 (31), 12601–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (146).Leslie-Barbick JE; Moon JJ; West JL Covalently-immobilized vascular endothelial growth factor promotes endothelial cell tubulogenesis in poly(ethylene glycol) diacrylate hydrogels. J. Biomater. Sci., Polym. Ed 2009, 20 (12), 1763–79. [DOI] [PubMed] [Google Scholar]
- (147).Moon JJ; Saik JE; Poche RA; Leslie-Barbick JE; Lee SH; Smith AA; Dickinson ME; West JL Biomimetic hydrogels with pro-angiogenic properties. Biomaterials 2010, 31 (14), 3840–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (148).Nguyen EH; Zanotelli MR; Schwartz MP; Murphy WL Differential effects of cell adhesion, modulus and VEGFR-2 inhibition on capillary network formation in synthetic hydrogel arrays. Biomaterials 2014, 35 (7), 2149–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (149).Turturro MV; Christenson MC; Larson JC; Young DA; Brey EM; Papavasiliou G MMP-sensitive PEG diacrylate hydrogels with spatial variations in matrix properties stimulate directional vascular sprout formation. PLoS One 2013, 8 (3), No. e58897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (150).Zisch AH; Lutolf MP; Ehrbar M; Raeber GP; Rizzi SC; Davies N; Schmokel H; Bezuidenhout D; Djonov V; Zilla P; Hubbell JA Cell-demanded release of VEGF from synthetic, biointeractive cell ingrowth matrices for vascularized tissue growth. FASEB J. 2003, 17 (15), 2260–2. [DOI] [PubMed] [Google Scholar]
- (151).Katata C; Sasaki JI; Li A; Abe GL; Nör JE; Hayashi M; Imazato S Fabrication of Vascularized DPSC Constructs for Efficient Pulp Regeneration. J. Dent. Res 2021, 100, 1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (152).Zhang D; Gao P; Li Q; Li J; Li X; Liu X; Kang Y; Ren L Engineering biomimetic periosteum with β-TCP scaffolds to promote bone formation in calvarial defects of rats. Stem Cell Res. Ther 2017, 8 (1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (153).Koike N; Fukumura D; Gralla O; Au P; Schechner JS; Jain RK Tissue engineering: creation of long-lasting blood vessels. Nature 2004, 428 (6979), 138–9. [DOI] [PubMed] [Google Scholar]
- (154).Zanotelli MR; Ardalani H; Zhang J; Hou Z; Nguyen EH; Swanson S; Nguyen BK; Bolin J; Elwell A; Bischel LL; Xie AW; Stewart R; Beebe DJ; Thomson JA; Schwartz MP; Murphy WL Stable engineered vascular networks from human induced pluripotent stem cell-derived endothelial cells cultured in synthetic hydrogels. Acta Biomater. 2016, 35, 32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (155).Roux BM; Akar B; Zhou W; Stojkova K; Barrera B; Brankov J; Brey EM Preformed vascular networks survive and enhance vascularization in critical sized cranial defects. Tissue Eng. Part A 2018, 24 (21–22), 1603–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (156).Das S; Browne KD; Laimo FA; Maggiore JC; Kaisaier H; Aguilar CA; Ali ZS; Moukioti F; Cullen DK, Pre-innervated Tissue Engineered Muscle Promotes a Pro-Regenerative Microenvironment Following Volumetric Muscle Loss. bioRxIV 2019. DOI: 10.1101/840124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (157).Wu Y; Jing D; Ouyang H; Li L; Zhai M; Li Y; Bi L; Guoxian P Pre-implanted sensory nerve could enhance the neurotization in tissue-engineered bone graft. Tissue Eng., Part A 2015, 21 (15–16), 2241–2249. [DOI] [PubMed] [Google Scholar]
- (158).Marrella A; Lee TY; Lee DH; Karuthedom S; Syla D; Chawla A; Khademhosseini A; Jang HL Engineering vascularized and innervated bone biomaterials for improved skeletal tissue regeneration. Mater. Today 2018, 21 (4), 362–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (159).García JR; Clark AY; García AJ Integrin-specific hydrogels functionalized with VEGF for vascularization and bone regeneration of critical-size bone defects. J. Biomed. Mater. Res., Part A 2016, 104 (4), 889–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (160).Leach JK; Kaigler D; Wang Z; Krebsbach PH; Mooney DJ Coating of VEGF-releasing scaffolds with bioactive glass for angiogenesis and bone regeneration. Biomaterials 2006, 27 (17), 3249–3255. [DOI] [PubMed] [Google Scholar]
- (161).Kaigler D; Wang Z; Horger K; Mooney DJ; Krebsbach PH VEGF scaffolds enhance angiogenesis and bone regeneration in irradiated osseous defects. J. Bone Miner. Res 2006, 21 (5), 735–744. [DOI] [PubMed] [Google Scholar]
- (162).Chen SY; Qin JJ; Wang L; Mu TW; Jin D; Jiang S; Zhao PR; Pei GX Different effects of implanting vascular bundles and sensory nerve tracts on the expression of neuropeptide receptors in tissue-engineered bone in vivo. Biomed. Mater 2010, 5 (5), 055002. [DOI] [PubMed] [Google Scholar]
- (163).Cui JD; Pei GX; Jiang S [A study of the different effect on the expression of calcitonin gene related peptide and neuropeptide Y in tissue engineered bone with vascular bundle graft in vivo and that with sensory nerve tract graft in vivo]. Zhonghua Wai Ke Za Zhi 2008, 46 (16), 1249–52. [PubMed] [Google Scholar]
- (164).Feng L; Lingling E; Liu H The effects of separating inferior alveolar neurovascular bundles on osteogenesis of tissue-engineered bone and vascularization. Biomed. Pap 2015, 159 (4), 637–41. [DOI] [PubMed] [Google Scholar]
- (165).Wu Y; Jing D; Ouyang H; Li L; Zhai M; Li Y; Bi L; Guoxian P Pre-implanted Sensory Nerve Could Enhance the Neurotization in Tissue-Engineered Bone Graft. Tissue Eng., Part A 2015, 21 (15–16), 2241–9. [DOI] [PubMed] [Google Scholar]
- (166).Gu Y; Zhu J; Xue C; Li Z; Ding F; Yang Y; Gu X Chitosan/silk fibroin-based, Schwann cell-derived extracellular matrix-modified scaffolds for bridging rat sciatic nerve gaps. Biomaterials 2014, 35 (7), 2253–2263. [DOI] [PubMed] [Google Scholar]
- (167).Freudenberg U; Hermann A; Welzel PB; Stirl K; Schwarz SC; Grimmer M; Zieris A; Panyanuwat W; Zschoche S; Meinhold D; et al. A star-PEG–heparin hydrogel platform to aid cell replacement therapies for neurodegenerative diseases. Biomaterials 2009, 30 (28), 5049–5060. [DOI] [PubMed] [Google Scholar]
- (168).Hsieh S-C; Chang C-J; Cheng W-T; Tseng T-C; Hsu S.-h. Effect of an epineurial-like biohybrid nerve conduit on nerve regeneration. Cell Transplant 2016, 25 (3), 559–574. [DOI] [PubMed] [Google Scholar]
- (169).Johnson BN; Lancaster KZ; Zhen G; He J; Gupta MK; Kong YL; Engel EA; Krick KD; Ju A; Meng F; et al. 3D printed anatomical nerve regeneration pathways. Adv. Funct. Mater 2015, 25 (39), 6205–6217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (170).García JR; García AJ Biomaterial-mediated strategies targeting vascularization for bone repair. Drug Delivery Transl. Res 2016, 6 (2), 77–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (171).Archibald SJ; Krarup C; Shefner J; Li ST; Madison RD A collagen-based nerve guide conduit for peripheral nerve repair: An electrophysiological study of nerve regeneration in rodents and nonhuman primates. J. Comp. Neurol 1991, 306 (4), 685–696. [DOI] [PubMed] [Google Scholar]
- (172).Guan S; Zhang X-L; Lin X-M; Liu T-Q; Ma X-H; Cui Z-F Chitosan/gelatin porous scaffolds containing hyaluronic acid and heparan sulfate for neural tissue engineering. J. Biomater. Sci., Polym. Ed 2013, 24 (8), 999–1014. [DOI] [PubMed] [Google Scholar]
- (173).Hanjaya-Putra D; Yee J; Ceci D; Truitt R; Yee D; Gerecht S Vascular endothelial growth factor and substrate mechanics regulate in vitro tubulogenesis of endothelial progenitor cells. Journal of cellular and molecular medicine 2010, 14 (10), 2436–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (174).Rosso G; Young P; Shahin V Mechanosensitivity of embryonic neurites promotes their directional extension and Schwann cells progenitors migration. Cell. Physiol. Biochem 2017, 44 (4), 1263–1270. [DOI] [PubMed] [Google Scholar]
- (175).Xu Y; Zhang Z; Chen X; Li R; Li D; Feng S A silk fibroin/collagen nerve scaffold seeded with a co-culture of Schwann cells and adipose-derived stem cells for sciatic nerve regeneration. PLoS One 2016, 11 (1), No. e0147184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (176).Arslantunali D; Dursun T; Yucel D; Hasirci N; Hasirci V Peripheral nerve conduits: technology update. Med. Devices: Evidence Res 2014, 7, 405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (177).Zaidi M; Alam A; Bax B; Shankar V; Bax C; Gill J; Pazianas M; Huang C-H; Sahinoglu T; Moonga B; et al. Role of the endothelial cell in osteoclast control: new perspectives. Bone 1993, 14 (2), 97–102. [DOI] [PubMed] [Google Scholar]
- (178).Lamalice L; Le Boeuf F; Huot J Endothelial cell migration during angiogenesis. Circ. Res 2007, 100 (6), 782–794. [DOI] [PubMed] [Google Scholar]
- (179).Evans GR; Brandt K; Katz S; Chauvin P; Otto L; Bogle M; Wang B; Meszlenyi RK; Lu L; Mikos AG; et al. Bioactive poly (L-lactic acid) conduits seeded with Schwann cells for peripheral nerve regeneration. Biomaterials 2002, 23 (3), 841–848. [DOI] [PubMed] [Google Scholar]
- (180).Schlosshauer B; Müller E; Schröder B; Planck H; Müller H-W Rat Schwann cells in bioresorbable nerve guides to promote and accelerate axonal regeneration. Brain Res. 2003, 963 (1–2), 321–326. [DOI] [PubMed] [Google Scholar]
- (181).Rajaram A 3D Bioprinted hydrogel scaffolds laden with schwann cells for use as nerve repair conduits, Thesis, University of Saskatchewan, 2015. [Google Scholar]
- (182).Santos MI; Tuzlakoglu K; Fuchs S; Gomes ME; Peters K; Unger RE; Piskin E; Reis RL; Kirkpatrick CJ Endothelial cell colonization and angiogenic potential of combined nano-and microfibrous scaffolds for bone tissue engineering. Biomaterials 2008, 29 (32), 4306–4313. [DOI] [PubMed] [Google Scholar]
- (183).Yu H; VandeVord PJ; Mao L; Matthew HW; Wooley PH; Yang S-Y Improved tissue-engineered bone regeneration by endothelial cell mediated vascularization. Biomaterials 2009, 30 (4), 508–517. [DOI] [PubMed] [Google Scholar]
- (184).Fedorovich NE; Haverslag RT; Dhert WJ; Alblas J The role of endothelial progenitor cells in prevascularized bone tissue engineering: development of heterogeneous constructs. Tissue Eng., Part A 2010, 16 (7), 2355–2367. [DOI] [PubMed] [Google Scholar]
- (185).Zhou LN; Wang JC; Zilundu PLM; Wang YQ; Guo WP; Zhang SX; Luo H; Zhou JH; Deng RD; Chen DF A comparison of the use of adipose-derived and bone marrow-derived stem cells for peripheral nerve regeneration in vitro and in vivo. Stem Cell Res. Ther 2020, 11, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (186).Yang E.-z.; Zhang G.-w.; Xu J.-g.; Chen S; Wang H; Cao L.-l.; Liang B; Lian X.-f. Multichannel polymer scaffold seeded with activated Schwann cells and bone mesenchymal stem cells improves axonal regeneration and functional recovery after rat spinal cord injury. Acta Pharmacol. Sin 2017, 38 (5), 623–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (187).Kaigler D; Krebsbach PH; West ER; Horger K; Huang Y-C; Mooney DJ Endothelial cell modulation of bone marrow stromal cell osteogenic potential. FASEB J. 2005, 19 (6), 1–26. [DOI] [PubMed] [Google Scholar]
- (188).Tsigkou O; Pomerantseva I; Spencer JA; Redondo PA; Hart AR; O’Doherty E; Lin Y; Friedrich CC; Daheron L; Lin CP; et al. Engineered vascularized bone grafts. Proc. Natl. Acad. Sci. U. S. A 2010, 107 (8), 3311–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (189).Mercado-Pagán ÁE; Kang Y; Park S; Yao J; Bishop J; Yang YP; et al. Synthesis and characterization of novel elastomeric poly (D, L-lactide urethane) maleate composites for bone tissue engineering. Eur. Polym. J 2013, 49 (10), 3337–3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (190).Kang Y; Ren L; Yang Y Engineering vascularized bone grafts by integrating a biomimetic periosteum and β-TCP scaffold. ACS Appl Mater. Interfaces 2014, 6 (12), 9622–9633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (191).Khayat A; Monteiro N; Smith EE; Pagni S; Zhang W; Khademhosseini A; Yelick PC GelMA-Encapsulated hDPSCs and HUVECs for Dental Pulp Regeneration. J. Dent. Res 2017, 96 (2), 192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (192).Dissanayaka WL; Zhu L; Hargreaves KM; Jin L; Zhang C Scaffold-free Prevascularized Microtissue Spheroids for Pulp Regeneration. J. Dent. Res 2014, 93 (12), 1296–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (193).Kingham PJ; Kalbermatten DF; Mahay D; Armstrong SJ; Wiberg M; Terenghi G Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp. Neurol 2007, 207 (2), 267–274. [DOI] [PubMed] [Google Scholar]
- (194).Wei Y; Gong K; Zheng Z; Liu L; Wang A; Zhang L; Ao Q; Gong Y; Zhang X Schwann-like cell differentiation of rat adipose-derived stem cells by indirect co-culture with Schwann cells in vitro. Cell Proliferation 2010, 43 (6), 606–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (195).Khaki M; Salmanian AH; Abtahi H; Ganji A; Mosayebi G Mesenchymal stem cells differentiate to endothelial cells using recombinant vascular endothelial growth factor–A. Rep. Biochem. Mol. Biol 2018, 6 (2), 144. [PMC free article] [PubMed] [Google Scholar]
- (196).Shang T; Li S; Zhang Y; Lu L; Cui L; Guo FF Hypoxia promotes differentiation of adipose-derived stem cells into endothelial cells through demethylation of ephrinB2. Stem Cell Res. Ther 2019, 10 (1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (197).Aguirre A; Planell J; Engel E Dynamics of bone marrow–derived endothelial progenitor cell/mesenchymal stem cell interaction in co-culture and its implications in angiogenesis. Biochem. Biophys. Res. Commun 2010, 400 (2), 284–291. [DOI] [PubMed] [Google Scholar]
- (198).Au P; Tam J; Fukumura D; Jain RK Bone marrow–derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood 2008, 111 (9), 4551–4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (199).Dimitriou R; Tsiridis E; Giannoudis PV Current concepts of molecular aspects of bone healing. Injury 2005, 36 (12), 1392–1404. [DOI] [PubMed] [Google Scholar]
- (200).Huang NF; Li S Mesenchymal stem cells for vascular regeneration. Regener. Med 2008, 3, 877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (201).Khayat A; Monteiro N; Smith E; Pagni S; Zhang W; Khademhosseini A; Yelick P GelMA-encapsulated hDPSCs and HUVECs for dental pulp regeneration. J. Dent. Res 2017, 96 (2), 192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (202).Lin W; Chen M; Hu C; Qin S; Chu C; Xiang L; Man Y; Qu Y Endowing ipsc-derived mscs with angiogenic and keratinogenic differentiation potential: A promising cell source for skin tissue engineering. BioMed Res. Int 2018, 2018, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (203).Lo B; Parham L Ethical issues in stem cell research. Endocr. Rev 2009, 30 (3), 204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (204).Lai W-H; Ho JC; Chan Y-C; Ng JH; Au K-W; Wong L-Y; Siu C-W; Tse H-F Attenuation of hind-limb ischemia in mice with endothelial-like cells derived from different sources of human stem cells. PLoS One 2013, 8 (3), No. e57876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (205).Faiz M; Nagy A Induced pluripotent stem cells and disorders of the nervous system: progress, problems, and prospects. Neuroscientist 2013, 19 (6), 567–577. [DOI] [PubMed] [Google Scholar]
- (206).Park TS; Bhutto I; Zimmerlin L; Huo JS; Nagaria P; Miller D; Rufaihah AJ; Talbot C; Aguilar J; Grebe R; et al. Vascular progenitors from cord blood-derived iPSC possess augmented capacity for regenerating ischemic retinal vasculature. Circulation 2014, 129 (3), 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (207).Kim KL; Song S-H; Choi K-S; Suh W Cooperation of endothelial and smooth muscle cells derived from human induced pluripotent stem cells enhances neovascularization in dermal wounds. Tissue Eng., Part A 2013, 19 (21–22), 2478–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (208).Huang C-W; Huang W-C; Qiu X; Da Silva FFF; Wang A; Patel S; Nesti LJ; Poo M-M; Li S The differentiation stage of transplanted stem cells modulates nerve regeneration. Sci. Rep 2017, 7 (1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (209).Csobonyeiova M; Polak S; Zamborsky R; Danisovic L iPS cell technologies and their prospect for bone regeneration and disease modeling: a mini review. Journal of advanced research 2017, 8 (4), 321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (210).Xu X; Liao L; Tian W Strategies of Prevascularization in Tissue Engineering and Regeneration of Craniofacial Tissues. Tissue Eng., Part B 2021. DOI: 10.1089/ten.teb.2021.0004 [DOI] [PubMed] [Google Scholar]
- (211).Radwan IA; Rady D; Abbass M; El Moshy S; AbuBakr N; Dorfer CE; Fawzy El-Sayed KM Induced pluripotent stem cells in dental and nondental tissue regeneration: a review of an unexploited potential. Stem Cells Int. 2020, 2020, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (212).Wang S; Cai L Polymers for fabricating nerve conduits. Int. J. Polym. Sci 2010, 2010, 1. [Google Scholar]
- (213).Das KK; Srivastava AK Nerve conduits as replacements of autografts in peripheral nerve surgery: Still a work in progress. Neurol. India 2019, 67 (7), 115. [DOI] [PubMed] [Google Scholar]
- (214).Heath CA; Rutkowski GE The development of bioartificial nerve grafts for peripheral-nerve regeneration. Trends Biotechnol. 1998, 16 (4), 163–168. [DOI] [PubMed] [Google Scholar]
- (215).Bellamkonda RV Peripheral nerve regeneration: an opinion on channels, scaffolds and anisotropy. Biomaterials 2006, 27 (19), 3515–3518. [DOI] [PubMed] [Google Scholar]
- (216).Evans GR Challenges to nerve regeneration, Semin Surg Oncol; Wiley Online Library, 2000; pp 312–318. [DOI] [PubMed] [Google Scholar]
- (217).Yao Y; Cui Y; Zhao Y; Xiao Z; Li X; Han S; Chen B; Fang Y; Wang P; Pan J; et al. Efect of longitudinally oriented collagen conduit combined with nerve growth factor on nerve regeneration after dog sciatic nerve injury. J. Biomed. Mater. Res., Part B 2018, 106 (6), 2131–2139. [DOI] [PubMed] [Google Scholar]
- (218).di Summa PG; Kalbermatten DF; Pralong E; Raffoul W; Kingham PJ; Terenghi G Long-term in vivo regeneration of peripheral nerves through bioengineered nerve grafts. Neuroscience 2011, 181, 278–291. [DOI] [PubMed] [Google Scholar]
- (219).Ceballos D; Navarro X; Dubey N; Wendelschafer-Crabb G; Kennedy WR; Tranquillo RT Magnetically aligned collagen gel filling a collagen nerve guide improves peripheral nerve regeneration. Exp. Neurol 1999, 158 (2), 290–300. [DOI] [PubMed] [Google Scholar]
- (220).Gonzalez-Perez F; Cobianchi S; Heimann C; Phillips JB; Udina E; Navarro X Stabilization, rolling, and addition of other extracellular matrix proteins to collagen hydrogels improve regeneration in chitosan guides for long peripheral nerve gaps in rats. Neurosurgery 2017, 80 (3), 465–474. [DOI] [PubMed] [Google Scholar]
- (221).Alhosseini SN; Moztarzadeh F; Mozafari M; Asgari S; Dodel M; Samadikuchaksaraei A; Kargozar S; Jalali N Synthesis and characterization of electrospun polyvinyl alcohol nanofibrous scaffolds modified by blending with chitosan for neural tissue engineering. Int. J. Nanomed 2012, 7, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (222).Gonzalez-Perez F; Cobianchi S; Geuna S; Barwig C; Freier T; Udina E; Navarro X Tubulization with chitosan guides for the repair of long gap peripheral nerve injury in the rat. Microsurg 2015, 35 (4), 300–308. [DOI] [PubMed] [Google Scholar]
- (223).Malafaya PB; Santos TC; van Griensven M; Reis RL Morphology, mechanical characterization and in vivo neovascularization of chitosan particle aggregated scaffolds architectures. Biomaterials 2008, 29 (29), 3914–3926. [DOI] [PubMed] [Google Scholar]
- (224).Deng W-S; Ma K; Liang B; Liu X-Y; Xu H-Y; Zhang J; Shi H-Y; Sun H-T; Chen X-Y; Zhang S Collagen scaffold combined with human umbilical cord-mesenchymal stem cells transplantation for acute complete spinal cord injury. Neural Regener. Res 2020, 15 (9), 1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (225).Shen YH; Shoichet MS; Radisic M Vascular endothelial growth factor immobilized in collagen scaffold promotes penetration and proliferation of endothelial cells. Acta Biomater. 2008, 4 (3), 477–489. [DOI] [PubMed] [Google Scholar]
- (226).Moore AM; Kasukurthi R; Magill CK; Farhadi HF; Borschel GH; Mackinnon SE Limitations of conduits in peripheral nerve repairs. Hand 2009, 4 (2), 180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (227).Wang X; Hu W; Cao Y; Yao J; Wu J; Gu X Dog sciatic nerve regeneration across a 30-mm defect bridged by a chitosan/PGA artificial nerve graft. Brain 2005, 128 (8), 1897–1910. [DOI] [PubMed] [Google Scholar]
- (228).Matsumoto K; Ohnishi K; Kiyotani T; Sekine T; Ueda H; Nakamura T; Endo K; Shimizu Y Peripheral nerve regeneration across an 80-mm gap bridged by a polyglycolic acid (PGA)–collagen tube filled with laminin-coated collagen fibers: a histological and electrophysiological evaluation of regenerated nerves. Brain Res. 2000, 868 (2), 315–328. [DOI] [PubMed] [Google Scholar]
- (229).Farzamfar S; Esmailpour F; Rahmati M; Vaez A; Mirzaii M; Garmabi B; Shayannia A; Ebrahimi E; Vahedi H; Salehi M Poly-lactic acid/gelatin nanofiber (PLA/GTNF) conduits containing platelet-rich plasma for peripheral nerve regeneration. International Journal of Health Studies 2017, 3 (2). DOI: 10.22100/IJHS.V3I2.236 [DOI] [Google Scholar]
- (230).Bini T; Gao S; Xu X; Wang S; Ramakrishna S; Leong KW Peripheral nerve regeneration by microbraided poly (L-lactide-co-glycolide) biodegradable polymer fibers. J. Biomed. Mater. Res 2004, 68 (2), 286–295. [DOI] [PubMed] [Google Scholar]
- (231).Luis AL; Rodrigues JM; Amado S; Veloso AP; Armada-Da-Silva PA; Raimondo S; Fregnan F; Ferreira AJ; Lopes MA; Santos JD; et al. PLGA 90/10 and caprolactone biodegradable nerve guides for the reconstruction of the rat sciatic nerve. Microsurgery: Official Journal of the International Microsurgical Society and the European Federation of Societies for Microsurgery 2007, 27 (2), 125–137. [DOI] [PubMed] [Google Scholar]
- (232).Oh SH; Kim JH; Song KS; Jeon BH; Yoon JH; Seo TB; Namgung U; Lee IW; Lee JH Peripheral nerve regeneration within an asymmetrically porous PLGA/Pluronic F127 nerve guide conduit. Biomaterials 2008, 29 (11), 1601–1609. [DOI] [PubMed] [Google Scholar]
- (233).Li Y; Yu Z; Men Y; Chen X; Wang B Laminin-chitosan-PLGA conduit co-transplanted with Schwann and neural stem cells to repair the injured recurrent laryngeal nerve. Exp. Ther. Med 2018, 16 (2), 1250–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (234).Peng S-W; Li C-W; Chiu M; Wang G-J Nerve guidance conduit with a hybrid structure of a PLGA microfibrous bundle wrapped in a micro/nanostructured membrane. Int. J. Nanomed 2017, 12, 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (235).Sundback CA; Shyu JY; Wang Y; Faquin WC; Langer RS; Vacanti JP; Hadlock TA Biocompatibility analysis of poly (glycerol sebacate) as a nerve guide material. Biomaterials 2005, 26 (27), 5454–5464. [DOI] [PubMed] [Google Scholar]
- (236).Vijayavenkataraman S; Kannan S; Cao T; Fuh JYH; Sriram G; Lu WF 3D-printed PCL/PPy conductive scaffolds as three-dimensional porous nerve guide conduits (ngcs) for peripheral nerve injury repair. Front. Bioeng. Biotechnol 2019, 7, 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (237).Frost HK; Andersson T; Johansson S; Englund-Johansson U; Ekström P; Dahlin LB; Johansson F Electrospun nerve guide conduits have the potential to bridge peripheral nerve injuries in vivo. Sci. Rep 2018, 8 (1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (238).Yu W; Zhao W; Zhu C; Zhang X; Ye D; Zhang W; Zhou Y; Jiang X; Zhang Z Sciatic nerve regeneration in rats by a promising electrospun collagen/poly (ε-caprolactone) nerve conduit with tailored degradation rate. BMC Neurosci. 2011, 12 (1), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (239).Pinho AC; Fonseca AC; Serra AC; Santos JD; Coelho JF Peripheral nerve regeneration: current status and new strategies using polymeric materials. Adv. Healthcare Mater 2016, 5 (21), 2732–2744. [DOI] [PubMed] [Google Scholar]
- (240).Lin C-C; Anseth KS PEG hydrogels for the controlled release of biomolecules in regenerative medicine. Pharm. Res 2009, 26 (3), 631–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (241).Wangensteen KJ; Kalliainen LK Collagen tube conduits in peripheral nerve repair: a retrospective analysis. Hand 2010, 5 (3), 273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (242).Bozkurt A; Claeys KG; Schrading S; Rodler JV; Altinova H; Schulz JB; Weis J; Pallua N; van Neerven SG Clinical and biometrical 12-month follow-up in patients after reconstruction of the sural nerve biopsy defect by the collagen-based nerve guide Neuromaix. Eur. J. Med.Res 2017, 22 (1), 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (243).Du J; Chen H; Qing L; Yang X; Jia X Biomimetic neural scaffolds: a crucial step towards optimal peripheral nerve regeneration. Biomater. Sci 2018, 6 (6), 1299–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (244).Park E-J; Kim E-S; Weber H-P; Wright RF; Mooney DJ Improved bone healing by angiogenic factor-enriched platelet-rich plasma and its synergistic enhancement by bone morphogenetic protein-2. Int. J. Oral Maxillofac. Implants 2008, 23 (5), 818. [PMC free article] [PubMed] [Google Scholar]
- (245).Sekiya N; Ichioka S; Terada D; Tsuchiya S; Kobayashi H Efficacy of a poly glycolic acid (PGA)/collagen composite nanofibre scaffold on cell migration and neovascularisation in vivo skin defect model. Journal of plastic surgery and hand surgery 2013, 47 (6), 498–502. [DOI] [PubMed] [Google Scholar]
- (246).Wernike E; Montjovent M-O; Liu Y; Wismeijer D; Hunziker EB; Siebenrock K-A; Hofstetter W; Klenke FM VEGF incorporated into calcium phosphate ceramics promotes vascularisation and bone formation in vivo. Eur. Cell Mater 2010, 19 (3), 30. [DOI] [PubMed] [Google Scholar]
- (247).Kampmann A; Lindhorst D; Schumann P; Zimmerer R; Kokemüller H; Rücker M; Gellrich N-C; Tavassol F Additive effect of mesenchymal stem cells and VEGF to vascularization of PLGA scaffolds. Microvasc. Res 2013, 90, 71–79. [DOI] [PubMed] [Google Scholar]
- (248).Kempen DH; Lu L; Heijink A; Hefferan TE; Creemers LB; Maran A; Yaszemski MJ; Dhert WJ Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials 2009, 30 (14), 2816–2825. [DOI] [PubMed] [Google Scholar]
- (249).Zhang Y; Yu T; Peng L; Sun Q; Wei Y; Han B Advancements in hydrogel-based drug sustained release systems for bone tissue engineering. Front. Pharmacol 2020, 11, 622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (250).Van Hove AH; Burke K; Antonienko E; Brown III E; Benoit DS Enzymatically-responsive pro-angiogenic peptide-releasing poly (ethylene glycol) hydrogels promote vascularization in vivo. J. Controlled Release 2015, 217, 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (251).Hoffman MD; Van Hove AH; Benoit DS Degradable hydrogels for spatiotemporal control of mesenchymal stem cells localized at decellularized bone allografts. Acta Biomater. 2014, 10 (8), 3431–3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (252).Van Hove AH; Beltejar M-JG; Benoit DS Development and in vitro assessment of enzymatically-responsive poly (ethylene glycol) hydrogels for the delivery of therapeutic peptides. Biomaterials 2014, 35 (36), 9719–9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (253).Van Hove AH; Antonienko E; Burke K; Brown E III; Benoit DS Temporally tunable, enzymatically responsive delivery of proangiogenic peptides from poly (ethylene glycol) hydrogels. Adv. Healthcare Mater 2015, 4 (13), 2002–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (254).Chato-Astrain J; Campos F; Roda O; Miralles E; Durand-Herrera D; Sáez-Moreno JA; García-García S; Alaminos M; Campos A; Carriel V In vivo evaluation of nanostructured fibrinagarose hydrogels with mesenchymal stem cells for peripheral nerve repair. Front. Cell. Neurosci 2018, 12, 501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (255).Evangelista MS; Perez M; Salibian AA; Hassan JM; Darcy S; Paydar KZ; Wicker RB; Arcaute K; Mann BK; Evans GR Single-lumen and multi-lumen poly (ethylene glycol) nerve conduits fabricated by stereolithography for peripheral nerve regeneration in vivo. J. Reconstr Microsurg 2015, 31 (05), 327–335. [DOI] [PubMed] [Google Scholar]
- (256).Tao J; Zhang J; Du T; Xu X; Deng X; Chen S; Liu J; Chen Y; Liu X; Xiong M; et al. Rapid 3D printing of functional nanoparticle-enhanced conduits for effective nerve repair. Acta Biomater. 2019, 90, 49–59. [DOI] [PubMed] [Google Scholar]
- (257).Stevens MM; Marini RP; Schaefer D; Aronson J; Langer R; Shastri VP In vivo engineering of organs: the bone bioreactor. Proc. Natl. Acad. Sci. U. S. A 2005, 102 (32), 11450–11455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (258).Hokugo A; Ozeki M; Kawakami O; Sugimoto K; Mushimoto K; Morita S; Tabata Y Augmented bone regeneration activity of platelet-rich plasma by biodegradable gelatin hydrogel. Tissue Eng. 2005, 11 (7–8), 1224–1233. [DOI] [PubMed] [Google Scholar]
- (259).Hokugo A; Sawada Y; Hokugo R; Iwamura H; Kobuchi M; Kambara T; Morita S; Tabata Y Controlled release of platelet growth factors enhances bone regeneration at rabbit calvaria. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology 2007, 104 (1), 44–48. [DOI] [PubMed] [Google Scholar]
- (260).Hoffman MD; Benoit DS Emulating native periosteum cell population and subsequent paracrine factor production to promote tissue engineered periosteum-mediated allograft healing. Biomaterials 2015, 52, 426–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (261).Baldwin J; Wagner F; Martine L; Holzapfel B; Theodoropoulos C; Bas O; Savi F; Werner C; De-Juan-Pardo E; Hutmacher D Periosteum tissue engineering in an orthotopic in vivo platform. Biomaterials 2017, 121, 193–204. [DOI] [PubMed] [Google Scholar]
- (262).Srouji S; Rachmiel A; Blumenfeld I; Livne E Mandibular defect repair by TGF-β and IGF-1 released from a biodegradable osteoconductive hydrogel. J. Cranio Maxill Surg 2005, 33 (2), 79–84. [DOI] [PubMed] [Google Scholar]
- (263).Sivashanmugam A; Charoenlarp P; Deepthi S; Rajendran A; Nair SV; Iseki S; Jayakumar R Injectable shear-thinning CaSO4/FGF-18-incorporated Chitin–PLGA hydrogel enhances bone regeneration in mice cranial bone defect model. ACS Appl. Mater. Interfaces 2017, 9 (49), 42639–42652. [DOI] [PubMed] [Google Scholar]
- (264).Madden LR; Mortisen DJ; Sussman EM; Dupras SK; Fugate JA; Cuy JL; Hauch KD; Laflamme MA; Murry CE; Ratner BD Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc. Natl. Acad. Sci U. S. A 2010, 107 (34), 15211–15216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (265).Oliviero O; Ventre M; Netti P Functional porous hydrogels to study angiogenesis under the effect of controlled release of vascular endothelial growth factor. Acta Biomater. 2012, 8 (9), 3294–3301. [DOI] [PubMed] [Google Scholar]
- (266).Vajda J; Milojević M; Maver U; Vihar B Microvascular Tissue Engineering—A Review. Biomedicines 2021, 9 (6), 589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (267).Evans G; Brandt K; Widmer M; Lu L; Meszlenyi R; Gupta P; Mikos A; Hodges J; Williams J; Gurlek A; et al. In vivo evaluation of poly (L-lactic acid) porous conduits for peripheral nerve regeneration. Biomaterials 1999, 20 (12), 1109–1115. [DOI] [PubMed] [Google Scholar]
- (268).Hsu S. h.; Chang WC; Yen CT Novel flexible nerve conduits made of water-based biodegradable polyurethane for peripheral nerve regeneration. J. Biomed. Mater. Res., Part A 2017, 105 (5), 1383–1392. [DOI] [PubMed] [Google Scholar]
- (269).Niu Y; Chen KC; He T; Yu W; Huang S; Xu K Scaffolds from block polyurethanes based on poly (ε-caprolactone)(PCL) and poly (ethylene glycol)(PEG) for peripheral nerve regeneration. Biomaterials 2014, 35 (14), 4266–4277. [DOI] [PubMed] [Google Scholar]
- (270).Saberianpour S; Heidarzadeh M; Geranmayeh MH; Hosseinkhani H; Rahbarghazi R; Nouri M Tissue engineering strategies for the induction of angiogenesis using biomaterials. J. Biol. Eng 2018, 12 (1), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (271).Rosso G; Young P; Shahin V Implications of Schwann cells biomechanics and mechanosensitivity for peripheral nervous system physiology and pathophysiology. Front. Mol. Neurosci 2017, 10, 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (272).Wu S; Xu R; Duan B; Jiang P Three-dimensional hyaluronic acid hydrogel-based models for in vitro human iPSC-derived NPC culture and differentiation. J. Mater. Chem. B 2017, 5 (21), 3870–3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (273).Lampe KJ; Antaris AL; Heilshorn SC Design of three-dimensional engineered protein hydrogels for tailored control of neurite growth. Acta Biomater. 2013, 9 (3), 5590–5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (274).Zuo Y Substrate Stress Relaxation Regulates Cell Migration; Johns Hopkins University, 2019. [Google Scholar]
- (275).Byfield FJ; Reen RK; Shentu T-P; Levitan I; Gooch KJ Endothelial actin and cell stiffness is modulated by substrate stiffness in 2D and 3D. J. Biomech 2009, 42 (8), 1114–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (276).Mason BN; Starchenko A; Williams RM; Bonassar LJ; Reinhart-King CA Tuning three-dimensional collagen matrix stiffness independently of collagen concentration modulates endothelial cell behavior. Acta Biomater. 2013, 9 (1), 4635–4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (277).Trappmann B; Baker BM; Polacheck WJ; Choi CK; Burdick JA; Chen CS Matrix degradability controls multicellularity of 3D cell migration. Nat. Commun 2017, 8 (1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (278).Wang L; Wu S; Cao G; Fan Y; Dunne N; Li X Biomechanical studies on biomaterial degradation and co-cultured cells: mechanisms, potential applications, challenges and prospects. J. Mater. Chem. B 2019, 7 (47), 7439–7459. [DOI] [PubMed] [Google Scholar]
- (279).Shih YC Effects of degradation on mechanical properties of tissue-engineering poly (glycolic acid) scaffolds, Thesis, Yale University, 2015. [Google Scholar]
- (280).Li Y; Hoffman MD; Benoit DS Matrix metalloproteinase (MMP)-degradable tissue engineered periosteum coordinates allograft healing via early stage recruitment and support of host neurovasculature. Biomaterials 2021, 268, 120535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (281).Du Y; Herath SC; Wang Q.-g.; Wang D.-a.; Asada HH; Chen PC Three-dimensional characterization of mechanical interactions between endothelial cells and extracellular matrix during angiogenic sprouting. Sci. Rep 2016, 6 (1), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (282).Rundhaug JE Matrix metalloproteinases and angiogenesis. J. Cell. Mol. Med 2005, 9 (2), 267–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (283).Dinglasan LAV The Role of Matrix Metalloproteinases in Axon Guidance and Neurite Outgrowth, Thesis, Yale University, 2008. [Google Scholar]
- (284).Moons LK; Buyens T; Lemmens K; Salinas-Navarro M; Behrendt N; Van Hove I; Gaublomme D; De Groef L MMP-2 and MT1-MMP as axonal outgrowth-promoting molecules in the neuroretina. Invest Ophth Vis Sci. 2014, 55 (13), 3515–3515. [Google Scholar]
- (285).Man AJ; Davis HE; Itoh A; Leach JK; Bannerman P Neurite outgrowth in fibrin gels is regulated by substrate stiffness. Tissue Eng., Part A 2011, 17 (23–24), 2931–2942. [DOI] [PubMed] [Google Scholar]
- (286).Woodruff MA; Hutmacher DW The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci 2010, 35 (10), 1217–1256. [Google Scholar]
- (287).Ivanovski S; Vaquette C; Gronthos S; Hutmacher DW; Bartold PM Multiphasic Scaffolds for Periodontal Tissue Engineering. J. Dent. Res 2014, 93 (12), 1212–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (288).Mohammad JA; Warnke PH; Pan Y; Shenaq S Increased axonal regeneration through a biodegradable amnionic tube nerve conduit: effect of local delivery and incorporation of nerve growth factor/hyaluronic acid media. Ann. Plast. Surg 2000, 44 (1), 59–64. [DOI] [PubMed] [Google Scholar]
- (289).Rich KM; Alexander TD; Pryor JC; Hollowell JP Nerve growth factor enhances regeneration through silicone chambers. Exp. Neurol 1989, 105 (2), 162–170. [DOI] [PubMed] [Google Scholar]
- (290).Lee AC; Vivian MY; Lowe III JB; Brenner MJ; Hunter DA; Mackinnon SE; Sakiyama-Elbert SE Controlled release of nerve growth factor enhances sciatic nerve regeneration. Exp. Neurol 2003, 184 (1), 295–303. [DOI] [PubMed] [Google Scholar]
- (291).Lin Y-C; Ramadan M; Hronik-Tupaj M; Kaplan DL; Philips BJ; Sivak W; Rubin JP; Marra KG spatially controlled delivery of neurotrophic factors in silk fibroin–based nerve conduits for peripheral nerve repair. Ann. Plast. Surg 2011, 67 (2), 147–155. [DOI] [PubMed] [Google Scholar]
- (292).Wood MD; Kim H; Bilbily A; Kemp SW; Lafontaine C; Gordon T; Shoichet MS; Borschel GH GDNF released from microspheres enhances nerve regeneration after delayed repair. Muscle Nerve 2012, 46 (1), 122–124. [DOI] [PubMed] [Google Scholar]
- (293).Chen Z-Y; Chai Y-F; Cao L; Lu C-L; He C Glial cell line-derived neurotrophic factor enhances axonal regeneration following sciatic nerve transection in adult rats. Brain Res. 2001, 902 (2), 272–276. [DOI] [PubMed] [Google Scholar]
- (294).Sanghvi AB; Murray ABJL Tissue engineering of peripheral nerve. Encyclopedia of biomaterials and biomedical engineering; CRC Press, 2008; pp 2811–2819. [Google Scholar]
- (295).Terris DJ; Toft KM; Moir M; Lum J; Wang M Brain-derived neurotrophic factor–enriched collagen tubule as a substitute for autologous nerve grafts. Arch. Otolaryngol., Head Neck Surg 2001, 127 (3), 294–298. [DOI] [PubMed] [Google Scholar]
- (296).Hollinger JO; Hart CE; Hirsch SN; Lynch S; Friedlaender GE Recombinant human platelet-derived growth factor: biology and clinical applications. JBJS 2008, 90 (Supplement_1), 48–54. [DOI] [PubMed] [Google Scholar]
- (297).Young S; Patel ZS; Kretlow JD; Murphy MB; Mountziaris PM; Baggett LS; Ueda H; Tabata Y; Jansen JA; Wong M; et al. Dose effect of dual delivery of vascular endothelial growth factor and bone morphogenetic protein-2 on bone regeneration in a rat critical-size defect model. Tissue Eng., Part A 2009, 15 (9), 2347–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (298).Pu LL; Syed SA; Reid M; Patwa H; Goldstein JM; Forman DL; Thomson JG; et al. Effects of nerve growth factor on nerve regeneration through a vein graft across a gap. Plast Reconstr Surg 1999, 104 (5), 1379–1385. [DOI] [PubMed] [Google Scholar]
- (299).Zieris A; Prokoph S; Levental KR; Welzel PB; Grimmer M; Freudenberg U; Werner C FGF-2 and VEGF functionalization of starPEG–heparin hydrogels to modulate biomolecular and physical cues of angiogenesis. Biomaterials 2010, 31 (31), 7985–7994. [DOI] [PubMed] [Google Scholar]
- (300).Khorsand B; Nicholson N; Do A-V; Femino JE; Martin JA; Petersen E; Guetschow B; Fredericks DC; Salem AK Regeneration of bone using nanoplex delivery of FGF-2 and BMP-2 genes in diaphyseal long bone radial defects in a diabetic rabbit model. J. Controlled Release 2017, 248, 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (301).Charles LF; Woodman JL; Ueno D; Gronowicz G; Hurley MM; Kuhn LT Effects of low dose FGF-2 and BMP-2 on healing of calvarial defects in old mice. Exp. Gerontol 2015, 64, 62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (302).Mastrullo V; Cathery W; Velliou E; Madeddu P; Campagnolo P Angiogenesis in tissue engineering: as nature intended? Front. Bioeng. Biotechnol 2020, 8, 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (303).Kuttappan S; Mathew D; Jo J.-i.; Tanaka R; Menon D; Ishimoto T; Nakano T; Nair SV; Nair MB; Tabata Y Dual release of growth factor from nanocomposite fibrous scaffold promotes vascularisation and bone regeneration in rat critical sized calvarial defect. Acta Biomater. 2018, 78, 36–47. [DOI] [PubMed] [Google Scholar]
- (304).Sharon J; Puleo D Immobilization of glycoproteins, such as VEGF, on biodegradable substrates. Acta Biomater. 2008, 4 (4), 1016–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (305).Budiraharjo R; Neoh KG; Kang E-T Enhancing bioactivity of chitosan film for osteogenesis and wound healing by covalent immobilization of BMP-2 or FGF-2. J. Biomater. Sci., Polym. Ed 2013, 24 (6), 645–662. [DOI] [PubMed] [Google Scholar]
- (306).Shekaran A; García JR; Clark AY; Kavanaugh TE; Lin AS; Guldberg RE; García AJ Bone regeneration using an alpha 2 beta 1 integrin-specific hydrogel as a BMP-2 delivery vehicle. Biomaterials 2014, 35 (21), 5453–5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (307).Sacchi V; Mittermayr R; Hartinger J; Martino MM; Lorentz KM; Wolbank S; Hofmann A; Largo RA; Marschall JS; Groppa E; et al. Long-lasting fibrin matrices ensure stable and functional angiogenesis by highly tunable, sustained delivery of recombinant VEGF164. Proc. Natl. Acad. Sci. U. S. A 2014, 111 (19), 6952–6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (308).Vardar E; Larsson HM; Allazetta S; Engelhardt EM; Pinnagoda K; Vythilingam G; Hubbell JA; Lutolf MP; Frey P Microfluidic production of bioactive fibrin micro-beads embedded in crosslinked collagen used as an injectable bulking agent for urinary incontinence treatment. Acta Biomater. 2018, 67, 156–166. [DOI] [PubMed] [Google Scholar]
- (309).Jha AK; Mathur A; Svedlund FL; Ye J; Yeghiazarians Y; Healy KE Molecular weight and concentration of heparin in hyaluronic acid-based matrices modulates growth factor retention kinetics and stem cell fate. J. Controlled Release 2015, 209, 308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (310).Martino MM; Briquez PS; Ranga A; Lutolf MP; Hubbell JA Heparin-binding domain of fibrin (ogen) binds growth factors and promotes tissue repair when incorporated within a synthetic matrix. Proc. Natl. Acad. Sci. U. S. A 2013, 110 (12), 4563–4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (311).Martino MM; Briquez PS; Güç E; Tortelli F; Kilarski WW; Metzger S; Rice JJ; Kuhn GA; Müller R; Swartz MA; et al. Growth factors engineered for super-affinity to the extracellular matrix enhance tissue healing. Science 2014, 343 (6173), 885–888. [DOI] [PubMed] [Google Scholar]
- (312).Comoglio PM; Boccaccio C; Trusolino L Interactions between growth factor receptors and adhesion molecules: breaking the rules. Curr. Opin. Cell Biol 2003, 15 (5), 565–571. [DOI] [PubMed] [Google Scholar]
- (313).Salmerón-Sánchez M; Dalby MJ Synergistic growth factor microenvironments. Chem. Commun 2016, 52 (91), 13327–13336. [DOI] [PubMed] [Google Scholar]
- (314).Subbiah R; Guldberg RE Materials science and design principles of growth factor delivery systems in tissue engineering and regenerative medicine. Adv. Healthcare Mater 2019, 8 (1), 1801000. [DOI] [PubMed] [Google Scholar]
- (315).Itoh S; Suzuki M; Yamaguchi I; Takakuda K; Kobayashi H; Shinomiya K; Tanaka J Development of a nerve scaffold using a tendon chitosan tube. Artif. Organs 2003, 27 (12), 1079–1088. [DOI] [PubMed] [Google Scholar]
- (316).Itoh S; Matsuda A; Kobayashi H; Ichinose S; Shinomiya K; Tanaka J Effects of a laminin peptide (YIGSR) immobilized on crab-tendon chitosan tubes on nerve regeneration. J. Biomed. Mater. Res., Part B 2005, 73 (2), 375–382. [DOI] [PubMed] [Google Scholar]
- (317).Sedaghati T; Jell G; Seifalian A Investigation of Schwann cell behaviour on RGD-functionalised bioabsorbable nanocomposite for peripheral nerve regeneration. New Biotechnol. 2014, 31 (3), 203–213. [DOI] [PubMed] [Google Scholar]
- (318).Zhu L; Wang K; Ma T; Huang L; Xia B; Zhu S; Yang Y; Liu Z; Quan X; Luo K; et al. Noncovalent bonding of RGD and YIGSR to an electrospun poly (ε-caprolactone) conduit through peptide self-assembly to synergistically promote sciatic nerve regeneration in rats. Adv. Healthcare Mater 2017, 6 (8), 1600860. [DOI] [PubMed] [Google Scholar]
- (319).Taite LJ; Yang P; Jun HW; West JL Nitric oxide-releasing polyurethane–PEG copolymer containing the YIGSR peptide promotes endothelialization with decreased platelet adhesion. J. Biomed. Mater. Res., Part B 2008, 84 (1), 108–116. [DOI] [PubMed] [Google Scholar]
- (320).Kibbey MC; Kleinman HK; Corcoran ML; Wahl LM Laminin SIKVAV peptide-induced angiogenesis in vivo is potentiated by neutrophils. J. Cell. Physiol 1994, 160 (1), 185–193. [DOI] [PubMed] [Google Scholar]
- (321).Li C; Hill A; Imran M In vitro and in vivo studies of ePTFE vascular grafts treated with P15 peptide. J. Biomater. Sci., Polym. Ed 2005, 16 (7), 875–891. [DOI] [PubMed] [Google Scholar]
- (322).Fittkau M; Zilla P; Bezuidenhout D; Lutolf M; Human P; Hubbell JA; Davies N The selective modulation of endothelial cell mobility on RGD peptide containing surfaces by YIGSR peptides. Biomaterials 2005, 26 (2), 167–174. [DOI] [PubMed] [Google Scholar]
- (323).Massia SP; Hubbell JA Vascular endothelial cell adhesion and spreading promoted by the peptide REDV of the IIICS region of plasma fibronectin is mediated by integrin alpha 4 beta 1. J. Biol. Chem 1992, 267 (20), 14019–14026. [PubMed] [Google Scholar]
- (324).Hamada Y; Yuki K; Okazaki M; Fujitani W; Matsumoto T; Hashida MK; Harutsugu K; Nokihara K; Daito M; Matsuura N; et al. Osteopontin-derived peptide SVVYGLR induces angiogenesis in vivo. Dent. Mater. J 2004, 23 (4), 650–655. [DOI] [PubMed] [Google Scholar]
- (325).Adini A; Adini I; Chi Z.-l.; Derda R; Birsner AE; Matthews BD; D’Amato RJ A novel strategy to enhance angiogenesis in vivo using the small VEGF-binding peptide PR1P. Angiogenesis 2017, 20 (3), 399–408. [DOI] [PubMed] [Google Scholar]
- (326).Miller JS; Stevens KR; Yang MT; Baker BM; Nguyen D-HT; Cohen DM; Toro E; Chen AA; Galie PA; Yu X; et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater 2012, 11 (9), 768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (327).Li S; Xiong Z; Wang X; Yan Y; Liu H; Zhang R Direct fabrication of a hybrid cell/hydrogel construct by a double-nozzle assembling technology. J. Bioact. Compat. Polym 2009, 24 (3), 249–265. [Google Scholar]
- (328).Lee S-J; Nowicki M; Harris B; Zhang LG Fabrication of a highly aligned neural scaffold via a table top stereolithography 3D printing and electrospinning. Tissue Eng., Part A 2017, 23 (11–12), 491–502. [DOI] [PubMed] [Google Scholar]
- (329).Grasman JM; Ferreira JA; Kaplan DL Tissue Models for Neurogenesis and Repair in 3D. Adv. Funct. Mater 2018, 28 (48), 1803822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (330).Jafarkhani M; Salehi Z; Aidun A; Shokrgozar MA Bioprinting in Vascularization Strategies. Iran. Biomed. J 2019, 23 (1), 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (331).Moreno Madrid AP; Vrech SM; Sanchez MA; Rodriguez AP Advances in additive manufacturing for bone tissue engineering scaffolds. Mater. Sci. Eng., C 2019, 100, 631. [DOI] [PubMed] [Google Scholar]
- (332).Lee VK; Kim DY; Ngo H; Lee Y; Seo L; Yoo S-S; Vincent PA; Dai G Creating perfused functional vascular channels using 3D bio-printing technology. Biomaterials 2014, 35 (28), 8092–8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (333).Hsiao D; Hsu S-H; Chen R-S; Chen M-H Characterization of designed directional polylactic acid 3D scaffolds for neural differentiation of human dental pulp stem cells. J. Formosan Med. Assoc 2020, 119 (1), 268–275. [DOI] [PubMed] [Google Scholar]
- (334).Kang H-W; Lee SJ; Ko IK; Kengla C; Yoo JJ; Atala A A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol 2016, 34 (3), 312–319. [DOI] [PubMed] [Google Scholar]
- (335).Jain RK; Au P; Tam J; Duda DG; Fukumura D Engineering vascularized tissue. Nat. Biotechnol 2005, 23 (7), 821–823. [DOI] [PubMed] [Google Scholar]
- (336).Asa’ad F; Pagni G; Pilipchuk SP; Giannì AB; Giannobile WV; Rasperini G 3D-Printed Scaffolds and Biomaterials: Review of Alveolar Bone Augmentation and Periodontal Regeneration Applications. Int. J. Dent 2016, 2016, 1239842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (337).Charbonneau AM; Kinsella JM; Tran SD 3D Cultures of Salivary Gland Cells in Native or Gelled Egg Yolk Plasma, Combined with Egg White and 3D-Printing of Gelled Egg Yolk Plasma. Materials 2019, 12 (21), 3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (338).Ferreira JN; Rungarunlert S; Urkasemsin G; Adine C; Souza GR Three-Dimensional Bioprinting Nanotechnologies towards Clinical Application of Stem Cells and Their Secretome in Salivary Gland Regeneration. Stem Cells Int. 2016, 2016, 7564689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (339).Chansaenroj A; Yodmuang S; Ferreira JN Trends in Salivary Gland Tissue Engineering: From Stem Cells to Secretome and Organoid Bioprinting. Tissue Eng., Part B 2021, 27 (2), 155–165. [DOI] [PubMed] [Google Scholar]
- (340).Barrows CML; Wu D; Farach-Carson MC; Young S Building a Functional Salivary Gland for Cell-Based Therapy: More than Secretory Epithelial Acini. Tissue Eng., Part A 2020, 26 (23–24), 1332–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (341).Bae H; Puranik AS; Gauvin R; Edalat F; Carrillo-Conde B; Peppas NA; Khademhosseini A Building vascular networks. Sci. Transl. Med 2012, 4 (160), 160ps23–160ps23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (342).Davis GE; Stratman AN; Sacharidou A; Koh W Molecular basis for endothelial lumen formation and tubulogenesis during vasculogenesis and angiogenic sprouting. International review of cell and molecular biology; Elsevier, 2011; Vol. 288, pp 101–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (343).Chan ZC-K; Oentaryo MJ; Lee CW MMP-mediated modulation of ECM environment during axonal growth and NMJ development. Neurosci. Lett 2020, 724, 134822. [DOI] [PubMed] [Google Scholar]
- (344).Elisseeff J; Puleo C; Yang F; Sharma B Advances in skeletal tissue engineering with hydrogels. Orthodontics & craniofacial research 2005, 8 (3), 150–161. [DOI] [PubMed] [Google Scholar]
- (345).Gunn JW; Turner SD; Mann BK Adhesive and mechanical properties of hydrogels influence neurite extension. J. Biomed. Mater. Res 2005, 72 (1), 91–97. [DOI] [PubMed] [Google Scholar]
- (346).Lutolf M; Hubbell J Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol 2005, 23 (1), 47–55. [DOI] [PubMed] [Google Scholar]
- (347).Culver JC; Hoffmann JC; Poché RA; Slater JH; West JL ; Dickinson ME Three-dimensional biomimetic patterning in hydrogels to guide cellular organization. Adv. Mater 2012, 24 (17), 2344–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (348).Klotz BJ; Gawlitta D; Rosenberg AJ; Malda J; Melchels FP Gelatin-methacryloyl hydrogels: towards biofabrication-based tissue repair. Trends Biotechnol. 2016, 34 (5), 394–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (349).Moore AN; Silva TLL; Carrejo NC; Marmolejo CAO; Li I-C; Hartgerink JD Nanofibrous peptide hydrogel elicits angiogenesis and neurogenesis without drugs, proteins, or cells. Biomaterials 2018, 161, 154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (350).Moshayedi P; Nih LR; Llorente IL; Berg AR; Cinkornpumin J; Lowry WE; Segura T; Carmichael ST Systematic optimization of an engineered hydrogel allows for selective control of human neural stem cell survival and differentiation after transplantation in the stroke brain. Biomaterials 2016, 105, 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (351).Shubin AD; Felong TJ; Schutrum BE; Joe DSL; Ovitt CE; Benoit DSW Encapsulation of primary salivary gland cells in enzymatically degradable poly(ethylene glycol) hydrogels promotes acinar cell characteristics. Acta Biomater. 2017, 50, 437–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (352).Song Y; Uchida H; Sharipol A; Piraino L; Mereness JA; Ingalls MH; Rebhahn J; Newlands SD; DeLouise LA; Ovitt CE; Benoit DSW Development of a functional salivary gland tissue chip with potential for high-content drug screening. Communications Biology 2021, 4 (1), 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (353).Luginbuehl V; Meinel L; Merkle HP; Gander B Localized delivery of growth factors for bone repair. Eur. J. Pharm. Biopharm 2004, 58 (2), 197–208. [DOI] [PubMed] [Google Scholar]
- (354).Sahoo S; Ang LT; Goh JCH; Toh SL Growth factor delivery through electrospun nanofibers in scaffolds for tissue engineering applications. J. Biomed. Mater. Res., Part A 2009, 93 (4), 1539–1550. [DOI] [PubMed] [Google Scholar]
- (355).Farokhi M; Mottaghitalab F; Shokrgozar MA; Ai J; Hadjati J; Azami M Bio-hybrid silk fibroin/calcium phosphate/PLGA nanocomposite scaffold to control the delivery of vascular endothelial growth factor. Mater. Sci Eng., C 2014, 35, 401–410. [DOI] [PubMed] [Google Scholar]
- (356).Wei G; Jin Q; Giannobile WV; Ma PX Nano-fibrous scaffold for controlled delivery of recombinant human PDGF-BB. J. Controlled Release 2006, 112 (1), 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (357).Whitehead TJ; Avila COC; Sundararaghavan HG Combining growth factor releasing microspheres within aligned nanofibers enhances neurite outgrowth. J. Biomed. Mater. Res., Part A 2018, 106 (1), 17–25. [DOI] [PubMed] [Google Scholar]
- (358).Grozdanic SD; Lazic T; Kuehn MH; Harper MM; Kardon RH; Kwon YH; Lavik EB; Sakaguchi DS Exogenous modulation of intrinsic optic nerve neuroprotective activity. Graefe’s Arch. Clin. Exp. Ophthalmol 2010, 248 (8), 1105–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (359).Kempen DH; Lu L; Hefferan TE; Creemers LB; Maran A; Classic KL; Dhert WJ; Yaszemski MJ Retention of in vitro and in vivo BMP-2 bioactivities in sustained delivery vehicles for bone tissue engineering. Biomaterials 2008, 29 (22), 3245–3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (360).Chiono V; Tonda-Turo C Trends in the design of nerve guidance channels in peripheral nerve tissue engineering. Prog. Neurobiol 2015, 131, 87–104. [DOI] [PubMed] [Google Scholar]
- (361).Vo TN; Kasper FK; Mikos AG Strategies for controlled delivery of growth factors and cells for bone regeneration. Adv. Drug Delivery Rev 2012, 64 (12), 1292–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (362).Santos MI; Reis RL Vascularization in bone tissue engineering: physiology, current strategies, major hurdles and future challenges. Macromol Biosci. 2010, 10 (1), 12–27. [DOI] [PubMed] [Google Scholar]
- (363).Baldwin J; Antille M; Bonda U; De-Juan-Pardo EM; Khosrotehrani K; Ivanovski S; Petcu EB; Hutmacher DW In vitro pre-vascularisation of tissue-engineered constructs A co-culture perspective. Vasc. Cell 2014, 6 (1), 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (364).Mercado-Pagán ÁE; Stahl AM; Shanjani Y; Yang Y Vascularization in bone tissue engineering constructs. Ann. Biomed. Eng 2015, 43 (3), 718–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (365).Doss MX; Sachinidis A Current challenges of iPSC-based disease modeling and therapeutic implications. Cells 2019, 8 (5), 403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (366).Rothe R; Hauser S; Neuber C; Laube M; Schulze S; Rammelt S; Pietzsch J Adjuvant drug-assisted bone healing: Advances and challenges in drug delivery approaches. Pharmaceutics 2020, 12 (5), 428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (367).Herten M; Jung RE; Ferrari D; Rothamel D; Golubovic V; Molenberg A; Hammerle CH; Becker J; Schwarz F Biodegradation of different synthetic hydrogels made of polyethylene glycol hydrogel/RGD-peptide modifications: an immunohistochemical study in rats. Clin Oral Implants Res. 2009, 20 (2), 116–25. [DOI] [PubMed] [Google Scholar]
- (368).Mammadov R; Mammadov B; Toksoz S; Aydin B; Yagci R; Tekinay AB; Guler MO Heparin mimetic peptide nanofibers promote angiogenesis. Biomacromolecules 2011, 12 (10), 3508–19. [DOI] [PubMed] [Google Scholar]
- (369).Miller JS; Shen CJ; Legant WR; Baranski JD; Blakely BL; Chen CS Bioactive hydrogels made from step-growth derived PEG-peptide macromers. Biomaterials 2010, 31 (13), 3736–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (370).Marrella A; Lee TY; Lee DH; Karuthedom S; Syla D; Chawla A; Khademhosseini A; Jang HL Engineering vascularized and innervated bone biomaterials for improved skeletal tissue regeneration. Mater. Today (Oxford, U. K.) 2018, 21 (4), 362–376. [DOI] [PMC free article] [PubMed] [Google Scholar]