Abstract
Aims
This study explored the association of coronary artery calcium (CAC) with incident cancer subtypes in the Multi-Ethnic Study of Atherosclerosis (MESA). CAC is an established predictor of cardiovascular disease (CVD), with emerging data also supporting independent predictive value for cancer. The association of CAC with risk for individual cancer subtypes is unknown.
Methods and results
We included 6271 MESA participants, aged 45–84 and without known CVD or self-reported history of cancer. There were 777 incident cancer cases during mean follow-up of 12.9 ± 3.1 years. Lung and colorectal cancer (186 cases) were grouped based on their strong overlap with CVD risk profile; prostate (men) and ovarian, uterine, and breast cancer (women) were considered as sex-specific cancers (in total 250 cases). Incidence rates and Fine and Gray competing risks models were used to assess relative risk of cancer-specific outcomes stratified by CAC groups or Log(CAC+1). The mean age was 61.7 ± 10.2 years, 52.7% were women, and 36.5% were White. Overall, all-cause cancer incidence increased with CAC scores, with rates per 1000 person-years of 13.1 [95% confidence interval (CI): 11.7–14.7] for CAC = 0 and 35.8 (95% CI: 30.2–42.4) for CAC ≥400. Compared with CAC = 0, hazards for those with CAC ≥400 were increased for lung and colorectal cancer in men [subdistribution hazard ratio (SHR): 2.2 (95% CI: 1.1–4.7)] and women [SHR: 2.2 (95% CI: 1.0–4.6)], but not significantly for sex-specific cancers across sexes.
Conclusion
CAC scores were associated with cancer risk in both sexes; however, this was stronger for lung and colorectal when compared with sex-specific cancers. Our data support potential synergistic use of CAC scores in the identification of both CVD and lung and colorectal cancer risk.
Keywords: coronary arterial calcium, cancer, cardiovascular disease, risk prediction, prevention
Graphical Abstract
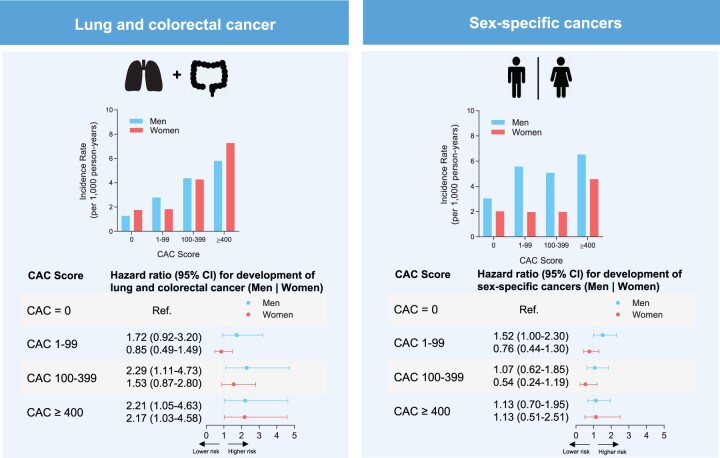
Association of coronary artery calcium with cancer subtypes. (Top left) Incidence rates per 1000 person-years for lung and colorectal cancer by sex. (Top right) Incidence rates per 1000 person-years for sex-specific cancers = prostate (men); ovarian, endometrial, and breast cancer (women). (Bottom left) Hazard ratios for development of lung and colorectal cancer and (Bottom right) sex-specific cancers by CAC score group and sex. CAC, coronary artery calcium score; CI, confidence interval.
Introduction
Coronary artery calcium (CAC) is a well-established tool for quantifying cardiovascular risk using non-contrast computed tomography.1–3 As a crude marker of total coronary atherosclerotic plaque burden, CAC reflects lifetime exposure to cardiovascular risk factors (known as well as unknown) and thus represents an excellent predictor for cardiovascular disease (CVD) outcomes.1,4,5 Increased CAC levels are also associated with an increased risk for incident cancer and cancer mortality.6–8 However, the extent to which CAC is associated with specific cancer subtypes is unknown.
Links between CVD and cancer are emerging.6,7,9,10 A substantial proportion of cancer deaths can be attributed to modifiable risk factors and, interestingly, many of the cancer-related risk factors overlap with CVD risk factors.11–13 Tobacco is related to the incidence of several malignancies and still represents the most common modifiable risk among all cancer cases.11,14 Similarly, obesity is a highly relevant modifiable risk factor that is associated with colorectal cancer prevalence. Since both CVD and cancer share numerous risk factors, we hypothesized that there may be a particular association of CAC with cancers related to modifiable risk factors. Thus, by categorizing cancers according to underlying associations with modifiable CVD/cancer risk factors (lung and colorectal cancers) and sex-specific entities with relation to hormonal processes (breast, ovarian, uterine and prostate cancer), we aimed to evaluate the predictive value of CAC for these cancer subgroups.15,16
Therefore, we evaluated whether baseline CAC scores in the well-characterized Multi-Ethnic Study of Atherosclerosis (MESA) cohort were predictive of risk for the respective cancer entities over long-term follow-up. We believe that results from our study might contribute to a better understanding of the cancer subtypes most strongly associated with CAC and may be helpful in exploring potential synergistic approaches to CVD and cancer risk assessment.
Methods
Study population
MESA is a prospectively observed cohort including 6814 individuals at 45–84 years of age without known CVD at enrolment that has been described in detail elsewhere.17 Participants were enrolled from July 2000 through September 2002 at 6 US field centres (Baltimore, MA; Chicago, IL; Forsyth County, NC; Los Angeles, CA; New York, NY; and St. Paul, MN). The study protocol was approved by the local institutional review boards and all subjects gave their informed consent. The data that support the findings of this study are available on reasonable request to the corresponding author.
The analysis was prespecified and included all MESA participants with Visit 1 CAC evaluation, no self-reported history of cancer at baseline, and available long-term follow-up for new cases of cancer (n = 6271). Also, we included two additional sensitivity analyses:
exclusion of participants with self-reported lung emphysema, liver disease, or prior blood clots, all of which might be suggestive of undiagnosed cancer at baseline (n = 419);
exclusion of participants with new cancer diagnoses within the first 180 days after baseline assessment (n = 54). Case numbers for individual cancer entities are summarized in Supplementary data online, Table S1.
Baseline characteristics and risk factors
Ethnicity was self-assessed as White, Black, Chinese, or Hispanic at the time of enrolment. Hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg and/or antihypertensive medication use. Diabetes mellitus was defined as fasting blood glucose ≥126 mg/dL or use of diabetes medication. Smoking status was determined by a self-completed questionnaire as published elsewhere.18 In brief, assessment was conducted to classify as current/former smoker or no smoker. In case of a positive smoking status, burden of smoking was calculated as pack-years. Family history of coronary heart disease was assessed using standardized questionnaires and defined as positive if any first-degree relative had any history of myocardial infarction with or without coronary revascularization. Physical activity was measured by using a detailed, semiquantitative questionnaire as reported previously.19 Healthy diet was defined as self-assessed dietary patterns with favourable impact on CVD incidence as previously published and guideline-recommended.20–22
CAC scoring and assessment
Non-contrast cardiac-gated computed tomography (CT) scanning and interpretation were performed as previously described.23 CAC was assessed at Visit 1 by using either a cardiac-gated electron-beam CT scanner (Chicago, Los Angeles, and New York Field Centres) or a multidetector CT system (Baltimore, Forsyth County, and St. Paul Field Centres). CAC was determined from two scans and was calculated for all analyses using the Agatston method.24 Further details on the MESA computed tomographic scanning protocol have been previously described.24 We defined the following CAC groups: CAC = 0, CAC 1–99, CAC 100–399, and CAC ≥400 as well as a log-transformed continuous (Log(CAC + 1)) form.
Outcome definitions and event ascertainment
Mean patient follow-up time was 12.9 years [95% confidence interval (CI): 8.0–17.8 years]. The outcomes of interest for the present study were time to incident cancer diagnosis. Cancer diagnosis was determined utilizing data gathered during prespecified annual phone calls after baseline examination inquiring about interim hospital admissions, CVD diagnoses, medical procedures, and deaths. For this purpose, International Classification of Diseases, 9th Revision (ICD-9) diagnosis codes from hospitalization records were ascertained. ICD-9 codes associated with cancer were extracted from the records and re-coded into the specific cancer entity (Supplementary data online, Table S2). The hospitalization date was defined as date of cancer diagnosis.
Based on the known predominant underlying risk factors from the cancer literature and due to the overlap with CVD risk profile, lung and colorectal cancer were grouped for the analyses. These two cancer entities are, based on large data sets from the Centers for Disease Control and Prevention and the National Cancer Institute, at the top of cancer deaths linked to modifiable risk factors.11 Additionally, prostate cancers in men were considered as ‘sex-specific cancers’; and accordingly ovarian, endometrial and breast cancers in women.25 The defined group term ‘All Cancers’ included all registered and defined cancer type groups within the MESA cohort, i.e. in addition to the above stated cancer entities, melanoma, bladder, thyroid, brain, non-melanoma skin cancer, liver, kidney, lymphoma, other gastrointestinal cancer, multiple myeloma, and other cancers.
Statistical analyses
Baseline characteristics were stratified by lung and colorectal cancer versus sex-specific cancers (main analysis) or sex (supplemental analysis) and shown as mean ± standard deviation for continuous variables, and as frequencies and percentages for categorical variables where appropriate. Cancer type-specific incidence rates were calculated and presented per 1000 person-years, both before and after stratification by sex.
Fine and Gray’s sub-distribution hazard models were used to assess the relative cancer-specific hazards across CAC groups, using CAC = 0 as the reference group, defining ‘other’ cancer and CVD as competing events. Fully adjusted model considered age, sex, ethnicity, and other conventional cardiovascular risk factors including body mass index, physical activity, socioeconomic status, education, health insurance, pack-years of smoking, and healthy diet. In order to further demonstrate the relative association of CAC groups with specific cancer type risk across age, sub-hazard distributions as a function of age (x-axis) were used with fully adjusted sub-distribution hazard ratios (y-axis). All calculations were performed in Stata version 15.0 software (Stata Corp., College Station, TX, USA).
Results
Baseline characteristics
Baseline characteristics of individuals meeting the inclusion criteria are shown in Table 1. Overall, the mean age of participants was 61.7 ± 10.2 years, 52.7% were women, 36.5% were White and 51.1% had CAC score = 0. In total, there were 777 new first cancer events during follow-up, of which 436 were grouped as either lung and colorectal cancer (n = 186) or sex-specific cancers (n = 250). The distribution of conventional cardiovascular risk factors in the entire cohort across cancer groups sexes is depicted in Table 1 and Supplementary data online, Table S3.
Table 1.
Baseline characteristics of cancer patients
| Characteristics | Total cohort (n = 6271) | Lung cancer + colorectal cancer (n = 186, 3.7%) | Sex-specific cancers (n = 250, 4.0%) |
|
|---|---|---|---|---|
| Men (n = 154, 100%) | Women (n = 93, 100%) | |||
| Age, mean ± SD (years) | 61.7 ± 10.2 | 66.2 ± 9.9 | 65.1 ± 9.1 | 64.3 ± 10.0 |
| Sex | ||||
| Men | 47.3 | 51.6 | 100 | |
| Women | 52.7 | 48.4 | 100 | |
| Ethnicity | ||||
| White-Caucasian | 36.5 | 37.1 | 40.3 | 37.6 |
| Chinese-American | 12.5 | 9.7 | 4.6 | 8.6 |
| African-American | 28.3 | 35.5 | 35.1 | 37.6 |
| Hispanic | 22.7 | 17.8 | 20.1 | 16.1 |
| High school education | 81.4 | 78.0 | 85.7 | 80.7 |
| Hypertension | 44.3 | 47.3 | 49.3 | 49.5 |
| Antihypertensive medication | 36.6 | 37.1 | 44.8 | 37.6 |
| Lipid lowering medication | 15.8 | 16.1 | 13.0 | 18.2 |
| Diabetes mellitus | 12.8 | 15.1 | 11.0 | 12.9 |
| Body mass index, mean ± SD | 28.4 ± 5.5 | 27.9 ± 5.0 | 28.1 ± 4.7 | 30.1 ± 6.8 |
| Healthy diet | 46.6 | 43.3 | 46.3 | 40.0 |
| Family history of heart attack | 42.0 | 42.6 | 39.9 | 47.2 |
| Cigarette smoking, pack-years ± SD | 11.0 ± 20.6 | 26.0 ± 31.8 | 30.8 ± 36.3 | 21.1 ± 25.7 |
| Mean CAC | 137.7 ± 402.0 | 310.5 ± 659.6 | 271.4 ± 558.3 | 91.7 ± 308.3 |
| CAC = 0 | 51.1 | 34.4 | 29.2 | 61.3 |
| CAC 1–99 | 26.1 | 24.2 | 35.1 | 21.5 |
| CAC 100–399 | 13.4 | 22.0 | 18.9 | 8.6 |
| CAC ≥400 | 9.3 | 19.4 | 16.9 | 8.6 |
Values are column percentages (%) or as indicated. Sex-specific cancers = prostate (men); ovarian, endometrial, and breast cancer (women).
CAC, coronary artery calcium score.
Incidence rates of distinct cancer subtypes by CAC scores
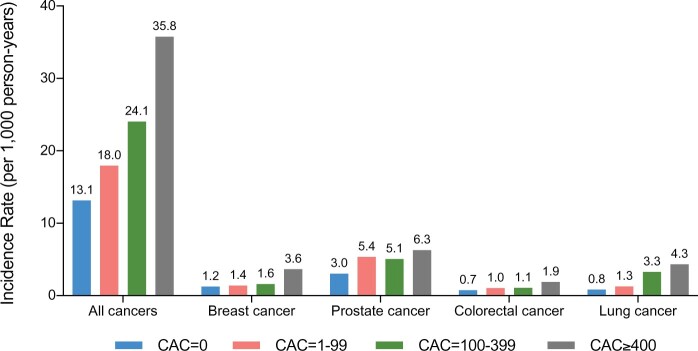
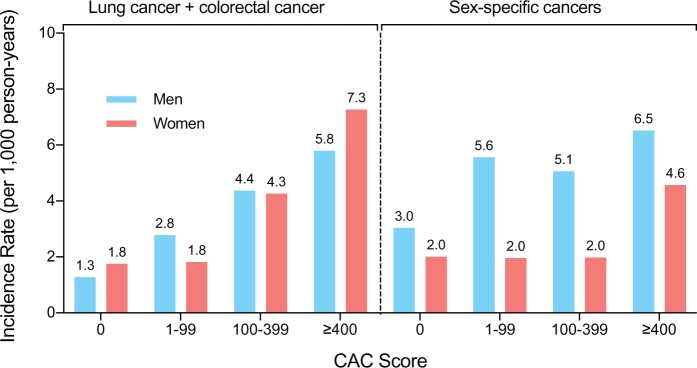
The incidence of cancer-specific outcomes increased with higher CAC scores as shown in Figures 1 and 2 and Graphical abstract. The incidences of lung and colorectal cancer increased progressively with CAC score (Figure 2 and Graphical abstract). For sex-specific cancers (prostate cancer, breast cancer, ovarian, cancer, and endometrial cancer), we did not observe such a trend of incidence rates across CAC score groups (Figure 2).
Figure 1.
Incidence rates for all cancers and distinct cancer subtypes by CAC score groups. Incidence rates per 1000 person-years for all cancers and distinct cancer subtypes. Rates increased with CAC score groups. Numbers indicate incidence rates. CAC, coronary artery calcium score.
Figure 2.
Incidence rates for lung and colorectal cancer, and sex-specific cancers by CAC score group and sex. Sex-specific cancers = prostate (men); ovarian, endometrial, and breast cancer (women). CAC, coronary artery calcium score.
A similar pattern could be detected for individual cancer entities (Figure 1). Lung and colorectal cancer incidences showed a clear increase with CAC in both sexes. The incidence rate per 1000-person years in women was 1.8 (95% CI: 1.3–2.3) in the CAC = 0 group and 7.3 (95% CI: 4.1–12.8) in the CAC ≥400 group when compared with men with 1.3 (95% CI: 0.8–2.0) and 5.8 (95% CI: 3.9–8.6), respectively (Figure 2). We observed similar results when excluding participants with additional potential indicators of undiagnosed cancer or self-reported cancer within the first 180 days after baseline assessment (Supplementary data online, Figure S1).
Unadjusted and multivariate-adjusted hazard ratios by CAC scores
The unadjusted hazards for lung and colorectal cancer, sex-specific cancers as well as for individual cancer entities with the reference group CAC = 0 are shown in Tables 2 and 3. These analyses showed a progressive increase in hazards across CAC score groups as well as for log-transformed CAC scores as a continuous variable, exclusively for lung and colorectal cancer. Adjusted for conventional cardiovascular risk factors, when compared with the CAC = 0 group, risks for lung and colorectal cancer in the CAC ≥400 group were significantly higher in all participants with a subdistribution hazard ratio (SHR) of 2.0 (95% CI: 1.2–3.4). Considering CAC as a continuous variable, there remained a statistically significant association with an SHR of 1.1 (95% CI: 1.0–1.2). The predictive value of CAC for lung and colorectal cancer could be detected for both men and women (Table 2). For sex-specific cancer risks, the adjusted model did not reveal a significant CAC-related association (Table 2). Additional analyses for the CAC related risks after removing those participants with potential indicators of undetected cancer at baseline or cancer diagnosis within the first 180 days of follow-up revealed similar results (Supplementary data online, Figure S1, Tables S4 and S5).
Table 2.
Hazard ratios for development of lung and colorectal cancer, and sex-specific cancers by CAC score group and sex.
Lung cancer + colorectal cancer
| CAC score, HR (95% CI) |
|||||
|---|---|---|---|---|---|
| CAC = 0 | CAC 1–99 | CAC 100–399 | CAC ≥ 400 | Log(CAC + 1) | |
| All | |||||
| Unadjusted | Ref. | 1.42 (0.97–2.09) | 2.59 (1.75–3.83) | 3.51 (2.33–5.28) | 1.20 (1.13–1.26) |
| Adjusteda | Ref. | 1.20 (0.81–1.78) | 1.87 (1.20–2.92) | 2.01 (1.20–3.35) | 1.11 (1.03–1.19) |
| Men | |||||
| Unadjusted | Ref. | 2.10 (1.18–3.76) | 3.20 (1.76–5.80) | 4.03 (2.21–7.35) | 1.22 (1.27–1.32) |
| Adjusteda | Ref. | 1.72 (0.92–3.20) | 2.29 (1.11–4.73) | 2.21 (1.05–4.63) | 1.12 (1.01–1.24) |
| Women | |||||
| Unadjusted | Ref. | 1.01 (0.58–1.78) | 2.27 (1.27–4.06) | 3.83 (2.03–7.23) | 1.18 (1.08–1.29) |
| Adjusteda | Ref. | 0.85 (0.49–1.49) | 1.56 (0.87–2.80) | 2.17 (1.03–4.58) | 1.09 (0.99–1.21) |
Table 3.
Hazard ratios for development of individual cancer entities by CAC score group
| Cancer types | CAC score groups, HR (95% CI) |
||||
|---|---|---|---|---|---|
| CAC = 0 | CAC 1–99 | CAC 100–399 | CAC ≥ 400 | Log(CAC + 1) | |
| Breast (n = 60) | |||||
| Unadjusted | Ref. | 1.12 (0.59–2.14) | 1.25 (0.52–3.00) | 2.76 (1.15–6.63) | 1.10 (0.98–1.23) |
| Adjusteda | Ref. | 0.98 (0.48–2.02) | 0.83 (0.32–2.15) | 1.91 (0.70–5.22) | 1.04 (0.98–1.05) |
| Prostate (n = 155) | |||||
| Unadjusted | Ref. | 1.73 (1.16–2.56) | 1.60 (1.01–2.56) | 1.89 (1.17–3.06) | 1.10 (1.04–1.17) |
| Adjusteda | Ref. | 1.53 (1.01–2.31) | 1.11 (0.64–1.90) | 1.17 (0.68–2.02) | 1.03 (0.96–1.10) |
| Colorectal (n = 71) | |||||
| Unadjusted | Ref. | 1.35 (0.77–2.39) | 1.35 (0.66–2.77) | 2.32 (1.16–4.64) | 1.09 (0.99–1.21) |
| Adjusteda | Ref. | 1.02 (0.59–1.77) | 0.77 (0.36–1.67) | 1.22 (0.58–2.53) | 1.00 (0.90–1.10) |
| Lung (n = 115) | |||||
| Unadjusted | Ref. | 1.52 (0.91–2.55) | 3.83 (2.36–6.21) | 4.87 (2.92–8.13) | 1.27 (1.19–1.37) |
| Adjusteda | Ref. | 1.44 (0.83–2.49) | 3.34 (1.88–5.94) | 3.22 (1.61–6.46) | 1.22 (1.11–1.34) |
Adjusted for: age, sex, race, body mass index, physical activity, income >40K, completed high school, health insurance, pack-years of smoking, healthy diet.
CAC, coronary artery calcium score; CI, confidence interval; HR, hazard ratio.
Sex-specific cancers
| CAC score, HR (95% CI) |
|||||
|---|---|---|---|---|---|
| CAC = 0 | CAC 1–99 | CAC 100–399 | CAC ≥ 400 | Log(CAC + 1) | |
| Men | |||||
| Unadjusted | Ref. | 1.77 (1.20–2.62) | 1.56 (0.98–2.49) | 1.88 (1.17–3.02) | 1.10 (1.03–1.16) |
| Adjusteda | Ref. | 1.52 (1.00–2.30) | 1.07 (0.62–1.85) | 1.13 (0.65–1.95) | 1.02 (0.95–1.10) |
| Women | |||||
| Unadjusted | Ref. | 0.95 (0.57–1.58) | 0.90 (0.43–1.88) | 1.93 (0.92–4.02) | 1.03 (0.94–1.13) |
| Adjusteda | Ref. | 0.76 (0.44–1.30) | 0.54 (0.24–1.19) | 1.13 (0.51–2.51) | 0.95 (0.86–1.05) |
Adjusted for: age, sex, race, body mass index, physical activity, income >40K, completed high school, health insurance, pack-years of smoking, healthy diet.
Sex-specific cancers = prostate (men); ovarian, uterine and breast cancer (women).
CAC, coronary artery calcium score; CI, confidence interval; HR, hazard ratio.
Exploratory analyses looking at individual cancer-specific hazards for breast, prostate, colorectal, and lung cancer are shown in Table 3. Here, unadjusted hazard ratios indicate an increased risk for the CAC ≥400 group for all entities which corresponds with respective incidence rates as shown in Figure 1. After adjusting for traditional risk factors, only lung cancer risk remained significantly increased for CAC ≥100 (Table 3).
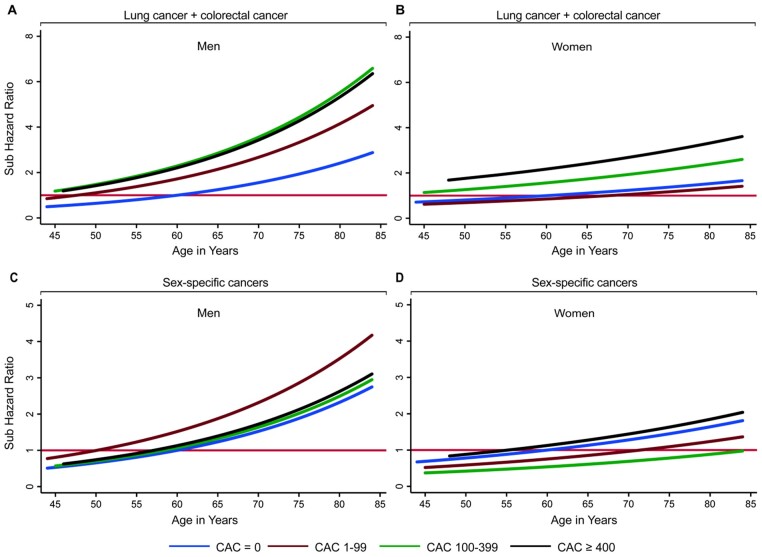
Figure 3 shows the relationship of CAC groups with cancer type-specific risk, considering age as the x-axis variable and age 60 years and CAC = 0 as the reference group. The multivariable-adjusted risks for lung and colorectal cancer increased with age in both men and women (Figure 3A and B). Curves for CAC score groups show a clear separation, thus indicating for the highest prevalence of these cancers in the CAC ≥400 group; for sex-specific cancers, the curves for CAC score groups did not clearly separate (Figure 3C and D).
Figure 3.
Subdistribution hazard ratio for lung and colorectal cancer, and sex-specific cancers as a function of age by CAC score group and sex. Graphed subdistribution hazard ratios (SHRs) as a function of age. SHR were adjusted for conventional risk factors (age, sex, race, body mass index, physical activity, income >40K, completed high school, health insurance, pack-years of smoking, healthy diet) for lung and colorectal cancer (A and B) and sex-specific (C and D) cancers. Sex-specific cancers = prostate (men); ovarian, endometrial, and breast cancer (women). SHR are stratified by CAC score group and refer to the CAC = 0 group at age 60 years. With increasing age and CAC score, cancer risk continues to increase exponentially for lung and colorectal cancer, but not for sex-specific cancers. CAC, coronary artery calcium score.
Discussion
There were three main findings in this study. First, we describe an independent association of CAC with incidence of lung and colorectal cancer, but not for sex-specific cancers such as prostate (men), breast, uterine and ovarian cancer (women). Second, the predictive value of CAC for lung and colorectal cancer was observed for both men and women (for women at CAC ≥400), and in exploratory analysis appeared strongest for lung cancer. Finally, we displayed the joint effects of CAC, age, and sex on the long-term risk of lung and colorectal cancer, underscoring the importance of considering all three of these variables in the prediction of cancer type-specific risks. Our findings support our hypothesis that CAC is predominately associated with lung and colorectal cancer, both of which relate to modifiable risk factors that in turn show strong overlap with CVD risk factor profiles.
While CVD mortality has declined over the past decades, cancer mortality has been predicted to become the leading cause of death.26 However, guideline recommendations for combined cancer and CVD prevention strategies are lacking. By dividing cancer entities into (i) cancers related to modifiable risk factors (lung and colorectal cancer) and (ii) sex-specific cancers, we believe we have further elucidated the potential predictive role of CAC in those cancers that are likely to be most responsive to preventive approaches. Modifiable risk factors such as tobacco use, obesity, alcohol consumption and physical activity account for almost half of cancer-related deaths in the USA.11,27,28 As colorectal and lung cancer are strongly linked to these modifiable risk factors that have a relevant overlap with the risk factor profile of CVD and as well account for a substantial proportion of cancer deaths, we grouped these entities accordingly.11,14,16 Islami et al.11 recently showed that these two specific cancer entities attributable to modifiable and thus preventable risk factors account for the largest proportion of overall cancer cases and deaths. Conversely, sex-specific cancers that are generally related to hormonal processes have a less clear lifestyle-dependent risk factor profile. Higher oestrogen levels exert a protective effect against CVD for instance in ovarian and breast cancer.29,30
Additionally, there is a growing body of literature pointing to chronic inflammation and related cellular processes, that are similarly found in cancer, including increased cellular proliferation, inhibition of cell death, and cell cycle progression, among the driving mechanisms for CVD progression.31,32 These overlapping mechanisms might further improve risk prediction models for both diseases and could eventually translate into combined therapeutic considerations.32
As reported previously, CAC may be predictive for incident cancer outcome because of (i) shared risk factors of CVD and cancer; (ii) CAC representing a marker of tissue vulnerability (or resilience) to risk factor-related injury.10,33–35 General associations of CAC with cancer incidence and mortality have been shown for the MESA as well as for the CAC Consortium.35,36 Indeed, while development of CAC has been shown to be associated with a diagnosis of cancer,10,37 absence of CAC is also predictive for a lower risk of cancer and other non-cardiovascular events; this indicates that CAC might be considered as a measure of vulnerability (or resilience) to risk factor-mediated organ damage.37 Given the shared risk factor profiles for both CVD and cancer, particularly smoking and adverse dietary patterns are of relevance for the development of lung and colorectal cancer.12,15 As such, CAC can be considered as indicative of an individual’s lifestyle, risk factor exposure, and overall health status and an increased CAC reflects cumulative exposure to key shared risk factors that then exerts a predictive value for both cancer and CVD risk. Thus, CAC reflects the risk factor-related damage on multiple organs that manifests as CVD and/or cancer. Although we observed an association for CAC with lung and colorectal cancer incidence (strongest for CAC ≥ 400), CAC represents a measure of biologic age and can be therapeutically influenced via attention to the underlying shared risk factors (although an exception is that statins decrease CVD and possibly risk but increase CAC scores).38,39 Additionally, radiation exposure due to CAC scanning for the MESA cohort has been previously reported to be comparable to mammography and thus less likely to substantially increase cancer incidence,40,41 while, however, patient-specific radiation doses for downstream cardiovascular and non-cardiovascular imaging procedures were not systematically collected. However, in the MESA study, limited reporting of CAC results was not accompanied by any routine referral for additional testing.
Our study contributes to a more comprehensive understanding of these associations by showing that CAC scores are particularly associated with cancer entities attributable to modifiable risk factors. We further show that risks of these cancers increased with CAC in both sexes and age, while sex-specific cancers with relation to hormonal processes appeared to have only limited CAC association.
For sex-specific cancers, the dependence on modifiable risk factors and overall risk factor-related organ damage is less strong.11 This likely explains the absence of an independent association with CAC. Additionally, some have pointed to the involved complex hormonal processes that might have favourable effects on the cardiovascular system that even lower CAC burden,42,43 although further research is needed in this field.
Based on the shared risk factor profiles and the pathophysiological overlap between CVD and specific cancer subtypes translating into increased CAC, CAC measurement could thus be helpful for synergistic risk assessment for distinct cancer entities and CVD.34,44,45 While at very low CAC scores or even CAC = 0 individuals had the lowest mortality risk (though with a larger proportion of deaths from cancer rather than from CVD), those with higher CAC scores had a generally higher mortality for both CVD and cancer.35,37 For very high CAC scores ≥1000, all-cause mortality including cancer-related mortality in the CAC Consortium population was significantly increased compared to CAC ≥400 underscoring the dose-dependent relationship of CAC and cancer outcome.7,46 Moreover, these risks substantially overlap with those for CVD and thus might offer future possibilities for synergistic risk assessment and potential combined preventive approaches.33–35
Study limitations
There are some relevant limitations to this study. First, the diagnosis of cancer and distinct cancer entities during the study follow-up is based on ICD-9 codes from hospitalizations or inpatient procedures. Therefore, participants who received care exclusively in the outpatient setting may not be fully captured. Second, while we have adjusted for pack-years or smoking and a healthy dietary pattern, these are complex exposures, and our analysis does not allow to exclude smoking and diet-related residual confounding. Third, we did not have any information on the histological subtype, cancer-related treatment, and whether radiation or surgery were also part of the treatment procedure. Fourth, it is possible that a few participants might have had undetected cancer at the time of the baseline MESA exam. To address this aspect, we excluded those participants with self-reported history of cancer at baseline, and furthermore, we performed additional analyses excluding participants by defining potential indicators of undiagnosed cancer at baseline. Finally, to address any concern that the baseline exam itself (which included CAC amongst many other lab and imaging tests) might have led to cancer ascertainment, we excluded those with a cancer diagnosis within 180 days after baseline. Despite these limitations, the MESA cohort represents one of the only settings to study the association of CAC with incident cancer in a comprehensively phenotyped community-based study cohort with ethnical diversity and detailed information on risk factors.
Conclusions
Our results demonstrate an association of CAC with increased risk of cancers attributable to modifiable risk factors. With respect to overlapping risk factors that are accessible to aggressive preventive action, we provide evidence that CAC score assessment might play a potential role for combined cancer and CVD risk evaluation. Future prospective studies investigating CAC and cancer risks are warranted.
Supplementary data
Supplementary data are available at European Heart Journal - Cardiovascular Imaging online.
Supplementary Material
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. This publication was developed under the Science to Achieve Results (STAR) research assistance agreements, no. RD831697 (MESA Air) and RD-83830001 (MESA Air Next Stage), awarded by the U.S Environmental Protection Agency. It has not been formally reviewed by the EPA. The views expressed in this document are solely those of the authors and the EPA does not endorse any products or commercial services mentioned in this publication.
Funding
This research was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS).
Conflict of interest: C.H.M.: BMS, Dava Oncology, McGraw Hill, Bayer Pharmaceuticals, Dendreon. The other authors report no conflicts of interest relevant to the content of this manuscript. The other authors report no conflicts of interest relevant to the content of this manuscript.
Contributor Information
Omar Dzaye, Johns Hopkins Ciccarone Center for Prevention of Cardiovascular Disease, Johns Hopkins University School of Medicine, Blalock 524D1, 600 N Wolfe St, Baltimore, MD 21287, USA; Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Philipp Berning, Department of Hematology and Oncology, University Hospital Muenster, Muenster, Germany.
Zeina A Dardari, Johns Hopkins Ciccarone Center for Prevention of Cardiovascular Disease, Johns Hopkins University School of Medicine, Blalock 524D1, 600 N Wolfe St, Baltimore, MD 21287, USA.
Martin Bødtker Mortensen, Johns Hopkins Ciccarone Center for Prevention of Cardiovascular Disease, Johns Hopkins University School of Medicine, Blalock 524D1, 600 N Wolfe St, Baltimore, MD 21287, USA; Department of Cardiology, Aarhus University Hospital, Aarhus, Denmark.
Catherine Handy Marshall, Johns Hopkins Ciccarone Center for Prevention of Cardiovascular Disease, Johns Hopkins University School of Medicine, Blalock 524D1, 600 N Wolfe St, Baltimore, MD 21287, USA.
Khurram Nasir, Division of Cardiovascular Prevention and Wellness, Houston Methodist DeBakey Heart & Vascular Center, Houston, TX, USA.
Matthew J Budoff, Department of Medicine, Lundquist Institute, Harbor-UCLA Medical Center, Torrance, CA, USA.
Roger S Blumenthal, Johns Hopkins Ciccarone Center for Prevention of Cardiovascular Disease, Johns Hopkins University School of Medicine, Blalock 524D1, 600 N Wolfe St, Baltimore, MD 21287, USA.
Seamus P Whelton, Johns Hopkins Ciccarone Center for Prevention of Cardiovascular Disease, Johns Hopkins University School of Medicine, Blalock 524D1, 600 N Wolfe St, Baltimore, MD 21287, USA.
Michael J Blaha, Johns Hopkins Ciccarone Center for Prevention of Cardiovascular Disease, Johns Hopkins University School of Medicine, Blalock 524D1, 600 N Wolfe St, Baltimore, MD 21287, USA.
References
- 1. Blaha MJ, Silverman MG, Budoff MJ. Is there a role for coronary artery calcium scoring for management of asymptomatic patients at risk for coronary artery disease?: clinical risk scores are not sufficient to define primary prevention treatment strategies among asymptomatic patients. Circ Cardiovasc Imaging 2014;7:398–408; discussion 408. [DOI] [PubMed] [Google Scholar]
- 2. Hecht HS. Coronary artery calcium scanning: past, present, and future. JACC Cardiovasc Imaging 2015;8:579–96. [DOI] [PubMed] [Google Scholar]
- 3. Dzaye O, Dudum R, Mirbolouk M, Orimoloye OA, Osei AD, Dardari ZA et al. Validation of the Coronary Artery Calcium Data and Reporting System (CAC-DRS): dual importance of CAC score and CAC distribution from the Coronary Artery Calcium (CAC) consortium. J Cardiovasc Comput Tomogr 2020;14:12–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blaha MJ, Cainzos-Achirica M, Dardari Z, Blankstein R, Shaw LJ, Rozanski A et al. All-cause and cause-specific mortality in individuals with zero and minimal coronary artery calcium: a long-term, competing risk analysis in the Coronary Artery Calcium Consortium. Atherosclerosis 2020;294:72–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dzaye O, Dudum R, Reiter-Brennan C, Kianoush S, Tota-Maharaj R, Cainzos-Achirica M et al. Coronary artery calcium scoring for individualized cardiovascular risk estimation in important patient subpopulations after the 2019 AHA/ACC primary prevention guidelines. Prog Cardiovasc Dis 2019;62:423–30. [DOI] [PubMed] [Google Scholar]
- 6. Chen WT, Huang JH, Hsieh MH, Chen YJ. Extremely high coronary artery calcium score is associated with a high cancer incidence. Int J Cardiol 2012;155:474–5. [DOI] [PubMed] [Google Scholar]
- 7. Peng AW, Mirbolouk M, Orimoloye OA, Osei AD, Dardari Z, Dzaye O et al. Long-term all-cause and cause-specific mortality in asymptomatic patients with CAC ≥1,000: results from the CAC Consortium. JACC Cardiovasc Imaging 2020;13:83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Whelton SP, Rifai MA, Marshall CH, Dardari Z, Shaw LJ, Al-Mallah MH et al. Coronary artery calcium and the age-specific competing risk of cardiovascular versus cancer mortality: the coronary artery calcium consortium. Am J Med 2020;133:e575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pursnani A, Massaro JM, D’Agostino RB, O'Donnell CJ, Hoffmann U. Guideline-based statin eligibility, cancer events, and noncardiovascular mortality in the Framingham Heart Study. JCO 2017;35:2927–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Whitlock MC, Yeboah J, Burke GL, Chen H, Klepin HD, Hundley WG. Cancer and its association with the development of coronary artery calcification: an assessment from the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc 2015;4:e002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Islami F, Goding Sauer A, Miller KD, Siegel RL, Fedewa SA, Jacobs EJ et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin 2018;68:31–54. [DOI] [PubMed] [Google Scholar]
- 12. Koene RJ, Prizment AE, Blaes A, Konety SH. Shared risk factors in cardiovascular disease and cancer. Circulation 2016;133:1104–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dzaye O, Reiter-Brennan C, Osei AD, Orimoloye OA, Uddin SMI, Mirbolouk M et al. The evolving view of coronary artery calcium: a personalized shared decision-making tool in primary prevention. Cardiol Res Pract 2019;2019:7059806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Batty GD, Kivimaki M, Gray L, Davey Smith G, Marmot MG, Shipley MJ. Cigarette smoking and site-specific cancer mortality: testing uncertain associations using extended follow-up of the original Whitehall study. Ann Oncol 2008;19:996–1002. [DOI] [PubMed] [Google Scholar]
- 15. Karahalios A, English DR, Simpson JA. Weight change and risk of colorectal cancer: a systematic review and meta-analysis. Am J Epidemiol 2015;181:832–45. [DOI] [PubMed] [Google Scholar]
- 16. Moghaddam AA, Woodward M, Huxley R. Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70,000 events. Cancer Epidemiol Biomarkers Prev 2007;16:2533–47. [DOI] [PubMed] [Google Scholar]
- 17. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–81. [DOI] [PubMed] [Google Scholar]
- 18. Al Rifai M, DeFilippis AP, McEvoy JW, Hall ME, Acien AN, Jones MR et al. The relationship between smoking intensity and subclinical cardiovascular injury: the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2017;258:119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bertoni AG, Whitt-Glover MC, Chung H, Le KY, Barr RG, Mahesh M et al. The association between physical activity and subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol 2008;169:444–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Polonsky TS, Ning H, Daviglus ML, Liu K, Burke GL, Cushman M et al. Association of cardiovascular health with subclinical disease and incident events: the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc 2017;6:e004894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nettleton JA, Polak JF, Tracy R, Burke GL, Jacobs DR Jr. Dietary patterns and incident cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr 2009;90:647–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Horn LV et al. Defining and setting national goals for cardiovascular health promotion and disease reduction. Circulation 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 23. Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, David R, Jacobs J et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and coronary artery risk development in young adults (CARDIA) study. Radiology 2005;234:35–43. [DOI] [PubMed] [Google Scholar]
- 24. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827–32. [DOI] [PubMed] [Google Scholar]
- 25. Henderson BE, Feigelson HS. Hormonal carcinogenesis. Carcinogenesis 2000;21:427–33. [DOI] [PubMed] [Google Scholar]
- 26. Weir HK, Anderson RN, Coleman KS, Soman A, Thompson TD, Hong Y et al. Heart disease and cancer deaths—trends and projections in the United States, 1969-2020. Prev Chronic Dis 2016;13:E157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gapstur SM, Drope JM, Jacobs EJ, Teras LR, McCullough ML, Douglas CE et al. A blueprint for the primary prevention of cancer: targeting established, modifiable risk factors. CA Cancer J Clin 2018;68:446–70. [DOI] [PubMed] [Google Scholar]
- 28. Goding Sauer A, Siegel RL, Jemal A, Fedewa SA. Current prevalence of major cancer risk factors and screening test use in the United States: disparities by education and race/ethnicity. Cancer Epidemiol Biomarkers Prev 2019;28:629–42. [DOI] [PubMed] [Google Scholar]
- 29. Trabert B, Brinton LA, Anderson GL, Pfeiffer RM, Falk RT, Strickler HD et al. Circulating estrogens and postmenopausal ovarian cancer risk in the Women's Health Initiative Observational Study. Cancer Epidemiol Biomarkers Prev 2016;25:648–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Travis RC, Key TJ. Oestrogen exposure and breast cancer risk. Breast Cancer Res 2003;5:239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alfaddagh A, Martin SS, Leucker TM, Michos ED, Blaha MJ, Lowenstein CJ et al. Inflammation and cardiovascular disease: from mechanisms to therapeutics. Am J Prev Cardiol 2020;4:100130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Narayan V, Thompson EW, Demissei B, Ho JE, Januzzi JL Jr, Ky B. Mechanistic biomarkers informative of both cancer and cardiovascular disease: JACC State-of-the-Art Review. J Am Coll Cardiol 2020;75:2726–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, Maisonneuve P. Smoking and colorectal cancer: a meta-analysis. JAMA 2008;300:2765–78. [DOI] [PubMed] [Google Scholar]
- 34. Mehta LS, Watson KE, Barac A, Beckie TM, Bittner V, Cruz-Flores S et al. ; American Heart Association Cardiovascular Disease in Women and Special Populations Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; and Council on Quality of Care and Outcomes Research. Cardiovascular disease and breast cancer: where these entities intersect: a scientific statement from the American Heart Association. Circulation 2018;137:e30–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Whelton SP, Al Rifai M, Dardari Z, Shaw LJ, Al-Mallah MH, Matsushita K et al. Coronary artery calcium and the competing long-term risk of cardiovascular vs. cancer mortality: the CAC Consortium. Eur Heart J Cardiovasc Imaging 2019;20:389–95. [DOI] [PubMed] [Google Scholar]
- 36. Dzaye O, Al RM, Dardari Z, Shaw LJ, Al-Mallah MH, Handy MC et al. Coronary artery calcium as a synergistic tool for the age- and sex-specific risk of cardiovascular and cancer mortality: the coronary artery calcium consortium. J Am Heart Assoc 2020;9:e015306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Handy CE, Desai CS, Dardari ZA, Al-Mallah MH, Miedema MD, Ouyang P et al. The association of coronary artery calcium with noncardiovascular disease: the Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc Imaging 2016;9:568–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Osei AD, Mirbolouk M, Berman D, Budoff MJ, Miedema MD, Rozanski A et al. Prognostic value of coronary artery calcium score, area, and density among individuals on statin therapy vs. non-users: the coronary artery calcium consortium. Atherosclerosis 2021;316:79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shaw LJ, Raggi P, Berman DS, Callister TQ. Coronary artery calcium as a measure of biologic age. Atherosclerosis 2006;188:112–9. [DOI] [PubMed] [Google Scholar]
- 40. Messenger B, Li D, Nasir K, Carr JJ, Blankstein R, Budoff MJ. Coronary calcium scans and radiation exposure in the multi-ethnic study of atherosclerosis. Int J Cardiovasc Imaging 2016;32:525–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Miglioretti DL, Lange J, van den Broek JJ, Lee CI, van Ravesteyn NT, Ritley D et al. Radiation-induced breast cancer incidence and mortality from digital mammography screening: a modeling study. Ann Intern Med 2016;164:205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Giordano S, Hage FG, Xing D, Chen Y-F, Allon S, Chen C et al. Estrogen and cardiovascular disease: is timing everything? Am J Med Sci 2015;350:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Murphy E. Estrogen signaling and cardiovascular disease. Circ Res 2011;109:687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Barish R, Lynce F, Unger K, Barac A. Management of cardiovascular disease in women with breast cancer. Circulation 2019;139:1110–20. [DOI] [PubMed] [Google Scholar]
- 45. Bhoo-Pathy N, Subramaniam S, Zaharah H, Kong YC, Taib NA, Deniel A et al. Baseline prevalence of cardiovascular disease (CVD) risk factors in women with breast cancer. Ann Oncol 2018;29:viii613. [Google Scholar]
- 46. Patel J, Blaha MJ, McEvoy JW, Qadir S, Tota-Maharaj R, Shaw LJ et al. All-cause mortality in asymptomatic persons with extensive Agatston scores above 1000. J Cardiovasc Comput Tomogr 2014;8:26–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.