Abstract
Introduction
A recent questionnaire-based study suggested that bruxism and painful temporomandibular disorders (TMD pain) may be more prevalent in patients with Parkinson’s disease (PD) compared with controls. The presence of both bruxism and TMD pain may negatively influence patients’ quality of life. The present study is designed to clinically and more objectively investigate the presence of bruxism and TMD pain in patients with PD. The secondary aim of the study is to identify factors associated with bruxism and TMD pain in patients with PD, such as disease severity and dopaminergic medication usage. Furthermore, the presence of tooth wear in patients with PD will be studied as this can be a major consequence of bruxism. Finally, deviations in saliva composition that may contribute to tooth wear will be studied.
Methods and analysis
This is a single-centre observational outpatient study at the Amsterdam University Medical Centres, location VUmc. All patients with a clinical diagnosis of PD will be eligible for inclusion. Participants will fill in a set of questionnaires. Subsequently, patients will be examined clinically for, among others, TMD pain, presence and severity of tooth wear, and deviations in saliva composition. Sleep-time registrations will take place for 5 nights with the GrindCare GC4 (ie, a portable, single-channel electromyographic recorder) to assess sleep bruxism and simultaneously by the use of the BruxApp for 5 days to assess awake bruxism. We will partly use data collected during standard clinical care to minimise patient burden.
Ethics and dissemination
The scientific and ethical aspects of this study protocol have been approved by the Medical Ethics Review Committee of the Amsterdam UMC, location VUmc; NL. 2019.143. Informed consent will be obtained from all participants. The results will be published in a peer-reviewed journal, if relevant presented at conferences, and published as part of a PhD thesis.
Trial registration number
NL8307.
Keywords: Parkinson's disease, epidemiology, motor neurone disease, oral medicine
Strengths and limitations of this study.
This observational study will provide accurate data on the presence of painful temporomandibular disorders and bruxism in patients with Parkinson’s disease (PD) attending the outpatient clinic for movement disorders of Amsterdam UMC, location VUmc, and their possible associated factors like disease severity and medication usage.
Novel information about tooth wear and saliva composition and quantity in patients with PD will be collected.
Since polysomnographic recordings for the assessment of definite sleep bruxism are not feasible in this study, a portable, single-channel electromyographic recorder is used instead.
Electromyographic recordings will be performed for several nights in a row, thus taking into account the fluctuating nature of sleep bruxism.
Because of the absence of a control group, no direct comparisons between individuals with PD and similar individuals without PD can be made.
Introduction
Parkinson’s disease (PD) is a neurodegenerative movement disorder characterised by motor symptoms, in particular rigidity, bradykinesia and tremor.1 2 Patients with PD experience motor symptoms, and non-motor symptoms like anxiety, depression, sleep problems and cognitive dysfunction.3 4 Besides, pain has been reported as one of the most troublesome non-motor symptoms in patients with PD, early in their disease, which could affect patients’ quality of life.5 6
Due to global ageing, the prevalence of PD is estimated to increase significantly in the near future. Ageing is associated with oral health-related issues, which may therefore occur more frequently in the near future as well.7 Dentists regularly see patients with bruxism in the dental office, which is an oral health-related issue that is not necessarily associated with systemic diseases. Bruxism is currently defined as ‘a repetitive jaw-muscle activity characterised by clenching or grinding of the teeth and/or by bracing or thrusting of the mandible’.8 It can occur during sleep, indicated as sleep bruxism, or during wakefulness, indicated as awake bruxism.8 Bruxism and its possible consequences, such as mechanical tooth wear and temporomandibular disorders (TMD), have hardly been studied in patients with PD. TMD is a collective term embracing disorders of the temporomandibular joint, masticatory muscles and adjacent anatomical structures.9 TMD can present as painful and non-painful conditions. Patients with TMD can report, for example, orofacial pain (including headache), limitations in the movement of the mandible and joint noises.9 Both tooth wear and TMD may affect the oral health-related quality of life.10
In a population with patients with PD, oral health was recently studied.11 It was shown that the oral health in patients with PD is deteriorated as compared with their peers without PD. Besides, medication usage can influence salivation production, which in turn influences the oral environment.12 Also, gastrointestinal problems are more frequently shown in patients with PD. In turn, this could influence the presence of tooth wear due to reflux.13 14
While oral health in PD has not been studied widely,11 oral (dys-)function in PD has been studied even less, even though PD, bruxism and TMD have been suggested to share several common characteristics (figure 1). Similar to PD, bruxism is considered to be regulated centrally and not peripherally.15 In addition, in the pathophysiology of both PD and bruxism, the brain dopamine system plays an important role.16–18 Besides, sleep disturbances19 that are present both in PD20 and in sleep bruxism, are associated with arousal activity.19 21 As a result of such arousal activity, sleep bruxism may occur more frequently in people with sleep disturbances than in those without.21 Also, in the prodromal phase of PD, a higher rhythmic masticatory muscle activity on polysomnography in non-rapid eye movement sleep has been observed, compared with controls.22 This is a characteristic that is also seen in patients with sleep bruxism.23 Furthermore, bruxism may be considered as a risk factor for TMD, depending on the assessment methods used.24 TMD itself shares some characteristics with PD. For example, musculoskeletal pain (of which TMD pain is a subtype) is frequently reported by patients with PD.3 25 Finally, suggestions have been put forward that alterations in the dopaminergic system are also present in patients with pain in the orofacial region,26 although this remains to be confirmed in patients with TMD pain.
Figure 1.
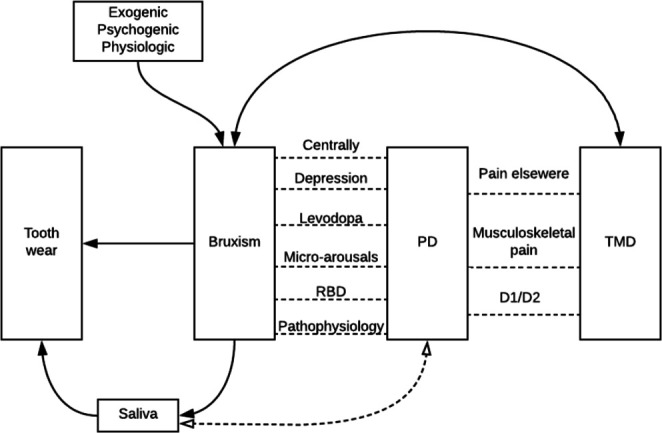
Visualisation of the possible interactions between the different research variables. Parkinson’s disease (PD) is associated with bruxism through different variables: the dopaminergic system (pathophysiology) plays a role in both PD and bruxism; the prodromal phase of PD (RBD) shows the same characteristics during sleep as does bruxism; sleep disturbances lead to micro-arousals that can lead to bruxism; dopaminergic medication (levodopa) can influence bruxism; depression is one of the risk factors for bruxism and is more prevalent in patients with PD and finally, both PD and bruxism are regulated centrally and not peripherally. PD is also associated with temporomandibular disorders (TMD): patients with PD experience pain in the entire body (ie, widespread pain), which is a risk factor for TMD pain; also, musculoskeletal pain (as in TMD pain) is frequently present and finally, alterations in the striatal dopaminergic system (D1/D2 ratio) could play a role in TMD pain. Bruxism itself is a risk factor for TMD pain and vice versa. Besides, tooth wear can be a consequence of bruxism. When bruxism occurs, saliva can be increased, which can be a protective factor for tooth wear. Also, saliva can be changed due to PD medication. Finally, several exogenic, psychogenic and physiological factors can be a risk for bruxism.
Recently, a questionnaire-based pilot study in 368 patients with PD and 340 controls suggested a higher prevalence of bruxism and TMD pain in patients with PD.27 Also, patients with PD reported a higher mean TMD pain intensity than controls.27 Besides, a large Taiwanese study showed a twofold increased risk of TMD in patients with PD as compared with controls.28 However, because of the limitations of the described studies (eg, questionnaire-based study27; no international validated clinical examination used; no detailed explanation of the clinical examination given and only patients with newly diagnosed TMD included),28 extrapolation of these findings requires further verification through clinical and instrumental data. Hence, to overcome some of the limitations, the present protocol was designed. The planned study will acquire more objective clinical and instrumental measures for awake and sleep bruxism and TMD pain, which can give more valid information on outcomes like the presence of bruxism in this population. Also, additional factors, such as the severity of PD and cognitive function, will be included as possible predictors for bruxism and/or TMD pain in patients with PD. Knowledge of the factors that can influence bruxism and/or TMD pain in patients with PD will help dentists and other oral healthcare providers to provide individualised care to prevent and/or alleviate symptoms of bruxism and/or TMD pain and their consequences in this vulnerable group of patients.
Based on the above-summarised evidence, the primary aim of this study is to investigate the presence of bruxism and TMD pain in patients with PD, through objective clinical and instrumental measurements. Based on our pilot study outcomes,27 we hypothesise that the prevalence of bruxism and TMD pain in the current population will be higher than in their peers without PD, as described in the literature.29 30
In addition, the secondary aims and their corresponding hypotheses are the following:
To identify which factors are associated with bruxism and TMD pain in patients with PD. We hypothesise that factors like medication usage,16 disease severity,15 17 psychosocial factors31–33 and lifestyle factors31 32 34 are influencing the studied associations.
To investigate whether the salivary flow, the pH and the buffer capacity of saliva in patients with PD are related to the severity of tooth wear. Our hypothesis is that in patients with PD, the saliva composition and salivary flow deviate from normal standards and that this is associated with the severity of tooth wear.14
To investigate with dopamine transporter single photon emission CT (DAT-SPECT) whether there is a relationship between the degree of presynaptic dopaminergic loss and the presence of bruxism in these patients. The hypothesis is that there is a difference in striatal dopaminergic deficit between patients with PD with and without bruxism, in which patients without bruxism show a smaller deficit.
Methods and analysis
The design of this study is a single-centre observational outpatient study that will take place at the Department of Neurology of the Amsterdam University Medical Centres (Amsterdam UMC), location VUmc. The data collection will take place for 2 years. Due to the COVID-19 pandemic, the start date is delayed. However, the estimated start and end dates will be January 2023 and January 2025, respectively.
Participants and eligibility
Patients already clinically diagnosed with PD or planned for an intake appointment with presumable PD at the outpatient clinic for movement disorders of the VUmc will be eligible to participate in the study. Yearly, about 100–120 new consultations for PD are seen in the outpatient clinic. In addition, patients already receiving treatment at the VUmc are eligible for participation as well. The inclusion and exclusion criteria are listed in table 1.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
| 1. ≥18 years of age | 1. Atypical parkinsonian syndromes |
| 2. ≥21 on the Montreal Cognitive Assessment53 | 2. For using the GrindCare: pacemaker |
| 3. Fulfil clinical diagnostic criteria for PD54 | 3. For using the BruxApp: no smartphone |
| 4. For the DAT-SPECT: no deep brain stimulation implant present |
When patients have a pacemaker, they cannot use the GrindCare GC4 (ie, a portable, single-channel electromyographic recorder to detect sleep bruxism) and will be excluded from that specific part of the study. When patients do not have a smartphone, participants cannot use the BruxApp (ie, an application on a smartphone to assess awake bruxism) and will be excluded from that specific part of the study.
DAT-SPECT, dopamine transporter single photon emission CT; PD, Parkinson’s disease.
Study procedure
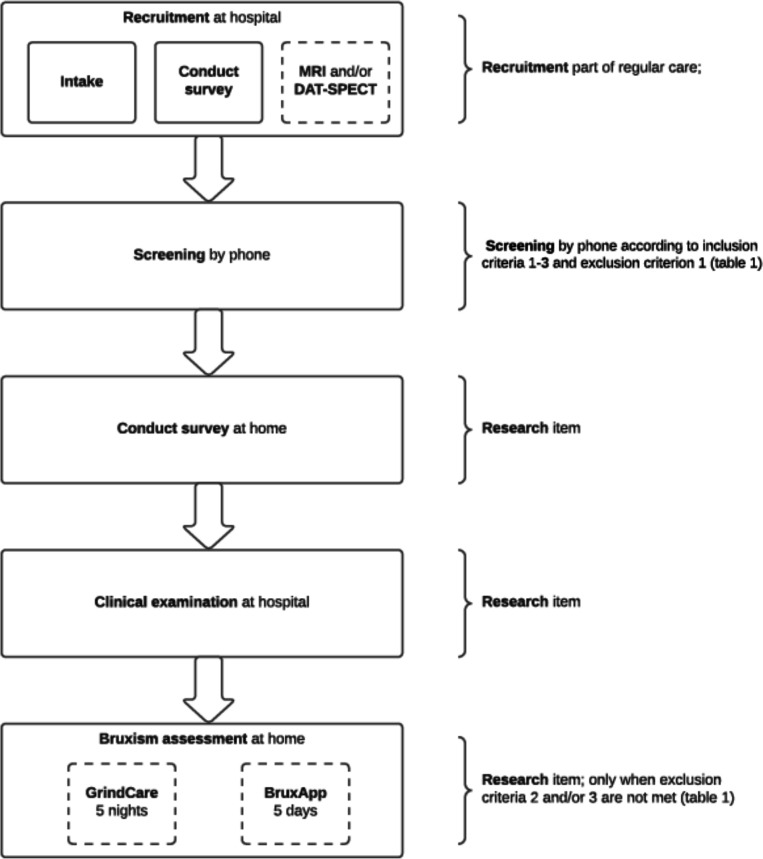
In figure 2, the study procedure is visualised. If patients agree to participate in the study, they will be asked to sign an informed consent. This study will be performed in parallel to the routine clinical care (box 1) at the Amsterdam UMC, location VUmc. When questionnaires/screenings were filled in ≥1 year ago, participants will be asked to repeat this. Specifically, for this study, additional information will be obtained in the form of a set of questionnaires that participants can fill in at home and of a clinical examination at the hospital (table 2). The neurologist will determine whether additional brain imaging (viz, MRI or DAT-SPECT) is necessary, mainly in cases of clinical doubt. The estimated percentage of additional brain imaging in newly referred patients is 40%.
Figure 2.
Flow chart of the study in which a distinction was made between the attendance of participants at the hospital and the study components at the participant’s home. The intake and first survey is part of the regular care at the hospital, and only followed by an additional MRI and/or dopamine transporter single photon emission CT (DAT-SPECT) scan when indicated (dashed line). When patients are eligible and consent to participate (screening by phone), the additional data are collected after the intake. First, a questionnaire is filled in by the participants. After that, the participant is invited for the clinical examination. When questionnaires/screenings that are part of the regular care were filled in ≥1 year ago, participants will be asked to repeat this procedure simultaneously with the additional questionnaire and/or clinical examination. Finally, participants will sleep for five complete registration nights with the GrindCare for the assessment of sleep bruxism (when exclusion criterion two was not met) and use the BruxApp for five complete registration days for the assessment of awake bruxism (when exclusion criterion 3 was not met).
Box 1. Questionnaires and clinical data collected as part of the regular care at the hospital, which is used in this observational study.
Variables standard care hospital:
Cognitive function (Montreal Cognitive Assessment35; Parkinson’s Disease Cognitive Functional Rating Scale).62
Disease stage (Hoehn and Yahr)63; disease severity (Unified Parkinson’s Disease Rating Scale-III).52
Dopaminergic medication (levodopa equivalent daily dose).64
Neuropsychiatric symptoms: depression (Beck Depression Inventory-ii)65; apathy (Apathy Evaluation Scale)66; anxiety (Parkinson Anxiety Scale)67; psychotic (Parkinson’s disease-adapted scale for assessment of positive symptoms)68; impulse control (Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease-Rating Scale).69
Presynaptic dopaminergic loss, when applicable (brain imaging) (dopamine transporter single photon emmission CT).42 44
Quality of sleep (Scales for Outcomes PD Sleep).70
Stimulants usage: alcohol (per unit, daily), drugs (per unit, daily), smoking (per unit, daily).
Table 2.
Additional research components, that is, performed in addition to the regular appointments at the hospital
| Questionnaires | 1. Reflux (GerdQ-NL)55 |
| 2. TMD pain (according to the DC/TMD)40 and intensity (graded chronic pain scale)56 | |
| 3. Tooth wear | |
| 4. Sleep (obstructive sleep apnoea, STOP-Bang NL)57 | |
| Clinical examination | 1. Intra-oral examination (positive symptoms of bruxism (viz, clenching marks in the soft tissues of the cheek, tongue or lip, mechanical tooth wear, hypertrophy of the masseter muscle))40 |
| 2. Quantitative tooth wear screening (part of the tooth wear evaluation system)58 | |
| 3. A brief screening of the dental prosthesis (when applicable) | |
| 4. Dry mouth screening (clinical oral dryness score)59 | |
| 5. Jaw-mobility examination (DC/TMD)40 | |
| 6. Joint noises examination (DC/TMD)40 | |
| 7. Palpation of masticatory muscles and temporomandibular joints (DC/TMD)40 | |
| 8. Dynamic/Static tests60 | |
| 9. Bruxoprovocation test60 | |
| 10. Saliva test (Saliva-Check Buffer)61 | |
| Registration | 1. BruxApp37 |
| 2. GrindCare GC435 36 |
See online supplemental appendix 1 for a description per questionnaire/instrument.
DC, diagnostic criteria; TMD, temporomandibular disorders.
bmjopen-2021-052329supp001.pdf (1.3MB, pdf)
Main study parameters
The main study parameters or end points are ‘presence of bruxism (sleep and/or awake)’ as well as ‘diagnosis of TMD pain’. For the assessment of sleep bruxism, patients will be asked to sleep five complete registration nights with a portable, single-channel electromyographic recorder, viz, the GrindCare GC4 (Sunstar Suisse, Etoy, Switzerland).35 36 For the assessment of awake bruxism, patients will use, for five complete registration days, the BruxApp,37 38 which is a mobile application for the recording of bruxism activity based on ecological momentary assessment.8 According to international consensus, a classification of the probability that bruxism is present can be made as follows: possible, probable and definite bruxism presence.39 In this research, all probabilities of bruxism presence can be determined, however, the highest probability will be used (viz, both probable and definite). When patients cannot use the GrindCare GC4 and/or BruxApp, and more certainty towards a definite presence is thus impossible, probable bruxism presence will be determined with the use of data from the clinical examination, based on the presence of positive symptoms of bruxism (viz, clenching marks in the soft tissues of the cheek, tongue or lip, mechanical tooth wear (attrition), and/or hypertrophy of the masseter muscle).39 Differences in PD symptoms between those who can, and those who cannot complete the instrumental assessments will be tested as to gain insight into the external validity or generalisability of the conclusions involving bruxism modelling.
The TMD pain diagnosis will be established according to the diagnostic criteria for TMD (DC/TMD),40 with the use of standardised questionnaires and clinical examination procedures. Based on the collected data, the following diagnoses can be set: myalgia (local myalgia, myofascial pain, myofascial pain with referral), arthralgia, headache attributed to TMD and non-painful joint disorders (disc displacement with reduction, disc displacement with reduction with intermitted locking, disc displacement without reduction with limited mouth opening, disc displacement without reduction without limited mouth opening, degenerative joint disease, subluxation). The main focus of this research protocol will be the TMD pain diagnosis, for the establishment of which the diagnostic flow chart of the DC/TMD will be used.40
Dentists making clinical assessments for bruxism or TMDs will blinded to the results of the instrumental assessments (ie, GrindCare GC4 and BruxApp for sleep bruxism and awake bruxism, respectively).
Secondary study parameters
To identify which factors are associated with bruxism and TMD pain in patients with PD, several variables will be evaluated (table 2, box 1), using different clinical/instrumental measures (online supplemental appendices 1-3). Most of these variables have already been reported as possible risk factors for bruxism32 and/or TMD41 in the general population.31–33 However, the variables dopaminergic medication usage and disease stage/severity of PD have not been studied yet in the association with bruxism or TMD pain in patients with PD. Finally, if DAT-SPECT imaging is available, we will compare the measured presynaptic striatal dopaminergic deficit between participants with and without bruxism.42
Sample size
According the pilot study, the prevalence of awake bruxism, sleep bruxism and TMD pain in patients with PD is 46%, 24% and 29.5%, respectively.23 Taking the cautious approach, we calculated the sample size for awake bruxism, sleep bruxism and TMD pain and chose the largest sample size. Aiming for a precision of 5% with a level of confidence of 95%, 246 participants are needed43 (see online supplemental appendix 2 for the sample size calculation). Furthermore, the approach to calculate the sample size for the most important secondary aim (viz, to identify which factors are associated with bruxism and TMD pain in patients with PD) is also shown in online supplemental appendix 2. The numbers are obtained when reaching the sample size for the primary aim.
Statistical approach
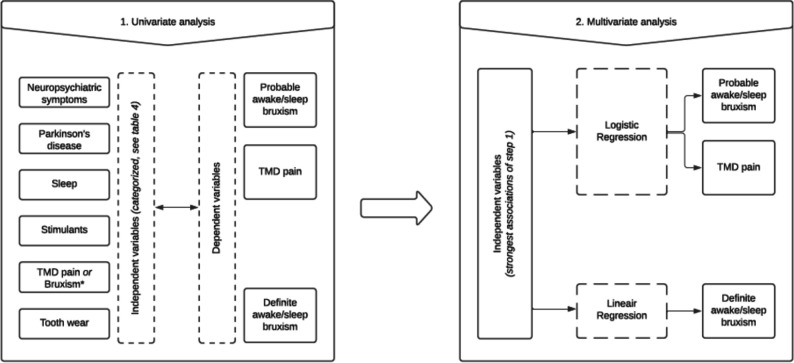
With the use of descriptive tests, demographic data will be summarised. In figure 3, it is shown how the dataset is analysed to give an answer on which factor is associated with the presence/absence of probable bruxism/TMD pain or with the frequency (ie, the number of bruxism events per hour) of definite bruxism. The forward selection procedure will be used for the (strongest) independent variables (box 2) until all variables in this regression model show a p value <0.05 (see step 2, figure 3). Finally, to analyse if there is an association between tooth wear and composition of saliva, Spearman’s correlation coefficient will be used. For the DAT-SPECT, a semi-quantitative analysis will be used. Ratios for specific versus non-specific binding will be calculated for the regions of interest (viz, left and right putamen and caudate nucleus, using the occipital cortex as a reference area) and analysed using the independent sample t-test.42 44
Figure 3.
Flow chart of the data analysis related to the first secondary aim: ‘to investigate which factors are influencing the presence of probable awake/sleep bruxism, temporomandibular disorders (TMD) pain and the frequency of definite awake/sleep bruxism’. All variables will be tested individually to see whether an association with the dependent variable (awake and sleep bruxism and TMD pain) exists, and to see how strong this association is (step 1). This dataset will be analysed for both probable bruxism and TMD pain with the use of logistic regression analyses. For definite bruxism, a linear regression analysis will be used (step 2). The forward selection procedure will be used for the (strongest) independent variables until all variables in this regression model show a p value <0.05 (step 2). In both step 1 and step 2, a distinction will be made between sleep and awake bruxism.
Box 2. The independent variables (categorised) that will be investigated for one of the secondary aims: which factors are associated with the presence of bruxism and temporomandibular disorders (TMD) pain in patients with Parkinson’s disease (PD)?
Independent variables (categorised)
Bruxism (when analysing which factors are associated with the presence of TMD pain in patients with PD).
Neuropsychiatric symptoms (depression, anxiety, apathy, psychosis, impulse disorders).
PD (disease stage, disease severity, medication usage, cognitive function).
Sleep (quality of sleep, obstructive sleep apnoea).
Stimulants usage (alcohol, smoking, drugs).
TMD pain (when analysing which factors are associated with the presence of bruxism in patients with PD).
Tooth wear related (reflux, saliva, dry mouth).
Patient and public involvement
Neither patients nor the community were involved in the design of this study. However, feedback from participants of the earlier pilot study23 was used to design this study. Patients with PD will be involved in the performance of the study. The burden for the participants will be kept as minimal as possible. On request, the outcomes of this study will be disseminated to the participants.
Discussion
The primary aim of this study is to objectively measure the presence of bruxism and TMD pain in a population of patients with PD. Furthermore, the three secondary aims are described as follows: (i) to identify which factors are associated with bruxism and TMD pain in patients with PD, (ii) to investigate whether the salivary flow, the pH and the buffer capacity of saliva in patients with PD are related to the severity of tooth wear and finally (iii) to investigate with DAT-SPECT whether there is a relationship between the degree of presynaptic dopaminergic loss and the presence of bruxism in these patients.
To the best of our knowledge, this is the first study that attempts to objectively measure the presence of awake bruxism, sleep bruxism and TMD pain in a population of patients with PD. Previous studies investigated the prevalence of awake bruxism in this population, however only few participants were included or only questionnaires were used.23 45 When quantifying bruxism with continuous data, recent insights showed a better quality of a definite bruxism diagnosis.39 Nevertheless, we used a dichotomous outcome in this protocol study to answer our first aim, that is, to investigate the presence of bruxism. Besides, we also included self-report and clinical data, which do not yield continuous outcomes. Despite this, in the present study, the use of the GrindCare GC4 and the BruxApp can give more certainty towards a definite establishment of sleep and awake bruxism, respectively.39 This enables the analysis of continuous outcomes, which has been suggested by several authors.46 47 However, as mentioned earlier, not every participant will be able to use the GrindCare GC4 and/or the BruxApp. Therefore, this protocol is designed to include all probability levels for the assessment of bruxism, which contributes to the feasibility of this protocol.39 Importantly, participants able to complete all assessments may differ from those who cannot complete instrumental assessments due to differences in severity of their PD symptoms. Fine motor problems which occur in PD create barriers for electrode placement and cell phone use as required for instrumental assessments of sleep and awake bruxism. Therefore, we will test for PD symptom differences between subgroups defined by comparing participants completing or not completing instrumental assessments. If differences are found, this will indicate limitations to the external validity or generalisability of conclusions involving bruxism modelling.
In addition, the clinical examination according to the DC/TMD40 enables setting a valid TMD pain diagnosis, making a distinction between several TMD complaints, and comparing the outcomes with other (inter-) national research. An important aspect of a TMD pain diagnosis according to the DC/TMD is that it considers the aspect of ‘familiar pain’ as part of the diagnostic algorithm. As such, PD-related pain characteristics like pain exacerbation due to ‘wearing off’ of dopaminergic medication and lower pain thresholds in individuals living with PD as compared with similar individuals without PD48 will be taken into account.
Because patients with PD are vulnerable and burdened with frequent visits to multiple caregivers (eg, their neurologist, physiotherapist and speech therapist), it is important to burden the participants as minimally as possible. Therefore, during the process of designing this study and collecting the data, a multidisciplinary approach was established between neurologists and dentists to enable an as efficient as possible usage of the patient’s time and energy.
The targeted number of inclusions will be a challenge. However, the calculated sample size is an estimation, because no clinical prevalences are known as yet. Like in otherwise healthy individuals, clenching and grinding are not always recognised by the patients themselves,49 50 thus the prevalence of sleep bruxism in the pilot study could have been underestimated. This means that the calculated sample size in this study might be higher than eventually required. Therefore, an interim analysis will be performed after 130 included participants or 6 months.
This study has no longitudinal character and therefore, no causal relations can be observed between the (in-) dependent variables. Also, polysomnography is the golden standard to detect sleep bruxism while in the present study, a portable electromyographic recorder will be used.39 However, since this device will be used for several nights in a row, the fluctuating character of sleep bruxism can be taken into account and is therefore considered a good proxy for definite sleep bruxism.36 It should be noted, however, that the portable recorder will fail to enable a distinction between jaw-muscle activities related to sleep bruxism and those related to other orofacial movement disorders like oral dyskinesia and oromandibular dystonia.51 This is an important issue, because such movement disorders can be present in patients with PD related to their medication usage. In fact, in their updated international consensus paper on bruxism, Lobbezoo et al added the phrase that bruxism is a masticatory muscle activity in ‘otherwise healthy individuals’.39 People living with PD are certainly not ‘otherwise healthy’. In the later stages of levodopa-treated PD, dyskinesias, including oral dyskinesias, commonly occur.51 Hence, the question could be raised if the masticatory muscle activity observed in people with PD is ‘bruxism’ at all. This calls for caution in the interpretation of the bruxism-related findings of this study. Fortunately, in the questionnaire and clinical examination of the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale-III52 (box 1), the presence of oral dyskinesia and oromandibular dystonia is included. Hence, it is possible to correct for their presence in the data analysis.
This study does not include a control group. This limits the interpretation of whether the prevalence of bruxism or TMDs is low or high in people with PD, which will only be possible by comparing the findings with prevalences as reported in the literature. In addition, since tooth wear in older people reflects a lifetime of factors, it will be also difficult to interpret the tooth wear findings in people with PD without having the possibility for a direct comparison with similar individuals without PD. Also in this case, comparisons should be sought with literature data. These issues should be considered limitations of this study.
In conclusion, this study will give more detailed information about the presence of bruxism and TMD pain in patients with PD, as well as about possible associated factors like medication usage and severity of the disease. Finally, more clinically relevant information will become available for dentists and other oral healthcare professionals about the amount of tooth wear and the composition of saliva in patients with PD.
Ethics and dissemination
This study protocol has been approved by the Medical Ethics Review Committee of Amsterdam UMC, location VUmc; NL. 2019.143. Informed consent will be obtained from all participants. A data monitor will meet annually to primarily concentrate on the safety of patients, and will be monitoring the collected data and informed consents. The results will be published in peer-reviewed journals, if relevant presented at conferences, and published as part of a PhD thesis.
Due to the sensitive nature of personal information, all data will be blinded and stored in secure environments. Only the executive researcher and the head of the department can reach the unblinded informed consents and the key for unblinding. These are stored separately. Digital data will be stored pseudonymised in a secure database using Castor EDC (CDISC, Amsterdam, The Netherlands). Detailed methods for data management and storage can be obtained by contacting the corresponding author.
Supplementary Material
Footnotes
Contributors: All authors were involved in designing this study. MCV obtained the approval of the Medical Ethics Review Committee and drafted the manuscript. Finally, all authors gave feedback on the draft and approved the final manuscript.
Funding: This work was partly supported by the foundation for Oral Health and Parkinson’s Disease (Stichting Mondzorg & Parkinson), the Dutch association for scientific dentistry (Nederlandse Wetenschappelijke Vereniging voor Tandheelkunde (NWVT)) and the Dutch association for Orofacial Pain, Dysfunction and Prosthetic Dentistry (Nederlandse Vereniging voor Gnathologie en Prothethische Tandheelkunde (NVGPT)).
Competing interests: FL reports grants and other from Sunstar Suisse, grants from Somnomed, Airway Management, Vivisol-Resmed, Health Holland/TKI, outside the submitted work.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
References
- 1.Kalia LV, Lang AE, Shulman G. Parkinson's disease. Lancet 2015;386:896–912. 10.1016/S0140-6736(14)61393-3 [DOI] [PubMed] [Google Scholar]
- 2.Opara J, Małecki A, Małecka E, et al. Motor assessment in Parkinson's disease. Ann Agric Environ Med 2017;24:411–5. 10.5604/12321966.1232774 [DOI] [PubMed] [Google Scholar]
- 3.Khoo TK, Yarnall AJ, Duncan GW, et al. The spectrum of nonmotor symptoms in early Parkinson disease. Neurology 2013;80:276–81. 10.1212/WNL.0b013e31827deb74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaudhuri KR, Healy DG, Schapira AHV, et al. Non-Motor symptoms of Parkinson's disease: diagnosis and management. Lancet Neurol 2006;5:235–45. 10.1016/S1474-4422(06)70373-8 [DOI] [PubMed] [Google Scholar]
- 5.Politis M, Wu K, Molloy S, et al. Parkinson's disease symptoms: the patient's perspective. Mov Disord 2010;25:1646–51. 10.1002/mds.23135 [DOI] [PubMed] [Google Scholar]
- 6.Silverdale MA, Kobylecki C, Kass-Iliyya L, et al. A detailed clinical study of pain in 1957 participants with early/moderate Parkinson’s disease. Parkinsonism Relat Disord 2018;56:27–32. 10.1016/j.parkreldis.2018.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ettinger RL. Oral health and the aging population. J Am Dent Assoc 2007;138 Suppl:S5–6. 10.14219/jada.archive.2007.0357 [DOI] [PubMed] [Google Scholar]
- 8.Lobbezoo F, Ahlberg J, Glaros AG, et al. Bruxism defined and graded: an international consensus. J Oral Rehabil 2013;40:2–4. 10.1111/joor.12011 [DOI] [PubMed] [Google Scholar]
- 9.de LR, Klasser GD. Orofacial pain: guidelines for assessment, diagnosis, and management. 5th ed. Chicago: Quintessence Publishing Co, 2013. [Google Scholar]
- 10.Papagianni CE, van der Meulen MJ, Naeije M, et al. Oral health-related quality of life in patients with tooth wear. J Oral Rehabil 2013;40:185–90. 10.1111/joor.12025 [DOI] [PubMed] [Google Scholar]
- 11.van SMAE, Marinus J, Van HJJ. Oral Health of Parkinson’s Disease Patients: A Case-Control Study. Parkinson Dis 2018:e9315285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villa A, Wolff A, Narayana N, et al. World workshop on oral medicine VI: a systematic review of Medication-induced salivary gland dysfunction. Oral Dis 2016;22:365–82. 10.1111/odi.12402 [DOI] [PubMed] [Google Scholar]
- 13.Maeda T, Nagata K, Satoh Y. High prevalence of gastroesophageal reflux disease in Parkinson’s disease: A questionnaire-based study. Parkinsons Dis 2013:e742128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wetselaar P, Manfredini D, Ahlberg J, et al. Associations between tooth wear and dental sleep disorders: a narrative overview. J Oral Rehabil 2019;46:765–75. 10.1111/joor.12807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lobbezoo F, Naeije M. Bruxism is mainly regulated centrally, not peripherally. J Oral Rehabil 2001;28:1085–91. 10.1046/j.1365-2842.2001.00839.x [DOI] [PubMed] [Google Scholar]
- 16.Lobbezoo F, Lavigne GJ, Tanguay R, et al. The effect of catecholamine precursor L-dopa on sleep bruxism: a controlled clinical trial. Mov Disord 1997;12:73–8. 10.1002/mds.870120113 [DOI] [PubMed] [Google Scholar]
- 17.Lobbezoo F, Soucy JP, Montplaisir JY, et al. Striatal D2 receptor binding in sleep bruxism: a controlled study with iodine-123-iodobenzamide and single-photon-emission computed tomography. J Dent Res 1996;75:1804–10. 10.1177/00220345960750101401 [DOI] [PubMed] [Google Scholar]
- 18.Lobbezoo F, Soucy JP, Hartman NG, et al. Effects of the D2 receptor agonist bromocriptine on sleep bruxism: report of two single-patient clinical trials. J Dent Res 1997;76:1610–4. 10.1177/00220345970760091401 [DOI] [PubMed] [Google Scholar]
- 19.Albers JA, Chand P, Anch AM. Multifactorial sleep disturbance in Parkinson's disease. Sleep Med 2017;35:41–8. 10.1016/j.sleep.2017.03.026 [DOI] [PubMed] [Google Scholar]
- 20.Menza M, Dobkin RD, Marin H, et al. Sleep disturbances in Parkinson's disease. Mov Disord 2010;25 Suppl 1:S117–22. 10.1002/mds.22788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kato T, Montplaisir JY, Guitard F, et al. Evidence that experimentally induced sleep bruxism is a consequence of transient arousal. J Dent Res 2003;82:284–8. 10.1177/154405910308200408 [DOI] [PubMed] [Google Scholar]
- 22.Abe S, Gagnon J-F, Montplaisir JY, et al. Sleep bruxism and oromandibular myoclonus in rapid eye movement sleep behavior disorder: a preliminary report. Sleep Med 2013;14:1024–30. 10.1016/j.sleep.2013.04.021 [DOI] [PubMed] [Google Scholar]
- 23.Lavigne GJ, Rompré PH, Poirier G, et al. Rhythmic masticatory muscle activity during sleep in humans. J Dent Res 2001;80:443–8. 10.1177/00220345010800020801 [DOI] [PubMed] [Google Scholar]
- 24.Manfredini D, Lobbezoo F. Relationship between bruxism and temporomandibular disorders: a systematic review of literature from 1998 to 2008. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010;109:e26–50. 10.1016/j.tripleo.2010.02.013 [DOI] [PubMed] [Google Scholar]
- 25.Tai Y-C, Lin C-H. An overview of pain in Parkinson's disease. Clin Park Relat Disord 2020;2:1–8. 10.1016/j.prdoa.2019.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagelberg N, Forssell H, Aalto S, et al. Altered dopamine D2 receptor binding in atypical facial pain. Pain 2003;106:43–8. 10.1016/S0304-3959(03)00275-6 [DOI] [PubMed] [Google Scholar]
- 27.Verhoeff MC, Lobbezoo F, Wetselaar P, et al. Parkinson's disease, temporomandibular disorders and bruxism: a pilot study. J Oral Rehabil 2018;45:854–63. 10.1111/joor.12697 [DOI] [PubMed] [Google Scholar]
- 28.Chen Y-Y, Fan H-C, Tung M-C, et al. The association between Parkinson's disease and temporomandibular disorder. PLoS One 2019;14:e0217763. 10.1371/journal.pone.0217763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wetselaar P, Vermaire EJH, Lobbezoo F, et al. The prevalence of awake bruxism and sleep bruxism in the Dutch adult population. J Oral Rehabil 2019;46:617–23. 10.1111/joor.12787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Visscher CM, Ligthart L, Schuller AA, et al. Comorbid disorders and sociodemographic variables in temporomandibular pain in the general Dutch population. J Oral Facial Pain Headache 2015;29:51–9. 10.11607/ofph.1324 [DOI] [PubMed] [Google Scholar]
- 31.van Selms MKA, Visscher CM, Naeije M, et al. Bruxism and associated factors among Dutch adolescents. Community Dent Oral Epidemiol 2013;41:353–63. 10.1111/cdoe.12017 [DOI] [PubMed] [Google Scholar]
- 32.Manfredini D, Lobbezoo F. Role of psychosocial factors in the etiology of bruxism. J Orofac Pain 2009;23:153–66. [PubMed] [Google Scholar]
- 33.Winocur E, Uziel N, Lisha T, et al. Self-reported bruxism - associations with perceived stress, motivation for control, dental anxiety and gagging. J Oral Rehabil 2011;38:3–11. 10.1111/j.1365-2842.2010.02118.x [DOI] [PubMed] [Google Scholar]
- 34.Castroflorio T, Bargellini A, Rossini G, et al. Sleep bruxism and related risk factors in adults: a systematic literature review. Arch Oral Biol 2017;83:25–32. 10.1016/j.archoralbio.2017.07.002 [DOI] [PubMed] [Google Scholar]
- 35.Yachida W, Arima T, Castrillon EE, et al. Diagnostic validity of self-reported measures of sleep bruxism using an ambulatory single-channel EMG device. J Prosthodont Res 2016;60:250–7. 10.1016/j.jpor.2016.01.001 [DOI] [PubMed] [Google Scholar]
- 36.Stuginski-Barbosa J, Porporatti AL, Costa YM, et al. Diagnostic validity of the use of a portable single-channel electromyography device for sleep bruxism. Sleep Breath 2016;20:695–702. 10.1007/s11325-015-1283-y [DOI] [PubMed] [Google Scholar]
- 37.Bracci A, Djukic G, Favero L, et al. Frequency of awake bruxism behaviours in the natural environment. A 7-day, multiple-point observation of real-time report in healthy young adults. J Oral Rehabil 2018;45:423–9. 10.1111/joor.12627 [DOI] [PubMed] [Google Scholar]
- 38.Manfredini D, Winocur E, Guarda-Nardini L, et al. Epidemiology of bruxism in adults: a systematic review of the literature. J Orofac Pain 2013;27:99–110. 10.11607/jop.921 [DOI] [PubMed] [Google Scholar]
- 39.Lobbezoo F, Ahlberg J, Raphael KG, et al. International consensus on the assessment of bruxism: report of a work in progress. J Oral Rehabil 2018;45:837–44. 10.1111/joor.12663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schiffman E, Ohrbach R, Truelove E, et al. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: recommendations of the International RDC/TMD Consortium Network* and orofacial pain special interest Group†. J Oral Facial Pain Headache 2014;28:6–27. 10.11607/jop.1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Durham J, Newton-John TRO, Zakrzewska JM. Temporomandibular disorders. BMJ 2015;350:h1154. 10.1136/bmj.h1154 [DOI] [PubMed] [Google Scholar]
- 42.van Dijk KD, Bidinosti M, Weiss A, et al. Reduced α-synuclein levels in cerebrospinal fluid in Parkinson's disease are unrelated to clinical and imaging measures of disease severity. Eur J Neurol 2014;21:388–94. 10.1111/ene.12176 [DOI] [PubMed] [Google Scholar]
- 43.Naing L, T. Winn, B.N. Rusli practical issues in calculating the sample size for prevalence studies. Archives of Orofacial Sciences 2006;1:9–14. [Google Scholar]
- 44.Berendse HW, Roos DS, Raijmakers P, et al. Motor and non-motor correlates of olfactory dysfunction in Parkinson’s disease. J Neurol Sci 2011;310:21–4. 10.1016/j.jns.2011.06.020 [DOI] [PubMed] [Google Scholar]
- 45.Ella B, Ghorayeb I, Burbaud P, et al. Bruxism in movement disorders: a comprehensive review. J Prosthodont 2017;26:599–605. 10.1111/jopr.12479 [DOI] [PubMed] [Google Scholar]
- 46.Manfredini D, Ahlberg J, Wetselaar P, et al. The bruxism construct: from cut-off points to a continuum spectrum. J Oral Rehabil 2019;46:991–7. 10.1111/joor.12833 [DOI] [PubMed] [Google Scholar]
- 47.Raphael KG, Santiago V, Lobbezoo F. Is bruxism a disorder or a behaviour? rethinking the International consensus on defining and grading of bruxism. J Oral Rehabil 2016;43:791–8. 10.1111/joor.12413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sung S, Vijiaratnam N, Chan DWC, et al. Pain sensitivity in Parkinson's disease: systematic review and meta-analysis. Parkinsonism Relat Disord 2018;48:17–27. 10.1016/j.parkreldis.2017.12.031 [DOI] [PubMed] [Google Scholar]
- 49.Goldstein RE, Auclair Clark W. The clinical management of awake bruxism. J Am Dent Assoc 2017;148:387–91. 10.1016/j.adaj.2017.03.005 [DOI] [PubMed] [Google Scholar]
- 50.Kawakami S, Kumazaki Y, Manda Y, et al. Specific diurnal EMG activity pattern observed in occlusal collapse patients: relationship between diurnal bruxism and tooth loss progression. PLoS One 2014;9:e101882. 10.1371/journal.pone.0101882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lobbezoo F. Taking up challenges at the interface of wear and tear. J Dent Res 2007;86:101–3. 10.1177/154405910708600201 [DOI] [PubMed] [Google Scholar]
- 52.Goetz CG, Tilley BC, Shaftman SR, et al. Movement disorder Society-sponsored revision of the unified Parkinson's disease rating scale (MDS-UPDRS): scale presentation and Clinimetric testing results. Mov Disord 2008;23:2129–70. 10.1002/mds.22340 [DOI] [PubMed] [Google Scholar]
- 53.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–9. 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 54.Postuma RB, Berg D, Stern M, et al. Mds clinical diagnostic criteria for Parkinson's disease. Mov Disord 2015;30:1591–601. 10.1002/mds.26424 [DOI] [PubMed] [Google Scholar]
- 55.Jonasson C, Wernersson B, Hoff DAL, et al. Validation of the GerdQ questionnaire for the diagnosis of gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2013;37:564–72. 10.1111/apt.12204 [DOI] [PubMed] [Google Scholar]
- 56.Von Korff M, Ormel J, Keefe FJ, et al. Grading the severity of chronic pain. Pain 1992;50:133–49. 10.1016/0304-3959(92)90154-4 [DOI] [PubMed] [Google Scholar]
- 57.Chung F, Abdullah HR, Liao P. Stop-Bang questionnaire: a practical approach to screen for obstructive sleep apnea. Chest 2016;149:631–8. 10.1378/chest.15-0903 [DOI] [PubMed] [Google Scholar]
- 58.Wetselaar P, Lobbezoo F. The tooth wear evaluation system: a modular clinical guideline for the diagnosis and management planning of worn dentitions. J Oral Rehabil 2016;43:69–80. 10.1111/joor.12340 [DOI] [PubMed] [Google Scholar]
- 59.Das P, Challacombe SJ. Dry mouth and clinical oral dryness scoring systems. Prim Dent J 2016;5:77–9. 10.1177/205016841600500110 [DOI] [PubMed] [Google Scholar]
- 60.Visscher CM, Naeije M, De Laat A, et al. Diagnostic accuracy of temporomandibular disorder pain tests: a multicenter study. J Orofac Pain 2009;23:108–14. [PubMed] [Google Scholar]
- 61.Maldupa I, Brinkmane A, Mihailova A. Comparative analysis of crt buffer, GC saliva check buffer tests and laboratory titration to evaluate saliva buffering capacity. Stomatologija 2011;13:55–61. [PubMed] [Google Scholar]
- 62.Kulisevsky J, Fernández de Bobadilla R, Pagonabarraga J, et al. Measuring functional impact of cognitive impairment: validation of the Parkinson's disease cognitive functional rating scale. Parkinsonism Relat Disord 2013;19:812–7. 10.1016/j.parkreldis.2013.05.007 [DOI] [PubMed] [Google Scholar]
- 63.Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality parkinsonism: onset, progression, and mortality. Neurology 1967;17:427–42. [DOI] [PubMed] [Google Scholar]
- 64.Tomlinson CL, Stowe R, Patel S, et al. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 2010;25:2649–53. 10.1002/mds.23429 [DOI] [PubMed] [Google Scholar]
- 65.Beck AT, Steer RA, Brown GK. Manual for the Beck depression inventory-II. San Antonio, TX Psychol Corp 1996;4:561–71. [Google Scholar]
- 66.Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the apathy evaluation scale. Psychiatry Res 1991;38:143–62. 10.1016/0165-1781(91)90040-V [DOI] [PubMed] [Google Scholar]
- 67.Leentjens AFG, Dujardin K, Pontone GM, et al. The Parkinson anxiety scale (PAS): development and validation of a new anxiety scale. Mov Disord 2014;29:1035–43. 10.1002/mds.25919 [DOI] [PubMed] [Google Scholar]
- 68.Voss T, Bahr D, Cummings J, et al. Performance of a shortened Scale for Assessment of Positive Symptoms for Parkinson’s disease psychosis. Parkinsonism Relat Disord 2013;19:295–9. 10.1016/j.parkreldis.2012.10.022 [DOI] [PubMed] [Google Scholar]
- 69.Weintraub D, Mamikonyan E, Papay K, et al. Questionnaire for Impulsive-Compulsive disorders in Parkinson's Disease-Rating scale. Mov Disord 2012;27:242–7. 10.1002/mds.24023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marinus J, Visser M, van Hilten JJ, et al. Assessment of sleep and sleepiness in Parkinson disease. Sleep 2003;26:1049–54. 10.1093/sleep/26.8.1049 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-052329supp001.pdf (1.3MB, pdf)