Abstract
Context
Reduced estrogen levels in postmenopausal women predispose them to metabolic side effects, including insulin resistance and type 2 diabetes; however, the cellular mechanisms are not well understood.
Objective
This work aimed to study the expression of estrogen receptors in adipose tissue from pre- and postmenopausal women and the effects of estradiol (E2) on glucose uptake of adipocytes.
Methods
Subcutaneous (SAT) and visceral adipose tissue (VAT) obtained from pre- and postmenopausal women (19-51 and 46-75 years old, respectively) were used to measure gene expression of ESR1 and ESR2. SAT tissue was incubated with E2, and glucose uptake and estrogen receptor levels were measured. Polymorphisms in ESR1 and ESR2 were addressed in public databases to identify single nucleotide polymorphisms associated with metabolic traits.
Results
ESR2 expression was lower in pre- vs postmenopausal women, corresponding to lower ESR1:ESR2 gene expression ratio in postmenopausal women. In premenopausal women, the expression of ESR1 was higher in VAT than in SAT. In both pre- and postmenopausal women, ESR2 expression was lower in VAT than in SAT. In late, but not pre- or early postmenopausal women, E2 reduced glucose uptake and GLUT4 protein and increased expression of ESR2. ESR1 polymorphisms were associated with weight, body fat distribution, and total cholesterol, and ESR2 polymorphisms were associated with total cholesterol and triglyceride levels and with body fat percentage.
Conclusion
E2 inhibits glucose utilization in human adipocytes in late postmenopausal women. Changes in glucose utilization over time since menopause may be explained by a lower ESR1:ESR2 ratio. This can have clinical implications on the timing of estrogen treatment in postmenopausal women.
Keywords: estradiol, adipose tissue, menopause, insulin resistance, type 2 diabetes
Estrogens are steroid hormones that play an important role in metabolic regulation. Their actions are mediated by estrogen receptors (ERs), such as ER alpha (ESR1) and ER beta (ESR2), which are encoded by the ESR1 and ESR2 genes, respectively. A decline in the circulating estrogen, 17β-estradiol (E2), due to either natural or surgical menopause is not only limited to reproductive health but can also impact metabolic homeostasis in women (1). For instance, E2 deficiency and its dysregulated cellular actions can result in central obesity, which is associated with an increased risk of insulin resistance, type 2 diabetes (T2D), dyslipidemia, and cardiovascular disease (1). Furthermore, the chances of developing an obesity-related disease is lower in premenopausal women than men; however, this difference is lost following menopause, highlighting the role of estrogen levels on metabolic regulation (2). The prevalence of disturbed glucose metabolism is lower in women, as it is apparent that women have greater protection against insulin resistance and T2D before menopause (3, 4). This may result from factors such as sexually dimorphic fat accumulation and differences in circulating hormones (4). Therefore, it is evident that estrogen has an important role in regulating systemic energy metabolism.
Adipose tissue has a central role in the regulation of metabolic homeostasis, as it functions as an endocrine organ and energy reservoir. Its actions can be regulated by the availability of nutrients, stress, and hormones (5). Human adipose tissue expresses both ESR1 and ESR2, which are differentially expressed during adipocyte differentiation (6). The expression of ESR1 and ESR2 in adipose tissue has long been discovered, but their impact on adipose tissue function is still not completely understood. To date, most studies have delineated the role of ESR1 and have suggested its protective role against metabolic disturbances. For instance, it has been shown that whole-body ESR1 knockout mice have metabolic alterations such as impaired glucose tolerance, insulin resistance, and adipocyte hyperplasia (7). The role of ESR2 in adipocytes and adipose tissue is less understood than ESR1; however, studies have suggested ESR2 to have a diabetogenic effect (8). The administration of an ESR2 agonist has been shown to reduce skeletal muscle GLUT4 levels, which plays an important role in glucose homeostasis (9). Furthermore, in tissues where ESR1 and ESR2 are both expressed, such as in muscle, it has been shown that it is not E2 that dictates effects but instead the ratio of ESR1 to ESR2 that determines what action is elicited (9). Therefore, understanding the different roles of ESR1and ESR2 in glucose metabolism and whether ligands of these receptors are diabetogenic under circumstances such as after menopause is crucial to further characterize the effects of estrogen on the development of insulin resistance and T2D.
There are age-related differences in the expression of genes corresponding to ERs in adipose tissue. For example, a reduction in ESR1 and ESR1:ESR2 gene ratio in adipose tissue was reported in subcutaneous adipose tissue (SAT) from postmenopausal women compared to premenopausal women (10). Moreover, time since menopause has also been suggested to effect E2 action and thereby whole-body insulin sensitivity (11, 12). For instance, 1 clinical study showed that E2 treatment in early menopausal women lead to improved glucose disposal, whereas administration in late postmenopausal women lead to a reduction (11). Nevertheless, the cellular mechanisms are not completely known. Taken together, we wanted to determine differences in expression of ERs in human SAT from pre- and postmenopausal women, which would be important in understanding differential effects carried out by E2. This, in turn, may help identify novel molecular targets for the treatment of metabolic diseases such as diabetes.
The objective of the present study was to characterize gene expression of ERs primarily in SAT from pre- and postmenopausal women with a wide range of body mass indices (BMIs) and investigate associations with different anthropometric characteristics and polymorphisms. In addition, we investigated the direct effects of E2 on glucose uptake capacity of adipocytes obtained from pre- and postmenopausal women and the underlying mechanisms. Moreover, we compared the impact of time since menopause on E2’s effect on adipocyte glucose uptake.
Subjects and Methods
Subjects
This study consisted of 67 premenopausal women and 79 postmenopausal women. Women were considered postmenopausal when more than 12 months had passed since the last menstruation, and the time was considered to be that of the last menstruation (13). Premenopause was defined in women who were not postmenopausal and having any menstruation during the last 12 months. We defined early postmenopausal when less than 6 years had elapsed after menopause based on previous literature (11, 14). Among postmenopausal women, 11 women were early postmenopausal (51-61 years old, BMI 25.7-31.6 kg/m2), and 42 were late postmenopausal (54-74 years old, BMI: 21.2-40.9 kg/m2). Data on years since the start of menopause was known for 53 subjects. The remaining postmenopausal women were not used for the subgroup analyses. Subjects with diabetes, polycystic ovarian syndrome (PCOS) and other endocrine disorders, cancer, or other major illnesses, as well as ongoing medication with beta-adrenergic blockers, systemic glucocorticoids, or immune-modulating therapies, were excluded from the study. Six postmenopausal women had previously undergone a hysterectomy before menopause and were defined menopausal due to age. Two subjects had previously undergone a bilat oophorectomy. One premenopausal woman had an intrauterine device (hormonal coil) and 1 late postmenopausal woman was on hormone replacement therapy (estriol).
Subjects arrived in the morning after an overnight fast at a clinical research unit at the respective hospital. Anthropometric measurements were performed, and fasting blood samples were taken to determine hemoglobin A1c (HbA1c), glucose, lipids, insulin, and C-peptide levels. Abdominal SAT was obtained by needle aspiration (56 premenopausal, 70 postmenopausal) after administration of local anesthetic lidocaine (Xylocain; AstraZeneca, Sweden) in the lower 2 quadrants of the abdomen. In addition, paired SAT and visceral adipose tissue (VAT) were obtained from 11 pre- and 9 postmenopausal women undergoing bariatric surgery or kidney donation at Uppsala and Gothenburg University Hospitals. Part of the adipose tissue was snap-frozen and stored at −80°C until used to analyze the expression of ERs, and part was used to perform metabolic assays. Not all subjects were used for all experiments due to limitations in adipose tissue quantity. Samples from 102 subjects were used for gene expression experiments, 30 for effects of E2 on glucose uptake, and 17 for protein analyses with Western blots. The anthropometric and biochemical characteristics of the subjects are presented in Table 1.
Table 1.
Anthropometric and clinical characteristics of study participants
| Premenopausal | Postmenopausala | P | |
|---|---|---|---|
| n | 67 | 79 | |
| Age, years | 34 (26, 45) | 66 (61, 70) | <0.001 |
| Time since menopause, years | NA | 16 (9, 20) | |
| BMI, kg/m2 | 33.2 (25.6, 43.8) | 27.9 (24.7, 32.0) | 0.004 |
| WHR | 0.85 (0.82, 0.89) | 0.90 (0.86, 0.94) | <0.001 |
| Body fat, % | 34.2 (27.4, 37.4) | 37.6 (34.1, 42.6) | 0.011 |
| Serum insulin, mU/L | 10.3 (7.0, 16.9) | 7.8 (6.1, 12.7) | 0.002 |
| Plasma glucose, mmol/L | 5.6 (5.3, 5.9) | 5.7 (5.5, 6.2) | 0.010 |
| HOMA-IR | 2.2 (1.5, 4.0) | 2.1 (1.4, 3.3) | 0.442 |
| Total cholesterol | 4.6 (3.7, 5.0) | 5.4 (4.9, 6.4) | <0.001 |
| Plasma HDL-cholesterol, mmol/L | 1.2 (0.9, 1.5) | 1.6 (1.3, 1.8) | <0.001 |
| Plasma triglycerides, mmol/L | 1.1 (0.8, 1.4) | 1.1 (0.9, 3.5) | 0.332 |
| Plasma LDL-cholesterol, mmol/L | 2.8 (2.2, 3.4) | 3.4 (2.8, 4.0) | <0.001 |
| HbA1c, mmol/mol | 33.0 (31.0, 35.0) | 37.0 (35.0, 38.0) | <0.001 |
Data are presented as median (interquartile range) or number of subjects.
Abbreviations: BMI, body mass index; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment for insulin resistance; LDL, low-density lipoprotein; NA, not applicable; WHR, waist-to-hip ratio.
aWomen with available data on years since menopause, n = 53. Among those, early postmenopausal n = 11 and late postmenopausal n = 42.
The Regional Ethics Review Board in Uppsala (Dnr 2013/330 and Dnr 2013-183/494) and Gothenburg (Dnr 336-07) approved the studies, and all participants gave their written informed consent.
Adipose Tissue Incubation
Adipose tissue was incubated for 24 hours at 37°C using phenol red-free Dulbecco’s modified Eagle’s medium containing 6 mM glucose (Invitrogen Corporation, Paisley, OR, USA), 10% charcoal-stripped fetal bovine serum (Invitrogen), and 1% penicillin-streptomycin (Invitrogen) in the presence or absence of E2, 0.01 to 100 nM (Sigma, Saint Louis, MO, USA) or with or without the specific ESR2 antagonist 4-[2-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol (1μM; Sigma). At the end of the incubation period, tissue was collected and snap-frozen for gene or protein expression analysis. Part of the incubated tissue was used to perform glucose uptake in isolated adipocytes.
Cell Viability
After adipose tissue incubation with E2 (0.01 to 100 nM) for 24 hours, adipocytes were isolated as described in the following discussion. The isolated adipocytes were then incubated with water soluble tetrazolium salts 1 reagent, and absorbance was measured as suggested by the manufacturer (WST-1 reagent, Roche, Indianapolis, IN, USA).
Isolation of Adipocytes and Ex Vivo Adipocyte Glucose Uptake
Glucose uptake in isolated adipocytes was performed as described previously (14, 15). In short, incubated adipose tissue was digested using collagenase A (Roche) in a shaking water bath at 105 RMP for 1 hour at 37°C in Medium 199 (Gibco, Life Technologies, Paisley, UK) supplemented with 6 mM glucose, 4% bovine serum albumin (Sigma) and 150 nM adenosine (Sigma); pH = 7.4. Thereafter, the cell suspension was passed through a 250 nM nylon mesh and collected into a falcon tube. Adipocytes were isolated from digestion media and washed 3 times at 5 minutes intervals in glucose-free KRH Krebs-Ringer bicarbonate medium supplemented with 4% bovine serum albumin and 150 nM adenosine; pH = 7.4. Next, adipocytes were incubated at 37°C in a shaking water bath at 65 RPM for 15 minutes in KRH Krebs-Ringer bicarbonate medium without (basal) or with 25 or 1000 µU/mL of insulin (Actrapid, Novo Nordisk, Bagsværd, Denmark). After 15 minutes, the cells were incubated with D-(U-14C) glucose (0.86 µM glucose, 0.26 mCi/mL; Perkin Elmer, Boston, MA, USA) for 45 minutes. The reaction was stopped by transferring the reaction tubes onto ice, and cells were immediately separated from the media by centrifugation through silicon fluid (WR Chemicals, Leuven, Belgium). Radioactivity associated with cells was then measured with a scintillation counter (Radiomatic series 500TR, Perkin Elmer Analytical Instruments). Glucose uptake was expressed as clearance of medium glucose per cell. Cell number was determined following measurements of triglyceride content (Doles extraction) (16), and cell size (AxioVision, Munich, Germany) (17).
Gene Expression
Total RNA was extracted from adipose tissue using the RNeasy lipid tissue mini kit (Qiagen, Hilden, Germany). The total RNA concentration and purity were measured with the Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, Rockford, IL, USA). RNA (400 ng) was then reverse-transcribed using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Thermo Fisher Scientific, CA, USA). The protocols were carried out as per manufacturer’s guidelines. TaqMan gene expression assays (Thermo Fisher) were used to study the expression of ESR1 (Hs01046816_m1), ESR2 (Hs01100353_m1), GLUT1 (SLC2A1; Dm01821912_g1), and GLUT4 (SLC2A4; Hs00168966_m1). Gene expression of ERs was denoted as ESR1 and ESR2, whereas protein expression was ESR1 and ESR2. The gene expression was detected using a QuantStudio 3 sequence detection system (Applied Biosystem). We analyzed gene expression data as described by Schmittgen and Livak (18). Messenger RNA expression was calculated using a 2-dCt method. The endogenous control was considered stable if the SD was <1, as described by Pfaffl et al (19). The results were normalized using GUSB (Hs99999908_m1) as an endogenous control. All samples were run in duplicates. Next-generation RNA sequencing was used to perform a targeted analysis of ESR1, ESR2, and GPER-1 gene expression in 6 premenopausal and 5 postmenopausal at Novogene (Cambridge, UK; https://novogene.com). A total amount of 1 μg of RNA per sample was used for RNA sample preparation. RNA purity was checked using NanoPhotometer® spectrophotometer (IMPLEN, CA, USA). RNA integrity and quantification were assessed using the RNA Nano 6000 Assay Kit of the Bioanalyzer 2100 system (Agilent Technologies, CA, USA). Sequencing libraries were generated with NEBNext® UltraTM RNA Library Prep Kit for Illumina® (NEB, USA) following manufacturer’s recommendations. Library quality was assessed on the Agilent Bioanalyzer 21000 system. Gene expression from RNA sequencing is expressed as fragments per kilobase of transcript per million. HTSeq v0.6.1 was used to count the read numbers mapped of each gene. Fragments per kilobase of transcript per million of each gene was calculated based on the length of the gene and reads count mapped to this gene.
Western Blot
Following incubation with or without E2, adipose tissue was washed 3 times with ice-cold phosphate buffer saline (Medicago, Uppsala, Sweden) and homogenized in ice-cold lysis buffer: 25 mM Tris-HCl (Sigma), pH 7.4; 0.5 mM ethyleneglycol-bis-(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (Sigma); 25 mM NaCl (Sigma); 1% Nonidet P-40; 1 mM Na3Vo4; 10 mM NaF; 100 nM okadaic acid (Alexis Biochemicals, Lausen, Switzerland); 1X Complete protease inhibitor cocktail (Roche); and 1 mM orthovanadate (Sigma). Next, the samples were rocked for 2 hours at 4°C and centrifuged at 12000 × g for 15 minutes at 4°C. The lysates were collected, and protein concentration was determined using a BCA protein assay kit (Pierce, Thermo Scientific).
Proteins (20 µg) were separated by sodium dodecyl sulfate-PAGE (5-8% gradient), transferred to nitrocellulose membranes and blocked with 0.05% Tween- phosphate-buffered saline (Medicago) with 5% nonfat dry milk (Biorad, Hercules, CA, USA). Membranes were incubated overnight with the primary antibody anti-GLUT4 (Invitrogen; RRID:AB_2191429; https://antibodyregistry.org/AB_2191429, 1:1000). Antiglyceraldehyde-3-phosphate dehydrogenase (Cell Signaling, Beverly, MA, USA; RRID:AB_561053; http://antibodyregistry.org/AB_561053, 1:2000) was used as a loading control protein. Membranes were then washed with 0.05% Tween-phosphate-buffered saline and incubated with appropriate horseradish peroxide-conjugated antimouse or antirabbit (Cell Signaling) secondary antibody. Visualization of protein bands was then performed using enhanced chemiluminescence with a high-resolution field and imaged with ChemiDocTM MP System (Biorad) and quantification with Image Lab Software.
Single Nucleotide Polymorphism Analysis
Associations of ESR1 and ESR2 single nucleotide polymorphisms (SNPs) with diabetes-related traits were assessed using Open Targets Genetics (https://genetics.opentargets.org/). We used publically available results from the UK Biobank and Global Lipids Genetics Consortium. We identified associations with four metabolic traits for ESR1 or ESR2, and therefore the P-values were Bonferroni-corrected with a factor of 4. The significance threshold for the resulting P-values in SNP analyses was P < 5 × 10−8.
Statistics
All data are presented as mean ± SD or SE of the mean, or median (interquartile range). A comparison between paired groups was made using a paired t-test or 1- or 2-way analysis of variance with repeated measures or mixed model effects, or Spearman’s correlation was used to test for bivariate analysis. For data where a statistical significance was found, a post hoc comparison was performed using Šidák correction or Benjamini, Krieger, and Yekutieli false discovery rate. A multilinear regression model has been used to predict the impact of clinical variables on the ESR1:ESR2 ratio. A P-value < 0.05 was considered statistically significant. All data were analyzed using GraphPad Prism 9.0.2 or IBM SPSS version 23.
Results
ERs Gene Expression in Human Adipose Tissue From Pre- and Postmenopausal Women
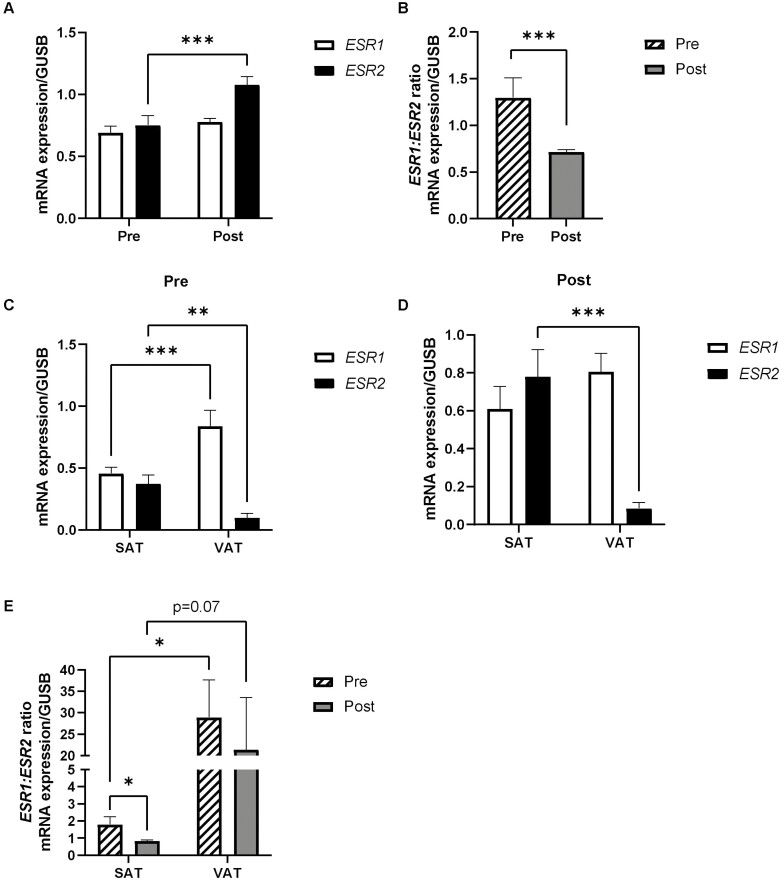
Gene expression of ESR1 and ESR2 genes were measured in adipose tissue from pre- and postmenopausal women. We found no differences in ESR1 expression in SAT between pre- and postmenopausal women (Fig. 1A). The expression of the ESR2 gene in SAT was approximately 53% higher in postmenopausal women compared to premenopausal women (P < 0.001) (Fig. 1A). The ratio of ESR1:ESR2 gene expression in SAT was significantly lower by approximately 45% in postmenopausal compared to premenopausal women (P < 0.001) (Fig. 1B). Moreover, we have obtained additional support from transcriptomics analyses by next-generation RNA sequencing in SAT in a separate subset of women, with a trend for higher ESR2 expression in post- vs premenopausal women (P = 0.18; n = 5 and n = 6, respectively). Accordingly, the ESR1:ESR2 ratio was numerically reduced by approximately 50% in the postmenopausal women (P = 0.14) (data not shown). When divided into early and late postmenopausal women, we found that there were no differences in ESR1 expression, but ESR2 was significantly higher in late postmenopausal women compared to premenopausal women (P < 0.05) (data not shown). In addition, we measured the expression of the ER G-protein coupled estrogen receptor 1 GPER-1 in adipose tissue of pre- and postmenopausal women and found no significant differences (data not shown).
Figure 1.
Gene expression of estrogen receptors in adipose tissue from pre- and postmenopausal women. (A) ESR1 and ESR2 genes were measured in SAT from pre- and postmenopausal women (n = 32 and n = 40, respectively) with real-time polymerase chain reaction. (B) ESR1:ESR2 gene expression ratio in SAT from pre- and postmenopausal women (n = 32 and n = 40, respectively). Comparison of ESR1 and ESR2 gene expression in paired samples of SAT and VAT from (C) pre- (n = 11) and (D) postmenopausal women (n = 9). (E) ESR1:ESR2 gene expression ratio in SAT and VAT of pre- (n = 11) and postmenopausal women (n = 9). Data represent mean ± SE of the mean. *P < 0.05, **P < 0.01, ***P < 0.001. Abbreviations: Post, postmenopausal women; Pre, premenopausal women; SAT, subcutaneous adipose tissue. VAT, visceral adipose tissue.
There were depot differences in the expression of ESRs. In premenopausal women, the expression of ESR1 was significantly higher in VAT compared to SAT (P < 0.001) (Fig. 1C). In both pre- and postmenopausal women, the expression of ESR2 was instead lower in VAT compared to SAT (P < 0.01) (Fig. 1C and 1D), respectively, and thus the ESR1:ESR2 ratio was higher (approximately 90%) in VAT compared to SAT (Fig. 1E).
Association Between ER Gene Expression and Clinical and Anthropometric Parameters
The association of the expression of ESR1, ESR2, and ESR1:ESR2 ratio in SAT (without estrogen treatment) was correlated with anthropometric and clinical characteristics of women (Table 2). In all women (pre- and postmenopausal), the expression of ESR1 correlated positively with high-density lipoprotein (HDL)-cholesterol and negatively with BMI, body fat percentage, waist-hip ratio, insulin, homeostatic model assessment for insulin resistance (HOMA-IR), and triglycerides. The expression of ESR2 showed a significant positive association with age and HDL-cholesterol, and negative with BMI and waist-hip ratio, insulin, HOMA-IR, and triglycerides. The ratio of ESR1:ESR2 gene expression in adipose tissue was positively associated with BMI, waist-to-hip ratio (WHR), insulin, HOMA-IR, and triglycerides, and negatively associated with age, HbA1c, total cholesterol, HDL, low-density lipoprotein, and body fat percentage (Table 2). A positive association between ESR1 expression and the Matsuda index for insulin sensitivity and a negative association between ESR2 expression and the WHR were shown in a separate cohort with pre- and postmenopausal women (data not shown).
Table 2.
Spearman’s correlations between expression of estrogen receptors 1 and 2 in subcutaneous adipose tissue and with anthropometric and clinical variables (n = 72)
| ESR1 | ESR2 | ESR1:ESR2 | ||||
|---|---|---|---|---|---|---|
| Variable | Rho | P | Rho | P | Rho | P |
| Age | 0.100 | 0.398 | 0.421 | <0.001 | −0.583 | <0.001 |
| BMI | −0.581 | <0.001 | −0.470 | <0.001 | 0.244 | 0.038 |
| WHR | −0.479 | <0.001 | −0.391 | 0.001 | 0.249 | 0.034 |
| Body fat, % | −0.373 | 0.006 | 0.012 | 0.935 | −0.348 | 0.012 |
| HbA1c | −0.174 | 0.141 | 0.068 | 0.568 | −0.301 | 0.010 |
| Plasma glucose | −0.108 | 0.362 | 0.002 | 0.987 | −0.148 | 0.210 |
| Serum insulin | −0.337 | 0.004 | −0.377 | 0.001 | 0.294 | 0.011 |
| HOMA-IR | −0.312 | 0.007 | −0.340 | 0.003 | 0.248 | 0.034 |
| Total cholesterol | 0.179 | 0.129 | 0.353 | 0.002 | −0.425 | 0.000 |
| Plasma triglyceride | −0.286 | 0.014 | −0.325 | 0.005 | 0.244 | 0.037 |
| Plasma HDL-cholesterol, mmol/L | 0.457 | 0.000 | 0.513 | 0.000 | −0.441 | 0.000 |
| Plasma LDL-cholesterol, mmol/L | 0.029 | 0.807 | 0.203 | 0.085 | −0.313 | 0.007 |
Abbreviations: BMI, body mass index; HbA1c, glycosylated hemoglobin; HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment for insulin resistance; LDL, low-density lipoprotein; WHR, waist-to-hip ratio.
Since age and menopausal status, including postmenopausal time, are strongly related to each other, our data do not allow for a detailed assessment of their respective contribution to variations in ESR1 and ESR2 expression and ratio. Nonetheless, to determine whether age or menopausal status is most important to predict the ESR1:ESR2 ratio, we assessed 2 multilinear regression models. Model A includes age, BMI, WHR, HbA1c and HOMA-IR; in model B, age is replaced by menopausal status (Table 3). Both models have nearly identical r2 values (0.490 and 0.466, respectively, P < 0.05) and both age (standard β = −0.361, P < 0.01) and menopausal status (standard for post vs premenopausal status: β = −0.308, P < 0.01) were negatively associated with ESR1:ESR2 ratio in the respective models (Table 3). Also, BMI was positively associated with ESR1:ESR2 ratio in both models (P < 0.001,Table 3). In addition, when Models A and B were applied separately to pre- and postmenopausal women, respectively, BMI was a predictor of ESR1:ESR2 ratio only in premenopausal women whereas there was no significant association for age; however, the number of subjects in the subgroup analysis was limited (data not shown).
Table 3.
Regression models for predicting the ESR1:ESR2 ratio in subcutaneous adipose tissue (n = 72)
| Model Aa | Model Bb | |||
|---|---|---|---|---|
| Standard β | P | Standard β | P | |
| BMI | 0.415 | 0.000 | 0.412 | 0.000 |
| WHR | −0.029 | 0.743 | −0.023 | 0.803 |
| HbA1c | −0.123 | 0.250 | −0.115 | 0.155 |
| HOMA-IR | 0.063 | 0.605 | 0.082 | 0.508 |
| Age | −0.361 | 0.001 | ||
| Menopausal status (postmenopausal) | −0.308 | 0.006 |
aBMI, WHR, HbA1c, HOMA-IR, age.
bBMI, WHR, HbA1c, HOMA-IR, menopausal status.
Abbreviations: BMI, body mass index; HbA1c: glycosylated hemoglobin; HOMA IR: homeostatic model assessment for insulin resistance; WHR, waist-to-hip ratio.
Association of ESR1 and ESR2 Polymorphisms With Adiposity and Dyslipidemia
We assessed whether polymorphisms in ESR1 or ESR2 are associated with adiposity and dyslipidemia. SNPs in ESR1 were associated with weight, body fat distribution, and total cholesterol (Table 4), whereas SNPs in ESR2 were associated with body fat percentage, total cholesterol, and triglyceride levels (Table 5).
Table 4.
Association of ESR1 polymorphisms with adiposity and dyslipidemia
| SNP | Effect allele | Other allele | Location | Beta | 95% CI | P-value | Adjusted P-value | na | |
|---|---|---|---|---|---|---|---|---|---|
| Weight | rs11968025 | T | G | chr6:151847839 | 0.28 | 0.22-0.35 | 2.5e-17 | 1.0e-16 | 360 116 |
| Weight | rs3853252 | G | A | chr6:151849112 | 0.28 | 0.22-0.35 | 2.2e-17 | 8.8e-17 | 354 838 |
| Body fat distribution (trunk fat ratio), females | rs2982708 | T | C | chr6:152035085 | 0.025 | 0.019-0.031 | 1.0e-14 | 4.0e-14 | 116 138 |
| Total cholesterol | rs4870044 | C | T | chr6:151580274 | 0.013 | 0.0085-0.017 | 2.0e-9 | 8-0e-9 | 297 824 |
Abbreviation: SNP, single nucleotide polymorphism.
aNumber of subjects in analysis.
Table 5.
Association of ESR2 Polymorphisms with adiposity and dyslipidemia
| SNP | Effect allele | Other allele | Location | Beta | 95% CI | P-value | Adjusted P-value | na | |
|---|---|---|---|---|---|---|---|---|---|
| Body fat, % | rs146131663 | C | T | chr14:64669886 | -0.41 | -0.56 - 0.26 | 4.3e-8 | 1.7e-7 | 354,628 |
| Total cholesterol | rs7157785 | G | T | chr14:63768838 | 0.021 | 0.014 - 0.027 | 2.9e-10 | 1.2e-9 | 297,626 |
| Triglyeride levels | rs7157785 | G | T | chr14:63768838 | 0.021 | 0.016 - 0.026 | 5.0e-15 | 2.5e-14 | 297,824 |
| Triglyceride levels | rs7148864 | A | G | chr14:63769473 | -0.027 | -0.036 - -0.018 | 2.0e-8 | 8.0e-8 | 87,819 |
Abbreviation: SNP, single nucleotide polymorphism.
aNumber of subjects in analysis.
Effect of E2 Treatment on Glucose Uptake Capacity of Adipocytes From Pre- and Postmenopausal Women
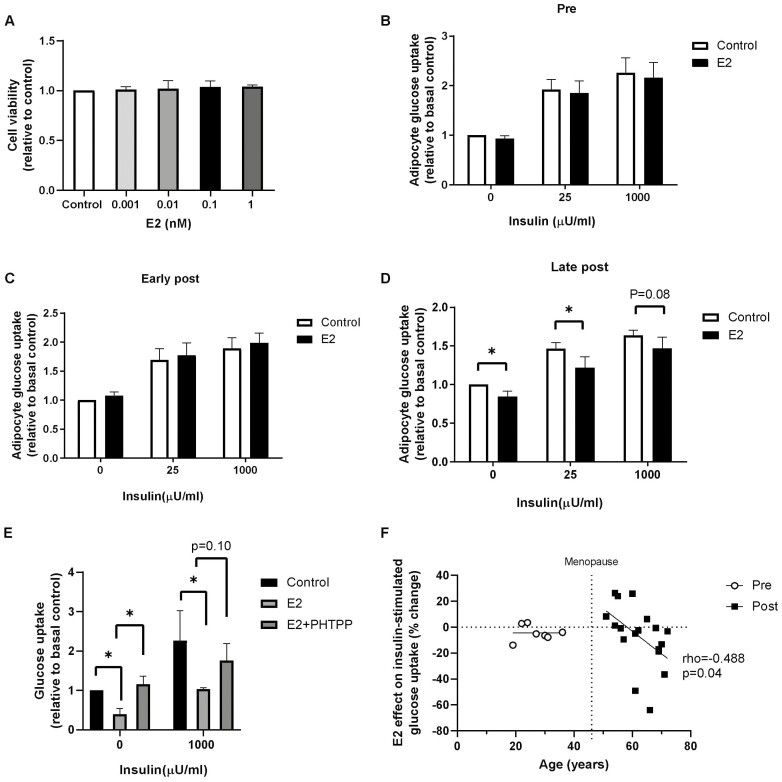
E2 treatment showed a dose-dependent decrease (0.001 nM to 1 nM) in basal and insulin-stimulated glucose uptake capacity of adipocytes from postmenopausal women, with the maximal effect observed at 0.1 nM (P < 0.05; data not shown) compared to the untreated control. Thus, we chose this concentration for the remainder of our experiments as it was also within the physiological levels (20) of free E2 in serum and SAT in pre- and postmenopausal women, and it did not affect the viability of isolated adipocytes (Fig. 2A).
Figure 2.
Effect of E2 on adipocyte glucose uptake in pre- and postmenopausal women. (A) Cell viability in adipocytes isolated from adipose tissue treated without (control) or with estradiol (E2) for 24 hours (0.001-1 nM). Subcutaneous adipose tissue was incubated with E2 (0.1 nM) or without (control) for 24 hours, and then adipocytes were isolated to measure glucose uptake in absence or presence of insulin (25 and 1000 µU/mL) in the (B) premenopausal group (n = 8), (C) early postmenopausal group (n = 7), and (D) late postmenopausal group (n = 10-12). (E) Basal and insulin-stimulated glucose uptake in adipocytes isolated from adipose tissue from late postmenopausal women incubated with E2 (0.1 nM) or E2 and PHTTP (1 µM) (n = 3). (F) Association between the age and the effect of E2 on insulin-stimulated glucose uptake (% change to control) in adipocytes in pre- (n = 7) and postmenopausal women (n = 25, P = 0.04). Data represent mean ± SE of the mean. *P < 0.05.
In premenopausal women, 24-hour E2 incubation did not affect basal or insulin-stimulated glucose uptake compared to untreated controls (Fig. 2B). When postmenopausal women were separated into early and late postmenopausal women, an inhibitory effect of E2 on glucose uptake was seen in adipocytes from late postmenopausal women only; E2 decreased the basal, submaximal, and maximal insulin-stimulated glucose uptake by 20% (P < 0.05), 25% (P < 0.05), and 15% (P = 0.08), respectively, compared to untreated controls (Fig. 2C and 2D). Furthermore, the effect of a physiological insulin concentration, 25 µU/mL on glucose uptake was significantly lower in late postmenopausal women than premenopausal women by about 22% (P < 0.05; data not shown). Since it has been previously proposed that ESR2 is involved in reduced GLUT4 activation (21) and insulin resistance and we found higher ESR2 expression in postmenopausal women, adipose tissue from late postmenopausal women was incubated with E2 in the presence or absence of the specific ESR2 antagonist 4-[2-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol (PHTTP). Interestingly, we found that the inhibiting-E2 effect on glucose uptake was prevented by this antagonist, supporting a role of ESR2 to modify glucose uptake (P < 0.05) (Fig. 2E). Next, we analyzed the association between age and the effect of E2 inhibiting glucose uptake, calculated as Δ-change of basal and insulin-stimulated glucose uptake in adipocytes in pre- and postmenopausal women (n = 25). Age was negatively correlated with Δ-change of basal (data not shown) and insulin-stimulated glucose uptake (rho = −0.488, P < 0.05) (Fig. 2F).
Effect of E2 Treatment on the Expression of Glucose Transporters on Adipose Tissue From Pre- and Postmenopausal Women
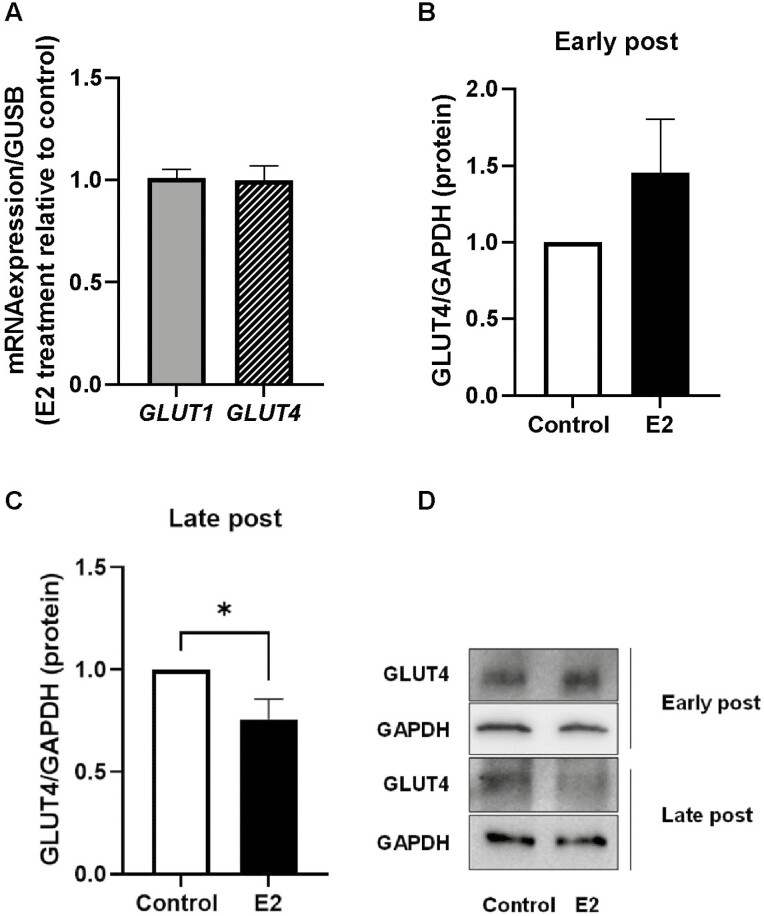
SAT was treated with 0.1 nM E2 for 24 hours, and gene and protein expression of glucose transporters, GLUT1 and GLUT4, were measured. E2 did not affect the expression of GLUT1 and GLUT4 genes irrespective of time since menopause (Fig. 3A). In early postmenopausal women, E2 treatment did not alter GLUT4 protein levels (Fig. 3B and 3D); however, in late postmenopausal women, GLUT4 protein levels were reduced by approximately 25% (P < 0.05) (Fig. 3C and 3D).
Figure 3.
Effect of estradiol (E2) on gene expression and protein levels of glucose transporters. Adipose tissue was incubated with or without E2 (0.1 nM) for 24 hours. (A) Gene expression of GLUT1 and GLUT4 were measured (n = 12, 4 early postmenopausal and 8 late postmenopausal). In addition, total protein was isolated and GLUT4 was measured with immunoblotting in adipose tissue from (B) early menopausal women (n = 7) or (C) late postmenopausal women (n = 10). (D) Representative Western blot with GLUT4 and glyceraldehyde-3-phosphate dehydrogenase. Data represent mean ± SE of the mean. *P < 0.05.
Effect of E2 Treatment on the Gene Expression of ERs in Adipose Tissue From Pre- and Postmenopausal Women
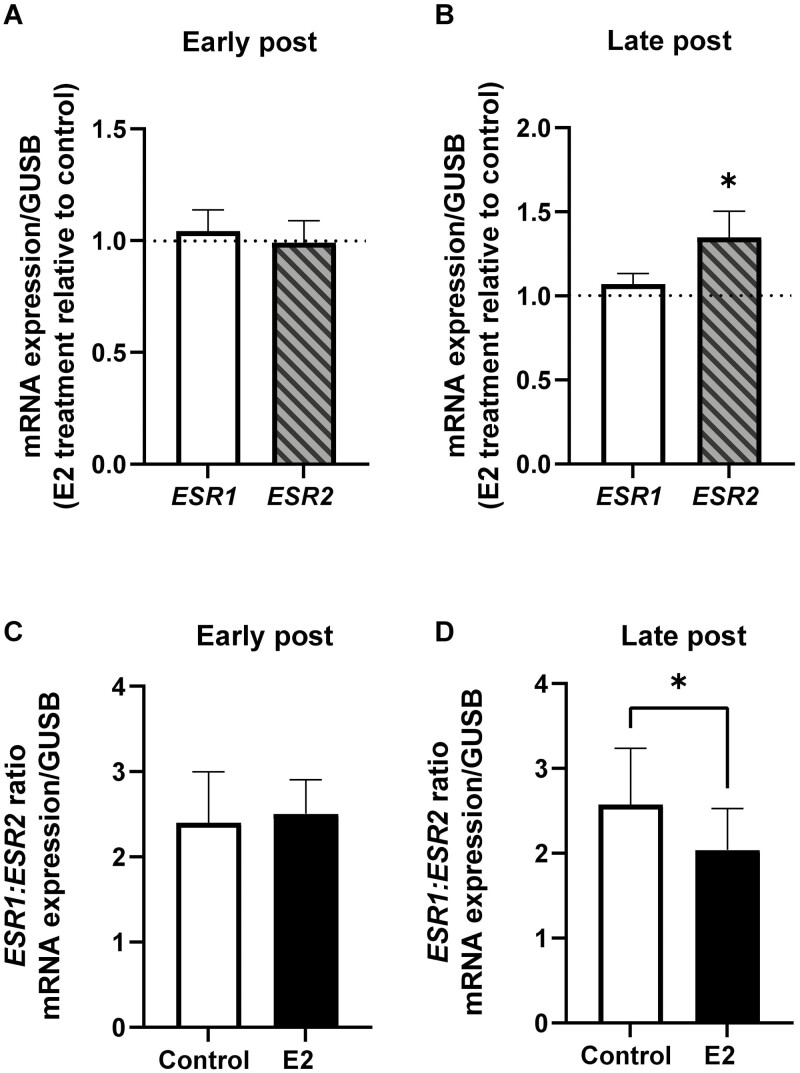
In early postmenopausal women, incubation of SAT with E2 did not affect the expression of the ESR1 or ESR2 gene (Fig. 4A). However, in late postmenopausal women the expression of ESR2 was increased by approximately 20% compared to untreated control (P < 0.05) (Fig. 4B). Additionally, E2 treatment decreased the ratio of ESR1:ESR2 by 20% in adipose tissue from the late postmenopausal women group (P < 0.05) (Fig. 4D), whereas no effect of E2 was seen on the ESR1:ESR2 ratio in samples from early menopausal women (Fig. 4C).
Figure 4.
Effect of estradiol (E2) on gene expression of estrogen receptors in SAT from postmenopausal women. Effect of 24-hour E2 treatment (0.1 nM, relative to control) on the expression of ESR1 and ESR2 genes in SAT from (A) early postmenopausal women and (B) late postmenopausal women. E2 effect on ESR1:ESR2 ratio in (C) early menopausal women and (D) late postmenopausal women. Early postmenopausal women, n = 4; late postmenopausal women, n = 7. Data represent mean ± SE of the mean. *P < 0.05.
Discussion
Previous research has often focused on the effects of estrogen replacement therapy to improve metabolic changes that occur after menopause. However, there is limited research on the tissue-specific effects of estrogens on ER levels and glucose metabolism. In this study, we wanted to characterize how menopause effects the expression of ERs in adipose tissue and determine whether there are any metabolic consequences. We provide novel insight on menopause-related changes on ER expression and the effects of E2 treatment in adipose tissue from pre- and postmenopausal women. In summary, we found that ESR1 gene expression was similar in pre- and postmenopausal women, whereas ESR2 was higher in postmenopausal women. To the best of our knowledge, this is the first study to demonstrate that the increase of adipose ESR2 expression occurring in postmenopausal women is linked to reduced glucose uptake in response to E2 treatment and that this is reversed by presence of an ESR2 antagonist. This corresponded to a reduction in GLUT4 protein levels in postmenopausal adipose tissue treated with E2. Altogether, the data suggest that E2 action mediated by ESR2 may contribute to the development of adipose tissue insulin resistance in postmenopausal women.
Adipose tissue expresses both ESR1 and ESR2 genes (6). As estrogen mediates its actions via these 2 receptors, which are known to elicit different metabolic responses, changes in their expression—for instance, due to age, menopause, or treatment—can result in differences in adipose tissue function (6, 10). In the present study, we observed differences in the expression of ERs in adipose tissue from pre- and postmenopausal women. We show that ESR1 expression is similar between pre- and postmenopausal women; however, ESR2 expression is enhanced in postmenopausal women, resulting in a significantly lower ESR1:ESR2 ratio compared to premenopausal women. In addition, we found that ESR2 is significantly higher in late postmenopausal women; however, due to the limited amount of biopsies from early postmenopausal women, these results must be taken with caution. Our data are in agreement with previous studies (10, 22). In contrast, the study by Shin et al showed no difference in the gene expression of these receptors in adipose tissue from pre- and postmenopausal women (23). Noticeably, previous studies included lean women, and therefore, the effect of adiposity, which may affect the expression of ERs and thus confound the interpretation, was not addressed. In our study, we included women with a wide range of BMI. We found that age, postmenopausal status, and BMI, are associated with ESR1:ESR2 ratio. Menopausal status and BMI appeared to be strong predictors of ESR1:ESR2 ratio, when analyzed using 2 multilinear regression models; however, this must be interpreted with caution as menopausal status and age are collinear and cannot independently predict ESR1:ESR2 ratio. In addition, it is important to acknowledge the wide age range used in this study, which may be a source of variability. Furthermore, the expression of the ESR2 gene and not ESR1 was associated positively with age. Studies have shown that ESR1 protects against the development of obesity and associated comorbidities (7, 24). In line with this, the ratio of ESR1:ESR2 in the present study was found to be negatively associated with body fat percentage and HbA1c and lipid profile in women. The role of ESR2, on the other hand, is not well understood. With some controversy in the actions of ESR2, studies have suggested either insulin-sensitizing or desensitizing effects (9, 25). Furthermore, we investigated whether polymorphisms in either ESR1 or ESR2 were associated with adiposity and dyslipidemia. Interestingly, we found that SNPs of ESR1 were associated with weight, body fat distribution, and total cholesterol, whereas polymorphisms in ESR2 were associated with body fat percentage, total cholesterol, and triglyceride levels.
In general, it is apparent that postmenopausal women are more likely to develop insulin resistance than premenopausal women (1). In this study, we also show depot-specific differences in the expression of ERs. Specifically, in premenopausal women, the expression of ESR1 was significantly higher in VAT compared to SAT, whereas in both pre- and postmenopausal women, the expression of ESR2 was lower in VAT compared to SAT. Interestingly, enhanced ESR2 expression seen in postmenopausal women was only seen in SAT and not VAT. The reasons behind depot-dependent differences in expression levels of ESR1 and ESR2 are not completely clear. In premenopausal women, higher levels of ESR1 in VAT may explain reduced visceral obesity and protection against insulin resistance. In mice, whole-body knockouts of ESR1 leads to increased visceral accumulation and metabolic syndrome (26). Furthermore, it was postulated that a higher ESR1:ESR2 ratio in VAT functions to limit adiposity in this depot. Moreover, it should be noted that lower ESR2 expression does not necessarily entail less detrimental effects of E2 in VAT, and other factors such as posttranslational modifications, expression of other ER types and tissue levels of E2 or cofactors may differ between the depots. This coincides well with the ratio of ERs in males (27), who have lower levels of ESR1 compared to ESR2 in VAT, which is consistent with the fact that males typically have more visceral fat accumulation (26). Although this study mainly focuses on SAT, further research is warranted to better characterize estrogen effects in VAT. Moreover, this study focuses on the effects of ER levels and E2 treatment on glucose metabolism and obesity. Our data suggest that reduced ESR1:ESR2 ratio in adipose tissue leads to a reduction in adipocyte glucose uptake; however, it is also possible that altered ER expression in adipose tissue can be a consequence of local insulin resistance.
In the present study, we show that E2 has divergent effects on modulating adipocyte glucose uptake capacity in pre-and postmenopausal women. In premenopausal women, E2 did not affect adipocyte glucose uptake. In contrast, in late postmenopausal women, E2 inhibited both basal and insulin-stimulated glucose uptake. The inhibitory effect on glucose uptake seems to be, at least partially, through a reduction in the main glucose transporter, GLUT4. This is supported by Barros et al, who demonstrated that activation of ESR2 with an agonist in muscle cells results in a reduction in GLUT4 protein levels (9). In addition, the E2 -effects on glucose uptake were lost when coincubated with an ESR2 antagonist, possibly indicating the role of ESR2 on the effects of E2 on glucose uptake in late postmenopausal women. Some studies have, in fact, shown evidence for insulin resistance with E2 treatment. In 1 study, it was shown that E2 activation of ESR1 increases GLUT4 and activation of ESR2 downregulates GLUT4 (21). In our study, we show that E2 downregulated GLUT4 in late postmenopausal women, whereas in premenopausal women there was a nominal increase. Surprisingly, a higher ratio of ESR1:ESR2 was shown to be positively associated with insulin resistance measured as HOMA-IR. However, multivariable analyses suggest that this association is explained by BMI, which is a strong covariate of HOMA-IR. Higher BMI is associated with a higher ESR1:ESR2 ratio, which, in turn, may explain higher HOMA-IR. Moreover, causality or its direction is not demonstrated, and obesity per se may possibly lead to an altered ESR1:ESR2 ratio. In addition, the length of time since the start of menopause and of estrogen deficiency has usually not been considered. Pereira et al investigated the effects of E2 therapy in early and late postmenopausal women and concluded that E2 effects may reduce the incidence of T2D in early postmenopausal women, but that this may be reversed in late postmenopausal women (11). The “timing hypothesis” of estrogen hormone therapy has been primarily focused on atherosclerotic disease in which it has been shown that E2 prevents progression of the disease only when it was administered early in the disease process. We found that the inhibitory effect of E2 seemed to be dependent on the time since menopause as there was a negative association between age and change of insulin-stimulated glucose uptake in adipocytes (11). In contrast to late postmenopausal women, E2 did not show any inhibitory effect on basal and insulin-stimulated glucose uptake in early postmenopausal women. Further research is needed to determine the optimal window for administering estrogen treatment, and our study provides some experimental support to previous literature on differences in the metabolic effects depending on timing of estrogen treatment.
The transition from the premenopausal to postmenopausal state in women coincides with a reduction in circulating estrogen levels. This can result in abdominal fat accumulation that can lead to decreased insulin sensitivity (28). Although tissue-specific effects in the context of metabolism are not well understood, accumulating evidence indicates that estrogenic signaling can play an important role in the development of obesity in postmenopausal women. Estrogen exerts its actions via ESR1 and ESR2; both are expressed in adipose tissue. In the context of metabolic effects, ESR1 is shown to be protective, whereas the role of ESR2 is not completely understood, although some studies have pointed to have its deleterious effect on insulin sensitivity (7, 9). For instance, preclinical studies of knockout models of ERs suggest that ESR1 but not ESR2 coincides positively with insulin-mediated glucose disposal and insulin sensitivity (29, 30). The opposite effects of these 2 receptors are also evident in other diseases, which implies that under certain circumstances ESR2 opposes the actions of ESR1 and that ESR1 and ESR2 produce a contrasting response in the presence of E2 (31).
The balance between ESR1 and ESR2 within the tissue also seems to be crucial in determining the actions of E2 on insulin sensitivity (12, 31). Our study found that E2 treatment did not affect the expression of the ESR1 gene but increased the expression of the ESR2, which also confirms our previous findings (32). This is also in line with a previous study reporting that E2 treatment increased the ESR1:ESR2 ratio in early postmenopausal women and decreased the ESR1:ESR2 ratio late postmenopausal women (10). E2 exposure caused a significant decrease in ESR1:ESR2 ratio, suggesting that the observed inhibitory effect of E2 on glucose uptake may be mediated through ESR2 signaling. This can be further supported by previous studies in which the activation of ESR1 signaling was shown to enhance glucose metabolism, while ESR2 activation had opposite effects (33). We show that both the postmenopausal state and E2 treatment upregulate ESR2 expression. This may be explained by several factors, such as the length of estrogen deficiency in postmenopausal women, which may alter ER balance and thus E2 response. This may also be explained by the length of estrogen treatment, since we incubate adipose tissue for 24 hours and longer incubations may lead to a different estrogen response. Preliminary data from our group using CRISPR/Cas9 knockouts in human preadipocytes show that knockout of ESR1 and ESR2 leads to a reduction in glucose uptake and altered differentiation (data not shown). This may highlight that ESR1 and ESR2 are involved in adipocyte glucose metabolism.
The study has some limitations. First, the ex vivo nature of the study does not take into account the cross-talk between other tissues regulating in vivo metabolism. In the future, it would be interesting to study the function of adipose tissue collected from women who are on E2 or hormone replacement therapy. Studies on ER knockouts and knock-ins in human adipose tissue and adipocyte glucose metabolism are warranted. In addition, in our study we measure gene expression of ESR1 and ESR2; however, it is of importance to also assess protein expression of ESR1 and ESR2. It has been reported by Andersson et al that there is a great discrepancy in measures of ESR2 messenger RNA and protein expression due to lack of adequate ESR2 antibodies (34). Specific ESR2 antibodies need to be validated before reliable assessments of this receptor type can be obtained. Moreover, the effect of E2 was investigated only after 24 hours of incubation. To reflect clinical estrogen replacement, longer term exposures would be of interest. Further, even shorter incubation times are warranted since E2 can mediate actions via nongenomic pathways. Furthermore, the women included in this study had a wide BMI range, and although PCOS was not reported by any of the subjects, some premenopausal women who were overweight or obese may have undiagnosed PCOS. Future studies investigating differences in BMI-matched pre- and postmenopausal women may be of interest. Furthermore, postmenopause was defined as loss of menstrual cycle for at least 1 year. However, due to the wide age range in this study, information on hormonal status such as follicle-stimulating hormone could be useful to provide additional confirmation on the menopausal state. Lastly, differences between pre- and postmenopausal women in adipose tissue expression of multiple genes in should also be assessed in a nontargeted manner to further elucidate pathways that are affected by the levels of circulating estrogen or by the relative expression of ESR1 and ESR2. These efforts may provide further insight on the mechanisms involved in development of insulin resistance and T2D in postmenopausal women.
Conclusion
In conclusion, this work provides new insights on the complex hormonal cross-talk regulating glucose homeostasis in human adipose tissue. We highlight the impact of estrogen, its 2 receptor types and menopausal status in women. In addition, we show novel effects of E2 to impair adipocyte glucose uptake in late postmenopausal but not in early post- or premenopausal women. Changes in glucose uptake may partly be explained by lower ESR1:ESR2 ratio in postmenopausal women. This supports that menopause itself and postmenopausal time play an important role for the effects of E2 in adipose tissue, and this may have clinical implications with respect to timing of estrogen treatment in postmenopausal women.
Acknowledgments
We warmly thank all participants in the study. We are indebted to our colleagues at Clinical Diabetes and Metabolism, Department of Medical Sciences, Uppsala University and at Uppsala University Hospital for their help in making this study possible. Part of the analyses was performed in a subcohort from a collaboration with AstraZeneca.
Funding
This work was supported by research grants from the Swedish Diabetes Foundation, European Commision via the Marie Sklodowska Curie Innovative Training Network TREATMENT (H2020-MSCA-ITN-721236), EXODIAB, the Ernfors Foundation, the P.O Zetterling Foundation, the Swedish Society for Medical Research, Novo Nordisk Foundation, and the Uppsala University Hospital ALF grants.
Disclosures
All authors have no potential conflict of interest to declare.
Data Availability
Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Meyer MR, Clegg DJ, Prossnitz ER, Barton M. Obesity, insulin resistance and diabetes: Sex differences and role of oestrogen receptors. Acta Physiologica. 2011;203(1):259-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mattsson C, Olsson T. Estrogens and glucocorticoid hormones in adipose tissue metabolism. Curr Med Chem. 2007;14(27):2918-2924. [DOI] [PubMed] [Google Scholar]
- 3. Louet JF, LeMay C, Mauvais-Jarvis F. Antidiabetic actions of estrogen: insight from human and genetic mouse models. Curr Atheroscler Rep. 2004;6(3):180-185. [DOI] [PubMed] [Google Scholar]
- 4. Macotela Y, Boucher J, Tran TT, Kahn CR. Sex and depot differences in adipocyte insulin sensitivity and glucose. Diabetes. 2009;58(4):803-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Choe SS, Huh JY, Hwang IJ, Kim JI, Kim JB. Adipose tissue remodeling: its role in energy metabolism and metabolic disorders. Front Endocrinol. 2016;7(APR):1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pedersen SB, Bruun JM, Hube F, Kristensen K, Hauner H, Richelsen B. Demonstration of estrogen receptor subtypes α and β in human adipose tissue: influences of adipose cell differentiation and fat depot localization. Mol Cell Endocrinol. 2001;182(1):27-37. [DOI] [PubMed] [Google Scholar]
- 7. Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-α knockout mice. Proc Natl Acad Sci USA. 2000;97(23):12729-12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Foryst-Ludwig A, Clemenz M, Hohmann S, et al. Metabolic actions of estrogen receptor beta (ERβ) are mediated by a negative cross-talk with PPARγ. PLoS Genet. 2008;4(6):e1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barros RPA, Machado UF, Warner M, Gustafsson JÅ. Muscle GLUT4 regulation by estrogen receptors ERβ and ERα. Proc Natl Acad Sci U S A. 2006;103(5):1605-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Park Y-M, Erickson C, Bessesen D, van Pelt RE, Cox-York K. Age- and menopause-related differences in subcutaneous adipose tissue estrogen receptor mRNA expression. Steroids 2017;121:17-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pereira RI, Casey BA, Swibas TA, Erickson CB, Wolfe P, van Pelt RE. Timing of estradiol treatment after menopause may determine benefit or harm to insulin action. J Clin Endocrinol Metab. 2015;100(12):4456-4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hevener AL, Clegg DJ, Mauvais-Jarvis F. Impaired estrogen receptor action in the pathogenesis of the metabolic syndrome. Mol Cell Endocrinol. 2015;418:306-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. WHO Scientific Group on Research on the Menopause, World Health Organization. Research on the Menopause: Report of the WHO Scientific Group. World Health Organization Technical Report Series no. 670. World Health Organization; 1981. [Google Scholar]
- 14. Park YM, Pereira RI, Erickson CB, Swibas TA, Cox-York KA, van Pelt RE. Estradiol-mediated improvements in adipose tissue insulin sensitivity are related to the balance of adipose tissue estrogen receptor α and β in postmenopausal women. PLoS One. 2017;12(5):e01764461-e01764415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pereira MJ, Thombare K, Sarsenbayeva A, et al. Direct effects of glucagon on glucose uptake and lipolysis in human adipocytes. Mol Cell Endocrinol. 2020;503:110696. [DOI] [PubMed] [Google Scholar]
- 16. Girolamo DI. A simple number method in four to determine mammalian fat cell size and species. Am J Physiol Endocrinol Metab. 1971;221(3):850-858. [DOI] [PubMed] [Google Scholar]
- 17. Lundgren M, Svensson M, Lindmark S, Renström F, Ruge T, Eriksson JW. Fat cell enlargement is an independent marker of insulin resistance and “hyperleptinaemia.” Diabetologia. 2007;50(3):625-633. [DOI] [PubMed] [Google Scholar]
- 18. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protocols. 2008;3(6):1101-1108. [DOI] [PubMed] [Google Scholar]
- 19. Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper – Excel-based tool using pair-wise correlations. Biotechnol Lett. 2004;26:509-515. [DOI] [PubMed] [Google Scholar]
- 20. Badeau M, Vihma V, Mikkola TS, Tiitinen A, Tikkanen MJ. Estradiol fatty acid esters in adipose tissue and serum of pregnant and pre- and postmenopausal women. J Clin Endocrinol Metab. 2007;92(11):4327-4331. [DOI] [PubMed] [Google Scholar]
- 21. Gregorio KCR, Laurindo CP, Machado UF. Estrogen and glycemic homeostasis: the fundamental role of. Cells. 2021;10(99):991-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McInnes KJ, Andersson TC, Šimonyte K, et al. Association of 11β-hydroxysteroid dehydrogenase type i expression and activity with estrogen receptor β in adipose tissue from postmenopausal women. Menopause. 2012;19(12):1347-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shin JH, Hur JY, Seo HS, et al. The ratio of estrogen receptor α to estrogen receptor β in adipose tissue is associated with leptin production and obesity. Steroids. 2007;72(6-7):592-599. [DOI] [PubMed] [Google Scholar]
- 24. Fatima LA, Campello RS, Santos RDS, et al. Estrogen receptor 1 (ESR1) regulates VEGFA in adipose tissue. Sci Rep. 2017;7(1):1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alonso-Magdalena P, Ropero AB, García-Arévalo M, et al. Antidiabetic actions of an estrogen receptor β selective agonist. Diabetes. 2013;62(6):2015-2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Davis KE, Neinast MD, Sun K, et al. The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis. Mol Metab. 2013;2(3):227-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Biff FP, Clegg DJ. The sexual dimorphism of obesity. Mol Cell Endocrinol. 2015;31(3):477-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lizcano F, Guzmán G. Estrogen deficiency and the origin of obesity during menopause. Biomed Res Int. 2014;2014:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ribas V, Nguyen MTA, Henstridge DC, et al. Impaired oxidative metabolism and inflammation are associated with insulin resistance in ERα-deficient mice. Am J Physiol Endocrinol Metab. 2010;298(2):304-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bryzgalova G, Gao H, Ahren B, et al. Evidence that oestrogen receptor-α plays an important role in the regulation of glucose homeostasis in mice: insulin sensitivity in the liver. Diabetologia. 2006;49(3):588-597. [DOI] [PubMed] [Google Scholar]
- 31. Barros RPA, Machado UF, Gustafsson JA. Estrogen receptors: new players in diabetes mellitus. Trends Mol Med. 2006;12(9):425-431. [DOI] [PubMed] [Google Scholar]
- 32. Kamble PG, Pereira MJ, Sidibeh CO, et al. Lipocalin 2 produces insulin resistance and can be upregulated by glucocorticoids in human adipose tissue. Mol Cell Endocrinol. 2016;427:124-132. [DOI] [PubMed] [Google Scholar]
- 33. Barros RPA, Gabbi C, Morani A, Warner M, Gustafsson JA. Participation of ERα and ERβ in glucose homeostasis in skeletal muscle and white adipose tissue. Am J Physiol Endocrinol Metab. 2009;297(1):124-133. [DOI] [PubMed] [Google Scholar]
- 34. Andersson S, Sundberg M, Pristovsek N, et al. Insufficient antibody validation challenges oestrogen receptor beta research. Nat Commun. 2017;8:15840. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.