Abstract
We compared glycoprotein E (gE)- and varicella zoster virus (VZV)-specific Th1 immunity in 160 adults, aged 50–85 years, randomized to receive live or recombinant zoster vaccine (RZV). gE-specific responses measured by interferon-γ (IFN-γ) and interleukin 2 (IL-2) dual-color Fluorospot were significantly higher at all time points postimmunization in RZV recipients. VZV-specific IL-2+ memory, but not IFN-γ+ or IFN-γ+IL-2+ effector responses, were higher in RZV recipients at ≥3 months postimmunization. Only RZV recipients maintained higher postvaccination gE-specific IL-2+ and IFN-γ+ and VZV-specific IL-2+ responses for 5 years. The 5-year persistence of VZV-specific memory and gE-specific Th1 immunity may underlie superior RZV efficacy.
Clinical Trials Registration NCT02114333.
Keywords: immune responses, immunogenicity, old adults, recombinant zoster vaccine, zoster vaccine live
The high efficacy of the recombinant adjuvanted glycoprotein E (gE) herpes zoster (HZ) vaccine (RZV), especially in vaccinees of advanced age at approximately 90%, contrasts with the efficacy of the live attenuated zoster vaccine (ZVL), which varies from 70% in individuals aged 50–60 years to 18% in those older than 80 years [1–4]. RZV-conferred protection is maintained at approximately 90% at 7 years postvaccination, whereas the efficacy of ZVL declines to approximately 40% at 5 years postvaccination [3, 5]. Based on this, RZV received preferential recommendations from the Advisory Committee on Immunization Practices in the United States and ZVL was withdrawn from the US market.
Protection against HZ relies on cell-mediated immunity (CMI), while antibodies play very little if any role [6, 7]. Comparing the CMI generated by the 2 HZ vaccines is important for (1) understanding the basis for their clinical differences; (2) seeking a correlate of protection; and (3) designing future vaccines for older people. We report on the completion of an experiment begun 3 years before the licensure of RZV with the intention of comparing the magnitude, and subsequent waning, of immune responses during 5 years after administration of the 2 HZ vaccines. Results from the first year of this trial have been published [8].
METHODS
Study Design
This study, NCT02114333, approved by the Colorado Multiple Institutional Review Board, enrolled 160 participants in good health except for treated chronic illnesses typical of the age of the vaccinees. All had prior varicella or resided in the United States at least 30 years; none had prior HZ. Exclusions from the study were immune suppression and recent blood products or other vaccines. Arms A and B (Supplementary Figure 1) included 90 total participants who had not previously received ZVL. Arms A and B were stratified by age (≥50–59 years, n=22 or ≥70–85 years, n=23), then randomly assigned to receive either ZVL followed by placebo 60 days later, or 2 doses of RZV at days 0 and 60. Arms C and D included an additional 70 participants who were ≥70–85 years old and had received ZVL ≥5 years previously. They were randomly assigned to receive either an additional dose of ZVL followed by placebo (arm C) or 2 doses of RZV (arm D). All vaccinations were blinded from the participant. Blood was obtained for immunologic assessment prevaccination and at 1, 3, 12, 24, and 60 months postvaccination from all participants and at 48 months from participants in arms A and B only.
Fluorospot Assay
Peripheral blood mononuclear cells (PBMC), plasma, and serum were cryopreserved within 4 hours of acquisition. PBMC were separated from heparinized blood on Ficoll-Hypaque gradients (Sigma) and frozen as previously described. Cryopreserved PBMC were thawed and rested overnight at 37°C and 5% CO2 at 106 PBMC/mL in growth medium consisting of RPMI 1640 (Mediatech) with l-glutamine (Gemini BioProducts), 10% human AB serum (Gemini BioProducts), 2% HEPES (Mediatech), and 1% penicillin-streptomycin (Gemini Bio-Products). After counting, PBMC were stimulated for 48 hours in 96-well dual-color interferon-γ (IFN-γ) and interleukin 2 (IL-2) Fluorospot plates (Mabtech) with preoptimized amounts of gE peptide pools (15-mer overlapping by 11-mer; gift from GlaxoSmithKline) or inactivated vaccine strain vOKA antigen in duplicate wells at 250000 PBMC/well. Medium-stimulated and phytohemagglutinin A controls were included. Assays were performed as per manufacturer’s instructions. Results were reported as the mean number of spot-forming cells (SFCs)/106 PBMC in varicella zoster virus (VZV)- or gE-stimulated wells after subtraction of the SFCs in mock-stimulated wells. An assay control of PBMC from a single leukopak with known performance characteristics was included in each run for validation. Assays were considered valid if the leukopak results were within preestablished bounds.
Statistical Analysis
To assess whether average VZV- and gE-stimulated response levels differed by vaccines, we used linear regression to model log10-transformed IFN-γ, IL-2, or IFN-γ/IL-2 double-positive SFC/106 PBMC postvaccination at time T (outcome) by vaccine indicator (predictor; RZV/VZV), adjusting for prevaccination log10-levels as a covariate. We corrected for multiple testing using a 5% Benjamini-Hochberg false discovery rate (t test, FDR <0.05; 42 total tests). We checked the impact of adjusting for age group (50–59 or 70–85 years old), sex (male or female), and prior ZVL (prior vaccination yes/no) on our conclusions. We also examined the potential for vaccine-by-age and vaccine-by-prior ZVL interactions.
We used 2 methods of evaluating the rate of longitudinal decline in T helper 1 (Th1) responses. First, we tested if recipients of a given vaccine had responses at postvaccination time T fall back to their baseline levels, by testing if average change in log10-responses at T minus prevaccination significantly differed from zero (t test, FDR <0.05; 72 total tests). Second, we calculated 5-year decline rates, defined as log10-responses at 5 years minus peak (ie, max observed response at 3 months for RZV, 1 month for ZVL) and tested if these changes differed by vaccine (t test, FDR <0.05). We also compared decline from 1 year to 5 years (12 total tests).
Lastly, we tested for differential vaccine response rates (VRR), defined as the fraction of recipients with ≥2-fold IL-2, IFN-γ, or double-positive SFCs at time T postvaccination divided by prevaccination levels [9]. We used permutation testing to examine whether the difference in VRR between RZV and ZVL recipients was significantly greater compared to that of random chance, represented by 10000 permutations (FDR <0.05; 42 total tests). Additional methodological details are available in the Supplementary Material.
RESULTS
Demographic Characteristics
The study enrolled 160 participants, including 124 who completed all visits. The mean age at enrollment was 70 years; 86 (54%) were women, 152 (97%) white, and 156 (98%) non-Hispanic (Supplementary Table 1). The demographic characteristics were similar between the 2 vaccine groups. We did not observe differences in dropout rates by group assignment or these demographic features: we observed 77% retention of the 50–59 age group at 5 years, and 76% retention of the 70–85 age group at 5 years.
Differential Effect of RZV and ZVL on the Persistence of VZV- and gE-Specific Th1 Responses
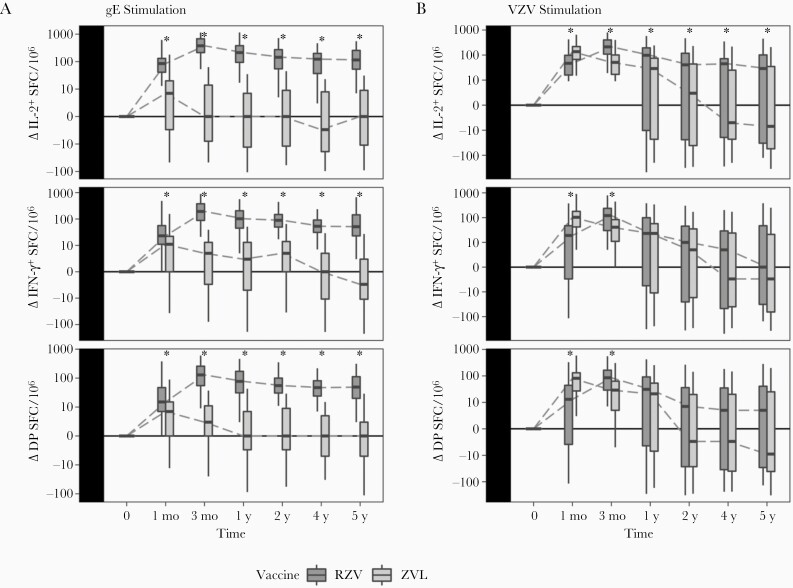
We previously showed that RZV and ZVL recipients differed in their gE- and VZV-specific Th1 responses within the first year of vaccination [8]. We continued to find differences up to 5 years postvaccination, with significantly higher average gE-specific IL-2, IFN-γ, and double-positive responses and VZV-specific IL-2 responses in RZV compared to ZVL recipients at 2, 4, and 5 years postvaccination (Figure 1 and Supplementary Table 2). Moreover, RZV recipients maintained average gE-specific IL-2, IFN-γ, and double-positive responses, and VZV-specific IL-2 responses significantly higher than prevaccination levels at all time points, while ZVL recipients did not (Supplementary Table 3). Notably, the rates of decline of gE- or VZV-specific Th1 responses from peak to 5 years and from 1 year to 5 years postvaccination did not significantly differ between the 2 groups, except for gE-IL-2, which declined faster in RZV than ZVL recipients (Supplementary Table 4).
Figure 1.
Kinetics of Th1 responses to gE and VZV after zoster vaccination. Data were derived from vaccinees ≥50 years of age. The numbers of ZVL recipients (light grey) contributing data at 1, 3, 12, 24, 48, and 60 months were 79, 79, 77, 75, 39 (group A only), and 61, respectively. Corresponding numbers for RZV recipients (dark grey) were 79, 79, 79, 76, 39 (group B only), and 63, respectively. Boxplots depict median and interquartile range responses to gE stimulation (A) or VZV stimulation (B) at the time points (log10 scale), after subtraction of prevaccination responses. Linear regression analyses showed significantly higher responses to gE stimulation in RZV compared with ZVL recipients for all markers and at all time points (FDR <0.05; significance indicated by asterisk). Responses to VZV significantly differed between RZV and VZV participants at 1 and 3 months with respect to all markers, then only by IL-2 VZV response at 12, 24, 48, and 60 months. Abbreviations: DP, double positive; FDR, false discovery rate; gE, glycoprotein E; IFN-γ, interferon-γ; IL-2, interleukin 2; RZV, recombinant zoster vaccine; SFC, spot-forming cell; Th1, T helper 1; VZV, varicella zoster virus; ZVL, zoster vaccine live.
We did not detect significant associations of VZV- and gE-responses with age, sex, or prior zoster vaccination. Adjusting for these covariates did not alter effect estimates (data not shown).
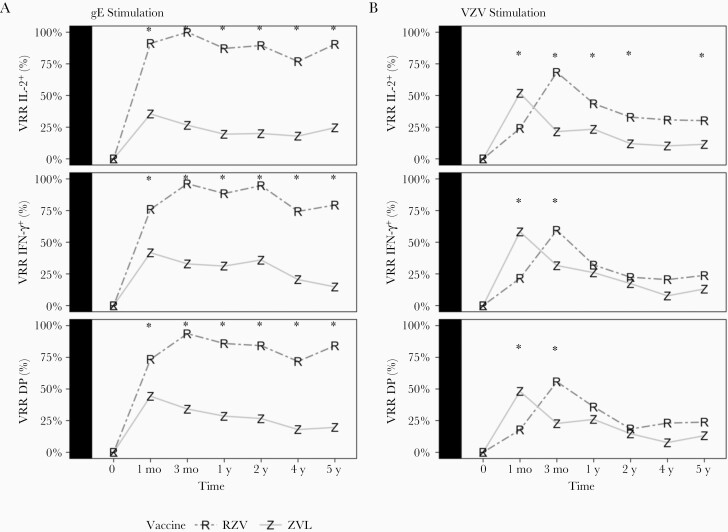
Vaccine Response Rate to gE and VZV in ZVL and RZV Recipients
We observed differences in VRR by vaccine type up to 5 years. Among RZV recipients, gE-VRR peaked at 94% to 100% 1 month after the second dose, then remained relatively constant at rates ≥74% (Figure 2A and Supplementary Table 5). In ZVL recipients, gE-specific VRR peaked at 35%–44% 1 month postvaccination and decreased to 15%–25% at 5 years. The VZV-specific VRR in RZV recipients was 58%–68% at peak response and decreased to 24%–30% at 5 years. In ZVL recipients, the VZV-specific VRR peaked at 48%–58% and decreased to 12%–13% at 5 years (Figure 2B). VZV-specific IL-2 VRR was significantly higher in RZV compared to ZVL recipients at all time points after peak response (3 months), but IFN-γ and double-positive VRR were not.
Figure 2.
VRRs in RZV and ZVL recipients during 5 years after vaccination. Data were derived from 160 participants ≥50 years of age. The numbers of ZVL recipients (Z) contributing data at 1, 3, 12, 24, 48, and 60 months were 79, 79, 77, 75, 39 (group A only), and 61, respectively. Corresponding numbers for RZV recipients (R) were 79, 79, 79, 76, 39 (group B only), and 63, respectively. VRR is the proportion of participants (0% to 100%) with ≥2-fold higher responses at the time indicated on the x-axis, compared to responses prevaccination. Differences in VRR to gE stimulation (A) were significantly higher in RZV compared with ZVL recipients at all time points, for all markers (FDR <0.05; significance indicated with asterisk). VZV-specific VRR (B) significantly differed by vaccine at 1 and 3 months for all markers, and only for IL-2 at 12, 24, and 60 months. Abbreviations: DP, double positive; gE, glycoprotein E; IFN-γ, interferon-γ; IL-2, interleukin 2; RZV, recombinant zoster vaccine; VRR, vaccine response rate; VZV, varicella zoster virus; ZVL, zoster vaccine live.
DISCUSSION
This was the first randomized study comparing the persistence of T-cell responses to RZV and ZVL. We showed that RZV recipients maintained VZV-specific memory IL-2 responses above prevaccination levels for at least 5 years after immunization, while VZV IL-2 responses in ZVL recipients did not significantly differ from prevaccination at 3 months postimmunization. VZV-specific effector T-cell responses did not differ from pre- to postvaccination after the first year postvaccination in either group. The difference in the VZV-specific memory responses generated by the 2 vaccines during 5 years expands our previous finding that peak VZV IL-2 responses mediated the RZV effect on 1-year persistence of Th1 immunity [8]. The VZV IL-2 memory may bear on the mechanism of RZV-conferred protection. HZ is the clinical manifestation of VZV reactivation that is no longer limited by the host protective immune responses. There is evidence that during their lifetime, VZV-infected individuals undergo multiple asymptomatic VZV reactivations, and it is likely that in most instances the immune system controls replication of VZV before the critical mass needed to generate symptoms (HZ) [10, 11]. The parallel between the duration of protection against HZ and the persistence of VZV-specific IL-2 responses that differentiate RZV from ZVL suggests that Th1 memory responses play an important role in the superior protection conferred by RZV.
The gE-specific Th1 responses were consistently higher in RZV than ZVL recipients. gE-specific IL-2, IFN-γ, and double-positive T-cell VRRs were detected in ≥74% of RZV recipients for the 5 years of our study compared with ≤58% of ZVL recipients. The persistence of gE-specific Th1 responses agrees with previous smaller studies that showed postvaccination responses are higher than prevaccination responses for 10 years [12]. The gE VRR in RZV recipients in this study was slightly higher than in a previous report that showed VRR <60% at 3 years postvaccination [8].
In contrast to almost 100% of RZV recipients who reached peak gE-specific VRR, gE VRR in ZVL recipients peaked at ≤44%. Although gE is the most abundant VZV glycoprotein, our data suggest that responses to gE may be immunodominant in <50% of the hosts. A previous study reported that IL-2 and IFN-γ T-cell responses in unvaccinated hosts were directed against the following VZV gene products in hierarchal order: immediate early (IE) 62, IE63, open reading frame (ORF) 9, gB, and gE [13]. ZVL administration increased IL-2 and IFN-γ T-cell responses, maintaining the same hierarchy [13]. These findings may explain the more limited response to gE in ZVL compared with RZV recipients.
The rate of decline of Th1 responses after vaccination did not appreciably differ between groups except for gE-specific IL-2, which declined faster in RZV recipients. This observation suggests that the magnitude of the peak responses generated by RZV accounts for the superior persistence of immune protection.
We did not find an effect of age or sex on responses to ZVL or RZV in this study. However, studying an age effect on responses to vaccines is greatly confounded by the difference between chronological and biological age. For this reason, large sample sizes are needed to demonstrate an effect of age. In a previous study that included 100 to 200 participants for each decade between 50 and >70 years, we showed a clear effect of age on responses to ZVL [14]. Likewise, Cunningham et al showed an effect of age on CD4+ T-cell responses to RZV in a cohort of 174 participants [9].
A limitation of this study was the use of antigens specific to each vaccine, including vOKA lysate and peptides based on the vaccine rgE amino acid sequence. Of note, a comparison of peptides based on the recombinant gE used in the GSK vaccine and vOKA sequence yielded similar Fluorospot results (not shown).
In conclusion, compared with ZVL, RZV generated longer persistence of memory T-cell responses against VZV and of both effector and memory responses to gE. These immune response differences between the 2 vaccines mirror the clinical differences previously described [1–3]. The adjuvant in RZV clearly plays a major role in the immunogenicity of the vaccine [15]. Whether focusing the immune response on a single glycoprotein, gE, adds anything to the improved immunogenicity and efficacy of RZV is unclear at this point, but warrants additional study as it may impact the design of future vaccines.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support. This work was supported by GlaxoSmithKline Investigator-Initiated Study Program (grant number 63007497, years 1 and 2); and by the National Institute of Allergy and Infectious Diseases (grant numbers U19 AI090023 and 1U01AI141919-01, years 3 to 5).
Potential conflicts of interest. A.W. receives grants from G.S.K. and Merck and personal fees from Merck; M.J.L. receives grants from G.S.K. and Merck and personal fees from G.S.K.
References
- 1. Cunningham AL, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med 2016; 375:1019–32. [DOI] [PubMed] [Google Scholar]
- 2. Lal H, Cunningham AL, Godeaux O, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015; 372:2087–96. [DOI] [PubMed] [Google Scholar]
- 3. Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352:2271–84. [DOI] [PubMed] [Google Scholar]
- 4. Boutry C, Hastie A, Diez-Domingo J, et al. The adjuvanted recombinant zoster vaccine confers long-term protection against herpes zoster: interim results of an extension study of the pivotal phase III clinical trials (ZOE-50 and ZOE-70) [published online ahead of print 20 July 2021]. Clin Infect Dis doi: 10.1093/cid/ciab629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morrison VA, Johnson GR, Schmader KE, et al. Long-term persistence of zoster vaccine efficacy. Clin Infect Dis 2015; 60:900–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arvin AM, Pollard RB, Rasmussen LE, Merigan TC.. Cellular and humoral immunity in the pathogenesis of recurrent herpes viral infections in patients with lymphoma. J Clin Invest 1980; 65:869–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Asada H. VZV-specific cell-mediated immunity, but not humoral immunity, correlates inversely with the incidence of herpes zoster and the severity of skin symptoms and zoster-associated pain: the SHEZ study. Vaccine 2019; 37:6776–81. [DOI] [PubMed] [Google Scholar]
- 8. Levin MJ, Kroehl ME, Johnson MJ, et al. Th1 memory differentiates recombinant from live herpes zoster vaccines. J Clin Invest 2018; 128:4429–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cunningham AL, Heineman TC, Lal H, et al. Immune responses to a recombinant glycoprotein E herpes zoster vaccine in adults aged 50 years or older. J Infect Dis 2018; 217:1750–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wilson A, Sharp M, Koropchak CM, Ting SF, Arvin AM.. Subclinical varicella-zoster virus viremia, herpes zoster, and T lymphocyte immunity to varicella-zoster viral antigens after bone marrow transplantation. J Infect Dis 1992; 165:119–26. [DOI] [PubMed] [Google Scholar]
- 11. Cohrs RJ, Mehta SK, Schmid DS, Gilden DH, Pierson DL.. Asymptomatic reactivation and shed of infectious varicella zoster virus in astronauts. J Med Virol 2008; 80:1116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hastie A, Catteau G, Enemuo A, et al. Immunogenicity of the adjuvanted recombinant zoster vaccine: persistence and anamnestic response to additional doses administered 10 years after primary vaccination [published online ahead of print 5 June 2020]. J Infect Dis doi: 10.1093/infdis/jiaa300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sei JJ, Cox KS, Dubey SA, et al. Effector and central memory poly-functional CD4+ and CD8+ T cells are boosted upon ZOSTAVAX(®) vaccination. Front Immunol 2015; 6:553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weinberg A, Pang L, Johnson MJ, et al. The effect of age on the immunogenicity of the live attenuated zoster vaccine is predicted by baseline regulatory T cells and varicella-zoster virus-specific T cell immunity. J Virol 2019; 93:e00305-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leroux-Roels G, Marchant A, Levy J, et al. Impact of adjuvants on CD4+ T cell and B cell responses to a protein antigen vaccine: results from a phase II, randomized, multicenter trial. Clin Immunol 2016; 169:16–27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.