Abstract
Context
Antiresorptive therapy significantly reduces fracture risk in patients with benign bone disease and skeletal-related events (SREs) in patients with bone metastases (BM). Osteonecrosis of the jaw (ONJ) is a rare but severe condition manifested as necrotic bone lesion or lesions of the jaws. ONJ has been linked to the use of potent antiresorptive agents, termed medication-related ONJ (MRONJ).
Objective
We aimed to identify the differences various aspects of MRONJ among distinct patient categories and provide recommendations on how to mitigate the risk and optimally manage MRONJ in each of them.
Methods
A working group of the European Calcified Tissue Society (ECTS) and 2 experts performed an updated detailed review of existing literature on MRONJ incidence, characteristics, and treatment applied in bone diseases with variable severity of skeletal insult, ranging from osteoporosis to prevention of cancer treatment–induced bone loss and SREs in cancer patients with BM.
Results
The risk for MRONJ is much higher in patients with advanced malignancies compared to those with benign bone diseases because of the higher doses and more frequent administration of antiresorptive agents in individuals with compromised general health, along with coadministration of other medications that predispose to MRONJ. The overall risk for MRONJ is considerably lower than the benefits in all categories of patients.
Conclusion
The risk for MRONJ largely depends on the underlying bone disease and the relevant antiresorptive regimen applied. Physicians and dentists should keep in mind that the benefits of antiresorptive therapy far outweigh the risk for MRONJ development.
Keywords: bisphosphonates, bone metastases, denosumab, osteonecrosis of the jaw, osteoporosis
An imbalance of bone turnover, with relatively increased osteoclast-mediated bone resorption rate, characterizes a broad spectrum of bone diseases, ranging from osteoporosis (OP) and other benign bone diseases to cancer treatment–induced bone loss (CTIBL; aromatase inhibitor (AI)-induced bone loss, and androgen deprivation–induced bone loss) and bone metastases (BM) in advanced malignancies. In all these conditions, targeting the osteoclast with antiresorptive agents is currently the cornerstone of treatment of the respective bone disease. Among them, bisphosphonates (BPs), and denosumab (Dmab) are the more potent and more frequently used agents in everyday clinical practice.
BPs are divided into oral, including alendronate (ALN), risedronate (RIS), clodronate (CLO), and ibandronate (IBN), and intravenous (IV) agents, including IBN, pamidronate (PAM), and zoledronate (ZOL). In general, oral BPs are preferred in OP and other benign bone diseases, whereas for prevention of CTIBL and the management of BM, IV BPs are mostly used (Table 1). Dmab binds the receptor activator of nuclear factor κ-Β ligand (RANKL), thus inhibiting osteoclast differentiation, function, and survival (1). BPs and Dmab both significantly decrease the risk of vertebral (by 41%-77% and 68%, respectively) and hip (by 30%-51% and 40%, respectively) fractures in OP patients (2, 3) and the incidence of skeletal-related events (SREs) in cancer patients (4, 5). The regimen of each BP and of Dmab varies depending on the disease (see Table 1).
Table 1.
Regimen of antiresorptive agents according to underlying bone disease
| Osteoporosis | CTIBL | Bone metastases | ||||
|---|---|---|---|---|---|---|
| Dose | Frequency | Dose | Frequency | Dose | Frequency | |
| Bisphosphonates | ||||||
| Alendronate | 70 mg by mouth | Weekly | 70 mg by mouth | Weekly | – | – |
| Risedronate | 35 mg (75 mg) by mouth | Weekly (2 consecutive d/mo) | 35 mg by mouth | Weekly | – | – |
| Ibandronate | 150 mg by mouth | Monthly | 150 mg by mouth | Monthly | 50 mg | Daily |
| 3 mg IV | Every 3 mo | – | – | 6 mg IV | Every 3-4 wk | |
| Pamidronate | – | – | 60 mg IV | Every 3 mo | 90 mg IV | Every 3-4 wk |
| Zoledronate | 5 mg IV | Yearly | 4 mg IV | Every 3-6 mo | 4 mg iv | Every 3-4 wka |
| Denosumab | 60 mg SC | Every 6 mo | 60 mg SC | Every 6 mo | 120 mg SC | Every 4 wk |
Abbreviations: CTIBL, cancer treatment–induced bone loss; IV, intravenously; SC, subcutaneously.
a At least for the first 3 to 6 months; de-escalation to doses every 12 weeks could be considered thereafter.
Osteonecrosis of the Jaw: Definitions and Staging
Description
Osteonecrosis of the jaw (ONJ) is a rare but serious condition manifested as one or more necrotic bone lesions that are exposed or can be probed through an intraoral or extraoral fistula in the maxillofacial region, and persist for at least 8 weeks without response to appropriate therapy (6, 7). ONJ is more commonly located in the mandible (8-10) but can be detected at both jaws (10). It may be accompanied by pain, inflammation, erythema, suppuration, and loose teeth. Although ONJ may occur spontaneously, in most cases it is the result of a dental procedure, for example, a tooth extraction or oral surgery (9, 11). Dentoalveolar infections may precede the appearance of necrotic bone (9). Lack of history of radiation therapy or metastatic disease to the jaws is required to confirm the diagnosis of ONJ (6).
Terminology
The occurrence of ONJ was initially associated with BP administration, and named bisphosphonate-related ONJ (BRONJ) (12). Later, ONJ was also attributed to Dmab (DRONJ), and named antiresorptive agent–related ONJ (ARONJ), and afterward to antiangiogenic medications and tyrosine kinase inhibitors (TKIs), transforming the term to the currently used medication-related ONJ (MRONJ) (6). To date, several other medications, mostly used in the oncology setting, have also been linked to the risk of MRONJ (9, 13) (Table 2).
Table 2.
Nonantiresorptive medications associated with osteonecrosis of the jaw development
| Class | Representatives |
|---|---|
| Glucocorticoids | |
| VEGF inhibitors | Bevacizumab, aflibercept |
| TKIs | Sunitinib, imatinib, cabozantinib, sorafenib, regorafenib, axitinib, pazopanib, dasatinib |
| mTORi | Everolimus, temsirolimus |
| BRAF inhibitors | Dabrafenib, trametinib |
| Monoclonal Abs against CD20 | Rituximab |
| Immune checkpoint inhibitors | Nivolumab, monoclonal Abs against CTLA-4 (ipilimumab) |
| Lenalidomide | |
| Chemotherapy regimens | Cytarabine, idarubicin, and daunorubicin; gemcitabine, vinorelbine, and doxorubicin; doxorubicin and cyclophosphamide; 5-azacitidine |
| Leflunomide | |
| Anti-TNF agents | Adalimumab |
Abbreviations: Abs, antibodies; BRAF, B-Raf; CTLA-4, cytotoxic T-lymphocyte–associated protein 4; TKIs, tyrosine kinase inhibitors; mTORi, inhibitors of mammalian target of rapamycin; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
Staging
Among several proposals for staging of ONJ (11), the staging of the American Association of Oral and Maxillofacial Surgeons (AAOMS) is now most commonly used (6) (Table 3). A nonexposed variant was also introduced (14), and not including this variant is reported to considerably underdiagnose ONJ (15).
Table 3.
Staging according to the American Association of Oral and Maxillofacial Surgeons and the alleged nonexposed variant
| Stage | Description |
|---|---|
| At risk | Patients treated with bone-modifying agents but without apparent necrotic bone (eg, all asymptomatic patients treated with antiresorptives) |
| Stage 0 | No clinical evidence of necrotic bone but nonspecific symptoms or clinical/radiographic findings |
| Stage 1 | Exposed and necrotic bone, or fistulae that probe to bone, that are asymptomatic with no evidence of significant adjacent or regional soft-tissue inflammation or infection |
| Stage 2 | Exposed and necrotic bone, or fistulae that probe to bone, associated with infection, evident by pain and adjacent or regional soft-tissue inflammatory swelling, with or without purulent drainage |
| Stage 3 | Exposed and necrotic bone, or fistulae that probe to bone, associated with pain and infection, and at least one of the following: (1) pathologic fracture, (2) extra-oral fistula, (3) oral-antral fistula, or (4) radiographic evidence of osteolysis extending to the inferior border of the mandible or floor of the maxillary sinus |
| Nonexposed variant (not widely adopted) | Presence of otherwise unexplained pain in the jaws, fistula, swelling, mobile teeth, or mandibular fracture diagnosed after excluding common diseases of the jaw known to cause similar manifestations |
Aim
The ECTS considers that there is confusion among clinicians and dentists regarding the occurrence and optimal prevention/management of ONJ, possibly owing to discrepancy regarding definition, incidence, characteristics, and treatment. This may in part relate to the underlying bone disease and consequent regimen (doses and frequency) of the antiresorptives administered. Therefore, we attempt to clarify various aspects of ONJ among patients with OP in comparison to patients at risk for CTIBL and patients with BM or multiple myeloma (MM). We provide recommendations on how to mitigate risk and optimally manage ONJ in each of these patient categories.
Methods
This review was performed under the auspices of the Clinical Action Group of the Policy and Consensus Committee of the ECTS. Detailed methodology is quoted in the Supplementary data (16). This review was not set up to make grade-based recommendations; given the lack of high-quality data, evidence would most likely be low, and this would render grade recommendations weak. The final manuscript has not been subjected to a membership hearing but has been approved for submission by the board of directors of the ECTS.
Pathophysiology
Several mechanisms have been proposed to contribute to MRONJ, with oversuppression of bone turnover considered the main mechanism attributed to antiresorptives. Infection and inflammation, as well as angiogenesis inhibition, are also important factors predisposing to ONJ development.
Inhibition of Bone Resorption and Turnover—Good for the Bone, Bad for the Jaw?
Bone turnover suppression is the main mechanism by which antiresorptives increase bone density and reduce fracture risk. However, osteoclast differentiation and functionality, and a degree of bone remodeling, is crucial for bone healing and repair of microdamage. Interestingly, “osteonecrosis” occurs primarily within the alveolar bone of the jaws and not in other skeletal sites. A comparatively higher remodeling rate in the jaws compared to other bones may play a role in this predisposition (17). Repetitive microtraumas from chewing may also contribute. Additionally, jaw osteoclasts may absorb higher amounts of BPs than long bone osteoclasts (18). Indirect evidence of the pivotal role of bone turnover suppression in MRONJ development derives from the facts that MRONJ has almost exclusively been attributed to the most potent antiresorptives and is far more common among BM or MM patients, for whom higher doses are administered in more frequent intervals, compared to OP. Additional, indirect, evidence comes from the improved extraction socket healing and resolution of ONJ when parathyroid hormone was administered in animals (19-21) and humans (22, 23).
Local inflammation and infections
Periodontal or periapical disease was common in initially reported cases (24). Subsequently, animal models proved that inflammation or bacterial infection combined with antiresorptives can induce ONJ (25, 26). A complex biofilm, composed of bacteria, especially Actinomyces species (10, 27), along with fungi and viruses (28), has been identified in biopsies of necrotic bone from ONJ lesions. Increased local infections and impaired oral mucosa healing with BPs suggested a synergistic role of antiresorptives with inflammation and infection (29, 30). BP administration has been associated with proliferation and adhesion to hydroxyapatite of oral bacteria (31). Furthermore, BPs may impair the immune response to infection through activation of γ δ T cells and altered production of proinflammatory cytokines (32).
Angiogenesis inhibition
Angiogenesis requires binding of signaling molecules, such as vascular endothelial growth factor (VEGF), to their receptors on endothelial cells, and is considered vital for tumor growth and metastases. VEGF inhibitors are used by oncologists to deter tumor growth. Osteonecrosis, which is an avascular necrosis, entails interruption of the blood supply in the lesion. Administration of antiangiogenic agents, for example, antibodies targeting VEGF and TKIs, has been linked with ONJ occurrence (11). Additionally, glucocorticoids, especially in the high doses used in the oncology setting, exert antiangiogenic effects (33). High doses of potent BPs, especially ZOL, have been consistently associated with decreased angiogenesis according to in vitro studies (34, 35) and decreased VEGF levels in human studies (36, 37). On the contrary, there is no evidence that Dmab exerts antiangiogenic effects (35).
Immune system dysfunction
Accumulated indirect evidence suggests that dysregulation of the immunological response may contribute to MRONJ development. The higher MRONJ occurrence when glucocorticoids are coadministered with BPs both in animals (38, 39) and humans (7) has been attributed to the immunomodulating effects of glucocorticoids on T-cell subpopulations (39), whereas immunotherapy with mesenchymal stem cells prevented and even cured MRONJ (39). Besides glucocorticoids, concurrent chemotherapy increases MRONJ risk (7), as do other conditions that adversely affect the immune system, including diabetes, inflammatory joint diseases, and even compromised general health (7). In addition, anti-VEGF agents can inhibit macrophage chemotaxis (40) while anti-TKIs inhibit the differentiation of cells of the monocyte/macrophage lineage, conditioning the local response of the immune system (41). Biologic immunomodulators, including anti–tumor necrosis factor α, anti-CD20, mammalian target of rapamycin inhibitors, and methotrexate have also been linked with ONJ development through their effects on immune system function (11). Finally, BPs and Dmab by themselves may affect monocyte and macrophage function and survival, although so far this has not been linked to systemic changes in immune function (41, 42).
Soft-tissue toxicity
Besides their effect on osteoclasts, BPs decrease proliferation and increase apoptosis of several cell lines in vitro, disrupting the mucosal barrier and favoring bacteria invasion (43-45). Furthermore, BPs can suppress the migration of oral epithelial cells, a step crucial for tooth socket closure (31). It has been proposed that BPs accumulate in the jawbone in concentrations toxic to the oral epithelium, deterring healing of soft-tissue injuries caused by invasive dental procedures or even subclinical trauma from dentures (46). In contrast, no soft-tissue toxicity has been reported with Dmab.
Vitamin D deficiency and osteomalacia
There is weak evidence for a role of vitamin D deficiency in MRONJ development. Studies have shown associations with chronic periodontitis or caries risk (47). Low vitamin D levels have been associated with ONJ in some (48, 49) but not all studies (50). Treatment with BPs in the presence of osteomalacia could exaggerate a mineralization defect (51). Such a mineralization defect has been described in ONJ patients (52).
Genetic predisposition
Variants of several genes, including the cytochrome P450 CYP2C8 (53, 54), VEGF (54, 55), peroxisome proliferator-activated receptor γ (56), aromatase (57), farnesyl pyrophosphate synthase (58), the RNA-binding motif, single-stranded-interacting protein 3 (RBMS3) gene (59), insulin-like growth factor-binding protein 7 (IGFBP7) (59), the ATP-binding cassette subfamily C member 4 (ABCC4) gene (59), and sirtuin 1 (SIRT1) (60) genes have been associated with increased MRONJ incidence in genome-wide association studies and whole-exome sequencing analyses conducted mainly in cancer patients treated with BPs. However, none of these studies determined which gene polymorphisms present some association (61), while subsequent candidate gene studies failed to replicate these results, thus questioning the predictive role of these polymorphisms in the risk for MRONJ development (62).
Pathophysiological mechanisms of medication-related osteonecrosis of the jaw related to underlying disease or antiresorptive agent
The pathogenesis of MRONJ is probably multifactorial. It is unclear if and which of the aforementioned mechanisms contribute the most to MRONJ development. Coexistence of more than one of the mechanisms seems to increase the risk. It is possible that the prevailing pathophysiological mechanism differs between BPs and Dmab, for example, the latter exerts no antiangiogenic effects or soft-tissue toxicity.
MRONJ is far more common in cancer patients compared to patients treated for benign bone diseases, such as OP (63). This is most likely a result of more intense and prolonged suppression of bone turnover by the higher and more frequent dosing, but may also be due to concurrent therapies (glucocorticoids, chemotherapy, antiangiogenic agents, immunomodulators) and decreased oral and general health in cancer patients (63).
Osteonecrosis of the jaw cases without antiresorptive therapy
ONJ has been rarely but increasingly described in patients not receiving bone-modifying agents (BMAs) (7, 9, 10, 13). Oral ulceration and benign sequestration, a benign, transient condition of presently unknown incidence, manifests as painful ulceration usually over the posterior lingual mandible in the absence of systemic disease or prior use of antiresorptive or antiangiogenic therapy (64). It resolves spontaneously in a few days to several months (65-68), although it may persist for more than 8 weeks and be misdiagnosed as ONJ.
The non-BMA agents that have been associated with ONJ development, mainly in patients with malignancies, are quoted in Table 2. Cases of ONJ in noncancer patients receiving none of the previously mentioned agents have been sparsely reported (69).
Incidence and Risk Factors
Medication-related Osteonecrosis of the Jaw Associated With Bisphosphonate Use
MRONJ real incidence is difficult to ascertain because diagnosis of BRONJ followed different definitions until 2007, when the AAOMS proposed a definition of MRONJ, partially revised in 2009 and again in 2014 (6, 70, 71).
As already mentioned, the incidence of BRONJ is considerably lower in OP patients compared to cancer patients. Of note, in such populations (mainly postmenopausal women) numerous clinical trials, corresponding to more than 60 000 patient-years, have reported no incidence of BRONJ (72). According to the latest consensus, BRONJ incidence in OP patients ranges from 0.01% to 0.06% (6, 73, 74), although the incidence in Asia may be higher (75-77), maybe due a higher prevalence of periodontal disease.
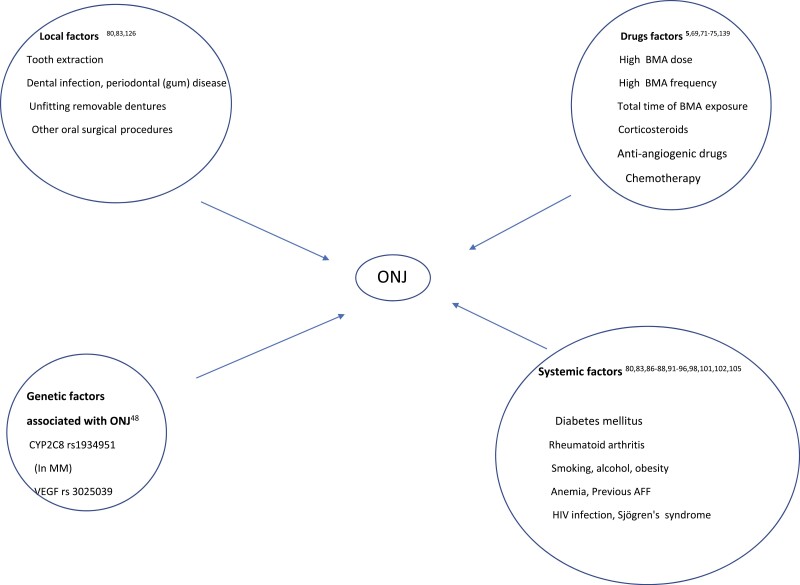
On the contrary, in cancer patients, in whom the use of BPs aims to prevent CTIBL, decrease the incidence of SREs, and reduce the risk of BM, the reported frequency for MRONJ after ZOL ranged from 1% to 8% when used for BM, and from 0% to 1.8% when administered as adjuvant treatment (78). Probably the most important factor for this difference is the higher (~ 12-15 times) dose of BPs used for metastatic bone disease compared to OP, including, in the case of ZOL, the higher number of infusions (79-87). A possible bias in interpreting the difference in incidences among studies may arise from the different duration of follow-up of each study, which may be a potential confounder. In addition, even among cancer patients, MRONJ incidence was quite different when ZOL was administered in doses used for CTIBL prevention, compared with the higher cumulative doses used for treatment of BM. Several local risk factors predispose to ONJ development (Fig. 1), the most common being tooth extraction. In cancer patients, tooth extraction not only after but also before initiation of BMA has been associated with MRONJ development (88). However, some studies suggest that preexisting dental disease, rather than tooth extraction, is the risk factor (88-93). In a recent systematic review, a 10% risk of MRONJ was noted in patients with periodontal disease treated with BPs (6). As expected, coexistence of local infection (periodontal/periapical inflammation) and tooth extraction further increases the risk of MRONJ (94). Systemic risk factors such as type of disease and concomitant therapies (eg, chemotherapy, glucocorticoids, antiangiogenic agents) also increase MRONJ risk (93, 95-110) (see Fig. 1). Among them, glucocorticoids, especially in the high doses used in oncology, alone or in combination with other medications, represent a prominent risk factor. Smoking (111-113), age (88, 97, 114), sex (74, 114), diabetes mellitus (112, 115), and obesity (111) have also been associated with ONJ risk, especially in oncology.
Figure 1.
Risk factors for antiresorptive agent-related osteonecrosis of the jaw. AFF, atypical femoral fracture; BMA, bone-modifying agents; MM, multiple myeloma; ONJ, osteonecrosis of the jaw; VEGF, vascular endothelial growth factor.
Despite the initial enthusiasm regarding the role of markers of bone resorption in the prediction of MRONJ risk, a systematic review suggested that no marker is useful in this setting (116).
Recently, a decline in the incidence of BRONJ has been reported, probably due to prevention of clinical risk factors. A 9-year (2009-2018) survey showed that the number of new MRONJ cases was stable from 2009 to 2015, with a mean of 51.3 cases per year declining during the years 2016 to 2018 to 33.3 cases per year (117). Increased clinician awareness as well as the reluctance of dental practitioners to perform dental procedures in patients on antiresorptives may have contributed to this decline in the incidence of ONJ.
Medication-related Osteonecrosis of the Jaw Associated With Denosumab Use
Similarly to BPs, the incidence in OP patients is considerably lower than in cancer patients and risk factors appear identical (63, 118-129). A systematic review of randomized controlled trials that directly compared Dmab with BPs in postmenopausal women with OP identified no reports of MRONJ (126), whereas in the FREEDOM and FREEDOM extension study the incidence of MRONJ was 5.2 per 10 000 patient-years (130). According to the FAERS database, Dmab-treated OP patients had an odds ratio of 0.63 (95% CI, 0.56-0.70; P < .001) to develop MRONJ compared to untreated OP patients, whereas in contrast the odds ratio was 4.9 (95% CI, 4.4-5.4; P < .001) for cancer patients treated with Dmab for prevention of SREs compared to untreated cancer patients (131). Similarly, according to the Multinational Association of Supportive Care in Cancer report, in cancer patients treated with Dmab, MRONJ frequency ranged from 0.7% to 6.9% for BM, whereas in the setting of CTIBL it was 0% (78).
Again, the incidence of MRONJ increases from cancer patients receiving Dmab for CTIBL, to those receiving Dmab for BM prevention, and finally those receiving it as BM therapy. In the CTIBL setting, where Dmab is administered in the same doses and frequency as administered for the treatment of OP, the incidence of MRONJ is similar to the incidence seen in OP patients, for example, no cases were reported in the latest double-blind, placebo-controlled trial, which included postmenopausal patients with early breast cancer (132). When Dmab was given monthly at a dose of 120 mg for the prevention of BM in prostate cancer patients, the incidence of MRONJ rose to 5% (133). The same incidence was reported in breast cancer patients in the D-Care trial (134).
Postmarketing surveillance data including 1 960 405 patient-years of Dmab exposure of 2 427 475 patients identified 47 adjudicated cases of MRONJ. All these individuals had at least one other risk factor for MRONJ development including concurrent glucocorticoid use, concurrent chemotherapy, prior BP use, or invasive dental procedures (74).
Frequently cancer patients switch BMA therapy, and a systematic review suggested an increased prevalence of MRONJ associated with sequential antiresorptive therapy with PAM and ZOL, and with BPs and Dmab when compared to single BMA therapy (135).
Differences Among Antiresorptive Agents
MRONJ has been rarely reported with antiresorptive agents other than BPs or Dmab. Since the degree of bone turnover suppression is considered one of the main mechanisms of MRONJ development, “milder” antiresorptives, such as selective estrogen receptor modulators, may be an alternative for women at increased risk of ONJ with OP. Following the same concept, women with early-stage breast cancer receiving AIs to prevent CTIBL, could replace AIs with tamoxifen, weighing however the superiority of tamoxifen over AIs on bone density/fracture risk (136) with its inferiority on breast cancer outcomes (137). Only 1 out of 1869 Taiwanese OP patients treated with raloxifene (RLX) experienced ONJ compared to the 39 out of 6485 treated with ALN (138). Two more cases of ONJ with RLX have been reported, 1 after 4 years of RLX in a previously treatment-naive OP woman (139) and another after 18 months of RLX treatment, but in a woman with several comorbidities who had previously been treated with ALN for 2 years (140). No case of ONJ has been reported with other selective estrogen receptor modulators, such as bazedoxifene or lasofoxifene to date. No case of ONJ has been reported with hormone replacement therapy, although estrogen exert a more potent antiresorptive effect than RLX, perhaps because of its limited current use. Notably, 2 cases of ONJ have been reported with romosozumab, a potent agent with both anabolic and antiresorptive effects, in OP patients with confounding factors (141).
In most (88, 121, 122, 128, 142) but not all (119, 124, 143) of the studies and meta-analyses in cancer patients with BM, the incidence of MRONJ was higher in those treated with Dmab than in BP-treated, mostly ZOL-treated, patients. A higher MRONJ incidence with Dmab was recently reported for OP patients as well (144). In the pivotal controlled, double-blind, double-dummy trials of ZOL vs Dmab, a preplanned pooled analysis of the 3 trials (breast, prostate, and other tumors), reported numerically more cases of ONJ after Dmab than after ZOL but the difference was not statistically significant (P = .13) (145). Consistently, in BM patients, replacing ZOL with Dmab was a risk factor for developing MRONJ (146-148). However, as reporting below, imaging characteristics of BRONJ and DRONJ may differ (63), potentially affecting the incidence reported for earlier stages, while including different type of cancers, some more prone to ONJ development than others, could modify the meta-analysis results. Importantly, MRONJ caused by Dmab has been reported to occur earlier than with BPs (149, 150) but also resolved faster (88). Of note, Dmab is more efficacious than BPs in preventing and delaying SREs in patients with solid tumors and MM (119, 128, 145).
Finally, the type of BP used may affect the risk of MRONJ development (96). MRONJ incidence is considerably higher with IV BPs compared to oral BPs (151) largely because oral BPs are mostly used for benign diseases whereas IV BPs are used for malignancies, in much higher and more frequently administered doses. In an in vitro study in human fibroblasts and osteoblasts, ALN suppressed cell viability and migration, and induced apoptosis more than IBN and similarly as ZOL (152). ZOL-treated cancer patients had a higher risk of MRONJ than those treated with PAM or sequentially with PAM and ZOL (96), or sequentially with ZOL and IBN (153). Similarly, in breast cancer patients receiving adjuvant BP therapy, MRONJ was higher with ZOL (1.26%) compared with oral CLO (0.36%) and IBN (0.77%) (154). Also in adjuvant breast cancer therapy, MRONJ was reported only with ZOL and not with CLO, PAM, RIS, or IBN (155). In OP patients, MRONJ was significantly more common with ALN than with RIS or PAM (8). Similarly, ALN was accountable for 77% of MRONJ cases attributed to oral BPs in a review of 2400 cases (10). Finally, a recent in vitro study suggested that the risk of MRONJ might be quite lower with nonamino-BPs than with amino-BPs because of their more potent antioxidant and anti-inflammatory effects (156).
Clinical Presentation and Imaging
Clinical Presentation
Signs and symptoms of MRONJ may include local pain, mucosal swelling, erythema, tooth mobility, tooth abscess, ulceration (15, 157), altered sensation because of compression of the nerves by surrounding inflammation (158, 159), and even paresthesia or anesthesia of the associated branch of the trigeminal nerve (159). However, exposed bone may remain asymptomatic for a long time and manifest clinical features only when inflammation of the surrounding tissue occurs (160).
Unfortunately, early signs of MRONJ include nonspecific sinus pain, odontalgia, and altered neurosensory function (71), which may prevent diagnosis and require a high index of suspicion when evaluating patients on antiresorptive and/or antiangiogenic agents.
Later findings could be fistulae, developed when necrotic jawbone tissue becomes infected (161), and chronic maxillary sinusitis with or without an oral-antral fistula secondary to maxilla osteonecrosis; the latter may rarely be the presenting feature in these patients (162, 163).
Oral and general quality of life (QoL) is affected in individuals who develop MRONJ (164, 165), and worsens with advancing of ONJ stage (165). The deterioration is mainly attributed to symptoms (mostly pain and chewing difficulties), feeling of uncertainty, speech difficulties, weight loss, and socializing issues (166). Pharmacological management and even more surgical intervention may improve QoL (166, 167).
Imaging
Imaging is critical both for diagnosis and the assessment of disease progression. The most used modality is panoramic radiography (or orthopantomography or OPG x-ray), which can identify early changes in the jaws, such as thickening of the lamina dura, widening of the periodontal ligament space, increased trabecular density of the alveolar bone, or even sequestration (168). In later stages findings vary, ranging from high bone density, dense woven bone, thickening of the periosteum, opacities, radiolucencies, and osteolysis (169), to diffuse osteosclerosis along with extended osteolysis of the surrounding bone tissue, and even pathologic fracture of the mandible in stage 3 of ONJ (161, 168).
Computed tomography and magnetic resonance imaging are considerably more sensitive than OPG x-ray and, despite their low sensitivity, can aid in diagnosis and provide qualitative assessment, with cone-beam computed tomography being the reference standard (170), and ultrashort echo-time magnetic resonance imaging recently proven equally useful (171). Bone scintigraphy could provide early detection of MRONJ in high-risk individuals (172).
Of note, although BP- and Dmab-induced ΜRONJ share a similar clinical presentation, their imaging characteristics may be quite different, although no characteristic is exclusively observed in 1 of them but rather the frequency and extent of each characteristic differs among these 2 entities (63, 173).
Prevention and Management
Medication-related Osteonecrosis of the Jaw Prevention
To determine the best approach for each individual patient, it is necessary to weigh the risks of MRONJ with the risk of fracture in OP patients and the risk of CTIBL and SREs in cancer patients. The MRONJ risk will be affected by comorbidities as well as the extent of the planned oral surgery.
To preserve oral health, coordination of care between the dentist and the bone specialist or the oncologist is strongly recommended.
Osteoporosis patients
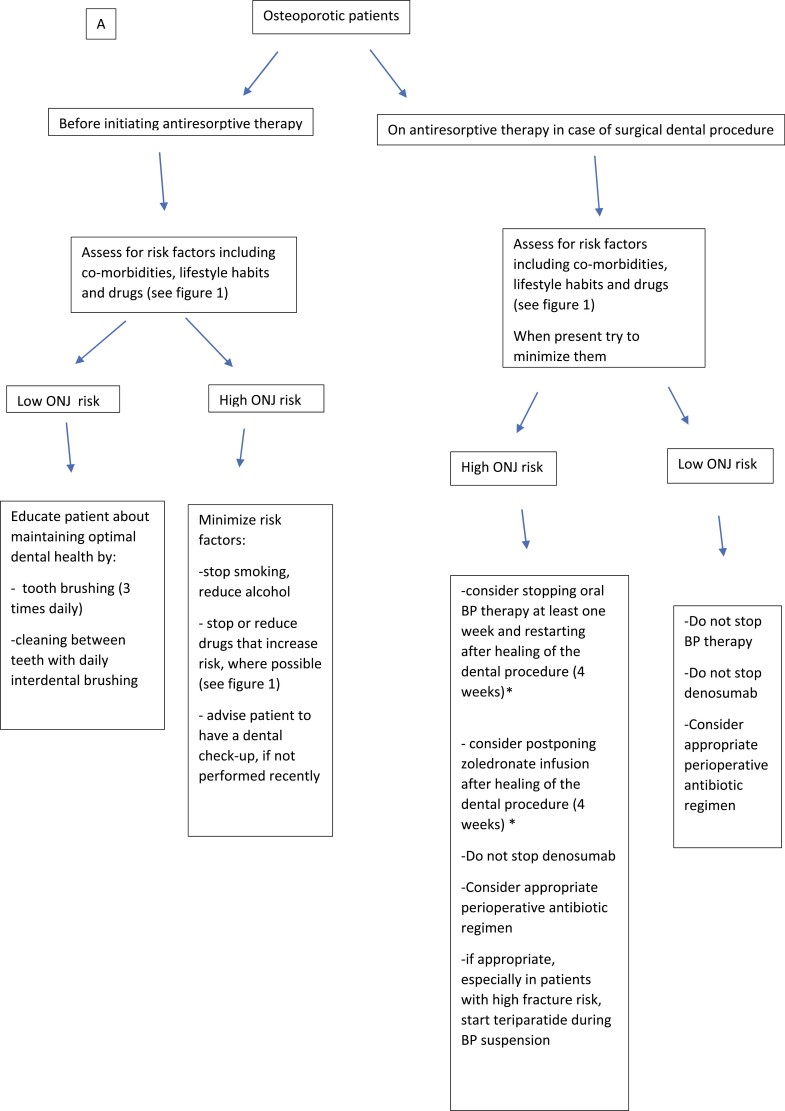
Although MRONJ risk is very low in these patients, primary prevention is recommended to restore and maintain good oral health (84). We emphasize the importance of usual oral hygiene. For patients not regularly checked by their dentist, we recommend a dental examination be performed at or shortly after the initiation of antiresorptive treatment. This may include radiologic tests as needed. We recommend that physicians and dentists be well educated in terms of ONJ. Primary prevention should be maintained throughout treatment, risk factors should be minimized, and regular dental follow-up is recommended (Fig. 2A). Any planned oral procedures or surgeries should be performed before or shortly after the antiresorptive treatment initiation (174-177). Appropriate oral health care should be planned with the oral hygienist and/or the dentist. Poorly fitting dentures should be replaced because local oral trauma is a risk factor for ONJ (98, 178, 179). In addition to general preventive measures, the following can be considered when extradental procedures are performed, especially after long-term treatment:
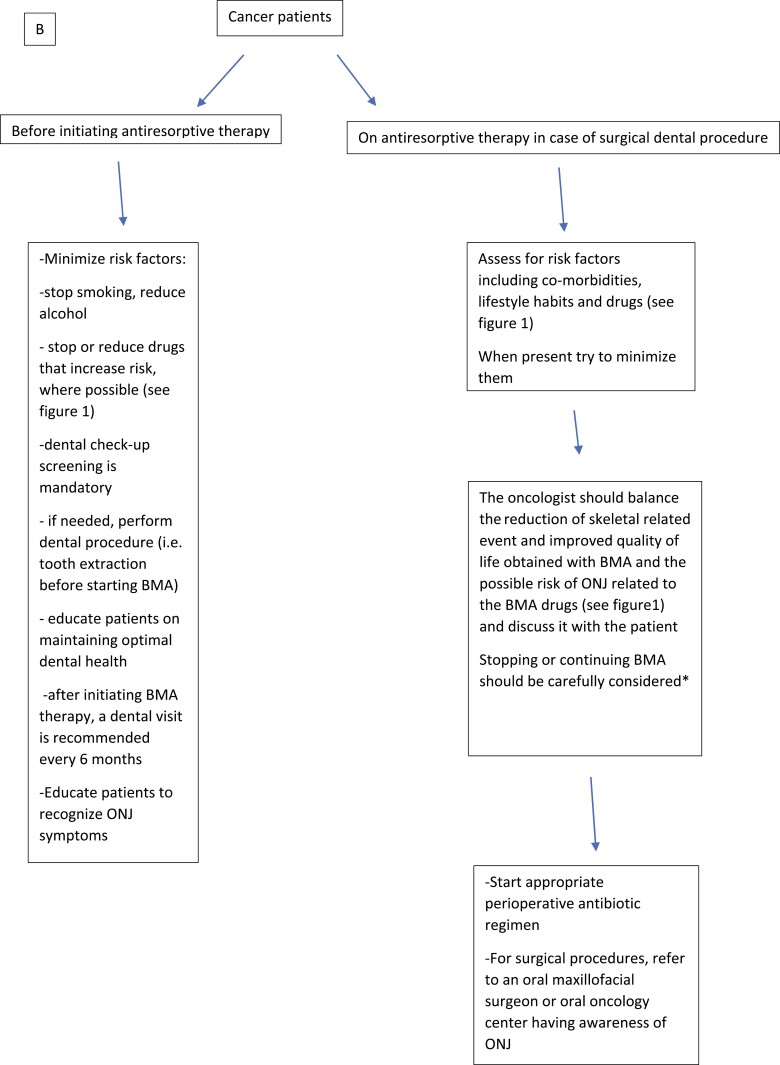
Figure 2.
Recommendations for prevention of antiresorptive agent-related osteonecrosis of the jaw in patients taking bone-modifying agents (BMA) due to A, osteoporosis, and B, cancer. ONJ, osteonecrosis of the jaw; RCT, randomized controlled trial. *In the absence of RCT.
antimicrobial mouth rinsing (180-182)
use of antibiotics before and/or after the procedure (175, 181-183). Although there is no consensus, the regimen most widely used in countries with widespread antibiotic resistance includes broad-spectrum antibiotics, such as amoxicillin/clavulanic acid, and clindamycin or metronidazole in combination or even as monotherapy. In contrast, narrow-spectrum antibiotics are preferably used in some of the ECTS membership countries where resistance to antibiotics is low. Consequently, this practice may differ among countries depending on the considerable differences in bacterial resistance across Europe. Furthermore, dosages and duration of therapy vary because of lack of precise guidelines.
Cancer patients
Since MRONJ risk is considerably higher in cancer patients than in OP patients, a thorough initial clinical and imaging dental evaluation is recommended, and oral procedures or surgeries should be performed before the initiation of antiresorptives (184) (Fig. 2B). Patients should be strictly educated on maintaining good oral health and on recognizing early ONJ signs and symptoms (see Fig. 2). Simple and low-cost preventive measures include the use of saline or sodium bicarbonate mouthwashes (185-187) and fluoridated toothpastes (188, 189). Frequent communication with patients is encouraged (see Fig. 2A). Attention should be paid to minimize modifiable risk factors such as poorly controlled diabetes (112), smoking (111-113, 190-192), ill-fitting dentures (98, 178, 179, 193), and poor dental and periodontal health (100, 176, 179, 193-198).
Prophylactic antibiotic treatment before dental procedures significantly decreases MRONJ risk (175, 176, 199). Nonurgent dentoalveolar surgery should be avoided during antiresorptive treatment at oncologic doses if possible. Otherwise, it should be planned based on cancer staging, risk assessment, and more appropriate timing.
Medication-related Osteonecrosis of the Jaw Management
Although several different guidelines have been published in the last 15 years, treatment strategies are based on limited evidence. In general, both in OP and cancer patients, MRONJ treatment depends on the stage of ONJ and the symptoms present and treatment providers should consider several clinical variables that will influence the outcome including the underlying disease, ONJ severity, age, sex, prognosis and life expectancy, comorbidities, symptoms, and estimated bone fragility, to define the optimal therapeutic approach.
Conservative management
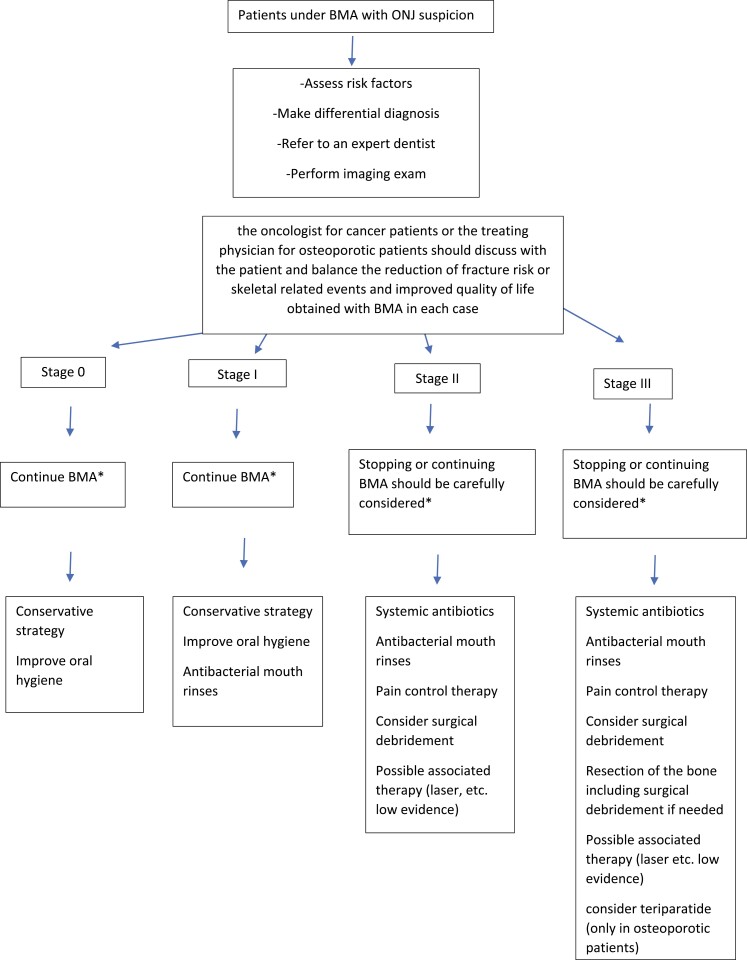
Conservative treatment is the basis of care of patients with ONJ (200-205) (Fig. 3). In many cases it is the only treatment needed and achieves long-term symptom relief (94, 206, 207). Cure rates with conservative management are higher for OP than for cancer patients (207). Management includes the following:
Figure 3.
Recommendations for management of antiresorptive agent-related osteonecrosis of the jaw in patients taking bone-modifying agents (BMA). Staging of antiresorptive agent-related osteonecrosis of the jaw according to the American Association of Oral and Maxillofacial Surgeons (2014). ONJ, osteonecrosis of the jaw; RCT, randomized controlled trial. *In the absence of RCT.
maintenance of optimal oral hygiene, including self and regular professional care;
treatment of any dental and periodontal disease;
antiseptic mouth rinses; and
systemic antibiotic therapy, if indicated. Dosages and duration of therapy varies depending on the stage of ONJ and the general condition of the patient.
Conservative management is mainly applied in the earlier stages of ONJ (see Fig. 3):
At-risk patients: Close clinical and radiographic monitoring is advised but there is no need for intervention. Nevertheless, patients must be informed about the possibility of bone exposure and necrosis and be able to early identify signs and symptoms
Stage 0: Since symptoms are not specific, the objective is to symptomatically control pain and infections, and closely monitor for signs of progression
Stage 1: Management with chlorhexidine mouthwash and regular follow-up. Neither antibiotic nor surgical intervention is required
Stage 2: Owing to necrosis and associated infection, an antibiotic regimen with an antimicrobial mouthwash and oral antibiotics is the treatment of choice
Teriparatide (TPTD) was helpful in ONJ management in several case reports (22, 23, 208-215). In a recent study in patients with established MRONJ, TPTD treatment for 8 weeks resulted in a significantly higher number of lesions healed and reduced bone defects by 52 weeks compared with placebo (216). Once-weekly TPTD was also effective (217). At present, TPTD treatment is contraindicated in patients with active malignancies or a history of BM or skeletal radiation. However, given the rapid turnover of TPTD, a brief exposure (eg, 8 weeks) will most likely not affect the “alleged” risk of osteosarcoma, reported in rats but never demonstrated in humans (218), or activate quiescent malignant cells.
Weak evidence suggests other experimental conservative treatments may be helpful (Table 4).
Table 4.
Adjuvant therapies applied in management of osteonecrosis of the jaw
| During conservative management | During surgical management |
|---|---|
| • Bone marrow stem cell intralesional transplantation (219) | • Laser-assisted surgical debridement (220, 221) |
| • Leukocyte and platelet rich fibrin membrane placement (183, 222, 223) | • Preoperative antibiotic treatment followed by laser and wound local treatment with platelet-rich plasma applications (224) |
| • Ozone (225) | • Surgical debridement in combination with platelet-derived growth factor (183) |
| • Pentoxifylline (226) | • Intraoperative fluorescence guidance (227, 228) |
| • Vitamin E (226) | • Longer-term preoperative antibiotics (229) |
| • Hyperbaric oxygen therapy | • Adjunctive therapy with hyperbaric oxygen combined with surgery (230, 231) |
Conservative therapy should be preferred over surgical management unless obvious progression of disease is observed or the pain is not controlled by conservative means.
Surgical management
Surgical treatment should be performed by experienced dentists or surgeons. Surgical management is applied in the latter stages of ONJ (see Fig. 3):
Stage 2: Besides the antibiotic regimen reported earlier, debridement is often needed (232-238)
Stage 3: Surgical management is indicated along with an antibiotic regimen. The surgical approach varies (232-238), ranging from limited debridement to complete resection with possible immediate reconstruction with plates or obturators (232, 239). A more conservative treatment should initially be considered, limiting operative therapy for cases not solved by this approach (238, 240, 241). However, the limited available data do not allow conclusive indications on surgical intervention. According to previous guidelines, removal of the necrotic bone with tension-free closure should be preferred, as it is considered to provide the most positive results.
Surgical success rates have been reported higher in OP and MM patients in comparison to patients with solid tumors (242).
Adjuvant treatments have also been proposed (243), although scientific evidence has often been controversial because of the lack of randomized controlled trials (Table 4).
Special Considerations
Table 5 presents the suggested approaches in i) dental procedures in low- and high-risk patients receiving antiresorptive treatment, and ii) patients receiving antiresorptives who develop ONJ.
Table 5.
Recommendations for dental management in specific conditions
| A.Dental procedures during antiresorptive therapy |
| Patients at low risk of ONJ |
| Conservative treatments (restorative treatment, non-surgical endodontic treatment, prosthodontic/orthodontic therapy) are safea |
| Elective dentoalveolar surgery, simple extractions, and procedures that do not involve osteotomy are considered to be of low risk (9) |
| Placement of dental implants entails small risk |
| Antimicrobial mouthwash before/after procedure is advised. Systemic antibiotics are also advised in nonconservative treatments (180) |
| Antiresorptive treatment management |
| Osteoporotic patients |
| Do not discontinue bisphosphonatesb |
| Do not discontinue denosumab—perform procedure preferably 5-6 mo following the last injection |
| Lower doses of antiresorptives (74)? No supporting evidence |
| Cancer patients |
| Do not discontinue bisphosphonatesb,c |
| Do not discontinue denosumab |
| Patients at high risk of ONJ |
| Mild conservative treatments (restorative treatment, removal of dental caries) are usually safe |
| Nonsurgical endodontic treatment has a small risk—could be an alternative to extraction (244)d |
| Root canal treatment and/or decoronation preferred over extraction (244) |
| Antimicrobial mouthwash, systemic antibiotics before/after the procedure, avoidance of anesthetic agents that contain vasoconstrictor, avoidance of gingival tissue damage |
| Denture wearing not prohibited (avoid exerting excessive pressure or friction) (79) |
| Antiresorptive treatment management |
| Osteoporotic patients |
| Bisphosphonates could be discontinued (at least 1 wk before and until surgical site healing) (84, 245)e |
| Do not discontinue denosumab—perform procedure preferably 5-6 months following last injection (245, 246)f—perform next denosumab injection 4-6 wk after the procedure but not > 4 wk later than it should be done |
| Consider replacing antiresorptives with teriparatideg |
| No data on romosozumab |
| Cancer patients |
| Personalized decision in agreement with treating oncologist, weighing risk of ONJ against risks of SREs |
| Bisphosphonates could be discontinued |
| Short-term denosumab discontinuation (eg, 3 wk before and 4-6 wk after dental procedure has been advised) (247)—no clear benefith |
| B.Antiresorptive treatment management in patients who develop ONJ |
| Consider discontinuing antiresorptives until complete soft-tissue closure after carefully weighing risk of ongoing ONJ with risk of fractures or SREsi |
| Consider teriparatide until complete soft-tissue closure (22) (in osteoporotic but probably not in cancer patients—individualized approach)j |
Abbreviations: ONJ, osteonecrosis of the jaw; SRE, skeletal-related event.
a A few, not well-documented cases of ONJ reported after nonsurgical endodontic procedures (248).
b Residual effect of bisphosphonates questions the effect of discontinuation on ONJ; in osteoporotic patients tooth extraction safely performed without bisphosphonate discontinuation (249); suspension of bisphosphonates not beneficial in animals (250) and humans (251, 252) who developed ONJ.
c Reduction in SRE risk is greater and the risk of ONJ lower in first years of bisphosphonate therapy (247).
d Soft-tissue damage during endodontic treatment has also been associated with initiation of ONJ process (253).
e Unknown optimal duration of off-treatment period.
f Based on denosumab pharmacokinetics, its effect on bone turnover is almost depleted around 6 months following the last injection (245, 246).
g Concerns: limited duration of teriparatide treatment (23); temporary decrease at least of hip bone mineral density (254); uncertain effect on rebound phenomenon after denosumab discontinuation (246).
h OPG-Fc discontinuation before tooth extraction ameliorated subsequent ONJ development in rodents (250); in contrast, in a multicenter retrospective Japanese study short-term denosumab discontinuation had no effect on ONJ risk (255).
i Concerns: denosumab discontinuation infers increased risk of multiple vertebral fractures (256, 257); discontinuation of either ZOL or OPG-Fc in rats with established ONJ did not lead to ONJ resolution (250).
j Teriparatide is theoretically contraindicated in cancer patients but a brief exposure (eg, 8 wk) should not activate quiescent malignant cells.
A balanced evaluation of the risk-to-benefit ratio of continuing or stopping antiresorptives should always be performed in such patients. A detailed medical history should be obtained, evaluating the duration and type of antiresorptives (current or past), concurrent pharmacological treatments, the presence of other risk factors for ONJ, and the risk of fracture or SRE. Clinical decision should also be made in relation to the invasiveness of the procedure.
Discontinuation of antiresorptive treatment in patients requiring dental procedures is particularly controversial because of low evidence to support strong recommendations. The following are the most accepted guidelines:
American Dental Association Guidelines in 2011 recognized the lower risk in OP patients and stated that discontinuation of oral BPs is not necessary before dental procedures (200)
The AAOMS, admitting the lack of scientific evidence, suggested in 2014 a drug holiday in patients who have been on BPs or Dmab for at least 4 years (6)
The US Food and Drug Administration stated there are “no substantial data available to guide decisions regarding the initiation or duration of a drug holiday” (258)
An ONJ International Task Force in 2015 recommended stopping antiresorptive treatment in patients needing extensive invasive surgery or presenting with significant ONJ risk factors (64) until soft-tissue healing has occurred.
Our recommendations on the subject, along with our concerns, are summarized in Table 5. Discontinuation until the surgical site heals could be considered in case of BPs, especially if the fracture risk is low and the MRONJ risk is high. Theoretically, since the uptake of BPs is comparatively increased at sites of local bone injury with high bone turnover, withholding BP treatment may reduce their local deposition in the jawbone. It has been proposed that BPs may be discontinued 1 week before the procedure and resumed when healing of oral mucosa is completed (84, 245), usually 2 to 4 weeks after the dental treatment (9, 74). However, the optimal duration of the off-treatment period is unknown and, furthermore, the efficacy of such an approach is questionable given the residual effect of BPs due to their long-term skeletal retention and the fact that no study results to date have confirmed that drug holidays are effective in preventing MRONJ. Recent evidence from a study in OP patients (249) suggests that tooth extraction can be safely performed without BP discontinuation while suspension of BP treatment in animals (250) and humans (251, 252) who developed MRONJ did not provide any significant benefit. Lower doses of antiresorptive therapy have also been proposed (74), but without supporting evidence. Especially in cancer patients, in whom the vast majority of MRONJ incidents occur, although it has been recommended to withhold antiresorptive therapy following dental procedures until soft-tissue healing has occurred (64), current evidence supporting this recommendation is limited and controversial, with some studies showing a benefit (242, 259) while others have shown a neutral effect on the outcome of the dental procedure (94, 260, 261).
In Dmab-treated OP patients, discontinuation should be discouraged in light of the risk for multiple vertebral fractures (256, 257, 262). Instead, based on the pharmacokinetics of Dmab, dental procedures could be planned at around 5 to 6 months following the last injection, when the effects of Dmab on bone turnover are depleted (245, 246). In cancer patients, Dmab could be withheld but only for a short period (3 wk before to 4-6 wk after the dental procedure) (247), because of the risk both of multiple vertebral fractures and SREs. However, there is no evidence that such a strategy reduces MRONJ risk and bone turnover will probably remain suppressed during this interval. In animals, discontinuation of OPG-Fc, a molecule resembling Dmab in action, before tooth extraction ameliorated subsequent ONJ development (250). On the contrary, in a multicenter retrospective Japanese study in cancer patients receiving oncologic doses of Dmab, short-term Dmab discontinuation had no effect on the risk of MRONJ (255).
Conclusions
Preventive measures, including maintenance of good oral health, completion of any required dental treatment before commencing antiresorptive therapy, at least in patients with metastatic bone disease, and use of antibiotics before and after a surgical procedure substantially reduce the risk of MRONJ. Conservative treatment is the mainstay of treatment and should be applied to the majority of MRONJ cases. Surgery remains the main treatment in nonresponsive cases of MRONJ. TPTD is a potential promising conservative therapeutic option in OP patients. However, this and other therapeutical approaches that have been proposed need further validation. Barriers to treatment, health care disparities, and underestimation of bone fragility by most health care providers should be considered.
The benefits of antiresorptive therapy far outweigh the potential risks, with major reductions in SREs in the oncology patient and fracture risk reduction in the OP patient, but ONJ can also severely affect QoL; therefore a personalized treatment plan with careful consideration of all aspects is of the utmost importance.
Acknowledgments
We would like to thank B. Abrahamsen, P. Hermann, and B. Langdahl for their valuable contribution to the finalization of the manuscript.
Glossary
Abbreviations
- AAOMS
American Association of Oral and Maxillofacial Surgeons
- AI
aromatase inhibitor
- ALN
alendronate
- ARONJ
antiresorptive agent–related osteonecrosis of the jaw
- BM
bone metastases
- BMA
bone-modifying agent
- BP
bisphosphonate
- BRONJ
bisphosphonate-related osteonecrosis of the jaw
- CLO
clodronate
- CTIBL
cancer treatment–induced bone loss
- Dmab
denosumab
- DRONJ
denosumab-related osteonecrosis of the jaw
- ECTS
European Calcified Tissue Society
- IBN
ibandronate
- IV
intravenous
- MM
multiple myeloma
- MRONJ
medication-related osteonecrosis of the jaw
- ONJ
osteonecrosis of the jaw
- OP
osteoporosis
- OPG
orthopantomography
- PAM
pamidronate
- QoL
quality of life
- RIS
risedronate
- RLX
raloxifene
- SRE
skeletal-related event
- TKI
tyrosine kinase inhibitor
- TPTD
teriparatide
- VEGF
vascular endothelial growth factor
- ZOL
zoledronate
Financial Support
The authors received no financial support for the research, authorship, and/or publication of this article.
Author Contributions
Literature research, data collection, and drafting of the first version of the manuscript: A.D.A., J.P., and N.N.; revising manuscript content: A.P., C.M., K.A.A., M.C.Z., and J.J.B.; approval of the final version of the manuscript: A.D.A., J.P., N.N., A.P., C.M., K.A.A., M.C.Z., and J.J.B.
Disclosures
A.D.A. has received lecture fees from Amgen, Eli Lilly, UCB, and VIANEX. J.P. has nothing to disclose. N.N. has received consultant fees from UCB, a speaker fee from Eli Lilly, and research support from Abiogen. A.P. has received honoraria for lectures from Amgen. C.M. has nothing to disclose. A.K. has received research funds received from Alexion, Amgen, Ascendis, Chugai, Radius, Takeda, and Ultragenyx. M.C.Z. has received honoraria for lectures or advice from Alexion, Amgen, Eli Lilly, Kyowa Kirin, Shire, and UCB. J.J.B. has received consulting fees from Cole Pharma, Sandoz, Takeda, and UCB.
Data Availability
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.
References
- 1. Kong YY, Yoshida H, Sarosi I, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397(6717):315-323. [DOI] [PubMed] [Google Scholar]
- 2. Bilezikian JP. Efficacy of bisphosphonates in reducing fracture risk in postmenopausal osteoporosis. Am J Med. 2009;122(2 Suppl):S14-S21. [DOI] [PubMed] [Google Scholar]
- 3. Cummings SR, San Martin J, McClung MR, et al. ; FREEDOM Trial . Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756-765. [DOI] [PubMed] [Google Scholar]
- 4. Rosen LS, Gordon D, Kaminski M, et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer. 2003;98(8):1735-1744. [DOI] [PubMed] [Google Scholar]
- 5. Zhang Z, Pu F, Shao Z. The skeletal-related events of denosumab versus zoledronic acid in patients with bone metastases: a meta-analysis of randomized controlled trials. J Bone Oncol. 2017;9:21-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ruggiero SL, Dodson TB, Fantasia J, et al. ; American Association of Oral and Maxillofacial Surgeons . American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J Oral Maxillofac Surg. 2014;72(10):1938-1956. [DOI] [PubMed] [Google Scholar]
- 7. McGowan K, McGowan T, Ivanovski S. Risk factors for medication-related osteonecrosis of the jaws: a systematic review. Oral Dis. 2018;24(4):527-536. [DOI] [PubMed] [Google Scholar]
- 8. Pazianas M, Miller P, Blumentals WA, Bernal M, Kothawala P. A review of the literature on osteonecrosis of the jaw in patients with osteoporosis treated with oral bisphosphonates: prevalence, risk factors, and clinical characteristics. Clin Ther. 2007;29(8):1548-1558. [DOI] [PubMed] [Google Scholar]
- 9. Nicolatou-Galitis O, Kouri M, Papadopoulou E, et al. ; MASCC Bone Study Group . Osteonecrosis of the jaw related to non-antiresorptive medications: a systematic review. Support Care Cancer. 2019;27(2):383-394. [DOI] [PubMed] [Google Scholar]
- 10. Filleul O, Crompot E, Saussez S. Bisphosphonate-induced osteonecrosis of the jaw: a review of 2,400 patient cases. J Cancer Res Clin Oncol. 2010;136(8):1117-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eguia A, Bagán-Debón L, Cardona F. Review and update on drugs related to the development of osteonecrosis of the jaw. Med Oral Patol Oral Cir Bucal. 2020;25(1):e71-e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yeo AC, Lye KW, Poon CY. Bisphosphonate-related osteonecrosis of the jaws. Singapore Dent J. 2005;27(1):36-40. [PubMed] [Google Scholar]
- 13. Teoh L, Moses G, Nguyen AP, McCullough MJ. Medication-related osteonecrosis of the jaw: analysing the range of implicated drugs from the Australian database of adverse event notifications. Br J Clin Pharmacol. 2021;87(7):2767-2776. [DOI] [PubMed] [Google Scholar]
- 14. Patel S, Choyee S, Uyanne J, et al. Non-exposed bisphosphonate-related osteonecrosis of the jaw: a critical assessment of current definition, staging, and treatment guidelines. Oral Dis. 2012;18(7):625-632. [DOI] [PubMed] [Google Scholar]
- 15. Fedele S, Bedogni G, Scoletta M, et al. Up to a quarter of patients with osteonecrosis of the jaw associated with antiresorptive agents remain undiagnosed. Br J Oral Maxillofac Surg. 2015;53(1):13-17. [DOI] [PubMed] [Google Scholar]
- 16. Anastasilakis AD, Pepe J, Napoli N, et al. Supplementary data for “Osteonecrosis of the jaw and antiresorptive agents in benign and malignant diseases: a critical review organized by the ECTS.” Posted December 3, 2021. https://figshare.com/articles/journal_contribution/jc_2021-02894_R2_supplemental_file_docx/17121251 [DOI] [PMC free article] [PubMed]
- 17. Allen MR, Burr DB. The pathogenesis of bisphosphonate-related osteonecrosis of the jaw: so many hypotheses, so few data. J Oral Maxillofac Surg. 2009;67(5 Suppl):61-70. [DOI] [PubMed] [Google Scholar]
- 18. Vermeer JA, Jansen ID, Marthi M, et al. Jaw bone marrow-derived osteoclast precursors internalize more bisphosphonate than long-bone marrow precursors. Bone. 2013;57(1):242-251. [DOI] [PubMed] [Google Scholar]
- 19. Kuroshima S, Mecano RB, Tanoue R, Koi K, Yamashita J. Distinctive tooth-extraction socket healing: bisphosphonate versus parathyroid hormone therapy. J Periodontol. 2014;85(1):24-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zandi M, Dehghan A, Zandipoor N, Amini P, Doulati S. Effect of different doses and durations of teriparatide therapy on resolution of medication-related osteonecrosis of the jaw: a randomized, controlled preclinical study in rats. J Craniomaxillofac Surg. 2018;46(3):466-472. [DOI] [PubMed] [Google Scholar]
- 21. Dayisoylu EH, Şenel FÇ, Üngör C, et al. The effects of adjunctive parathyroid hormone injection on bisphosphonate-related osteonecrosis of the jaws: an animal study. Int J Oral Maxillofac Surg. 2013;42(11):1475-1480. [DOI] [PubMed] [Google Scholar]
- 22. Bashutski JD, Eber RM, Kinney JS, et al. Teriparatide and osseous regeneration in the oral cavity. N Engl J Med. 2010;363(25):2396-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Subramanian G, Cohen HV, Quek SYP. A model for the pathogenesis of bisphosphonate-associated osteonecrosis of the jaw and teriparatide’s potential role in its resolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112(6):744-753. [DOI] [PubMed] [Google Scholar]
- 24. Ficarra G, Beninati F, Rubino I, et al. Osteonecrosis of the jaws in periodontal patients with a history of bisphosphonates treatment. J Clin Periodontol. 2005;32(11):1123-1128. [DOI] [PubMed] [Google Scholar]
- 25. Aguirre JI, Akhter MP, Kimmel DB, et al. Oncologic doses of zoledronic acid induce osteonecrosis of the jaw-like lesions in rice rats (Oryzomys palustris) with periodontitis. J Bone Miner Res. 2012;27(10):2130-2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kang B, Cheong S, Chaichanasakul T, et al. Periapical disease and bisphosphonates induce osteonecrosis of the jaws in mice. J Bone Miner Res. 2013;28(7):1631-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sedghizadeh PP, Kumar SK, Gorur A, Schaudinn C, Shuler CF, Costerton JW. Identification of microbial biofilms in osteonecrosis of the jaws secondary to bisphosphonate therapy. J Oral Maxillofac Surg. 2008;66(4):767-775. [DOI] [PubMed] [Google Scholar]
- 28. Boff RC, Salum FG, Figueiredo MA, Cherubini K. Important aspects regarding the role of microorganisms in bisphosphonate-related osteonecrosis of the jaws. Arch Oral Biol. 2014;59(8):790-799. [DOI] [PubMed] [Google Scholar]
- 29. Hikita H, Miyazawa K, Tabuchi M, Kimura M, Goto S. Bisphosphonate administration prior to tooth extraction delays initial healing of the extraction socket in rats. J Bone Miner Metab. 2009;27(6):663-672. [DOI] [PubMed] [Google Scholar]
- 30. Ravosa MJ, Ning J, Liu Y, Stack MS. Bisphosphonate effects on the behaviour of oral epithelial cells and oral fibroblasts. Arch Oral Biol. 2011;56(5):491-498. [DOI] [PubMed] [Google Scholar]
- 31. Kobayashi Y, Hiraga T, Ueda A, et al. Zoledronic acid delays wound healing of the tooth extraction socket, inhibits oral epithelial cell migration, and promotes proliferation and adhesion to hydroxyapatite of oral bacteria, without causing osteonecrosis of the jaw, in mice. J Bone Miner Metab. 2010;28(2):165-175. [DOI] [PubMed] [Google Scholar]
- 32. Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M. Stimulation of gammadelta T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood. 2000;96(2):384-392. [PubMed] [Google Scholar]
- 33. Martens B, Drebert Z. Glucocorticoid-mediated effects on angiogenesis in solid tumors. J Steroid Biochem Mol Biol. 2019;188:147-155. [DOI] [PubMed] [Google Scholar]
- 34. Wood J, Bonjean K, Ruetz S, et al. Novel antiangiogenic effects of the bisphosphonate compound zoledronic acid. J Pharmacol Exp Ther. 2002;302(3):1055-1061. [DOI] [PubMed] [Google Scholar]
- 35. Misso G, Porru M, Stoppacciaro A, et al. Evaluation of the in vitro and in vivo antiangiogenic effects of denosumab and zoledronic acid. Cancer Biol Ther. 2012;13(14):1491-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ferretti G, Fabi A, Carlini P, et al. Zoledronic-acid-induced circulating level modifications of angiogenic factors, metalloproteinases and proinflammatory cytokines in metastatic breast cancer patients. Oncology. 2005;69(1):35-43. [DOI] [PubMed] [Google Scholar]
- 37. Santini D, Vincenzi B, Galluzzo S, et al. Repeated intermittent low-dose therapy with zoledronic acid induces an early, sustained, and long-lasting decrease of peripheral vascular endothelial growth factor levels in cancer patients. Clin Cancer Res. 2007;13(15 Pt 1):4482-4486. [DOI] [PubMed] [Google Scholar]
- 38. Sonis ST, Watkins BA, Lyng GD, Lerman MA, Anderson KC. Bony changes in the jaws of rats treated with zoledronic acid and dexamethasone before dental extractions mimic bisphosphonate-related osteonecrosis in cancer patients. Oral Oncol. 2009;45(2):164-172. [DOI] [PubMed] [Google Scholar]
- 39. Kikuiri T, Kim I, Yamaza T, et al. Cell-based immunotherapy with mesenchymal stem cells cures bisphosphonate-related osteonecrosis of the jaw-like disease in mice. J Bone Miner Res. 2010;25(7):1668-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Aldridge SE, Lennard TW, Williams JR, Birch MA. Vascular endothelial growth factor receptors in osteoclast differentiation and function. Biochem Biophys Res Commun. 2005;335(3):793-798. [DOI] [PubMed] [Google Scholar]
- 41. Pazianas M. Osteonecrosis of the jaw and the role of macrophages. J Natl Cancer Inst. 2011;103(3):232-240. [DOI] [PubMed] [Google Scholar]
- 42. Tseng HC, Kanayama K, Kaur K, et al. Bisphosphonate-induced differential modulation of immune cell function in gingiva and bone marrow in vivo: role in osteoclast-mediated NK cell activation. Oncotarget. 2015;6(24):20002-20025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Landesberg R, Cozin M, Cremers S, et al. Inhibition of oral mucosal cell wound healing by bisphosphonates. J Oral Maxillofac Surg. 2008;66(5):839-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pabst AM, Ziebart T, Koch FP, Taylor KY, Al-Nawas B, Walter C. The influence of bisphosphonates on viability, migration, and apoptosis of human oral keratinocytes–in vitro study. Clin Oral Investig. 2012;16(1):87-93. [DOI] [PubMed] [Google Scholar]
- 45. Magopoulos C, Karakinaris G, Telioudis Z, et al. Osteonecrosis of the jaws due to bisphosphonate use. A review of 60 cases and treatment proposals. Am J Otolaryngol. 2007;28(3):158-163. [DOI] [PubMed] [Google Scholar]
- 46. Reid IR, Bolland MJ, Grey AB. Is bisphosphonate-associated osteonecrosis of the jaw caused by soft tissue toxicity? Bone. 2007;41(3):318-320. [DOI] [PubMed] [Google Scholar]
- 47. Dalle Carbonare L, Mottes M, Valenti MT. Medication-related osteonecrosis of the jaw (MRONJ): are antiresorptive drugs the main culprits or only accomplices? The triggering role of vitamin D deficiency. Nutrients. 2021;13(2):561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Heim N, Warwas FB, Wilms CT, Reich RH, Martini M. Vitamin D (25-OHD) deficiency may increase the prevalence of medication-related osteonecrosis of the jaw. J Craniomaxillofac Surg. 2017;45(12):2068-2074. [DOI] [PubMed] [Google Scholar]
- 49. Demircan S, Isler SC. Changes in serological bone turnover markers in bisphosphonate induced osteonecrosis of the jaws: a case control study. Niger J Clin Pract. 2020;23(2):154-158. [DOI] [PubMed] [Google Scholar]
- 50. Danila MI, Outman RC, Rahn EJ, et al. Evaluation of a multimodal, direct-to-patient educational intervention targeting barriers to osteoporosis care: a randomized clinical trial. J Bone Miner Res. 2018;33(5):763-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Boyce BF, Adamson BB, Gallacher SJ, Byars J, Ralston SH, Boyle IT. Mineralisation defects after pamidronate for Paget’s disease. Lancet. 1994;343(8907):1231-1232. [DOI] [PubMed] [Google Scholar]
- 52. Bedogni A, Saia G, Bettini G, et al. Osteomalacia: the missing link in the pathogenesis of bisphosphonate-related osteonecrosis of the jaws? Oncologist. 2012;17(8):1114-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhong DN, Wu JZ, Li GJ. Association between CYP2C8 (rs1934951) polymorphism and bisphosphonate-related osteonecrosis of the jaws in patients on bisphosphonate therapy: a meta-analysis. Acta Haematol. 2013;129(2):90-95. [DOI] [PubMed] [Google Scholar]
- 54. Guo Z, Cui W, Que L, Li C, Tang X, Liu J. Pharmacogenetics of medication-related osteonecrosis of the jaw: a systematic review and meta-analysis. Int J Oral Maxillofac Surg. 2020;49(3):298-309. [DOI] [PubMed] [Google Scholar]
- 55. Choi H, Lee JH, Kim HJ, Park W, Lee JH, Kim JH. Genetic association between VEGF polymorphisms and BRONJ in the Korean population. Oral Dis. 2015;21(7):866-871. [DOI] [PubMed] [Google Scholar]
- 56. Di Martino MT, Arbitrio M, Guzzi PH, et al. A peroxisome proliferator-activated receptor gamma (PPARG) polymorphism is associated with zoledronic acid-related osteonecrosis of the jaw in multiple myeloma patients: analysis by DMET microarray profiling. Br J Haematol. 2011;154(4):529-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. La Ferla F, Paolicchi E, Crea F, et al. An aromatase polymorphism (g.132810C > T) predicts risk of bisphosphonate-related osteonecrosis of the jaw. Biomark Med. 2012;6(2):201-209. [DOI] [PubMed] [Google Scholar]
- 58. Marini F, Tonelli P, Cavalli L, et al. Pharmacogenetics of bisphosphonate-associated osteonecrosis of the jaw. Front Biosci (Elite Ed). 2011;3:364-370. [DOI] [PubMed] [Google Scholar]
- 59. Nicoletti P, Cartsos VM, Palaska PK, Shen Y, Floratos A, Zavras AI. Genomewide pharmacogenetics of bisphosphonate-induced osteonecrosis of the jaw: the role of RBMS3. Oncologist. 2012;17(2):279-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yang G, Hamadeh IS, Katz J, et al. SIRT1/HERC4 locus associated with bisphosphonate-induced osteonecrosis of the jaw: an exome-wide association analysis. J Bone Miner Res. 2018;33(1):91-98. [DOI] [PubMed] [Google Scholar]
- 61. Bastida-Lertxundi N, Leizaola-Cardesa IO, Hernando-Vázquez J, et al. Pharmacogenomics in medication-related osteonecrosis of the jaw: a systematic literature review. Eur Rev Med Pharmacol Sci. 2019;23(23):10184-10194. [DOI] [PubMed] [Google Scholar]
- 62. Yang G, Singh S, Chen Y, et al. Pharmacogenomics of osteonecrosis of the jaw. Bone. 2019;124:75-82. [DOI] [PubMed] [Google Scholar]
- 63. Querrer R, Ferrare N, Melo N, et al. Differences between bisphosphonate-related and denosumab-related osteonecrosis of the jaws: a systematic review. Support Care Cancer. 2021;29(6):2811-2820. [DOI] [PubMed] [Google Scholar]
- 64. Khan AA, Morrison A, Hanley DA, et al. ; International Task Force on Osteonecrosis of the Jaw . Diagnosis and management of osteonecrosis of the jaw: a systematic review and international consensus. J Bone Miner Res. 2015;30(1):3-23. [DOI] [PubMed] [Google Scholar]
- 65. Farah CS, Savage NW. Oral ulceration with bone sequestration. Aust Dent J. 2003;48(1):61-64. [DOI] [PubMed] [Google Scholar]
- 66. Sonnier KE, Horning GM. Spontaneous bony exposure: a report of 4 cases of idiopathic exposure and sequestration of alveolar bone. J Periodontol. 1997;68(8):758-762. [DOI] [PubMed] [Google Scholar]
- 67. Peters E, Lovas GL, Wysocki GP. Lingual mandibular sequestration and ulceration. Oral Surg Oral Med Oral Pathol. 1993;75(6):739-743. [DOI] [PubMed] [Google Scholar]
- 68. Scully C. Oral ulceration: a new and unusual complication. Br Dent J. 2002;192(3):139-140. [DOI] [PubMed] [Google Scholar]
- 69. Black DM, Delmas PD, Eastell R, et al. ; HORIZON Pivotal Fracture Trial . Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356(18):1809-1822. [DOI] [PubMed] [Google Scholar]
- 70. Advisory Task Force on Bisphosphonate-Related Ostenonecrosis of the Jaws, American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2007;65(3):369-376. [DOI] [PubMed] [Google Scholar]
- 71. Ruggiero SL, Dodson TB, Assael LA, Landesberg R, Marx RE, Mehrotra B; American Association of Oral and Maxillofacial Surgeons . American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws—2009 update. J Oral Maxillofac Surg. 2009;67(5 Suppl):2-12. [DOI] [PubMed] [Google Scholar]
- 72. Bilezikian JP. Osteonecrosis of the jaw—do bisphosphonates pose a risk? N Engl J Med. 2006;355(22):2278-2281. [DOI] [PubMed] [Google Scholar]
- 73. Mavrokokki T, Cheng A, Stein B, Goss A. Nature and frequency of bisphosphonate-associated osteonecrosis of the jaws in Australia. J Oral Maxillofac Surg. 2007;65(3):415-423. [DOI] [PubMed] [Google Scholar]
- 74. Khan AA, Morrison A, Kendler DL, et al. ; International Task Force on Osteonecrosis of the Jaw . Case-based review of osteonecrosis of the jaw (ONJ) and application of the international recommendations for management from the International Task Force on ONJ. J Clin Densitom. 2017;20(1):8-24. [DOI] [PubMed] [Google Scholar]
- 75. Yoneda T, Hagino H, Sugimoto T, et al. Bisphosphonate-related osteonecrosis of the jaw: position paper from the Allied Task Force Committee of Japanese Society for Bone and Mineral Research, Japan Osteoporosis Society, Japanese Society of Periodontology, Japanese Society for Oral and Maxillofacial Radiology, and Japanese Society of Oral and Maxillofacial Surgeons. J Bone Miner Metab. 2010;28(4):365-383. [DOI] [PubMed] [Google Scholar]
- 76. Urade M, Tanaka N, Furusawa K, et al. Nationwide survey for bisphosphonate-related osteonecrosis of the jaws in Japan. J Oral Maxillofac Surg. 2011;69(11):e364-e371. [DOI] [PubMed] [Google Scholar]
- 77. Japanese Allied Committee on Osteonecrosis of the Jaw; Yoneda T, Hagino H, Sugimoto T, et al. Antiresorptive agent-related osteonecrosis of the jaw: position paper 2017 of the Japanese Allied Committee on Osteonecrosis of the Jaw. J Bone Miner Metab. 2017;35(1):6-19. [DOI] [PubMed] [Google Scholar]
- 78. Yarom N, Shapiro CL, Peterson DE, et al. Medication-related osteonecrosis of the jaw: MASCC/ISOO/ASCO clinical practice guideline. J Clin Oncol. 2019;37(25):2270-2290. [DOI] [PubMed] [Google Scholar]
- 79. Marx RE, Sawatari Y, Fortin M, Broumand V. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg. 2005;63(11):1567-1575. [DOI] [PubMed] [Google Scholar]
- 80. Morgan GJ, Davies FE, Gregory WM, et al. ; National Cancer Research Institute Haematological Oncology Clinical Study Group . First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial. Lancet. 2010;376(9757):1989-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Grbic JT, Black DM, Lyles KW, et al. The incidence of osteonecrosis of the jaw in patients receiving 5 milligrams of zoledronic acid: data from the Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly clinical trials program. J Am Dent Assoc. 2010;141(11):1365-1370. [DOI] [PubMed] [Google Scholar]
- 82. Kühl S, Walter C, Acham S, Pfeffer R, Lambrecht JT. Bisphosphonate-related osteonecrosis of the jaws—a review. Oral Oncol. 2012;48(10):938-947. [DOI] [PubMed] [Google Scholar]
- 83. Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a Task Force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2016;31(1):16-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Himelstein AL, Foster JC, Khatcheressian JL, et al. Effect of longer-interval vs standard dosing of zoledronic acid on skeletal events in patients with bone metastases: a randomized clinical trial. JAMA. 2017;317(1):48-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bal O, Oksuzoglu B, Dogan M, et al. Long-term outcomes of prolonged bisphosphonates more than 2 years in bone metastatic breast cancer: risk vs benefit. Ir J Med Sci. 2020;189(3):805-810. [DOI] [PubMed] [Google Scholar]
- 86. Van Poznak CH, Unger JM, Darke AK, et al. Association of osteonecrosis of the jaw with zoledronic acid treatment for bone metastases in patients with cancer. JAMA Oncol. 2021;7(2):246-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yang M, Yu X. Management of bone metastasis with intravenous bisphosphonates in breast cancer: a systematic review and meta-analysis of dosing frequency. Support Care Cancer. 2020;28(6):2533-2540. [DOI] [PubMed] [Google Scholar]
- 88. Ikesue H, Mouri M, Tomita H, et al. Associated characteristics and treatment outcomes of medication-related osteonecrosis of the jaw in patients receiving denosumab or zoledronic acid for bone metastases. Support Care Cancer. 2021;29(8):4763-4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lo JC, O’Ryan FS, Gordon NP, et al. ; Predicting Risk of Osteonecrosis of the Jaw with Oral Bisphosphonate Exposure (PROBE) Investigators . Prevalence of osteonecrosis of the jaw in patients with oral bisphosphonate exposure. J Oral Maxillofac Surg. 2010;68(2):243-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wang Q, Liu J, Qi S, Liao X, Liu D, Pan J. Clinical analysis of medication related osteonecrosis of the jaws: a growing severe complication in China. J Dent Sci. 2018;13(3):190-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Soutome S, Hayashida S, Funahara M, et al. Factors affecting development of medication-related osteonecrosis of the jaw in cancer patients receiving high-dose bisphosphonate or denosumab therapy: is tooth extraction a risk factor? PLoS One. 2018;13(7):e0201343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hasegawa T, Hayashida S, Kondo E, et al. ; Japanese Study Group of Co-operative Dentistry with Medicine (JCDM) . Medication-related osteonecrosis of the jaw after tooth extraction in cancer patients: a multicenter retrospective study. Osteoporos Int. 2019;30(1):231-239. [DOI] [PubMed] [Google Scholar]
- 93. Kizub DA, Miao J, Schubert MM, et al. Risk factors for bisphosphonate-associated osteonecrosis of the jaw in the prospective randomized trial of adjuvant bisphosphonates for early-stage breast cancer (SWOG 0307). Support Care Cancer. 2021;29(5):2509-2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Saad F, Brown JE, Van Poznak C, et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann Oncol. 2012;23(5):1341-1347. [DOI] [PubMed] [Google Scholar]
- 95. Fortuna G, Ruoppo E, Pollio A, et al. Multiple myeloma vs. breast cancer patients with bisphosphonates-related osteonecrosis of the jaws: a comparative analysis of response to treatment and predictors of outcome. J Oral Pathol Med. 2012;41(3):222-228. [DOI] [PubMed] [Google Scholar]
- 96. Bamias A, Kastritis E, Bamia C, et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol. 2005;23(34):8580-8587. [DOI] [PubMed] [Google Scholar]
- 97. Jadu F, Lee L, Pharoah M, Reece D, Wang L. A retrospective study assessing the incidence, risk factors and comorbidities of pamidronate-related necrosis of the jaws in multiple myeloma patients. Ann Oncol. 2007;18(12):2015-2019. [DOI] [PubMed] [Google Scholar]
- 98. Vahtsevanos K, Kyrgidis A, Verrou E, et al. Longitudinal cohort study of risk factors in cancer patients of bisphosphonate-related osteonecrosis of the jaw. J Clin Oncol. 2009;27(32):5356-5362. [DOI] [PubMed] [Google Scholar]
- 99. Silverman SL, Landesberg R. Osteonecrosis of the jaw and the role of bisphosphonates: a critical review. Am J Med. 2009;122(2 Suppl):S33-S45. [DOI] [PubMed] [Google Scholar]
- 100. Guarneri V, Miles D, Robert N, et al. Bevacizumab and osteonecrosis of the jaw: incidence and association with bisphosphonate therapy in three large prospective trials in advanced breast cancer. Breast Cancer Res Treat. 2010;122(1):181-188. [DOI] [PubMed] [Google Scholar]
- 101. Gimsing P, Carlson K, Turesson I, et al. Effect of pamidronate 30 mg versus 90 mg on physical function in patients with newly diagnosed multiple myeloma (Nordic Myeloma Study Group): a double-blind, randomised controlled trial. Lancet Oncol. 2010;11(10):973-982. [DOI] [PubMed] [Google Scholar]
- 102. Vestergaard P, Schwartz K, Rejnmark L, Mosekilde L, Pinholt EM. Oral bisphosphonate use increases the risk for inflammatory jaw disease: a cohort study. J Oral Maxillofac Surg. 2012;70(4):821-829. [DOI] [PubMed] [Google Scholar]
- 103. Khan A, Morrison A, Cheung A, Hashem W, Compston J. Osteonecrosis of the jaw (ONJ): diagnosis and management in 2015. Osteoporos Int. 2016;27(3):853-859. [DOI] [PubMed] [Google Scholar]
- 104. Graves LL, Bukata SV, Aghazadehsanai N, Chang TI, Garrett NR, Friedlander AH. Patients receiving parenteral bisphosphonates for malignant disease and having developed an atypical femoral fracture are at risk of concomitant osteonecrosis of the jaw: an evidence-based review. J Oral Maxillofac Surg. 2016;74(12):2403-2408. [DOI] [PubMed] [Google Scholar]
- 105. Eiken PA, Prieto-Alhambra D, Eastell R, Abrahamsen B. Surgically treated osteonecrosis and osteomyelitis of the jaw and oral cavity in patients highly adherent to alendronate treatment: a nationwide user-only cohort study including over 60,000 alendronate users. Osteoporos Int. 2017;28(10):2921-2928. [DOI] [PubMed] [Google Scholar]
- 106. Chan BH, Yee R, Puvanendran R, Ang SB. Medication-related osteonecrosis of the jaw in osteoporotic patients: prevention and management. Singapore Med J. 2018;59(2):70-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kang MH, Lee DK, Kim CW, Song IS, Jun SH. Clinical characteristics and recurrence-related factors of medication-related osteonecrosis of the jaw. J Korean Assoc Oral Maxillofac Surg. 2018;44(5):225-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Shibahara T, Morikawa T, Yago K, Kishimoto H, Imai Y, Kurita K. National survey on bisphosphonate-related osteonecrosis of the jaws in Japan. J Oral Maxillofac Surg. 2018;76(10):2105-2112. [DOI] [PubMed] [Google Scholar]
- 109. Liao MT, Chien WC, Wang JC, Chung CH, Chu SJ, Tsai SH. Increased risk of bisphosphonate-related osteonecrosis of the jaw in patients with Sjögren’s syndrome: nationwide population-based cohort study. BMJ Open. 2019;9(2):e024655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Qi WX, Zhao S, Chen J. Risk factors for developing osteonecrosis of jaw in advanced cancer patients underwent zoledronic acid treatment. Future Oncol. 2019;15(30):3503-3511. [DOI] [PubMed] [Google Scholar]
- 111. Wessel JH, Dodson TB, Zavras AI. Zoledronate, smoking, and obesity are strong risk factors for osteonecrosis of the jaw: a case-control study. J Oral Maxillofac Surg. 2008;66(4):625-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Thumbigere-Math V, Tu L, Huckabay S, et al. A retrospective study evaluating frequency and risk factors of osteonecrosis of the jaw in 576 cancer patients receiving intravenous bisphosphonates. Am J Clin Oncol. 2012;35(4):386-392. [DOI] [PubMed] [Google Scholar]
- 113. Katz J, Gong Y, Salmasinia D, et al. Genetic polymorphisms and other risk factors associated with bisphosphonate induced osteonecrosis of the jaw. Int J Oral Maxillofac Surg. 2011;40(6):605-611. [DOI] [PubMed] [Google Scholar]
- 114. Borromeo GL, Brand C, Clement JG, et al. A large case-control study reveals a positive association between bisphosphonate use and delayed dental healing and osteonecrosis of the jaw. J Bone Miner Res. 2014;29(6):1363-1368. [DOI] [PubMed] [Google Scholar]
- 115. Khamaisi M, Regev E, Yarom N, et al. Possible association between diabetes and bisphosphonate-related jaw osteonecrosis. J Clin Endocrinol Metab. 2007;92(3):1172-1175. [DOI] [PubMed] [Google Scholar]
- 116. Lorenzo-Pouso AI, Pérez-Sayáns M, González-Palanca S, Chamorro-Petronacci C, Bagán J, García-García A. Biomarkers to predict the onset of biphosphonate-related osteonecrosis of the jaw: a systematic review. Med Oral Patol Oral Cir Bucal. 2019;24(1):e26-e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Fusco V, Cabras M, Erovigni F, et al. A multicenter observational study on medication-related osteonecrosis of the jaw (MRONJ) in advanced cancer and myeloma patients of a cancer network in North-Western Italy. Med Oral Patol Oral Cir Bucal. 2021;26(4):e466-e473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Taylor KH, Middlefell LS, Mizen KD. Osteonecrosis of the jaws induced by anti-RANK ligand therapy. Br J Oral Maxillofac Surg. 2010;48(3):221-223. [DOI] [PubMed] [Google Scholar]
- 119. Sun L, Yu S. Efficacy and safety of denosumab versus zoledronic acid in patients with bone metastases: a systematic review and meta-analysis. Am J Clin Oncol. 2013;36(4):399-403. [DOI] [PubMed] [Google Scholar]
- 120. Peddi P, Lopez-Olivo MA, Pratt GF, Suarez-Almazor ME. Denosumab in patients with cancer and skeletal metastases: a systematic review and meta-analysis. Cancer Treat Rev. 2013;39(1):97-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Qi WX, Tang LN, He AN, Yao Y, Shen Z. Risk of osteonecrosis of the jaw in cancer patients receiving denosumab: a meta-analysis of seven randomized controlled trials. Int J Clin Oncol. 2014;19(2):403-410. [DOI] [PubMed] [Google Scholar]
- 122. Boquete-Castro A, Gómez-Moreno G, Calvo-Guirado JL, Aguilar-Salvatierra A, Delgado-Ruiz RA. Denosumab and osteonecrosis of the jaw. A systematic analysis of events reported in clinical trials. Clin Oral Implants Res. 2016;27(3):367-375. [DOI] [PubMed] [Google Scholar]
- 123. de Boissieu P, Kanagaratnam L, Mahmoudi R, Morel A, Dramé M, Trenque T. Adjudication of osteonecrosis of the jaw in phase III randomized controlled trials of denosumab: a systematic review. Eur J Clin Pharmacol. 2017;73(5):517-523. [DOI] [PubMed] [Google Scholar]
- 124. Raje N, Terpos E, Willenbacher W, et al. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: an international, double-blind, double-dummy, randomised, controlled, phase 3 study. Lancet Oncol. 2018;19(3):370-381. [DOI] [PubMed] [Google Scholar]
- 125. Hayes AR, Brungs D, Pavlakis N. Osteoclast inhibitors to prevent bone metastases in men with high-risk, non-metastatic prostate cancer: a systematic review and meta-analysis. PLoS One. 2018;13(1):e0191455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Chandran T, Venkatachalam I. Efficacy and safety of denosumab compared to bisphosphonates in improving bone strength in postmenopausal osteoporosis: a systematic review. Singapore Med J. 2019;60(7):364-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Watts NB, Grbic JT, Binkley N, et al. Invasive oral procedures and events in postmenopausal women with osteoporosis treated with denosumab for up to 10 years. J Clin Endocrinol Metab. 2019;104(6):2443-2452. [DOI] [PubMed] [Google Scholar]
- 128. Chen J, Zhou L, Liu X, Wen X, Li H, Li W. Meta-analysis of clinical trials to assess denosumab over zoledronic acid in bone metastasis. Int J Clin Pharm. 2020;43(1):2-10. [DOI] [PubMed] [Google Scholar]
- 129. Jakob T, Tesfamariam YM, Macherey S, et al. Bisphosphonates or RANK-ligand-inhibitors for men with prostate cancer and bone metastases: a network meta-analysis. Cochrane Database Syst Rev. 2020;12:CD013020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5(7):513-523. [DOI] [PubMed] [Google Scholar]
- 131. Zhang X, Hamadeh IS, Song S, et al. Osteonecrosis of the jaw in the United States Food and Drug Administration’s Adverse Event Reporting System (FAERS). J Bone Miner Res. 2016;31(2):336-340. [DOI] [PubMed] [Google Scholar]
- 132. Gnant M, Pfeiler G, Steger GG, et al. ; Austrian Breast and Colorectal Cancer Study Group . Adjuvant denosumab in postmenopausal patients with hormone receptor-positive breast cancer (ABCSG-18): disease-free survival results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(3):339-351. [DOI] [PubMed] [Google Scholar]
- 133. Smith MR, Saad F, Coleman R, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012;379(9810):39-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Coleman R, Finkelstein DM, Barrios C, et al. Adjuvant denosumab in early breast cancer (D-CARE): an international, multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2020;21(1):60-72. [DOI] [PubMed] [Google Scholar]
- 135. Srivastava A, Nogueras Gonzalez GM, Geng Y, et al. Prevalence of medication related osteonecrosis of the jaw in patients treated with sequential antiresorptive drugs: systematic review and meta-analysis. Support Care Cancer. 2021;29(5):2305-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Pineda-Moncusí M, Garcia-Giralt N, Diez-Perez A, et al. Increased fracture risk in women treated with aromatase inhibitors versus tamoxifen: beneficial effect of bisphosphonates. J Bone Miner Res. 2020;35(2):291-297. [DOI] [PubMed] [Google Scholar]
- 137. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386(10001):1341-1352. [DOI] [PubMed] [Google Scholar]
- 138. Chiu WY, Chien JY, Yang WS, Juang JM, Lee JJ, Tsai KS. The risk of osteonecrosis of the jaws in Taiwanese osteoporotic patients treated with oral alendronate or raloxifene. J Clin Endocrinol Metab. 2014;99(8):2729-2735. [DOI] [PubMed] [Google Scholar]
- 139. Pontes HAR, Souza LL, Uchôa DCC, Cerqueira JMM. Mandibular osteonecrosis associated with raloxifene. J Craniofac Surg. 2018;29(3):e257-e259. [DOI] [PubMed] [Google Scholar]
- 140. Baur DA, Altay MA, Teich S, Schmitt Oswald M, Quereshy FA. Osteonecrosis of the jaw in a patient on raloxifene: a case report. Quintessence Int. 2015;46(5):423-428. [DOI] [PubMed] [Google Scholar]
- 141. Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375(16):1532-1543. [DOI] [PubMed] [Google Scholar]
- 142. Limones A, Sáez-Alcaide LM, Díaz-Parreño SA, Helm A, Bornstein MM, Molinero-Mourelle P. Medication-related osteonecrosis of the jaws (MRONJ) in cancer patients treated with denosumab vs. zoledronic acid: a systematic review and meta-analysis. Med Oral Patol Oral Cir Bucal. 2020;25(3):e326-e336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Coleman R, Hadji P, Body JJ, et al. ; ESMO Guidelines Committee. . Bone health in cancer: ESMO clinical practice guidelines. Ann Oncol. 2020;31(12):1650-1663. [DOI] [PubMed] [Google Scholar]
- 144. Everts-Graber J, Lehmann D, Burkard JP, et al. Risk of osteonecrosis of the jaw under denosumab compared to bisphosphonates in patients with osteoporosis. J Bone Miner Res. Published online November 17, 2021. doi:10.1002/jbmr.4472 [DOI] [PubMed] [Google Scholar]
- 145. Lipton A, Fizazi K, Stopeck AT, et al. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer. 2012;48(16):3082-3092. [DOI] [PubMed] [Google Scholar]
- 146. Loyson T, Van Cann T, Schöffski P, et al. Incidence of osteonecrosis of the jaw in patients with bone metastases treated sequentially with bisphosphonates and denosumab. Acta Clin Belg. 2018;73(2):100-109. [DOI] [PubMed] [Google Scholar]
- 147. Higuchi T, Soga Y, Muro M, et al. Replacing zoledronic acid with denosumab is a risk factor for developing osteonecrosis of the jaw. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125(6):547-551. [DOI] [PubMed] [Google Scholar]
- 148. Ikesue H, Doi K, Morimoto M, et al. Switching from zoledronic acid to denosumab increases the risk for developing medication-related osteonecrosis of the jaw in patients with bone metastases. Cancer Chemother Pharmacol. 2021;87(6):871-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Diz P, López-Cedrún JL, Arenaz J, Scully C. Denosumab-related osteonecrosis of the jaw. J Am Dent Assoc. 2012;143(9):981-984. [DOI] [PubMed] [Google Scholar]
- 150. Poubel VLDN, Silva CAB, Mezzomo LAM, De Luca Canto G, Rivero ERC. The risk of osteonecrosis on alveolar healing after tooth extraction and systemic administration of antiresorptive drugs in rodents: a systematic review. J Craniomaxillofac Surg. 2018;46(2):245-256. [DOI] [PubMed] [Google Scholar]
- 151. King AE, Umland EM. Osteonecrosis of the jaw in patients receiving intravenous or oral bisphosphonates. Pharmacotherapy. 2008;28(5):667-677. [DOI] [PubMed] [Google Scholar]
- 152. Jung J, Park JS, Righesso L, et al. Effects of an oral bisphosphonate and three intravenous bisphosphonates on several cell types in vitro. Clin Oral Investig. 2018;22(7):2527-2534. [DOI] [PubMed] [Google Scholar]
- 153. Dimopoulos MA, Kastritis E, Anagnostopoulos A, et al. Osteonecrosis of the jaw in patients with multiple myeloma treated with bisphosphonates: evidence of increased risk after treatment with zoledronic acid. Haematologica. 2006;91(7):968-971. [PubMed] [Google Scholar]
- 154. Gralow JR, Barlow WE, Paterson AHG, et al. Phase III randomized trial of bisphosphonates as adjuvant therapy in breast cancer: S0307. J Natl Cancer Inst. 2020;112(7):698-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Mauri D, Valachis A, Polyzos IP, Polyzos NP, Kamposioras K, Pesce LL. Osteonecrosis of the jaw and use of bisphosphonates in adjuvant breast cancer treatment: a meta-analysis. Breast Cancer Res Treat. 2009;116(3):433-439. [DOI] [PubMed] [Google Scholar]
- 156. Suzuki K, Takeyama S, Murakami S, et al. Structure-dependent effects of bisphosphonates on inflammatory responses in cultured neonatal mouse calvaria. Antioxidants (Basel). 2020;9(6):503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Sharma D, Ivanovski S, Slevin M, et al. Bisphosphonate-related osteonecrosis of jaw (BRONJ): diagnostic criteria and possible pathogenic mechanisms of an unexpected anti-angiogenic side effect. Vasc Cell. 2013;5(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Otto S, Hafner S, Grötz KA. The role of inferior alveolar nerve involvement in bisphosphonate-related osteonecrosis of the jaw. J Oral Maxillofac Surg. 2009;67(3):589-592. [DOI] [PubMed] [Google Scholar]
- 159. Zadik Y, Benoliel R, Fleissig Y, Casap N. Painful trigeminal neuropathy induced by oral bisphosphonate-related osteonecrosis of the jaw: a new etiology for the numb-chin syndrome. Quintessence Int. 2012;43(2):97-104. [PubMed] [Google Scholar]
- 160. Allen MR, Ruggiero SL. Higher bone matrix density exists in only a subset of patients with bisphosphonate-related osteonecrosis of the jaw. J Oral Maxillofac Surg. 2009;67(7):1373-1377. [DOI] [PubMed] [Google Scholar]
- 161. Phal PM, Myall RW, Assael LA, Weissman JL. Imaging findings of bisphosphonate-associated osteonecrosis of the jaws. AJNR Am J Neuroradiol. 2007;28(6):1139-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Maurer P, Sandulescu T, Kriwalsky MS, et al. Bisphosphonate-related osteonecrosis of the maxilla and sinusitis maxillaris. Int J Oral Maxillofac Surg. 2011;40(3):285-291. [DOI] [PubMed] [Google Scholar]
- 163. Voss PJ, Vargas Soto G, Schmelzeisen R, et al. Sinusitis and oroantral fistula in patients with bisphosphonate-associated necrosis of the maxilla. Head Face Med. 2016;12:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Murphy J, Mannion CJ. Medication-related osteonecrosis of the jaws and quality of life: review and structured analysis. Br J Oral Maxillofac Surg. 2020;58(6):619-624. [DOI] [PubMed] [Google Scholar]
- 165. Miksad RA, Lai KC, Dodson TB, et al. Quality of life implications of bisphosphonate-associated osteonecrosis of the jaw. Oncologist. 2011;16(1):121-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Moll S, Mueller S, Meier JK, Reichert TE, Ettl T, Klingelhöffer C. Patients’ quality of life improves after surgical intervention of stage III medication-related osteonecrosis of the jaw. Oral Maxillofac Surg. 2021;25(3):359-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Oteri G, Trifirò G, Peditto M, et al. Treatment of medication-related osteonecrosis of the jaw and its impact on a patient’s quality of life: a single-center, 10-year experience from Southern Italy. Drug Saf. 2018;41(1):111-123. [DOI] [PubMed] [Google Scholar]
- 168. He L, Sun X, Liu Z, Qiu Y, Niu Y. Pathogenesis and multidisciplinary management of medication-related osteonecrosis of the jaw. Int J Oral Sci. 2020;12(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Ramaglia L, Guida A, Iorio-Siciliano V, Cuozzo A, Blasi A, Sculean A. Stage-specific therapeutic strategies of medication-related osteonecrosis of the jaws: a systematic review and meta-analysis of the drug suspension protocol. Clin Oral Investig. 2018;22(2):597-615. [DOI] [PubMed] [Google Scholar]
- 170. Guggenberger R, Koral E, Zemann W, Jacobsen C, Andreisek G, Metzler P. Cone beam computed tomography for diagnosis of bisphosphonate-related osteonecrosis of the jaw: evaluation of quantitative and qualitative image parameters. Skeletal Radiol. 2014;43(12):1669-1678. [DOI] [PubMed] [Google Scholar]
- 171. Huber FA, Schumann P, von Spiczak J, et al. Medication-related osteonecrosis of the jaw-comparison of bone imaging using ultrashort echo-time magnetic resonance imaging and cone-beam computed tomography. Invest Radiol. 2020;55(3):160-167. [DOI] [PubMed] [Google Scholar]
- 172. O’Ryan FS, Khoury S, Liao W, et al. Intravenous bisphosphonate-related osteonecrosis of the jaw: bone scintigraphy as an early indicator. J Oral Maxillofac Surg. 2009;67(7):1363-1372. [DOI] [PubMed] [Google Scholar]
- 173. Aoki K, Matsunaga S, Ito S, et al. Persistent bone resorption lacunae on necrotic bone distinguish bisphosphonate-related osteonecrosis of jaw from denosumab-related osteonecrosis. J Bone Miner Metab. 2021;39(5):737-747. [DOI] [PubMed] [Google Scholar]
- 174. Vandone AM, Donadio M, Mozzati M, et al. Impact of dental care in the prevention of bisphosphonate-associated osteonecrosis of the jaw: a single-center clinical experience. Ann Oncol. 2012;23(1):193-200. [DOI] [PubMed] [Google Scholar]
- 175. Montefusco V, Gay F, Spina F, et al. Antibiotic prophylaxis before dental procedures may reduce the incidence of osteonecrosis of the jaw in patients with multiple myeloma treated with bisphosphonates. Leuk Lymphoma. 2008;49(11):2156-2162. [DOI] [PubMed] [Google Scholar]
- 176. Ripamonti CI, Maniezzo M, Campa T, et al. Decreased occurrence of osteonecrosis of the jaw after implementation of dental preventive measures in solid tumour patients with bone metastases treated with bisphosphonates. The experience of the National Cancer Institute of Milan. Ann Oncol. 2009;20(1):137-145. [DOI] [PubMed] [Google Scholar]
- 177. Bantis A, Zissimopoulos A, Sountoulides P, et al. Bisphosphonate-induced osteonecrosis of the jaw in patients with bone metastatic, hormone-sensitive prostate cancer. Risk factors and prevention strategies. Tumori. 2011;97(4):479-483. [DOI] [PubMed] [Google Scholar]
- 178. Kyrgidis A, Vahtsevanos K, Koloutsos G, et al. Bisphosphonate-related osteonecrosis of the jaws: a case-control study of risk factors in breast cancer patients. J Clin Oncol. 2008;26(28):4634-4638. [DOI] [PubMed] [Google Scholar]
- 179. Tsao C, Darby I, Ebeling PR, et al. Oral health risk factors for bisphosphonate-associated jaw osteonecrosis. J Oral Maxillofac Surg. 2013;71(8):1360-1366. [DOI] [PubMed] [Google Scholar]
- 180. Lodi G, Sardella A, Salis A, Demarosi F, Tarozzi M, Carrassi A. Tooth extraction in patients taking intravenous bisphosphonates: a preventive protocol and case series. J Oral Maxillofac Surg. 2010;68(1):107-110. [DOI] [PubMed] [Google Scholar]
- 181. Schubert M, Klatte I, Linek W, et al. The Saxon Bisphosphonate Register—therapy and prevention of bisphosphonate-related osteonecrosis of the jaws. Oral Oncol. 2012;48(4):349-354. [DOI] [PubMed] [Google Scholar]
- 182. Ferlito S, Puzzo S, Liardo C. Preventive protocol for tooth extractions in patients treated with zoledronate: a case series. J Oral Maxillofac Surg. 2011;69(6):e1-e4. [DOI] [PubMed] [Google Scholar]
- 183. Mozzati M, Gallesio G, Arata V, Pol R, Scoletta M. Platelet-rich therapies in the treatment of intravenous bisphosphonate-related osteonecrosis of the jaw: a report of 32 cases. Oral Oncol. 2012;48(5):469-474. [DOI] [PubMed] [Google Scholar]
- 184. Laimer J, Hechenberger M, Müller D, et al. Dental pathologies in tumor patients with bone metastases or multiple myeloma scheduled for antiresorptive therapy. Future Oncol. 2021;17(21):2705-2711. [DOI] [PubMed] [Google Scholar]
- 185. Segelman AE, Doku HC. Treatment of the oral complications of leukemia. J Oral Surg. 1977;35(6):469-477. [PubMed] [Google Scholar]
- 186. Maurer J. Providing optimal oral health. Nurs Clin North Am. 1977;12(4):671-685. [PubMed] [Google Scholar]
- 187. Trowbridge JE, Carl W. Oral care of the patient having head and neck irradiation. Am J Nurs. 1975;75(12):2146-2149. [PubMed] [Google Scholar]
- 188. Jensen SB, Pedersen AM, Vissink A, et al. ; Salivary Gland Hypofunction/Xerostomia Section; Oral Care Study Group; Multinational Association of Supportive Care in Cancer (MASCC)/International Society of Oral Oncology (ISOO) . A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: management strategies and economic impact. Support Care Cancer. 2010;18(8):1061-1079. [DOI] [PubMed] [Google Scholar]
- 189. McGuire DB, Fulton JS, Park J, et al. ; Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) . Systematic review of basic oral care for the management of oral mucositis in cancer patients. Support Care Cancer. 2013;21(11):3165-3177. [DOI] [PubMed] [Google Scholar]
- 190. Rabelo GD, Assunção JN Jr, Chavassieux P, Soares HA, Alves FA, Lemos CA Jr. Bisphosphonate-related osteonecrosis of the jaws and its array of manifestations. J Maxillofac Oral Surg. 2015;14(3):699-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Clarke BM, Boyette J, Vural E, Suen JY, Anaissie EJ, Stack BC Jr. Bisphosphonates and jaw osteonecrosis: the UAMS experience. Otolaryngol Head Neck Surg. 2007;136(3):396-400. [DOI] [PubMed] [Google Scholar]
- 192. Quispe D, Shi R, Burton G. Osteonecrosis of the jaw in patients with metastatic breast cancer: ethnic and socio-economic aspects. Breast J. 2011;17(5):510-513. [DOI] [PubMed] [Google Scholar]
- 193. Schiodt M, Vadhan-Raj S, Chambers MS, et al. A multicenter case registry study on medication-related osteonecrosis of the jaw in patients with advanced cancer. Support Care Cancer. 2018;26(6):1905-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Estilo CL, Van Poznak CH, Wiliams T, et al. Osteonecrosis of the maxilla and mandible in patients with advanced cancer treated with bisphosphonate therapy. Oncologist. 2008;13(8):911-920. [DOI] [PubMed] [Google Scholar]
- 195. Hoff AO, Toth BB, Altundag K, et al. Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J Bone Miner Res. 2008;23(6):826-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Lesclous P, Abi Najm S, Carrel JP, et al. Bisphosphonate-associated osteonecrosis of the jaw: a key role of inflammation? Bone. 2009;45(5):843-852. [DOI] [PubMed] [Google Scholar]
- 197. Boonyapakorn T, Schirmer I, Reichart PA, Sturm I, Massenkeil G. Bisphosphonate-induced osteonecrosis of the jaws: prospective study of 80 patients with multiple myeloma and other malignancies. Oral Oncol. 2008;44(9):857-869. [DOI] [PubMed] [Google Scholar]
- 198. Marx RE, Cillo JE Jr, Ulloa JJ. Oral bisphosphonate-induced osteonecrosis: risk factors, prediction of risk using serum CTX testing, prevention, and treatment. J Oral Maxillofac Surg. 2007;65(12):2397-2410. [DOI] [PubMed] [Google Scholar]
- 199. Dimopoulos MA, Kastritis E, Bamia C, et al. Reduction of osteonecrosis of the jaw (ONJ) after implementation of preventive measures in patients with multiple myeloma treated with zoledronic acid. Ann Oncol. 2009;20(1):117-120. [DOI] [PubMed] [Google Scholar]
- 200. Hellstein JW, Adler RA, Edwards B, et al. ; American Dental Association Council on Scientific Affairs Expert Panel on Antiresorptive Agents . Managing the care of patients receiving antiresorptive therapy for prevention and treatment of osteoporosis: executive summary of recommendations from the American Dental Association Council on Scientific Affairs. J Am Dent Assoc. 2011;142(11):1243-1251. [DOI] [PubMed] [Google Scholar]
- 201. Ruggiero SL, Dodson TB, Assael LA, Landesberg R, Marx RE, Mehrotra B; Task Force on Bisphosphonate-Related Osteonecrosis of the Jaws, American Association of Oral and Maxillofacial Surgeons . American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaw—2009 update. Aust Endod J. 2009;35(3):119-130. [DOI] [PubMed] [Google Scholar]
- 202. Khan AA, Sándor GK, Dore E, et al. ; Canadian Association of Oral and Maxillofacial Surgeons . Canadian consensus practice guidelines for bisphosphonate associated osteonecrosis of the jaw. J Rheumatol. 2008;35(7):1391-1397. [PubMed] [Google Scholar]
- 203. Khan AA, Sándor GK, Dore E, et al. ; Canadian Taskforce on Osteonecrosis of the Jaw . Bisphosphonate associated osteonecrosis of the jaw. J Rheumatol. 2009;36(3):478-490. [DOI] [PubMed] [Google Scholar]
- 204. Moretti F, Pelliccioni GA, Montebugnoli L, Marchetti C. A prospective clinical trial for assessing the efficacy of a minimally invasive protocol in patients with bisphosphonate-associated osteonecrosis of the jaws. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112(6):777-782. [DOI] [PubMed] [Google Scholar]
- 205. Ortega C, Montemurro F, Faggiuolo R, et al. Osteonecrosis of the jaw in prostate cancer patients with bone metastases treated with zoledronate: a retrospective analysis. Acta Oncol. 2007;46(5):664-668. [DOI] [PubMed] [Google Scholar]
- 206. Ji X, Pushalkar S, Li Y, Glickman R, Fleisher K, Saxena D. Antibiotic effects on bacterial profile in osteonecrosis of the jaw. Oral Dis. 2012;18(1):85-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Kaibuchi N, Hoshi K, Yamazaki A, Miyamoto-Sangu N, Akagi Y, Okamoto T. The progress of medication-related osteonecrosis of the jaw with conservative initial treatment: a 12-year retrospective study of 129 patients. Bone Rep. 2021;14:101072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Lee JJ, Cheng SJ, Jeng JH, Chiang CP, Lau HP, Kok SH. Successful treatment of advanced bisphosphonate-related osteonecrosis of the mandible with adjunctive teriparatide therapy. Head Neck. 2011;33(9):1366-1371. [DOI] [PubMed] [Google Scholar]
- 209. Neuprez A, Rompen E, Crielaard JM, Reginster JY. Teriparatide therapy for denosumab-induced osteonecrosis of the jaw in a male osteoporotic patient. Calcif Tissue Int. 2014;95(1):94-96. [DOI] [PubMed] [Google Scholar]
- 210. Cheung A, Seeman E. Teriparatide therapy for alendronate-associated osteonecrosis of the jaw. N Engl J Med. 2010;363(25):2473-2474. [DOI] [PubMed] [Google Scholar]
- 211. Iwamoto J, Yago K, Sato Y, Matsumoto H. Teriparatide therapy for bisphosphonate-associated osteonecrosis of the jaw in an elderly Japanese woman with severe osteoporosis. Clin Drug Investig. 2012;32(8):547-553. [DOI] [PubMed] [Google Scholar]
- 212. Narongroeknawin P, Danila MI, Humphreys LG Jr, Barasch A, Curtis JR. Bisphosphonate-associated osteonecrosis of the jaw, with healing after teriparatide: a review of the literature and a case report. Spec Care Dentist. 2010;30(2):77-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Lau AN, Adachi JD. Resolution of osteonecrosis of the jaw after teriparatide [recombinant human PTH-(1-34)] therapy. J Rheumatol. 2009;36(8):1835-1837. [DOI] [PubMed] [Google Scholar]
- 214. Harper RP, Fung E. Resolution of bisphosphonate-associated osteonecrosis of the mandible: possible application for intermittent low-dose parathyroid hormone [rhPTH(1-34)]. J Oral Maxillofac Surg. 2007;65(3):573-580. [DOI] [PubMed] [Google Scholar]
- 215. Kakehashi H, Ando T, Minamizato T, et al. Administration of teriparatide improves the symptoms of advanced bisphosphonate-related osteonecrosis of the jaw: preliminary findings. Int J Oral Maxillofac Surg. 2015;44(12):1558-1564. [DOI] [PubMed] [Google Scholar]
- 216. Sim IW, Borromeo GL, Tsao C, et al. Teriparatide promotes bone healing in medication-related osteonecrosis of the jaw: a placebo-controlled, randomized trial. J Clin Oncol. 2020;38(26):2971-2980. [DOI] [PubMed] [Google Scholar]
- 217. Kim JY, Park JH, Jung HD, Jung YS. Treatment of medication-related osteonecrosis of the jaw around the dental implant with a once-weekly teriparatide: a case report and literature review. J Oral Implantol. 2019;45(5):403-407. [DOI] [PubMed] [Google Scholar]
- 218. Gilsenan A, Midkiff K, Harris D, Kellier-Steele N, McSorley D, Andrews EB. Teriparatide did not increase adult osteosarcoma incidence in a 15-year US postmarketing surveillance study. J Bone Miner Res. 2021;36(2):244-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Cella L, Oppici A, Arbasi M, et al. Autologous bone marrow stem cell intralesional transplantation repairing bisphosphonate related osteonecrosis of the jaw. Head Face Med. 2011;7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Vescovi P, Merigo E, Meleti M, Manfredi M, Guidotti R, Nammour S. Bisphosphonates-related osteonecrosis of the jaws: a concise review of the literature and a report of a single-centre experience with 151 patients. J Oral Pathol Med. 2012;41(3):214-221. [DOI] [PubMed] [Google Scholar]
- 221. Atalay B, Yalcin S, Emes Y, et al. Bisphosphonate-related osteonecrosis: laser-assisted surgical treatment or conventional surgery? Lasers Med Sci. 2011;26(6):815-823. [DOI] [PubMed] [Google Scholar]
- 222. Tenore G, Zimbalatti A, Rocchetti F, et al. Management of medication-related osteonecrosis of the jaw (MRONJ) using leukocyte- and platelet-rich fibrin (L-PRF) and photobiomodulation: a retrospective study. J Clin Med. 2020;9(11):3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Maluf G, Caldas RJ, Silva Santos PS. Use of leukocyte- and platelet-rich fibrin in the treatment of medication-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2018;76(1):88-96. [DOI] [PubMed] [Google Scholar]
- 224. Martins MA, Martins MD, Lascala CA, et al. Association of laser phototherapy with PRP improves healing of bisphosphonate-related osteonecrosis of the jaws in cancer patients: a preliminary study. Oral Oncol. 2012;48(1):79-84. [DOI] [PubMed] [Google Scholar]
- 225. Ripamonti CI, Napoli N. Are we ready to use teriparatide to treat medication-related osteonecrosis of the jaw in clinical practice? J Clin Oncol. 2020;38(26):2949-2951. [DOI] [PubMed] [Google Scholar]
- 226. Epstein MS, Wicknick FW, Epstein JB, Berenson JR, Gorsky M. Management of bisphosphonate-associated osteonecrosis: pentoxifylline and tocopherol in addition to antimicrobial therapy. An initial case series. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110(5):593-596. [DOI] [PubMed] [Google Scholar]
- 227. Pautke C, Bauer F, Otto S, et al. Fluorescence-guided bone resection in bisphosphonate-related osteonecrosis of the jaws: first clinical results of a prospective pilot study. J Oral Maxillofac Surg. 2011;69(1):84-91. [DOI] [PubMed] [Google Scholar]
- 228. Otto S, Schnödt EM, Haidari S, et al. Autofluorescence-guided surgery for the treatment of medication-related osteonecrosis of the jaw (MRONJ): a retrospective single-center study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2021;131(5):519-526. [DOI] [PubMed] [Google Scholar]
- 229. Hoefert S, Eufinger H. Relevance of a prolonged preoperative antibiotic regime in the treatment of bisphosphonate-related osteonecrosis of the jaw. J Oral Maxillofac Surg. 2011;69(2):362-380. [DOI] [PubMed] [Google Scholar]
- 230. Bedogni A, Saia G, Bettini G, et al. Long-term outcomes of surgical resection of the jaws in cancer patients with bisphosphonate-related osteonecrosis. Oral Oncol. 2011;47(5):420-424. [DOI] [PubMed] [Google Scholar]
- 231. Freiberger JJ, Padilla-Burgos R, McGraw T, et al. What is the role of hyperbaric oxygen in the management of bisphosphonate-related osteonecrosis of the jaw: a randomized controlled trial of hyperbaric oxygen as an adjunct to surgery and antibiotics. J Oral Maxillofac Surg. 2012;70(7):1573-1583. [DOI] [PubMed] [Google Scholar]
- 232. Mücke T, Koschinski J, Deppe H, et al. Outcome of treatment and parameters influencing recurrence in patients with bisphosphonate-related osteonecrosis of the jaws. J Cancer Res Clin Oncol. 2011;137(5):907-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Stockmann P, Vairaktaris E, Wehrhan F, et al. Osteotomy and primary wound closure in bisphosphonate-associated osteonecrosis of the jaw: a prospective clinical study with 12 months follow-up. Support Care Cancer. 2010;18(4):449-460. [DOI] [PubMed] [Google Scholar]
- 234. Graziani F, Vescovi P, Campisi G, et al. Resective surgical approach shows a high performance in the management of advanced cases of bisphosphonate-related osteonecrosis of the jaws: a retrospective survey of 347 cases. J Oral Maxillofac Surg. 2012;70(11):2501-2507. [DOI] [PubMed] [Google Scholar]
- 235. Carlson ER, Basile JD. The role of surgical resection in the management of bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2009;67(5 Suppl):85-95. [DOI] [PubMed] [Google Scholar]
- 236. Stanton DC, Balasanian E. Outcome of surgical management of bisphosphonate-related osteonecrosis of the jaws: review of 33 surgical cases. J Oral Maxillofac Surg. 2009;67(5):943-950. [DOI] [PubMed] [Google Scholar]
- 237. Eckardt AM, Lemound J, Lindhorst D, Rana M, Gellrich NC. Surgical management of bisphosphonate-related osteonecrosis of the jaw in oncologic patients: a challenging problem. Anticancer Res. 2011;31(6):2313-2318. [PubMed] [Google Scholar]
- 238. El-Rabbany M, Sgro A, Lam DK, Shah PS, Azarpazhooh A. Effectiveness of treatments for medication-related osteonecrosis of the jaw: a systematic review and meta-analysis. J Am Dent Assoc. 2017;148(8):584-594.e2. [DOI] [PubMed] [Google Scholar]
- 239. Ngamphaiboon N, Frustino JL, Kossoff EB, Sullivan MA, O’Connor TL. Osteonecrosis of the jaw: dental outcomes in metastatic breast cancer patients treated with bisphosphonates with/without bevacizumab. Clin Breast Cancer. 2011;11(4):252-257. [DOI] [PubMed] [Google Scholar]
- 240. Ferlito S, Puzzo S, Palermo F, Verzì P. Treatment of bisphosphonate-related osteonecrosis of the jaws: presentation of a protocol and an observational longitudinal study of an Italian series of cases. Br J Oral Maxillofac Surg. 2012;50(5):425-429. [DOI] [PubMed] [Google Scholar]
- 241. Scoletta M, Arduino PG, Dalmasso P, Broccoletti R, Mozzati M. Treatment outcomes in patients with bisphosphonate-related osteonecrosis of the jaws: a prospective study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110(1):46-53. [DOI] [PubMed] [Google Scholar]
- 242. Wutzl A, Pohl S, Sulzbacher I, et al. Factors influencing surgical treatment of bisphosphonate-related osteonecrosis of the jaws. Head Neck. 2012;34(2):194-200. [DOI] [PubMed] [Google Scholar]
- 243. de Souza Tolentino E, de Castro TF, Michellon FC, et al. Adjuvant therapies in the management of medication-related osteonecrosis of the jaws: systematic review. Head Neck. 2019;41(12):4209-4228. [DOI] [PubMed] [Google Scholar]
- 244. Song M. Dental care for patients taking antiresorptive drugs: a literature review. Restor Dent Endod. 2019;44(4):e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Stopeck AT, Warner DJ. Response to letter to the editors—safety of long-term denosumab therapy. Support Care Cancer. 2017;25(2):353-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Tsourdi E, Zillikens MC, Meier C, et al. Fracture risk and management of discontinuation of denosumab therapy: a systematic review and position statement by ECTS. J Clin Endocrinol Metab. 2021;106(1):264-281. [DOI] [PubMed] [Google Scholar]
- 247. Campisi G, Mauceri R, Bertoldo F, et al. Medication-related osteonecrosis of jaws (MRONJ) prevention and diagnosis: Italian consensus update 2020. Int J Environ Res Public Health. 2020;17(16):59-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Fedele S, Kumar N, Davies R, Fiske J, Greening S, Porter S. Dental management of patients at risk of osteochemonecrosis of the jaws: a critical review. Oral Dis. 2009;15(8):527-537. [DOI] [PubMed] [Google Scholar]
- 249. Lesclous P, Cloitre A, Catros S, et al. Alendronate or zoledronic acid do not impair wound healing after tooth extraction in postmenopausal women with osteoporosis. Bone. 2020;137:115412. [DOI] [PubMed] [Google Scholar]
- 250. Hadaya D, Soundia A, Gkouveris I, et al. Antiresorptive-type and discontinuation-timing affect ONJ burden. J Dent Res. 2021;100(7):746-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Yoshida T, Watanabe T, Akizuki S, et al. Adverse events caused by the discontinuation of anti-resorptive agents during treatment for anti-resorptive agent-related osteonecrosis of the jaw: a single-center cohort study. J Oral Maxillofac Surg Med Pathol. 2021;33(2):115-119. [Google Scholar]
- 252. Hasegawa T, Kawakita A, Ueda N, et al. ; Japanese Study Group of Cooperative Dentistry with Medicine (JCDM) . A multicenter retrospective study of the risk factors associated with medication-related osteonecrosis of the jaw after tooth extraction in patients receiving oral bisphosphonate therapy: can primary wound closure and a drug holiday really prevent MRONJ? Osteoporos Int. 2017;28(8):2465-2473. [DOI] [PubMed] [Google Scholar]
- 253. Gallego L, Junquera L, Pelaz A, Díaz-Bobes C. Rubber dam clamp trauma during endodontic treatment: a risk factor of bisphosphonate-related osteonecrosis of the jaw? J Oral Maxillofac Surg. 2011;69(6):e93-e95. [DOI] [PubMed] [Google Scholar]
- 254. Anastasilakis AD, Polyzos SA, Yavropoulou MP, Makras P. Combination and sequential treatment in women with postmenopausal osteoporosis. Expert Opin Pharmacother. 2020;21(4):477-490. [DOI] [PubMed] [Google Scholar]
- 255. Hasegawa T, Ueda N, Yamada SI, et al. ; Japanese Study Group of Co-operative Dentistry with Medicine (JCDM) . Denosumab-related osteonecrosis of the jaw after tooth extraction and the effects of a short drug holiday in cancer patients: a multicenter retrospective study. Osteoporos Int. 2021;32(11):2323-2333. [DOI] [PubMed] [Google Scholar]
- 256. Niimi R, Kono T, Nishihara A, Hasegawa M, Kono T, Sudo A. Rebound-associated vertebral fractures after discontinuation of denosumab for the treatment of maxillitis. Osteoporos Int. 2018;29(3):769-772. [DOI] [PubMed] [Google Scholar]
- 257. Leaney A, Sztal-Mazer S. Rebound vertebral fracture in the dental chair during a tooth extraction whilst on a treatment holiday from denosumab to avoid ONJ! Bone. 2018;108:43. [DOI] [PubMed] [Google Scholar]
- 258. US Food and Drug Administration. Background document for meeting of advisory committee for reproductive health drugs and drug safety and risk management advisory committee. September 9, 2011.
- 259. Martins AS, Correia JA, Salvado F, et al. Relevant factors for treatment outcome and time to healing in medication-related osteonecrosis of the jaws—a retrospective cohort study. J Craniomaxillofac Surg. 2017;45(10):1736-1742. [DOI] [PubMed] [Google Scholar]
- 260. Bodem JP, Schaal C, Kargus S, et al. Surgical management of bisphosphonate-related osteonecrosis of the jaw stages II and III. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;121(4):367-372. [DOI] [PubMed] [Google Scholar]
- 261. Nicolatou-Galitis O, Papadopoulou E, Sarri T, et al. Osteonecrosis of the jaw in oncology patients treated with bisphosphonates: prospective experience of a dental oncology referral center. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112(2):195-202. [DOI] [PubMed] [Google Scholar]
- 262. Tripto-Shkolnik L, Fund N, Rouach V, Chodick G, Shalev V, Goldshtein I. Fracture incidence after denosumab discontinuation: real-world data from a large healthcare provider. Bone. 2020;130:115-150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.