Abstract
Context
Currently there are no assays that can simultaneously quantify serum levels of the third-generation aromatase inhibitors (AIs): letrozole, anastrozole, and exemestane, and the ultra-low levels of estrogens in postmenopausal breast cancer patients on AI treatment. Such measurements may be pivotal for the determination of optimal and individualized treatment regimens. We aimed at developing a liquid chromatography–tandem mass spectrometry (MS/MS) method for simultaneous assessment of letrozole, anastrozole, exemestane, and 17-hydroxyexemestane as well as subpicomolar levels of estradiol and estrone.
Methods
Internal standards, calibrators, serum samples, and quality controls were in fully automated steps transferred to a deep-well plate for a 2-step liquid-liquid extraction. The extracts were reconstituted and analytes were separated chromatographically using 2 serially coupled columns, then subject to MS/MS in electrospray ionization mode. The method was thoroughly validated and is traceable to 2 accredited estrogen methods.
Results
The measurement range for estrone and estradiol was 0.2 to 12 000 pmol/L and 0.8 to 13 000 pmol/L, and covered the expected therapeutic range for the AIs. All analytes had a precision of less than or equal to 13%, and accuracies within 100 ± 8%. As proof of concept, AI and estrogen levels were determined in serum samples from postmenopausal breast cancer patients under treatment.
Conclusion
We present here an assay suitable for the simultaneous measurement of serum levels of all third-generation AIs and ultra-low levels of estrogens, providing a powerful new tool to study drug efficacy and compliance. The method is highly valuable for postmenopausal patients whose pretreatment estradiol levels are below the threshold of detection for most routine assays, but still require suppression.
Keywords: estradiol, exemestane, letrozole, anastrozole, breast cancer, aromatase inhibitors
Breast cancer is the most common malignancy affecting women worldwide, and the leading cause of death among women in Europe in the age group of 30 to 59 years (1). Approximately 75% express the estrogen and/or progesterone receptor, making them potential candidates for endocrine therapy (2). In postmenopausal women estrogens are produced by peripheral aromatization of circulating androgens (3). Subject to introduction of the third-generation aromatase inhibitors (AIs: letrozole [LET], anastrozole [ANA], and exemestane [EXE]), aromatase inhibition has become the recommended first-line endocrine therapy in postmenopausal breast cancer patients (4). While AIs in concert with luteinizing hormone–releasing hormone (LHRH) analogues are used in premenopausal women, careful monitoring is required because of the risk of ovarian suppression escape (5).
Anastrozole and letrozole are nonsteroidal, so-called type-II inhibitors, whereas EXE is a steroidal compound acting as a type-I aromatase inactivator, binding irreversibly to the substrate binding site of the aromatase enzyme. Despite their structural differences, AIs appear to have comparable treatment efficacies with regard to clinical outcome (2, 6, 7), even though LET seems to be somewhat more potent concerning estrogen suppression when compared head to head with ANA (6,8). There are fewer data on blood estrogen levels in patients treated with EXE because estrogen measurements by immunochemical methods are subject to assay interaction from EXE metabolites (9), and may require extensive prepurification before analysis (10). However, a recent evaluation of the “overall estrogenic activity” during treatment with LET and EXE suggested a lower estrogenic activity using LET when compared to EXE (11). This is in accordance with results from our recently published liquid chromatography–tandem mass spectrometry (LC-MS/MS) method, determining estradiol (E2) and estrone (E1) blood levels in a limited number of patients treated with either LET or EXE (12). The main metabolite of EXE is 17-hydroxyexemestane (17HEXE), which may be as efficient in inhibiting aromatase as EXE itself (13). For ANA and LET, inhibition of aromatase is mainly effected by the parent compound, as their main metabolites are inactive (14, 15).
The optimal efficacy of aromatase inhibition depends on the degree of estrogen suppression (16). Thus, Ingle et al (17) showed in a clinical case-control study an increased risk of an early breast cancer event for postmenopausal women on AI, not reaching sufficiently suppressed levels of E2 and E1. Commonly used clinical routine methods lack the sensitivity and specificity to detect the low levels of estrogens that are expected during AI treatment, often below 1 pmol/L (12, 17, 18). Thus, we (12) and others (19) have reported LC-MS/MS assays with the required sensitivity for estrogens combined with the robustness to function in a routine laboratory test. The specificity offered by LC-MS/MS assays at the low-serum E2 levels is also crucial for accurate measurements, hence EXE interference has been shown to result in falsely elevated E2 concentrations in some immunoassays (9).
LC-MS/MS assays for the analysis of AIs in blood have been developed to investigate pharmacokinetics (20) or as potential therapeutic drug monitoring tools (21, 22). These assays are often complex research methods (23), or focus on measuring one single AI, although assays measuring combinations of AIs have been reported (21, 24).
Combined measurements of blood AI levels and estrogens are useful in the clinic as a tool to monitor individual drug efficacy, treatment failure, and compliance. This may relate to premenopausal patients in particular, with a substantial number of patients escaping from estrogen suppression on combined treatment with an LHRH analogue in concert with an AI (5). Concurrent assessments both of AI and estrogen blood levels have so far involved analysis by separate methods.
Here we have developed a robust and sensitive method for the measurement of all of the third-generation AIs simultaneously with E2 and E1 in subpicomolar levels. The assay should meet the requirements to be run in a clinical routine laboratory. To demonstrate the performance of the method, blood samples obtained from patients on treatment with ANA, LET, or EXE were analyzed.
Materials and Methods
Patient Samples
Serum samples from postmenopausal patients with estrogen receptor (ER)-positive breast cancer receiving standard-dose AI treatment were obtained from the Prospective Breast Cancer Biobank, a population-based, general research biobank comprising early-stage breast cancer patients from Haukeland University Hospital and Stavanger University Hospital in Norway (25). All participants provided written informed consent before enrolling in the Prospective Breast Cancer Biobank. The biobank and its use for the present project were approved by the Norwegian Regional Ethical Committee (2010/1957 and 2011/2161, 172 359, and 255 133). Furthermore, we reanalyzed 16 serum samples from postmenopausal breast cancer patients (8 on LET and 8 on EXE) used in previous studies (12, 26). These samples were also used for method correlation (explained later).
Chemicals
ANA (A2736, PHR1783), LET (L6545, PHR1540), E2 (E1024, E060), E2-2,3,4-13C3 (E-117), E1 (E9750, E075), E1-2,3,4-13C3 (802 921), EXE (PZ0006, PHR1634), EXE-3,4,6-13C (809 802), and 2-propanol (34 965) were from Sigma Aldrich. ANA-d12 (A637427), LET-d4 (L330102), and 17-β-hydroxyexemestane (H942340) were from Toronto Research Chemicals Inc. All AI and estrogen analytes had a chemical and isotopic purity greater than 98%.
Hexane (No. 327890010) from Fisher Scientific. Methanol (A456-212), ammonium hydroxide (153312K), and methyl tert-butyl ether (MTBE) (1.01845) were from VWR. Steroid-depleted human serum (SDHS) (SF236-7) were from BBI solutions. Intralipid (No. 000641) was from Fresenius Kabi.
Calibrators, Internal Standard, and Quality Controls
Stock solutions of all analytes were prepared in methanol. The stock solutions were further serially diluted. The individual calibrator levels were prepared by adding these serial dilutions to SDHS, making 7 or 8 calibrator levels. An additional high-concentration calibrator was used for high AI levels to extend the measurement range. When this calibrator was used, the injection volumes were reduced from 50 to 10 µL. Internal standard (IS) was prepared in 50% methanol.
Sample Workup
Automated sample handling and liquid-liquid extraction was performed using a Hamilton Microlab STAR Liquid Handling System. The robotic mixing procedure allows for an easy, precise, and high-throughput sample flow and facilitates significant improvement of the liquid-extraction step, which was crucial to yield sensitivity goals set for this assay as well as our previously reported estrogen method (12). A total of 10 µL of IS was pipetted into a 2-mL 96–deep-well plate before 500 µL of calibrators, serum samples, or quality controls (QCs) were added and mixed before 1 hour incubation at room temperature. A total of 1000-µL extraction solvent (hexane:MTBE, 75:25, v:v) was added and mixed at 500 µL/s × 25 following the liquid surface. After mixing, 75-µL separation solvent (hexane:2-propanol 75:25, v:v) was added to ensure distinct separation of the 2 liquid phases, ensuring clean extracts without reducing the extraction efficacy. The plate was gently swirled for 5 seconds and centrifuged for 2 minutes at 4000g. Then, 700 µL of the organic phase was transferred into a second well plate containing glass vials and evaporated under nitrogen gas at 40 °C. The samples were reconstituted in 70-µL water:methanol (75:25, v:v). The plate was stored at 5 °C overnight before analysis, as we have previously observed that storage increases the analyte signal and lowers the baseline noise for E2 and E1 (12). The sensitivity increase resulting from this protocol is approximately 20% to 25% both for E2 and E1.
Liquid Chromatography–Tandem Mass Spectrometry Conditions
A UPLC: Shimadzu Nexera LC systems with a phenyl column (Waters, No. 186002884) connected in series with a C8 column (Waters, No. 186002877) at 60°C achieved the desired chromatographic separation. Owing to the low flow, the back pressure was well within the operating limits. Injection volume was 10 or 50 µL depending on the required concentration range. The mobile phases were water with 0.08% ammonium hydroxide (phase A) and methanol with 0.02% ammonium hydroxide (phase B). The linear gradient of phase B from 40% to 82% in 5.95 minutes was at a flow rate of 0.250 mL/min. A freshly made mobile phase is essential because of the low stability of ammonium hydroxide. Thus, safety caps were used instead of connection to the ventilation system. MS analysis: QTRAP 6500 + (SCIEX) was operated in positive electrospray ionization (ESI) mode for ANA, EXE, and 17HEXE and negative ESI mode for LET, E2, and E1. The temperature was set at 500 °C and entrance potential was –10 or 10 V for all analytes; additional LC-MS/MS parameters are available on request. The problem of ANA and LET coelution was solved by using polarity switching with settling time at 30 ms.
Assay Validation
Measurement Range, Accuracy, and Precision
The linearity of the calibration curves were assessed by residual plots and regression analysis. Accuracy against nominal concentrations and the coefficient of variation (CV) of the slope for the calibration curve was calculated (n = 6). Weighting of 1/x2 and linear regression was used for the calibrator curve of LET, E2, and E1. Quadratic regression with weighting of 1/x was used for ANA, EXE, and 17HEXE. Quality goals for calibrator precision were set to a CV of 20% for the lowest calibration point and 15% for the remaining levels. Measurement range was defined from the lower limit of quantification (LLOQ) to the upper limit of quantification (ULOQ).
We defined LLOQ as the lowest concentration with a CV equal to or below 20%. The lowest calibration point on the calibration curve was set as the LLOQ and verified using a spiked SDHS pool (n = 5). The highest calibration point on the calibration curve was set as the ULOQ. The limit of detection (LOD) was defined as the lowest concentration at which analytes could be detected and separated from blank samples (SDHS) (n = 10).
Accuracy for ANA, LET, E2, E1, and EXE was determined against certified reference materials (CRMs) PHR1783, PHR1540, E-060, E-075, and PHR1634, which were added to SDHS at 6 levels. Each level was analyzed in triplicate in 3 runs (n = 9). Currently no commercial CRM is available for 17HEXE. To estimate analytical precision, QCs at 4 levels were analyzed in quadruplicates over 6 days, 1 run per day (n = 24).
Selectivity and Interference
Pooled serum samples from postmenopausal women were spiked with high concentrations (100-2000 nmol/L) of several potentially interfering compounds. Because of similar retention time for ANA and LET, spectral interference between the 2 was studied by analyzing SDHS spiked with concentration close to detector saturation of either ANA or LET. Interference from lipemic samples was tested by adding intralipid in increasing amounts to a pool of samples from postmenopausal women, up to an L index of 1735 mg/dL. Carryover was assessed by analysis of 2 blanks after the highest calibrator concentration in every validation run. The chromatograms of more than 400 patient samples were inspected for interfering peaks.
Matrix Effects
Add-in recovery was used to evaluate matrix effects. Ten unique patient pools from postmenopausal women and SDHS were analyzed before and after spiking with known amounts of analytes. Recovery (%) was calculated as postspiked value × 100/expected value (endogen + added concentration).
Stability
Stability in patient samples was examined at 5 °C over a period of 7 days, samples stored at room temperature over a period of 7 days, and freeze/thaw-stability was tested over 3 freeze/thaw cycles. All experiments used 5 patient samples, and spiking with AIs was performed on day 0. Analytes with a deviation of less than 10% from day 0 and no apparent trend were considered stable.
Method Comparison
Method comparison for E2 and E1 was performed against an ultra-sensitive, accredited in-house–validated routine LC-MS/MS method (measuring range, 0.6-224 pmol/L E2; 0.3-234 pmol/L E1) (12) using serum from 60 unique patient pools and individual samples, including 16 patients samples from a former study (12,26) (see “Patient Samples” in “Materials and Methods”). E2 was also compared to another accredited in-house–validated routine LC-MS/MS method for E2 included in the NEQAS program (measuring range, 13-2722 pmol/L) using serum from 40 unique patient pools.
Results
Measurement Range, Accuracy, and Precision
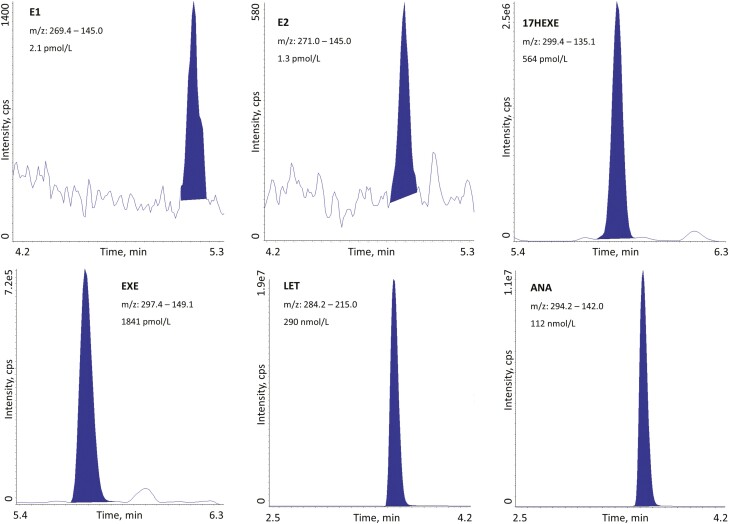
Measurement ranges were based on the residual plots, regression analysis and quality goals for precision (Table 1); all curves had R2 greater than or equal to 0.997. Mean accuracy against nominal concentrations was 100 ± 5% for all calibration points. The CV of the slope of the regression line was below 5% for all analytes. The upper measurement range can be expanded to 12 000 pmol/L (E1), 13 000 pmol/L (E2), 125 nmol/L (17HEXE), 203 nmol/L (EXE), 701 nmol/L (LET), and 1500 nmol/L (ANA) by adding an extra calibration point. In Fig. 1, chromatograms of all analytes are shown to demonstrate the methods’ performance.
Table 1.
Measurement range and method sensitivity
| LOD pmol/L | LLOQ pmol/L | ULOQ pmol/L | |
|---|---|---|---|
| E1 | 0.17 | 0.2 | 2400 |
| E2 | 0.56 | 0.8 | 2594 |
| 17HEXE | 2.3 | 8.0 | 25 077 |
| EXE | 5.8 | 13 | 40 520 |
| LET | 9.8 | 14 | 140 000 |
| ANA | 32 | 95 | 300 000 |
LLOQ was defined as the lowest concentration with a CV equal to or below 20% and with an accuracy of ± 20%. Measuring range: LLOQ – ULOQ. LOD assessed by the ability to differentiate blank samples spiked to concentrations close to projected LODs and blank samples.
Abbreviations: 17HEXE, 17-hydroxyexemestane; ANA, anastrozole; CV, coefficient of variation; E1, estrone; E2, estradiol; EXE, exemestane; LET, letrozole; LLOQ, lower limit of quantification; LOD, limit of detection; ULOQ, upper limit of quantification.
Figure 1.
Liquid chromatography–tandem mass spectrometry chromatograms of the lowest quality control (E2) and serum samples from postmenopausal breast cancer patients. 17HEXE, 17-hydroxyexemestane; ANA, anastrozole; E1, estrone; E2, estradiol, EXE, exemestane; LET, letrozole.
Accuracies were within 100 ± 8% at all levels, while precision values were within ± 13% (Table 2).
Table 2.
Method precision
| QC-level 1 | QC-level 2 | QC-level 3 | QC-level 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Mean, pmol/L | Total CV% | Mean, pmol/L | Total CV% | Mean, pmol/L | Total CV% | Mean, pmol/L | Total CV% | |
| E1 | 3.6 | 6 | 20 | 5 | 747 | 6 | 1055 | 5 |
| E2 | 1.3 | 13 | 22 | 5 | 711 | 2 | 1100 | 4 |
| 17HEXE | 13 | 8 | 155 | 4 | 4960 | 5 | 6680 | 4 |
| EXE | 52 | 4 | 503 | 2 | 22 940 | 2 | 43 230 | 4 |
| LET | 597 | 2 | 21 100 | 2 | 67 340 | 2 | 146 630 | 3 |
| ANA | 786 | 5 | 30 400 | 6 | 91 960 | 4 | 211 120 | 8 |
Total precision for QCs. QCs were analyzed in quadruplicates over 6 days, 1 run per day (N = 24). QCs made from spiked serum depleted human serum and/or spiked patient pools.
Abbreviations: 17HEXE, 17-hydroxyexemestane; ANA, anastrozole; CV, coefficient of variation; E1, estrone; E2, estradiol; EXE, exemestane; LET, letrozole; QC, quality control.
Selectivity and Interference
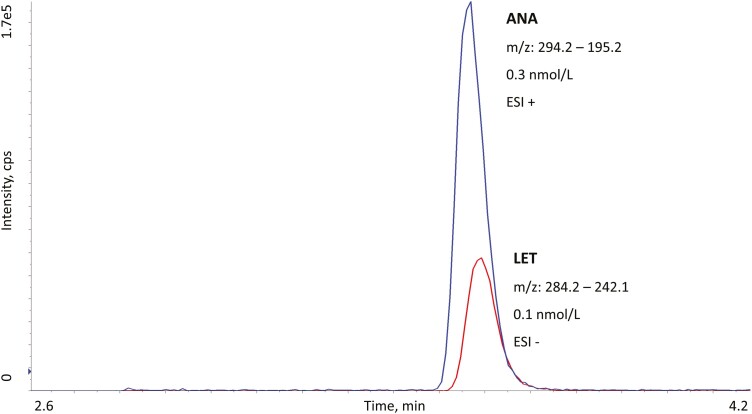
At a lipid index of 1735 mg/dL, the measured value of 17HEXE was increased by + 14%. None of the other analytes was affected. High concentrations (2000 nmol/L) of 17-α-estradiol and ethinylestradiol peaks were found to interfere with low-concentration E2 and E1 peaks. Owing to coelution, spectral interference between ANA and LET was investigated, but even at concentrations as high as detector saturation no interference was observed. Fig. 2 shows a chromatogram of ANA and LET overlaid (analyzed with different ESI polarities). Carryover was not detected for any of the analytes.
Figure 2.
Overlaid liquid chromatography–tandem mass spectrometry chromatograms of anastrozole (ANA) in electrospray ionization (ESI)+ (blue line) and letrozole (LET) in ESI– (red line). Because of similar retention time, polarity switching was used. We observed no spectral interferences between the 2 analytes.
Matrix Effects
Mean add-in recovery was 102 ± 2% for ANA, 101 ± 2% for LET, 95 ± 4% for E2, 102 ± 2% for E1, 101 ± 2% for EXE, and 96 ± 11% for 17HEXE, indicating negligible matrix effects for all analytes.
Stability
All analytes were found to be stable at 5 °C for minimum 7 days, with a mean deviation of ± 5%. At room temperature, EXE dropped to a mean deviation of –11% at day 3, and further to –14% at day 7. All other analytes were stable at room temperature for a minimum of 7 days, with the mean deviation staying between ± 3% and no apparent trend. All analytes were stable through 3 freeze/thaw cycles, with a mean deviation of –1% to 6%.
Method Comparison
Method comparison against the accredited (ISO 15189:2012) in-house LC-MS/MS method for E2 gave a mean difference of 2% and R2 equal to 0.996 (n = 40). Compared to the ultrasensitive accredited LC-MS/MS method for E2 and E1 (12), a mean deviation of less than 6% was found for both compounds and R2 greater than 0.997. We had no alternative method for comparison of the AI blood levels.
Measurement of Aromatase Inhibitors and Estrogens in Breast Cancer Patients
To verify the method’s utility, serum samples from 51 postmenopausal patients with breast cancer on AI treatment were analyzed. All patients were registered to receive standard dosage of the AI. LET treatment caused suppression of E1 and E2 to subpicomolar levels in all but one patient (Table 3). In the EXE-treated group, only one patient had detectable E2. E1 levels were also low, although all but one had quantifiable peaks. In the 4 patients who received ANA, all patients had quantifiable peaks for E1 and 2 had relatively high E2 levels (2.3 and 4.5 pmol/L, respectively). In the LET-treated group, 23 of 26 patients had drug levels in the range of 180 to 380 nmol/L. Drug concentrations were detected in the range of 0.6 to 90.2 nmol/L for EXE and 0.3 to 8.4 nmol/L for 17HEXE. EXE and 17HEXE correlated strongly with an R equal to 0.88, and an average EXE/17HEXE ratio of 6:1. No correlation was observed between EXE and E1 levels. The measured ANA concentrations were in the range of 6.5 to 112 nmol/L.
Table 3.
Estrogen and aromatase inhibitor levels in breast cancer patients
| Treated with letrozole | Treated with exemestane | |||||
|---|---|---|---|---|---|---|
| LET | E2 | E1 | EXE | 17HEXE | E2 | E1 |
| 290 | 1.2 | 1.5 | 5.1 | 1.2 | 1.1 | 4.1 |
| 193 | <LOQ | 0.5 | 0.8 | 0.4 | <LOQ | 2.4 |
| 252 | <LOQ | 0.4 | 0.6 | 0.3 | <LOQ | 2.3 |
| 238 | <LOQ | 0.3 | 7.0 | 1.0 | <LOQ | 2.3 |
| 361 | <LOQ | <LOQ | 90.2 | 7.0 | <LOQ | 1.7 |
| 349 | <LOQ | <LOQ | 2.0 | 0.5 | <LOQ | 1.5 |
| 326 | <LOQ | <LOQ | 30.1 | 4.7 | <LOQ | 1.4 |
| 245 | <LOQ | <LOQ | 3.3 | 0.7 | <LOQ | 1.1 |
| 329 | <LOQ | <LOQ | 45.4 | 4.7 | <LOQ | 0.6 |
| 372 | <LOQ | <LOQ | 63.2 | 8.4 | <LOQ | 0.5 |
| 869 | <LOQ | <LOQ | 38.7 | 4.9 | <LOQ | 0.4 |
| 262 | <LOQ | <LOQ | 34.1 | 7.9 | <LOQ | 0.4 |
| 241 | <LOQ | <LOQ | 1.8 | 0.6 | <LOQ | 0.3 |
| 269 | <LOQ | <LOQ | 27.4 | 4.5 | <LOQ | 0.3 |
| 263 | <LOQ | <LOQ | 6.1 | 1.4 | <LOQ | <LOQ |
| 323 | <LOQ | <LOQ | Treated with anastrozole | |||
| 275 | <LOQ | <LOQ | ||||
| 290 | <LOQ | <LOQ | ANA | E2 | E1 | |
| 211 | <LOQ | <LOQ | 6.5 | 4.5 | 25.3 | |
| 181 | <LOQ | <LOQ | 109 | 2.3 | 5.5 | |
| 341 | <LOQ | <LOQ | 112 | <LOQ | 2.1 | |
| 376 | <LOQ | <LOQ | 81.6 | <LOQ | 1.2 | |
| 268 | <LOQ | <LOQ | ||||
| 742 | <LOQ | <LOQ | ||||
| 30.4 | <LOQ | <LOQ | ||||
| 251 | <LOQ | <LOQ |
E2 and E1 concentrations pmol/L. LET, ANA, EXE, and 17HEXE are given in nmol/L.
Abbreviations: 17HEXE, 17-hydroxyexemestane; ANA, anastrozole; E1, estrone; E2, estradiol; EXE, exemestane; LET, letrozole; LOQ, limit of quantification.
Discussion
Suppression of estrogen levels by third-generation AIs is a recommended adjuvant therapy for many postmenopausal women with ER-positive breast cancer, while a combination of AI + LHRH analogue may be used in premenopausal patients at high risk of recurrence (27, 28). To date no methods can simultaneously quantify all third-generation AIs and subpicomolar levels of estrogen. Very few routine applicable methods reach the level of sensitivity needed for estrogen analysis in postmenopausal breast cancer patients (12, 19). Here we report a novel, thoroughly validated LC-MS/MS method that combines the measurement of serum AI levels of all relevant third-generation compounds (LET, ANA, EXE) and, at the same time, the measurement of ultra-low suppressed levels of E2 and E1 in serum samples.
The reported method has a wide measuring range for all analytes, and performed with excellent accuracy and precision at all levels. For E2 and E1 the LLOQs were obtained in the required subpicomolar levels (0.8 and 0.2 pmol/L, respectively), in accordance with our previously reported and accredited method (12), while the ULOQ was in the high nanomolar range (12-13 nmol/L). The measurement range for the AI levels was set to nanomolar concentrations to cover the therapeutic ranges, but the method also demonstrated excellent precision at low levels to accommodate dose-response studies with lower dosages than standard. To our knowledge, this is the most sensitive method for E1, ANA, LET, EXE, and 17HEXE so far reported, and comparable in sensitivity for E2 to our previously reported method (12). We have implemented several original steps to increase method performance, such as the use of a separation solvent, serial column coupling, and polarity switching to improve selectivity.
We measured 51 serum samples from postmenopausal breast cancer patients receiving AI treatment to confirm the validity of the method. In Norway LET is the predominantly used AI; accordingly, 26 of the samples were from patients who received treatment with LET. Fifteen patients received EXE and 4 were treated with ANA. Although the number of samples analyzed are too low to draw conclusions on drug efficacy in this study, some differences between LET, EXE, and ANA were observed. Hence, based on the measured estrogen-suppression levels, LET appeared to be a more efficient AI, which is in accordance with previous studies (12, 17, 18). However, the FACE study showed that LET was not superior to ANA in postmenopausal patients with respect to clinical outcome (29). We have previously obtained reference ranges for E2 and E1 in healthy, postmenopausal women: for E2 3.8 to 36 pmol/L with a median level of 13.9 pmol/L, and for E1 22 to 122 pmol/L with a median level of 57.2 pmol/L (12). In the present study the suppressed levels of estrogens, with one exception for ANA, were found to be well below the expected levels for postmenopausal women, demonstrating the method’s clinical potential. In premenopausal patients who have their ovarian function still intact, it is still vital to determine whether the ovarian suppression obtained by gonadotropin-releasing hormone analogues is sufficient (28). Clearly, more studies involving higher numbers of patients on AI treatment should be performed for conclusions on drug efficacy and dosage.
The levels of EXE and the active metabolite 17HEXE were positively correlated, and the mean ratio of EXE:17HEXE was 6:1, in accordance with previous studies (30). However, the absolute amounts of 17HEXE that are reported should be interpreted with some caution. There is currently no commercial CRM available for this compound for accuracy determination and traceability. In addition, the available deuterated 17HEXE was, from our experience, not isotopically pure enough to be used as an IS. Furthermore, EXE is extensively metabolized to several metabolites with similar structures (31), which may interfere with the assay. We also observed interference in several of the 17HEXE multiple reaction monitoring transitions.
To ensure compliance with treatment, measurements of the AIs in itself represents a valuable QC preceding clinical decisions. This is also crucial in clinical studies, especially when the efficacy of different AIs are compared to each other. In addition to compliance, measurement of AI verifies use of the registered drug, or may identify the correct drug in use when the patient has been switched to one of the alternative drugs under treatment without proper registration. In a routine setting as well as for clinical studies, the advantages of a method that offers simultaneous monitoring of all third-generation AIs, E2, and E1 are evident. For instance, as preanalytical and analytical procedures are identical, patient samples can be subjected to the same workflow and analyzed randomly without presorting according to treatment regimens and the AI in use.
In conclusion, we here present an LC-MS/MS method that aids simultaneous control of both drug levels and the corresponding ultra-low serum estrogen levels in breast cancer patients on AI treatment. The method is cost-effective and customized for routine use, and all sample preparation steps are fully automated. The method may prove useful in the clinical setting, especially for premenopausal patients, as well as for clinical studies, to monitor drug efficacy on an individual basis and to monitor patient compliance with treatment. The ability to determine the adherence of AIs also makes it possible to conduct studies to assess the influence of nonadherence on relapse and survival in breast cancer patients.
Glossary
Abbreviations
- 17HEXE
17-hydroxyexemestane
- AI
aromatase inhibitor
- ANA
anastrozole
- CRM
certified reference material
- CV
coefficient of variation
- E1
estrone
- E2
estradiol
- ER
estrogen receptor
- ESI
electrospray ionization
- EXE
exemestane
- IS
internal standard
- LC
liquid chromatography
- LET
letrozole
- LHRH
luteinizing hormone–releasing hormone
- LLOQ
lower limit of quantification
- LOD
limit of detection
- MS/MS
tandem mass spectrometry
- QC
quality control
- SDHS
steroid-depleted human serum
- ULOQ
upper limit of quantification
Financial Support
This work was supported by the Department of Medical Biochemistry and Pharmacology, Haukeland University Hospital, Bergen, Norway. We thank Trond Mohn for generous financial support of the LC-MS/MS instrumentation.
Disclosures
B.E.B., K.V., T.H., M.H., H.S., J.G., T.H.L., J.V.S., G.M., and B.A. have nothing to disclose. P.E.L. has received unrestrictive research grants from Pfizer and Astra Zeneca (other projects); holds stocks in and is chair of Cytovation; and has received consulting fees from ROVI.
Data Availability
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Vardell E. Global Health Observatory Data Repository. Med Ref Serv Q. 2020;39(1):67-74. [DOI] [PubMed] [Google Scholar]
- 2. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386(10001):1341-1352. [DOI] [PubMed] [Google Scholar]
- 3. Lønning PE, Dowsett M, Powles TJ. Postmenopausal estrogen synthesis and metabolism: alterations caused by aromatase inhibitors used for the treatment of breast cancer. J Steroid Biochem. 1990;35(3-4):355-366. [DOI] [PubMed] [Google Scholar]
- 4. Lønning PE. The potency and clinical efficacy of aromatase inhibitors across the breast cancer continuum. Ann Oncol. 2011;22(3):503-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bellet M, Gray KP, Francis PA, et al. Twelve-Month Estrogen Levels in Premenopausal Women With Hormone Receptor-Positive Breast Cancer Receiving Adjuvant Triptorelin Plus Exemestane or Tamoxifen in the Suppression of Ovarian Function Trial (SOFT): the SOFT-EST substudy. J Clin Oncol. 2016;34(14):1584-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Geisler J, Haynes B, Anker G, Dowsett M, Lonning PE. Influence of letrozole and anastrozole on total body aromatization and plasma estrogen levels in postmenopausal breast cancer patients evaluated in a randomized, cross-over study. J Clin Oncol. 2002;20(3):751-757. [DOI] [PubMed] [Google Scholar]
- 7. Geisler J, King N, Anker G, et al. In vivo inhibition of aromatization by exemestane, a novel irreversible aromatase inhibitor, in postmenopausal breast cancer patients. Clin Cancer Res. 1998;4(9):2089-2093. [PubMed] [Google Scholar]
- 8. Dixon JM, Renshaw L, Young O, et al. Letrozole suppresses plasma estradiol and estrone sulphate more completely than anastrozole in postmenopausal women with breast cancer. J Clin Oncol. 2008;26(10):1671-1676. [DOI] [PubMed] [Google Scholar]
- 9. Mandic S, Kratzsch J, Mandic D, et al. Falsely elevated serum oestradiol due to exemestane therapy. Ann Clin Biochem. 2017;54(3):402-405. [DOI] [PubMed] [Google Scholar]
- 10. Johannessen DC, Engan T, Di Salle E, et al. Endocrine and clinical effects of exemestane (PNU 155971), a novel steroidal aromatase inhibitor, in postmenopausal breast cancer patients: a phase I study. Clin Cancer Res. 1997;3(7):1101-1108. [PubMed] [Google Scholar]
- 11. Bahrami N, Chang G, Kanaya N, et al. Changes in serum estrogenic activity during neoadjuvant therapy with letrozole and exemestane. J Steroid Biochem. 2020;200:105641. doi: 10.1016/j.jsbmb.2020.105641 [DOI] [PubMed] [Google Scholar]
- 12. Bertelsen BE, Kellmann R, Viste K, et al. An ultrasensitive routine LC-MS/MS method for estradiol and estrone in the clinically relevant sub-picomolar range. J Endocr Soc. 2020;4(6):bvaa047. doi: 10.1210/jendso/bvaa047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sun D, Chen G, Dellinger RW, Sharma AK, Lazarus P. Characterization of 17-dihydroexemestane glucuronidation: potential role of the UGT2B17 deletion in exemestane pharmacogenetics. Pharmacogenet Genomics. 2010;20(10):575-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ingle JN, Buzdar AU, Schaid DJ, et al. Variation in anastrozole metabolism and pharmacodynamics in women with early breast cancer. Cancer Res. 2010;70(8):3278-3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bhatnagar AS. The discovery and mechanism of action of letrozole. Breast Cancer Res Treat. 2007;105(Suppl 1):7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lønning PE. Aromatase inhibitors in breast cancer. Endocr Relat Cancer. 2004;11(2):179-189. [DOI] [PubMed] [Google Scholar]
- 17. Ingle JN, Cairns JM, Suman VJ, et al. Anastrozole has an association between degree of estrogen suppression and outcomes in early breast cancer and is a ligand for estrogen receptor α. Clin Cancer Res. 2020;26(12):2986-2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Geisler J, Ekse D, Helle H, Duong NK, Lønning PE. An optimised, highly sensitive radioimmunoassay for the simultaneous measurement of estrone, estradiol and estrone sulfate in the ultra-low range in human plasma samples. J Steroid Biochem. 2008;109(1-2):90-95. [DOI] [PubMed] [Google Scholar]
- 19. Handelsman DJ, Gibson E, Davis S, Golebiowski B, Walters KA, Desai R. Ultrasensitive serum estradiol measurement by liquid chromatography-mass spectrometry in postmenopausal women and mice. J Endocr Soc. 2020;4(9):bvaa086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Precht JC, Ganchev B, Heinkele G, Brauch H, Schwab M, Mürdter TE. Simultaneous quantitative analysis of letrozole, its carbinol metabolite, and carbinol glucuronide in human plasma by LC-MS/MS. Anal Bioanal Chem. 2012;403(1):301-308. [DOI] [PubMed] [Google Scholar]
- 21. van Nuland M, Venekamp N, de Vries N, de Jong KAM, Rosing H, Beijnen JH. Development and validation of an UPLC-MS/MS method for the therapeutic drug monitoring of oral anti-hormonal drugs in oncology. J Chromatogr B Analyt Technol Biomed Life Sci. 2019;1106-1107:26-34. [DOI] [PubMed] [Google Scholar]
- 22. Shao R, Yu LY, Lou HG, Ruan ZR, Jiang B, Chen JL. Development and validation of a rapid LC-MS/MS method to quantify letrozole in human plasma and its application to therapeutic drug monitoring. Biomed Chromatogr. 2016;30(4):632-637. [DOI] [PubMed] [Google Scholar]
- 23. Abubakar MB, Gan SH. A review of chromatographic methods used in the determination of anastrozole levels. Indian J Pharm Sci. 2016;78(2):173-181. [Google Scholar]
- 24. Beer B, Schubert B, Oberguggenberger A, Meraner V, Hubalek M, Oberacher H. Development and validation of a liquid chromatography-tandem mass spectrometry method for the simultaneous quantification of tamoxifen, anastrozole, and letrozole in human plasma and its application to a clinical study. Anal Bioanal Chem. 2010;398(4):1791-1800. [DOI] [PubMed] [Google Scholar]
- 25. Lunde S, Helland T, Jonassen J, et al. (2018).. A prospective, longitudinal, breast cancer biobank (PBCB) in western Norway. Antwerp: In Eurpe Biobank Week (EBW), Poster. doi: 10.13140/RG.2.2.16266.11201. [DOI] [Google Scholar]
- 26. Geisler J, Helle H, Ekse D, et al. Letrozole is superior to anastrozole in suppressing breast cancer tissue and plasma estrogen levels. Clin Cancer Res. 2008;14(19):6330-6335. [DOI] [PubMed] [Google Scholar]
- 27. Smith I, Yardley D, Burris H, et al. Comparative efficacy and safety of adjuvant letrozole versus anastrozole in postmenopausal patients with hormone receptor-positive, node-positive early breast cancer: final results of the randomized phase III Femara Versus Anastrozole Clinical Evaluation (FACE) Trial. J Clin Oncol. 2017;35(10):1041-1048. [DOI] [PubMed] [Google Scholar]
- 28. Francis PA, Pagani O, Fleming GF, et al. SOFT and TEXT Investigators and the International Breast Cancer Study Group . Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med. 2018;379(2):122-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brower V. Letrozole not superior to anastrozole for early breast cancer. Lancet Oncol. 2017;18(3):e138. [DOI] [PubMed] [Google Scholar]
- 30. Wang LZ, Goh SH, Wong AL, et al. Validation of a rapid and sensitive LC-MS/MS method for determination of exemestane and its metabolites, 17β-hydroxyexemestane and 17βa-hydroxyexemestane-17-O-β-D-glucuronide: application to human pharmacokinetics study. PLoS One. 2015;10(3):e0118553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peterson A, Xia ZP, Chen G, Lazarus P. Exemestane potency is unchanged by common nonsynonymous polymorphisms in CYP19A1: results of a novel anti-aromatase activity assay examining exemestane and its derivatives. Pharmacol Res Perspect. 2017;5(3):e00313. doi: 10.1002/prp2.313 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.