Abstract
Context
Diagnosis of primary aldosteronism (PA) for many patients depends on positive results for the saline infusion test (SIT). Plasma aldosterone is often measured by immunoassays, which can return inaccurate results.
Objective
This study aimed to establish whether differences in aldosterone measurements by immunoassay versus mass spectrometry (MS) might impact confirmatory testing for PA.
Methods
This study, involving 240 patients tested using the SIT at 5 tertiary care centers, assessed discordance between immunoassay and MS-based measurements of plasma aldosterone.
Results
Plasma aldosterone measured by Liaison and iSYS immunoassays were respectively 86% and 58% higher than determined by MS. With an immunoassay-based SIT cutoff for aldosterone of 170 pmol/L, 78 and 162 patients had, respectivel, negative and positive results. All former patients had MS-based measurements of aldosterone < 117 pmol/L, below MS-based cutoffs of 162 pmol/L. Among the 162 patients with pathogenic SIT results, MS returned nonpathologic results in 62, including 32 under 117 pmol/L. Repeat measurements by an independent MS method confirmed nonpathogenic results in 53 patients with discordant results. Patients with discordant results showed a higher (P < 0.0001) prevalence of nonlateralized than lateralized adrenal aldosterone production than patients with concordant results (83% vs 28%). Among patients with nonlateralized aldosterone production, 66% had discordant results. Discordance was more prevalent for the Liaison than iSYS immunoassay (32% vs 16%; P = 0.0065) and was eliminated by plasma purification to remove interferents.
Conclusion
These findings raise concerns about the validity of immunoassay-based diagnosis of PA in over 60% of patients with presumed bilateral disease. We provide a simple solution to minimize immunoassay inaccuracy-associated misdiagnosis of PA.
Keywords: aldosterone, renin, adrenal cortex, mass spectrometry, diagnosis, interference
Diagnosis and subtype classification of primary aldosteronism (PA) involves a multistep process first involving measurement of the aldosterone to renin ratio (ARR), which should usually be followed by confirmatory testing (1, 2). Diagnostic performance of the ARR is highly variable and dependent on numerous preanalytical and analytical factors including selected cutoffs for determining a positive result (3-6). Cutoffs for screening are best set to maximize diagnostic sensitivity and minimize a missed diagnosis (7, 8). This, however, is at the expense of suboptimal diagnostic specificity that requires follow-up testing to further confirm or exclude the diagnosis for the majority of cases where clinical presentation and initial tests are equivocal.
Among several follow-up procedures the saline infusion test (SIT) is the most commonly used test for disease confirmation and exclusion. As for the ARR, there are several preanalytical factors that can impact SIT performance and interpretation; among these, conducting the test in the seated position is now recognized to provide superior diagnostic accuracy than testing in the supine position (9). For the seated SIT, a cutoff for plasma aldosterone of 170 pmol/L (61 ng/L) has been outlined in the Endocrine Society guideline (1), although it is also acknowledged that cutoffs should be established according to methods of analyses. Thus, for liquid chromatography with tandem mass spectrometry (LC-MS/MS) Stowasser and colleagues established an optimal cutoff of 162 pmol/L (58 ng/L) for the seated SIT, with higher cutoffs indicated for immunoassay methods (9, 10).
Once confirmatory testing indicates PA, the next step is adrenal venous sampling (AVS) to establish unilateral disease, which may be cured by adrenalectomy, versus bilateral disease, which is optimally treated by mineralocorticoid receptor antagonist therapy (1-4). It should be recognized throughout these processes that diagnosis of bilateral PA depends on confirmatory tests. For patients with unilateral PA who undergo surgical intervention, follow-up to confirm biochemical cure provides the currently accepted method for disease confirmation (11). Thereby, as in initial confirmatory testing, confirmation of unilateral PA according to biochemical cure depends on diagnostic test accuracy.
Recently we documented a patient in whom interference with immunoassay measurements of plasma aldosterone resulted in up to 10-fold higher concentrations than measured by LC-MS/MS (12). According to immunoassay measurements the patient showed highly pathologic results for the SIT and even lateralization during AVS. The patient escaped an unneeded adrenalectomy by inclusion in a prospective clinical protocol and exhaustive follow-up testing to establish continuously inaccurate measurements by the immunoassay due to analytical interference.
In order to establish whether the aforementioned patient represented an isolated case or was part of a broader problem impacting diagnosis of PA, this study further assessed the presence of discordant results of immunoassay and LC-MS/MS measurements of plasma aldosterone during the SIT.
Methods
Patient Recruitment
The 254 patients who underwent the SIT were recruited at 5 European tertiary care centers under the PROspective study on the diagnostic value of Steroid profiling in primary ALDOsteronism (PROSALDO) according to details provided in the supplement (13). The primary objective of this ongoing clinical trial is to prospectively evaluate use of steroid profiling to streamline diagnostic decision-making in patients with suspected PA. The protocol allows for substudies with secondary objectives, including evaluation of routine diagnostic procedures, such as the SIT, the focus of the present report. All patients provided written informed consent under the PROSALDO protocol, which was approved by the ethics committees at each center.
Clinical Test Procedures
Patients were selected to undergo a SIT on the basis of an elevated ARR according to reference intervals used at each participating center or according to positive results of LC-MS/MS-based steroid profiling as established elsewhere (14). On this basis, 254 patients underwent a SIT between October 14, 2019, and September 25, 2021. Among these, results for both routine immunoassay and LC-MS/MS measurements of aldosterone were available for 240 patients who were included in the analysis (Supplemental Figure 1 (13)).
The SIT involved intravenous infusion of 2 liters of 0.9% saline over 4 hours, initiated in the morning with patients in the sitting position throughout. Blood samples were collected for aldosterone, renin, and cortisol before and at the end of infusions. Antihypertensive medications known to interfere with aldosterone and renin measurements were withdrawn and replaced with alpha-adrenoceptor blockers (eg, doxazosine, prazosine), vasodilators (eg, hydralazine) or non-dihydropyridine calcium channel blockers (eg, verapamil). A SIT was interpreted to confirm PA if at the end of the infusion, plasma aldosterone measured by immunoassays remained above 170 pmol/L (61 ng/L), a cutoff defined by Endocrine Society guidelines (1). For LC-MS/MS measurements of aldosterone, confirmation of PA required a plasma aldosterone above 162 pmol/L (58 ng/L), as defined by Stowasser et al (9).
Unless adrenal imaging had already been carried out, all patients with positive results of the SIT according to routine immunoassay procedures underwent imaging studies (preferably with computed tomography). These patients were also invited to undergo AVS carried out without use of corticotropin and according to willingness to undergo adrenalectomy if AVS revealed lateralized aldosterone secretion. Adrenalectomies were also carried out on a case-by-case basis in some younger patients or when there was a failure of AVS selectivity, but only when a single adrenal mass identified by imaging and clinical findings strongly supported the diagnosis of PA. The PROSALDO protocol includes patient follow-up as described in the supplement (13), which for those who undergo surgical resection of adrenals follows previously established procedures (11).
Measurements of Plasma Aldosterone
Blood samples (10 mL) drawn into EDTA collection tubes before and at the end of SITs were centrifuged and plasma separated into aliquots for analyte measurements and biobanking. Routine measurements of plasma aldosterone at all 5 participating centers were by chemiluminescence immunoassays, including the DiaSorin Liaison immunoassay (AB 2889867) at 3 centers and the Immunodiagnostic Systems iSYS immunoassay (IS3300, AB 2892056) at 2 centers. Separate samples of plasma from the same blood collection tubes underwent measurements of plasma aldosterone at a single center according to a previously established LC-MS/MS procedure (15). A Chromsystems LC-MS/MS kit method (MassChrom) employed at either of 2 centers was used for additional measurements of aldosterone to confirm discordant results between immunoassays and LC-MS/MS-based measurements (Supplemental Figure 1 (13)). Lower limits of quantification ranged from 22 to 103 pmol/L depending on the assay method while interassay coefficients of variation ranged from 2.4% to 12.8% depending on the method and with lower precision at lower concentrations (Supplemental Table 1 and Supplemental Figure 2A (13)).
Immunoassay Measurements After Plasma Purification
Immunoassays were repeated in 21 plasma samples from 7 patients with discordant results (in whom there was sufficient remaining banked plasma) after purification of plasma to remove possible interfering macromolecules (eg, heterophile antibodies) as detailed in the supplement (13).
Histopathology
Hematoxylin and eosin staining and CYP11B2 immunostaining were performed in resected adrenals of selected patients to ascertain classical versus nonclassical histopathological features. This was according to the HISTALDO (histopathology of primary aldosteronism) consensus (16), as outlined previously (17).
Statistical Analysis
Statistical analysis utilized the JMP statistics software package version 15 (SAS Institute Inc, Cary, NC). Data that were not normally distributed were either analyzed by nonparametric methods or normalized by logarithmic transformation before parametric statistics. Differences in proportions were assessed using Chi-square and Fisher’s exact tests. Data are displayed as arithmetic or geometric means and 95% confidence intervals where relevant in brackets. Relationships between variables were examined after logarithmic transformation where appropriate. Logistic regression was used to generate receiver-operating characteristic (ROC) curves.
Results
Patient Flow Through Study Procedures
The final study population included 129 female and 111 male participants with a median age of 51 years (range, 23-75). Among these 240 patients, analyses of data focused on discordant versus concordant positive and negative test results for the SIT by immunoassay versus LC-MS/MS; in subsets of patients this led to AVS and/or adrenalectomy with follow-up to confirm postoperative cure in 17 patients to date (Supplemental Figure 1 and Supplemental Table 2 (13)).
Discordance Between Initial Analytical Methods
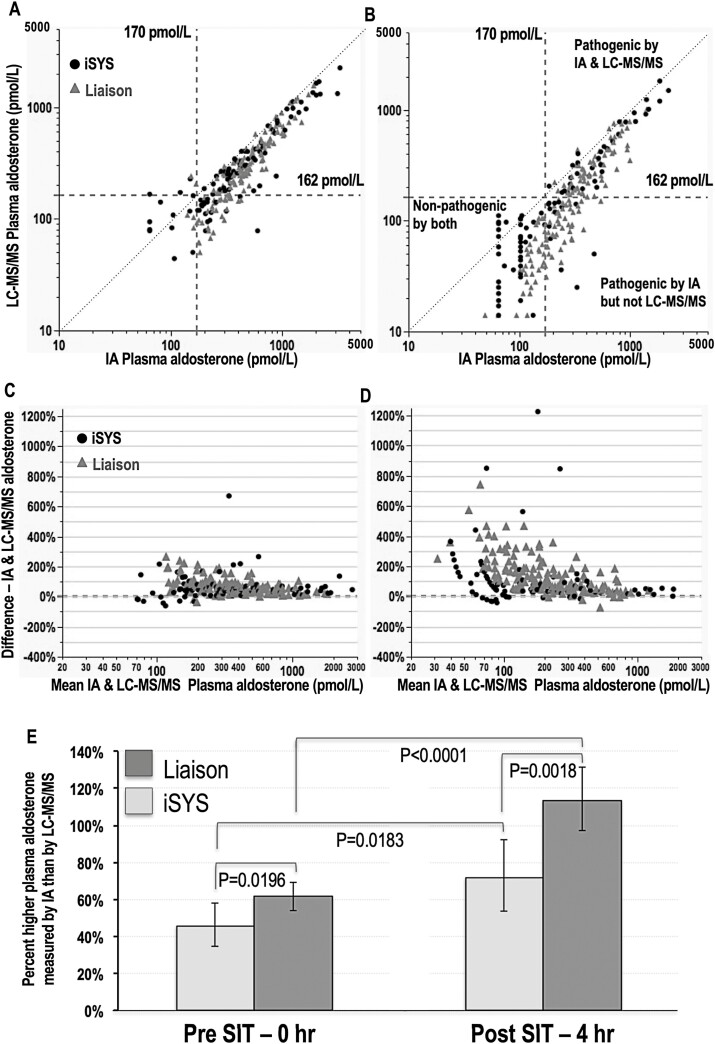
Routine concentrations of aldosterone during the SIT were initially measured singly by the Liaison and iSYS immunoassays in 147 and 93 patients, respectively. As shown by relationships of plasma aldosterone measured by immunoassays versus LC-MS/MS, measurements by the former methods were invariably higher than by the latter and there was a high degree of scatter particularly prominent for measurements of the lower concentrations at the end of the SIT (Fig. 1, panels A-D). Differences in concentrations of aldosterone measured by immunoassays and by LC-MS/MS were significantly larger for blood samples drawn at the end compared to before the SIT and for the Liaison compared to the iSYS immunoassay (Fig. 1, panel E).
Figure 1.
Relationships between plasma concentrations of aldosterone measured by immunoassay versus LC-MS/MS at baseline (A) and at 4 hours after the SIT (B), modified Bland-Altman graphs to illustrate assay bias at baseline (C) and at 4 hours after the SIT (D) and bar graphs to illustrate differences between immunoassay and LC-MS/MS measured concentrations for the Liaison and iSYS immunoassays and at baseline (0 hours) and 4 hours after the SIT (E). Dashed vertical and horizontal lines in panels A and B show cutoffs for the SIT by both immunoassay (IA) and LC-MS/MS. The dotted lines illustrate lines of identity. For panels A-D results for the iSYS IA are shown as solid dots and for the Liaison IA as gray triangles. Results in panel E are shown as geometric means with CIs, and with statistical significance of percent differences determined by respective paired and unpaired t tests for comparisons of pre- and post-SIT percent differences and of Liaison versus iSYS immunoassays.
With a cutoff of 170 pmol/L (61 ng/L) for post-SIT immunoassay measurements of aldosterone, 78 (32.5%) of the 240 tested patients had negative results and 162 (67.5%) had positive results (Supplemental Figure 1 (13)). All 78 patients with negative results for immunoassays had LC-MS/MS measurements of aldosterone at or below 117 pmol/L (42 ng/L) and well below the defined cutoff of 162 pmol/L (58 ng/L) for LC-MS/MS (Fig. 1, panel B). However, among the 162 patients with pathogenic results according to immunoassays, 62 (38%) had LC-MS/MS measurements below 162 pmol/L. Among these 62 patients, 32 had concentrations at or below 117 pmol/L, the highest concentration measured by LC-MS/MS in patients with nonpathologic results by immunoassays.
In line with larger magnitudes of differences between immunoassay and LC-MS/MS measurements for the Liaison than for the iSYS immunoassay (Fig. 1, panel E), discordant results were twice as prevalent (32% vs 16%; P = 0.0065) for the Liaison than the iSYS immunoassay (Fig. 1, panel B).
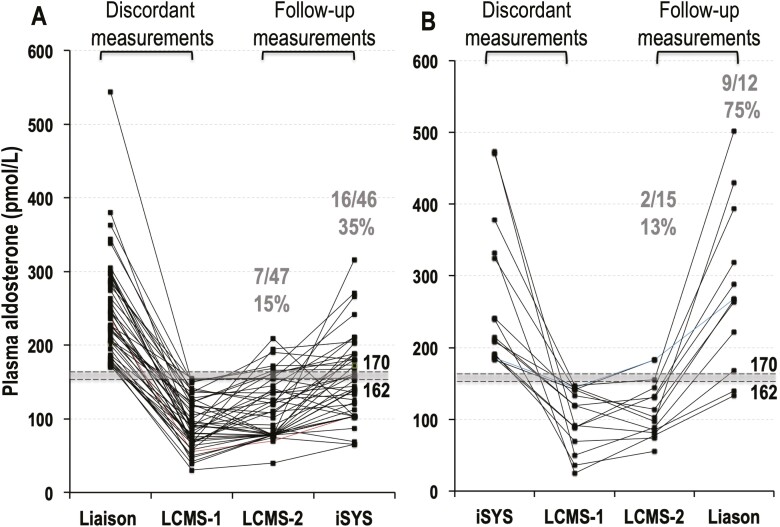
Confirmation of Discordance
For the 62 patients with discordant results for the SIT, additional single measurements of plasma aldosterone were performed by a second LC-MS/MS method and the alternative iSYS or Liaison immunoassay. For discordant results in 47 patients with the Liaison immunoassay, nonpathologic results were returned in 85% of cases by the second LC-MS/MS method and in 65% by the iSYS immunoassay (Fig. 2A). For discordant results in 15 patients with the iSYS immunoassay, nonpathologic results were returned in 87% of patients by LC-MS/MS, but in only 25% of cases for the Liaison immunoassay (Fig. 2B), again confirming more prevalent discordance with the Liaison than the iSYS immunoassay. Among the 9 patients with negative results for the first LC-MS/MS and positive results for the second LC-MS/MS, there were 2 patients who had negative results for the second immunoassay. Details of those 9 patients and data for all 62 patients with discordant results are provided in Supplemental Table 3 (13).
Figure 2.
Plasma concentrations of aldosterone for samples taken at the end of the SIT in 62 patients with discordant positive results (>170 pmol/L) for immunoassays and negative results (<162 pmol/L) for the initial LC-MS/MS method (LC-MS/MS 1). Data are shown separately according to whether initial immunoassays were by Liaison (A) versus iSYS methods (B). For the 47 patients with discordant results by the Liaison immunoassay and the initial LC-MS/MS method (A), there were 40 patients in whom a second LC-MS/MS method confirmed nonpathologic results, including 30 in whom repeat iSYS immunoassays were also nonpathologic. For the 15 patients with discordant by the iSYS immunoassay and the initial LC-MS/MS method (B), there were 13 patients in whom a second LC-MS/MS method confirmed nonpathologic results, but only 3 showed nonpathologic results by repeat Liaison immunoassays.
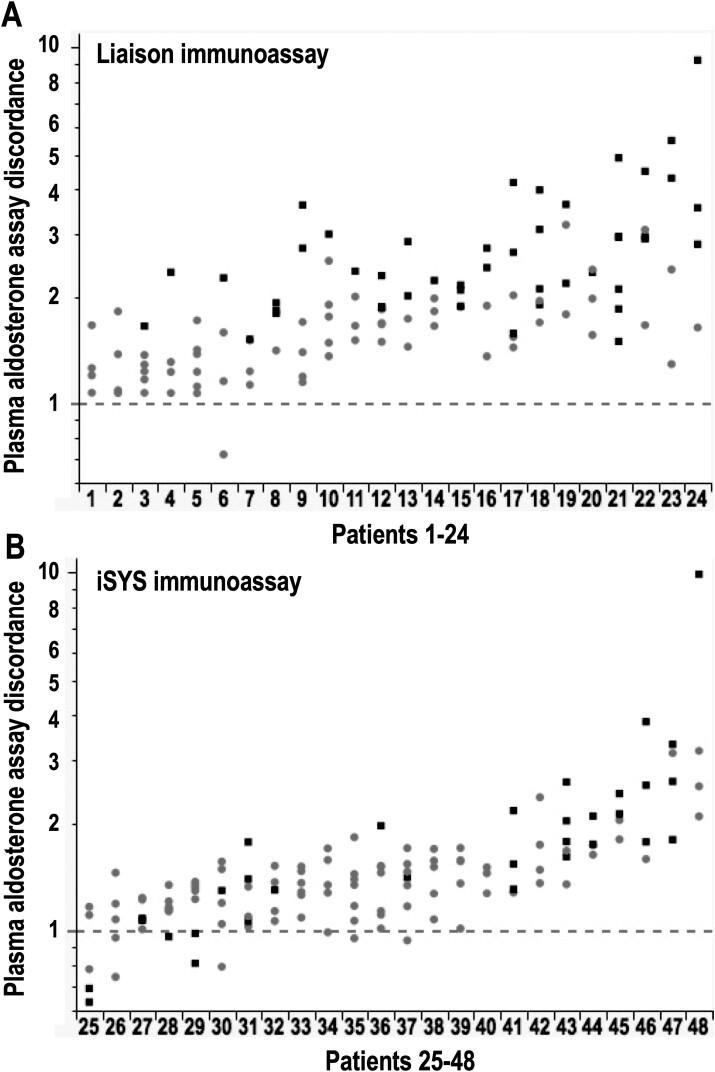
Patient-Specific Discordance
From comparisons of fold differences in immunoassay over LC-MS/MS-based measured plasma aldosterone in 48 patients in whom there were more than 4 separate peripheral plasma measurements available, it was apparent that discordance varied substantially between different patients (Fig. 3). With multivariable analyses that included LC-MS/MS measured concentrations, patient-specific discordance was established for both Liaison (P = 0.0018) and iSYS (P < 0.0001) immunoassays; discordance was inversely impacted by plasma concentrations of aldosterone for the Liaison (P < 0.0001) but not the iSYS immunoassay (P = 0.0931). Thus, for the Liaison immunoassay discordance was most severe for low concentrations of aldosterone, thereby accounting for the wider variance of within-patient differences for the Liaison than the iSYS immunoassay.
Figure 3.
Patient-specific discordance of immunoassay versus LC-MS/MS measurements of aldosterone according to 4 or more measurements of plasma aldosterone in each of 48 patients, including 24 in whom measurements included the Liaison immunoassay (A) and 24 for the iSYS immunoassay (B). Discordance in y-axes is represented by fold differences in immunoassay over LC-MS/MS measured concentrations of aldosterone. The 4 or more separate measurements for each patient are from peripheral blood samples taken at screening, before and during the SIT, and during AVS for some patients who progressed to that procedure. Different symbols for the differences are according to whether LC-MS/MS measured aldosterone concentrations were below (■) or above (●) 170 pmol/L and serve to illustrate contributions of lower plasma aldosterone concentrations to measured differences for the Liaison and not the iSYS immunoassay.
AVS Lateralization According to Discordant and Concordant SIT Results
Among the 162 patients with positive results of the SIT according to immunoassays of aldosterone, 102 had undergone AVS and 83 of these had selective sampling of both adrenal veins, among whom 19 had undergone adrenalectomy by the time of this report. Six patients with small solitary adrenal masses and nonselective sampling (n = 5) or young age and no AVS (n = 1) also underwent adrenalectomy, all with biochemical cure according to PASO criteria at 6 to 12 months after adrenalectomy. These latter 6 patients with confirmed unilateral PA were combined with the 83 who had selective sampling (Supplemental Figure 1 (13)). Among those 89 patients, 31 had discordant results for immunoassay versus LC-MS/MS-based results of the SIT (Table 1). Among the patients with concordant results 78% had evidence of lateralized aldosterone secretion supportive of unilateral disease, a larger (P < 0.0001) proportion than the 19% of patients with lateralized aldosterone secretion in the discordant group; those latter 6 patients had lower (P < 0.005) lateralization and contralateral suppression indexes compared to patients of the concordant group (Table 1). Among the 38 patients without lateralized aldosterone secretion, 66% had discordant results.
Table 1.
Demographics, baseline aldosterone and renin biochemistry, and AVS interpretations in 89 patients with and without discordant results for saline infusion tests who underwent AVS with selective sampling or who were adrenalectomized and showed biochemical cure without selective AVS a
| Concordant | Discordant | P value | ||
|---|---|---|---|---|
| Demographics | ||||
| N | 58 | 31 | ||
| Female/ Male | 23/ 35 | 18/ 13 | 0.1204 | |
| Age (y) | 53 (43-57) | 50 (42-57) | 0.5669 | |
| Baseline values for aldosterone, renin, and ARR | ||||
| IA method—Liaison/ iSYS | 30/28 | 22/9 | 0.1139 | |
| IA Aldosterone (pmol/L) | 768 (452-1241) | 408 (338-547) | <0.0001 | |
| MS Aldosterone | 474 (287-963) | 219 (175-316) | <0.0001 | |
| Renin (ng/L)b | 2.1 (1.1-4.7) | 1.7 (1.1-3.9) | 0.7076 | |
| ARR IA Aldosterone (pmol/ng) | 329 (168-560) | 210 (97-325) | 0.0155 | |
| ARR MS Aldosterone (pmol/ng) | 214 (118-373) | 100 (62-183) | 0.0003 | |
| AVS resultsa | ||||
| Lateralized/nonlateralizeda | 45/ 13 | 6/ 25 | <0.0001 | |
| Lateralized—right/left | 17/ 28 | 5/ 1 | 0.0729 | |
| Unilateral lateralization index | 21.8 (7.2-42.9) | 6.0 (4.1-7.0) | 0.0033 | |
| Unilateral contralateral suppression index | 0.33 (0.15-0.77) | 2.6 (0.9-5.4) | 0.0031 |
Abbreviations: ARR, aldosterone renin ratio; AVS, adrenal venous sampling; IA, immunoassay; MS, mass spectrometry.
a Results for AVS with selective sampling were available from 83 patients, while adrenalectomies were performed without lateralization data in the other 6 patients, all resulting in biochemical cure at follow-up. Those 6 patients were considered to have unilateral disease and thus were included as lateralized.
b Values of renin reported in mU/L were converted to ng/L. Continuous data are reported as medians and interquartiles.
Histopathology
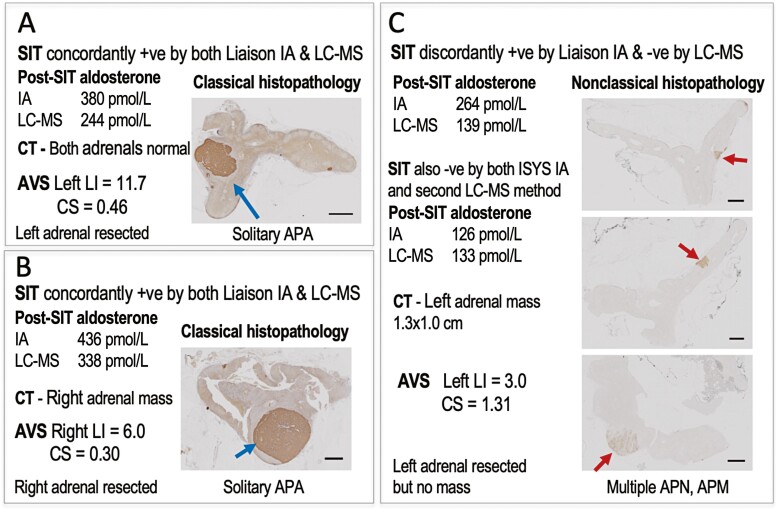
Among the 6 patients in the aforementioned discordant group with evidence of lateralized aldosterone secretion, there was 1 patient in whom computed tomography suggested the presence of a 1.3 × 1.0 cm adenoma in the same adrenal that showed evidence of lateralized aldosterone secretion. That adrenal was resected before results of other measurements of aldosterone for the SIT became available. No adenoma was found. With CYP11B2 immunostaining the results indicated nonclassical histopathology (Fig. 4). As detailed in the supplement (13), follow-up according to PASO criteria and using immunoassay measurements of aldosterone showed positive test results for the SIT.
Figure 4.
Histopathological analyses of resected adrenals in 3 patients, 2 of whom had concordant positive results of initial saline infusions tests (A & B) and the third patient with positive results for the Liaison immunoassay and negative results for the initial LC-MS/MS methods (C). Data in panels include results of saline infusion tests (SIT), computed tomography (CT) imaging of the adrenals, and adrenal venous sampling (AVS) studies. Abbreviations: APA, aldosterone-producing adenoma; APN, APM, aldosterone-producing nodules or micronodules; CS, contralateral suppression index; IA, immunoassay; LC-MS, liquid chromatography with mass spectrometry; LI, lateralization index.
Immunoassay Interference
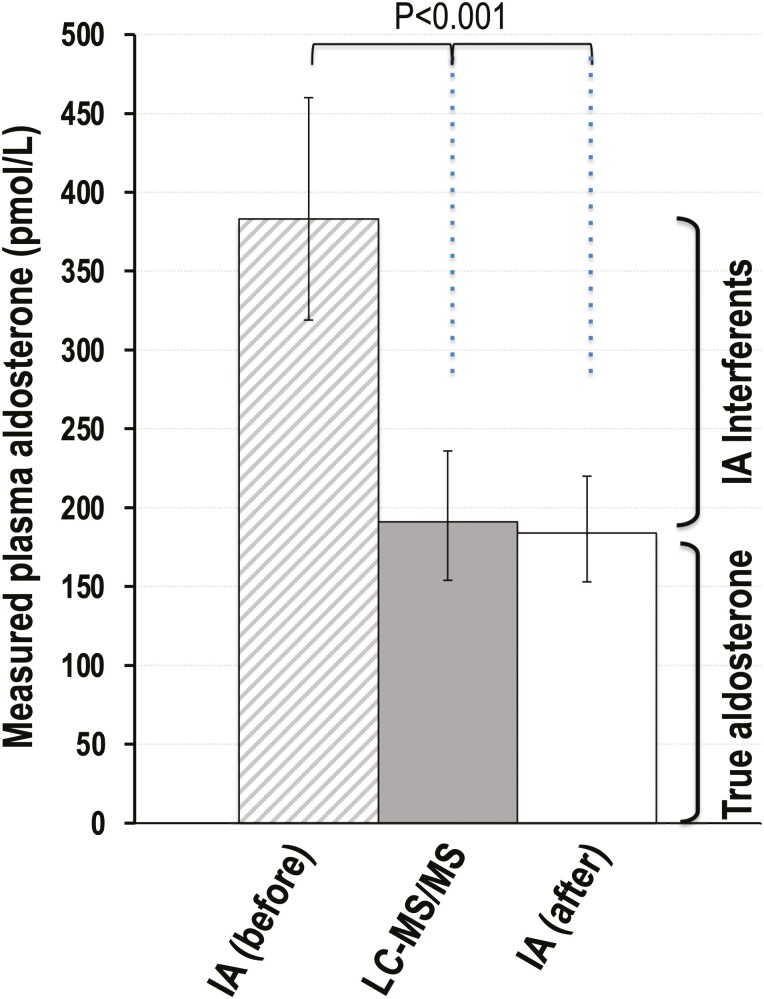
For the 7 patients in whom repeat immunoassays were performed after processing plasma to remove macromolecular interferents, all cases of discordant results for the SIT became concordantly nonpathologic after removal of interferents (Supplemental Table 4 (13)). Plasma concentrations of aldosterone measured by immunoassays after processing were similar to those by LC-MS/MS, but less (P < 0.001) than half the concentrations measured before removal of interferents (Fig. 5).
Figure 5.
Bar graph display of measured plasma concentrations (geometric means with confidence intervals) of aldosterone by LC-MS/MS and immunoassays before (IA before) and after (IA after) sample processing to remove interferents. As described in the supplement (13), plasma concentrations of aldosterone after removal of interferents were below the limits of quantification (LOQ) of immunoassays for most samples. Thus, data for this display are restricted to samples with measurable concentrations (ie, >LOQ). The full dataset is available in Supplemental Table 4 (13).
ROC Curve-Based Reassessments of Cutoffs for the SIT
As outlined in Supplemental Figures 3 and 4 and the associated text (13), ROC curve–derived optimal cutoffs for the SIT varied from 144 to 180 pmol/L (52-65 ng/L) for LC-MS/MS measurements and from 243 to 313 pmol/L (88-113 ng/L) for immunoassay measurements.
Discussion
This study demonstrates the presence of considerable inaccuracies in measurements of plasma aldosterone by immunoassays of 2 manufacturers that can potentially contribute to significant overdiagnosis of PA. From examination of patients with discordant SIT results for immunoassay versus LC-MS/MS measurements it can be estimated that over 60% of patients with nonlateralized aldosterone secretion may be incorrectly diagnosed with bilateral PA.
There were also some discordant results for the SIT between the 2 LC-MS/MS methods, although in 2 of those 9 cases, the second immunoassay was concordant with the first LC-MS/MS method. These discordances were largely limited to post-SIT concentrations closely surrounding cutoffs, and as clarified in the supplement can be largely explained by assay imprecision at those concentrations (13). In contrast, assay imprecision cannot explain the more substantial and extensive nature of the discordances between immunoassays and LC-MS/MS methods.
Although it is well established that immunoassays yield overall higher measurements of plasma aldosterone than LC-MS/MS, a result particularly prominent at lower concentrations (18-21), it has not been established until now whether this might broadly impact diagnostic accuracy in patients with suspected PA. Interferences from macromolecules leading to falsely elevated plasma aldosterone by both the iSYS and Liaison immunoassays have been established by us for 1 previous patient (12), but it was not clear at that time whether this was an isolated case. That particular patient not only had highly false-positive results by immunoassay for the SIT, but also evidence of lateralized aldosterone secretion; the patient was spared unnecessary surgery only after results of LC-MS/MS revealed that all tests by immunoassay measurements had yielded erroneous findings.
Among the 31 patients of the present series who underwent AVS despite discordant results of the SIT, 6 were found to show evidence of lateralized aldosterone secretion; however, all 6 had relatively low lateralization ratios and/or no contralateral suppression. These cases, along with our previously published index case (12) and other cases of discordant AVS results (22), illustrate the possibility that immunoassay inaccuracy might not only lead to an incorrect diagnosis of PA, but also unnecessary surgical intervention in occasional patients with lateralized aldosterone secretion. Indeed, 1 of those 6 patients did undergo adrenalectomy and while that patient showed no adenoma, CYP11B immunostaining demonstrated presence of multiple aldosterone-producing micronodules (APMs) consistent with nonclassical histopathological features (16). Findings of CYP11B-positive APMs (previously referred to as aldosterone-producing cell clusters) have been described as a common feature in autopsy series without known PA (23, 24). Although there are significant differences in both numbers and size of APMs between normal and diseased adrenals, there is also considerable overlap so that these features cannot be used alone to definitively diagnose PA (25).
Our findings that the extent and prevalence of discordance for SIT results were both higher in magnitude with the Liaison than the iSYS immunoassay are in line with other observations of extensive variation in measurements by different immunoassays (21, 26). For the Liaison immunoassay, Rehan et al (4) documented measurements by one LC-MS/MS method that while less than half those of the immunoassay were near identical with another LC-MS/MS method. More recently Thuzar et al (10) reexamined cutoffs for seated SIT plasma aldosterone measured using the Liaison immunoassay. They established that while a cutoff of 171 pmol/L could be used to reliably exclude PA, this resulted in too many false-positives. Since confirmatory tests should provide high positive predictive value, they proposed a cutoff of 217 pmol/L for the immunoassay. However, even at that cutoff, specificity remained suboptimal at 86.7% for a sensitivity of 86.2%. In our series, ROC curve–derived cutoffs for immunoassays of 243 and 313 pmol/L provided similar sensitivities but higher specificities than indicated by Thuzar et al (10).
As emphasized previously (4, 5, 10), highly variable and poorly harmonized analytical methods mandate use of method-specific reference intervals, which are rarely established by manufacturers of kit methods. Cutoffs indicated by others for immunoassay measurements of aldosterone for the SIT vary considerably from 140 to 444 pmol/L (27-30), likely reflecting not only different analytical methods but also preanalytical factors such as supine versus seated sampling, differing emphasis on diagnostic specificity versus sensitivity and lastly less than ideal methods for disease classification. Although mass spectrometric methods are not immune from analytical interferences (31), they do provide higher analytical specificity than immunoassays and thereby offer opportunities for improved harmonization of reference intervals. In the present study the optimal cutoffs for LC-MS/MS measurements of aldosterone during the SIT were between 144 to 180 pmol/L, precisely bridging that of 162 pmol/L indicated for LC-MS/MS by Stowasser et al (9).
While LC-MS/MS methods offer a solution to improve both accuracy of analytical test results and diagnostic performance, these methods are not available to many clinicians. For those who continue to use immunoassays, it must be appreciated that improved diagnostic performance is not simply a matter of optimized method-specific reference intervals. As shown here, inaccuracy of immunoassays varies considerably according to variable patient-specific interferences that likely involve circulating macromolecules; these can include heterophile antibodies that impact reagent binding (32, 33). Impact appears larger at lower plasma concentrations of analytes such as for aldosterone during the SIT. Both clinicians and laboratory scientists should be aware of these sources of interferences so that when they are suspected, measures can be taken to investigate and avoid unnecessary invasive procedures (ie, AVS) or even surgical interventions. Given the high prevalence of immunoassay inaccuracy, all positive results should ideally be followed up by LC-MS/MS; if that is not possible, one simple solution indicated in this report is to process plasma to remove interferents before immunoassay. With such solutions available it may also be possible achieve improved harmonization of diagnostic cutoffs, and ultimately more satisfactory outcomes for patients.
This study raises questions concerning findings of previous publications—including our own—that depended on immunoassay-based measurements and interpretations of plasma aldosterone. For example, our previously reported machine learning algorithms for diagnosis of PA (14) are likely compromised by incorrect classifications of disease. Also, most studies suggest that bilateral forms outnumber unilateral forms of PA, including some indicating a more than 2-fold higher prevalence (34); however, for patients in the present series with concordantly positive results of the SIT there was a more than 3-fold higher prevalence of lateralized than nonlateralized aldosterone secretion (ie, presumed bilateral PA).
As covered in the supplement (13), there are some limitations of the present study, the most important being that there is no sound independent method other than the biochemical tests themselves to exclude PA; even for follow-up confirmation of disease in a patient who has undergone adrenalectomy, this requires repeat biochemical tests according to the same procedures required during screening and confirmation testing (11). Normalization of biochemical test results provides a valid means to confirm unilateral disease. However, confirmation of bilateral disease or disease exclusion even in patients with presumed PA remains problematic. Usually in the end this again depends on confirmatory tests. Captopril challenge and fludrocortisone suppression tests have been used by others as alternative means of confirmation (9, 10), but these tests still require measurements of plasma aldosterone and potentially suffer the same measurement inaccuracy limitation as for the SIT. Thus, for the present analysis all that was possible was for exhaustive repeated measurements by alternative analytical methods to the initial LC-MS/MS and immunoassay methods used in confirmatory testing.
Measurements of urinary aldosterone or its metabolites may provide an alternative to plasma measurements (35), but as yet do not provide an established “gold standard” for diagnosis. Nevertheless, measurements in urine would not be expected to be impacted by the same interferences as in plasma. Without alternative “gold standard” methods for diagnosis it is not possible to conclusively confirm bilateral disease or even exclude PA in other patients; the former in particular remains a presumed diagnosis simply based on AVS evidence of nonlateralized aldosterone secretion. This is far from ideal. Nevertheless, even with the aforementioned caveats in mind, our study has identified patient-specific inaccuracies in routine testing procedures for PA that differ according to the particular immunoassay. Without employment of the suggested solutions outlined in this report, these inaccuracies can potentially lead to overdiagnosis of the disease with potentially less than optimal patient management that in some cases may even involve unnecessary harm.
Acknowledgments
Thanks are extended to Katharina Langton, Carola Kunath, Jimmy Rusdian Masjkur, and Ramona Walter-Ruckstetter for support with patients, to Thomas Baumgartner for coordination of Zurich patient specimens and data handling, and to Martin Fassnacht for helpful review of the manuscript.
Glossary
Abbreviations
- APM
aldosterone-producing micronodule
- ARR
aldosterone to renin ratio
- AVS
adrenal vein sampling
- LC-MS/MS
liquid chromatography–tandem mass spectrometry
- PA
primary aldosteronism
- PROSALDO
PROspective study on the diagnostic value of Steroid profiling in primary ALDOsteronism
- ROC
receiver-operating characteristic
- SIT
saline infusion test
Financial Support
This study was supported by the Deutsche Forschungsgemeinschaft (314061271-TRR/CRC 205-1/2 to G.E., M.K., M.P., F.B., T.A.W., M.R., and M.B.), by the Else Kröner-Fresenius Stiftung (2019_A104; Else-Kröner Hyperaldosteronismus—German Conn Registry) to M.R. and by the European Research Council under the European Union’s Horizon 2020 research and innovation program (grant agreement No 694913) to M.R.
Disclosure
The authors have nothing to disclose.
Data Availability
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(5):1889-9166. [DOI] [PubMed] [Google Scholar]
- 2. Mulatero P, Monticone S, Deinum J, et al. Genetics, prevalence, screening and confirmation of primary aldosteronism: a position statement and consensus of the working group on endocrine hypertension of the European society of hypertension. J Hypertens. 2020;38(10):1919-1928. [DOI] [PubMed] [Google Scholar]
- 3. Stowasser M, Taylor PJ, Pimenta E, Ahmed AH, Gordon RD. Laboratory investigation of primary aldosteronism. Clin Biochem Rev. 2010;31(2):39-56. [PMC free article] [PubMed] [Google Scholar]
- 4. Rehan M, Raizman JE, Cavalier E, Don-Wauchope AC, Holmes DT. Laboratory challenges in primary aldosteronism screening and diagnosis. Clin Biochem. 2015;48(6):377-387. [DOI] [PubMed] [Google Scholar]
- 5. O’Shea PM, Griffin TP, Denieffe S, Fitzgibbon MC. The aldosterone to renin ratio in the diagnosis of primary aldosteronism: promises and challenges. Int J Clin Pract. 2019;73(7):e13353. [DOI] [PubMed] [Google Scholar]
- 6. Hung A, Ahmed S, Gupta A, et al. Performance of the aldosterone-to-renin ratio as a screening test for primary aldosteronism: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2021;106(8):2423-2435. [DOI] [PubMed] [Google Scholar]
- 7. Bernini G, Moretti A, Orlandini C, et al. Plasma and urine aldosterone to plasma renin activity ratio in the diagnosis of primary aldosteronism. J Hypertens. 2008;26(5):981-988. [DOI] [PubMed] [Google Scholar]
- 8. Vorselaars W, Valk GD, Vriens MR, Westerink J, Spiering W. Case detection in primary aldosteronism: high-diagnostic value of the aldosterone-to-renin ratio when performed under standardized conditions. J Hypertens. 2018;36(7):1585-1591. [DOI] [PubMed] [Google Scholar]
- 9. Stowasser M, Ahmed AH, Cowley D, et al. Comparison of seated with recumbent saline suppression testing for the diagnosis of primary aldosteronism. J Clin Endocrinol Metab. 2018;103(11):4113-4124. [DOI] [PubMed] [Google Scholar]
- 10. Thuzar M, Young K, Ahmed AH, et al. Diagnosis of primary aldosteronism by seated saline suppression test-variability between immunoassay and HPLC-MS/MS. J Clin Endocrinol Metab. 2020;105(3):e477-e483. [DOI] [PubMed] [Google Scholar]
- 11. Williams TA, Lenders JWM, Mulatero P, et al. Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol. 2017;5(9):689-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Constantinescu G, Bidlingmaier M, Gruber M, et al. Mass spectrometry reveals misdiagnosis of primary aldosteronism with scheduling for adrenalectomy due to immunoassay interference. Clin Chim Acta. 2020;507:98-103. [DOI] [PubMed] [Google Scholar]
- 13. Eisenhofer G, Kurlbaum M, Peitzsch M, et al. The saline infusion test for primary aldosteronism: implications of immunoassay inaccuracy [supplemental file]. Zenodo Digital Repository. Posted online November 12, 2021. 10.5281/zenodo.5776348 [DOI] [PMC free article] [PubMed]
- 14. Eisenhofer G, Duran C, Cannistraci CV, et al. Use of steroid profiling combined with machine learning for identification and subtype classification in primary aldosteronism. JAMA Netw Open. 2020;3(9):e2016209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Peitzsch M, Dekkers T, Haase M, et al. An LC-MS/MS method for steroid profiling during adrenal venous sampling for investigation of primary aldosteronism. J Steroid Biochem Mol Biol. 2015;145:75-84. [DOI] [PubMed] [Google Scholar]
- 16. Williams TA, Gomez-Sanchez CE, Rainey WE, et al. International histopathology consensus for unilateral primary aldosteronism. J Clin Endocrinol Metab. 2021;106(1):42-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Meyer LS, Handgriff L, Lim JS, et al. Single-center prospective cohort study on the histopathology, genotype, and postsurgical outcomes of patients with primary aldosteronism. Hypertension. 2021;78(3):738-746. [DOI] [PubMed] [Google Scholar]
- 18. Turpeinen U, Hamalainen E, Stenman UH. Determination of aldosterone in serum by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;862(1-2):113-118. [DOI] [PubMed] [Google Scholar]
- 19. Hinchliffe E, Carter S, Owen LJ, Keevil BG. Quantitation of aldosterone in human plasma by ultra high performance liquid chromatography tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;913-914:19-23. [DOI] [PubMed] [Google Scholar]
- 20. Ray JA, Kushnir MM, Palmer J, Sadjadi S, Rockwood AL, Meikle AW. Enhancement of specificity of aldosterone measurement in human serum and plasma using 2D-LC-MS/MS and comparison with commercial immunoassays. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;970:102-107. [DOI] [PubMed] [Google Scholar]
- 21. Baron S, Amar L, Faucon AL, et al. Criteria for diagnosing primary aldosteronism on the basis of liquid chromatography-tandem mass spectrometry determinations of plasma aldosterone concentration. J Hypertens. 2018;36(7):1592-1601. [DOI] [PubMed] [Google Scholar]
- 22. El Ghorayeb N, Mazzuco TL, Bourdeau I, et al. Basal and post-ACTH aldosterone and its ratios are useful during adrenal vein sampling in primary aldosteronism. J Clin Endocrinol Metab. 2016;101(4):1826-1835. [DOI] [PubMed] [Google Scholar]
- 23. Nishimoto K, Tomlins SA, Kuick R, et al. Aldosterone-stimulating somatic gene mutations are common in normal adrenal glands. Proc Natl Acad Sci USA. 2015;112(33):E4591-E4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Omata K, Anand SK, Hovelson DH, et al. Aldosterone-producing cell clusters frequently harbor somatic mutations and accumulate with age in normal adrenals. J Endocr Soc. 2017;1(7):787-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Omata K, Satoh F, Morimoto R, et al. Cellular and genetic causes of idiopathic hyperaldosteronism. Hypertension. 2018;72(4):874-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schirpenbach C, Seiler L, Maser-Gluth C, Beuschlein F, Reincke M, Bidlingmaier M. Automated chemiluminescence-immunoassay for aldosterone during dynamic testing: comparison to radioimmunoassays with and without extraction steps. Clin Chem. 2006;52(9):1749-1755. [DOI] [PubMed] [Google Scholar]
- 27. Rossi GP, Belfiore A, Bernini G, et al. Prospective evaluation of the saline infusion test for excluding primary aldosteronism due to aldosterone-producing adenoma. J Hypertens. 2007;25(7):1433-1442. [DOI] [PubMed] [Google Scholar]
- 28. Monticone S, Burrello J, Tizzani D, et al. Prevalence and clinical manifestations of primary aldosteronism encountered in primary care practice. J Am Coll Cardiol. 2017;69(14):1811-1820. [DOI] [PubMed] [Google Scholar]
- 29. Wu CH, Wu V, Yang YW, et al. Plasma aldosterone after seated saline infusion test outperforms captopril test at predicting clinical outcomes after adrenalectomy for primary aldosteronism. Am J Hypertens. 2019;32(11):1066-1074. [DOI] [PubMed] [Google Scholar]
- 30. Fuss CT, Brohm K, Kurlbaum M, et al. Confirmatory testing of primary aldosteronism with saline infusion test and LC-MS/MS. Eur J Endocrinol. 2021;184(1):167-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vogeser M, Seger C. Pitfalls associated with the use of liquid chromatography-tandem mass spectrometry in the clinical laboratory. Clin Chem. 2010;56(8):1234-1244. [DOI] [PubMed] [Google Scholar]
- 32. Tate J, Ward G. Interferences in immunoassay. Clin Biochem Rev. 2004;25(2):105-120. [PMC free article] [PubMed] [Google Scholar]
- 33. Sturgeon CM, Viljoen A. Analytical error and interference in immunoassay: minimizing risk. Ann Clin Biochem. 2011;48(Pt 5):418-432. [DOI] [PubMed] [Google Scholar]
- 34. Fukumoto T, Umakoshi H, Ogata M, et al. Significance of discordant results between confirmatory tests in diagnosis of primary aldosteronism. J Clin Endocrinol Metab. 2021;106(2):e866-e874. [DOI] [PubMed] [Google Scholar]
- 35. Brown JM, Siddiqui M, Calhoun DA, et al. The unrecognized prevalence of primary aldosteronism. Ann Intern Med. 2020;73(1):10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.