Abstract
Background
Schistosomiasis is a major global health problem caused by blood-dwelling parasitic worms, which is currently tackled primarily by mass administration of the drug praziquantel. Appropriate drug treatment strategies are informed by diagnostics that establish the prevalence and intensity of infection, which, in regions of low transmission, should be highly sensitive.
Methods
To identify sensitive new serological markers of Schistosoma mansoni infections, we have compiled a recombinant protein library of parasite cell-surface and secreted proteins expressed in mammalian cells.
Results
Together with a time series of sera samples from volunteers experimentally infected with a defined number of male parasites, we probed this protein library to identify several markers that can detect primary infections with as low as 10 parasites and as early as 5 weeks postinfection.
Conclusions
These new markers could be further explored as valuable tools to detect ongoing and previous S mansoni infections, including in endemic regions where transmission is low.
Keywords: antibodies, Schistosoma mansoni, schistosomiasis, serology
A library of recombinant S mansoni cell-surface and secreted proteins was produced in mammalian cells for functional and epidemiological studies. Using human and mouse sera from experimentally controlled infections, we identified molecular markers of infection as early as 5 weeks.
Schistosomiasis is a neglected tropical disease affecting more than 200 million people in 52 countries and is one of the world’s major health problems causing 200 000 deaths per year. In 2015, the impact of the disease was estimated at 3.5 million disability-adjusted life years, putting a huge socioeconomic burden on many low- and middle-income countries [1]. In humans, schistosomiasis is caused by 5 species of platyhelminth parasites belonging to the genus Schistosoma. Their geographical distribution is restricted by the presence of species-specific freshwater snails that act as intermediate hosts, with Schistosoma mansoni having the most widespread distribution, encompassing both Africa and South America [2]. Infected snails shed cercariae, a free-swimming larva, which penetrates human skin to initiate infection. Parasite maturation within its host takes several weeks: cercarial heads first remodel their surface to form schistosomula [3], which migrate through the dermis for several days before entering the host bloodstream. Eventually, young adult male and female S mansoni worms pair up in the liver before moving to the mesenteric blood vessels, where each pair can release over 300 eggs per day from 5 weeks postinfection [4]. The symptoms of schistosomiasis are caused by progressive accumulation of eggs within host tissues, eliciting host-derived immune responses that can eventually lead to liver fibrosis, portal hypertension, and, if left untreated, death [5]. In the absence of a licenced vaccine, treatment relies on the use of a single drug, praziquantel.
The exact prevalence of schistosomiasis worldwide may be underestimated due to limitations of routine methods of detection [6]. Diagnosis of S mansoni infections and subsequent decisions on mass drug administration mainly rely on observing parasite eggs in patients’ stools with the Kato-Katz test. Although this method can detect current infections, it is not sensitive enough to diagnose low levels of infection present in low-endemicity areas or in recently treated populations [7]. In these instances, detection of parasite antigens such as the circulating cathodic antigen or circulating anodic antigen (CAA) in patients’ sera or urine by sensitive field-applicable or laboratory-based assays is highly useful [8–10]. In areas where elimination has been achieved, the detection of antiparasite serum antibodies could support epidemiological surveillance that informs of any risk of resurgence. Host antibody responses to S mansoni parasites is often measured against whole parasite extracts such as soluble egg antigens (SEA) or soluble worm antigen preparation (SWAP), which are not molecularly defined and can lead to cross-reactivity with other helminth species. Although a diagnostic test based on a recombinant protein could mitigate these problems, only very few have been used to test patient antibody responses to Schistosoma infections [11]. The development of new, more sensitive diagnostic tools would therefore help to improve the detection and early treatment of schistosomiasis.
Extracellular antigens released by or displayed at the surface of the parasite at the initial stage of infection [3] can be valuable early immunodiagnostic markers because they are directly exposed to the host humoral system. The identification of such antigens has been aided by the sequencing and annotation of the S mansoni genome [12], and several proteomics [13–20], transcriptomics, and in silico analyses [21–23] have identified genes expressed by the schistosomula and adult worm. Despite their value, extracellular proteins pose challenges for recombinant expression because they contain structurally important posttranslational modifications such as glycosylation and especially disulphide bonds required to produce informative antibody epitopes. To address this, we have previously developed protein expression approaches in mammalian cells to compile large panels of parasite recombinant ectodomains that retain their binding activity and immunogenicity [24], enabling the identification of host-parasite receptor-ligand interactions and humoral markers of protection against malaria [25].
To identify new markers for S mansoni infections, we created a panel of 115 recombinant proteins representing secreted and membrane-tethered S mansoni proteins mostly enriched at the schistosomula stage. Using human and mouse sera from experimentally controlled S mansoni infections, we determined the kinetics of humoral responses to this panel of antigens in the context of primary infections and identified several early serological markers.
MATERIAL AND METHODS
Identification of Schistosoma mansoni Cell-Surface and Secreted Proteins
Genes encoding cell-surface and secreted proteins from cercarial and adult S mansoni were identified using published proteomic and transcriptional data [13–16, 19–21]. To further enrich for genes transcribed at the schistosomula stage, we identified 1302 transcripts upregulated at 48 versus 3 hours posttransformation [26] using EdgeR [27] as well as the 1000 most abundant transcripts at 48 hours as assessed by reads per kilobase mapped, resulting in 1977 unique genes, 274 of which encoded secreted and cell-surface proteins identified using signal peptide and transmembrane domain prediction software [28, 29]. All RNA-seq data are available in the European Nucleotide Archive (ENA) under study PRJEB3190 and run accession numbers ERR411525, ERR411535, and ERR411541 for the 3-hour time point and ERR411522, ERR411527, and ERR411538 for the 48-hour time point. Ninety-four mitochondrial, endoplasmic reticulum, or multipass proteins, which are difficult to express as a contiguous ectodomain, were subsequently excluded, leaving a short list of 180 proteins. Gene structures were manually refined by mapping transcriptome data to the genome sequence, and genes spanning gaps in the genome sequence or with ambiguity in structure were removed, resulting in a final list of 115 candidates numbered in Table 1.
Table 1.
Details of 115 Cell-Surface and Secreted Proteins From Schistosoma mansonia
| Number | Accession No. | Name | Boundaries | MM | Domain/Protein Similarity | Level | Ref. |
|---|---|---|---|---|---|---|---|
| Cell Surface | |||||||
| 1 | Smp_195190 | Sm13 | E18-T80 | 37 | High | [17] | |
| 2 | Smp_081920 | SmLy6I (Cd59.5) | L26-T104 | 45 | Medium | [17] | |
| 3 | Smp_166340 | SmLy6F (Cd59.4) | L26-S98 | 47 | Medium | [17] | |
| 4 | Smp_017730 | Sm200 | D20-S1662 | 257 | Medium | [14] | |
| 5 | Smp_127820 | V18-S760 | 132 | Medium | [14] | ||
| 6 | Smp_194920 | D17-T592 | 98 | T-cell immunomodulatory protein | Low | [14] | |
| 7 | Smp_011680 | L30-P348 | 87 | Cd36-like class B scavenger receptor | Low | ||
| 8 | Smp_054070 | D31-S210 | 71 | TM2 domain-containing protein 3 | High | ||
| 9 | Smp_073400 | R25-T214 | 78 | LAMP-like protein | Medium | ||
| 10 | Smp_105220 | SmLyB (Cd59.2) | I20-P99 | 42 | High | [15] | |
| 11 | Smp_019350 | SmLy6A (Cd59.1) | H28-T102 | 38 | High | [15] | |
| 12 | Smp_021220 | E24-S119 | 53 | High | |||
| 13 | Smp_031880 | R18-S240 | 58 | Ig domain-containing protein, basigin-related | High | ||
| 14 | Smp_009830 | D20-S149 | 51 | Translocon-associated protein subunit beta | Low | ||
| 15 | Smp_032520 | Y19-R200 | 74 | LAMP-like protein | High | ||
| 16 | Smp_074000 | I19-I232 | 56 | Low | |||
| 17 | Smp_102480 | N29-D66 | 37 | High | |||
| 18 | Smp_124000 | MEG-14 | T27-D110 | 38 | High | ||
| 19 | Smp_156270 | S23-P144 | 47 | Post-GPI attachment to protein factor | Low | ||
| 20 | Smp_174580 | S17-T275 | 59 | Vesicular integral membrane protein | High | ||
| 21 | Smp_176020 | MEG-11 | D23-P55 | 34 | High | ||
| 22 | Smp_048380 | L20-S283 | 73 | Medium | |||
| 23 | Smp_060570 | Y21-S428 | 85 | Low | |||
| 24 | Smp_075280 | V19-T222 | 77 | LAMP-like protein | High | ||
| 25 | Smp_129840 | G25-S1161 | 206 | Medium | |||
| 26 | Smp_133270 | E34-T759 | 120 | Sel1-like protein | - | ||
| 27 | Smp_145420 | H30-I1733 | 282 | Plexin A3 | - | ||
| 28 | Smp_149740 | F26-T560 | 130 | Alzheimer disease beta-amyloid related | Medium | ||
| 29 | Smp_155810 | L29-T1100 | 184 | Protocadherin 11 | Medium | ||
| 30 | Smp_162520 | N20-P1070 | 162 | Protocadherin fat4 | Low | ||
| 31 | Smp_164760 | S22-P1160 | 184 | IgSF | Low | ||
| 32 | Smp_166300 | N27-I356 | 94 | High | |||
| 33 | Smp_168240 | Q25-T263 | 68 | High | |||
| 34 | Smp_171460 | L19-S841 | 161 | IgSF | Medium | ||
| 35 | Smp_176540 | V31-T956 | 146 | Protocadherin 18 | Medium | ||
| 36 | Smp_157070 | I19-P397 | 83 | EGF-domain-containing protein | Low | ||
| 37 | Smp_165440 | Q25-P363 | 74 | Netrin receptor unc5 | Low | ||
| 38 | Smp_136690 | N26-T674 | 118 | Acetylcholinesterase | Medium | [17] | |
| 39 | Smp_061970 | F21-P518 | 95 | GPI ethanolamine phosphate transferase 2 | - | ||
| 40 | Smp_153390 | SmNNP5 | S19-S428 | 95 | Medium | [14] | |
| 41 | Smp_072190 | SmLy6D (Sm29) | V27-T168 | 57 | High | [14] | |
| 42 | Smp_064430 | A22-S155 | 53 | Medium | [19] | ||
| Secreted Adhesion/Growth Factor/Metabolite Binding | |||||||
| 43 | Smp_194840 | E18-I146 | 47 | NPC-like cholesterol-binding protein | High | [50] | |
| 44 | Smp_194910 | N22-I180 | 51 | Saposin B domain-containing protein | Medium | [20] | |
| 45 | Smp_063530 | E26-R193 | 49 | Apoferritin | Medium | [50] | |
| 46 | Smp_141680 | I33-A659 | 110 | Fasciclin domain-containing protein | High | [14] | |
| 47 | Smp_043650 | Q23-L81 | 37 | Prohormone npp-28 | Low | ||
| 48 | Smp_170550 | N27-Y928 | 164 | Granulin | Medium | ||
| 49 | Smp_035040 | N24-L241 | 63 | IgSF | Low | ||
| 50 | Smp_052660 | I22-R488 | 97 | Matabotropic glutamate receptor | - | ||
| 51 | Smp_128590 | K21-S260 | 69 | Laminin gamma3 | Low | ||
| 52 | Smp_135210 | I21-Q1584 | 220 | EGF-domain-containing protein | Low | ||
| 53 | Smp_136320 | H21-K154 | 48 | GSK3beta-interacting protein | Low | ||
| 54 | Smp_144130 | L26-V553 | 118 | Septate junction protein | Low | ||
| 55 | Smp_154760 | Q30-M2155 | 298 | EGF-domain-containing protein | Low | ||
| 56 | Smp_171780 | Q19-K260 | 61 | SPARC | High | ||
| 57 | Smp_178740 | Q31-V533 | 97 | Tesmin-related protein | Low | ||
| 58 | Smp_180600 | S22-S292 | 72 | IGF-binding protein | High | ||
| 59 | Smp_181220 | N18-I173 | 50 | C1q-binding protein | Medium | ||
| 60 | Smp_211020 | L32-Y703 | 122 | Discoidin domain-containing protein | - | ||
| 61 | Smp_016490 | E21-Q194 | 53 | SaposinB domain-containing protein | Medium | ||
| 62 | Smp_130100 | F20-I128 | 45 | Saposin domain-containing protein | Low | [20] | |
| 63 | Smp_105420 | I19-S196 | 53 | Saposin domain-containing protein | High | ||
| 64 | Smp_105450 | Y19-C126 | 58 | Saposin domain-containing protein | Medium | [50] | |
| 65 | Smp_202610 | V19-T135 | 46 | Saposin domain-containing protein | Medium | ||
| Secreted Proteases | |||||||
| 66 | Smp_090100 | E24-Q582 | 98 | Invadolysin | Low | [16] | |
| 67 | Smp_067060 | H18-N340 | 73 | Cathepsin B1, isotype2 | High | [50] | |
| 68 | Smp_103610 | SmCB1 (Sm31) | H18-N340 | 73 | Cathepsin B1, isotype1 | Low | [50] |
| 69 | Smp_019030 | D21-L455 | 91 | Cathepsin C/Dipeptylpeptidase 1 | Low | [50] | |
| 70 | Smp_002600 | L17-G498 | 111 | Lysosomal Pro X carboxypeptidase | Medium | [50] | |
| 71 | Smp_071610 | I24-L472 | 99 | Dipeptidyl-peptidase II | Medium | [50] | |
| 72 | Smp_089670 | S19-N2127 | 409 | Alpha-2 macroglobulin | Medium | [15] | |
| 73 | Smp_112090 | SmCE2a.3 | F19-I263 | 56 | Cercarial elastase 2a | - | [16] |
| 74 | Smp_119130 | SmCE1a.2 | W25-I263 | 55 | Cercarial elastase 1a | - | [16] |
| 75 | Smp_002150 | SmSP2 | S25-F501 | 97 | Trypsin-like serine protease | Low | |
| 76 | Smp_141610 | SmCB2 | N23-N347 | 72 | Cathepsin B | Medium | |
| 77 | Smp_147730 | SmKI-1 | Y21-K146 | 47 | Kunitz-type protease inhibitor | Medium | |
| 78 | Smp_034420 | Sm12.8 | N20-S148 | 56 | Cystatin | Medium | |
| 79 | Smp_075800 | Q20-G429 | 83 | Hemoglobinase | Low | ||
| 80 | Smp_210500 | SmCL3 | D17-V370 | 75 | Cathepsin L3 | Medium | |
| 81 | Smp_132480 | E16-S393 | 79 | Subfamily A1A unassigned peptidase | Low | ||
| 82 | Smp_166280 | C20-L337 | 79 | Glutaminyl cyclase | Medium | ||
| 83 | Smp_187 140 | K21-F342 | 74 | Cathepsin L | Low | ||
| 84 | Smp_006510 | SmCE2a.2 | W25-I263 | 53 | Cercarial elastase 2a | - | [19] |
| 85 | Smp_090110 | E24-I591 | 103 | Invadolysin | - | [19] | |
| Other Secreted Enzymes | |||||||
| 86 | Smp_145920 | Y30-F340 | 71 | Protein tyrosine sulfotransferease | Low | ||
| 87 | Smp_040790 | E24-E213 | 54 | Peptidyl prolyl cis-trans isomerase B | Medium | ||
| 88 | Smp_008320 | D18-D325 | 72 | Pap-inositol-1,4-phosphatase | Low | ||
| 89 | Smp_021730 | G30-D220 | 52 | Cytochrome c oxydase subunit Vb | Low | ||
| 90 | Smp_078800 | E25-S192 | 49 | DnaJ subfamily B | Low | ||
| 91 | Smp_007450 | R22-G160 | 48 | Heat-shock 67b2 | Medium | ||
| 92 | Smp_026930 | T51-V442 | 85 | Acetylglucosaminyltransferase | Medium | ||
| 93 | Smp_059910 | D28-Q274 | 70 | Ser-Thr protein phosphatase | - | ||
| 94 | Smp_089240 | K43-Y498 | 93 | Acetylglucosaminyltransferase | - | ||
| 95 | Smp_134800 | K20-T1590 | 242 | Tyr protein kinase | Low | ||
| 96 | Smp_018760 | N25-L991 | 152 | Alpha glucosidase | Low | [19] | |
| Secreted VALs/MEGs | |||||||
| 97 | Smp_194860 | Sm8.7 | E20-E92 | 39 | Medium | [17] | |
| 98 | Smp_138080 | MEG-3.1 | A21-S146 | 50 | High | [17] | |
| 99 | Smp_194830 | SmKK7 | K21-D79 | 37 | High | [16] | |
| 100 | Smp_001890 | VAL18 | K27-Y194 | 55 | Low | [16] | |
| 101 | Smp_002070 | VAL4 | K22-E181 | 57 | Low | [16] | |
| 102 | Smp_138060 | MEG-3.3 | A21-G151 | 46 | High | ||
| 103 | Smp_180620 | MEG-17 | N17-R65 | 41 | High | ||
| 104 | Smp_123540 | VAL12 | I22-L24 | 57 | Low | ||
| 105 | Smp_123550 | VAL8 | Q24-K261 | 66 | Medium | ||
| Putative Secreted Proteins | |||||||
| 106 | Smp_181070 | K25-Q114 | 43 | Low | |||
| 107 | Smp_004710 | M26-S127 | 42 | High | |||
| 108 | Smp_061310 | Y20-E140 | 65 | Low | |||
| 109 | Smp_005060 | E17-I146 | 45 | - | |||
| 110 | Smp_141500 | N27-L121 | 56 | High | |||
| 111 | Smp_006060 | P35-P380 | 92 | High | |||
| 112 | Smp_063330 | K24-F182 | 48 | High | |||
| 113 | Smp_096 790 | T21-E94 | 38 | Medium | |||
| 114 | Smp_201730 | E24-R92 | 38 | Medium | |||
| 115 | Smp_019000 | D28-D236 | 53 | Medium | [19] |
Abbreviations: EGF, epidermal growth factor; GPI, glycosylphosphatidylinositol; GSK3beta, glycogen synthase kinase 3 beta; Ig, immunoglobulin; IGF, insulin-like growth factor; IgSF, Ig superfamily; LAMP, lysosome-associated membrane glycoprotein; MEGs, micro-exon genes; MM, molecular mass; NPC, Niemann-Pick C; NPP, ectonucleotide pyrophosphatase/phosphodiesterase; Ref., reference; SPARC, secreted protein acidic and rich in cysteine; VAL, venom allergen-like.
aFor each protein, we show the accession number, alternative name, boundaries of the extracellular domain expressed in mammalian cells, expected MM in kilodaltons, domain similarities, and level of expression as determined by enzyme-linked immunosorbent assay (ELISA). References to previous proteomics studies where this protein was identified are provided where available. The expected MM of each protein was calculated by adding 3 kDa per predicted glycosylation site (the average mass of a N-linked glycan) to the expected mass of the protein. Levels of expression were determined from each ELISA profile as detailed under Material and Methods.
Recombinant Protein Expression Using a Mammalian Expression System
The entire ectodomain of membrane-anchored proteins was selected, their signal peptide removed using predictions from SignalP v3.0, and the corresponding cDNAs were made by gene synthesis after codon-optimization for expression in human cells (GeneArt; Invitrogen). The ectodomains were flanked by unique NotI and AscI restriction sites and subcloned into an expression plasmid containing the mouse VK7–33 signal peptide [24], and a C-terminal tag containing rat Cd4d3 + 4 domain, a BirA monobiotinylation sequence, and 6-His tag (Addgene plasmid no. 50 803) [30]. All expression constructs are available at www.addgene.org (plasmids nos. 120 590 to 120 704). Plasmids were transiently cotransfected with BirA in HEK293-E or -6E cells, supernatants were collected, and recombinant proteins were detected by Western blotting with 0.02 µg/mL streptavidin-HRP (Jackson ImmunoResearch) as previously described [24]. When proteins showed signs of proteolytic cleavages, transfections were repeated in the presence of a protease inhibitor cocktail (Sigma).
Human Samples From Endemic Regions and Experimentally Controlled Infections
To initially characterize the immunoreactivity of the proteins, we used plasma pools from 10 nonexposed European controls and 10 Ugandan adults from a cohort living in a high transmission area [31, 32]. Individuals were selected from those who had a soluble worm antigen immunoglobulin (Ig)G1 response in the upper third for the cohort with a median egg count of 876 eggs per gram (epg) (interquartile range, 345–1967 epg). All patient samples were collected in accordance with the Uganda National Council for Science and Technology and the Cambridge Local Research Ethics Committee. Sera or plasma from volunteers experimentally infected percutaneously with 10–30 male cercariae were collected weekly in accordance with the LUMC Institutional Medical Ethical Research Committee (P16.111), as previously described [33]. All volunteers were treated with praziquantel 12 weeks after infection.
Sera From Experimental Mouse Infections
The life cycle of the NMRI (Puerto Rican) strain of S mansoni was maintained by routine infections of mice and susceptible Biomphalaria glabrata snails under the UK Home Office Project Licence nos. P77E8A062 and PD3DA8D1F; all protocols were approved by the local Animal Welfare and Ethical Review Body (AWERB). Seven-week-old BALB/c female mice were infected percutaneously by tail immersion in water containing 200 cercariae for 40 minutes under general anaesthesia or by injection of 350 cercariae intraperitoneally. Blood samples were collected at 8, 21, and 42 days postinfection.
Enzyme-Linked Immunosorbent Assays
Protein expression was quantified by enzyme-linked immunosorbent assay (ELISA) as previously described [24]. Briefly, serial dilutions of biotinylated proteins were captured on streptavidin-coated microtitre plates and detected by mouse antirat Cd4 OX68 antibody (AbD Serotec), followed by an alkaline-phosphatase-conjugated antimouse secondary antibody (Sigma), and proteins were classified into high (typically >5 µg/mL transfection supernatant), medium (between 1 and 5 µg/mL), and low (<1 µg/mL) levels of expression. To determine the presence of heat-labile epitopes, biotinylated proteins were captured on streptavidin-coated plates either untreated or after heat treatment for 10 minutes at 80°C before incubation with sera at 1:1000 dilution in HBST/2% bovine serum albumin (HBST/2%BSA). Statistical analysis was performed in GraphPad Prism using the Holm-Sidak method for multiple t tests. Sera from human volunteers or experimentally infected mice were diluted 1:250 or 1:1000, respectively, in HBST/2%BSA; binding was detected with horseradish peroxidase-conjugated antihuman or antimouse secondary antibodies (Sigma), respectively, recognizing IgA, IgM, and IgG. For each individual, the optical density (OD) value of the preinfection samples was reference-subtracted from the OD readings at all subsequent time points. The OD values for each protein were compared with that of the rat Cd4d3 + 4 tag used as a negative control, and seropositivity was defined as ODprotein > ODcontrol + 3SDcontrol.
RESULTS
Selection and Expression of a Panel of 115 Secreted and Cell-Surface Proteins From Schistosoma mansoni
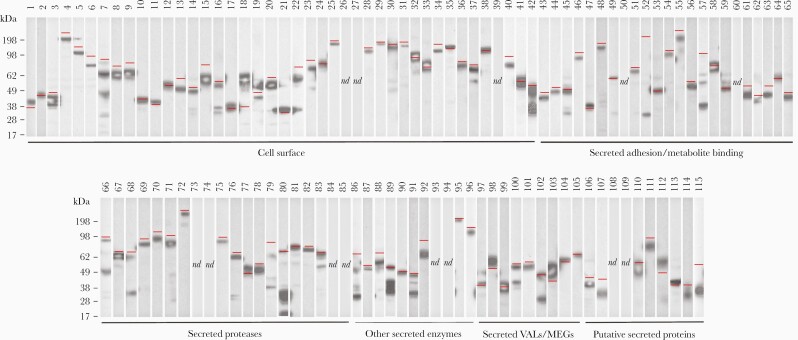
To compile a library of recombinant S mansoni proteins, we used published proteomics data to identify 40 proteins predicted to be located on the surface or secreted by the parasite (Table 1). Because membrane and secreted proteins of the schistosomula stage were underrepresented in proteomics datasets, we supplemented our library using transcriptomics data. To identify genes transcribed early after infection, we selected 1302 transcripts enriched at 48 versus 3 hours postinfection and the 1000 most abundant transcripts in the 48-hour schistosomula. Proteins likely to be secreted or membrane-anchored were identified, resulting in a list of 274 genes. Of those, mitochondrial, endoplasmic reticulum proteins, and those with incomplete open reading frames were excluded so that 75 new candidates were added for a total of 115 genes (Table 1). One third encoded single-pass transmembrane or GPI-anchored proteins, and the remaining secreted proteins were divided into 5 broad categories (Table 1). Proteins were expressed in HEK293 cells, quantified by ELISA, and their integrity was determined by Western blotting (Table 1, Figure 1). Most proteins were detected at their expected size including the very large proteins Sm200 (257 kDa, protein 4) and α 2-macroglobulin (409 kDa, protein 72) (Figure 1), and only 12 (10%) could not be detected (Table 1, Figure 1). This library of recombinant S mansoni surface and secreted proteins represents a valuable resource for immunological and functional studies.
Figure 1.
A library of 115 recombinant cell-surface and secreted proteins from Schistosoma mansoni expressed as secreted enzymatically monobiotinylated recombinant proteins in HEK293 cells. Supernatants were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions, blotted, and detected with streptavidin-conjugated horseradish peroxidase. Approximately one third of the protein library consists of membrane-tethered surface proteins, whereas the remainder of the library corresponds to secreted proteins as indicated. Their predicted molecular mass is indicated by a red line. Three proteins (52, 57, 86) migrated faster than expected; 6 proteins (42, 68, 80, 89, 91, 102) exhibited evidence of partial processing; 13 proteins were not detected by Western blotting.
Schistosoma mansoni Recombinant Proteins Expressed in Mammalian Cells Contain Heat-Labile Conformational Epitopes
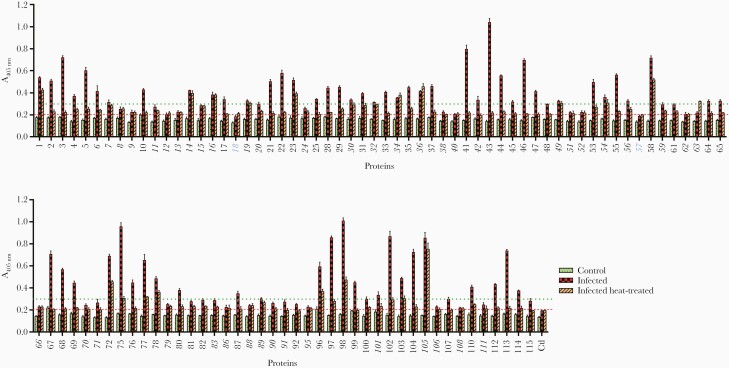
Many antibodies elicited in the context of a natural infection recognize conformational epitopes present on the native protein. To determine the fraction of proteins containing conformational epitopes, we compared the immunoreactivity of pooled plasma from chronically exposed individuals to untreated and heat-treated proteins. All except proteins 18 and 57 were seropositive, and 66 of the expressed proteins (64%) were highly immunoreactive (A280 > 0.3) (Figure 2). The majority of proteins showed moderate to strong loss of immunoreactivity after heat treatment; only 16 of the highly reactive proteins showed no statistically significant loss of reactivity, suggesting that they were either natively unstructured, misfolded, or contained heat-stable domains (Figure 2). Overall, the recombinant proteins were therefore immunoreactive to plasma from individuals living in high-endemicity areas and contained heat-labile epitopes.
Figure 2.
The majority of recombinant proteins are immunoreactive to sera from individuals living in schistosomiasis-endemic areas and contain heat-labile conformational epitopes. Recombinant proteins were probed with pooled sera from individuals living in schistosomiasis-endemic areas (“infected,” red checked bars) or individuals from the United Kingdom who have never been infected (“control,” green dotted bars). To test for the presence of heat-labile epitopes, recombinant proteins were also heat-treated (80°C, 10 minutes) before being exposed to immune sera (“infected heat treated,” orange hatched bars). All except 2 proteins (18 and 57, shown in blue) were seropositive, as determined by Aprotein > Acontrol + 3SDcontrol ( = 0.201) (red dashed line), where control is the rat Cd4d3 + 4 protein tag. High immunoreactivity was determined as Aprotein > 0.3 (green dotted line). Proteins that exhibited little or no loss of reactivity after heat treatment are shown in italics, including 16 highly reactive proteins (6, 7, 14, 16, 19, 30, 32, 34, 36, 42, 49, 54, 56, 89, 101, 105). All measurements were performed in triplicate; error bars = standard deviation.
Serological Profiles of Human Experimental Schistosoma mansoni Infections
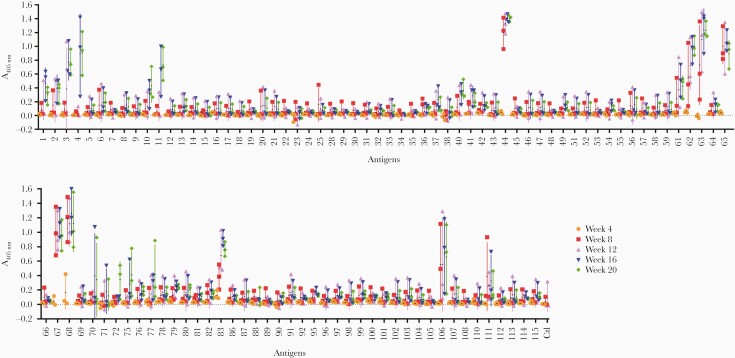
The recent establishment of controlled human S mansoni infections [33, 34] provided a unique opportunity to identify early serological markers of infection and the kinetics of the host antibody response in an experimentally controlled setting. We initially tested plasma taken at 4 weekly intervals from 3 volunteers, each infected with 30 male cercariae, against our 103 expressed S mansoni proteins. As expected, compared with high-endemicity plasma, fewer antigens were immunoreactive, but several were immunopositive in all participants, and the number of positive antigens increased over time (Figure 3). Five antigens (44, 63, 65, 67, and 68) showed consistently strong responses in all volunteers from 8 weeks postinfection. At 12 weeks, 4 additional antigens (61, 62, 83, and 106), which were already positive in 2 of 3 individuals at 8 weeks, were detected in all participants (Figure 3). It is remarkable that 5 of the 9 antigens seropositive by week 12 contained a saposin domain (proteins 44, 61, 62, 63, and 65), whereas proteins 67, 68, and 83 all belong to the cathepsin family of proteases. The number of antigens seropositive in all individuals increased over time up to week 20, so, in total, 20 antigens were immunoreactive in all volunteers for at least 1 time point, whereas another 17 were observed in at least 2 participants.
Figure 3.
Identification of early markers of infection in human volunteers experimentally infected with Schistosoma mansoni male cercariae. Three individuals were each infected with 30 male cercariae, and their antibody response to 103 Schistosoma antigens were monitored every 4 weeks over a period of 20 weeks. The number of positive antigens increased over time with the most highly immunoreactive antigens containing saposin domains (proteins 44, 61, 62, 63, and 65) or belonging to the cathepsin family of proteases (proteins 67, 68, and 83). Colored symbols represent time point readings for each individual. Data points represent mean ± standard deviation; n = 3.
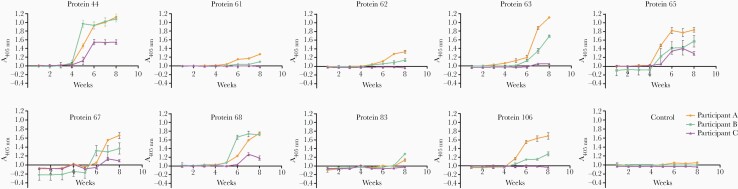
Early diagnosis of infection before the onset of symptoms would be very valuable; therefore, we analyzed weekly samples from all 3 individuals until week 8 against the 9 antigens positive in all volunteers at the 12-week time point (Figure 4). Immunoreactivity was observed in all volunteers as early as 5 weeks postinfection for proteins 44 and 65, 6 weeks for protein 68, and 7 weeks for proteins 63 and 67. As observed previously, only 2 of the 3 participants were reactive to proteins 61, 62, 83, and 106 between 5 and 8 weeks postinfection. In the case of antigens 44, 65, 68, and 106, seropositivity increased sharply between weeks 4 and 6 before plateauing at later time points.
Figure 4.
Kinetics of human antibody response to early markers of infection using sera from experimental infections by Schistosoma mansoni. Immunoreactivity to S mansoni antigens were quantified on a weekly basis in 3 individuals infected with 30 male cercariae. Reactivity to proteins 44 and 65 could be detected in all volunteers as early as 5 weeks postinfection. The control antigen corresponds to the rat Cd4d3 + 4 protein tag. Data points represent mean ± standard deviation; n = 3.
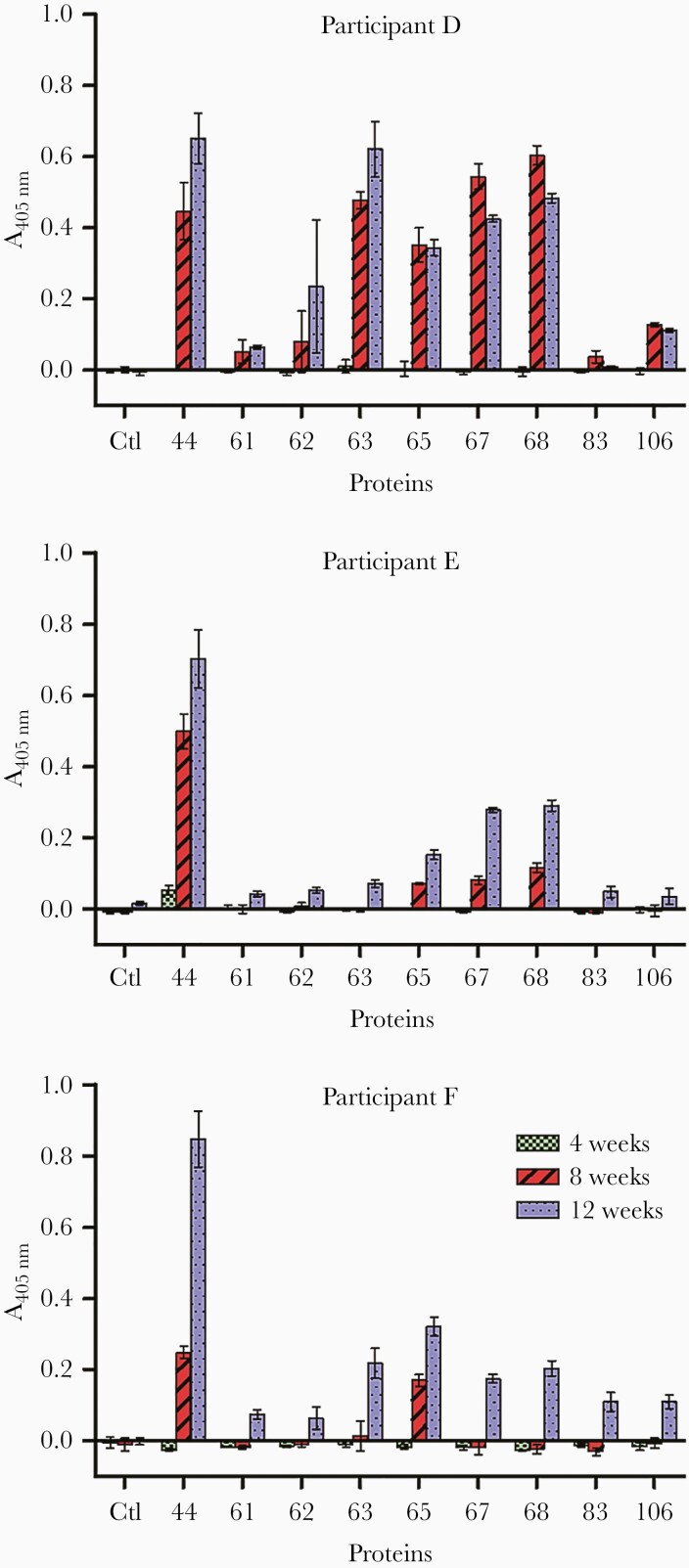
To further determine the sensitivity of detection, we quantified the immunoreactivity in 3 volunteers exposed to only 10 cercariae (Figure 5). At 4 weeks, only 1 individual (participant E) was weakly seropositive for protein 44. All 3 volunteers were seropositive for proteins 44 and 65 at 8 weeks and reacted to all other antigens, except protein 83 by 12 weeks. Although immunoreactivity was lower than with the 30-cercariae dose and interindividual variability was present, proteins 44 and 65 were again the most immunogenic antigens. In conclusion, individuals experimentally infected with small numbers of S mansoni cercariae showed seropositivity to 5 antigens as early as 5 to 7 weeks postinfection.
Figure 5.
Early reactivity to Schistosoma mansoni antigens is detectable in individuals challenged with just 10 cercariae. The immune response from 3 human volunteers infected with 10 male S mansoni cercariae was monitored at 4, 8, and 12 weeks postinfection. Reactivity to antigens 44 and 65 was detected in all participants at 8 weeks and as early as 4 weeks in the case of participant E and antigen 44. With the exception of protein 83, all volunteers were immunoreactive for the antigens tested at 12 weeks postinfection. Data points represent mean ± standard deviation; n = 3.
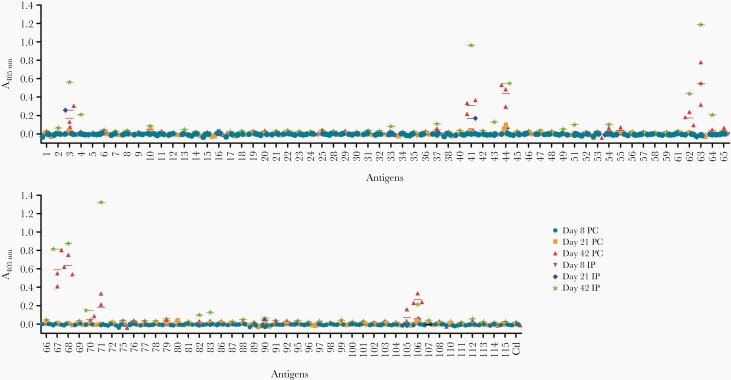
The Humoral Response Elicited by Mixed-Sex Infections in Mice Is Broadly Similar to That of Humans
Although sera from experimental infections with male carcariae identified valuable diagnostic antigens, any female-restricted antigens would not have been identified. To address this in a controlled experimental setting, we used mice as an animal model of infection. Sera were collected from mice infected either percutaneously with 200 cercariae or through intraperitoneal injection of 350 cercariae, and their serological responses were quantified (Figure 6). None of the antigens showed consistent reactivity across samples at 8 days postinfection, as expected; however, reactivity to antigen 44, and to a lesser extent, antigen 3, could be detected across all samples from day 21. At 42 days, an additional 8 antigens showed reactivity across all sera tested. Overall, reactivity with the pool of sera from mice infected intraperitoneally was stronger than individual mice infected percutaneously, possibly due to the higher number of cercariae used. Just like in human samples, proteins 44, 62, 63, 67, 68, and 106 were the most immunoreactive. In addition, reactivity to the Ly6 family members Ly6F, Ly6B, and Ly6D (proteins 3, 10, and 41, respectively) was detected at an earlier time point in mice than it was in humans. It is interesting to note that proteins 61 and 65, which were positive between 5 and 6 weeks postinfection in humans, did not react with murine samples, whereas conversely protein 71 generated a stronger response in mice.
Figure 6.
Analysis of the acquired antibody response to Schistosoma mansoni antigens in experimentally infected mice. Individual sera from 3 mice infected percutaneously (PC) or pooled sera from 3 mice infected intraperitoneally (IP) were analyzed at 8, 21, and 42 days postinfection. The aim was to try and capture the antibody reactivity at different stages of parasite maturation: schistosomule at 8 days, immature adult at 21 days, and mature adult at 42 days. Proteins 44 and 3 were immunopositive at day 21, and 8 additional proteins (10, 41, 62, 63, 64, 67, 68, 106) were immunopositive at day 42. Each data point represents the average of triplicate experiments; error bars = standard deviation.
DISCUSSION
Despite its widespread distribution and high morbidity rate, schistosomiasis remains a neglected tropical disease whose true incidence and health impact remains underestimated [6]. In this study, we have described a recombinant protein library containing secreted and cell-surface proteins from different developmental stages of S mansoni, and together with sera obtained from a recently established controlled human infection model [33] we used it to determine the kinetics of the humoral response to S mansoni infection. Although other large arrays of parasite proteins have been used for diagnostic and immuno-epidemiological purposes, they have mostly relied on cell-free or bacterial expression systems [35, 36]. Mammalian expression systems are more suitable for the addition of structurally important posttranslational modifications found on extracellular proteins and thereby preserve conformational epitopes that can be recognized by antibodies. Using this approach, we successfully expressed 103 proteins, the majority of which were observed at their expected size. Although the 12 proteins we could not express were equally distributed among the different protein families represented in the library, 3 of them corresponded to elastases. Using pooled plasma from patients living in a high-transmission region of Uganda, we observed that 64% of the expressed proteins were strongly immunoreactive (A280 > 0.3) with the majority (82%) showing sensitivity to heat treatment, an indicator of tertiary folding. Although the lower immunoreactivity for 37 proteins may suggest incorrect folding, they could also be weakly immunogenic in humans or not directly exposed to the host immune system. Of the 16 most immunoreactive proteins observed, 11 had already been identified in the surface and secreted proteome of S mansoni; by contrast, only 6 of the 37 proteins with low serum reactivity (16%) have already been described in proteomics studies. The use of a single mammalian expression system for the production of large panels of proteins is particularly attractive for the systematic comparison of antigens in diagnostic, immuno-epidemiological, or vaccination studies, and we have used this approach previously to identify potentially protective antibodies against malaria [24, 25, 30].
Mass administration of praziquantel to schoolchildren has been the mainstay of control programs against schistosomiasis, and this has proved relatively successful in reducing parasite burdens and contributing to elimination from some areas [37]. Continued surveillance and early detection of new cases remains critical to avoid any risk of resurgence; however, the commonly used Kato-Katz method is not sensitive enough to detect low levels of parasitemia, resulting in the underestimation of the real number of cases [7]. Detection of parasite-derived glycans such as CAA in the urine or serum of patients by lateral-flow test is currently considered the most sensitive assay for the detection of current Schistosoma infections because it can detect very low infection levels and reactivity disappears rapidly after praziquantel treatment [8, 38]. In areas where schistosomiasis has been eliminated or is close to elimination, alternative methods of surveillance might be needed. By persisting several months or even years after infection [39], antibody responses provide a useful historical measure of parasite exposure to monitor populations at risk of resurgence. Currently, most host antibody responses are measured against crude parasite preparations such SEA or SWAP, which may suffer from considerable cross-reactivity with other helminths antigens. Therefore, species-specific recombinant proteins as diagnostic tools could be more reliable.
A striking feature of this study is the relatively small number of antigens eliciting a patent immune response in the few weeks after a primary infection. In humans, almost no IgM/IgG response could be detected before 4 to 5 weeks postinfection: some antigens might be more highly expressed at later stages of parasite development, or the parasite could evade the host immune response. Subsequently, antibody responses were almost exclusively directed at secreted proteins belonging to the saposin and cathepsin families. Of the 9 proteins reactive in all human volunteers before 12 weeks, 5 contained saposin domains and 3 were cathepsins. It is interesting to note that several saposin domain-containing proteins from Schistosoma japonicum have been proposed as markers of infections in mice and humans [40–42], whereas proteins 66 and 67, 2 isoforms of the cathepsin B1 protease, seem to have built-in adjuvanticity [43]. Both saposins and cathepsins are produced by the schistosome’s alimentary tract and have been involved in the digestion of lipids and proteins [44, 45]. Their reactivity to the host humoral system suggests they are produced by the parasite at an early stage. Although 5 of the 6 saposin-domain-containing proteins present in the library were immunoreactive (although reactivity of protein 83 remained modest up to 12 weeks), they share only 9% to 28% sequence identity, making cross-reactivity unlikely. The dynamics of the host response to the recombinant saposins closely parallels the detection of the parasite CAA glycans in the sera of infected patients, which is first detected at 4 weeks [38].
Reactivity to members of the uPAR/Ly6 domain-containing family, which are expressed at the somule and adult stages [46–48], was also consistently observed across human and murine samples. In our study, reactivity to Ly6F, Ly6B, and Ly6D (proteins 3, 10, and 41, respectively) was observed in both human and mouse serum samples, whereas reactivity to Ly6A and Ly6I (proteins 11 and 2, respectively) was only detected in human samples. Three of these proteins (Ly6B, Ly6D, and Ly6F) have been shown to elicit strong antibody responses in rat, mouse, and human sera [48]. Although they share some homology with the complement-inactivating Cd59 protein, they do not seem to have conserved the same function [46].
CONCLUSIONS
By producing recombinant proteins in a mammalian expression system, we have paid particular attention to their correct folding, and thus this new resource could be used in a wide range of cellular and molecular assays such as vaccine screening [49], cellular assays looking at immunomodulatory functions, immunoepidemiological studies, or the identification of host binding partners by receptor-ligand screening. We envisage these proteins will be useful to the wider scientific community to further understand Schistosoma biology.
Notes
Acknowledgments. We thank Catherine McCarthy for technical help and David Dunne for helpful discussions on the study design.
Financial support. This work was funded by the Wellcome Trust (Grant 206194).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Presented in part: Joint Spring Meeting of the British Society for Parasitology, April 2019, Manchester, UK.
References
- 1. World Health Organization. Schistosomiasis and soil-transmitted helminthiases: number of people treated in 2016. Wkly Epidemiol Rec 2017; 92:749–60. [PubMed] [Google Scholar]
- 2. Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet 2014; 383:2253–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cousin CE, Stirewalt MA, Dorsey CH. Schistosoma mansoni: ultrastructure of early transformation of skin- and shear-pressure-derived schistosomules. Exp Parasitol 1981; 51:341–65. [DOI] [PubMed] [Google Scholar]
- 4. Cheever AW, Macedonia JG, Mosimann JE, Cheever EA. Kinetics of egg production and egg excretion by Schistosoma mansoni and S. japonicum in mice infected with a single pair of worms. Am J Trop Med Hyg 1994; 50:281–95. [DOI] [PubMed] [Google Scholar]
- 5. Burke ML, Jones MK, Gobert GN, Li YS, Ellis MK, McManus DP. Immunopathogenesis of human schistosomiasis. Parasite Immunol 2009; 31:163–76. [DOI] [PubMed] [Google Scholar]
- 6. Colley DG, Andros TS, Campbell CH Jr. Schistosomiasis is more prevalent than previously thought: what does it mean for public health goals, policies, strategies, guidelines and intervention programs? Infect Dis Poverty 2017; 6:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ogongo P, Kariuki TM, Wilson RA. Diagnosis of Schistosomiasis mansoni: an evaluation of existing methods and research towards single worm pair detection. Parasitology 2018; 145:1355–66. [DOI] [PubMed] [Google Scholar]
- 8. Corstjens PL, Nyakundi RK, de Dood CJ, et al. Improved sensitivity of the urine CAA lateral-flow assay for diagnosing active Schistosoma infections by using larger sample volumes. Parasit Vectors 2015; 8:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clements MN, Corstjens PLAM, Binder S, et al. Latent class analysis to evaluate performance of point-of-care CCA for low-intensity Schistosoma mansoni infections in Burundi. Parasit Vectors 2018; 11:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Dam GJ, de Dood CJ, Lewis M, et al. A robust dry reagent lateral flow assay for diagnosis of active schistosomiasis by detection of Schistosoma circulating anodic antigen. Exp Parasitol 2013; 135:274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hinz R, Schwarz NG, Hahn A, Frickmann H. Serological approaches for the diagnosis of schistosomiasis - A review. Mol Cell Probes 2017; 31:2–21. [DOI] [PubMed] [Google Scholar]
- 12. Berriman M, Haas BJ, LoVerde PT, et al. The genome of the blood fluke Schistosoma mansoni. Nature 2009; 460: 352–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Braschi S, Borges WC, Wilson RA. Proteomic analysis of the schistosome tegument and its surface membranes. Mem Inst Oswaldo Cruz 2006; 101(Suppl 1):205–12. [DOI] [PubMed] [Google Scholar]
- 14. Braschi S, Wilson RA. Proteins exposed at the adult schistosome surface revealed by biotinylation. Mol Cell Proteomics 2006; 5:347–56. [DOI] [PubMed] [Google Scholar]
- 15. Castro-Borges W, Dowle A, Curwen RS, Thomas-Oates J, Wilson RA. Enzymatic shaving of the tegument surface of live schistosomes for proteomic analysis: a rational approach to select vaccine candidates. PLoS Negl Trop Dis 2011; 5:e993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Curwen RS, Ashton PD, Sundaralingam S, Wilson RA. Identification of novel proteases and immunomodulators in the secretions of schistosome cercariae that facilitate host entry. Mol Cell Proteomics 2006; 5:835–44. [DOI] [PubMed] [Google Scholar]
- 17. Wilson RA. Proteomics at the schistosome-mammalian host interface: any prospects for diagnostics or vaccines? Parasitology 2012; 139:1178–94. [DOI] [PubMed] [Google Scholar]
- 18. Knudsen GM, Medzihradszky KF, Lim KC, Hansell E, McKerrow JH. Proteomic analysis of Schistosoma mansoni cercarial secretions. Mol Cell Proteomics 2005; 4: 1862–75. [DOI] [PubMed] [Google Scholar]
- 19. Sotillo J, Pearson M, Becker L, Mulvenna J, Loukas A. A quantitative proteomic analysis of the tegumental proteins from Schistosoma mansoni schistosomula reveals novel potential therapeutic targets. Int J Parasitol 2015; 45:505–16. [DOI] [PubMed] [Google Scholar]
- 20. Sotillo J, Pearson M, Potriquet J, et al. Extracellular vesicles secreted by Schistosoma mansoni contain protein vaccine candidates. Int J Parasitol 2016; 46:1–5. [DOI] [PubMed] [Google Scholar]
- 21. Collins JJ 3rd, Wendt GR, Iyer H, Newmark PA. Stem cell progeny contribute to the schistosome host-parasite interface. Elife 2016; 5:e12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Farias LP, Tararam CA, Miyasato PA, et al. Screening the Schistosoma mansoni transcriptome for genes differentially expressed in the schistosomulum stage in search for vaccine candidates. Parasitol Res 2011; 108:123–35. [DOI] [PubMed] [Google Scholar]
- 23. Parker-Manuel SJ, Ivens AC, Dillon GP, Wilson RA. Gene expression patterns in larval Schistosoma mansoni associated with infection of the mammalian host. PLoS Negl Trop Dis 2011; 5:e1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Crosnier C, Wanaguru M, McDade B, et al. A library of functional recombinant cell-surface and secreted P. falciparum merozoite proteins. Mol Cell Proteomics 2013; 12:3976–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Osier FH, Mackinnon MJ, Crosnier C, et al. New antigens for a multicomponent blood-stage malaria vaccine. Sci Transl Med 2014; 6:247ra102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Protasio AV, van Dongen S, Collins J, et al. MiR-277/4989 regulate transcriptional landscape during juvenile to adult transition in the parasitic helminth Schistosoma mansoni. PLoS Negl Trop Dis 2017; 11:e0005559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Robinson MD, Oshlack A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol 2010; 11:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol 2004; 340:783–95. [DOI] [PubMed] [Google Scholar]
- 29. Sonnhammer EL, von Heijne G, Krogh A. A hidden Markov model for predicting transmembrane helices in protein sequences. Proc Int Conf Intell Syst Mol Biol 1998; 6:175–82. [PubMed] [Google Scholar]
- 30. Zenonos ZA, Rayner JC, Wright GJ. Towards a comprehensive Plasmodium falciparum merozoite cell surface and secreted recombinant protein library. Malar J 2014; 13:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kabatereine NB, Vennervald BJ, Ouma JH, et al. Adult resistance to Schistosomiasis mansoni: age-dependence of reinfection remains constant in communities with diverse exposure patterns. Parasitology 1999; 118 (Pt 1):101–5. [DOI] [PubMed] [Google Scholar]
- 32. Naus CW, Booth M, Jones FM, et al. The relationship between age, sex, egg-count and specific antibody responses against Schistosoma mansoni antigens in a Ugandan fishing community. Trop Med Int Health 2003; 8:561–8. [DOI] [PubMed] [Google Scholar]
- 33. Langenberg MCC, Hoogerwerf MA, Koopman JPR, et al. A controlled human Schistosoma mansoni infection model to advance novel drugs, vaccines and diagnostics. Nat Med 2020; 26:326–32. [DOI] [PubMed] [Google Scholar]
- 34. Janse JJ, Langenberg MCC, Kos-Van Oosterhoud J, et al. Establishing the production of male Schistosoma mansoni cercariae for a controlled human infection model. J Infect Dis 2018; 218:1142–6. [DOI] [PubMed] [Google Scholar]
- 35. Gaze S, Driguez P, Pearson MS, et al. An immunomics approach to schistosome antigen discovery: antibody signatures of naturally resistant and chronically infected individuals from endemic areas. PLoS Pathog 2014; 10:e1004033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. de Assis RR, Ludolf F, Nakajima R, et al. A next-generation proteome array for Schistosoma mansoni. Int J Parasitol 2016; 46:411–5. [DOI] [PubMed] [Google Scholar]
- 37. Deol AK, Fleming FM, Calvo-Urbano B, et al. Schistosomiasis - Assessing progress toward the 2020 and 2025 global goals. N Engl J Med 2019; 381:2519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. van Grootveld R, van Dam GJ, de Dood C, et al. Improved diagnosis of active Schistosoma infection in travellers and migrants using the ultra-sensitive in-house lateral flow test for detection of circulating anodic antigen (CAA) in serum. Eur J Clin Microbiol Infect Dis 2018; 37:1709–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yong MK, Beckett CL, Leder K, Biggs BA, Torresi J, O’Brien DP. Long-term follow-up of schistosomiasis serology post-treatment in Australian travelers and immigrants. J Travel Med 2010; 17:89–93. [DOI] [PubMed] [Google Scholar]
- 40. Cai P, Weerakoon KG, Mu Y, et al. A parallel comparison of antigen candidates for development of an optimized serological diagnosis of Schistosomiasis japonica in the Philippines. EBioMedicine 2017; 24:237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu S, Zhou X, Piao X, et al. Saposin-like proteins, a multigene family of schistosoma species, are biomarkers for the immunodiagnosis of Schistosomiasis japonica. J Infect Dis 2016; 214:1225–34. [DOI] [PubMed] [Google Scholar]
- 42. Xu X, Zhang Y, Lin D, et al. Serodiagnosis of Schistosoma japonicum infection: genome-wide identification of a protein marker, and assessment of its diagnostic validity in a field study in China. Lancet Infect Dis 2014; 14:489–97. [DOI] [PubMed] [Google Scholar]
- 43. El Ridi R, Tallima H, Selim S, et al. Cysteine peptidases as schistosomiasis vaccines with inbuilt adjuvanticity. PLoS One 2014; 9:e85401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Don TA, Bethony JM, Loukas A. Saposin-like proteins are expressed in the gastrodermis of Schistosoma mansoni and are immunogenic in natural infections. Int J Infect Dis 2008; 12:e39–47. [DOI] [PubMed] [Google Scholar]
- 45. Skelly PJ, Da’dara AA, Li XH, Castro-Borges W, Wilson RA. Schistosome feeding and regurgitation. PLoS Pathog 2014; 10:e1004246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Farias LP, Krautz-Peterson G, Tararam CA, et al. On the three-finger protein domain fold and CD59-like proteins in Schistosoma mansoni. PLoS Negl Trop Dis 2013; 7:e2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chalmers IW, Fitzsimmons CM, Brown M, et al. Human IgG1 responses to surface localised Schistosoma mansoni Ly6 family members drop following praziquantel treatment. PLoS Negl Trop Dis 2015; 9:e0003920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Krautz-Peterson G, Debatis M, Tremblay JM, et al. Schistosoma mansoni infection of mice, rats and humans elicits a strong antibody response to a limited number of reduction-sensitive epitopes on five major tegumental membrane proteins. PLoS Negl Trop Dis 2017; 11:e0005306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Crosnier C, Brandt C, Rinaldi G, et al. Systematic screening of 96 Schistosoma mansoni cell-surface and secreted antigens does not identify any strongly protective vaccine candidates in a mouse model of infection. Wellcome Open Res 2019; 4:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hall SL, Braschi S, Truscott M, Mathieson W, Cesari IM, Wilson RA. Insights into blood feeding by schistosomes from a proteomic analysis of worm vomitus. Mol Biochem Parasitol 2011; 179:18–29. [DOI] [PubMed] [Google Scholar]