Abstract
Aims
Currently, no head-to-head data are available comparing semaglutide 2.0 mg with dulaglutide 3.0 mg or 4.5 mg. We conducted an indirect treatment comparison (ITC) of their effects on glycated hemoglobin (HbA1c) and body weight in patients with type 2 diabetes.
Materials and methods
Multilevel network meta-regression was conducted, based on a connected evidence network of published results from the A Study of the Efficacy and Safety of Dulaglutide (LY2189265) in Participants With Type 2 Diabetes 11 trial and individual patient data from the A Research Study to Compare Two Doses of Semaglutide Taken Once Weekly in People With Type 2 Diabetes (SUSTAIN) and SUSTAIN 7 trials.
Results
Semaglutide 2.0 mg significantly reduced HbA1c vs dulaglutide 3.0 mg and 4.5 mg, with estimated treatment differences (ETDs) of –0.44% points (95% credible interval [CrI], –0.68 to –0.19) and –0.28% points (95% CrI, –0.52 to –0.03), respectively. Semaglutide 2.0 mg also significantly reduced body weight vs dulaglutide 3.0 mg and 4.5 mg with ETDs of –3.29 kg (95% CrI, –4.62 to −1.96) and –2.57 kg (95% CrI, –3.90 to –1.24), respectively. Odds of achieving HbA1c < 7.0% were significantly greater for semaglutide 2.0 vs dulaglutide 3.0 mg (odds ratio [OR]: 2.23 [95% CrI, 1.15-3.90]), whereas this did not reach significance for semaglutide 2.0 mg vs dulaglutide 4.5 mg (OR: 1.58 [95% CrI, 0.82-2.78]). Sensitivity analyses supported the main analysis findings.
Conclusions
This ITC demonstrated significantly greater reductions from baseline in HbA1c and body weight with semaglutide 2.0 mg vs dulaglutide 3.0 mg and 4.5 mg. The findings of this study provide important comparative effectiveness information until randomized head-to-head studies become available.
Keywords: semaglutide, dulaglutide, HbA1c, body weight, diabetes, multilevel network meta regression
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) provide an effective treatment option for people with type 2 diabetes (T2D), offering improved glycemic control alongside weight management and cardiovascular benefits (1). Current treatment guidelines recommend GLP-1 RAs across the treatment pathway for patients with T2D not adequately controlled on metformin alone and as a preferred option for those with established cardiovascular disease regardless of glycated hemoglobin (HbA1c) level (2). Semaglutide and dulaglutide are both long-acting GLP-1 RAs, which are available for once-weekly administration via subcutaneous injection. However, they differ in molecular structure, resulting in potential differences in their metabolic effects (3-5). Understanding the impact of these differences on clinical outcomes is important for clinicians.
Semaglutide and dulaglutide have both been investigated extensively as part of the A Research Study to Compare Two Doses of Semaglutide Taken Once Weekly in People With Type 2 Diabetes (SUSTAIN) and A Study of the Efficacy and Safety of Dulaglutide (LY2189265) in Participants With Type 2 Diabetes (AWARD) clinical trial programs, respectively. A head-to-head trial, SUSTAIN 7, compared the efficacy and safety of semaglutide and dulaglutide at lower doses (semaglutide 0.5 mg vs dulaglutide 0.75 mg) and higher doses (semaglutide 1.0 mg vs dulaglutide 1.5 mg) in patients with T2D inadequately controlled with metformin (4). Superiority of semaglutide compared with dulaglutide was demonstrated regarding reducing HbA1c and body weight at week 40 (4). The 2 treatments had similar safety profiles, with similar proportions of patients experiencing gastrointestinal adverse events and discontinuing treatment because of adverse events (4).
Semaglutide was initially approved for use at maintenance doses of 0.5 mg and 1.0 mg by the US Food and Drug Administration (FDA) in 2017 (6) and European Medicines Agency (EMA) in 2018 (7); and dulaglutide was initially approved at doses of 0.75 mg and 1.5 mg by the FDA and EMA in 2014 (8, 9). However, T2D is a progressive disease, and treatment intensification is often required for maintenance of glycemic control and minimization of complications (10). The results of SUSTAIN 7 and other trials in the SUSTAIN and AWARD programs indicate that higher doses of GLP-1 RAs are associated with greater reductions in HbA1c, although there is a need to balance treatment benefits with adverse events, particularly gastrointestinal adverse events (4). Therefore, higher maintenance doses of semaglutide and dulaglutide have recently been investigated to provide potential treatment options for patients already receiving GLP-1 RAs who require further treatment intensification. Dulaglutide 3.0 mg and 4.5 mg were investigated in AWARD-11 (11) and subsequently approved in 2020 and 2021 by the FDA and EMA, respectively (8, 12). Semaglutide 1.0 mg has previously been compared with dulaglutide 3.0 mg and 4.5 mg using a Bucher indirect treatment comparison (ITC) (13). Semaglutide 2.0 mg has also been investigated in SUSTAIN FORTE (14) and is currently under review by the FDA and EMA.
Although SUSTAIN 7 provides a head-to-head comparison between lower doses of semaglutide and dulaglutide (4), there are currently no head-to-head data comparing the higher doses of the 2 treatments. The objective of this study was to compare the efficacy of semaglutide 2.0 mg with dulaglutide 3.0 mg and 4.5 mg doses using an ITC based on aggregate and individual patient data (IPD) from SUSTAIN FORTE (14) and SUSTAIN 7 (4) and published aggregate data from AWARD-11 (11).
Methods
A multilevel network meta-regression (ML-NMR) was conducted in a Bayesian framework (15) to compare efficacy outcomes of dulaglutide 3.0 mg and 4.5 mg with semaglutide 2.0 mg using data from the SUSTAIN FORTE, SUSTAIN 7, and AWARD-11 randomized controlled trials. ML-NMR has been recently introduced as a methodology for conducting ITCs when IPD are available for some, but not all, included trials in a connected evidence network (15, 16). When implemented in a Bayesian framework, ML-NMR retains the flexibility and extensibility of Bayesian network meta-analysis, allowing prior information to be used, data types with differing likelihoods to be included, and the analysis to be embedded in a probabilistic cost-effectiveness framework (15). Unlike other methods for ITC that allow for population adjustment (eg, matching-adjusted indirect comparison [MAIC] and simulated treatment comparison methods), ML-NMR enables comparisons across multiple studies. It also allows comparison in any target population within a given covariate distribution, meaning that comparisons can be made in each of the included trial populations in a connected evidence network (15, 16). A shared effect modifier assumption (17, 18) was chosen to make the model estimable. Therefore, it was assumed that the potential effect modifier interaction coefficients are identical for all dulaglutide dose levels. This assumption was necessary because data for the high doses of dulaglutide (3.0 mg and 4.5 mg) were only available on aggregate level and for 1 trial (AWARD-11).
Data Sources
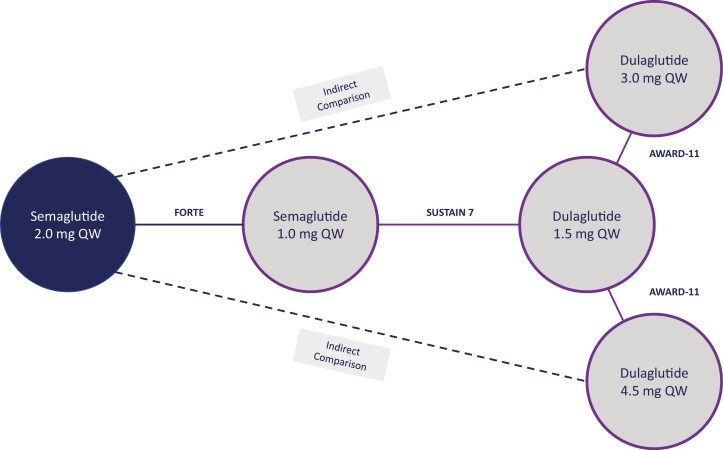
Published aggregate data were available from AWARD-11, and aggregate and IPD were available from SUSTAIN FORTE and SUSTAIN 7. SUSTAIN FORTE reported a comparison between semaglutide 1.0 mg and semaglutide 2.0 mg, SUSTAIN 7 compared semaglutide 1.0 mg with dulaglutide 1.5 mg, and AWARD-11 compared dulaglutide 3.0 mg and 4.5 mg with dulaglutide 1.5 mg. Therefore, the 3 trials formed the connected evidence network shown in Fig. 1, in which SUSTAIN 7 was used as an anchoring trial. Missing data in the studies for which IPD were available were imputed using multiple imputation and the multiply imputed data sets were incorporated into the ML-NMR model as described in Gelman et al (19).
Figure 1.
Network of evidence for semaglutide vs high-dose dulaglutide. Abbreviations: QW, once weekly.
Outcomes Assessed
Outcomes assessed were change from baseline in HbA1c and change from baseline in body weight, corresponding with the primary and secondary confirmatory outcomes in all included trials. These outcomes were assessed at 40 weeks in SUSTAIN FORTE and SUSTAIN 7 and at 36 weeks in AWARD-11. For these continuous outcomes, mean treatment differences with associated 95% credible intervals (CrIs) were calculated. Proportions of patients achieving HbA1c < 7.0% were also assessed and, for this dichotomous outcome, treatment odds ratios (ORs) with associated 95% CrIs were calculated. Statistical significance was determined as Crl not crossing 0.
Study and Patient Characteristics
Study characteristics and inclusion criteria of the SUSTAIN FORTE, SUSTAIN 7, and AWARD-11 trials used in the analysis are shown in Table 1. SUSTAIN FORTE and SUSTAIN 7 were multinational, phase 3b trials: SUSTAIN FORTE was a double-blind trial and SUSTAIN 7 was open-label. AWARD-11 was a double-blind, multinational, phase 3 trial. There were some differences in terms of trial design and inclusion criteria among the studies; notably, there were differences in HbA1c inclusion criteria among the trials.
Table 1.
Overview of study characteristics and inclusion criteria of included trials
| Study name (primary reference) NCT number | Study design | Inclusion criteria | Duration of follow-up | Randomized treatment | No. of patients randomized | Completion date | |||
|---|---|---|---|---|---|---|---|---|---|
| HbA1c | BMI | T2D duration | Background medication | ||||||
| SUSTAIN FORTE(14) NCT03989232 |
Phase 3b, double-blind, RCT Multinational |
8.0%-10.0% | No restriction | At least 6 mo | Metformin ≥ 1500 mg/d (or a maximal tolerated dose) ≥ 90 d with or without sulphonylurea (≥ half the maximum approved dose according to local label or maximum tolerated or effective dose) | 40 wk | Semaglutide 2.0 mg |
480 | November 2020 |
| Semaglutide 1.0 mg |
481 | ||||||||
| SUSTAIN 7(4)a NCT02648204 |
Phase 3b, open-label, RCT Multinational |
7.0%-10.5% | No restriction | Not specified | Metformin ≥ 1500 mg/d (or a maximal tolerated dose) ≥ 90 d | 40 wk | Semaglutide 1.0 mg |
300 | May 2017 |
| Dulaglutide 1.5 mg |
299 | ||||||||
| AWARD-11(11) NCT03495102 |
Phase 3, double-blind, RCT Multinational |
7.5%-11.0% | ≥25 kg/m2 | At least 6 mo | Metformin ≥ 1500 mg/d for ≥ 3 months | 36 wk | Dulaglutide 3.0 mg |
616 | May 2019 |
| Dulaglutide 4.5 mg |
614 | ||||||||
| Dulaglutide 1.5 mg |
612 |
Abbreviations: AWARD, A Study of the Efficacy and Safety of Dulaglutide (LY2189265) in Participants With Type 2 Diabetes; BMI, body mass index; HbA1c, glycated hemoglobin; RCT, randomised controlled trial; SUSTAIN, A Research Study to Compare Two Doses of Semaglutide Taken Once Weekly in People With Type 2 Diabetes; T2D, type 2 diabetes.
a SUSTAIN 7 also included semaglutide 0.5 mg and dulaglutide 0.75 mg; however, these were not treatments of interest for the current analysis.
Although baseline characteristics were generally similar among the trials (Table 2), the analysis was conducted using a connected network in which SUSTAIN 7 was used as an anchoring trial, making robust analysis possible despite some differences. Although there is little evidence to suggest that differences in baseline characteristics (age, sex, diabetes duration, glycemic control, and body mass index [BMI]) result in effect modification for semaglutide vs dulaglutide (20), ML-NMR takes into account both potential effect modifiers and prognostic factors across trials, allowing adjustment for baseline covariates; therefore, in the main analysis, baseline HbA1c, BMI, and diabetes duration were adjusted for as potential effect modifiers. An unadjusted analysis was also conducted as a sensitivity analysis. ML-NMR also allows comparison in each of the included trial populations in a connected evidence network. For the main analysis, the patient population considered was that of AWARD-11, which is in line with the approach that would be used in other population-adjusted methods (eg, MAIC), in which IPD would be used to match baseline summary statistics to aggregate data (21). The impact of using different populations was explored in sensitivity analyses.
Table 2.
Characteristics of enrolled patients reported in SUSTAIN FORTE, SUSTAIN 7, and AWARD-11
| Trial name | SUSTAIN FORTE | SUSTAIN 7a | AWARD-11 |
|---|---|---|---|
| Treatment (pooled treatment arms) | Semaglutide 2.0 mg and semaglutide 1.0 mg | Semaglutide 1.0 mg and dulaglutide 1.5 mg | Dulaglutide 1.5 mg, dulaglutide 3.0 mg, and dulaglutide 4.5 mg |
| N | 961 | 599 | 1842 |
| Mean age (SD), y | 58.0 (10.0) | 55.6 (10.6) | 57.1 (10.0) |
| Female, % | 41.4 | 44.4 | 48.8 |
| Race, n (%) | |||
| White | 847 (88) | 463 (77) | 1580 (86) |
| Asian | 69 (7) | 93 (16) | 45 (2.4) |
| Black or African American | 43 (4) | 36 (6) | 82 (4.5) |
| American Indian or Alaska Native | 1 (<1) | 0 | 88 (4.8) |
| Native Hawaiian or Pacific Islander | 0 | 0 | 5 (<1) |
| Other | 1 (<1) | 7 (1) | 0 |
| Multiple | 0 | 0 | 42 (2.3) |
| Mean HbA1c (SD), % | 8.9 (0.6) | 8.2 (0.9) | 8.6 (1.0) |
| Mean body weight (SD), kg | 99.3 (23.5) | 94.5 (21.4) | 95.7 (20.3) |
| Mean BMI (SD), kg/m2 | 34.6 (7.0) | 33.3 (6.5) | 34.2 (6.3) |
| Mean T2D duration (SD), y | 9.5 (6.2) | 7.5 (5.7) | 7.6 (5.7) |
| Mean fasting glucoseb (SD), mg/dL | 194.4 (50.0) | 174.8 (44.0) | 184.1 (51.5) |
| Mean DBP (SD), mmHg | 80.9 (9.4) | 80.8 (8.9) | 78.7 (9.0) |
| Mean SBP (SD), mmHg | 134.4 (14.0) | 132.6 (14.1) | 131.8 (14.1) |
| Mean pulse rate (SD), beats/minute | 75.9 (10.1) | 75.1 (10.5) | 75.5 (10.0) |
| Mean eGFR (SD), mL/min/1.73 m2 | 93.4 (16.9) | 97.2 (16.0) | 93.5 (18.1) |
| Mean lipid levels (SD), mg/dL | |||
| Total cholesterol | Not collected | 183.2 (44.7) | 177.5 (NR)c |
| HDL cholesterol | Not collected | 45.0 (10.8) | 45.6 (NR)c |
| LDL cholesterol | Not collected | 103.8 (38.8) | 93.4 (NR)c |
| VLDL cholesterol | Not collected | Not collected | 34.5 (NR)c |
| Triglycerides | Not collected | 183.3 (112.6) | 202.4 (NR)c |
Abbreviations: AWARD, A Study of the Efficacy and Safety of Dulaglutide (LY2189265) in Participants With Type 2 Diabetes; BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NR, not reported; SUSTAIN, A Research Study to Compare Two Doses of Semaglutide Taken Once Weekly in People With Type 2 Diabetes; T2D, type 2 diabetes; VLDL, very low-density lipoprotein.
a Note SUSTAIN 7 also included semaglutide 0.5 mg and dulaglutide 0.75 mg; however, these were not treatments of interest for the current analysis.
b Fasting plasma glucose in SUSTAIN FORTE and SUSTAIN 7; fasting serum glucose in AWARD-11.
c Pooled data are not presented in the AWARD-11 publication. Mean values have been calculated as weighted average.
Analyses were based on results for the trial product estimand in SUSTAIN FORTE and SUSTAIN 7, and the efficacy estimand in AWARD-11. These estimands were similar in that they were defined in all trials as including patients on treatment without rescue therapy. This means that they target the treatment effect when all patients continue to use the treatment for the planned duration of the trial without rescue medication, thus reflecting effect when treatment is used as intended (22).
Sensitivity Analyses
Three sensitivity analyses were conducted to assess the findings of the main analysis. For the first sensitivity analysis (sensitivity analysis 1), the impact of using the SUSTAIN 7 patient population as the target population was explored. In another analysis (sensitivity analysis 2), the impact of using the patient population from SUSTAIN FORTE was explored. To explore the impact of adjustment for potential effect modifiers, an unadjusted analysis (sensitivity analysis 3) using standard Bayesian network meta-analysis methodology was also conducted.
To account for the difference in timing of assessments between AWARD-11 (36 weeks) and the SUSTAIN trials (40 weeks), an additional sensitivity analysis was conducted using SUSTAIN 7 and SUSTAIN FORTE results corresponding to week 36 (obtained from linear interpolation between the week 28 and week 40 visits). Sensitivity analyses 1 and 2, exploring the impact of using the SUSTAIN 7 and SUSTAIN FORTE patient populations, respectively, as the target populations, were also conducted using the interpolated 36-week data from SUSTAIN 7 and SUSTAIN FORTE.
Results
In total, 1842 patients were randomly assigned to a dulaglutide dose in AWARD-11 (3.0 mg, n = 616; 4.5 mg, n = 614; 1.5 mg, n = 612). In SUSTAIN 7, 599 of 1199 patients were randomly assigned to the highest available doses of semaglutide or dulaglutide (semaglutide 1.0 mg, n = 300; dulaglutide 1.5 mg, n = 299). In SUSTAIN FORTE, 961 patients were randomly assigned to either dose of semaglutide (semaglutide 1.0 mg, n = 481; semaglutide 2.0 mg, n = 480).
Change in HbA1c
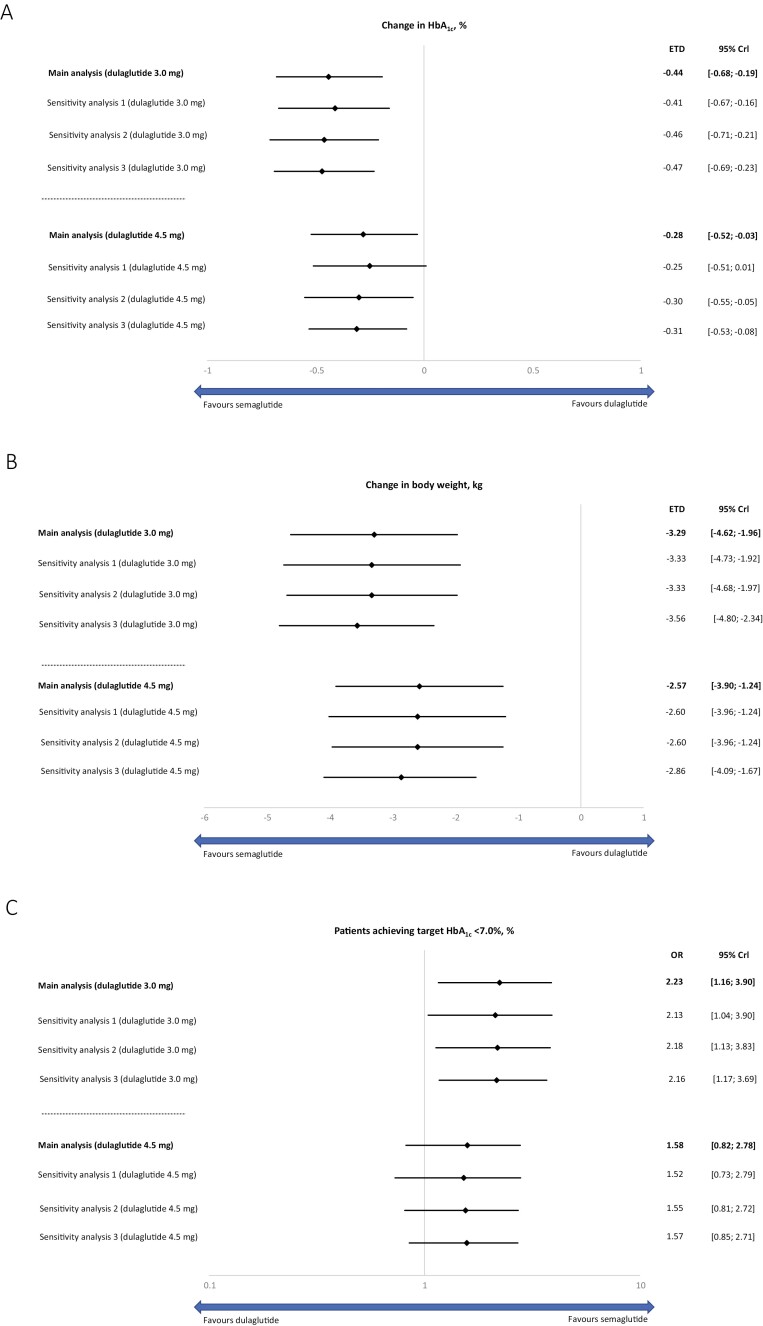
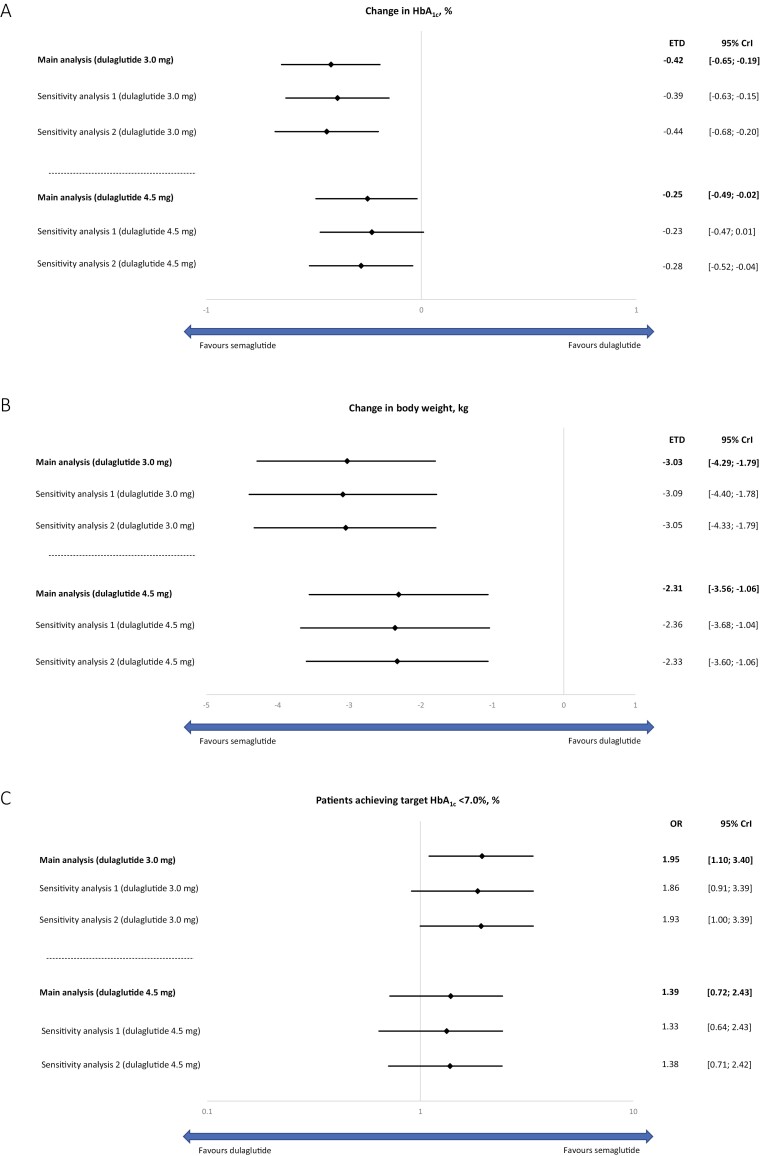
In the main analysis, semaglutide 2.0 mg was significantly more effective at reducing HbA1c from baseline compared with dulaglutide 3.0 mg and 4.5 mg with estimated treatment differences of –0.44% points (95% CrI, –0.68 to –0.19) and –0.28% points (95% CrI, –0.52 to –0.03), respectively (Fig. 2A). Sensitivity analyses adjusting for different target populations (sensitivity analyses 1 and 2) and without adjustment for potential effect modification (sensitivity analysis 3) supported the findings of the main analysis (Fig. 2A), although in sensitivity analysis 1, the difference between treatment did not reach statistical significance. Sensitivity analyses conducted using SUSTAIN 7 and SUSTAIN FORTE results corresponding to week 36 also supported the findings of the main analysis (Fig. 3A).
Figure 2.
Efficacy outcomes of semaglutide 2.0 mg vs dulaglutide 3.0 mg and 4.5 mg. Change in HbA1c from baseline (A); change in bodyweight (kg) from baseline (B); proportion of patients achieving target HbA1c (C). Main analysis, sensitivity analyses 1-3. Abbreviations: Crl, credible interval; ETD, estimated treatment difference; HbA1c, glycated hemoglobin; OR, odds ratio.
Figure 3.
Efficacy outcomes of semaglutide 2.0 mg vs dulaglutide 3.0 mg and 4.5 mg using SUSTAIN 7 results corresponding to week 36. Change in HbA1c from baseline (A); change in bodyweight (kg) from baseline (B); proportion of patients achieving target HbA1c (C). Main analysis, sensitivity analyses 1 and 2. Abbreviations: Crl, credible interval; ETD, estimated treatment difference; HbA1c, glycated hemoglobin; OR, odds ratio.
Change in Body Weight
In the main analysis, semaglutide 2.0 mg was significantly more effective at reducing body weight from baseline compared with dulaglutide 3.0 mg and 4.5 mg with ETDs of –3.29 kg (95% CrI, –4.62 to −1.96) and –2.57 kg (95% CrI, –3.90 to –1.24), respectively (Fig. 2B). Sensitivity analyses adjusting for different target populations (sensitivity analyses 1 and 2) and without adjustment for potential effect modification (sensitivity analysis 3) supported the findings of the main analysis (Fig. 2B). Sensitivity analyses conducted using SUSTAIN 7 and SUSTAIN FORTE results corresponding to week 36 also supported the findings of the main analysis (Fig. 3B).
Patients Achieving HbA1c < 7.0%
In the main analysis, the proportion of patients achieving HbA1c < 7.0% was significantly greater for semaglutide 2.0 compared with dulaglutide 3.0 mg, with an OR of 2.23 (95% CrI, 1.16-3.90), whereas this did not reach significance for semaglutide 2.0 mg vs dulaglutide 4.5 mg (OR: 1.58 [95% CrI, 0.82-2.78]) (Fig. 2C). Sensitivity analyses adjusting for different target populations (sensitivity analyses 1 and 2) and without adjustment for potential effect modification (sensitivity analysis 3) supported the findings of the main analysis (Fig. 2C). Sensitivity analyses conducted using SUSTAIN 7 and SUSTAIN FORTE results corresponding to week 36 also supported the findings of the main analysis (Fig. 3C).
Discussion
This study indirectly compared the effect of 2 high-dose GLP-1 RAs (semaglutide 2.0 mg vs dulaglutide 3.0 mg and 4.5 mg), on reducing HbA1c and body weight from baseline in patients with T2D using ML-NMR. In this study, semaglutide 2.0 mg provided significantly greater reductions from baseline in HbA1c and body weight vs dulaglutide 3.0 mg and 4.5 mg. In addition, significantly greater proportions of patients achieving HbA1c < 7.0% with semaglutide 2.0 mg vs dulaglutide 3.0 mg, and comparable proportions of patients achieved HbA1c < 7.0% with semaglutide 2.0 mg and dulaglutide 4.5 mg. Results of the main analysis were supported by sensitivity analyses and consistent regardless of the target population used in the ML-NMR.
Semaglutide 1.0 mg has previously been compared with dulaglutide 3.0 mg and 4.5 mg using a Bucher ITC. In the analysis, significantly greater reductions in HbA1c were demonstrated with semaglutide 1.0 mg vs dulaglutide 3.0 mg, and comparable reductions in HbA1c were shown for semaglutide 1.0 mg and dulaglutide 4.5 mg. Significantly greater reductions in body weight were also demonstrated with semaglutide 1.0 mg vs both doses of dulaglutide (13). However, in the absence of a head-to-head trial comparing semaglutide 2.0 mg with dulaglutide 3.0 mg and 4.5 mg, this is the first comparison of these doses of semaglutide and dulaglutide. The results provide comparison of 2 high-dose GLP-1 RAs, which have been shown in clinical trials to provide greater reductions in HbA1c and body weight than their lower dose equivalents (11), thereby providing additional options for patients who require treatment intensification.
Strengths of this study include the comparison method used. As discussed in a recent report by the National Institute for Heath and Care Excellence Decision Support Unit, ML-NMR provides a means of conducting a population-adjusted analysis using a connected evidence network, as opposed to only 2 trials, in situations where IPD are available for some, but not all included trials (15, 16). The report recommends development of a new Technical Support Document recommending ML-NMR as the preferred population-adjusted approach for anchored comparisons (16). Although there were some differences in baseline characteristics of patients enrolled across the three included trials, based on subgroup analyses of SUSTAIN 7, there is little evidence to suggest that differences in baseline characteristics (age, sex, diabetes duration, glycemic control, and BMI) result in effect modification for semaglutide vs dulaglutide (20). The main analysis adjusted for baseline HbA1c, BMI, and diabetes duration as potential effect modifiers, thereby accounting for differences in baseline characteristics among trials. However, a sensitivity analysis conducted without adjustment for potential effect modifiers supported the findings of the main analysis, confirming no appreciable impact of effect modification on the results of the analysis. The ML-NMR approach allows comparison in any target population within a given covariate distribution, meaning that comparisons can be made in each of the included trial populations in a connected evidence network (15, 16). Comparisons were conducted using the AWARD-11 population in the main analysis. Because only published aggregate data were available for AWARD-11, this is in line with the approach that would be used in other population-adjusted methods, such as MAIC, in which IPD would be used to match baseline summary statistics to aggregate data (21). This is also aligned with the MAIC conducted as a supplementary analysis in the recent publication indirectly comparing semaglutide 1.0 mg with dulaglutide 3.0 mg and 4.5 mg (13). The impact of using the SUSTAIN FORTE and SUSTAIN 7 populations was explored in supplementary analyses, all of which supported the findings of the main analysis.
The current study focused only on efficacy outcomes and did not evaluate differences between treatments in terms of safety outcomes, which are highly relevant to clinical decision making. However, a quantitative ITC of safety outcomes is intrinsically more difficult because of cross-trial differences in the assessment of adverse events. In all included trials, a similar safety profile was observed across treatment arms; adverse event rates were comparable between semaglutide 1.0 mg and dulaglutide 1.5 mg treatment arms in SUSTAIN 7 (4); dulaglutide 1.5 mg, 3.0 mg, and 4.5 mg treatment arms in AWARD-11 (11); and semaglutide 1.0 mg and 2.0 mg treatment arms in SUSTAIN FORTE (14). Furthermore, the current study did not evaluate differences in cardiovascular outcomes between treatments. Although cardiovascular outcomes trials have demonstrated cardiovascular benefits of semaglutide 0.5 mg and 1.0 mg (23) and dulaglutide 1.5 mg (24) among people with T2D at high cardiovascular risk, cardiovascular outcomes for semaglutide 2.0 mg and dulaglutide 3.0 mg and 4.5 mg are yet to be fully established.
A limitation of this study is comparison of estimands across trials because there are subtle differences in the handling of intercurrent events, with different criteria for initiation of rescue medication. Furthermore, it was not possible to conduct an analysis based on the results of the treatment policy estimand in SUSTAIN FORTE (14) and SUSTAIN 7 (4), similar to the treatment-regimen estimand in AWARD-11 (11), which included efficacy data for patients regardless of treatment discontinuation or rescue medication. This was because confidence intervals and SEs for estimates on treatment level for the treatment-regimen estimand from AWARD-11 have not been published. However, the trial product estimand may be seen as the more relevant estimand for comparisons between treatments, as it provides estimates of treatment effects without potential confounding because of intercurrent events.
Despite the limitations, the ML-NMR approach allowed for a robust ITC of changes in HbA1c and body weight associated with semaglutide 2.0 mg compared with dulaglutide 3.0 mg and 4.5 mg, and showed consistent results regardless of the target population used in analyses. As for any ITC, a head-to-head trial would be required to fully validate the findings.
Conclusion
This ITC demonstrated significantly greater reductions from baseline in HbA1c and body weight with semaglutide 2.0 mg vs dulaglutide 3.0 mg and 4.5 mg. The findings of this study, and particularly the results provided in different trial populations, provide important comparative effectiveness information until randomized head-to-head studies become available.
Acknowledgments
The authors thank Sophie Doran, PhD, from DRG Abacus (part of Clarivate), for providing medical writing support, which was funded by Novo Nordisk A/S.
Glossary
Abbreviations
- AWARD
A Study of the Efficacy and Safety of Dulaglutide (LY2189265) in Participants With Type 2 Diabetes
- BMI
body mass index
- CrI
95% credible interval
- EMA
European Medicines Agency
- FDA
US Food and Drug Administration
- GLP-1 RA
glucagon-like peptide-1 receptor agonist
- HbA1c
glycated hemoglobin
- IPD
individual patient data
- ITC
indirect treatment comparison
- MAIC
matching-adjusted indirect comparison
- ML-NMR
multilevel network meta-regression
- OR
odds ratio
- SUSTAIN
A Research Study to Compare Two Doses of Semaglutide Taken Once Weekly in People With Type 2 Diabetes
- T2D
type 2 diabetes
Funding
This study was funded by Novo Nordisk A/S.
Author Contributions
All authors contributed to the conception, drafting, and critical editing of the manuscript. R.B. conducted the statistical analyses.
Disclosures
I.L. has received research funding, advisory/consulting fees, and/or other support from Novo Nordisk, Eli Lilly, Sanofi, AstraZeneca, Boehringer Ingelheim, Janssen, Intercept, Intarcia, TARGETPharma, Merck, Pfizer, Novartis, GI Dynamics, Mylan, Mannkind, Valeritas, Bayer, and Zealand Pharma. R.P. reports consulting fees from AstraZeneca; consulting fees from Glytec, LLC; grants from Hanmi Pharmaceutical Co.; grants and consulting fees from Janssen; consulting fees from Merck; grants from Metavention; consulting fees from Mundipharma; grants, speaker fees, and consulting fees from Novo Nordisk; consulting fees from Pfizer; grants from Poxel SA; grants and consulting fees from Sanofi; consulting fees from Scohia Pharma Inc.; consulting fees from Sun Pharmaceutical Industries; and personal consulting fees from Sanofi US Services, Inc. Except for consulting fees in February 2018 and June 2018 from Sanofi US Services, Inc., R.P.’s services were paid for directly to AdventHealth, a nonprofit organization. J.B.-K., J.L., and R.B. are employees of Novo Nordisk A/S.
Data Availability
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Leiter LA, Nauck MA. Efficacy and safety of GLP-1 receptor agonists across the spectrum of type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2017;125(7):419-435. [DOI] [PubMed] [Google Scholar]
- 2. Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of diabetes (EASD). Diabetes Care. 2020;43(2):487-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lau J, Bloch P, Schäffer L, et al. Discovery of the once-weekly glucagon-like peptide-1 (GLP-1) analogue semaglutide. J Med Chem. 2015;58(18):7370-7380. [DOI] [PubMed] [Google Scholar]
- 4. Pratley RE, Aroda VR, Lingvay I, et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6(4):275-286. [DOI] [PubMed] [Google Scholar]
- 5. Dalsgaard NB, Vilsbøll T, Knop FK. Effects of glucagon-like peptide-1 receptor agonists on cardiovascular risk factors: a narrative review of head-to-head comparisons. Diabetes Obes Metab. 2018;20(3):508-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. FDA. Ozempic (semaglutide). Highlights of prescribing information. 2017. Accessed December 24, 2021.https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209637lbl.pdf.
- 7. EMA. Ozempic (semaglutide). Summary of product characteristics. 2020. Accessed December 24, 2021.https://www.ema.europa.eu/en/documents/product-information/ozempic-epar-product-information_en.pdf.
- 8. EMA. Trulicity (dulaglutide). Summary of product characteristics. 2021. Accessed December 21, 2021.https://www.ema.europa.eu/en/documents/product-information/trulicity-epar-product-information_en.pdf
- 9. FDA. Trulicity (dulaglutide). Summary of product characteristics. 2014. Accessed December 24, 2021.https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/125469Orig1s000Lbl.pdf.
- 10. Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes Care. 2009;32 Suppl 2(Suppl 2):S151-S156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Frias JP, Bonora E, Nevarez Ruiz L, et al. Efficacy and safety of dulaglutide 3.0 mg and 4.5 mg versus dulaglutide 1.5 mg in metformin-treated patients with type 2 diabetes in a randomized controlled trial (AWARD-11). Diabetes Care. 2021;44(3):765-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. FDA. Trulicity (dulaglutide). Summary of product characteristics. 2020. Accessed December 24, 2021.https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125469s036lbl.pdf.
- 13. Pratley RE, Catarig A-M, Lingvay I, et al. An indirect treatment comparison of the efficacy of semaglutide 1.0 mg versus dulaglutide 3.0 and 4.5 mg. Diabetes Obes Metab. 2021;23(11):2513-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Frías JP, Auerbach P, Bajaj HS, et al. Efficacy and safety of once-weekly semaglutide 2.0 mg versus 1.0 mg in patients with type 2 diabetes (SUSTAIN FORTE): a double-blind, randomised, phase 3B trial. Lancet Diabetes Endocrinol. 2021;9(9):563-574. [DOI] [PubMed] [Google Scholar]
- 15. Phillippo DM, Dias S, Ades AE, et al. Multilevel network meta-regression for population-adjusted treatment comparisons. J R Stat Soc Ser A Stat Soc. 2020;183(3):1189-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. National Institute for Health and Care Excellence Decision Support Unit. CHTE2020 Sources and synthesis of evidence; update to evidence synthesis methods. 2020.
- 17. Phillippo DM, Ades AE, Dias S, Palmer S, Abrams KR, Welton NJ. NICE DSU technical support document 18: methods for population-adjusted indirect comparisons in submissions to NICE.2016. Accessed December 24, 2021. http://nicedsu.org.uk/wp-content/uploads/2017/05/Population-adjustment-TSD-FINAL.pdf.
- 18. Phillippo DM, Ades AE, Dias S, Palmer S, Abrams KR, Welton NJ. Methods for population-adjusted indirect comparisons in health technology appraisal. Med Decis Making. 2018;38(2):200-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gelman A, Carlin JB, Stern HS, Dunson DB, Vehtari A, Rubin DB. Bayesian data analysis. Third edition (with errors fixed as of 15 February 2021). Accessed December 24, 2021. http://www.stat.columbia.edu/~gelman/book/BDA3.pdf.
- 20. Pratley RE, Aroda VR, Catarig A-M, et al. Impact of patient characteristics on efficacy and safety of once-weekly semaglutide versus dulaglutide: SUSTAIN 7 posthoc analyses. BMJ Open. 2020;10(11):e037883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Signorovitch JE, Sikirica V, Erder MH, et al. Matching-adjusted indirect comparisons: a new tool for timely comparative effectiveness research. Value Health. 2012;15(6):940-947. [DOI] [PubMed] [Google Scholar]
- 22. Aroda VR, Saugstrup T, Buse JB, Donsmark M, Zacho J, Davies MJ. Incorporating and interpreting regulatory guidance on estimands in diabetes clinical trials: the PIONEER 1 randomized clinical trial as an example. Diabetes Obes Metab. 2019;21(10):2203-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834-1844. [DOI] [PubMed] [Google Scholar]
- 24. Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 2019;394(10193):121-130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References.