Abstract
Purpose
Little is known about the relations between changes in circulating microRNA-122 (miR-122) and liver fat in response to weight-loss interventions. We aimed to investigate the association between miR-122 and changes of hepatic fat content during 18-month diet and physical activity interventions.
Methods
The CENTRAL trial is an 18-month randomized, controlled trial among adults with abdominal obesity or dyslipidemia. Subjects were randomly assigned to a low-fat diet or a Mediterranean/low-carbohydrate diet. After 6 months of dietary intervention, each diet group was further randomized into added physical activity groups or no added physical activity groups for the following 12 months of intervention. The current study included 220 participants at baseline and 134 participants with repeated measurements on serum miR-122 and hepatic fat content over 18 months.
Results
Serum miR-122 significantly increased from baseline to 18 months, while no difference was observed across the 4 intervention groups. We found a significant association between miR-122 and hepatic fat content at baseline, as per unit increment in log-transformed miR-122 was associated with 3.79 higher hepatic fat content (P < 0.001). Furthermore, we found that higher elevations in miR-122 were associated with less reductions in hepatic fat percentage during 18-month interventions (β = 1.56, P = 0.002). We also found a significant interaction between changes in miR-122 and baseline fasting plasma glucose with hepatic fat content changes in 18 months (P interaction = 0.02).
Conclusions
Our data indicate that participants with higher elevation in serum miR-122 may benefit less in reduction of hepatic fat content in response to diet and physical activity interventions.
Keywords: microRNA-122, hepatic fat, weight-loss interventions, randomized controlled trial
Compelling evidence has implicated obesity-related ectopic fat deposition in the liver in the development of a variety of disorders such as cardiovascular disease, diabetes, and cancer, independent of overall adiposity (1,2). MicroRNAs (miRNAs) are a group of small, noncoding, highly conserved RNAs that pair to target messenger RNAs and modulate gene expression through posttranscriptional repression (3). Recently emerging studies suggest that circulating miRNAs may play a critical role in linking hepatic fat and obesity related cardiometabolic complications (4,5).
MiR-122 is a liver-specific miRNA, which accounts for approximately 70% of the total miRNA pool in the organ (6). MiR-122 is released into the circulation constantly and circulating miR-122 level is highly correlated with hepatic miR-122 expression (4,7). MiR-122 has been reported to be involved in the regulation of liver lipid and glucose metabolism (4) and associated with obesity (8). Recent epidemiological studies found that circulating miR-122 levels were associated with liver fat deposition and the development of fatty liver disease (9). In addition, data from the animal studies suggest that miR-122 expression in the liver may be changed by a high-fat diet or physical activity intervention (10,11); however, thus far no study has assessed whether diet and lifestyle interventions may affect the temporal changes in circulating miR-122, and whether such changes are related to hepatic fat distribution.
In our 18-month randomized diet and physical activity intervention study, the CENTRAL trial, we found that interventions with weight-loss diets and improved physical activity led to reduction of intrahepatic fat (1). In addition, the cardiometabolic benefits of the Mediterranean/low-carbohydrate (MED/LC) diet over low-fat (LF) diet might be mediated by the decrease in hepatic fat content (12). In the current study, we analyzed whether the levels of miR-122 in blood were altered during the interventions and assessed the association between changes in miR-122 and hepatic fat content in response to the interventions in the CENTRAL trial.
Methods
Study Design and Participants
The CENTRAL trial is a randomized clinical trial conducted between October 2012 and April 2014 in Israel. The study design and sample collection have been described elsewhere (1). In brief, 278 subjects with abdominal obesity or dyslipidemia were randomly assigned to 2 equal-caloric diets of a LF diet (n = 139) or a MED/LC diet (n = 139). For male participants, abdominal obesity was defined as waist circumference (WC) > 102 cm, and dyslipidemia was defined as serum triglycerides > 150 mg/dL and high-density lipoprotein cholesterol (HDL-c) < 40 mg/dL. The corresponding criteria for women were abdominal obesity > 88 cm, serum triglycerides > 150 mg/dL, and HDL-c < 50 mg/dL. After 6 months of dietary intervention, each diet group was further randomized into added physical activity groups (LFPA+, MED/LCPA+) or no added physical activity groups (LFPA−, MED/LCPA−) for the following 12 months of intervention. The main exclusion criteria in the trial included serum creatinine ≥ 2 mg/dL, impaired liver function (≥3-fold the upper level of alanine amino transferase and aspartate amino transferase), active cancer, pregnancy or lactation, being physically active or unable to participate in physical activity, or participation in another trial (1). All participants provided written informed consent, and the study was approved by the Medical Ethics Board and Helsinki Committee of the Soroka University Medical Center.
In the current study, 227 participants had measurements of circulating miR-122 at baseline, and 157 of the participants had circulating miR-122 data after 18 months. After excluding those with missing data on liver fat content or covariates including WC, physical activity, triglycerides, and HDL-c, 220 participants at baseline and 134 participants at 18 months were included in the final analysis. The flowchart of the participants selection is shown in Supplementary Figure 1 (13).
Assessments of microRNA
We extracted total serum RNA using the RNeasy Serum/Plasma Advanced kit (Qiagen, Hilden Germany). We used up to 20 ng of total serum RNA in the small RNA protocol of the NEBNext® Small RNA Library Prep Set for Illumina according to instructions of the manufacturer (NEB, Ipswich, MA, USA). The barcoded libraries between 140 and 165 bp were purified and quantified using the Library Quantification Kit-Illumina/Universal (KAPA Biosystems, Wilmington, MA, USA). In addition, the size distribution of the miRNA libraries was visualized on a fragment analyzer (Agilent, Santa Clara, CA, USA). A total of 384 samples (227 at baseline; 157 at 18 months) were of sufficient quality to proceed to sequence. A pool of up to 100 libraries was used for cluster generation at a concentration of 1.5 pM followed by sequencing of 75 bp using an Illumina NextSeq 550 sequencer at the sequencing core facility of the University Leipzig (Faculty of Medicine) employing version 2.5 flowcell and chemistry according to the instructions of the manufacturer (Illumina, San Diego, CA, USA). Demultiplexing of raw reads, adapter trimming, and quality filtering were conducted using Illumina bcl2fastq conversion (v2.20.0) and cutadapt software (v1.18) (14,15). Mapping against the human reference genome (hg38) and miRbase reference sequences (v22) were conducted using Bowtie2 (16). Read counts were calculated with the Rsamtools R bioconductor package and normalized using the DESeq2 (17) and EdgeR (18) R bioconductor packages. A total of 2656 miRNAs entries are present in the current miRBase (v22) and can be annotated if the homologous sequences are present. To get a profile for the serum samples, we counted the numbers of miRNA entities of miRBase for which we found homologous sequences in the libraries. We detected 1145 miRNAs with at least 10 counts in at least 1 sample. The median of miRNA entities per individual sample is 419. This represents a considerable diversity of miRNAs in our serum samples and compares very well to data from literature (19). To reduce noise from the data before final comparison, we removed outliers: (1) samples that do not have at least 200 detectable entities of miRNAs (7 samples) and (2) miRNA entities that are detectable in less than 20 samples.
Measurements of Hepatic Fat Content
Body fat depots/deposits were measured using 45-minute 3-Tesla magnetic resonance imaging (Ingenia 3.0 T, Philips Healthcare) scans at baseline and after 18 months in the CENTRAL trial (20). The validation of the measurements has been described previously (1). The percentage of hepatic fat was quantified using PRIDE software from Philips Medical Systems. We calculated the mean percentage from four 2-dimensional slices using the region of interest approach, which is based on measurements of tissue densities (fat/fat + water) with the fat ratio calculation (21). More specifically, each slice was divided into quarters (3-cm intervals), and regions of interest in each of the 4 quarters were chosen to represent the entire liver. The mean percentage of fat for each slice and quarter was determined to calculate the mean value of the whole liver fat percentage.
Other Measurements
Information on age and sex was collected at baseline. Physical activity was assessed using metabolic equivalents (METs) per week. Baseline height and monthly body weight were measured by standard wall-mounted stadiometer. Body mass index (BMI) was calculated by dividing weight in kilograms by the square of height in meters. Furthermore, WC was measured using an anthropometric measuring tape. Blood pressure was measured twice with an automatic blood pressure monitor (Datascope Accutorr 4, Datascope). In addition, biochemical analyses were performed using fasting blood samples taken at baseline and at 18 months at the laboratories of the University of Leipzig, Germany. Serum total cholesterol, HDL-c, low-density lipoprotein cholesterol (LDL-c), and triglycerides were measured with a Cobas 6000 automatic analyzer (Roche). Fasting plasma glucose was measured by Roche GLUC 3 (hexokinase method). Plasma insulin was measured with an enzyme immunometric assay (Immulite automated analyzer, Diagnostic Products). Homeostasis model assessment of insulin resistance was calculated based on the equation: insulin (U/mL) × fasting plasma glucose (mmol/L)/22.5.
Statistical Analysis
Data on miR-122 were log-transformed to improve normality. Generalized linear models were used to compare the trajectories of miR-122 at baseline and at 18 months across the intervention groups. The Kruskal-Wallis test and Chi-square test were used to assess differences in continuous variables and categorical variables across miR-122 groups, respectively. We used the generalized linear model to compute β (SE) for the association between miR-122 and hepatic fat content at the baseline measurement with adjustment for age (continuous), sex (men, women), and baseline abdominal obesity status (yes/no) in Model 1. We further adjusted for baseline physical activity (MET/week, continuous), serum HDL-c (mg/dL, continuous), serum triglycerides (mg/dL, continuous), and fasting glucose levels (mg/dL, continuous) in Model 2.
Changes in miR-122 during the 18-month intervention were calculated to further examine the effect of each log-transformed change in the biomarker on hepatic fat content change in 18 months. In Model 1, we adjusted for age (continuous), sex (men, women), intervention groups (LFPA+, MED/LCPA+, LFPA−, MED/LCPA−), concurrent weight change percentage [weight change percentage = (weight at 18 month/weight at baseline) − 1*100], liver fat content at baseline, and circulating miR-122 levels at baseline. In Model 2, we additionally adjusted for concurrent changes in physical activity (MET/week, continuous), serum HDL-c (mg/dL, continuous), serum triglycerides (mg/dL, continuous), and fasting glucose levels (mg/dL, continuous). The linear trend was tested by modeling log-transformed miR-122 continuously. We also conducted stratified analyses by baseline age (<55 vs ≥55 years), BMI (<30 vs ≥30 kg/m2), abdominal obesity (yes vs no), and tertile groups of physical activity, HDL-c, LDL-c, triglycerides, and fasting plasma glucose levels. The multiplicative interactions between miR-122 and stratified factors were tested by including cross-product interaction terms of miR-122 changes and the baseline variables in Model 2.
We defined nonalcoholic fatty liver disease (NAFLD) as hepatic fat content > 5% to evaluate whether circulating levels of miR-122 could predict NAFLD at baseline. In total, 117 NAFLD cases and 103 NAFLD-free participants were identified. Two thirds (68 cases, 79 NAFLD-free participants) and one third (49 cases, 24 NAFLD-free participants) of the participants were selected as the training set and testing set, respectively. A logistic regression model with adjustment for age (continuous), sex (men, women), baseline abdominal obesity status (yes/no), baseline physical activity (MET/week, continuous), serum HDL-c (mg/dL, continuous), serum triglycerides (mg/dL, continuous), and fasting glucose levels (mg/dL, continuous) was adopted for the prediction.
Statistical analyses were performed using SAS, version 9.4 (SAS Institute, Cary, NC, USA). All statistical comparisons were 2-sided, and a P-value < 0.05 was considered statistically significant.
Results
The baseline characteristics of the participants according to the tertiles of baseline log-transformed serum miR-122 levels are shown in Table 1. The log-transformed median (interquartile range) miR-122 at baseline measurement was 9.0 (0.6), 9.9 (0.3), and 10.8 (0.6) in the tertile groups, respectively. We did not find significant differences for baseline age, sex, BMI, weight, WC, physical activity, total cholesterol, HDL-c, LDL-c, fasting plasma glucose, and hemoglobin A1c across the miR-122 tertile groups. Further, elevation in serum miR-122 level was associated with higher serum triglycerides (P = 0.01) and homeostasis model assessment of insulin resistance (P = 0.04). In addition, hepatic fat content was significantly higher among participants with elevated serum miR-122 levels (P < 0.001).
Table 1.
Baseline characteristics of the participants according to the tertiles of miR-122
| Tertile 1 | Tertile 2 | Tertile 3 | P-value | |
|---|---|---|---|---|
| n | 74 | 73 | 73 | |
| Age, years | 48.1 (16.9) | 48.1 (16.0) | 44.1 (17.7) | 0.47 |
| Male, n (%) | 60 (81.1) | 67 (91.8) | 66 (90.4) | 0.10 |
| Liver fat content, % | 3.4 (8.9) | 5.2 (11.1) | 15.0 (20.9) | <0.001 |
| BMI, kg/m2 | 30.3 (5.3) | 30.4 (4.2) | 30.2 (5.0) | 0.95 |
| Weight, kg | 88.7 (16.0) | 90.6 (16.1) | 91.4 (16.0) | 0.65 |
| Waist circumference, cm | ||||
| Men | 106.0 (11.5) | 107.0 (13.0) | 105.7 (10.0) | 0.61 |
| Women | 92.5 (9.0) | 94.0 (12.0) | 102.5 (8.0) | 0.15 |
| Physical activity, MET/week | 28.2 (41.6) | 22.8 (24.2) | 23.7 (29.9) | 0.57 |
| Total cholesterol, mg/dL | 197.7 (57.5) | 206.2 (49.0) | 200.4 (53.3) | 0.11 |
| Serum HDL-c, mg/dL | ||||
| Men | 42.1 (15.1) | 41.3 (15.1) | 39.0 (12.7) | 0.11 |
| Women | 48.6 (19.3) | 61.4 (19.7) | 45.9 (17.4) | 0.30 |
| Serum LDL-c, mg/dL | 117.4 (41.7) | 125.1 (43.2) | 118.9 (47.5) | 0.36 |
| Serum triglycerides, mg/dL | 121.7 (76.1) | 149.6 (115.1) | 154.0 (92.0) | 0.01 |
| Fasting plasma glucose, mg/dL | 101.4 (16.9) | 104.1 (15.0) | 102.5 (13.2) | 0.81 |
| HbA1c, % | 5.5 (0.5) | 5.5 (0.5) | 5.5 (0.5) | 0.91 |
| HOMA-IR | 3.4 (2.3) | 3.8 (3.1) | 3.9 (3.5) | 0.04 |
Data are median (interquartile range) unless otherwise indicated. Tertiles are based on log-transformed miR-122 levels at baseline.
Abbreviations: BMI, body mass index; HbA1c, hemoglobin A1c; HDL-c, high-density lipoprotein cholesterol; HOMA-IR, homeostatic model of insulin resistance; LDL-c, low-density lipoprotein cholesterol; MET, metabolic equivalents; miR-122, microRNA-122.
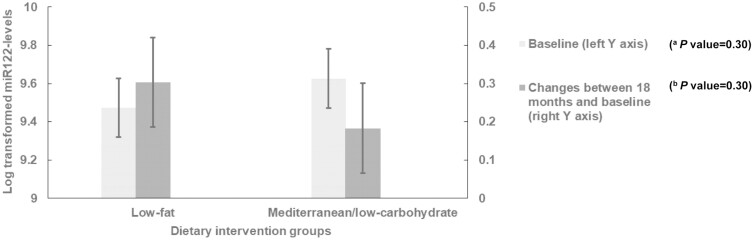
Log-transformed miR-122 levels among different dietary intervention groups at baseline and during 18 months for participants with measurements at both time points are shown in Figure 1. There was no significant difference in serum miR-122 levels between LF and MED/LC groups at baseline (P = 0.30). In the 2 dietary intervention groups, we observed higher miR-122 levels at 18-month compared with the measurement at baseline (P < 0.05 for both groups vs baseline). However, serum miR-122 change between 18 months and baseline did not show significant difference between the 2 dietary intervention groups with adjustment for physical activity interventions (P = 0.30).
Figure 1.
Log transformed microRNA-122 (miR-122) levels between different dietary intervention groups. Values were expressed as adjusted least square means ± SE for miR-122 levels at baseline and changes between 18 months and baseline. (A) Adjusted for age, sex, and abdominal obesity status. (B) Adjusted for age, sex, concurrent weight change percentage, physical activity intervention groups, and baseline miR-122 levels. The number of participants was 70 for low-fat group and 64 for Mediterranean/low-carbohydrate group at both baseline and 18 months.
We found a significant association between log-transformed miR-122 and hepatic fat percentage at baseline (Table 2). We observed higher hepatic fat percentages in tertile 2 and tertile 3 groups of miR-122 when compared with tertile 1 group (P for trend < 0.001), as the median (interquartile range) of hepatic fat content in the tertile groups of miR-122 was 3.4 (8.9), 5.2 (11.1), and 15.0 (20.9), respectively. In the model adjusted for age, sex, and abdominal obesity status, we observed that per unit increment in log-transformed miR-122 was associated with 4.11 higher hepatic fat percentages (P trend < 0.001). After further adjustment for physical activity, serum HDL-c, serum triglycerides, and fasting glucose levels, the association between miR-122 and hepatic fat content remained significant (β = 3.79, P trend < 0.001).
Table 2.
The association between liver fat content and log-transformed miR-122 levels at baseline
| Tertiles categories of miR-122 | β (SE) per log-transformed miR-122 increment | P for trend | |||
|---|---|---|---|---|---|
| T1 | T2 | T3 | |||
| n | 74 | 73 | 73 | ||
| miR-122a | 9.0 [8.7, 9.3] | 9.9 [9.7, 10.0] | 10.8 [10.4, 11.0] | ||
| Hepatic fat contenta | 3.4 [1.0, 9.8] | 5.2 [2.1, 13.1] | 15.0 [3.7, 24.6] | ||
| Model 1b | Reference | 0.56 (1.48) | 7.92 (1.48) | 4.11 (0.73) | <0.001 |
| Model 2c | Reference | 0.09 (1.48) | 7.25 (1.47) | 3.79 (0.73) | <0.001 |
Abbreviation: miR-122, microRNA-122.
aData are given as median [25th, 75th].
bAdjusted for age, sex, abdominal obesity status.
cAdjusted for age, sex, abdominal obesity status, physical activity, serum high-density lipoprotein cholesterol, serum triglycerides, and fasting glucose levels.
Additionally, the results showed that miR-122 levels could predict NAFLD at baseline. We observed a P-value (testing global null hypothesis: β = 0) of <0.05 in the model adjusted for age, sex, abdominal obesity status, physical activity, serum HDL-c, serum triglycerides, and fasting glucose levels. Furthermore, miR-122 can predict liver steatosis with an acceptable accuracy (73%) and sensitivity (84%) but with a low specificity (50%).
At 18 months, the median (25th, 75th) change in miR-122 was 0.23 (−0.28, 0.84). The correlation between change in miR-122 and change in hepatic fat content is shown in Supplementary Figure 2 (13). We further assessed the dose-response association between change in log-transformed miR-122 and change in hepatic fat content during 18-month interventions using the restricted cubic spline analysis. The results showed that a nonlinear relationship between change in log-transformed miR-122 and change in hepatic fat content during 18-month interventions, where the change in hepatic fat percentage during 18-month interventions was the strongest when the change in log-transformed miR-122 was 0.6 [Supplementary Figure 3 (13)]. The associations between changes in miR-122 and hepatic fat percentage changes are shown in Table 3. In the model adjusted for age, sex, intervention groups, concurrent weight change percentage, baseline miR-122, and baseline liver fat content, we found that a higher elevation in miR-122 during 18 months was associated with a less loss in hepatic fat percentage at 18 months (β = 1.45, P trend = 0.005). The association remained significant with additional adjustment for concurrent changes in physical activity, serum HDL-c, serum triglycerides, and fasting glucose levels, as per unit elevation in log-transformed miR-122 was associated with 1.56 less percentage in hepatic fat reductions (P trend = 0.002).
Table 3.
The association between changes in liver fat content and log-transformed miR-122 between 18 months and baseline
| Tertiles categories of miR-122 change | β (SE) per log-transformed miR-122 change | P for trend | |||
|---|---|---|---|---|---|
| T1 | T2 | T3 | |||
| n | 44 | 46 | 44 | ||
| MiR-122 changea | −0.6 [−1.0, −0.3] | 0.2 [0.1, 0.4] | 1.1[0.8, 1.3] | ||
| Hepatic fat content changea | −5.4 [−13.2, −0.5] | −1.3 [−4.9, 0.5] | −0.8[−3.4, 0.3] | ||
| Model 1b | Reference | 2.66 (0.85) | 2.47 (0.97) | 1.45 (0.51) | 0.005 |
| Model 2c | Reference | 3.06 (0.83) | 2.91 (0.95) | 1.56 (0.50) | 0.002 |
Abbreviation: miR-122, microRNA-122.
aData were shown as median [25th, 75th].
bAdjusted for age, sex, intervention groups, concurrent weight change percentage, baseline miR-122 levels, and baseline liver fat content.
cAdjusted for age, sex, intervention groups, concurrent weight change percentage, baseline miR-122 levels, baseline liver fat content, concurrent physical activity change, concurrent serum high-density lipoprotein cholesterol change, concurrent serum triglycerides change, and concurrent fasting glucose change.
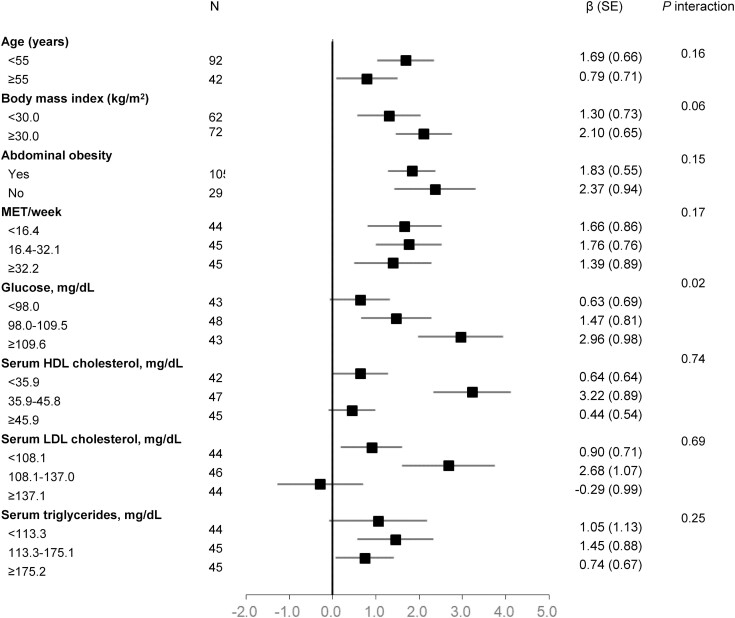
The stratified analyses showed that the associations between changes in miR-122 and hepatic fat content changes during 18-month interventions were consistently significant across the strata of baseline age, BMI, abdominal obesity status, physical activity, serum HDL-c, serum LDL-c, or serum triglycerides (all P interaction > 0.05) (Fig. 2). However, we found a significant interaction between changes in miR-122 and baseline fasting plasma glucose with hepatic fat content changes (P interaction = 0.02). The association between changes in miR-122 and hepatic fat content changes appeared to be stronger in the tertile 3 group of fasting plasma glucose (β = 2.96, P trend = 0.003) compared with the tertile 2 group (β = 1.47, P trend = 0.07) and the tertile 1 group (β = 0.63, P trend = 0.36).
Figure 2.
Association between changes in liver fat content and log-transformed microRNA-122 (miR-122) levels between 18 months and baseline, stratified by baseline risk factors. Adjusted for age, sex, intervention groups, concurrent weight change percentage, concurrent physical activity change, concurrent serum high-density lipoprotein cholesterol change, concurrent serum triglycerides change, concurrent fasting glucose levels change, baseline miR-122 levels, and baseline liver fat content.
Discussion
In the current study of participants with abdominal obesity or dyslipidemia from an 18-month randomized, controlled diet and physical activity intervention trial, we observed that serum miR-122 levels were positively associated with hepatic fat percentage at baseline. In addition, we found that a greater elevation in miR-122 from baseline to 18 months was associated with a greater reduction in hepatic fat content during the 18-month intervention process. Furthermore, we found that the association of changes in miR-122 with hepatic fat content changes was modified by baseline fasting plasma glucose, as the association was stronger among participants with higher fasting plasma glucose levels.
In the present study, we observed that higher serum levels of miR-122 were positively associated with hepatic fat content. The result was consistent with previous epidemiological investigations that showed evident correlation between high circulating miR-122 and fatty liver disease (hepatic fat content > 5%) (9,22-25). In addition, the prediction model showed that miR-122 levels could predict NAFLD at baseline to some extent. Our data indicate that miR-122 might be a potential marker to assess the severity of hepatic fat accumulation. However, the internal and external validity of the prediction model might be interpreted with caution due to the limited sample size in the analysis. Further studies with larger sample size are needed to verify our findings. A novel observation is that changes in serum miR-122 were significantly associated with hepatic fat content changes in response to diet and physical activity interventions, independent of concurrent weight loss and the types of interventions. We observed that a greater elevation in miR-122 was associated with a lower loss in hepatic fat content. The findings suggest that a higher magnitude of increase in serum miR-122 was associated with less hepatic fat reductions during interventions. Our findings may provide new insight into the molecular biology of liver fat deposition during diet and physical activity interventions, and miR-122 might be a potential marker to evaluate treatment responses to interventions.
In the current intervention study, we observed an elevation in serum miR-122 after 18-month LF or MED/LC diet with/without physical activity interventions. Similarly, several animal studies have shown a significant downregulation of miR-122 in response to high-fat diet (26,27). In addition, high-intensity interval training may increase miR-122 expression in rats (28). However, the miR-122 expression under diet and physical activity interventions in the current study showed different results from previous population-based studies. For example, a 12-week weight-loss intervention trial showed that serum miR-122 decreased in response to fiber supplement intervention (29). In addition, the Physical Activity Following Surgery Induced Weight Loss study indicated that plasma miR-122 reduced in both exercise and control groups during 6 months following Roux-en-Y gastric bypass surgery-induced weight loss (30). Discrepancies in this sense may arise from differences regarding intervention methods, intervention period, or blood sources of miR-122 across studies. Notably, there was no difference in miR-122 changes between LF and MED/LC intervention groups with adjustment for physical activity interventions in the current analysis, which could be partially due to the limited sample size.
Growing evidence has implicated miR-122 expression in regulation of hepatic fat content. For example, several experimental studies have shown that miR-122 might promote hepatic lipogenesis through upregulating the expression of lipid biosynthesis genes and downregulating hepatic fatty acid oxidation (11,31,32). In addition, antagonism of miR-122 in nonhuman primates is related to lower plasma cholesterol levels (33,34). The mechanisms of kinetic variation of miR-122 and liver fat deposition have not been thoroughly understood. We did not find any significant association of changes in miR-122 with serum biomarkers changes, such as lipids or glucose. Previous studies have demonstrated that participants with mild fatty liver disease may have higher hepatic and serum miR-122 compared those with severe fatty liver disease (27), which may partially explain the association between changes in serum miR-122 and hepatic fat content changes in the current analysis. Further studies are needed to explore the role of miR-122 in liver fat deposition during diet and physical activity interventions.
Intriguingly, we found a significant interaction between baseline fasting plasma glucose and changes in miR-122 in relation to hepatic fat content changes during 18-month interventions. The association between elevation in miR-122 and hepatic fat percentage reduction was strengthened by higher fasting plasma glucose levels. Although the potential mechanisms underlying the observed interaction between miR-122 changes and fasting plasma glucose remain unknown at this point, existing evidence suggests that the interaction might be biologically plausible. For instance, several epidemiological studies have shown that participants with type 2 diabetes had higher circulating miR-122 levels compared those without diabetes (35,36). In addition, experimental studies demonstrated that miR-122 was significantly elevated in type 2 diabetic mice compared to controls (37,38). In addition, the level of hepatocyte nuclear factor 4 alpha was higher in the liver of diabetic animals compared to the controls, and the expression of hepatocyte nuclear factor 4 alpha was correlated with an increased expression of miR-122 (37,39). Given the complex pathophysiological mechanisms underlying hepatic fat distribution in diabetes, further investigations are needed to explore the relations between miR-122 and hepatic fat content in patients with diabetes and the complex interaction with glycemic control.
To the best of our knowledge, this is the first prospective study to assess the association between changes in serum miR-122 and hepatic fat content changes in response to diet and physical activity interventions using data from a long-term randomized clinical trial. The major strengths of our study included analyses of dynamic changes with repeated measurements of serum miR-122 and hepatic fat content over 18 months’ diet and physical activity interventions. In addition, hepatic fat percentage was assessed by 3-Tesla magnetic resonance imaging, which has been validated against liver biopsy (21). Nevertheless, several limitations warrant consideration. First, we did not replicate our observed associations in other trials; thus, further research is needed to confirm these findings. Second, although our study is a large trial with repeated miRNA measurements, the relatively small sample size of the subgroups may limit the power to detect potential interactions. Third, the causality could not be determined since the changes in hepatic fat content are accompanied by serum miR-122 changes during the same time. Finally, our results might not be generalized to other populations since most of the participants are males.
Conclusions
In conclusion, we found that participants with a greater elevation in serum miR-122 levels had a less loss in hepatic fat content during diet/physical activity-induced weight loss. We also observed a significant interaction between miR-122 changes and baseline fasting plasma glucose on changes in hepatic fat content, suggesting a strengthened association with higher fasting plasma glucose levels. Our findings provide novel evidence that miRNAs may be useful biomarkers for better understanding of adipose tissues and deposits in response to distinct lifestyles interventions.
Funding
This work was supported by grants from the National Heart, Lung, and Blood Institute (HL071981, HL034594, HL126024), the National Institute of Diabetes and Digestive and Kidney Diseases (DK115679, DK091718, DK100383, DK078616), and the Fogarty International Center (TW010790). L.Q. is a recipient of the American Heart Association Scientist Development Award (0730094N). L.Q. is also supported by the National Institute of General Medical Sciences (P20GM109036). The study is also funded by China Postdoctoral Science Foundation (BX2021021).
Clinical Trial Registration
ClinicalTrials.gov: NCT01530724.
Disclosures
The authors declare no conflict of interest.
Data Availability
Data described in the manuscript, code book, and analytic code will be made available upon reasonable request.
References
- 1. Gepner Y, Shelef I, Schwarzfuchs D, et al. Effect of distinct lifestyle interventions on mobilization of fat storage pools: CENTRAL magnetic resonance imaging randomized controlled trial. Circulation. 2018;137(11):1143-1157. [DOI] [PubMed] [Google Scholar]
- 2. Despres JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126(10):1301-1313. [DOI] [PubMed] [Google Scholar]
- 3. Friedman RC, Farh KKH, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19(1):92-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Willeit P, Skroblin P, Kiechl S, Fernandez-Hernando C, Mayr M. Liver microRNAs: potential mediators and biomarkers for metabolic and cardiovascular disease? Eur Heart J. 2016;37(43):3260-3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang YS, Jin PP, Liu JJ, Xie X. Exosomal microRNA-122 mediates obesity-related cardiomyopathy through suppressing mitochondria ADP-ribosylation factor-like 2. Clin Sci. 2019;133(17):1871-1881. [DOI] [PubMed] [Google Scholar]
- 6. Jopling C. Liver-specific microRNA-122: biogenesis and function. RNA Biol. 2012;9(2):137-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miyaaki H, Ichikawa T, Kamo Y, et al. Significance of serum and hepatic microRNA-122 levels in patients with non-alcoholic fatty liver disease. Liver Int. 2014;34(7):e302-e307. [DOI] [PubMed] [Google Scholar]
- 8. Ortega FJ, Mercader JM, Catalan V, et al. Targeting the circulating microRNA signature of obesity. Clin Chem. 2013;59(5):781-792. [DOI] [PubMed] [Google Scholar]
- 9. Pirola CJ, Gianotti TF, Castano GO, et al. Circulating microRNA signature in non-alcoholic fatty liver disease: from serum non-coding RNAs to liver histology and disease pathogenesis. Gut. 2015;64(5):800-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kalaki-Jouybari F, Shanaki M, Delfan M, Gorgani-Firouzjae S, Khakdan S. High-intensity interval training (HIIT) alleviated NAFLD feature via miR-122 induction in liver of high-fat high-fructose diet induced diabetic rats. Arch Physiol Biochem. 2020;126(3):242-249. [DOI] [PubMed] [Google Scholar]
- 11. Esau C, Davis S, Murray SF, et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006;3(2):87-98. [DOI] [PubMed] [Google Scholar]
- 12. Gepner Y, Shelef I, Komy O, et al. The beneficial effects of Mediterranean diet over low-fat diet may be mediated by decreasing hepatic fat content. J Hepatol. 2019;71(2):379-388. [DOI] [PubMed] [Google Scholar]
- 13. Wang M, Xue Q, Li X, et al. Supplementary information for: Circulating levels of microRNA-122 and hepatic fat change in response to weight-loss interventions: CENTRAL Trial. FigShare. Deposited January 12, 2022. 10.6084/m9.figshare.17156342.v2 [DOI]
- 14. Stokowy T, Eszlinger M, Swierniak M, et al. Analysis options for high-throughput sequencing in miRNA expression profiling. BMC Res Notes. 2014;7:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. 2011. 2011;17(1):3. [Google Scholar]
- 16. Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9(4):357-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McCarthy DJ, Chen Y, Smyth GK. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 2012;40(10):4288-4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yeri A, Courtright A, Reiman R, et al. Total extracellular small RNA profiles from plasma, saliva, and urine of healthy subjects. Sci Rep. 2017;7:44061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thomas EL, Fitzpatrick JA, Malik SJ, Taylor-Robinson SD, Bell JD. Whole body fat: content and distribution. Prog Nucl Mag Res Sp. 2013;73:56-80. [DOI] [PubMed] [Google Scholar]
- 21. Schuchmann S, Weigel C, Albrecht L, et al. Non-invasive quantification of hepatic fat fraction by fast 1.0, 1.5 and 3.0 T MR imaging. Eur J Radiol. 2007;62(3):416-422. [DOI] [PubMed] [Google Scholar]
- 22. Cermelli S, Ruggieri A, Marrero JA, Ioannou GN, Beretta L. Circulating microRNAs in patients with chronic hepatitis C and non-alcoholic fatty liver disease. PLoS One. 2011;6(8):e23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jampoka K, Muangpaisarn P, Khongnomnan K, Treeprasertsuk S, Tangkijvanich P, Payungporn S. Serum miR-29a and miR-122 as potential biomarkers for non-alcoholic fatty liver disease (NAFLD). Microrna. 2018;7(3):215-222. [DOI] [PubMed] [Google Scholar]
- 24. Raitoharju E, Seppala I, Lyytikainen LP, et al. Blood hsa-miR-122-5p and hsa-miR-885-5p levels associate with fatty liver and related lipoprotein metabolism-the young Finns study. Sci Rep. 2016;6:38262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62(1 suppl):S47-S64. [DOI] [PubMed] [Google Scholar]
- 26. Alisi A, Da Sacco L, Bruscalupi G, et al. Mirnome analysis reveals novel molecular determinants in the pathogenesis of diet-induced nonalcoholic fatty liver disease. Lab Invest. 2011;91(2):283-293. [DOI] [PubMed] [Google Scholar]
- 27. Su Q, Kumar V, Sud N, Mahato RI. MicroRNAs in the pathogenesis and treatment of progressive liver injury in NAFLD and liver fibrosis. Adv Drug Deliv Rev. 2018;129:54-63. [DOI] [PubMed] [Google Scholar]
- 28. Kalaki-Jouybari F, Shanaki M, Delfan M, Gorgani-Firouzjae S, Khakdan S. High-intensity interval training (HIIT) alleviated NAFLD feature via miR-122 induction in liver of high-fat high-fructose diet induced diabetic rats. Arch Physiol Biochem. 2020;126(3):242-249. [DOI] [PubMed] [Google Scholar]
- 29. Hess AL, Larsen LH, Udesen PB, Sanz Y, Larsen TM, Dalgaard LT. Levels of circulating miR-122 are associated with weight loss and metabolic syndrome. Obesity (Silver Spring). 2020;28(3):493-501. [DOI] [PubMed] [Google Scholar]
- 30. Lopez YON, Coen PM, Goodpaster BH, Seyhan AA. Gastric bypass surgery with exercise alters plasma microRNAs that predict improvements in cardiometabolic risk. Int J Obesity. 2017;41(7):1121-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Krutzfeldt J, Rajewsky N, Braich R, et al. Silencing of microRNAs in vivo with “antagomirs.” Nature. 2005;438(7068):685-689. [DOI] [PubMed] [Google Scholar]
- 32. Long JK, Dai W, Zheng YW, Zhao SP. miR-122 promotes hepatic lipogenesis via inhibiting the LKB1/AMPK pathway by targeting Sirt1 in non-alcoholic fatty liver disease. Mol Med. 2019;25(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Elmen J, Lindow M, Schutz S, et al. LNA-mediated microRNA silencing in non-human primates. Nature. 2008;452(7189):896-899. [DOI] [PubMed] [Google Scholar]
- 34. Lanford RE, Hildebrandt-Eriksen ES, Petri A, et al. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science. 2010;327(5962):198-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Willeit P, Skroblin P, Moschen AR, et al. Circulating MicroRNA-122 is associated with the risk of new-onset metabolic syndrome and type 2 diabetes. Diabetes. 2017;66(2):347-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. de Candia P, Spinetti G, Specchia C, et al. A unique plasma microRNA profile defines type 2 diabetes progression. PLoS One. 2017;12(12):e0188980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wei S, Zhang M, Yu Y, et al. HNF-4alpha regulated miR-122 contributes to development of gluconeogenesis and lipid metabolism disorders in type 2 diabetic mice and in palmitate-treated HepG2 cells. Eur J Pharmacol. 2016;791:254-263. [DOI] [PubMed] [Google Scholar]
- 38. Delic D, Eisele C, Schmid R, Luippold G, Mayoux E, Grempler R. Characterization of micro-RNA changes during the progression of type 2 diabetes in Zucker diabetic fatty rats. Int J Mol Sci. 2016;17(5):665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li ZY, Xi Y, Zhu WN, et al. Positive regulation of hepatic miR-122 expression by HNF4alpha. J Hepatol. 2011;55(3):602-611. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon reasonable request.