Abstract
Context
Salt sensitivity of blood pressure (SSBP) is associated with increased cardiovascular risk, especially in individuals of African descent, although the underlying mechanisms remain obscure. Lysine-specific demethylase 1 (LSD1) is a salt-sensitive epigenetic regulator associated with SSBP and aldosterone dysfunction. An LSD1 risk allele in humans is associated with SSBP and lower aldosterone levels in hypertensive individuals of African but not European descent. Heterozygous knockout LSD1 mice display SSBP and aldosterone dysregulation, but this effect is modified by age and biological sex. This might explain differences in cardiovascular risk with aging and biological sex in humans.
Objective
This work aims to determine if LSD1 risk allele (rs587618) carriers of African descent display a sex-by-age interaction with SSBP and aldosterone regulation.
Methods
We analyzed 297 individuals of African and European descent from the HyperPATH cohort. We performed multiple regression analyses for outcome variables related to SSBP and aldosterone.
Results
LSD1 risk allele carriers of African (but not European) descent had greater SSBP than nonrisk homozygotes. Female LSD1 risk allele carriers of African descent had greater SSBP, mainly relationship-driven by women with low estrogen (postmenopausal). There was a statistically significant LSD1 genotype-sex interaction in aldosterone response to angiotensin II stimulation in individuals aged 50 years or younger, with female carriers displaying decreased aldosterone responsiveness.
Conclusion
SSBP associated with LSD1 risk allele status is driven by women with a depleted estrogen state. Mechanisms related to a resistance to develop SSBP in females are uncertain but may relate to an estrogen-modulating effect on mineralocorticoid receptor (MR) activation and/or LSD1 epigenetic regulation of the MR.
Keywords: lysine-specific demethylase 1, salt sensitivity of blood pressure, aldosterone, sex, age
Hypertension is a common condition and contributor to increased cardiovascular risk. Heritability, lifestyle, and environmental factors all contribute to the development of hypertension. Among the genes associated with hypertension is lysine-specific demethylase 1 (LSD1) (1-3). LSD1, also known as KDM1A, is a flavin-containing protein based on its ability to bind flavin-adenine dinucleotide. LSD1 can act as either a coactivator or corepressor of gene transcription based on the histone amino acid position where demethylation occurs; if it demethylates K9, it promotes transcription, whereas if it demethylates K4 site, it acts as a corepressor (4, 5). Interest in LSD1’s connection with hypertension spawned from its earlier described control over transcription of steroid receptors (androgen and estrogen receptors) (6-9), which we hypothesize would extend to the mineralocorticoid receptor (MR).
As such, we have documented the association between LSD1 deficiency, dietary salt intake, and salt-sensitive hypertension. In humans, we studied the relationship of the LSD1 genotype and salt sensitivity of blood pressure (SSBP) in a cohort of the International Hypertensive Pathotype (HyperPATH) consortium in which individuals are well characterized, and factors affecting the renin-angiotensin-aldosterone-system (RAAS) and blood pressure (BP) are tightly controlled. Polymorphic variants of LSD1 (including the tagging single-nucleotide variation rs587168) are associated with greater SSBP in hypertensive people of African descent and Hispanics, but not in those of European descent (1). To elucidate the mechanism of SSBP and LSD1 deficiency, we used an LSD1 heterozygous knockout (LSD1+/–) mouse (homozygous knockout is embryonically lethal) (10-12). LSD1 levels are reduced in response to increased salt intake. LSD1+/– male mice display enhanced vascular contractility, reduced relaxation via the nitric oxide–cyclic guanosine monophosphate (NO-cGMP) pathway, and increased BP (10). Aldosterone dysregulation is present both in LSD1+/– male and female mice. However, only LSD1+/– male mice showed greater SSBP (11). The mechanism responsible for the apparent protective effects of female mice from having increased BP in the LSD1-deficient state is uncertain. Estrogen is a logical contributor since it can modulate MR activity. Recently, we showed increased MR expression in LSD1+/– male mice fed a liberal salt (LIB) diet (12). MR target gene expression, the epithelial sodium channel (ENac) α and γ subunits, increased in the absence of elevated aldosterone levels, posing a ligand-independent MR activation in mice with genetic LSD1 deficiency.
We hypothesized that LSD1 gene variants in humans would also lead to aldosterone dysregulation in response to an increase in dietary salt intake leading to SSBP in a race-, sex-, and age-specific manner. To test the hypothesis, we assessed the association of the LSD1 polymorphic variant (rs587168) and SSBP in both cohorts of African descent and European descent regardless of hypertensive state. Then, we evaluated the modulating effect of biological sex on the relationship of LSD1 genotype and SSBP. Finally, we assessed the effect of presumed menopausal state (based on age and hormonal levels) on the interaction between LSD1 and SSBP to explore the role estrogen may play.
Materials and Methods
Study Population
This study is based on data present in the HyperPATH cohort. The HyperPATH consortium is an ongoing, international program to study the genetic underpinnings of the hormonal mechanism of hypertension and cardiovascular disease. Its strengths include an enhanced signal-to-noise ratio through rigorous control of factors that influence BP, including medication washout, body positioning, diurnal variation, and dietary salt. Participants had been recruited and studied in an inpatient clinical research center at 5 sites (Brigham and Women’s Hospital, Boston, Massachusetts, USA; University of Utah Medical Center, Salt Lake City, Utah, USA; Vanderbilt University, Nashville, Tennessee, USA; Hospital Broussais, Paris, France, and University La Sapienza, Rome, Italy). The institutional review boards of each institution approved the original study protocol. All participants gave written informed consent before enrollment and underwent identical protocols at each study site. All laboratory assessments were processed and analyzed at one site (Boston, Massachusetts, USA). We included all individuals with available data for the LSD1 polymorphic variant (rs587168) and SSBP from those of African descent for this analysis. We then expanded our analyses by using the subcohort of European descent. Those of European descent were matched to the African descent at a ratio of 2:1, using the following parameters: sex, age, disease states (normotensive or hypertensive), body mass index (BMI), and diastolic BP (DBP).
Although other results from the HyperPATH cohort have been previously reported, the present analyses are original. Inclusion and exclusion criteria for the HyperPATH protocol are described elsewhere (1, 13, 14). In brief, all participants had screening history and physical and laboratory examinations. All participants were between ages 18 and 65 years. Race was self-defined. Normotension was defined as a seated DBP less than or equal to 80 mm Hg on 3 different dates at least 1 month apart with 3 replicates at each date. Hypertension was defined as seated DBP greater than or equal to100 mm Hg without antihypertensive medication, greater than or equal to 90 mm Hg on 1 or more antihypertensive medications, or treatment with 2 or more antihypertensive medications. Participants on 4 or more antihypertensive medications were excluded for safety reasons. Participants with known or suspected secondary hypertension, coronary artery disease, stroke, overt renal insufficiency (serum creatinine > 1.5 mg/dL), diabetes mellitus, current tobacco or illicit drug use, moderate alcohol use, or other significant medical or psychiatric illnesses were excluded. At the screening examination, participants with abnormal electrolytes, thyroid, and liver function tests or electrocardiographic evidence of heart block, ischemia, or prior coronary events were excluded.
Study Protocol
Details of the study protocol have been described previously (1, 13, 14). In brief, to minimize the interference of medication with our assessment of RAAS activity, all angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers, or MR antagonists were discontinued 3 months before the study. Amlodipine was given if BP control was needed. All calcium channel blockers, β-blockers, and diuretics were discontinued at least 2 weeks before the study initiation. Hence, individuals were studied off all antihypertensive agents.
Participants were studied under 2 dietary conditions, at 5 to 7 days apart: LIB diet (200 mmol/d sodium) and a restricted salt (RES) diet (10 mmol/d sodium). Both diets also contained 100 mmol/day potassium to fix the influence of potassium on aldosterone, 20 mmol/day calcium, and were isocaloric. In the present analyses, participants had verified urinary sodium excretion of less than 25 mmol/day on the RES diet and more than 160 mmol/day on the LIB diet based on 24-hour urine collection. Participants were admitted to the clinical research center at their respective institutions and kept in supine postures overnight. After 12 hours of supine, fasting posture, an automated device (Dinamap, Critikon) was used to obtain BPs. The mean of 3 grouped readings from 10 baseline values separated by 5 minutes each was used for analyses. SSBP was recorded as a continuous variable representing the change in systolic BP (SBP) in response to dietary salt manipulation; SBP on a LIB diet minus SBP on a RES diet. Blood was obtained at 8 AM to measure electrolytes, plasma renin activity (PRA), serum aldosterone and cortisol. Angiotensin II (ANGII) (3 ng/kg/min) was infused for 60 minutes, and repeated blood samples were obtained. Aldosterone level response to ANGII infusion was calculated by the change in the absolute aldosterone level after ANGII stimulation and baseline aldosterone level. Since HyperPATH did not document the age of onset of menopause, and there were available estradiol and follicle-stimulating hormone (FSH) values in some participants, individuals were classified according to presumed menopausal status. We used the cutoff of 50 years as the average age at which women enter natural menopause (15, 16). All HyperPATH samples were analyzed at a central laboratory. Aldosterone levels were measured using Coat-A-Count radioimmunoassay (RIA) kit (Siemens); PRA by RIA assay (Diasorin). Serum cortisol was measured with the Access Chemiluminescent Immunoassay (Beckman Coulter).
Statistical Analyses
All statistical analyses were performed using STATA/SE version 16.1. All figures were created using Prism version 9.0.1. Hardy-Weinberg equilibrium was assessed using the χ 2 test. Continuous variables are presented as SEM. Categorical variables are presented as a percentage of the total sample. Baseline analyses included the t test and Wilcoxon rank sum test. Binary variables were compared using the Fisher exact test. Outcome variables, such as SSBP and serum aldosterone level response to ANGII infusion, are presented as least square mean and SEM. We performed a multiple linear regression model on the association between the LSD1 rs587168 genotype and outcome variables, adjusted for age, BMI, disease states (hypertension or normotension), and study sites. In addition, for SSBP and serum aldosterone level response to ANGII infusion, we tested the interaction between sex and LSD1 genotype in those of African descent (whole population and separately according to different age groups). A P value of less than .05 was considered statistically significant.
Results
Of those of African descent, but not European descent, rs587168 risk allele carriers had greater SSBP than the nonrisk homozygotes regardless of hypertensive states.
Baseline characteristics of African descent and European descent classified by LSD1 genotype are shown in Table 1. Sex, disease states, BMI, PRA, and aldosterone-to-renin ratio (ARR) did not differ between the LSD1 genotypes of participants of African and European descent. However, risk allele carriers displayed lower aldosterone levels on a LIB diet than nonrisk homozygotes in those of African descent (see Table 1). In the HyperPATH cohort, the genotypes were in Hardy-Weinberg equilibrium (χ 2P = .99). The distribution of risk allele carriers was higher in those of African descent than European descent (52% vs 36%; P = .01). Age, sex, disease states, BMI, BP on a LIB diet, and 24-hour urine sodium excretion did not differ by race (Supplementary Table 1) (17). Consistent with our previous report, those of African descent had lower aldosterone (posture or upright), PRA, and higher ARR levels than those of European descent (see Table 1) (18).
Table 1.
Baseline characteristics and selected biochemical values among those of African descent and matched European subcohort in HyperPATH
| Characteristics | African descent | P | European descent | P | P a | ||
|---|---|---|---|---|---|---|---|
| Risk allele carriers | Nonrisk homozygotes | Risk allele carriers | Nonrisk homozygotes | ||||
| No. (%) | 51 (52) | 48 (48) | – | 71 (36) | 127 (64) | – | .01 |
| Female (n, %) | 27 (53) | 32 (67) | .22 | 43 (61) | 75 (59) | .88 | ≥ .999 |
| Age, y | 43.04 ± 1.58 | 44.42 ± 1.47 | .52 | 46.28 ± 1.24 | 42.67 ± 0.97 | .02 | .85 |
| SBP on LIB diet, mm Hg | 139 ± 3 | 141 ± 3 | .58 | 139 ± 3 | 137 ± 2 | .47 | .47 |
| DBP on LIB diet, mm Hg | 82 ± 2 | 83 ± 2 | .75 | 82 ± 2 | 82 ± 1 | .73 | .79 |
| Disease state | |||||||
| Hypertensive (n) | 33 | 35 | .67 | 49 | 88 | ≥ .999 | ≥ .999 |
| Normotensive (n) | 18 | 13 | 22 | 39 | |||
| BMI | 29.23 ± 0.66 | 28.01 ± 0.68 | .20 | 28.39 ± 0.52 | 27.56 ± 0.34 | .17 | .14 |
| Aldosterone on LIB diet, ng/dL | 3.63 ± 0.26 | 5.08 ± 0.58 | .02 | 4.88 ± 0.57 | 4.56 ± 0.31 | .36 | .35 |
| Aldosterone on RES diet, ng/dL | 14.93 ± 1.16 | 15.22 ± 1.31 | .96 | 15.80 ± 1.09 | 18.41 ± 1.16 | .34 | .24 |
| Posture aldosterone level, ng/dL | 34.72 ± 3.05 | 35.89 ± 3.34 | .81 | 47.49 ± 3.33 | 52.81 ± 3.44 | .57 | < .05 |
| PRA on LIB diet, ng/mL/h | 0.30 ± 0.05 | 0.32 ± 0.05 | .87 | 0.43 ± 0.05 | 0.57 ± 0.05 | .09 | < .05 |
| PRA on RES diet, ng/mL/h | 2.16 ± 0.90 | 1.95 ± 0.36 | .78 | 3.64 ± 1.33 | 2.81 ± 0.22 | .28 | .001 |
| Posture PRA, ng/mL/h | 5.73 ± 0.90 | 5.21 ± 0.87 | .63 | 7.45 ± 6.55 | 8.23 ± 0.60 | .36 | < .05 |
| ARR on LIB diet, ng/dL per ng/mL/h | 30.71 ± 6.45 | 34.20 ± 6.90 | .20 | 26.79 ± 6.12 | 16.66 ± 1.75 | .19 | < .05 |
| ARR on RES diet, ng/dL per ng/mL/h | 20.91 ± 4.64 | 22.73 ± 7.94 | .57 | 15.30 ± 4.24 | 13.65 ± 2.56 | .63 | 002 |
| 24-h urine sodium excretion on LIB diet, mmol/24 h | 229.64 ± 11.27 | 207.01 ± 12.43 | .18 | 230.45 ± 8.69 | 220.54 ± 6.90 | .38 | .58 |
| 24-h urine sodium excretion on RES diet, mmol/24 h | 19.94 ± 2.48 | 16.67 ± 1.57 | .70 | 16.00 ± 1.62 | 15.13 ± 1.57 | .45 | .05 |
Data represent mean ± SEM. T test, Wilcoxon rank sum test, or Fisher exact test was used in the analysis.
Abbreviations: ARR, aldosterone-to-renin ratio; BMI, body mass index; DBP, diastolic blood pressure; HyperPATH, International Hypertensive Pathotype; LIB, liberal salt; PRA, plasma renin activity; RES, restricted salt; SBP, systolic blood pressure.
a Statistical differences between African and European descents.
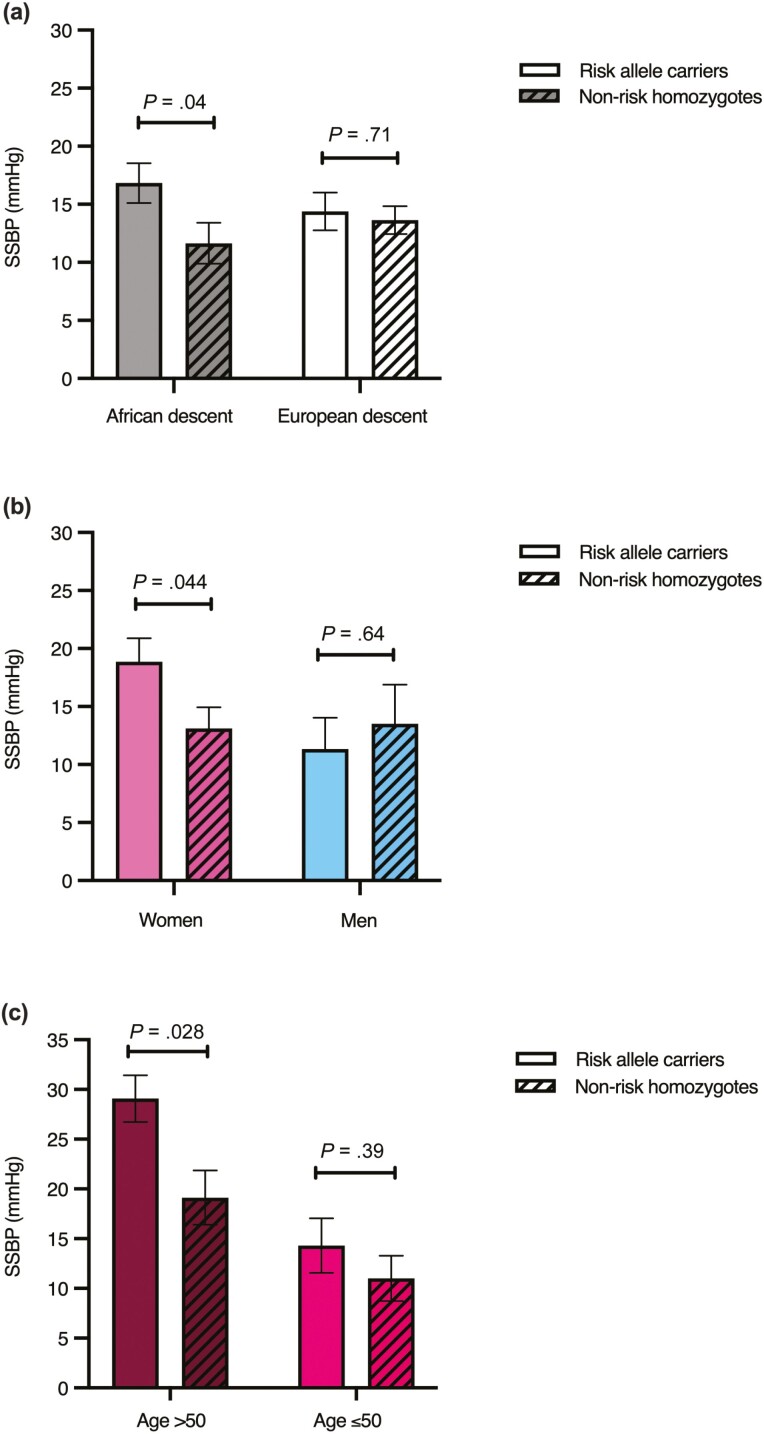
We then assessed SSBP in participants of African descent and European descent (normotensives and hypertensives). In those of African descent, there was a statistically significantly greater SSBP in the risk allele carriers than the nonrisk homozygotes (17 ± 2 mm Hg vs 12 ± 2 mm Hg; P = .04). The difference in the SSBP between the risk allele carriers and nonrisk homozygotes was not observed in those of European descent (14 ± 2 mm Hg vs 14 ± 1; P = .71) (Fig. 1A).
Figure 1.
A, Salt sensitivity of blood pressure (SSBP). SSBP in those of African (n = 99) and European descent (n = 198). B, SSBP in women (n = 59) and men (n = 40). C, SSBP in women older than 50 (n = 16) and aged 50 years or younger (n = 43). Multiple regression analysis for SSBP was adjusted for age, body mass index, sites, and disease status. Data represent mean ± SEM.
Consistent with our previous reports, only risk allele carriers of hypertensive African descent had greater SSBP than nonrisk homozygotes (20 ± 2 mm Hg vs 12 ± 2; P = .014). There was no association between the rs587168 risk allele and SSBP in the hypertensive individuals of European descent (P = .99) (data not shown).
In those of African descent, female risk allele carriers, but not males, had greater SSBP than the nonrisk homozygotes.
Baseline characteristics of individuals of African descent are shown by the rs587168 genotype in women and men (Table 2). The distribution of disease states, age, BMI, and 24-hour urine sodium excretion did not differ significantly by the rs587168 genotype, as shown in Table 2.
Table 2.
Baseline characteristics of the 99 individuals of African descent
| Characteristics | Women | P | Men | P | ||
|---|---|---|---|---|---|---|
| Risk allele carriers | Nonrisk homozygotes | Risk allele carriers | Nonrisk homozygotes | |||
| No. | 27 | 32 | 24 | 16 | .22 | |
| Age, y | 43.48 ± 2.21 | 43.97 ± 1.87 | .87 | 42.54 ± 2.30 | 45.33 ± 2.42 | .42 |
| Disease state | ||||||
| Hypertensive (n) | 18 | 24 | .57 | 15 | 11 | .75 |
| Normotensive (n) | 9 | 8 | 9 | 5 | ||
| BMI | 29.26 ± 1.00 | 27.92 ± 0.92 | .33 | 29.20 ± 0.86 | 28.20 ± 0.90 | .42 |
| SBP on LIB diet, mm Hg | 140 ± 5 | 143 ± 4 | .63 | 137 ± 4 | 138 ± 5 | .44 |
| DBP on LIB diet, mm Hg | 83 ± 3 | 83 ± 2 | .90 | 81 ± 2 | 81 ± 11 | .94 |
| Aldosterone on LIB diet, ng/dL | 3.70 ± 0.42 | 4.82 ± 0.62 | .04 | 3.56 ± 0.29 | 5.63 ± 1.25 | .20 |
| PRA on LIB diet, ng/mL/h | 0.29 ± 0.06 | 0.35 ± 0.07 | .69 | 0.31 ± 0.07 | 0.24 ± 0.05 | .96 |
| ARR on LIB diet, ng/dL per ng/mL/h | 38.43 ± 11.59 | 30.50 ± 6.36 | .64 | 21.98 ± 3.81 | 42.15 ± 17.17 | .21 |
| 24-h urine sodium excretion on LIB diet, mmol/24 h | 215.29 ± 18.20 | 206.25 ± 15.32 | .70 | 245.78 ± 12.01 | 208.63 ± 21.93 | .90 |
| 24-h urine sodium excretion on RES diet, mmol/24 h | 19.06 ± 3.83 | 15.88 ± 1.97 | .91 | 20.93 ± 3.11 | 18.26 ± 2.61 | .99 |
Data represent mean ± SEM. T test, Wilcoxon rank sum test, or Fisher exact test was used in the analysis.
Abbreviations: ARR, aldosterone-to-renin ratio; BMI, body mass index; DBP, diastolic blood pressure; LIB, liberal salt; PRA, plasma renin activity; RES, restricted salt; SBP, systolic blood pressure.
We next assessed SSBP in women and men separately. Women who carried the rs587168 minor risk allele showed greater SSBP than noncarriers (19 ± 2 mm Hg vs 13 ± 2 mm Hg; P = .044). Men who carried the rs587168 minor risk allele did not show a statistically significantly greater SSBP than the nonrisk homozygotes (11 ± 3 vs 14 ± 3; P = .64) (Fig. 1B). However, there was no significant sex-LSD1 genotype interaction for SSBP in the whole population of African descent (P = .61).
Since SSBP represents the change in SBP in response to dietary salt, SBP on LIB and RES were assessed (Supplementary Table 2) (17). In women, risk allele carriers showed lower SBP on a RES diet than nonrisk homozygotes (121 ± 2 mm Hg vs 129 ± 2 mm Hg: P = .024). During a LIB diet, SBP was not different in the risk allele carriers and nonrisk homozygotes (140 ± 3 mm Hg vs 142 ± 3 mm Hg; P = .59). In men, SBP on either a LIB or RES diet did not differ by rs587168 genotype.
Female risk allele carriers older than 50 years, but not younger than 50 years, had greater SSBP than the nonrisk homozygotes.
We assessed SSBP in younger and older women, using the age cutoff of 50 years. Only women older than 50 years who carried risk alleles had statistically significantly greater SSBP than the nonrisk homozygotes (29 ± 2 mm Hg vs 19 ± 3 mm Hg; P = .028) (Fig. 1C). There was no statistically significant difference in SBP on a RES diet between risk allele carriers and nonrisk homozygotes in women older than 50 years (117 ± 4 mm Hg vs 126 ± 4 mm Hg; P = .15). SBP on a LIB diet was also not statistically significantly different between the risk allele carriers and nonrisk homozygotes (146 ± 5 mm Hg vs 145 ± 5 mm Hg; P = .90) (see Supplementary Table 2) (17).
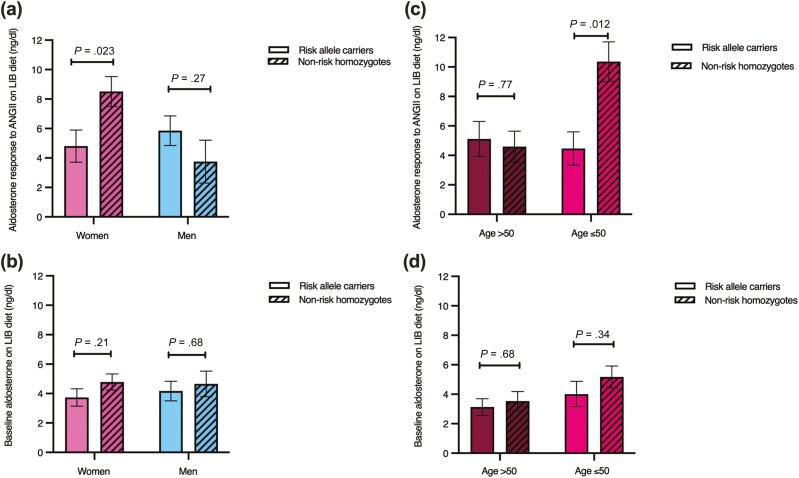
In those of African descent, female risk allele carriers had lower aldosterone level response to ANGII infusion on a LIB diet. The sex-LSD1 genotype interaction was also observed for aldosterone level response to ANGII infusion on a LIB diet, but only in the younger age group.
Women who carried the rs587168 minor risk allele had statistically significantly lower aldosterone level response to ANGII infusion on a LIB diet than the noncarriers (4.81 ± 1.09 ng/dL vs 8.51 ± 1.02 ng/dL; P = .023). In men, risk allele carriers did not show statistically significantly different aldosterone level response to ANGII infusion on a LIB diet than the nonrisk homozygotes (5.85 ± 1.01 ng/dL vs 3.75 ± 1.45 ng/dL; P = .27) (Fig. 2A). Baseline aldosterone levels on a LIB diet were not statistically significantly different between LSD1 genotypes in both sexes (Fig. 2B). The sex-LSD1 genotype interaction for aldosterone level response to ANGII infusion on a LIB diet was observed only in individuals aged 50 years or younger (P = .007) (Table 3). There was no statistically significant difference between basal serum cortisol and serum levels after ANGII stimulation between LSD1 genotype in both sexes (data not shown).
Figure 2.
Aldosterone response to an angiotensin II (ANGII) infusion on a liberal salt (LIB) diet and baseline aldosterone level on a LIB diet in those of African descent. A, Aldosterone level response to ANGII infusion on a LIB diet in women (n = 32) and men (n = 29). B, Aldosterone level response to ANGII infusion on a LIB diet in women older than 50 (n = 9) and aged 50 years or younger (n = 23). C, Baseline aldosterone level on a LIB diet in women (n = 58) and men (n = 39). D, Baseline aldosterone level on a LIB diet in women older than 50 (n = 16) and aged 50 years or younger (n = 42). Multiple regression analyses for aldosterone level response to ANGII infusion and baseline aldosterone level were adjusted for age, body mass index, sites, and disease status. Data represent mean ± SEM.
Table 3.
The sex-LSD1 genotype interaction for aldosterone level response to angiotensin II infusion on a liberal salt diet in those of African descent
| Populations | Sex | β-coefficient | 95% CI | Risk allele carriers, ng/dL | Nonrisk homozygotes, ng/dL | P interaction |
|---|---|---|---|---|---|---|
| A whole cohort of African descent (n = 61) | Female | –3.70 | –6.85 to –0.56 | 4.81 ± 1.09 | 8.51 ± 1.02 | .023 |
| Male | 2.10 | –1.78 to 5.99 | 5.85 ± 1.01 | 3.75 ± 1.45 | ||
| Age > 50 y (n = 19) | Female | 0.52 | –4.17 to 5.22 | 5.11 ± 1.19 | 4.59 ± 1.05 | .63 |
| Male | –2.84 | –17.62 to 11.93 | 5.23 ± 2.74 | 8.08 ± 3.63 | ||
| Age ≤ 50 y (n = 42) | Female | –5.9 | –10.34 to –1.47 | 4.64 ± 1.43 | 10.36 ± 1.34 | .007 |
| Male | 2.35 | –1.68 to 6.38 | 5.21 ± 1.00 | 2.86 ± 1.51 |
Data represent least square mean ± SEM. Multiple regression analysis for aldosterone level response to angiotensin II infusion was adjusted for age, body mass index, sites, and disease status.
Abbreviation: LSD1, lysine-specific demethylase 1.
The lower aldosterone level response to ANGII infusion on a LIB diet in women with the risk allele was driven by the younger participants. In women aged 50 years or younger, risk allele carriers had lower aldosterone level response to ANGII infusion on a LIB diet than the nonrisk homozygotes (4.46 ± 1.43 ng/dL vs 10.36 ± 1.34 ng/dL; P = .012). Aldosterone level response to ANGII infusion on a LIB diet did not differ by rs587168 genotype in the older women (5.11 ± 1.19 ng/dL vs 4.59 ± 1.05 ng/dL; P = .77) (Fig. 2C). Baseline aldosterone levels were also not statistically significantly different between LSD1 genotypes in both groups (Fig. 2D). We then assessed the difference in estradiol and FSH levels between the younger and older women. Women aged 50 years or younger had statistically significantly higher estradiol (55.73 ± 6.81 pg/mL vs 32.67 ± 11.06 pg/mL; P = .0008) and lower FSH level (14.53 ± 2.66 mIU/mL vs 58.88 ± 5.45 mIU/mL; P < .0001) compared with the older women.
Discussion
The major findings from this study were first, we demonstrated greater SSBP in the LSD1 risk allele carriers than the nonrisk homozygotes of African descent. At the same time, there was no statistically significant difference in SSBP between the LSD1 genotype in those of European descent. Second, the difference in SSBP between LSD1 genotypes among those of African descent was driven by females, especially women of low estrogen (age > 50 y). Third, we identified a statistically significant LSD1 genotype-sex interaction in aldosterone response to stimulation in younger individuals (age ≤ 50 y) but not older individuals. These data support biological sex differences and age effects in modifying the response of LSD1 polymorphic variants and increased dietary salt intake.
Our study showed that the association between the LSD1 genotype and SSBP was found only in those of African descent. In addition, consistent with previous studies, we found that individuals of African descent had lower serum aldosterone, PRA, and higher ARR levels than those of European descent (18). In the United States, hypertension prevalence and hypertension-related death rates were highest among individuals of African descent than other ethnic groups (19, 20). Various factors are responsible for the difference in hypertension etiology and phenotypes in those of African descent, including the difference in RAAS. For example, compared with those of European descent, aldosterone and renin levels are lower in people of African descent despite a nonstatistically significant difference in prorenin levels (21-24). People of African descent have a higher ARR and sodium-modulated-aldosterone-suppression-stimulation index, indicating more aldosterone dysregulation (18, 25), which are associated with an increased risk of having metabolic syndrome and high Framingham risk scores (26, 27). Further, individuals of African descent are more sensitive to aldosterone, both physiologically or fludrocortisone experimentally (28, 29). In addition, aldosterone levels declined as age increased in both ethnic groups (African and European descent). However, the association of SBP and aldosterone was found only in participants of African descent (28, 30). Finally, people of African descent had suppressed RAAS in response to sodium retention by the kidney. Following short-term sodium restriction, those of African descent had a greater reduction in SBP than those of European descent regardless of hypertensive status (31). The difference in SBP response after salt restriction may explain why individuals of African descent display a greater hypotensive response to thiazide diuretics (22, 32). In this study, we demonstrated a greater reduction of BP after RES in LSD1 risk allele carrier females of African descent and not in European descent or the entire cohort of both ethnicities (data not shown). The reasons for this observed racial genetic dimorphism are not clear at present but do provide an interesting foundation to explore well-described clinical differences in sensitivity to salt and cardiovascular risk between individuals of African and European descents. Perhaps counterregulatory measures surrounding volume homeostasis developed along different pathways based on genetic pressures as populations either remained in or migrated from the African continent. Future studies should explore differences in salt handling, excretion, and related pathways to provide additional mechanistic clues to unravel these differences to uncover vulnerabilities or exploit protective factors.
Since the discovery of LSD1, it has been linked to various biological processes ranging from embryology to cancer (33-36). Recent evidence found that LSD1 also plays a role in the pathogenesis of bilateral macronodular adrenal hyperplasia and Cushing syndrome. However, according to the methods of cortisol evaluation in our HyperPATH cohort, we found no statistically significant difference between baseline serum cortisol levels and cortisol levels after ANGII stimulation among the LSD1 genotypes (36). Further, LSD1 can modulate the effects of sex hormones on gene expression and has been implicated as an important regulator of aging (37). Regarding salt-sensitive hypertension, we reported the association of SSBP and LSD1 genotype (rs587168) in hypertensive individuals of African descent (1). In addition, Krug et al (3) showed an association of LSD1 genotype (rs7548692) and increased BP in response to dietary salt intake with increasing age in normotensive individuals of European descent. However, no study has addressed the interaction of LSD1 and the effect of sex and age on BP phenotypes in humans, which we previously reported in the LSD1+/– mice (11). In this study, not only did we replicate findings of greater SSBP in LSD1 risk allele carriers (rs587168) of African descent, but we also found that female risk allele carriers had greater SSBP than nonrisk homozygotes. The greater SSBP in women with the LSD1 risk allele was driven by women older than 50 years classified as estrogen-deplete (low estradiol levels). Biological sex differences and the role of estrogen in BP regulation have been extensively studied (38-40). Compared with age-matched premenopausal women, men have higher BP and are at higher risk for cardiovascular and renal complications. Following menopause, BP rises in women (19, 20, 39); the loss in estrogen when women enter menopause may explain the higher risk of developing hypertension in postmenopausal women. Estrogen is associated with higher circulating angiotensinogen levels and lower circulating renin, ACE, angiotensin type 1 receptor (AT1R) density, and aldosterone levels (40-43). In animal models, estrogen increases renal angiotensin type 2 receptor (AT2R) expression and decreases renal AT1R synthesis (44). It modulates the expression of RAAS components by shifting toward the AT2R–ACE2–angiotensin (1-7)–MAS1 protooncogene axis, which opposes the pressor effect of AT1R (44-46). Further, AT2R expression in the kidneys decreases after ovariectomy and increases after 17β-estradiol administration (44, 47), and the production of angiotensin (1-7) increases following estrogen administration (48, 49). Estradiol inhibited aldosterone-stimulated transcription of important MR targets in an in vitro study. In the presence of estradiol, there was no aldosterone-stimulated increase in intracellular adhesion molecule-1 (50). Finally, our group previously showed that premenopausal women with the polymorphic variant of the estrogen receptor β gene (ESR2) had SSBP and increased aldosterone secretion as their ability to mediate aldosterone secretion may be altered. In addition, estrogen may decrease binding to the receptor and cannot cause vasodilation resulting in SSBP in a sex- and age-specific manner (51).
Higher aldosterone dysregulation has been reported with aging (30, 52). Even though baseline aldosterone and stimulated aldosterone levels were lower as age increased, ARR and sodium-modulated–aldosterone-suppression-stimulation index increased (52, 53). Despite the lower baseline values, women had higher aldosterone levels in response to ANGII infusion than men. As women aged, their aldosterone levels in response to ANGII infusion decreased (52). This study found a blunted aldosterone response to ANGII stimulation in young females carrying the LSD1 risk allele, suggesting dysregulated aldosterone physiology at a premature age. Estrogen may protect against BP increase as we did not observe the greater SSBP in these premenopausal women with the LSD1 risk allele.
Hypertension is one of the major risk factors leading to cardiovascular morbidity and mortality. According to current guidelines, the effect of genetic predisposition is a concern but requires further investigation linking genotype with underlying pathophysiological mechanisms. In terms of ethnic differences, initial treatment in patients of African descent is either thiazide-type diuretics or calcium channel blockers (54). In this study, we identified the association of the LSD1 polymorphic variant with SSBP of African-descent participants, in which postmenopausal females drove the relationship. We previously provided supporting evidence in LSD1+/– mice, in which they had increased MR expression (MR messenger RNA) and downstream MR effectors and statistically significantly lowered BP and albuminuria after MR antagonists treatment (12). Our data suggest that LSD1 female African descent risk allele carriers, especially if postmenopausal, may specifically benefit from MR antagonist treatment.
Strengths of this study include 1) the precision and quality of phenotypic data in the HyperPATH cohort; 2) extensive control of known environmental factors affecting RAAS and BP; 3) the study of participants off antihypertensive agents; 4) the use of a centralized laboratory; and 5) confirmation of previous findings with an extension of age and sex interactions with LSD1 genotype state. There are also limitations to our study. First, it is important to emphasize that tagging single-nucleotide variation association studies cannot confirm causality. Although our association analyses confirm our original findings, they are post hoc in nature and would require the rigor of a randomized clinical trial to provide stronger evidence of clinical utility. Second, in our cohort, aldosterone and cortisol were measured by immunoassay, in which interfering steroids could have affected hormonal levels. Aldosterone and cortisol levels measured by immunoassay are typically higher than liquid chromatography–tandem mass spectrometry, which is a more specific method (55, 56). A third limitation relates to incomplete data available from study participants in a large cohort data set. We used age as a surrogate of menopausal status and divided women into presumed estrogen-deplete and estrogen-replete states. However, in participants with available estrogen and FSH levels (56% had available hormonal data and 41% had complete hormonal data), the results were as expected for the premenopausal and postmenopausal women. The average age of postmenopausal values was 54 years. It supported the validity of this approach. Future studies that focus on the roles of female sex hormones in protecting against the LSD1-mediated hypertensive phenotype are needed; we will perform a study in ovariectomized LSD1+/– female mice. In humans, we will also address whether LSD1 risk allele carriers have increased MR expression and the overall effect of MR blockade in individuals of African descent.
In conclusion, in humans, the LSD1 gene variant (rs587168) is associated with SSBP and aldosterone dysregulation in response to dietary salt intake. The presence of the LSD1 risk variant impairs the aldosterone and MR response to salt intake, resulting in SSBP in a race-, sex-, and age-specific manner. Our results suggest one mechanism that underlies the biological sex difference in primary hypertension and may suggest therapeutic candidates for MR antagonists.
Acknowledgments
We acknowledge Dr Gordon H. Williams and Dr Xavier Jeunemaitre and all supporting staffs from the HyperPATH cohort.
Glossary
Abbreviations
- ACE
angiotensin-converting enzyme
- ANGII
angiotensin II
- ARR
aldosterone-to-renin ratio
- AT1R
angiotensin type 1 receptor
- AT2R
angiotensin type 2 receptor
- BMI
body mass index
- BP
blood pressure
- DBP
diastolic blood pressure
- FSH
follicle-stimulating hormone
- HyperPATH
International Hypertensive Pathotype
- LIB
liberal salt
- LSD1
lysine-specific demethylase
- MR
mineralocorticoid receptor
- PRA
plasma renin activity
- RAAS
renin-angiotensin-aldosterone-system
- RES
restricted salt
- RIA
radioimmunoassay
- SBP
systolic blood pressure
- SSBP
salt sensitivity of blood pressure
Financial Support
This work was supported by the National Institutes of Health (grant Nos. R01-HL-127146, R01-HL-104032, DP50HL055000 [HyperPATH Cohort], and T32HL007609); and the American Heart Association (grant No. GRNT20500000).
Disclosures
The authors have nothing to disclose.
Clinical Trial Information
Trial registration No. NCT03029806 (registered January 24, 2017).
Data Availability
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Williams JS, Chamarthi B, Goodarzi MO, et al. Lysine-specific demethylase 1: an epigenetic regulator of salt-sensitive hypertension. Am J Hypertens. 2012;25(7):812-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Manosroi W, Williams GH. Genetics of human primary hypertension: focus on hormonal mechanisms. Endocr Rev. 2019;40(3):825-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Krug AW, Tille E, Sun B, et al. Lysine-specific demethylase-1 modifies the age effect on blood pressure sensitivity to dietary salt intake. Age (Dordr). 2013;35(5):1809-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shi Y, Lan F, Matson C, et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119(7):941-953. [DOI] [PubMed] [Google Scholar]
- 5. Baudrand R, Pojoga LH, Romero JR, Williams GH. Aldosterone’s mechanism of action: roles of lysine-specific demethylase 1, caveolin and striatin. Curr Opin Nephrol Hypertens. 2014;23(1):32-37. [DOI] [PubMed] [Google Scholar]
- 6. Lee KH, Kim BC, Jeong SH, et al. Histone demethylase LSD1 regulates kidney cancer progression by modulating androgen receptor activity. Int J Mol Sci . 2020;21(17):6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cai C, He HH, Gao S, et al. Lysine-specific demethylase 1 has dual functions as a major regulator of androgen receptor transcriptional activity. Cell Rep. 2014;9(5):1618-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bennesch MA, Segala G, Wider D, Picard D. LSD1 engages a corepressor complex for the activation of the estrogen receptor α by estrogen and cAMP. Nucleic Acids Res. 2016;44(18):8655-8670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garcia-Bassets I, Kwon YS, Telese F, et al. Histone methylation-dependent mechanisms impose ligand dependency for gene activation by nuclear receptors. Cell. 2007;128(3):505-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pojoga LH, Williams JS, Yao TM, et al. Histone demethylase LSD1 deficiency during high-salt diet is associated with enhanced vascular contraction, altered NO-cGMP relaxation pathway, and hypertension. Am J Physiol Heart Circ Physiol. 2011;301(5):H1862-H1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang Y, Ting PY, Yao TM, et al. Histone demethylase LSD1 deficiency and biological sex: impact on blood pressure and aldosterone production. J Endocrinol. 2019;240(2):111-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Treesaranuwattana T, Wong KYH, Brooks DL, et al. Lysine-specific demethylase-1 deficiency increases agonist signaling via the mineralocorticoid receptor. Hypertension. 2020;75(4):1045-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rao AD, Sun B, Saxena A, et al. Polymorphisms in the serum- and glucocorticoid-inducible kinase 1 gene are associated with blood pressure and renin response to dietary salt intake. J Hum Hypertens. 2013;27(3):176-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vaidya A, Forman JP, Underwood PC, et al. The influence of body mass index and renin-angiotensin-aldosterone system activity on the relationship between 25-hydroxyvitamin D and adiponectin in Caucasian men. Eur J Endocrinol. 2011;164(6):995-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chan S, Gomes A, Singh RS. Is menopause still evolving? Evidence from a longitudinal study of multiethnic populations and its relevance to women’s health. BMC Womens Health. 2020;20(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Palacios S, Henderson VW, Siseles N, Tan D, Villaseca P. Age of menopause and impact of climacteric symptoms by geographical region. Climacteric. 2010;13(5):419-428. [DOI] [PubMed] [Google Scholar]
- 17. Parksook WW, Heydarpour M, Gholami SK, et al. Supplementary data for “ Salt sensitivity of blood pressure and aldosterone: interaction between lysine-specific demethylase 1 gene, sex, and age.” Deposited January 17, 2021 10.6084/m9.figshare.16734865 [DOI] [PMC free article] [PubMed]
- 18. Tan JW, Gupta T, Manosroi W, et al. Dysregulated aldosterone secretion in persons of African descent with endothelin-1 gene variants. JCI Insight 2017;2(23):e95992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ostchega Y, Fryar CD, Nwankwo T, Nguyen DT. Hypertension prevalence among adults aged 18 and over: United States, 2017-2018. NCHS Data Brief. 2020;( 364):1-8. [PubMed] [Google Scholar]
- 20. Benjamin EJ, Blaha MJ, Chiuve SE, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics–2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146-e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tu W, Eckert GJ, Decker BS, Howard Pratt J. Varying influences of aldosterone on the plasma potassium concentration in Blacks and Whites. Am J Hypertens. 2017;30(5):490-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lindhorst J, Alexander N, Blignaut J, Rayner B. Differences in hypertension between blacks and whites: an overview. Cardiovasc J Afr. 2007;18(4):241-247. [PMC free article] [PubMed] [Google Scholar]
- 23. Tu W, Eckert GJ, Pratt JH, Jan Danser AH. Plasma levels of prorenin and renin in blacks and whites: their relative abundance and associations with plasma aldosterone concentration. Am J Hypertens. 2012;25(9):1030-1034. [DOI] [PubMed] [Google Scholar]
- 24. Rifkin DE, Khaki AR, Jenny NS, et al. Association of renin and aldosterone with ethnicity and blood pressure: the Multi-Ethnic Study of Atherosclerosis. Am J Hypertens. 2014;27(6):801-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grim CE, Cowley AW Jr, Hamet P, et al. Hyperaldosteronism and hypertension: ethnic differences. Hypertension. 2005;45(4):766-772. [DOI] [PubMed] [Google Scholar]
- 26. Vaidya A, Underwood PC, Hopkins PN, et al. Abnormal aldosterone physiology and cardiometabolic risk factors. Hypertension. 2013;61(4):886-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brown JM, Underwood PC, Ferri C, et al. Aldosterone dysregulation with aging predicts renal vascular function and cardiovascular risk. Hypertension. 2014;63(6):1205-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tu W, Eckert GJ, Hannon TS, et al. Racial differences in sensitivity of blood pressure to aldosterone. Hypertension. 2014;63(6):1212-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Funder JW. Sensitivity to aldosterone: plasma levels are not the full story. Hypertension. 2014;63(6):1168-1170. [DOI] [PubMed] [Google Scholar]
- 30. Tu W, Li R, Bhalla V, Eckert GJ, Pratt JH. Age-related blood pressure sensitivity to aldosterone in blacks and whites. Hypertension. 2018;72(1):247-252. [DOI] [PubMed] [Google Scholar]
- 31. Graudal NA, Hubeck-Graudal T, Jurgens G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst Rev. 2020;12(12):CD004022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Veterans Administration Cooperative Study Group on Antihypertensive Agents. Comparison of propranolol and hydrochlorothiazide for the initial treatment of hypertension. I. Results of short-term titration with emphasis on racial differences in response. JAMA. 1982;248(16):1996-2003. [PubMed] [Google Scholar]
- 33. Stazi G, Zwergel C, Valente S, Mai A. LSD1 inhibitors: a patent review (2010-2015). Expert Opin Ther Pat. 2016;26(5):565-580. [DOI] [PubMed] [Google Scholar]
- 34. Hosseini A, Minucci S. A comprehensive review of lysine-specific demethylase 1 and its roles in cancer. Epigenomics. 2017;9(8):1123-1142. [DOI] [PubMed] [Google Scholar]
- 35. Zhu D, Hölz S, Metzger E, et al. Lysine-specific demethylase 1 regulates differentiation onset and migration of trophoblast stem cells. Nat Commun. 2014;5:3174. [DOI] [PubMed] [Google Scholar]
- 36. Chasseloup F, Bourdeau I, Tabarin A, et al. Loss of KDM1A in GIP-dependent primary bilateral macronodular adrenal hyperplasia with Cushing’s syndrome: a multicentre, retrospective, cohort study. Lancet Diabetes Endocrinol. Published online October 13, 2021. doi:10.1016/S2213-8587(21)00236-9 [DOI] [PubMed] [Google Scholar]
- 37. Duteil D, Tosic M, Schüle R. Lsd1, a metabolic sensor of environment requirements that prevents adipose tissue from aging. Adipocyte. 2017;6(4):298-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sabbatini AR, Kararigas G. Estrogen-related mechanisms in sex differences of hypertension and target organ damage. Biol Sex Differ. 2020;11(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Colafella KMM, Denton KM. Sex-specific differences in hypertension and associated cardiovascular disease. Nat Rev Nephrol. 2018;14(3):185-201. [DOI] [PubMed] [Google Scholar]
- 40. Ahmed S, Hu R, Leete J, Layton AT. Understanding sex differences in long-term blood pressure regulation: insights from experimental studies and computational modeling. Am J Physiol Heart Circ Physiol. 2019;316(5):H1113-H1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fischer M, Baessler A, Schunkert H. Renin angiotensin system and gender differences in the cardiovascular system. Cardiovasc Res. 2002;53(3):672-677. [DOI] [PubMed] [Google Scholar]
- 42. Komukai K, Mochizuki S, Yoshimura M. Gender and the renin-angiotensin-aldosterone system. Fundam Clin Pharmacol. 2010;24(6):687-698. [DOI] [PubMed] [Google Scholar]
- 43. Schunkert H, Danser AH, Hense HW, Derkx FH, Kürzinger S, Riegger GA. Effects of estrogen replacement therapy on the renin-angiotensin system in postmenopausal women. Circulation. 1997;95(1):39-45. [DOI] [PubMed] [Google Scholar]
- 44. Baiardi G, Macova M, Armando I, Ando H, Tyurmin D, Saavedra JM. Estrogen upregulates renal angiotensin II AT1 and AT2 receptors in the rat. Regul Pept. 2005;124(1-3):7-17. [DOI] [PubMed] [Google Scholar]
- 45. Roesch DM, Tian Y, Zheng W, Shi M, Verbalis JG, Sandberg K. Estradiol attenuates angiotensin-induced aldosterone secretion in ovariectomized rats. Endocrinology. 2000;141(12):4629-4636. [DOI] [PubMed] [Google Scholar]
- 46. Nickenig G, Strehlow K, Wassmann S, et al. Differential effects of estrogen and progesterone on AT(1) receptor gene expression in vascular smooth muscle cells. Circulation. 2000;102(15):1828-1833. [DOI] [PubMed] [Google Scholar]
- 47. Brosnihan KB, Hodgin JB, Smithies O, Maeda N, Gallagher P. Tissue-specific regulation of ACE/ACE2 and AT1/AT2 receptor gene expression by oestrogen in apolipoprotein E/oestrogen receptor-alpha knock-out mice. Exp Physiol. 2008;93(5):658-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mompeón A, Lázaro-Franco M, Bueno-Betí C, et al. Estradiol, acting through ERα, induces endothelial non-classic renin-angiotensin system increasing angiotensin 1-7 production. Mol Cell Endocrinol. 2016;422:1-8. [DOI] [PubMed] [Google Scholar]
- 49. Brosnihan KB, Li P, Ganten D, Ferrario CM. Estrogen protects transgenic hypertensive rats by shifting the vasoconstrictor-vasodilator balance of RAS. Am J Physiol. 1997;273(6):R1908-R1915. [DOI] [PubMed] [Google Scholar]
- 50. Barrett Mueller K, Lu Q, Mohammad NN, et al. Estrogen receptor inhibits mineralocorticoid receptor transcriptional regulatory function. Endocrinology. 2014;155(11):4461-4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Manosroi W, Tan JW, Rariy CM, et al. The association of estrogen receptor-β gene variation with salt-sensitive blood pressure. J Clin Endocrinol Metab. 2017;102(11):4124-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shukri MZ, Tan JW, Manosroi W, et al. Biological sex modulates the adrenal and blood pressure responses to angiotensin II. Hypertension. 2018;71(6):1083-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nanba K, Vaidya A, Williams GH, Zheng I, Else T, Rainey WE. Age-related autonomous aldosteronism. Circulation. 2017;136(4):347-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e136-e139. [DOI] [PubMed] [Google Scholar]
- 55. Javorsky BR, Raff H, Carroll TB, et al. New cutoffs for the biochemical diagnosis of adrenal insufficiency after ACTH stimulation using specific cortisol assays. J Endocr Soc. 2021;5(4):bvab022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Guo Z, Poglitsch M, McWhinney BC, et al. Aldosterone LC-MS/MS assay-specific threshold values in screening and confirmatory testing for primary aldosteronism. J Clin Endocrinol Metab. 2018;103(11):3965-3973. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.