Abstract
Context
Hypersexual disorder (HD) involves excessive, persistent sexual behaviors related to various mood states and the diagnosis compulsive sexual behavior disorder is included as an impulse control disorder in the 11th revision of the International Classification of Diseases. Although the neurobiology behind the disorder is not clear, some studies suggest dysregulated hypothalamic-pituitary-adrenal axis. Oxytocin acts as counterregulatory neuroendocrine hormone to cortisol and is also involved in sexual behavior.
Objective
We hypothesized that oxytocin may play a role in the pathophysiology of HD with compensatory actions to cortisol.
Design
Longitudinal.
Setting
ANOVA clinic (Karolinska University Hospital).
Patients or other participants
64 males with HD and 38 age-matched healthy volunteers.
Main Outcome Measures
Plasma oxytocin levels, measured with radioimmunoassay; Hypersexual Disorder Screening Inventory; and Hypersexual Disorder: Current Assessment Scale for assessing hypersexual symptoms.
Interventions
A patient subgroup (n = 30) completed the manual-based group-administered cognitive-behavioral therapy (CBT) program for HD, and posttreatment oxytocin levels were measured.
Results
Hypersexual men (n = 64) exhibited significantly higher oxytocin plasma levels (mean ± SD: 31.0 ± 9.9 pM) compared with healthy volunteers (16.9 ± 3.9 pM; P < 0.001). There were significant positive correlations between oxytocin levels and the rating scales measuring hypersexual behavior. Patients who completed CBT treatment (n = 30) had a significant reduction of oxytocin plasma levels from pretreatment (30.5 ± 10.1 pM) to posttreatment (20.2 ± 8.0 pM; P < 0.001).
Conclusions
The results suggest that the hyperactive oxytocinergic system in hypersexual men may be a compensatory mechanism to attenuate hyperactive stress.
Keywords: oxytocin, hypersexual disorder, compulsive sexual behavior disorder, cognitive behavioral therapy
Introduction
Hypersexual disorder (HD) is considered a nonparaphilic sexual desire disorder that involves excessive and persistent sexual behaviors in relation to various mood states, with an impulsivity component and experienced loss of control (1). HD was originally suggested as a diagnostic entity for the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (2), and a similar disorder is now included in the 11th revision of the International Classification of Diseases (ie, compulsive sexual behavior disorder, which is classified as an impulse control disorder) (3). Although the inclusion of compulsive sexual behavior disorder was a significant step forward for the identification and treatment of suffering individuals, there is still a lack of knowledge regarding the pathophysiology underlying the disorder. Sexual behavior is a complex process and under the control of neuroendocrine regulation, the limbic system, and inhibitory frontal lobe activity (4,5). Although sexual response studies have implicated dopamine, serotonin, neuropeptides, glutamate, and gamma aminobutyric acid (6,7), little is known how they influence patients with excessive sexual behavior/HD.
Insights into the pathophysiology of HD have been described recently, such as endocrine dysregulation and differences in the brain’s functional systems (8). Chatzittofis et al demonstrated that men diagnosed with HD showed evidence of neuroendocrine dysregulation, using the dexamethasone suppression test as a proxy variable for a dysregulated hypothalamic-pituitary-adrenal (HPA) axis, compared to healthy subjects (9).The hypothalamic-pituitary-gonadal axis was also reported to be dysregulated in HD. Men with HD had higher luteinizing hormone plasma levels, but testosterone levels did not differ compared to healthy controls (10). In addition, epigenetic changes in the CRH gene, an essential gene involved in the cortisol stress responses in the brain, were reported in men with HD (11). Another important neuroendocrine system that might be implicated in the pathophysiology of HD is oxytocin. Oxytocin is involved in social bonding, sexual behavior stress regulation, and the reward pathway (6,12,13). Existing findings suggest that central oxytocin release contributes to the modulation and maintenance of cortisol levels that favor a rapid return to prestress baseline states as well as having a neuromodulatory role on the reward pathway with inhibitory effects on addictive behaviors (14). Moreover, a recent epigenetics study implicated the involvement of microRNA-4456, believed to regulate genes involved in the oxytocin signaling pathway, in HD (15). However, other than this epigenetic study, no study to date has evaluated oxytocin levels in HD and their possible relationship with hypersexual symptoms.
Based on previous research reporting a hyperactive HPA axis and the preliminary epigenetic data implicating the oxytocinergic system in the pathophysiology of HD, we hypothesized an involvement of the oxytocinergic system as a counterregulator of the cortisol stress system in HD.
In this study, we aimed to examine plasma oxytocin levels in men diagnosed with HD and age-matched healthy controls. Further, we aimed to examine the correlations between plasma oxytocin levels and dimensional symptoms of HD using hypersexual behavior rating scales and whether cognitive behavioral therapy (CBT) treatment for HD symptoms influences plasma oxytocin levels.
Materials and Methods
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. The study protocols and informed consent documents were approved by the Regional Ethical Review Board in Stockholm (registration IDs: 2010/5:3 and 2013/1335-31/2). Written informed consent was obtained from all participants at the initial screening.
Setting
This is a single-center, longitudinal study between 2013 and 2014. The study took place at the ANOVA clinic at Karolinska University Hospital, which is a multidisciplinary center for research, assessment, and treatment in the fields of andrology, sexual, and transgender medicine.
Participants
Patients
Patients (n = 64) were a subgroup of help-seeking men diagnosed with HD, included in a randomized clinical study of a manual-based, group-administered CBT (CBGT) treatment program for HD. The inclusion criteria were (1) age > 18 years; (2) clinically endorsed HD diagnosis; (3) stable in medication regimen of psychoactive compounds (≥3 months); and (4) informed consent to participate in the study. The exclusion criteria were (1) paraphilias of pedophilia, voyeurism, exhibitionism, frotteurism, sadism, and sexual coercion and (2) severe psychiatric comorbidity (ie, severe anxiety, depression, other contraindicating psychiatric conditions, or substance abuse). Participants using dopamine agonists were also excluded. The participants applied for the study by submitting informed consent and valid contact information on a secure Internet platform. They were then instructed to complete an online screening battery consisting of 23 structured questionnaires on sociodemographics, overall psychiatric status, and hypersexual behavior/symptoms. Following the initial online screening, eligible participants were assessed in clinical interviews with a psychiatrist and a clinical psychologist, both experienced within the field of sexual medicine. The interviews aimed to establish the participants’ HD diagnostic status in accordance with the proposed HD criteria (1) and to assess their overall psychiatric comorbidity using the Mini International Neuropsychiatric Interview 6.0 (16). In total, 64 (n = 64) HD-diagnosed participants were included in the present study. A subgroup (n = 30) of the included patients were randomized to take part of a manual-based CBGT program for HD. The remaining patients (n = 34) were randomized and assigned to a waitlist condition.
Controls
For the control group, healthy volunteers were recruited from the Karolinska Trial Alliance database. Exclusion criteria for the healthy controls were (1) previous or current psychiatric illness; (2) severe physical illness; (3) first-degree relative diagnosed with bipolar disorder, completed suicide, or schizophrenia; (4) previous exposure to serious trauma; and (5) positive screening pedophilia or pedophilic disorder. The volunteers were prescreened via telephone interviews and, if found eligible, instructed by the study coordinators to log into the Internet platform and submit valid personal information, their preliminary informed consent to participate in the study and responses to the previously presented online screening battery. The controls were matched to the included patients regarding age and equivalent blood sample collection times in the fall or spring to control for possibly interfering seasonal variations. In total, 38 healthy male volunteers were included in the study. Before the baseline blood samples were taken, the controls submitted informed consent for participation in the study. For a thorough description of the study population and study design, see Chatzittofis et al (9).
Assessments
All participants were assessed by diagnostic clinical interview using the Mini International Neuropsychiatric Interview 6.0 (16) and self-rating scales by an Internet-based platform using the following self-rating measures.
Self-rating measures
The Hypersexual Disorder Screening Inventory (HDSI) (17) is a tool for screening of a possible HD diagnosis (17). The inventory consists of 7 items following the A (n = 2) and B (n = 5) criteria for HD during the past 6 months. The items are rated along 5 graded Likert scales (0-4 points), ranging from “never true” to “almost always true,” The total sum score ranges from 0 to 28. For a possible HD diagnosis, a minimum score of 3 is required on 4 out of 5 A criteria, and a minimum score of 3 or 4 points on at least 1 for the 2 B criteria items. The inventory has a high internal consistency (α = 0.88-0.96) and high test-retest reliability (r = 0.81) over a 2-week period. HDSI was used for baseline measurement of HD symptoms and for assessment of the likelihood of positive HD diagnosis over the last 6 months.
Hypersexual Disorder: Current Assessment Scale (HD:CAS) (18) was used to assesses the HD symptom severity over the recent 2-week period. HD:CAS consists 7 items. The first (A1) examines the type as well as the number of sexual behaviors specifiers (masturbation, pornography consumption, sex behaviors with consenting adults, cybersex, telephone sex, visits to venues for sexual entertainment, and other sexual behaviors). Items 2 to 7 quantify the symptoms during the recent 2-week period. Items 2 to 7 are rated on a 5-point Likert scale (0-4 points) rendering a total score range of 0 through 24 points. HD:CAS was used as a dimensional measurement of hypersexual behavior. HD:CAS is not validated but has been used to measure the severity of HD symptomatology in studies on HD (9,19-22).
The Childhood Trauma Questionnaire (CTQ) is a self-assessment tool for examination of childhood trauma. The questionnaire consists of 28 items. Twenty-five of the items are divided into 5 subscales measuring emotional abuse, physical abuse, sexual abuse, emotional neglect and physical neglect. The 3 remaining items constitute a minimization/denial scale that pertains to identify individuals who may be underreporting traumatic childhood events (23). CTQ has exhibited good test-retest reliability (r = 0.80) and a high internal consistency for the 4 subscales sexual abuse (α = 0.93-0.95), emotional neglect (α = 0.88-92), emotional abuse (α = 0.84-0.89), and physical abuse (α = 0.81-0.86).
CBT intervention
The CBGT program consisted of 7 CBT-based modules, presented in group sessions through lectures and written materials over a period of 7 weeks. For a thorough description of the CBGT program, see Hallberg et al (21). Hallberg et al reported significantly greater decrease of HD symptoms for the treatment group when compared to the waitlist condition (19).
Blood Sample Collection and Analysis
Morning blood samples were collected from all study participants between 2013 and 2014 (n = 102), according to standard procedures. After preparation of serum and plasma, samples were stored at −80°C until assayed. All participants were fasting at the time for the blood sample collection. For the subgroup of HD-diagnosed patients who underwent the CBGT program, blood samples were also collected following their final CBT session (approximately 2 months after the baseline blood samples). Routine blood analysis was performed by the Clinical Chemistry Laboratory at the Karolinska University Hospital using standard procedures. The oxytocin level in blood plasma was determined by radioimmunoassay (Antibody: RRID:AB_2894933; https://antibodyregistry.org/search.php?q=AB_2894933) at the Clinical Neurochemistry Laboratory, Sahlgrenska University Hospital, Mölndal, Sweden. Further assay details were published previously (20). The assay’s lower limit of detection was 5 pM. The intra- and interassay coefficients of variation were 14% and 8%, 10% and 15%, and 12% and 10% at 12, 31, and 60 pM, respectively.
Statistical Analysis
All statistical analyses were performed using SigmaSTAT statistical analysis tool within Sigmaplot Software (version 13, Systat Software Inc, London, UK). Skewness and kurtosis of the distribution of continuous variables were evaluated by the Shapiro-Wilk test. Oxytocin levels were normally distributed both in patients with HD and healthy volunteers. Unpaired Student t-test was used to investigate group differences in oxytocin plasma levels between patients with HD and healthy volunteers. Correlation analyses were performed using Spearman’s rho to determine associations between the clinical and biologic variables. Paired t-test was used to compare mean plasma oxytocin levels in HD male patients (n = 30) before and after CBT intervention. Correlational analysis using Pearson’s r was performed between changes in clinical ratings (HD:CAS) and plasma oxytocin levels before and after CBT. All statistical tests were 2-tailed. The P-value for significance was < 0.05.
Role of the Funding Source
The funding source had no involvement in study design, collection, analysis, interpretation of data, writing, or the decision to submit the paper for publication.
Results
Oxytocin Levels in HD-diagnosed Men and Healthy Controls
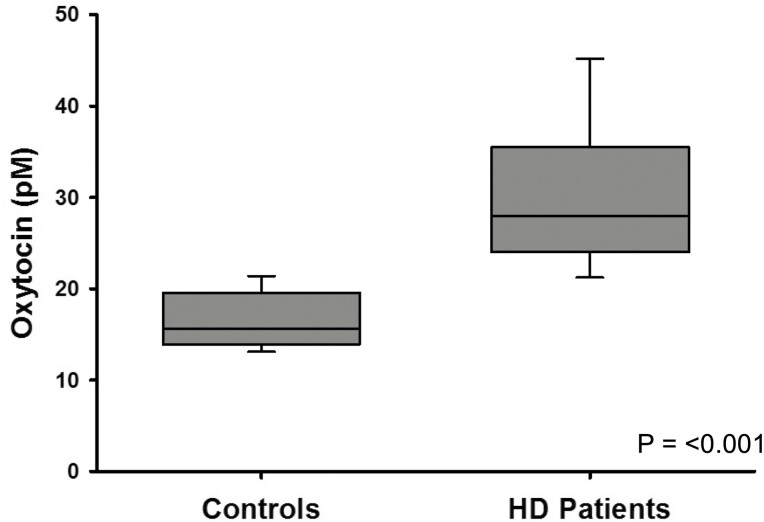
The mean plasma oxytocin level in healthy controls was (mean ± SD) 16.9 ± 3.9 pM (median 16.0, range 8-27, n = 38), whereas plasma oxytocin levels in the HD-diagnosed patients were significantly higher at 31.0 ± 9.0 pM (median 28.0, range, 17-55, n = 64; P < 0.001) (Fig. 1).
Figure 1.
Boxplot of mean plasma oxytocin levels in hypersexual disorder male patients(n = 64) and healthy male controls (n = 38). P-value was calculated by t-test. (line = median, box = Q1-Q3 interquartile range, bars = minimum and maximum).
Correlations Between Oxytocin, HD Symptom Severity, and Adverse Childhood Events
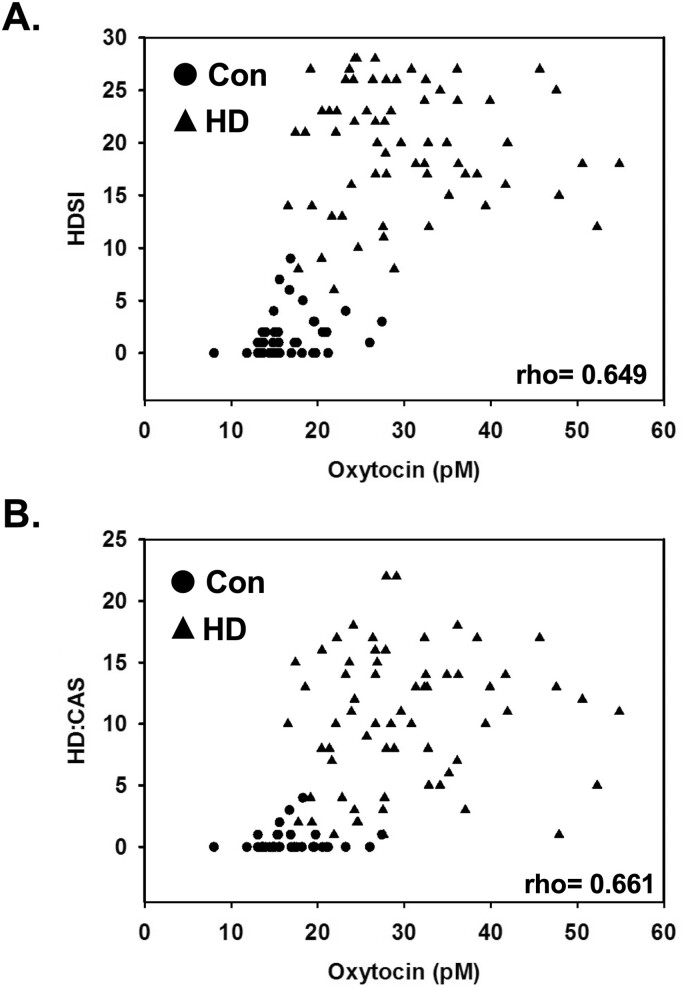
Next, correlation analyses (Spearman’s rho) were performed to examine the relationship between plasma oxytocin levels and HD symptoms. Both the HDSI scores and HD:CAS scores showed significant positive correlations with baseline plasma oxytocin levels (rho = 0.649, P = 0.0000002; rho = 0.661, P = 0.0000002) in the study participants combined (Fig. 2). The correlations between plasma oxytocin levels and CTQ scores were nonsignificant.
Figure 2.
Scatterplots of plasma oxytocin levels and self-rating scales measuring hypersexual behavior in hypersexual disorder male patients (represented by solid black triangles) and healthy male controls (represented by solid black circles). (A) Hypersexual Disorder Screening Inventory (HDSI) and (B) Hypersexual Disorder: Current Assessment Scale (HD:CAS). Spearman correlation rho values are indicated in each graph and all correlations had P-values = <0.0001.
Effect of CBT Intervention on Plasma Oxytocin Levels in Patients With HD
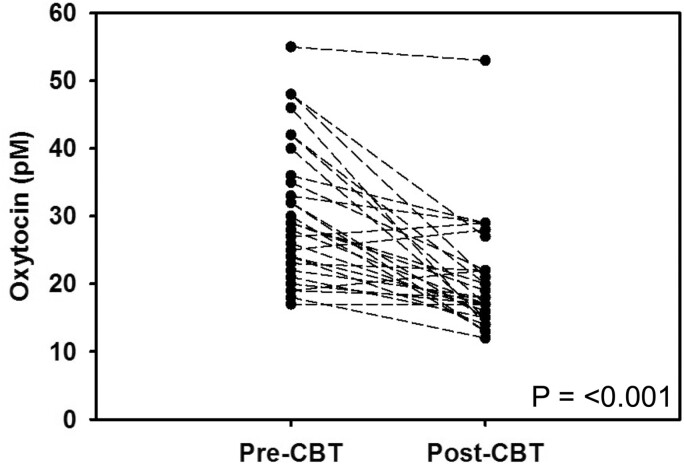
Patients diagnosed with HD were offered to participate in a 7-week CBT intervention with the goal of reducing their hypersexual behavior symptoms. The CBT treatment had reducing effects on the symptoms in the patients (19). As demonstrated in Figure 3, a paired t-test revealed that patients with HD who completed CBT treatment exhibited a significant reduction of oxytocin levels from pretreatment (30.5 ± 10.1 pM) to posttreatment (20.2 ± 8.0 pM; P = 0.0000019). Patients with HD had a significantly positive correlation of their changes in HD:CAS with plasma oxytocin levels before and after CBT (r = 0.388, P = 0.0344).
Figure 3.
Mean plasma oxytocin levels in hypersexual disorder male patients(n = 30) before and after cognitive behavioral therapy intervention. P-value was calculated by paired t-test.
Discussion
In this study, we discovered that male patients with HD had significantly higher oxytocin levels compared with healthy volunteers. Furthermore, the oxytocin levels were significantly positively correlated with the sum scores of rating scales measuring hypersexual behavior. Using a manual-based CBGT program for HD, we demonstrated that patients who completed treatment had a significant reduction in their posttreatment oxytocin levels and the changes in oxytocin levels before and after treatment were significantly positively correlated with changes in HD:CAS sum scores. To our knowledge, this is the first report to indicate a role for oxytocin’s involvement in HD and the discovery of oxytocin as a potential biomarker for diagnosis and potential drug target for treatment.
It is important to interpret the results bearing in mind the effects of oxytocin on sexual behavior, stress regulation, and psychopathology. Oxytocin, besides associated with childbirth and lactation, may exert additional regulatory functions in male sexuality. For example, oxytocin is a key inducer of penile erection in animal studies (24) with oxytocin neurons projecting from the paraventricular nucleus (PVN) to extrahypothalamic regions in the brain and spinal cord (25). In support, oxytocin was implicated in ejaculation (26-28), with a significant activation of PVN oxytocin neurons after stimulus of the dorsal penile nerve, leading to the hypothalamic release of oxytocin at ejaculation (29). This increase in oxytocin may exert an effect on sexual behavior (30), with plasma oxytocin levels being associated with orgasm intensity in both sexes (31) and naloxone-induced oxytocin suppression has been shown to decrease sexual satiety (32), while not inhibiting ejaculation. Moreover, in animal studies, cerebrospinal fluid (CSF) oxytocin levels are greatly increased shortly after ejaculation (33). This effect is completely subdued by the induced damage to the parvocellular neurosecretory neurons of the PVN (33), underlying the key role of this brain region in facilitating oxytocin activity. Intriguingly, oxytocin is important in pair bonding and monogamous behavior in animal studies, and variation of oxytocin receptor gene may also be of importance in pair bonding in women and men (34,35).
Apart from its purported role in sexuality, the oxytocinergic system is implicated in regulating complex social behaviors acting in concert with other neurotransmitters (35-39). Interactions with serotonin may influence social reward processing in the nucleus accumbens and dopamine interactions regulate social behaviors (38,39). In addition, by modulating HPA axis activity and amygdala responsiveness (37), oxytocin has been shown to reduce anxiety and partly inhibit stress responses (36,37,40,41).
Thus, the HPA axis-mediated response to stressful stimuli is proposed to be partly regulated by oxytocin activity (42). In support of this downstream effects of the oxytocin receptor activity were reported to induce delayed transcription of the corticotropin-releasing factor in the PVN (43). Moreover, exogenous oxytocin administration has been shown to dampen the cortisol response to stressful stimuli (40,44-46). In fact, it has been proposed that the prosocial behavioral effects of oxytocin could be an indirect consequence of oxytocin acting to reduce the responsiveness of the HPA axis (42). Animal studies further suggest the oxytocin and HPA axis systems may share partly intertwined trajectories, exhibiting both coordinated and autonomous activity, the details of which are not yet known comprehensively (47). In support of contextual independent action, Thomas and Larkin demonstrated that rumination in human subjects was associated with elevated cortisol and reduced oxytocin levels (48).
The role of oxytocin has been investigated in other psychopathologies that exhibit severe impulsivity dysregulation similar to HD, such as borderline personality disorder (BPD) and addiction. While reduced oxytocin plasma levels have been reported in BPD compared to healthy controls (49-51), the exogenous administration of oxytocin in subjects with BPD reported partly inconsistent results (52). A majority of studies reported on improved social skills after oxytocin administration and generally different effects in patients and controls. The authors speculated that childhood trauma, which is commonly present in subjects with BPD, may cause an adverse reaction to oxytocin with increased antisocial behavior. Moreover, the oxytocinergic system has been widely implicated in addiction pathophysiology, purportedly acting to abate drug-seeking behavior, craving, and relapse from some of the most commonly abused substances, including cocaine, marijuana, and alcohol (53-55).
Another defining feature of HD is compulsivity. In this regard, obsessive-compulsive disorder (OCD) may share some common etiology with HD. Studies investigating oxytocin levels in OCD have been mostly inconclusive, and the literature on the therapeutic effect of exogenous oxytocin administration has not reported consistent results (56). Of interest, a study demonstrated that the debut age of OCD was inversely associated with oxytocin levels (57). In support of this hypothesis, gene polymorphisms of the oxytocin receptor gene (OXTR) were associated with late-onset OCD (58).
Thus, our results of high oxytocin levels, together with previously reported noninhibition and hyperactivity of the HPA axis, are in line with previous research reporting compensatory effects of oxytocin to the HPA function. This function of oxytocin has been proposed both from addiction research as well as stress research.
Most human therapeutic studies targeting oxytocin utilize intranasal administration of oxytocin (52). While oxytocin receptor antagonists have traditionally been researched in the context of childbirth (59), emerging animal studies investigating the effects of oxytocin receptor antagonism report effects on social behavior and, to a lesser extent, on HPA responses (60) whereas the administration of oxytocin receptor antagonism to male rats, resulted in reduced sexual drive (61).
In addition, to the best of our knowledge, no studies have hitherto investigated the effects of CBT on plasma oxytocin levels. A recent study indicated that baseline plasma levels of oxytocin predict psychotherapeutic treatment outcome in chronic depression (62). However, the study did not measure oxytocin levels postintervention. The present study has several strengths. First, a representative and well-characterized patient population of subjects with HD and controls contributed to minimizing cohort bias. Second, the longitudinal and interventional study set up considered the dynamic quality of oxytocin. Thus, our study more specifically elucidated the role of oxytocin in HD compared to a case-control study set up by also analyzing the effects of CBT intervention. Third, previous studies that have implicated effects of exogenous oxytocin administration may vary by sex in specific cases. As such, the present study pertained only to male subjects to exclude sex as a potential source of bias in the association analysis between HD and oxytocin levels.
Limitations of the present study include the lack of oxytocin levels measurements from a waitlist or control group as well as the use of radioimmunoassay instead of the gold-standard stable isotope dilution mass spectrometry. There are many potential confounding factors on the association analysis between HD and plasma oxytocin levels before and after CBT intervention. The study group was made as reasonably homogenous as possible, and individuals were excluded if they exhibited addictive or psychotic illnesses and other severe physical illness. However, other possible confounding factors cannot be excluded completely, (eg, dietary patterns, ethnicity). For example, a systematic review noted a significant effect on oxytocin plasma levels of diet and dietary behaviors in clinical samples but not in nonclinical samples, which suggests that even meal consumption patterns were a potential factor unaccounted for bias (63). Moreover, while considerable effort was put into ensuring the blood measurements were as standardized and homogenous as possible, effects of timing from physical exercise prior to sampling, perceived stress, social interactions, and intra-individual variability, as well as sample storage, cannot be excluded (64). However, controlling the time of sample collection and processing samples immediately, with storage at −80°C minimizes possible effects (65). Especially, the present study did not consider potential sexual activity prior to oxytocin measurements. As ejaculation is known to significantly increase plasma oxytocin levels in the short term after ejaculation, future studies should address and properly account for this potential source of bias. However, it was reported that plasma oxytocin levels fell to basal concentrations 30 minutes after ejaculation (27). This relatively short time frame, combined with the procedures for blood sampling, suggest this as a less probable source of confounding factors. Moreover, as oxytocin levels were measured in plasma, it can be disputed whether the observed alterations reflect pathophysiological alterations in CSF, where the key biological effects of oxytocin are believed to occur. Although there is no clear consensus at this point (66), previous studies support the use of oxytocin plasma levels as a surrogate variable for CSF oxytocin activity (67). However, replicating our findings in CSF oxytocin would be valuable. Finally, replicating our findings in independent study groups using larger sample sizes would be useful in confirming the presented results.
In summary, this is the first study to investigate oxytocin in HD using a longitudinal study approach. We present our novel findings in demonstrating elevated plasma oxytocin levels in cases of HD compared to controls. Moreover, we provide evidence that these excessive oxytocin levels are effectively normalized after CBT treatment. As such, oxytocin holds promise as a potential biomarker for HD diagnostics and as a measure of disease severity. Taken together, these findings motivate further research to elucidate the explicit role of oxytocin in HD pathophysiology. Importantly, additional studies excluding potential confounding factors from recent sexual activity are needed before causality can be inferred.
Funding
Funding for this study was provided through a regional agreement between Umeå University and Västerbotten County Council (ALF) and by grants provided by the Stockholm County Council (ALF) (Jussi Jokinen); and by Swedish Research Council Grant number (Jussi Jokinen, 2020-01183).
Author Contributions
J.F, A.C., S.A., and J.J. conceptualized the study. All the authors were responsible for the design of the study. A.C., J.H., K.G.Ö., and J.F. were responsible for the data collection. A.E.B., J.F., and J.J carried out the statistical analyses and verified the underlying data. All the authors were responsible for the interpretation of the data. J.F. wrote the first draft of the manuscript. All authors revised the paper critically for important intellectual content and gave final approval of the version to be published.
Disclosures
The authors have nothing to disclose.
Data Availability
Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Kafka MP. Hypersexual disorder: a proposed diagnosis for DSM-V. Arch Sex Behav. 2010;39(2):377-400. [DOI] [PubMed] [Google Scholar]
- 2. Kafka MP. What happened to hypersexual disorder? Arch Sex Behav. 2014;43(7):1259-1261. [DOI] [PubMed] [Google Scholar]
- 3. Kraus SW, Krueger RB, Briken P, et al. Compulsive sexual behaviour disorder in the ICD-11. World Psychiatr. 2018;17(1):109-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goldey KL, van Anders SM. Sexual thoughts: links to testosterone and cortisol in men. Arch Sex Behav. 2012;41(6):1461-1470. [DOI] [PubMed] [Google Scholar]
- 5. Ragan PW, Martin PR. The psychobiology of sexual addiction. Sex Addict Compulsivity J Treatment Prevent. 2000;7(3):161-175. [Google Scholar]
- 6. Bancroft J. The endocrinology of sexual arousal. J Endocrinol. 2005;186(3):411-427. [DOI] [PubMed] [Google Scholar]
- 7. Saleh FM, Berlin FS. Sex hormones, neurotransmitters, and psychopharmacological treatments in men with paraphilic disorders. J Child Sex Abus. 2003;12(3-4):233-253. [DOI] [PubMed] [Google Scholar]
- 8. Kraus SW, Voon V, Potenza MN. Should compulsive sexual behavior be considered an addiction? Addiction 2016;111(12):2097-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chatzittofis A, Arver S, Öberg K, Hallberg J, Nordström P, Jokinen J. HPA axis dysregulation in men with hypersexual disorder. Psychoneuroendocrinology 2016;63:247-253. [DOI] [PubMed] [Google Scholar]
- 10. Chatzittofis A, Boström AE, Öberg KG, et al. Normal testosterone but higher luteinizing hormone plasma levels in men with hypersexual disorder. Sex Med 2020;8(2):243-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jokinen J, Bostrom AE, Chatzittofis A, et al. Methylation of HPA axis related genes in men with hypersexual disorder. Psychoneuroendocrinology 2017;80:67-73. [DOI] [PubMed] [Google Scholar]
- 12. Heinrichs M, von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Front Neuroendocrinol. 2009;30(4):548-557. [DOI] [PubMed] [Google Scholar]
- 13. Burri A, Heinrichs M, Schedlowski M, Kruger TH. The acute effects of intranasal oxytocin administration on endocrine and sexual function in males. Psychoneuroendocrinology 2008;33(5):591-600. [DOI] [PubMed] [Google Scholar]
- 14. Sundar M, Patel D, Young Z, Leong K-C. Oxytocin and addiction: potential glutamatergic mechanisms. Int J Mol Sci. 2021;22(5):2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boström AE, Chatzittofis A, Ciuculete DM, et al. Hypermethylation-associated downregulation of microRNA-4456 in hypersexual disorder with putative influence on oxytocin signalling: a DNA methylation analysis of miRNA genes. Epigenetics 2020;15(1-2):145-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22-33;quiz 34. [PubMed] [Google Scholar]
- 17. American Psychiatric Association’s DSM-5 Workgroup on Sexual and Gender Identity Disorders Hypersexual Disorder Screening Inventory. American Psychiatric Association; 2010. [Google Scholar]
- 18. American Psychiatric Association’s DSM-5 Workgroup on Sexual and Gender Identity Disorders. Hypersexual Disorder: Current Assessment Scale. American Psychiatric Association; 2010. [Google Scholar]
- 19. Hallberg J, Kaldo V, Arver S, Dhejne C, Jokinen J, Öberg KG. A randomized controlled study of group-administered cognitive behavioral therapy for hypersexual disorder in men. J Sex Med. 2019;16(5):733-745. [DOI] [PubMed] [Google Scholar]
- 20. Nielsen EI, Al-Saqi SH, Jonasson AF, Uvnäs-Moberg K. Population pharmacokinetic analysis of vaginally and intravenously administered oxytocin in postmenopausal women. J Clin Pharmacol. 2017;57(12):1573-1581. [DOI] [PubMed] [Google Scholar]
- 21. Hallberg J. Hypersexual Disorder: Clinical Presentation and Treatment. Department of Medicine. Vol Doctoral. Karolinska Institutet; 2019. [Google Scholar]
- 22. Nair D, Pawar A, Kalra G, Shah N. An Indian study of hypersexual disorder in patients with anxiety and mood disorders. Sex Addict Compulsivity. 2013;20(4):292-305. [Google Scholar]
- 23. Bernstein DP, Stein JA, Newcomb MD, et al. Development and validation of a brief screening version of the childhood trauma questionnaire. Child Abuse Negl. 2003;27(2):169-190. [DOI] [PubMed] [Google Scholar]
- 24. Argiolas A. Oxytocin stimulation of penile erection: pharmacology, site, and mechanism of action. Ann N Y Acad Sci. 1992;652(1):194-203. [DOI] [PubMed] [Google Scholar]
- 25. Melis MR, Argiolas A, Gessa GL. Oxytocin-induced penile erection and yawning: site of action in the brain. Brain Res. 1986;398(2):259-265. [DOI] [PubMed] [Google Scholar]
- 26. Carmichael MS, Humbert R, Dixen J, Palmisano G, Greenleaf W, Davidson JM. Plasma oxytocin increases in the human sexual response. J Clin Endocrinol Metab. 1987;64(1):27-31. [DOI] [PubMed] [Google Scholar]
- 27. Murphy MR, Seckl JR, Burton S, Checkley SA, Lightman SL. Changes in oxytocin and vasopressin secretion during sexual activity in men. J Clin Endocrinol Metab. 1987;65(4):738-741. [DOI] [PubMed] [Google Scholar]
- 28. Ogawa S, Kudo S, Kitsunai Y, Fukuchi S. Increase in oxytocin secretion at ejaculation in male. Clin Endocrinol (Oxf) 1980;13(1):95-97. [DOI] [PubMed] [Google Scholar]
- 29. Yanagimoto M, Honda K, Goto Y, Negoro H. Afferents originating from the dorsal penile nerve excite oxytocin cells in the hypothalamic paraventricular nucleus of the rat. Brain Res. 1996;733(2):292-296. [DOI] [PubMed] [Google Scholar]
- 30. Thackare H, Nicholson HD, Whittington K. Oxytocin--its role in male reproduction and new potential therapeutic uses. Hum Reprod Update. 2006;12(4):437-448. [DOI] [PubMed] [Google Scholar]
- 31. Carmichael MS, Warburton VL, Dixen J, Davidson JM. Relationships among cardiovascular, muscular, and oxytocin responses during human sexual activity. Arch Sex Behav. 1994;23(1):59-79. [DOI] [PubMed] [Google Scholar]
- 32. Murphy MR, Checkley SA, Seckl JR, Lightman SL. Naloxone inhibits oxytocin release at orgasm in man. J Clin Endocrinol Metab. 1990;71(4):1056-1058. [DOI] [PubMed] [Google Scholar]
- 33. Corona G, Jannini EA, Vignozzi L, Rastrelli G, Maggi M. The hormonal control of ejaculation. Nat Rev Urol. 2012;9(9):508-519. [DOI] [PubMed] [Google Scholar]
- 34. Walum H, Lichtenstein P, Neiderhiser JM, et al. Variation in the oxytocin receptor gene is associated with pair-bonding and social behavior. Biol Psychiatr.. 2012;71(5):419-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7(10):1048-1054. [DOI] [PubMed] [Google Scholar]
- 36. Kirsch P, Esslinger C, Chen Q, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25(49):11489-11493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30(4):534-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Baskerville TA, Douglas AJ. Dopamine and oxytocin interactions underlying behaviors: potential contributions to behavioral disorders. CNS Neurosci Ther. 2010;16(3):e92-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dölen G, Darvishzadeh A, Huang KW, Malenka RC. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 2013;501(7466):179-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biol Psychiatr. 2009;65(9):728-731. [DOI] [PubMed] [Google Scholar]
- 41. Winter J, Jurek B. The interplay between oxytocin and the CRF system: regulation of the stress response. Cell Tissue Res. 2019;375(1):85-91. [DOI] [PubMed] [Google Scholar]
- 42. Flanagan JC, Fischer MS, Nietert PJ, et al. Effects of oxytocin on cortisol reactivity and conflict resolution behaviors among couples with substance misuse. Psychiatry Res. 2018;260:346-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jurek B, Slattery DA, Hiraoka Y, et al. Oxytocin regulates stress-induced Crf gene transcription through CREB-regulated transcription coactivator 3. J Neurosci. 2015;35(35):12248-12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cardoso C, Ellenbogen MA, Orlando MA, Bacon SL, Joober R. Intranasal oxytocin attenuates the cortisol response to physical stress: a dose-response study. Psychoneuroendocrinology 2013;38(3):399-407. [DOI] [PubMed] [Google Scholar]
- 45. Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54(12):1389-1398. [DOI] [PubMed] [Google Scholar]
- 46. Linnen AM, Ellenbogen MA, Cardoso C, Joober R. Intranasal oxytocin and salivary cortisol concentrations during social rejection in university students. Stress 2012;15(4):393-402. [DOI] [PubMed] [Google Scholar]
- 47. Samuni L, Preis A, Deschner T, Wittig RM, Crockford C. Cortisol and oxytocin show independent activity during chimpanzee intergroup conflict. Psychoneuroendocrinology 2019;104:165-173. [DOI] [PubMed] [Google Scholar]
- 48. Thomas SJ, Larkin T. Cognitive distortions in relation to plasma cortisol and oxytocin levels in major depressive disorder. Front Psychiatry. 2019;10:971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bertsch K, Schmidinger I, Neumann ID, Herpertz SC. Reduced plasma oxytocin levels in female patients with borderline personality disorder. Horm Behav. 2013;63(3):424-429. [DOI] [PubMed] [Google Scholar]
- 50. Ebert A, Edel MA, Gilbert P, Brüne M. Endogenous oxytocin is associated with the experience of compassion and recalled upbringing in borderline personality disorder. Depress Anxiety. 2018;35(1):50-57. [DOI] [PubMed] [Google Scholar]
- 51. Striepens N, Kendrick KM, Maier W, Hurlemann R. Prosocial effects of oxytocin and clinical evidence for its therapeutic potential. Front Neuroendocrinol. 2011;32(4):426-450. [DOI] [PubMed] [Google Scholar]
- 52. Peled-Avron L, Abu-Akel A, Shamay-Tsoory S. Exogenous effects of oxytocin in five psychiatric disorders: a systematic review, meta-analyses and a personalized approach through the lens of the social salience hypothesis. Neurosci Biobehav Rev. 2020;114:70-95. [DOI] [PubMed] [Google Scholar]
- 53. Carson DS, Hunt GE, Guastella AJ, et al. Systemically administered oxytocin decreases methamphetamine activation of the subthalamic nucleus and accumbens core and stimulates oxytocinergic neurons in the hypothalamus. Addict Biol. 2010;15(4):448-463. [DOI] [PubMed] [Google Scholar]
- 54. McRae-Clark AL, Baker NL, Maria MM, Brady KT. Effect of oxytocin on craving and stress response in marijuana-dependent individuals: a pilot study. Psychopharmacology (Berl) 2013;228(4):623-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pedersen CA, Smedley KL, Leserman J, et al. Intranasal oxytocin blocks alcohol withdrawal in human subjects. Alcohol Clin Exp Res. 2013;37(3):484-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cochran DM, Fallon D, Hill M, Frazier JA. The role of oxytocin in psychiatric disorders: a review of biological and therapeutic research findings. Harv Rev Psychiatry 2013;21(5):219-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Humble MB, Uvnäs-Moberg K, Engström I, Bejerot S. Plasma oxytocin changes and anti-obsessive response during serotonin reuptake inhibitor treatment: a placebo controlled study. BMC Psychiatry 2013;13:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kang JI, Kim HW, Kim CH, Hwang EH, Kim SJ. Oxytocin receptor gene polymorphisms exert a modulating effect on the onset age in patients with obsessive-compulsive disorder. Psychoneuroendocrinology 2017;86:45-52. [DOI] [PubMed] [Google Scholar]
- 59. Kim SH, Riaposova L, Ahmed H, et al. Oxytocin receptor antagonists, atosiban and nolasiban, inhibit prostaglandin F(2α)-induced contractions and inflammatory responses in human myometrium. Sci Rep. 2019;9(1):5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hodges TE, Eltahir AM, Patel S, Bredewold R, Veenema AH, McCormick CM. Effects of oxytocin receptor antagonism on social function and corticosterone release after adolescent social instability in male rats. Horm Behav. 2019;116:104579. [DOI] [PubMed] [Google Scholar]
- 61. Blitzer DS, Wells TE, Hawley WR. Administration of an oxytocin receptor antagonist attenuates sexual motivation in male rats. Horm Behav. 2017;94:33-39. [DOI] [PubMed] [Google Scholar]
- 62. Jobst A, Sabaß L, Hall D, et al. Oxytocin plasma levels predict the outcome of psychotherapy: a pilot study in chronic depression. J Affect Disord. 2018;227:206-213. [DOI] [PubMed] [Google Scholar]
- 63. Skinner JA, Garg ML, Dayas CV, Fenton S, Burrows TL. Relationship between dietary intake and behaviors with oxytocin: a systematic review of studies in adults. Nutr Rev. 2018;76(5):303-331. [DOI] [PubMed] [Google Scholar]
- 64. Martins D, Gabay AS, Mehta M, Paloyelis Y. Salivary and plasmatic oxytocin are not reliable trait markers of the physiology of the oxytocin system in humans. Elife 2020;9:e62456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Schaebs FS, Wirobski G, Marshall-Pescini S, Range F, Deschner T. Validation of a commercial enzyme immunoassay to assess urinary oxytocin in humans. Endocr Connect 2021;10(3):290-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lefevre A, Mottolese R, Dirheimer M, Mottolese C, Duhamel JR, Sirigu A. A comparison of methods to measure central and peripheral oxytocin concentrations in human and non-human primates. Sci Rep. 2017;7(1):17222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Carson DS, Berquist SW, Trujillo TH, et al. Cerebrospinal fluid and plasma oxytocin concentrations are positively correlated and negatively predict anxiety in children. Mol Psychiatry. 2015;20(9):1085-1090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.