Abstract
Context
Supplementation with vitamin D has the potential to both reduce and increase risk of falling, and parathyroid hormone (PTH) may contribute to fall risk.
Objective
To assess the associations of intra-trial mean circulating levels of 25-hydroxyvitamin D [25(OH)D] and PTH on incident falls in healthy older adults.
Design
Observational within a clinical trial.
Setting
The Bone Metabolism Laboratory at the USDA Nutrition Center at Tufts University.
Participants
410 men and women age ≥65 years who participated in the 3-year Boston STOP IT trial to determine the effect of supplementation with 700 IU of vitamin D3 plus calcium on incident falls (secondary endpoint). Intra-trial exposures of 25(OH)D and PTH were calculated as the mean of biannual measures up to and including the first fall.
Main outcome measures
Incidence of first fall.
Results
Intra-trial mean 25(OH)D was significantly associated with risk of falling in a U-shaped pattern; the range associated with minimal risk of falling was approximately 20 to 40 ng/mL. PTH was not significantly associated with risk of falling.
Conclusions
The findings highlight the importance of maintaining the circulating 25(OH)D level between 20 and 40 ng/mL, the range that is also recommended for bone health. At PTH levels within the normal range, there was no detectible independent association of PTH with fall risk.
Keywords: 25(OH)D, PTH, falls, vitamin D supplementation
In observational studies in older adults (1), low circulating levels of 25-hydroxyvitamin D [25(OH)D] have been associated with increased risk of falling, but the evidence that supplementation with vitamin D lowers that risk is inconsistent. Several recent clinical trials found that supplementation did not reduce fall risk (2-4), although in D-Health, there was a increased fall risk in the subset of participants with a body mass index < 25 kg/m2 (4). Low 25(OH)D levels have traditionally been thought to adversely affect fall risk through adverse effects on muscle performance and balance, but a recent meta-analysis of 54 controlled trials found no significant effects of vitamin D supplementation on any of a variety of muscle and balance measures (5). In contrast, evidence is emerging that high levels of 25(OH)D and/or bolus dosing with vitamin D may increase fall risk in older adults at high risk for falling (6-8). There is no consensus currently on the specific range of 25(OH)D levels at which fall risk is minimal in older adults.
Higher parathyroid hormone (PTH) levels have been associated with poor muscle performance (9-11) and with falls (11) in an observational cohort study. It is not clear whether PTH has an effect on risk of falls that is independent of its inverse association with circulating 25(OH)D levels. Several observations support an independent role for PTH. Adults with primary hyperparathyroidism have symptoms of muscle weakness (12). In a different report, knee flexion strength and maximal force production improved modestly 7 months after successful parathyroid surgery, although knee extension was unchanged (13).
The Boston Site Testing Osteoporosis Prevention/Intervention Treatment (STOP IT) study was a clinical trial in older adults recruited from the general population. They were treated with vitamin D3 plus calcium or placebo daily for 3 years. Plasma 25(OH)D and serum PTH levels and falls were assessed every 6 months throughout the study. We have previously shown that vitamin D and calcium supplementation in STOP IT significantly reduced risk of falling in the women [odds ratio (OR) 0.54, 95% CI 0.30-0.97] but not in the men (OR 0.93, 95% CI 0.50-1.72) (14). The objectives of this analysis are to describe the associations of intra-trial mean circulating concentrations of 25(OH)D and PTH with risk of falling over the 3 years in STOP IT participants.
Materials and Methods
Overview of the STOP IT Study
The STOP IT study was a randomized, double-blind, placebo-controlled trial to test whether treatment with 700 IU of vitamin D3 and 500 mg of calcium as calcium citrate malate vs placebo daily for 3 years decreased bone loss from the hip (15). In the trial, 445 men and women age ≥65 years were enrolled over a 12.5-month period. Exclusion criteria, described in detail elsewhere (15), included osteoporosis or treatment with osteoporosis drugs or steroids and history of renal disease and kidney stones. Participants agreed not to take personal calcium or vitamin D supplements during the study. The protocol was approved by the Investigational Review Committee at Tufts University, and all participants gave written informed consent. Study visits took place at 0 (baseline), 6, 12, 18, 24, 30, and 36 months. Falls were assessed on each visit. Blood was drawn after an 8-hour overnight fast on each visit except the 30-month visit.
Measurements Used in the Current Analysis
The STOP IT participants who had assessment of falls and at least one 25(OH)D and PTH measurement after baseline (n = 410) are included in this analysis.
Intra-study Measurements
Falls were defined as unintentionally coming to rest on the ground, floor, or other lower level. Participants were asked to keep a fall diary documenting the date, location, and details of any falls in the last 6 months. They were also asked to send a postcard to the center after every fall, which was then followed up by a telephone call by staff to the participant to assess the circumstances of the fall. Falls over the last 6 month were also assessed on each intra-study visit. The primary analysis examines participants with any first fall (fallers) and those without any reported falls (nonfallers).
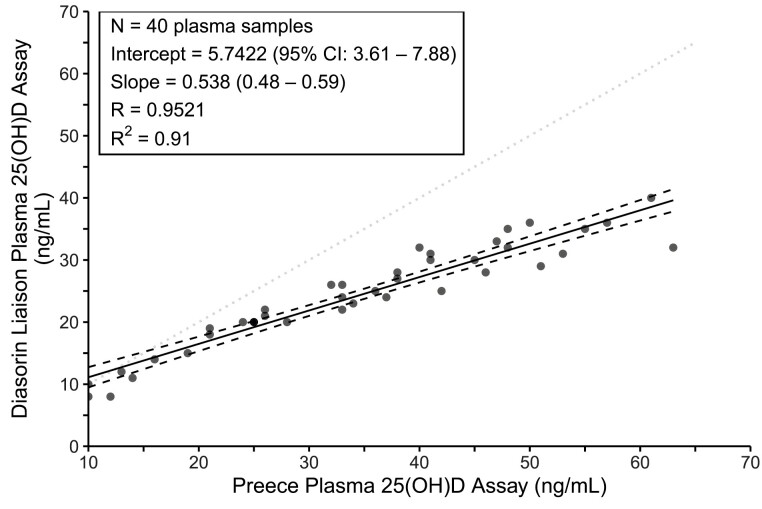
Plasma 25(OH)D was assessed by the competitive binding protein method of Preece (16) and subsequently the 25(OH)D values were converted to Diasorin equivalent values. The formula for converting these values to those of the Diasorin was based on a calibration study in which 40 samples were measured by both methods. All 25(OH)D values reported herein are estimated Diasorin values (D), which were calculated from the competitive binding protein values (C) in ng/mL as D = 5.742 + 0.538(C), as reported earlier (17). Figure 1 displays the plot of the 40 results used in our calibration study along with the R2, a measure of the regression model fit. Serum intact PTH was assessed by Allegro Intact PTH kits from Nichols Institute (San Juan Capistrano, CA, USA), with a coefficient of variation of 6.6% and reference range of 10 to 69 pg/mL. Samples were analyzed as they were collected.
Figure 1.
Correlation of the older Preece plasma 25(OH)D assay (x-axis) with the Diasorin plasma 25(OH)D assay in 40 samples. The dotted line is the line of identity. The calibration equation and R2 values are shown in the box.
Baseline Measurements
Calcium intake from diet was assessed by the Walter Willett food frequency questionnaire and physical activity by the Physical Activity Scale for the Elderly, as described previously (15). Grip strength was assessed as the higher of 2 serial measurements made for the nondominant hand with a digital handgrip dynamometer. Tandem standing balance was assessed over 5 seconds with feet in the full tandem position. Scoring was 1 if unable to perform the tandem stand, 2 if some staggering, swaying or moving arms, and 3 if able to hold a tandem stand for 5 seconds.
Statistical Analysis
The primary endpoint was any first fall after baseline. The primary modeling strategy was to use intra-trial mean 25(OH)D and PTH levels as predictor variables in a logistic regression analysis. Intra-trial mean 25(OH)D and PTH levels were calculated for each individual as the mean of all values starting at month 6 and measured up to and including the time of the first fall. Plasma 25(OH)D and serum PTH levels were not measured at month 30, and intra-trial mean 25(OH)D and PTH concentrations for participants who reported a first fall at month 30 were calculated as the mean of values measured at 6, 12, 18, and 24 months.
To determine whether the supplementation of vitamin D and calcium modified the effect of 25(OH)D on falls, we tested the interaction between treatment group and intra-trial mean 25(OH)D through a likelihood-ratio test. The interaction was not significant (P = 0.60), and so participants in the 2 treatment arms were analyzed together. Univariable and multivariable logistic regression were used to assess the association between intra-trial mean 25(OH)D and PTH levels and the risk of falling (18). Age (in 5-year intervals), sex, and total calcium intake were included as covariates in the 25(OH)D and PTH models. To avoid over adjustment, we included total calcium intake, rather than treatment group, as a covariate in the models. Total calcium intake was calculated as the sum of dietary intake and the 500 mg of supplemental calcium (regardless of their pill compliance) or no calcium (placebo) from the STOP IT intervention
Logistic regression models showed evidence of nonlinearity; thus, to examine nonlinear associations of 25(OH)D and risk of falls, polynomial and piecewise regression models were utilized. Quadratic regression was the best-fit model over linear and cubic models based on likelihood ratio tests (chi-square tests). We further used 3-segment piecewise regression models to aid in interpretation of the nonlinearity and identify ranges of 25(OH)D levels associated with odds of falls. Model fit was examined based on a combination of Akaike information criterion and visual agreement with the quadratic regression models. The change points in piecewise regressions were estimated in R with the segmented package v 1.3-4 (19,20).
Even though term for the interaction of sex with intra-trial mean 25(OH)D levels on fall risk was not statistically significant (P-values > 0.26), previous results on falls showed evidence of sex differences (14). Thus, we describe the proportion of fallers among men and women in the three intra-trial mean 25(OH)D segments.
The independent effect of intra-trial mean PTH on fall risk was assessed by logistic regression with the presence of 25(OH)D in its piecewise function with the change points estimated from the fully adjusted 25(OH)D model. The model was adjusted for age, sex, and total calcium intake.
Summary statistics are reported as mean ± SD unless otherwise specified. Comparisons between the fallers and nonfallers were made with 2-sample t-tests. Analyses were conducted in R 4.1 (21). Significance level of 0.05 was used and all P-values are 2-sided.
Results
The clinical characteristics of the 410 fallers and nonfallers at baseline are shown in Table 1. Participants excluded from the analysis were 1 year older and had a 125 mg lower daily calcium intake but had no other known significant differences. There were no statistically significant differences in baseline 25(OH)D or PTH levels or in grip strength or tandem stand tests or in any other characteristic between the fallers and nonfallers (Table 1). Of the 219 participants who fell at least once during the trial, 73 first falls occurred in months 0 to 6, 33 in month 6 to 12, 27 in months 12 to 18, 17 in months 18 to 24, 27 in months 24 to 30, and 42 in months 30 to 36.
Table 1.
Baseline characteristics by intra-trial fall status
| n | Fallers | Nonfallers | |
|---|---|---|---|
| Sex, n | |||
| Male | 187 | 94 | 93 |
| Female | 223 | 125 | 98 |
| Age, years | 410 | 71.2 ± 4.9 | 71.0 ± 4.5 |
| BMI, kg/m2 | 410 | 27.0 ± 4.4 | 26.8 ± 3.8 |
| Dietary calcium intake, mg | 410 | 757 ± 352 | 703 ± 343 |
| Total calcium intake, mg | 410 | 988 ± 417 | 965 ± 421 |
| PASE score | 407 | 117 ± 54 | 112 ± 54 |
| Grip, nondominant, kg | 406 | 24.6 ± 9.8 | 25.7 ± 9.6 |
| Tandem stand, sec | 410 | 2.64 ± 0.69 | 2.75 ± 0.57 |
| Thiazide use, % | 410 | 10.5 | 8.9 |
| Plasma 25(OH)D, ng/mL | 409a | 21.3 ± 7.6 | 22.1 ± 6.9 |
| Serum PTH, pg/mL | 409a | 38.9 ± 17.2 | 37.3 ± 16.1 |
Data are given as mean ± SD unless otherwise specified.
Abbreviations: BMI, body mass index; PASE, Physical Activity Scale for the Elderly; PTH, parathyroid hormone.
aBaseline 25(OH)D and PTH measure are missing in one faller, but this participant had 25(OH)D and PTH measures from 6 months up to the first fall.
Intra-trial Mean 25(OH)D Level and Risk of Falling
The intra-trial mean 25(OH)D level was 26.7 ± 9.1 ng/mL in the 219 fallers and 26.8 ± 7.5 ng/mL in the 191 nonfallers (P = 0.87).
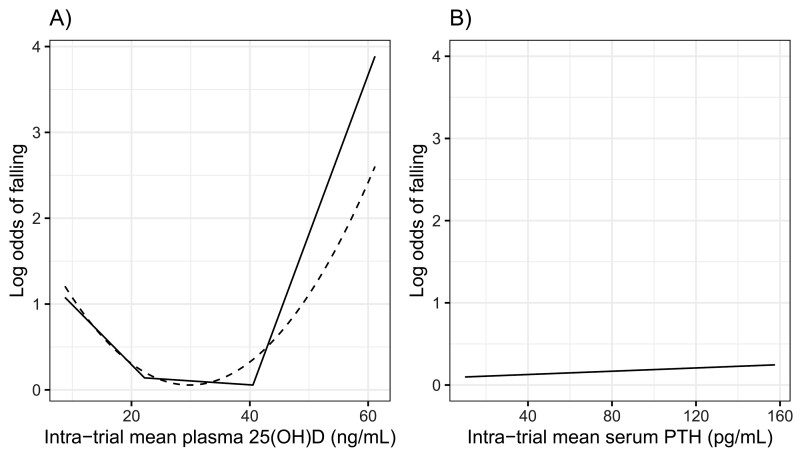
In the logistic models in the 410 participants, there was an association of intra-trial mean 25(OH)D level with risk of falling that appeared to be U-shaped. The quadratic model showed a nonlinear association of intra-trial mean 25(OH)D in both unadjusted and adjusted models (Table 2). To further examine the nonlinearity, piecewise regression models were fit. The associated estimated change points were at intra-trial mean 25(OH)D levels of 22.2 ± 6.0 (SE) and 40.5 ± 3.7 (SE) ng/mL. The association of intra-trial mean 25(OH)D with risk of falling is inverse at 25(OH)D levels < 22.2 ng/mL, flat for middle values between change points (22.2 and 40.5 ng/mL), and positive for 25(OH)D levels > 40.5 ng/mL. In our sample, there are 134 participants with intra-trial mean 25(OH)D < 22.2 ng/mL, 252 participants with intra-trial mean 25(OH)D between 22.2 and 40.5 ng/mL, and 24 participants with intra-trial 25(OH)D > 40.5 ng/mL. The proportion of fallers in these groups were 57%, 50%, and 67%, respectively. The pattern among men and women was similar (53.3%, 48.5%, and 62.5%, respectively, in the men and 58.4%, 52.5%, and 68.8%, respectively, in the women). The estimated log odds from piecewise and quadratic regressions in all participants, adjusted for age, sex, and total calcium intake are shown in Figure 2A.
Table 2.
Estimates of nonlinear associations of intra-trial plasma 25(OH)D and risk of falling from quadratic regressions
| Unadjusted model | Adjusted modela | |||
|---|---|---|---|---|
| β (SE) | P-value | β (SE) | P-value | |
| Linear 25(OH)D | −0.15 (0.064) | 0.017 | −0.15 (0.069) | 0.025 |
| Quadratic 25(OH)D | 0.003 (0.001) | 0.018 | 0.003 (0.001) | 0.027 |
| Overallb | — | 0.031 | — | 0.048 |
aAdjusted for age, sex, and total calcium intake.
bOverall P-value tests both linear and quadratic terms based on the likelihood ratio test (chi-squared).
Figure 2.
Intra-trial mean plasma 25(OH)D (A) and serum parathyroid hormone (PTH) (B) and risk of falling, adjusted for age (half decades), sex, and calcium intake, estimated from quadratic and piecewise logistic regression. In (A), dashed line is a quadratic and solid line is a 3-piecewise regression. The 2 estimated change points are 22.2 ± 6.0 (SE) and 40.5 ± 3.7 (SE) ng/mL. In (B), estimated log odds are shown from multivariable logistic regression. There was no significant association of intra-trial mean PTH with risk of falling (odds ratio 1.00, 95% CI 0.99-1.02).
Intra-trial Mean PTH Level and Risk of Falling
The intra-trial mean PTH level was 36.8 ± 17.4 pg/mL in the 219 fallers and 35.8 ± 13.3 pg/mL in the 191 nonfallers (P = 0.52).
To assess the independent effect of PTH level on the risk of falling, intra-trial mean PTH was added to the models. Intra-trial mean PTH did not show an independent effect on the risk of falling (OR 1.00, 95% CI 0.99-1.02). The result of logistic regression adjusted for all covariates is shown in Figure 2B.
Discussion
In this secondary analysis of a vitamin D and calcium intervention trial conducted in community-dwelling adults age ≥65 years, the mean intra-trial 25(OH)D level, whether attained by supplementation or other means (diet and sun exposure), was a significant predictor of falling whereas the intra-trial mean PTH level was not an independent predictor of falling. More first falls occurred in the 0- to 6-month period than in subsequent 6-month periods. This was expected because the most vulnerable participants likely fell early, leaving less vulnerable participants in the pool, and because the pool size diminished with each passing 6-month interval. In all participants, the intra-trial mean 25(OH)D level was significantly associated with risk of falling in a U-shaped pattern. Risk of falling was higher in those with 25(OH)D levels < 22 ng/mL and it was progressively higher at intra-trial mean 25(OH)D values > 40.5 ng/mL. Lower 25(OH)D levels are thought to impair muscle performance and balance and thereby increase risk of falling. The basis for increased fall risk at higher 25(OH)D levels is unclear, but it may be related to the observation that 25(OH)D levels > 40 ng/mL are associated with increased circulating levels of fibroblast growth factor 23 (22,23), a compound that not only activates 24-hydroxylation (CYP24A1), thereby increasing the catabolism of 1,25(OH)2D, but also reduces its production (24). Based on fall risk, the optimal 25(OH)D range for older adults appears to be approximately 20 to 40 ng/mL. This is the range that is recommended for optimal bone health by the National Academy of Medicine, formerly the Institute of Medicine (25).
Our lower threshold is consistent with prior studies reporting that supplementation of adults with 25(OH)D levels <20 ng/mL lowers incidence of falling (26,27). Recent trials that recruited participants with mean 25(OH)D levels in the “minimal risk” range of 20 to 40 ng/mL were not well positioned to determine with certainty whether adults with 25(OH)D levels < 20 ng/mL benefited from supplementation because of limited numbers of participants with baseline levels < 20 ng/mL (2,3,4,28).
There is now a substantial body of evidence that in older adults who are at increased risk of falling based on having had a fall in the last year, supplementation at doses that result in high 25(OH)D levels adversely affects fall risk. In adults aged ≥70 years with a recent history of falling, monthly treatment with 60 000 IU of vitamin D3 resulting in a 25(OH)D level of 40 ng/mL was associated with more falls than treatment with 24 000 IU per month resulting in a 25(OH)D level of 32 ng/mL (8). In nursing home residents, a population that is generally at high risk for falling, treatment with 100 000 IU of vitamin D3 monthly doubled the risk of falling, compared with placebo (6). In another study, community-dwelling older adults at high risk for falls were treated for 2 years with either 200, 1000, 2000, or 4000 IU per day (29). There were more falls in the 2 higher dose groups than in the 200 IU control group, whereas the 1000 IU dose reduced risk of falling (29). Over the ensuing 2 years, participants taking 200 IU per day continued on this dose, and the others continued or were transitioned to a dose of 1000 IU per day (29). There was no significant difference in fall risk in the 200 and 1000 IU dose groups after 2 years. Notably, the achieved 25(OH)D levels of both the 200 IU and 1000 IU dose groups, 28 and 38 ng/mL, respectively, were within our observed minimal-risk range of 20 to 40 ng/mL.
Several vitamin D intervention trials have assessed fall risk in community-dwelling older adults not selected on the basis of their fall risk. The VITAL study did not identify an effect of 2000 IU per day of vitamin D on fall risk (3). This cohort had a 25(OH)D level of 30.8 ng/mL at baseline and 41.6 ng/mL on supplementation and were thus largely in the range of minimal fall risk both before and during treatment. In the D-HEALTH study, a dose of 60 000 IU per month, when compared with placebo, was not associated with increased fall risk in 16 000 participants; however, increased risk was observed in the subset of participants with body mass index < 25 kg/m2 (4). In this pragmatic trial, 25(OH)D levels, measured only in a random subset of participants during the trial, were 31 ng/mL in the placebo group and 46 ng/mL in the supplemented group. Thus the study population was, for the most part, in the optimal range at entry but achieved levels above the optimal range on treatment (4). It is tempting to speculate that, compared with overweight and obese participants, the normal-weight participants achieved higher 25(OH)D levels on treatment and that higher intra-trial 25(OH)D levels contributed to their increased fall risk. In a smaller, multiple dose trial in postmenopausal women with mean age 67 years and with initial 25(OH)D levels < 20 ng/mL, fall risk declined as 25(OH)D levels rose to 40 ng/mL, but at intra-trial 25(OH)D levels > 41 ng/mL, fall risk increased in Caucasian women but not in African American women (30). Our estimated desirable 25(OH)D range of 20 to 40 ng/mL for minimal risk of falling pertains to community-dwelling and largely Caucasian adults aged ≥65 years at average risk of falling. Importantly, it may not apply to younger adults or to other race groups.
In our earlier report, the STOP IT intervention reduced risk of falling in the women but not in the men (14). The sex difference may have resulted in part from the fact that the men had higher starting 25(OH)D levels than the women (33 vs 25 ng/mL), and thus fewer men than women had initial 25(OH)D levels < 20 ng/mL at baseline. Even though terms for the interaction of sex with intra-trial mean 25(OH)D were not statistically significant in the current analysis, we examined the association of falls with 25(OH)D in men and women separately and found that in both sexes, there was a U-shaped pattern with higher proportions of fallers in the lower and higher ranges of 25(OH)D and a nadir in the 20 to 40 ng/mL range. Thus, the optimal range of 20 to 40 ng/mL to minimize risk of falling appears to apply to both men and women.
In this study of older adults at average risk for falling, we did not find that intra-trial mean PTH is associated with falling, after taking into account the U-shaped association of 25(OH)D with fall risk, age, sex, and calcium intake. This is in contrast to observational studies reporting that higher PTH levels were associated with more falls in older adults at high risk for falling (11,31). In the Sambrook study in 637 adults, mean age 86 years and residing in intermediate-care hostels or nursing homes (11), median PTH levels were 64.8 pg/mL in the fallers and 57.0 pg/mL in the nonfallers. These levels were considerably higher than the baseline mean PTH levels in STOP IT of 38.9 pg/mL in the fallers and 37.3 pg/mL in the nonfallers. We hypothesize that a PTH level higher than that of the STOP IT participants is needed to trigger a detectable adverse effect on risk of falling, if in fact PTH is an independent determinant of falling. An independent effect of PTH on fall risk has indirect support from early observations, that (1) patients with primary hyperparathyroidism complained of generalized muscle weakness (12) and (2) isokinetic knee flexion strength and maximal force production in patients with primary hyperparathyroidism improved 7 months after successful parathyroid surgery, and the improvement was correlated with the preoperative serum calcium level, although knee extension did not change in that study (13). Thus, our observation that PTH is not an independent risk factor for falling applies to older adults with PTH levels near the middle of the reference range of 10 to 69 pg/mL but not to populations with frankly elevated PTH levels.
Our study has strengths and limitations. Among the strengths is the comprehensive falls assessments involving frequent staff interaction, as opposed to self-report via annual mail-out questionnaire and other methods. The availability of multiple intra-trial 25(OH)D and PTH measurements up to and including the time of the first fall allowed for a relatively robust assessment of intra-trial mean levels. While this analysis did not rely directly on the randomization, the STOP IT trial allowed for the assessment of fall risk at higher 25(OH)D levels than are often seen in observational studies.
A limitation is that the 25(OH)D measurements, made over 25 years ago, have been calibrated to the Diasorin assay but not to an assay certified by the Vitamin D Standardization Program (VDSP) as standardized (32). Notably, Smith et al reported both Diasorin values and VSDP-standardized liquid chromatography-tandem mass spectrometry (LC-MS/MS) values above which fall risk was increased (30). Those levels were 38 ng/mL by Diasorin and 41 ng/mL by LC-MS/MS (30). Based on the similarity of these values (30), our proposed optimal range of 20 to 40 ng/mL may be a reasonable approximation of the optimal range determined by a VDSP-standardized LC-MS/MS assay. Confirmation in studies that employed a VDSP-standardized 25(OH)D assay is important. Another limitation is the relatively small number of participants with intra-trial mean 25(OH)D levels > 40.2 ng/mL (n = 24). Finally, we did not examine associations of circulating 25(OH)D levels with all falls. Doing so would require a different statistical approach, but it could be informative.
In conclusion, this analysis indicates that adults aged ≥65 in the general population are likely at increased risk of falling when their circulating 25(OH)D levels are <20 ng/mL and when they are >40 ng/mL. The 25(OH)D range we identified as associated with minimal fall risk is also the range considered optimal for bone health. We identified no role for the intra-trial mean PTH level as an independent predictor of falling in older men and women with intra-trial mean PTH levels near the middle of the reference range; however, this finding does not apply to populations with higher PTH levels.
Acknowledgments
This study was supported by grant AG10353 from the National Institutes of Health (Bethesda, MD, USA) and by the USDA Agricultural Research Service under Cooperative Agreement No. 58-1950-7-707. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views of the USDA or the National Institutes of Health.
Disclosures
B.D-H., J.W., K.B., C.T.S., R.A.D-A., L.C. have nothing to disclose. H.B-F. received investigator-initiated funding from DSM Nutritional Products, Wild, Vifor, Nestlé, Streuli, and Pfizer for vitamin D–related research.
Data Availability
Data will be made available to external researchers on a selective basis upon request.
References
- 1. Snijder MB, van Schoor NM, Pluijm SM, van Dam RM, Visser M, Lips P. Vitamin D status in relation to one-year risk of recurrent falling in older men and women. J Clin Endocrinol Metab. 2006;91(8):2980-2985. [DOI] [PubMed] [Google Scholar]
- 2. Khaw KT, Stewart AW, Waayer D, et al. Effect of monthly high-dose vitamin D supplementation on falls and non-vertebral fractures: secondary and post-hoc outcomes from the randomised, double-blind, placebo-controlled ViDA trial. Lancet Diab Endocrinol. 2017;5(6):438-447. [DOI] [PubMed] [Google Scholar]
- 3. LeBoff MS, Murata EM, Cook NR, et al. VITamin D and OmegA-3 trial (VITAL): effects of vitamin D supplements on risk of falls in the US population. J Clin Endocrinol Metab. 2020;105(9):2929-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Waterhouse M, Sanguineti E, Baxter C, et al. Vitamin D supplementation and risk of falling: outcomes from the randomized, placebo-controlled D-health trial. J. Cachexia Sarcopenia Muscle. 2021;12(6):1428-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bislev LS, Grove-Laugesen D, Rejnmark L. Vitamin D and muscle health: a systematic review and meta-analysis of randomized placebo-controlled trials. J Bone Miner Res. 2021;36(9):1651-1660. [DOI] [PubMed] [Google Scholar]
- 6. Ginde AA, Blatchford P, Breese K, et al. High-dose monthly vitamin D for prevention of acute respiratory infection in older long-term care residents: a randomized clinical trial. J Am Geriatr Soc. 2017;65(3):496-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303(18):1815-1822. [DOI] [PubMed] [Google Scholar]
- 8. Bischoff-Ferrari HA, Dawson-Hughes B, Orav EJ, et al. Monthly high-dose vitamin D treatment for the prevention of functional decline: a randomized clinical trial. JAMA Intern Med. 2016;176(2):175-183. [DOI] [PubMed] [Google Scholar]
- 9. Visser M, Deeg DJ, Lips P. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the longitudinal aging study Amsterdam. J Clin Endocrinol Metab. 2003;88(12):5766-5772. [DOI] [PubMed] [Google Scholar]
- 10. Bislev LS, Langagergaard Rodbro L, Sikjaer T, Rejnmark L. Effects of elevated parathyroid hormone levels on muscle health, postural stability and quality of life in vitamin D-insufficient healthy women: a cross-sectional study. Calcif Tissue Int. 2019;105(6):642-650. [DOI] [PubMed] [Google Scholar]
- 11. Sambrook PN, Chen JS, March LM, et al. Serum parathyroid hormone is associated with increased mortality independent of 25-hydroxy vitamin d status, bone mass, and renal function in the frail and very old: a cohort study. J Clin Endocrinol Metab. 2004;89(11):5477-5481. [DOI] [PubMed] [Google Scholar]
- 12. Patten BM, Bilezikian JP, Mallette LE, Prince A, Engel WK, Aurbach GD. Neuromuscular disease in primary hyperparathyroidism. Ann Intern Med. 1974;80(2):182-193. [DOI] [PubMed] [Google Scholar]
- 13. Joborn C, Joborn H, Rastad J, Akerstrom G, Ljunghall S. Maximal isokinetic muscle strength in patients with primary hyperparathyroidism before and after parathyroid surgery. Br J Surg. 1988;75(1):77-80. [DOI] [PubMed] [Google Scholar]
- 14. Bischoff-Ferrari HA, Orav EJ, Dawson-Hughes B. Effect of cholecalciferol plus calcium on falling in ambulatory older men and women: a 3-year randomized controlled trial. Arch Intern Med. 2006;166(4):424-430. [DOI] [PubMed] [Google Scholar]
- 15. Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997;337(10):670-676. [DOI] [PubMed] [Google Scholar]
- 16. Preece MA, O’Riordan JL, Lawson DE, Kodicek E. A competitive protein-binding assay for 25-hydroxycholecalciferol and 25-hydroxyergocalciferol in serum. Clin Chim Acta. 1974;54(2):235-242. [DOI] [PubMed] [Google Scholar]
- 17. Durazo-Arvizu RA, Dawson-Hughes B, Sempos CT, et al. Three-phase model harmonizes estimates of the maximal suppression of parathyroid hormone by 25-hydroxyvitamin D in persons 65 years of age and older. J Nutr. 2010;140(3): 595-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kahn HA, Sempos CT.. Statistical Methods in Epidemiology. Oxford University Press; 1989. [Google Scholar]
- 19. Muggeo VMR. Segmented: an R package to fit regression models with broken line relationships. R News. 2008;8(1):20-25. [Google Scholar]
- 20. Muggeo VM. Estimating regression models with unknown break-points. Stat Med. 2003;22(19):3055-3071. [DOI] [PubMed] [Google Scholar]
- 21.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2021. [Google Scholar]
- 22. Zittermann A, Berthold HK, Pilz S. The effect of vitamin D on fibroblast growth factor 23: a systematic review and meta-analysis of randomized controlled trials. Eur J Clin Nutr. 2021;75(6):980-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zittermann A, Berthold HK, Pilz S. Correction: the effect of vitamin D on fibroblast growth factor 23: a systematic review and meta-analysis of randomized controlled trials. Eur J Clin Nutr. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bacchetta J, Sea JL, Chun RF, et al. Fibroblast growth factor 23 inhibits extrarenal synthesis of 1,25-dihydroxyvitamin D in human monocytes. J Bone Miner Res. 2013;28(1):46-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cangussu LM, Nahas-Neto J, Orsatti CL, et al. Effect of isolated vitamin D supplementation on the rate of falls and postural balance in postmenopausal women fallers: a randomized, double-blind, placebo-controlled trial. Menopause. 2016;23(3):267-274. [DOI] [PubMed] [Google Scholar]
- 27. LeBlanc ES, Chou R. Vitamin D and falls-fitting new data with current guidelines. JAMA Intern Med. 2015;175(5):712-713. [DOI] [PubMed] [Google Scholar]
- 28. Aaltonen T, Adelman J, Akimoto T, et al. Search for a Higgs boson decaying to two W bosons at CDF. Phys Rev Lett. 2009;102(2):021802. [DOI] [PubMed] [Google Scholar]
- 29. Appel LJ, Michos ED, Mitchell CM, et al. The effects of four doses of vitamin D supplements on falls in older adults: a response-adaptive, randomized clinical trial. Ann Intern Med. 2021;174(2):145-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smith LM, Gallagher JC, Suiter C. Medium doses of daily vitamin D decrease falls and higher doses of daily vitamin D3 increase falls: a randomized clinical trial. J Steroid Biochem Mol Biol. 2017;173:317-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stein MS, Wark JD, Scherer SC, et al. Falls relate to vitamin D and parathyroid hormone in an Australian nursing home and hostel. J Am Geriatr Soc. 1999;47(10):1195-1201. [DOI] [PubMed] [Google Scholar]
- 32. Sempos CT, Heijboer AC, Bikle DD, et al. Vitamin D assays and the definition of hypovitaminosis D: results from the first international conference on controversies in vitamin D. Br J Clin Pharmacol. 2018;84(10):2194-2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available to external researchers on a selective basis upon request.