Abstract
In some chronic primary pain conditions such as temporomandibular disorder (TMD) and fibromyalgia syndrome (FMS), mild or chronic stress enhances pain. TMD and FMS often occur together, but the underlying mechanisms are unclear. The purpose of this study was to investigate the role of cholecystokinin (CCK) in the spinal cord in somatic hyperalgesia induced by orofacial inflammation combined with stress. Somatic hyperalgesia was detected by the thermal withdrawal latency and mechanical withdrawal threshold. The expression of CCK1 receptors, CCK2 receptors, ERK1/2 and p-ERK1/2 in the spinal cord was examined by Western blot. After the stimulation of orofacial inflammation combined with 3 day forced swim, the expression of CCK2 receptors and p-ERK1/2 protein in the L4-L5 spinal dorsal horn increased significantly, while the expression of CCK1 receptors and ERK1/2 protein remained unchanged. Intrathecal injection of the CCK2 receptor antagonist YM-022 or mitogen-activated protein kinase (MAPK) kinase (MEK) inhibitor PD98059 blocked somatic hyperalgesia induced by orofacial inflammation combined with stress. Intrathecal administration of the MEK inhibitor blocked somatic sensitization caused by the CCK receptor agonist CCK8. The CCK2 receptor antagonist YM-022 significantly reduced the expression of p-ERK1/2. These data indicate that upregulation of CCK2 receptors through the MAPK pathway contributes to somatic hyperalgesia in this comorbid pain model. Thus, CCK2 receptors and MAPK pathway may be potential targets for the treatment of TMD comorbid with FMS.
Keywords: Hyperalgesia, Cholecystokinin receptor, ERK1/2, Temporomandibular disorder, Fibromyalgia syndrome, Comorbidity
1. Introduction
Chronic primary pain (CPP) is a chronic clinical condition manifesting a variety of unexplained somatic symptoms with females at higher risk, which has been proposed as an inclusive nosology encompassing individual syndromes, such as temporomandibular disorder (TMD) and fibromyalgia syndrome (FMS). TMD has a series of manifestations characterized by pain and functional disorder in the temporomandibular joint and masticatory muscles (Scrivani et al., 2008), and ranks fourth in oral epidemiology (Landi et al., 2004, 2005). FMS is a disease characterized chiefly by diffuse pain and clinical features including wide-spreading pain, hyperalgesia, mood disorders and anxiety (Sugerman, 2014). TMD and FMS have a high degree of similarity and co-occurrence, but the mechanisms of comorbidity are unclear (Rabhi et al., 2010). It has been shown that the occurrence and development of TMD and FMS are closely associated with stress such as mental and psychological factors (Furness et al., 2018; Giannakopoulos et al., 2010). Our previous studies have shown that 3 day forced swim (FS) stress induces mechanical alloydnia and thermal hyperalgesia in hindpaws (Li et al., 2019; Xu et al., 2020). Central sensitization in the spinal dorsal horn and the descending facilitation contributed to the development of wide-spreading hyperalgesia in the model of comorbid TMD and FMS (Li et al., 2020). In the study, injection of estradiol (E2) to ovariectomized rats, Complete Freund’s Adjuvant (CFA) into the masseter muscles or application of FS stress alone could not induce somatic hyperalgesia of the hindpaw. All of three factors were essential and necessary for the development of long-lasting and wide-spreading hyperalgesia (Li et al., 2020).
Cholecystokinin (CCK) is a gut-brain peptide that participates in the pain facilitation system (Noble et al., 1999). The roles of CCK closely related to digestion, pain, stress and depression are mediated by CCK1 and CCK2 receptor subtypes. A few studies have shown that CCK contributes to the descending pain facilitatory system from the periaqueductal gray (PAG) and rostral ventromedial medulla (RVM). CCK mediates anxiety-induced hyperalgesia and hyperalgesia associated with peripheral neuropathy by activating the descending pro-nociceptive facilitatory pathways (Lovick, 2008a). CCK activates ON-cells in the RVM selectively and produces behavioral hyperalgesia (Heinricher and Neubert, 2004). Microinjection of CCK into the RVM produces hyperalgesia via activation of CCK2 receptors, which can be reversed by CCK2 receptor antagonists (Marshall et al., 2012). Blockade of CCK2 receptors reduces anxiety-induced hyperalgesia in humans (Benedetti et al., 2006). It has been demonstrated that CCK expressing cells in the dorsal horn of the spinal cord have important roles in mechanical allodynia in neuropathic pain states (Gutierrez-Mecinas et al., 2019). However, whether spinal CCK contributes to the development of the comorbidity of TMD and FMS remains unclear.
A few studies have shown that CCK2 receptors can activate the extracellular signal-regulated kinase 1/2 (ERK1/2) signal transduction pathway in the digestive system (Grossini et al., 2012; Mao et al., 2014). ERK1/2 is activated in neurons in the dorsal horn of spinal cord in response to stimulation of nociceptors and is involved in nociceptive plasticity (Ji et al., 1999; Liao et al., 2020). However, whether the upregulation of CCK2 receptors contributes to hyperalgesia via the activation of ERK1/2 is unknown. In our previous study, we demonstrated that ERK activation contributed to visceral hypersensitivity evoked by orofacial inflammation combined with stress (Zhao et al., 2018). Thus, the purpose of this study was to explore the independent and joint contributions of CCK2 receptors and ERK1/2 in the spinal cord in the somatic hyperalgesia induced by orofacial inflammation combined with stress using an established animal model for comorbidity of TMD and FMS.
2. Materials and methods
2.1. Animals
Female Sprague-Dawley rats weighing 200–250 g (about 10 weeks of age) were used in the present study. The animals were provided by Xi’an Jiaotong University Laboratory Animal Center (Xi’an, Shaanxi, China) and housed in a climate-controlled room on a 12-h light/dark cycle (lights on at 7 a.m.), with food and water available ad libitum. All experiments were approved by the Institutional Animal Care and Use Committees of Xi’an Jiaotong University Health Science Center, China (approved number: 2019-950), and adhered to guidelines for experimental pain in animals published by the International Association for the Study of Pain.
2.2. Experimental protocol
The experimental scheme is shown in Fig. 1. Rats were anesthetized with isoflurane (3%) and ovariectomized (OVx) by a dorsolateral approach (Traub et al., 2014). Ten days after OVx, rats were given a subcutaneous injection of 17-β-estradiol (E2, 50 μg in 100 μL safflower oil, Sigma, St Louis, MO, USA) or safflower oil (vehicle, 100 μL). The E2 injection was repeated every 4 days to mimic the estrous cycle of the rats so that the E2 levels of OVx rats were similar at the same time and the effect of the natural estrous cycle of the rats on the experimental results was excluded (Ji et al., 2003; Traub et al., 2014). Complete Freund’s adjuvant (CFA, 150 μL, 1:1 in saline) or saline (control for CFA injection) was injected into the bilateral masseter muscles. Stress was induced by a 3 day FS paradigm starting the day following CFA injection (Li et al., 2020; Traub et al., 2014). Rats were forced to swim (10 min on the first day, 20 min on the next 2 days) in a cylindrical container (30 cm diameter) filled to 20 cm with 25–26 °C water (Imbe and Kimura, 2015; Imbe et al., 2014; Li et al., 2019; Quintero et al., 2000). After each swim session, the rats were dried with a towel and placed in a cage partially heated with a fan before being returned to their home cage. The control rats for FS remained undisturbed in their home cages. The day after the last FS was designated Day 1 post-stress. All studies employed randomized and vehicle-controlled designs with observers blind to treatment allocation.
Fig. 1.
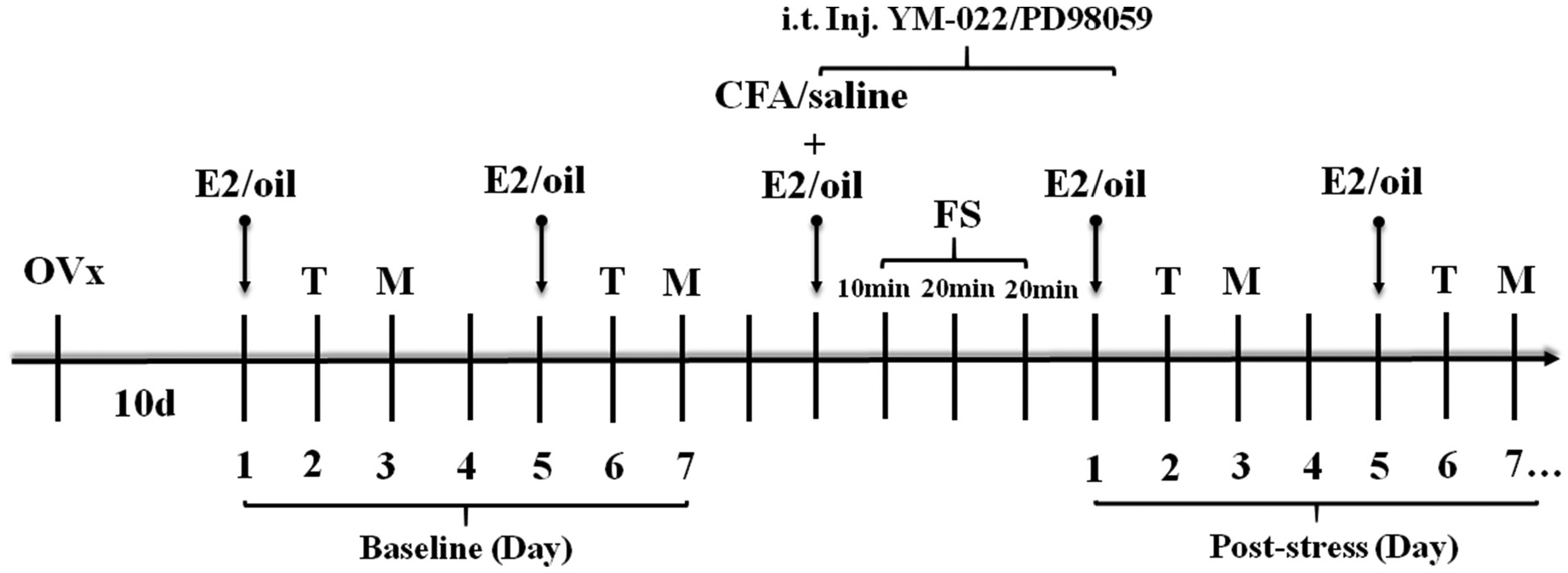
Experimental design. All rats were ovariectomized (Ovx). After 10 days of recovery, 17-β-estradiol (E2) or oil was injected subcutaneously every 4 days. The baselines of thermal withdrawal latency (T) and mechanical withdrawal threshold (M) were tested twice to ensure the reproducibility. The average of two baseline values was used for analysis. The same day as an E2/oil injection the bilateral masseter muscles were injected with complete Freund’s adjuvant (CFA) and a 3 day forced swim (FS) protocol was initiated the following day. E2/oil injections continued every 4th day starting one day after the last FS. The thermal withdrawal latency and mechanical withdrawal threshold were measured 1 and 2 days following each E2/oil injection, respectively. The measurements were continued until the thermal withdrawal latency or mechanical withdrawal threshold returning to the baseline level.
2.3. Experimental groups
The expression of CCK receptors and ERK1/2 was investigated in E2 replaced rats having orofacial inflammation combined with stress (E2 + CFA + FS group, i.e. comorbid pain condition, n = 4) and the control group (oil + saline + non-FS group, n = 6). The effects of CCK2 receptor antagonist YM-022 (E2 + CFA + FS + YM-022 vs. E2 + CFA + FS + saline, n = 7 for each group) and MEK inhibitor PD98059 (E2 + CFA + FS + PD98059 vs. E2 + CFA + FS + DMSO, n = 9 for each group) on the somatic hyperalgesia in the comorbid pain condition were examined. Whether blocking CCK2 receptor affected the expression of p-ERK1/2 in the spinal cord was also investigated in the comorbid pain condition (E2 + CFA + FS + YM-022 vs. E2 + CFA + FS + saline, n = 6 and 4, respectively). Lastly, whether inhibiting ERK1/2 activation blocked somatic hyperalgesia induced by intrathecal injection of CCK receptor agonist CCK8 was examined (E2 + CCK8 + PD98059 vs. E2 + CCK8 + DMSO, n = 8 and 10, respectively).
2.4. Behavioral tests
Thermal withdrawal latency and mechanical withdrawal threshold were tested 1 and 2 days, respectively, following each E2 or oil injection. This corresponded to days 2–19 after the end of FS (see Fig. 1). Thermal sensitivity was measured as the latency to withdraw from radiant heat directed at the hindpaw (Hargreaves et al., 1988). Rats were placed on a glass plate, individually covered with an inverted clear plastic cage and allowed to acclimate for 30 min before testing. Radiant heat was directed to the plantar surface of the right hindpaw and the stimulus ceased when the rat withdrew its hindpaw and the latency was recorded using a plantar thermal test device (Ugo Basile, Gemonio, Italy). Rats were tested three times with an inter-stimulus interval of 5 min, and the mean value was determined. A cutoff of 20 s was set to avoid tissue damage.
The mechanical withdrawal threshold was measured with a series of calibrated von Frey filaments. Rats were individually placed in a plastic cage with a wire mesh bottom which allowed full access to the hindpaw. After 30 min of adaptation, the von Frey filaments (Stoelting, Wood Dale, IL, USA) with logarithmically incremental stiffness (0.41–26 g) were applied from underneath the wire mesh floor to the plantar surface of the left hindpaw. The cutoff force was 26 g to prevent tissue damage. The mechanical withdrawal threshold was determined by sequentially increasing and decreasing the stimulus strength (“up and down” method) (Chaplan et al., 1994; Marshall et al., 2012).
2.5. Intrathecal injection
Rats were anesthetized with isoflurane and placed in a prone position with a round tube underneath the abdomen. A portion of the caudal half of the rat’s back was shaved and scrubbed with povidone iodine solution. Then a disposable 30-gauge needle connected to a 25-μl Hamilton syringe was slowly inserted into the intervertebral space between the L4-L5 spinous process and the needle was allowed to penetrate the dura. A quick flick of the tail or hindlimb was a sign that the needle entered the intrathecal space. The CCK2 receptor antagonist YM-022 (30 μg/10 μl), MEK inhibitor PD98059 (2 μg/10 μl) or their vehicle was injected in the E2 + CFA + FS rats. The intrathecal injection was administered 30 min before the CFA injection and each FS. In the CCK-induced hyperalgesia experiments, the MEK inhibitor PD98059 (2 μg/10 μl) or its vehicle was intrathecally injected 30 min before the administration of a CCK receptor agonist (CCK-8, 3 μg/10 μl) daily for 5 days. Drug doses were chosen based on the literature (Fukazawa et al., 2007; Yamamoto and Nozaki-Taguchi, 1996; Zhao et al., 2018) and our preliminary studies.
2.6. Western blot
Two days after the last FS, rats in the Western blot experiments group were anesthetized with isoflurane (5%) and quickly decapitated. The spinal cord was flushed out with ice-cold saline (Cao et al., 2015). The L4 to L5 section of the spinal cord was rapidly isolated and immediately snap frozen on dry ice. The dorsal part of the spinal cord was separated and stored at −80 °C until use. Tissues were homogenized in solubilization buffer (1% NP-40, 1% Sodium deoxycholate, 0.1% SDS) and protease inhibitor cocktail. The homogenates were centrifuged at 10, 000 rpm for 10 min at 4 °C. The supernatant was collected and protein concentration was determined using the bicinchoninic acid (BCA) method. Protein samples (18 μg) were separated on 10% SDS-PAGE and blotted onto PVDF membranes after denaturing. The blots were blocked with 5% non-fat milk in Tris-buffered saline (TBS) and incubated with primary antibody directed against CCK1 receptor (1:1000, Bioss, bs-11514R, Beijing, China), CCK2 receptor (1:1000, Bioss, bs-1777R), p-ERK1/2 (1:1000, Cell Signaling Technology, Danvers, MA, USA) and GAPDH (1:4000, Boster, BA2913, Wuhan, China) at 4 °C overnight. The membranes were washed with TBST and incubated with goat anti-rabbit secondary antibody (Boster, BA1054) at 1:4000 dilution. The immunoreactivity was detected using an enhanced chemiluminescence (ECL) detection system (Thermo Scientific, Waltham, MA, USA). The immunoreactive band densities were analyzed using Image J software. The blot of p-ERK1/2 was stripped for 30 min and reprobed with antibodies against ERK1/2 (1:1000, Cell Signaling Technology, Danvers, MA, USA) which served as the internal control for p-ERK1/2 and then GAPDH for ERK1/2.
2.7. Drugs
All drugs were dissolved to a final concentration in 10 μL vehicle. The CCK2 receptor antagonist YM-022 (30 μg, Santa Cruz Biotechnology, Santa Cruz, US) was dissolved in saline. The CCK receptor agonist cholecystokinin octapeptide (CCK-8, 3 μg, Med Chem Express, Monmouth Junction, NJ, USA) and the MEK inhibitor PD98059 (2 μg, Sigma, St Louis, MO, USA) were dissolved in 4% DMSO. Saline and DMSO have no significant effect on the nociceptive information transmission in the spinal cord.
2.8. Data analysis
All data are presented as mean ± SEM. Statistical and figure analyses were performed using GraphPad Prism 6 software. The data of protein expression of CCK receptors, ERK1/2, and p-ERK1/2 between two groups were compared using unpaired t-test. Two-way repeated measures analysis of variance (ANOVA) followed by Sidak post hoc test was used for thermal withdrawal latency and mechanical withdrawal threshold. P < 0.05 was considered significant.
3. Results
3.1. CCK2 receptors are involved in the somatic hyperalgesia in the comorbid pain condition
3.1.1. The comorbid pain condition significantly increases the expression of CCK2 receptors
We previously reported that E2, CFA or FS alone had no effect on somatic hyperalgesia relative to baseline, but E2 + CFA + FS induced thermal and mechanical hyperalgesia in the hindpaws (Li et al., 2020). Therefore, the control group for comparison was the oil + saline + non-FS group.
To determine whether spinal CCK receptors mediate somatic hyperalgesia in the comorbid pain condition, we examined the protein expression of CCK1 and CCK2 receptors in the L4-L5 spinal dorsal horn. There were no significant differences in the expression of CCK1 receptors in the E2 + CFA + FS group compared with the oil + saline + non-FS group (P = 0.7161, Fig. 2A). In contrast, the expression of CCK2 receptors significantly increased in the E2 + CFA + FS group (P = 0.0279 vs. the oil + saline + non-FS group, n = 4 and 6, respectively, Fig. 2B), suggesting that CCK2 receptors, but not CCK1 receptors, are upregulated in the spinal cord in the comorbid pain condition.
Fig. 2.
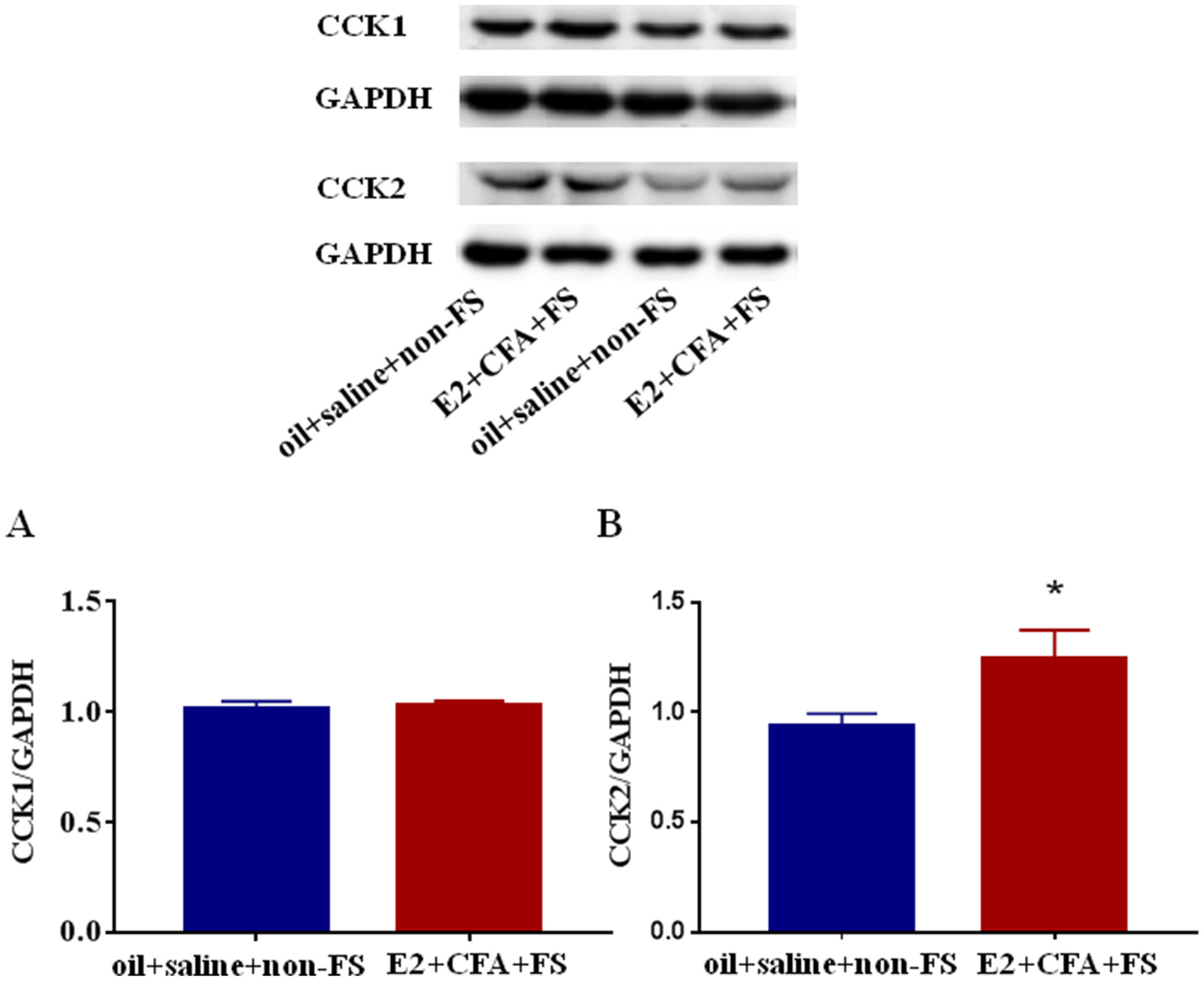
The expression of CCK1 receptors (A) and CCK2 receptors (B) in the L4-L5 spinal dorsal horn in the comorbid pain condition. The expression of CCK2 receptors in the E2 + CFA + FS group (n = 4) was significantly increased compared with the oil + saline + non-FS group (n = 6). The expression of CCK1 receptors in the E2 + CFA + FS group did not significantly change. *P < 0.05 vs. the oil + saline + non-FS group.
3.1.2. CCK2 receptor antagonist YM-022 blocks the hyperalgesia in the comorbid pain condition
Next, it was determined if a CCK2 receptor antagonist could prevent the somatic hyperalgesia in the comorbid pain condition. YM-022 or saline was injected intrathecally for 5 consecutive days (before CFA injection, before each FS and before injection of E2). Two way ANOVA revealed a significant effect of YM-022 on the thermal withdrawal latency compared with saline treated rats (F5,60 = 8.311, P < 0.0001 for interaction; F5,60 = 6.611, P < 0.0001 for time factor; F1,12 = 15.15, P = 0.0021 for group factor, n = 7 for each group, Fig. 3A) and mechanical withdrawal threshold (F5,60 = 4.427, P = 0.0017 for interaction; F5,60 = 12.16, P < 0.0001 for time factor; F1,12 = 3.83, P = 0.0740 for group factor, Fig. 3B). In the E2 + CFA + FS + saline group, thermal hyperalgesia persisted from Day 2 to Day 6 post FS (P < 0.0001 and P = 0.0204 for Day 2 and Day 6 vs. Baseline, respectively, Fig. 3A), and mechanical hyperalgesia lasted from Day 3 to Day 11 post FS (P < 0.0001 for Day 3 and Day 7 vs. Baseline, P = 0.0045 for Day 11 vs. Baseline, Fig. 3B).
Fig. 3.
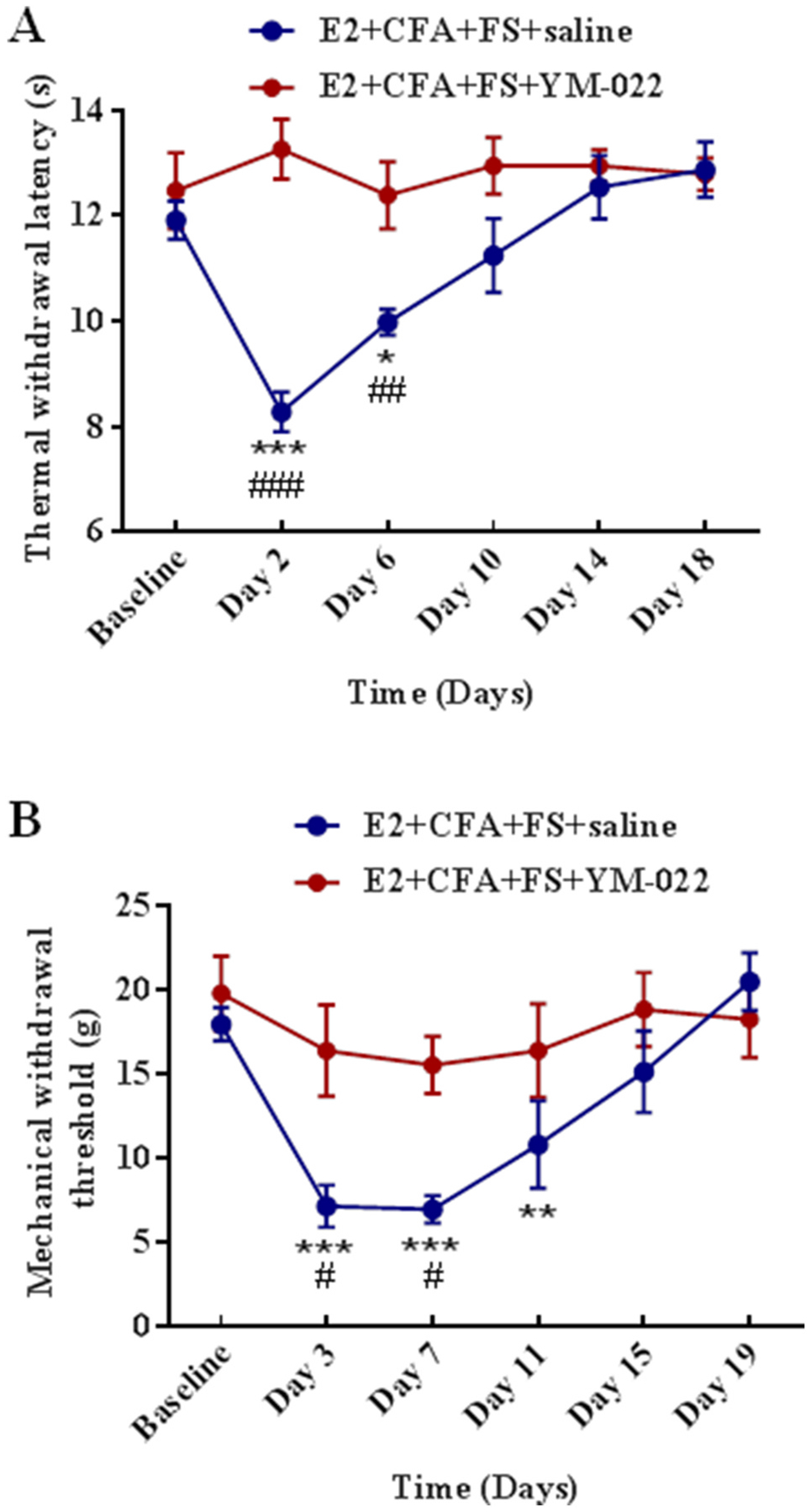
CCK2 receptors are involved in the somatic hyperalgesia in the comorbid pain condition. (A) YM-022 inhibited the thermal hyperalgesia. The thermal withdrawal latency significantly decreased at Day 2 and Day 6 post FS in saline treated rats. *, ***P < 0.05, 0.001 vs. Baseline. YM-022 blocked the thermal hyperalgesia compared with saline. ##, ###P < 0.01, 0.001 vs. the E2 + CFA + FS + YM-022 group at the same time point. n = 7 for each group. (B) YM-022 inhibited mechanical hyperalgesia. The mechanical withdrawal threshold significantly decreased at Day 3, 7 and 11 post FS in saline treated rats. **, ***P < 0.01, 0.001 vs. Baseline. YM-022 blocked the mechanical hyperalgesia compared with saline. #P < 0.05 vs. the E2 + CFA + FS + YM-022 group at the same time point.
In contrast, YM-022 blocked the reduction in the thermal withdrawal latency in the E2 + CFA + FS + YM-022 group compared with the E2 + CFA + FS + saline group (P > 0.05 for each time point vs. Baseline). Comparing the YM-022 group vs. saline at each time point, the thermal withdrawal latency (P < 0.0001 and P = 0.0089 for Day 2 and Day 6, respectively, Fig. 3A) and mechanical withdrawal threshold (P = 0.0147 and 0.0286 for Day 3 and Day 7, respectively, Fig. 3B) in hindpaws in the E2 + CFA + FS + YM-022 group were significantly different from that of the E2 + CFA + FS + saline group from Day 1 to Day 7 after FS stress. These results showed that CCK2 receptor antagonist YM-022 prevented the hyperalgesia in the comorbid pain condition, suggesting that CCK2 receptors in the spinal cord contribute to the hyperalgesia.
3.2. Activation of ERK1/2 is involved in the somatic hyperalgesia in the comorbid pain condition
3.2.1. The comorbid pain condition significantly increases the expression of p-ERK1/2
To explore the role of ERK1/2 in the somatic hyperalgesia, we examined the protein expression of ERK1/2, p-ERK1/2 in the L4-L5 spinal dorsal horn in the comorbid pain condition. There were no significant differences in the expression of ERK1 (P = 0.7450) and ERK2 (P = 0.6870) between the E2 + CFA + FS and oil + saline + non-FS groups (n = 4 and 6 respectively, Fig. 4A and B). However, E2 + CFA + FS significantly increased the expression of p-ERK1 (P = 0.0081, Fig. 4C) and p-ERK2 (P = 0.0017, Fig. 4D) in the spinal cord compared with the oil + saline + non-FS group.
Fig. 4.
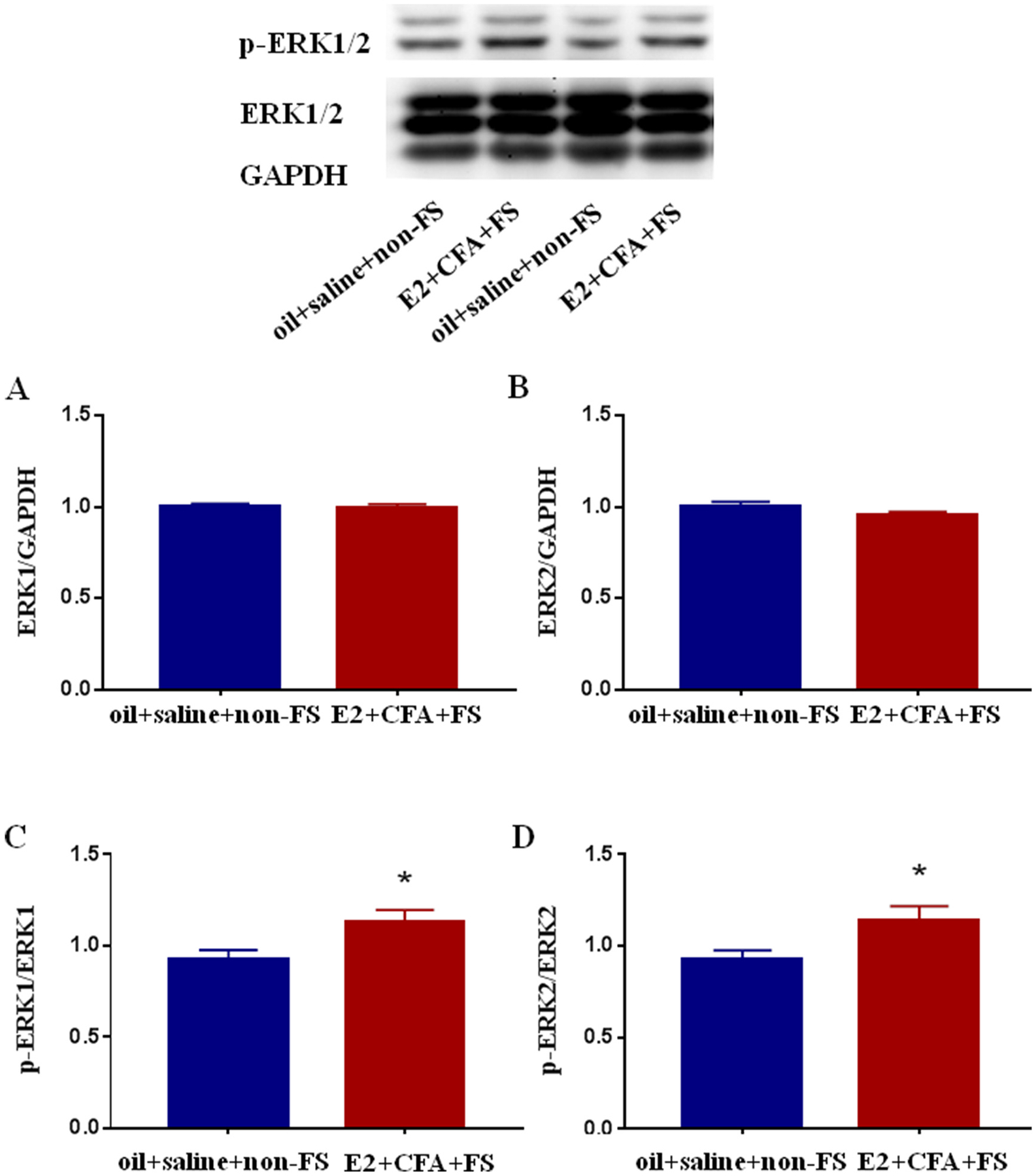
The expression of ERK1/2 (A, B) and p-ERK1/2 (C, D) in the L4-L5 spinal dorsal horn in the comorbid pain condition. The expression of ERK1/2 in the E2 + CFA + FS group (n = 4) did not significantly change compared with the oil + saline + non-FS group (n = 6). The expression of p-ERK1/2 in the E2 + CFA + FS group significantly increased compared with the oil + saline + non-FS group. Data were normalized to the oil + saline + non-FS group. *P < 0.05 vs. the oil + saline + non-FS group.
3.2.2. MEK inhibitor PD98059 prevents somatic hyperalgesia in the comorbid pain condition
To further verify the role of ERK1/2 in the somatic hyperalgesia, we explored whether MEK inhibitor PD98059 could prevent somatic hyperalgesia in the comorbid pain condition through blocking the activation of ERK1/2. There were significant differences between the E2 + CFA + FS + PD98059 group and E2 + CFA + FS + DMSO group in the thermal withdrawal latency (F5,80 = 3.219, P = 0.0107 for interaction; F5,80 = 1.122, P = 0.3553 for time factor; F1,16 = 3.617, P = 0.0754 for group factor, n = 9 for each group, Fig. 5A) and mechanical withdrawal threshold (F5,75 = 5.319, P = 0.0003 for interaction; F5,75 = 4.255, P = 0.0018 for time factor; F1,15 = 25.2, P = 0.0002 for group factor, Fig. 5B). Post-hoc tests showed that the thermal hyperalgesia persisted over 6 days post FS compare with Baseline (P = 0.0031 for Day 2, P = 0.0398 for Day 6, Fig. 5A) and the mechanical allodynia persisted over 15 days (P = 0.0001 for Day 3, P < 0.0001 for Day 7, P = 0.0004 for Day 11, P = 0.0003 for Day 15, Fig. 5B) in the E2 + CFA + FS + DMSO group. No thermal hypersensitivity or mechanical allodynia was observed compared with Baseline in the E2 + CFA + FS + PD98059 group (P > 0.05 for each time point compared with Baseline). In addition, when comparing the values in each time point between the two groups, the mechanical withdrawal threshold in the E2 + CFA + FS + DMSO group was significantly lower than that in the E2 + CFA + FS + PD98059 group from day 3 to day 15 post FS (P < 0.0001 for all time points, Fig. 5B). These results indicate that ERK1/2 activation participates in the somatic hyperalgesia in the comorbid pain condition.
Fig. 5.
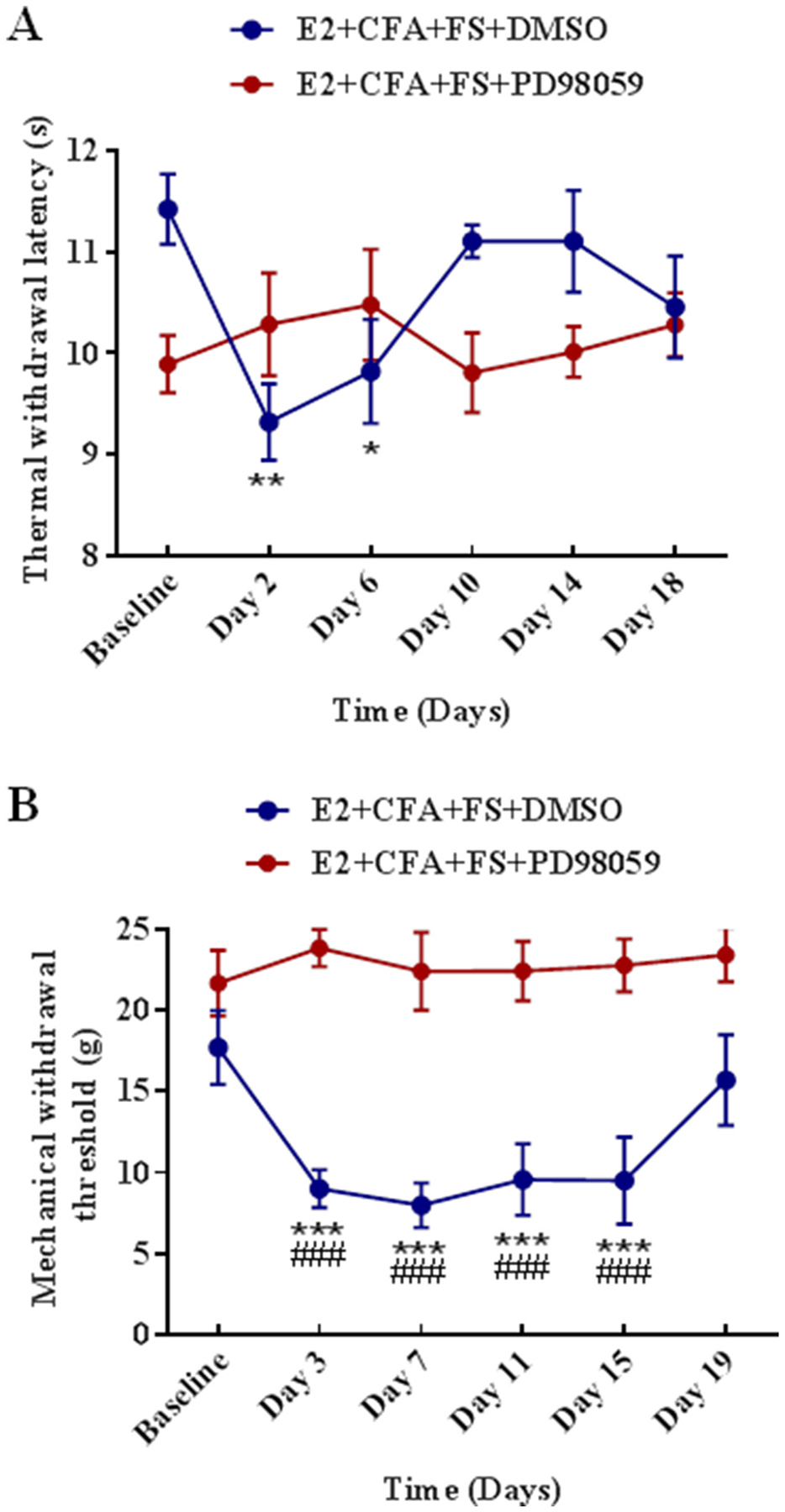
The activation of ERK1/2 is involved in the somatic hyperalgesia in the comorbid pain condition. (A) PD98059 inhibited the thermal hyperalgesia. n = 9 for each group. *, **P < 0.05, 0.01 vs. Baseline. (B) PD98059 inhibited the mechanical hyperalgesia. ***P < 0.001 vs Baseline. The mechanical withdrawal threshold significantly decreased at Day 3, 7, 11, 15 post stress in DMSO treated rats compared with PD98059 treated. ###P < 0.001 vs. the E2 + CFA + FS + PD98059 group at the same time point.
3.3. ERK1/2 signaling pathway mediates CCK-induced somatic hyperalgesia in the spinal cord
3.3.1. CCK receptor agonist CCK8 induces somatic hyperalgesia and the hyperalgesia is blocked by inhibiting ERK1/2 activation
Since the expressions of CCK2 receptors and p-ERK1/2 in the L4-L5 spinal dorsal horn were significantly up-regulated in the E2 + CFA + FS group compared with the oil + saline + non-FS group, we hypothesized that there existed a CCK-ERK signaling pathway in which CCK2 receptors might activate ERK1/2 to produce somatic hyperalgesia in the comorbid pain condition. We examined whether the MEK inhibitor PD98059 could block ERK1/2 signaling cascade induced by the CCK receptor agonist CCK8. There were significant differences in the thermal withdrawal latency (F5,80 = 26.42, P < 0.0001 for interaction; F5,80 = 35.6, P < 0.0001 for time factor; F1,16 = 112.7, P < 0.0001 for group factor, Fig. 6A) and mechanical withdrawal threshold (F5,80 = 4.508, P = 0.0011 for interaction; F5,80 = 2.283, P = 0.0541 for time factor; F1,16 = 0.1008, P = 0.7550 for group factor, Fig. 6B) between the E2 + CCK8 + DMSO (n = 10) and E2 + CCK8 + PD98059 groups (n = 8). Intrathecal injection of CCK8 daily for 5 days produced thermal hyperalgesia starting from Day 2 to Day 10 (P < 0.0001 for Day 2 and Day 6 vs. Baseline, P = 0.0423 for Day 10 vs. Baseline, Fig. 6A) and mechanical allodynia appeared on day 3 (P < 0.0001 vs. Baseline, Fig. 6B) in the E2 + CCK8 + DMSO group. No thermal hyperalgesia or mechanical allodynia was observed in the E2 + CCK8 + PD98059 group in which PD98059 was injected 30 min before each CCK8 injection (P > 0.05 for all time points vs. Baseline). In addition, when comparing the values in each time point between the two groups, the thermal withdrawal latency (P < 0.0001 for Day 2 and Day 6, P = 0.0024 for Day 10 and P = 0.0197 for Day 14, Fig. 6A) and mechanical withdrawal threshold (P < 0.0001 for Day 2, Fig. 6B) in the E2 + CCK8 + PD98059 group significantly increased compared with the E2 + CCK8 + DMSO group from Day 2 to Day 14. These findings suggest that spinal administration of the CCK receptor agonist CCK8 induces somatic hyperalgesia and the hyperalgesia can be blocked by inhibiting ERK1/2 activation.
Fig. 6.
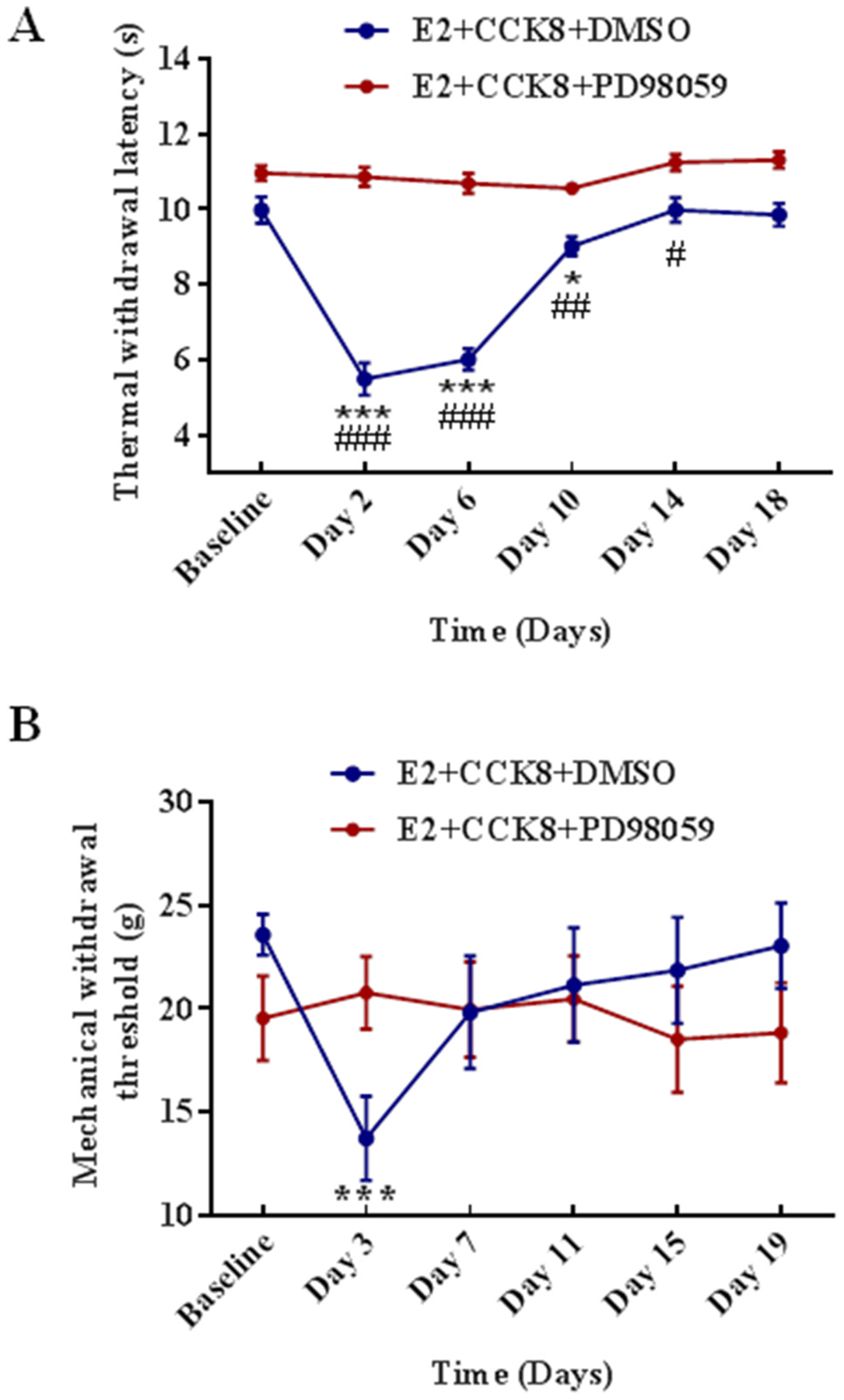
The effect of ERK1/2 signaling cascade blocking on the function of CCK receptor agonist. (A) PD98059 inhibited thermal hyperalgesia induced by CCK8. CCK8 decreased the thermal withdrawal latency for 10 days in the E2 + CCK8 + DMSO rats (n = 10) compared with Baseline, and PD98059 (n = 8) blocked the decrease in the thermal withdrawal latency. *, ***P < 0.05, 0.001 vs. Baseline. The thermal withdrawal latency significantly decreased at Day 2, 6, 10, 14 post injection of CCK8 in the E2 + CCK8 + DMSO rats compared with PD98059 treated. #, ##, ###P < 0.05, 0.01, 0.001 vs. the E2 + CCK8 + PD98059 group at the same time point. (B) PD98059 inhibited mechanical hyperalgesia induced by CCK8. CCK8 decreased the mechanical withdrawal threshold for 3 days in the E2 + CCK8 + DMSO group compared with Baseline, and PD98059 blocked the decrease in the mechanical withdrawal threshold. ***P < 0.001 vs. Baseline.
3.3.2. Intrathecal injection of CCK2 receptor antagonist YM-022 decreases p-ERK1/2 expression in the spinal cord
The effects of intrathecal injection of CCK2 receptor antagonist on the activation of ERK1/2 in the L4-L5 spinal cord were examined. The Western blot data showed that there were no significant differences in the expression of ERK1 (P = 0.2060) and ERK2 (P = 0.0988) in the E2 + CFA + FS + YM-022 group (n = 4) compared with the E2 + CFA + FS + saline group (n = 6, Fig. 7A and B). However, intrathecal injection of CCK2 receptor antagonist YM-022 caused a significant reduction of p-ERK1 and p-ERK2 expression (P = 0.0089 and 0.0298, respectively, Fig. 7C and D) in the L4-L5 spinal cord in the E2 + CFA + FS + YM-022 group compared with the E2 + CFA + FS + saline group. The behavioral results combined with the Western blot data indicate that CCK-induced somatic hyperalgesia is mediated by the ERK1/2 signaling pathway in the spinal cord.
Fig. 7.
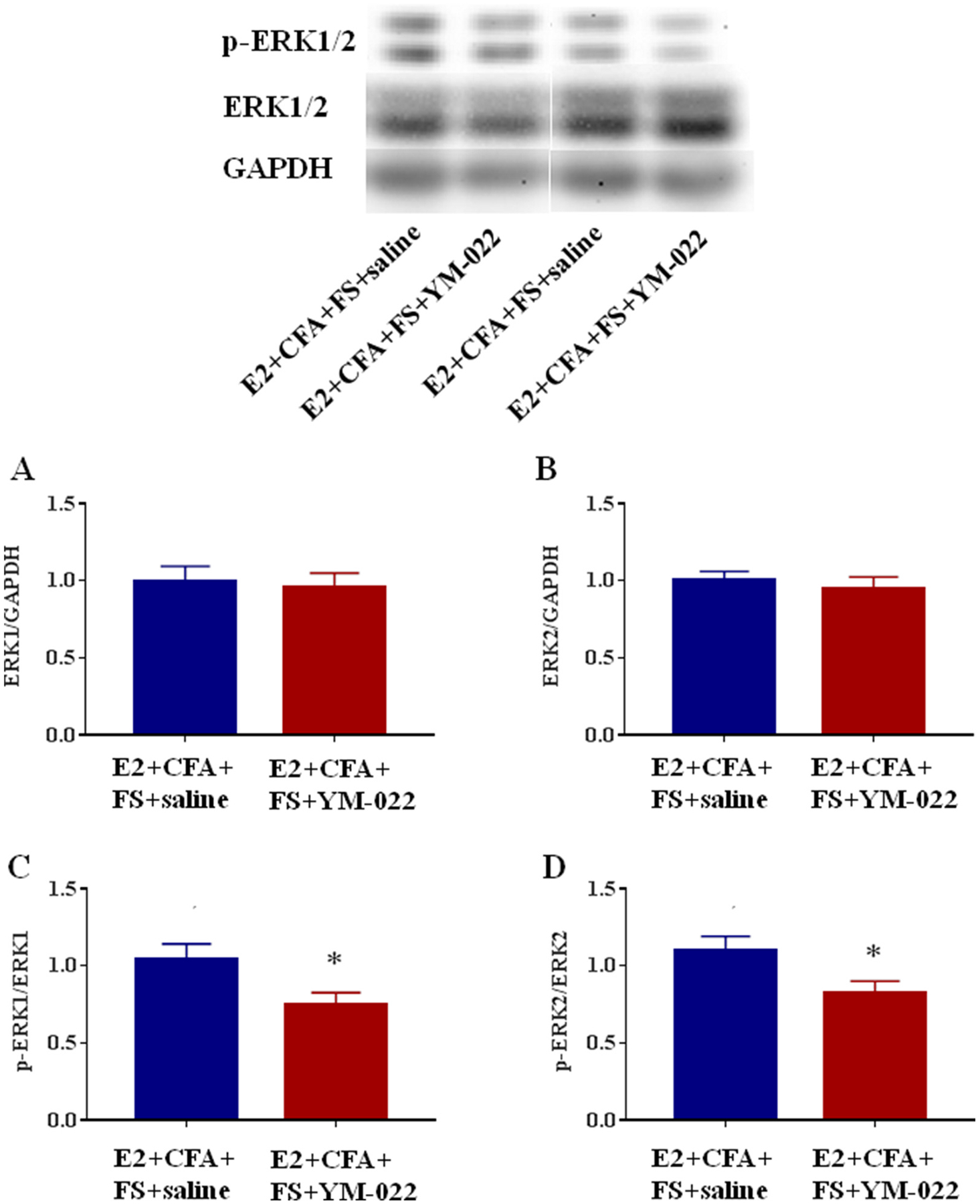
The effect of intrathecal injection of CCK2 receptor antagonist on the activation of ERK1/2 in the spinal cord. The expression of ERK1/2 in the L4-L5 spinal dorsal horn in the E2 + CFA + FS + YM-022 group (n = 4) did not significantly change compared with the E2 + CFA + FS + saline group (n = 6) (A, B), but the expression of p-ERK1/2 in the E2 + CFA + FS + YM-022 group significantly decreased than that in the E2 + CFA + FS + saline group (C, D). *P < 0.05 vs. the E2 + CFA + FS + saline group.
4. Discussion
In the present study, we aimed to analyze the independent and joint contributions of spinal CCK2 receptors and ERK1/2 activation to the somatic hyperalgesia in the comorbid pain condition. We demonstrated that CCK2 receptors in the spinal cord were involved in the regulation of central sensitization in the comorbid pain condition by activating the ERK1/2 pathway.
In our previous study, we found that stress induced visceral hypersensitivity and mechanical allodynia in the lower back of female rats with preexisting orofacial inflammation (Traub et al., 2014). We used the same inflammation plus stress paradigm in the current study to simulate TMD and FMS comorbidity (Li et al., 2020). The perception of pain is a dynamic process that is subject to ongoing modulation by descending inhibition and descending facilitation. Accumulating evidence shows that descending facilitation of spinal nociception is the main factor in central sensitization and secondary hyperalgesia (Baron et al., 2013; Liu et al., 2018). Descending facilitation has been studied and characterized in animal models of pain and hyperalgesia/allodynia. Therefore, the somatic hyperalgesia caused by orofacial inflammation combined with repeated FS stress may be associated with a dysregulation in descending pain modulation, both inhibitory and facilitatory, from the midbrain and brainstem. Disrupting the balance of descending modulatory circuits to favor facilitation and impair inhibition may promote and maintain the spreading pain (Li et al., 2020; Xue et al., 2020; Xu et al., 2020). Our results are agreement with the previous studies. For example, repeated stimulation of meningeal nociceptors by injecting an inflammatory soup in rats, an animal model of chronic migraine, leads to facial and hindpaw mechanical allodynia. Such wide-spreading central sensitization have been demonstrated to require both the activation of descending facilitation from the RVM (Edelmeyer et al., 2009) and impairment of diffuse noxious inhibitory controls (Boyer et al., 2014).
The descending facilitation system consists of the PAG, RVM, anterior cingulate cortex (ACC), hypothalamus, amygdala, and nucleus of the solitary tract and there are reports indicating that CCK plays an important role in the descending facilitatory pathway. There is a close relationship between CCK and a few brain nuclei involved in the composition and regulation of the descending facilitation pathway. For example, pain-related behaviors and CCK release in the ACC significantly increased in rats with carrageenan-induced arthritis compared with the control animals (Heilborn et al., 2007). In addition, the PAG of both rodents and primates is rich in CCK2 receptors and CCK-containing neuronal cell bodies (Mercer et al., 2000). CCK2 receptor agonists excite PAG neurons by inhibiting K+ channels and increasing calcium influx, which in turn leads to increased glutamate release and eventually hyperalgesia (Yang et al., 2006). Similar to the PAG, the nociceptive facilitatory system may modulate tactile allodynia and thermal hyperalgesia, through increased activity of CCK within the RVM (Kovelowski et al., 2000). Both direct injection into the RVM and intrathecal injection of CCK2 receptor antagonists produce anti-nociception in neuropathic pain rats, presumably by interrupting transmission in the pain facilitatory pathway (Coudoré-Civiale et al., 2000; Inoue et al., 2017; Kovelowski et al., 2000; Xie et al., 2005). In the spinal dorsal horn, CCK is found mainly in interneurons and terminals of descending fibers, and receptor binding sites for CCK are located throughout the spinal dorsal horn with the highest density in the superficial laminae (Ghilardi et al., 1992). After spinal hemisection at the T13 level, CCK mRNA expression increased on both sides of the L4-L5 segments of spinal cord and intrathecal injection of a CCK2 receptor antagonist dose-dependently increased the paw withdrawal threshold in both paws (Kim et al., 2009). The present study showed that the expression of CCK2 receptors, but not CCK1 receptors, significantly increased in the L4-L5 spinal dorsal horn following orofacial inflammation plus stress. Intrathecal administration of the selective CCK2 receptor antagonist significantly reduced the thermal hyperalgesia and mechanical allodynia in the hindpaws, suggesting that CCK2 receptors mediate the somatic hyperalgesia.
It has been shown that activation of CCK2 receptor related signaling leads to ERK phosphorylation (Grossini et al., 2012; Mao et al., 2014). ERK1/2 mediates intracellular signal transduction in response to various stimuli. Some evidence suggests that ERK1/2 is activated in neurons in the spinal dorsal horn in response to the stimulation of nociceptors and that ERK signaling is involved in nociceptive plasticity (Pezet et al., 2002a, 2002b). Acute noxious stimuli, such as formalin or capsaicin, induce ERK phosphorylation in spinal dorsal horn neurons (Ji et al., 1999). Intrathecal injection of the MEK inhibitor PD98059 reduces acute pain behavior after subcutaneous injection of formalin (Karim et al., 2001). In the previous study, we demonstrated that orofacial inflammation followed by 3 day FS stress induced ERK1/2 activation in the lumbosacral spinal segments in female rats, and intrathecal administration of the MEK inhibitor PD98059 blocked the visceral hypersensitivity induced by orofacial inflammation followed by stress, indicating that ERK1/2 activation contributes to the visceral hypersensitivity (Zhao et al., 2018). Therefore, we investigated whether ERK1/2 activation in the spinal cord contributed to the somatic hyperalgesia in this comorbid pain condition. We found that the expression of p-ERK1/2 in the spinal dorsal horn of rats in the E2 + CFA + FS group was significantly higher than that in the oil + saline + non-FS group. Intrathecal injection of a MEK inhibitor blocked the somatic hyperalgesia in the comorbid pain condition, suggesting that spinal ERK1/2 activation plays an important role in the development of somatic hyperalgesia in the comorbidity of TMD and FMS.
Since both CCK2 receptors and ERK1/2 activation in the spinal cord are involved in the regulation of somatic hyperalgesia in the comorbid pain condition, we speculate whether there is a close relationship between CCK and ERK1/2 in the spinal cord. To determine the effect of ERK1/2 activation on the function of CCK2 receptors, rats were intrathecally injected with the CCK receptor agonist CCK8 in the E2 + CCK8 + DMSO group. The resulting thermal hyperalgesia and mechanical allodynia in the hindpaws were similar to the model of TMD and FMS comorbidity. The CCK-8-induced hyperalgesia was blocked by intrathecal injection of the MEK inhibitor PD98059, inhibiting the activation of ERK1/2. These results are consistent with the fact that pharmacological inhibition of ERK1/2 reverses the increase of BDNF expression evoked by CCK-8 (Hwang et al., 2013), suggesting that CCK exerts pro-nociceptive properties by binding to the CCK2 receptors and ERK1/2 activation may participate in the reaction as a downstream signaling molecule of CCK. To investigate the effect of CCK2 receptors on ERK1/2 activation, rats in the comorbid pain group were intrathecally injected with the CCK2 receptor antagonist YM-022. It was found that the expression of p-ERK1/2 in the L4-L5 segments of the spinal cord was significantly reduced compared with the E2 + CFA + FS + saline group, which further confirmed that CCK2 receptors enhanced the activation of ERK1/2 and participated in the process of somatic hyperalgesia in the comorbid pain condition. Thus, in the spinal cord, there is a CCK-ERK1/2 signaling pathway leading to hyperalgesia.
Taken together, we hypothesize that orofacial inflammation combined with FS stress activates the descending facilitation system. Complex networks of pathways projected from various brain structures integrate to affect the spinal processing of nociceptive input. The descending facilitatory system from the midbrain and brainstem promotes CCK binding to CCK2 receptors on the membrane of neurons in the spinal cord, and further stimulates the activation of ERK1/2 through Ras-Raf-MEK1/2-ERK1/2 pathway, increasing spinal excitability causing somatic hyperalgesia.
5. Conclusion
In the current study, we demonstrated that orofacial inflammation combined with stress affected spinal plasticity and led to a decrease in the thermal withdrawal latency and mechanical nociceptive threshold by activating CCK2 receptors. Intrathecal injection of CCK2 receptor antagonist prevented the somatic hyperalgesia by affecting the activation of ERK1/2. Therefore, these data may provide a therapeutic target to treat TMD and FMS comorbidity in the clinic.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81971049, 81671097), the Social Development Program of Shaanxi Province (2020SF-018), and Key Laboratory of Anesthesiology of Zhejiang Province, China (KLAZJ2101) to DYC and partially by the National Institutes of Health, USA (R01 NR015472) to RJT.
Footnotes
CRediT authorship contribution statement
Lu-Lu Duan: Investigation, Methodology, Validation, Writing – original draft. Xin-Yi Qiu: Investigation, Methodology, Writing – original draft. Si-Qi Wei: Investigation. Han-Yu Su: Investigation. Fu-Rong Bai: Investigation. Richard J. Traub: Writing – review & editing. Qin Zhou: Investigation, Writing – review & editing, Supervision. Dong-Yuan Cao: Investigation, Resources, Conceptualization, Writing – review & editing, Supervision.
Declaration of competing interest
None of the authors has any financial interest or conflict of interest related to the manuscript.
References
- Baron R, Hans G, Dickenson AH, 2013. Peripheral input and its importance for central sensitization. Ann. Neurol 74, 630–636. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Amanzio M, Vighetti S, Asteggiano G, 2006. The biochemical and neuroendocrine bases of the hyperalgesic nocebo effect. J. Neurosci 26, 12014–12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer N, Dallel R, Artola A, Monconduit L, 2014. General trigeminospinal central sensitization and impaired descending pain inhibitory controls contribute to migraine progression. Pain 155, 1196–1205. [DOI] [PubMed] [Google Scholar]
- Cao DY, Bai G, Ji Y, Traub RJ, 2015. Epigenetic upregulation of metabotropic glutamate receptor 2 in the spinal cord attenuates oestrogen-induced visceral hypersensitivity. Gut 64, 1913–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL, 1994. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 53, 55–63. [DOI] [PubMed] [Google Scholar]
- Coudoré-Civiale M-A, Courteix C, Fialip J, Boucher M, Eschalier A, 2000. Spinal effect of the cholecystokinin-B receptor antagonist CI-988 on hyperalgesia, allodynia and morphine-induced analgesia in diabetic and mononeuropathic rats. Pain 88, 15–22. [DOI] [PubMed] [Google Scholar]
- Edelmayer RM, Vanderah TW, Majuta L, Zhang ET, Fioravanti B, Felice MD, Chichorro JG, Ossipov MH, King T, Lai J, Kori SH, Nelsen AC, Cannon KE, Heinricher MM, Porreca F, 2009. Medullary pain facilitating neurons mediate allodynia in headache-related pain. Ann. Neurol 65, 184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukazawa Y, Maeda T, Kiguchi N, Tohya K, Kimura M, Kishioka S, 2007. Activation of spinal cholecystokinin and neurokinin-1 receptors is associated with the attenuation of intrathecal morphine analgesia following electroacupuncture stimulation in rats. J. Pharmacol. Sci 104, 159–166. [DOI] [PubMed] [Google Scholar]
- Furness PJ, Vogt K, Ashe S, Taylor S, Haywood-Small S, Lawson K, 2018. What causes fibromyalgia? An online survey of patient perspectives. Health Psychol Open 5, 2055102918802683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghilardi JR, Allen CJ, Vigna S, McVey DC, Mantyh P, 1992. Trigeminal and dorsal root ganglion neurons express CCK receptor binding sites in the rat, rabbit, and monkey: possible site of opiate-CCK analgesic interactions. J. Neurosci 12, 4854–4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakopoulos NN, Keller L, Rammelsberg P, Kronmuller KT, Schmitter M, 2010. Anxiety and depression in patients with chronic temporomandibular pain and in controls. J. Dent 38, 369–376. [DOI] [PubMed] [Google Scholar]
- Grossini E, Caimmi P, Molinari C, Uberti F, Mary D, Vacca G, 2012. CCK receptors-related signaling involved in nitric oxide production caused by gastrin 17 in porcine coronary endothelial cells. Mol. Cell. Endocrinol 350, 20–30. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Mecinas M, Bell AM, Shepherd F, Polgár E, Watanabe M, Furuta T, Todd AJ, 2019. Expression of cholecystokinin by neurons in mouse spinal dorsal horn. J. Comp. Neurol 527, 1857–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J, 1988. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32, 77–88. [DOI] [PubMed] [Google Scholar]
- Heilborn U, Rost BR, Arborelius L, Brodin E, 2007. Arthritis-induced increase in cholecystokinin release in the rat anterior cingulate cortex is reversed by diclofenac. Brain Res. 1136, 51–58. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Neubert MJ, 2004. Neural basis for the hyperalgesic action of cholecystokinin in the rostral ventromedial medulla. J. Neurophysiol 92, 1982–1989. [DOI] [PubMed] [Google Scholar]
- Hwang CK, Kim DK, Chun HS, 2013. Cholecystokinin-8 induces brain-derived neurotrophic factor expression in noradrenergic neuronal cells. Neuropeptides 47, 245–250. [DOI] [PubMed] [Google Scholar]
- Imbe H, Kimura A, 2015. Repeated forced swim stress prior to complete Freund’s adjuvant injection enhances mechanical hyperalgesia and attenuates the expression of pCREB and DeltaFosB and the acetylation of histone H3 in the insular cortex of rat. Neuroscience 301, 12–25. [DOI] [PubMed] [Google Scholar]
- Imbe H, Kimura A, Donishi T, Kaneoke Y, 2014. Repeated forced swim stress enhances CFA-evoked thermal hyperalgesia and affects the expressions of pCREB and c-Fos in the insular cortex. Neuroscience 259, 1–11. [DOI] [PubMed] [Google Scholar]
- Inoue S, Johanek LM, Sluka KA, 2017. Lack of analgesic synergy of the cholecystokinin receptor antagonist proglumide and spinal cord stimulation for the treatment of neuropathic pain in rats. Neuromodulation 20, 534–542. [DOI] [PubMed] [Google Scholar]
- Ji RR, Baba H, Brenner GJ, Woolf CJ, 1999. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat. Neurosci 2, 1114–1119. [DOI] [PubMed] [Google Scholar]
- Ji Y, Murphy AZ, Traub RJ, 2003. Estrogen modulates the visceromotor reflex and responses of spinal dorsal horn neurons to colorectal stimulation in the rat. J. Neurosci 23, 3908–3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim F, Wang C-C, Gereau RW, 2001. Metabotropic glutamate receptor subtypes 1 and 5 are activators of extracellular signal-regulated kinase signaling required for inflammatory pain in mice. J. Neurosci 21, 3771–3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kim JH, Kim Y, Cho HY, Hong SK, Yoon YW, 2009. Role of spinal cholecystokinin in neuropathic pain after spinal cord hemisection in rats. Neurosci. Lett 462, 303–307. [DOI] [PubMed] [Google Scholar]
- Kovelowski CJ, Ossipov MH, Sun H, Lai J, Malan TP, Porreca F, 2000. Supraspinal cholecystokinin may drive tonic descending facilitation mechanisms to maintain neuropathic pain in the rat. Pain 87, 265–273. [DOI] [PubMed] [Google Scholar]
- Landi N, Lombardi I, Manfredini D, Casarosa E, Biondi K, Gabbanini M, Bosco M, 2005. Sexual hormone serum levels and temporomandibular disorders. A preliminary study. Gynecol. Endocrinol 20, 99–103. [DOI] [PubMed] [Google Scholar]
- Landi N, Manfredini D, Lombardi I, Casarosa E, Bosco M, 2004. 17-beta-estradiol and progesterone serum levels in temporomandibular disorder patients. Minerva Stomatol. 53, 651–660. [PubMed] [Google Scholar]
- Li JH, Yang JL, Wei SQ, Li ZL, Collins AA, Zou M, Wei F, Cao DY, 2020. Contribution of central sensitization to stress-induced spreading hyperalgesia in rats with orofacial inflammation. Mol. Brain 13, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZL, Xue Y, Tao ZY, Du WZ, Jiang YG, Cao DY, 2019. Spinal 5-HT3 receptor contributes to somatic hyperalgesia induced by sub-chronic stress. Mol. Pain 15, 1744806919859723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao WT, Tseng CC, Wu CH, Lin CR, 2020. Early high-frequency spinal cord stimulation treatment inhibited the activation of spinal mitogen-activated protein kinases and ameliorated spared nerve injury-induced neuropathic pain in rats. Neurosci. Lett 721, 134763. [DOI] [PubMed] [Google Scholar]
- Liu Y, Latremoliere A, Li X, Zhang Z, Chen M, Wang X, Fang C, Zhu J, Alexandre C, Gao Z, 2018. Touch and tactile neuropathic pain sensitivity are set by corticospinal projections. Nature 561, 547–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovick TA, 2008. Pro-nociceptive action of cholecystokinin in the periaqueductal grey: a role in neuropathic and anxiety-induced hyperalgesic states. Neurosci. Biobehav. Rev 32, 852–862. [DOI] [PubMed] [Google Scholar]
- Mao JD, Wu P, Huang JX, Wu J, Yang G, 2014. Role of ERK-MAPK signaling pathway in pentagastrin-regulated growth of large intestinal carcinoma. World J. Gastroenterol 20, 12542–12550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall TM, Herman DS, Largent-Milnes TM, Badghisi H, Zuber K, Holt SC, Lai J, Porreca F, Vanderah TW, 2012. Activation of descending pain-facilitatory pathways from the rostral ventromedial medulla by cholecystokinin elicits release of prostaglandin-E(2) in the spinal cord. Pain 153, 86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer LD, Le VQ, Nunan J, Jones NM, Beart PM, 2000. Direct visualization of cholecystokinin subtype2 receptors in rat central nervous system using anti-peptide antibodies. Neurosci. Lett 293, 167–170. [DOI] [PubMed] [Google Scholar]
- Noble F, Wank SA, Crawley JN, Bradwejn J, Seroogy KB, Hamon M, Roques BP, 1999. International Union of Pharmacology. XXI. Structure, distribution, and functions of cholecystokinin receptors. Pharmacol. Rev 51, 745–781. [PubMed] [Google Scholar]
- Pezet S, Cunningham J, Patel J, Grist J, Gavazzi I, Lever IJ, Malcangio M, 2002a. BDNF modulates sensory neuron synaptic activity by a facilitation of GABA transmission in the dorsal horn. Mol. Cell. Neurosci 21, 51–62. [DOI] [PubMed] [Google Scholar]
- Pezet S, Malcangio M, Lever IJ, Perkinton MS, Thompson SW, Williams RJ, McMahon SB, 2002b. Noxious stimulation induces Trk receptor and downstream ERK phosphorylation in spinal dorsal horn. Mol. Cell. Neurosci 21, 684–695. [DOI] [PubMed] [Google Scholar]
- Quintero L, Moreno M, Avila C, Arcaya J, Maixner W, Suarez-Roca H, 2000. Long-lasting delayed hyperalgesia after subchronic swim stress. Pharmacol. Biochem. Behav 67, 449–458. [DOI] [PubMed] [Google Scholar]
- Rabhi M, Ennibi K, Chaari J, Toloune F, 2010. [Functional somatic syndromes]. Rev. Med. Interne 31, 17–22. [DOI] [PubMed] [Google Scholar]
- Scrivani SJ, Keith DA, Kaban LB, 2008. Temporomandibular disorders. N. Engl. J. Med 359, 2693–2705. [DOI] [PubMed] [Google Scholar]
- Sugerman DT, 2014. JAMA patient page. Fibromyalgia. J. Am. Med. Assoc 311, 1577. [DOI] [PubMed] [Google Scholar]
- Traub RJ, Cao DY, Karpowicz J, Pandya S, Ji Y, Dorsey SG, Dessem D, 2014. A clinically relevant animal model of temporomandibular disorder and irritable bowel syndrome comorbidity. J. Pain : official journal of the American Pain Society 15, 956–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie JY, Herman DS, Stiller CO, Gardell LR, Ossipov MH, Lai J, Porreca F, Vanderah TW, 2005. Cholecystokinin in the rostral ventromedial medulla mediates opioid-induced hyperalgesia and antinociceptive tolerance. J. Neurosci 25, 409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu GZ, Xue Y, Wei SQ, Li JH, Traub RJ, Wang MD, Cao DY, 2020. Valproate reverses stress-induced somatic hyperalgesia and visceral hypersensitivity by up-regulating spinal 5-HT2C receptor expression in female rats. Neuropharmacology 165, 107926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Wei SQ, Wang PX, Wang WY, Liu EQ, Traub RJ, Cao DY, 2020. Down-regulation of spinal 5-HT2A and 5-HT2C receptors contributes to somatic hyperalgesia induced by orofacial inflammation combined with stress. Neuroscience 440, 196–209. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Nozaki-Taguchi N, 1996. The effects of intrathecally administered FK480, a cholecystokinin-A receptor antagonist, and YM022, a cholecystokinin-B receptor antagonist, on the formalin test in the rat. Anesth. Analg 83, 107–113. [DOI] [PubMed] [Google Scholar]
- Yang YM, Chung JM, Rhim H, 2006. Cellular action of cholecystokinin-8S-mediated excitatory effects in the rat periaqueductal gray. Life Sci. 79, 1702–1711. [DOI] [PubMed] [Google Scholar]
- Zhao YJ, Li JH, Hu B, Wang Y, Chang XF, Traub RJ, Cao DY, 2018. Extracellular signal-regulated kinase activation in the spinal cord contributes to visceral hypersensitivity induced by craniofacial injury followed by stress. Neuro Gastroenterol. Motil 30, e13161. [DOI] [PubMed] [Google Scholar]


