Abstract
Background:
Following a prostate cancer (PC) diagnosis, treatment-related symptoms may result in diminished quality of life (QoL). Improved diet and increased exercise may improve QoL in men with PC.
Methods:
We conducted a 4-arm pilot randomized trial to assess feasibility and acceptability of a 3-month web-based diet and exercise intervention, among men (>18 years of age) with PC (reported elsewhere). The purpose of this study is to describe the change in QoL measured by surveys (eg, QLQ-C30, PROMIS Fatigue) at enrollment and following the intervention. Men were randomized 1:1:1:1 to increasing levels of web-based behavioral support: Level 1: website; Level 2: Level 1 plus personalized diet and exercise prescription; Level 3: Levels 1-2 plus Fitbit and text messages; Level 4: Levels 1-3 plus 2 30-minute coaching calls. T-tests were used to compare pre-post change in mean QoL scores between each Level and Level 1.
Results:
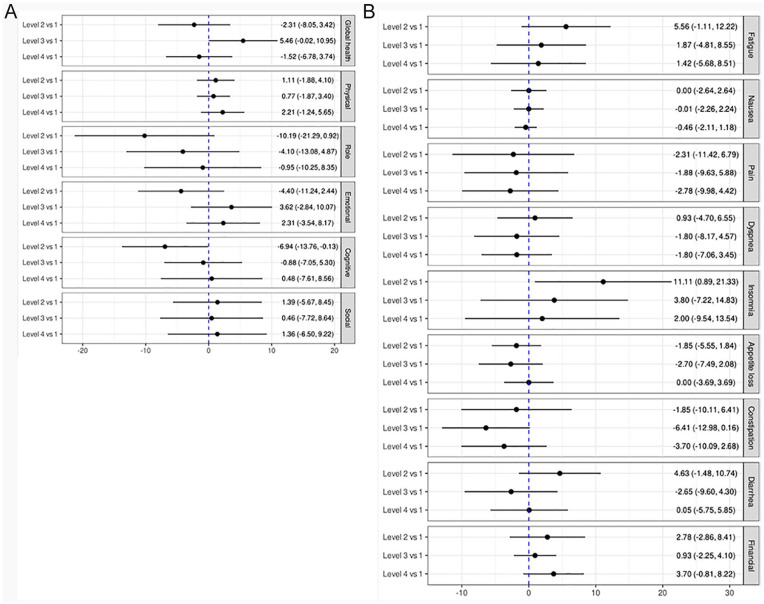
Two hundred and two men consented and were randomized (n = 49, 51, 50, 52 for Levels 1-4, respectively). Men were predominantly white (93%), with a median age of 70 years (Intra-quartile Range [IQR]: 65,75) and 3 years (IQR: 1,9) post primary treatment for mostly localized disease (74% with T1-2). There were no meaningful changes in QoL, but there were notable trends. Level 3 participants had small improvements in QLQ-C30 Global Health (5.46; 95% CI: −0.02, 10.95) compared to Level 1. In contrast, Level 2 participants trended toward decreasing Global QoL (−2.31, 95% CI: −8.05, 3.42), which may reflect declines in function (eg, Cognitive: −6.94, 95% CI: −13.76, −0.13) and higher symptom burden (eg, Diarrhea: 4.63, 95% CI: −1.48, 10.74).
Conclusions:
This short, web-based intervention did not appear to have an impact on PC survivors’ QoL. Most men were several years past treatment for localized disease; the potential for this approach to reduce symptoms and improve QoL in men who have worse health may still be warranted.
Keywords: cancer survivorship, diet, physical activity, exercise, patient-reported outcomes
Background
There are more than 3.1 million prostate cancer survivors in the United States. 1 A prostate cancer diagnosis is most common in men aged 65 to 74 years and roughly 98% of those diagnosed will survive 10 years or more. 2 During this time, men may experience decreased quality of life (QoL) due to the combined effects of the cancer diagnosis, concurrent co-morbidities, as well as aging and side effects of prostate cancer treatment, including incontinence, impotence, bowel dysfunction, fatigue, muscle loss, poor sleep quality, depression, isolation and loneliness, and increased frailty.3-10 Prior research suggests treatment-related insults to QoL may subside in as little as 1 year after treatment, particularly for men undergoing radical prostatectomy.11,12 It is less clear how long declines in physical, functional, emotional, and social well-being may last, especially considering they may be compounded by age-related declines.
An increasing body of evidence suggests that regular exercise, defined as 150 minutes per week of aerobic exercise and ≥2 sessions per week of strength training activity, 13 may improve prostate cancer clinical outcomes and QoL following diagnosis and treatment.13-20 However, few men met these recommendations even when the evidence supporting them is strong. For example, in addition to reducing the risk of prostate cancer progression17,20,21 and mortality,17,20,22-26 participating in regular exercise, including aerobic and strength training activities, has been shown to offset age-related declines and relieve treatment side-effects, thereby improving prostate cancer survivors’ QoL.14-16 Yet, <25% of prostate cancer survivors meet aerobic exercise recommendations and only 4% meet those for resistance exercise. 27
Evidence also suggests that changing certain dietary habits may improve disease-specific outcomes.20,28-37 Further, prior reports have identified an association between healthy dietary patterns and higher levels or health-related QoL in the general population, 38 though less is known about the role of diet and QoL following a prostate cancer diagnosis. However, age-associated changes themselves, including declining muscle mass and changes in metabolism and physical functioning, can result in dietary shifts and ultimately decreased enjoyment of or interest in dietary intake.39,40 In turn, poor nutrition among older adults has also been linked to decreased appetite and diminished capability in performing activities of daily living. 41
Recognizing that a prostate cancer diagnosis may be a teachable moment—affording an opportunity to improve adherence to behavioral recommendations42-45 —we conducted a pilot randomized trial to assess the feasibility and acceptability of a web-based intervention designed to support evidence-based dietary and exercise behaviors shown to improve prostate cancer-specific outcomes, 46 such as progression and disease-specific mortality. 20 Prior analyses showed the intervention to be feasible with no serious adverse effects and suggested it may modestly improve diet as well as exercise behaviors, particularly among men who were not meeting physical activity recommendations at baseline. 47 Additional data collected during the pilot study are relevant to understanding the impact of such technology-based approaches on other outcomes important to prostate cancer survivors. In this secondary analysis, we evaluate whether this practical and scalable technology-based intervention can also improve PC survivors’ QoL.
Methods
Study Population
We conducted a multi-center 4-arm pilot randomized trial of a 3-month intervention among men (>18 years of age) with a self-reported diagnosis of prostate cancer (clinicaltrials.gov NCT03406013). There were no restrictions on stage of cancer or time since diagnosis. Men were identified through hospital cancer registry databases and from the Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) registry, 48 and mailed a letter and study brochure directing men to contact research staff to learn about the study. Study brochures were also placed in clinic waiting and exam rooms. To participate, men were required to speak English, have an email address, and have a personal device with internet and text messaging capability. All participants provided written consent and all study-related activities were done in accordance with and under the supervision of each study site’s Institutional Review Board. Additional details have been reported elsewhere. 46
Interventions
Men were block randomized 1:1:1:1 to increasing levels of web-based behavioral support. Participants randomized to Level 1 received general educational information regarding exercise and diet, including a resource directory and study-specific guidelines posted on the study website. Participants randomized to Level 2 received Level 1 resources plus personalized written diet and exercise recommendations, videos demonstrating recommended exercises, and a weekly e-log to track progress toward meeting recommendations. Participants randomized to Level 3 received Level 1 to 2 resources plus text messages to support achieving diet and exercise recommendations (average: 4 texts/week, no response required) and a Fitbit Alta that integrated with the study website to display physical activity reports back to the participant. Participants randomized to Level 4 received Level 1 to 3 resources plus access to two 30-minute coaching calls, 1 with an exercise trainer and 1 with a registered dietician. Participants were told they would be randomly assigned to receive access to websites with different tools and resources but were unaware of the resources they received relative to other participants. Additional details on randomization, interventions, and material content have been reported previously. 46
Quality of Life Assessments
Patient-reported outcomes were assessed using both general population and cancer-specific validated questionnaires. All QoL assessments were obtained at study enrollment, at the end of intervention (3 months) and at 3-month follow-up after intervention ended (6 months).
QLQ-C30
The European Organization for the Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire-Core 30 (QLQ-C30) is a cancer-specific 30-item questionnaire used to measure health-related QoL. 49 The QLQ-C30 includes 5 functioning scales (physical, role, cognitive, emotional, and social functioning), 3 symptom scales (fatigue, pain, nausea and vomiting), 6 symptom items (dyspnea, insomnia, appetite loss, constipation, diarrhea, financial difficulties), and a global health status/QoL scale. Each scale’s score ranges from 0 to 100 points; a higher score for global QoL and functioning reflect better health-related QoL or functioning, while a higher symptom scale score reflects higher symptom burden (eg, more severe pain). The QLQ-C30 scoring manual was used to calculate the scores. 50 Per scoring instructions, participants who responded to less than half of the scale components did not have a score calculated for that scale (ie, treated as missing; n = 57 for emotional functioning and financial difficulties subscales, n = 56 for all others). We used guidelines published in 2012 for interpreting longitudinal QoL score differences to quantify a meaningful change (see Table 4 in Cocks et al 51 ), which expanded on the 1998 published guidelines. 52
PROMIS fatigue
In addition to the fatigue symptoms assessed by the QLQ-C30, we used the Patient Reported Outcomes Measurement Information System (PROMIS) Fatigue 8a – Adult v1.0 questionnaire to assess participants’ fatigue. 53 Consistent with the PROMIS scoring manual, responses to all questions were summed and standardized using the T-Score Conversion Table. 54 The standardized score ranges from 33.1 to 77.8 (corresponding to a raw score of 8 to 40); a higher score is associated with greater symptoms of fatigue. We used the cancer calibration cohort within the HealthMeasures 55 Scoring Service (HM-SS) to impute responses for men who failed to answer at least one, but not all, PROMIS Fatigue questions. Men who failed to respond to at least 1 question were excluded from PROMIS analyses (n = 57). We followed the general threshold of a 5-point change to represent a minimally important difference, defined a priori.
PSQI
Sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI) validated questionnaire.56,57 The PSQI questionnaire evaluates 7 component scores (sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medications, daytime dysfunction). Component scores range from 0 (no difficulty) to 3 (severe difficulty) and are summed to generate a Global PSQI Score ranging from 0 (no difficulty) to 21 (severe difficulty in all areas). Men were considered non-responders and were excluded from the PSQI analysis (n = 70) if they failed to respond to ≥1 of the questions. A global score <5 reflects good sleep quality. 56
Statistical Analysis
Assessing QoL was a stated secondary objective of our pilot randomized trial. 46 Descriptive statistics of participants’ socio-demographics, clinical characteristics, and randomization details are provided for each of the main QoL scores. We compared change in patient-reported QoL measures between enrollment and end of intervention for Levels 2, 3, and 4 compared to Level 1 via t-tests. Where differences were observed, we planned to report change in QoL measures between enrollment and 6 months to assess if effects were maintained after the intervention ended. Results are reported as mean change with 95% confidence intervals (CI). Paired t-tests were also used to compare the 3-month QoL measures to the baseline QoL measures within each level of intervention (see Supplemental Table 1).
As secondary trial endpoints, all QoL analyses were conducted among men with complete follow-up data. Though we report formal tests for change, our focus is on patterns of change, rather than statistical significance, consistent with guidelines for pilot trials. 58 All analyses were conducted in R version 3.6.3 using two-sided hypothesis testing and an alpha level of .05 to assess statistical significance.
Results
A total of 202 men with prostate cancer were consented and randomized to increasing levels of web-based behavioral support and provided access to the intervention: 49 assigned to Level 1, 51 to Level 2, 50 to Level 3, and 52 to Level 4. Of the 202 men randomized, 161 (80%) accessed their intervention (38 in Level 1, 38 in Level 2, 42 in Level 3, and 42 in Level 4); 35 of the 202 men (17%) were lost to follow-up at 3 months and an additional 11 (5%) were lost to follow-up at 6 months. Attrition was similar across levels. More details about study recruitment and retention were reported previously.46,47
Men were predominantly white (93%) and well-educated (83% with 4-year college degree or more). A total of 30 (15%) men reported receiving hormone therapy for the treatment of their prostate cancer. The overall median (IQR) age at enrollment was 70 (65-75) and was similar across all levels. However, by chance, men in Level 1 were farther out from their prostate cancer diagnosis compared to the group as a whole (median [IQR]: 9 years [4, 14] vs 4 years [2,10]). Table 1 shows median baseline QoL scores by participant characteristics. Men diagnosed with higher T-stage (T3-T4) reported lower median QLQ-C30 Global Health at baseline (75, IQR: 67, 83) compared to men diagnosed with stage T1-T2 (83, IQR: 75, 92), though scores were similar across other characteristics.
Table 1.
Median Baseline QoL Scores by Sociodemographic and Clinical Characteristics of 202 Prostate Cancer Survivors Participating in A Technology-Supported Exercise and Dietary Intervention.
| Characteristic | n (%) a | QLQ-C30 global health b | PROMIS fatigue c | PSQI c |
|---|---|---|---|---|
| All participants | 202 (100) | 83.3 (75.0, 91.7) | 46.4 (41.0, 49.2) | 5.0 (3.0, 7.0) |
| Age | ||||
| <65 | 56 (27.7) | 83.3 (75.0, 91.7) | 46.7 (42.6, 51.2) | 6.0 (4.0, 8.0) |
| ≥65 | 146 (72.3) | 83.3 (75.0, 91.7) | 46.4 (41.0, 49.0) | 5.0 (3.0, 7.0) |
| Race | ||||
| White | 187 (92.6) | 83.3 (75.0, 91.7) | 46.4 (41.0, 49.2) | 5.0 (3.0, 7.0) |
| Other | 13 (6.4) | 83.3 (81.2, 85.4) | 48.7 (44.0, 51.7) | 8.0 (6.0, 8.5) |
| Education | ||||
| ≤ High school | 15 (7.4) | 83.3 (83.3, 100.0) | 47.7 (41.3, 48.7) | 5.5 (4.0, 7.0) |
| 2- or 4- year college | 78 (38.6) | 83.3 (75.0, 91.7) | 46.4 (41.0, 51.2) | 5.0 (3.0, 7.0) |
| Grad/prof degree | 109 (54.0) | 83.3 (75.0, 91.7) | 46.4 (41.0, 48.7) | 5.0 (3.0, 8.0) |
| PSA d | ||||
| ≤10 ng/mL | 129 (63.9) | 83.3 (75.0, 91.7) | 46.6 (41.2, 49.2) | 5.0 (3.0, 8.0) |
| >10 ng/mL | 48 (23.8) | 83.3 (75.0, 91.7) | 46.4 (40.9, 48.8) | 5.0 (3.2, 7.0) |
| T-stage | ||||
| T1-T2 | 149 (73.8) | 83.3 (75.0, 91.7) | 45.4 (35.2, 48.7) | 5.0 (3.0, 7.0) |
| T3-T4 | 40 (19.8) | 75.0 (66.7, 83.3) | 48.7 (46.7, 53.0) | 6.0 (4.5, 8.5) |
| Gleason | ||||
| <7 | 38 (18.8) | 83.3 (83.3, 97.9) | 46.4 (41.3, 49.1) | 6.0 (2.0, 7.0) |
| 7 | 80 (39.6) | 83.3 (66.7, 91.7) | 46.4 (41.0, 48.7) | 5.0 (3.0, 7.2) |
| >7 | 46 (22.8) | 83.3 (66.7, 83.3) | 46.7 (44.1, 50.1) | 5.0 (4.0, 8.5) |
| ADT e | 30 (14.9) | 83.3 (75.0, 100) | 44.1 (35.1, 49.5) | 4.0 (3.0, 5.8) |
| Levels | ||||
| Level 1 | 49 (24.3) | 83.3 (75.0, 95.8) | 46.4 (44.0, 49.1) | 5.0 (3.0, 7.0) |
| Level 2 | 51 (25.2) | 83.3 (75.0, 91.7) | 47.7 (41.4, 51.2) | 5.0 (3.8, 8.0) |
| Level 3 | 50 (24.8) | 83.3 (75.0, 91.7) | 44.2 (36.5, 48.7) | 5.0 (2.0, 7.0) |
| Level 4 | 52 (25.7) | 83.3 (66.7, 91.7) | 46.7 (38.2, 49.8) | 5.0 (4.0, 7.0) |
Abbreviations: ADT, androgen deprivation therapy; PROMIS, patient reported outcomes measurement system; PSA, prostate-specific antigen; PSQI, Pittsburgh sleep quality index; QLQ-C30, Quality of Life Questionnaire-Core 30; QoL, quality of life.
Percentages may not sum 100% due to missingness: 2 men with unknown race, 25 men with unknown diagnostic PSA, 13 with unknown T-stage, 38 with unknown Gleason.
Higher score reflects better QoL.
Lower score reflects less fatigue/symptom burden.
Median PSA value at diagnosis was 6.4 ng/mL.
Reflects the number of men on active ADT treatment at enrollment. Two men reported active chemotherapy and 1 man reported active radiation therapy; counts were too low to summarize across scores.
QLQ-C30
Mean changes in the QLQ-C30 are shown in Figure 1. Generally, most confidence intervals crossed 0 (ie, included the null), but there were notable trends. Compared to Level 1, men assigned to Level 2 tended to report worsening role, emotional, and cognitive functioning at 3 months, and increasing burden of fatigue, insomnia, diarrhea, and financial difficulties (Figure 1), resulting in a decreasing Global Health measure at 3 months (−2.31, 95% CI: −8.05, 3.42). Level 4 participants also reported a trend toward decreasing Global Health status compared to Level 1 (−1.52, 95% CI: −6.78, 3.74), though the only notable decline reported was an increase in financial difficulties. Conversely, there was a trend toward improving Global Health Status for men in Level 3 versus 1 (5.46, 95% CI: −0.02, 10.95), reflecting a small, but meaningful change. 51 Correspondingly, Level 3 reported improving emotional function and symptoms, including declining appetite loss, constipation, and diarrhea. The within-level mean change from baseline to 3-months is provided in Supplemental Table 1.
Figure 1.
Mean change in QLQ-C30 sub-scales compared to level 1. (A) QLQ-C30 global health and function scales (a positive change score reflects better health/functioning comparing the level to the referent level). (B) QLQ-C30 symptoms scales and items (a negative change score reflects lower symptom burden comparing the level to the referent level).
PROMIS Fatigue Score
Mean changes in PROMIS Fatigue scores are shown in Figure 2. Level 2 participants had increasing fatigue symptoms compared to Level 1 (3.24, 95% CI: 0.77, 5.71), with an upward trend also observed among Level 4 versus Level 1 participants (2.11, 95% CI: −0.82, 5.04). However, neither point estimate reached our a priori threshold of meaningful change (5 points). Level 3 participants did not demonstrate differences from men in Level 1 (0.39, 95% CI: −2.32, 3.11). The within-level mean change from baseline to 3-months is provided in Supplemental Table 1.
Figure 2.

Mean change in PROMIS fatiguea and PSQI sleep indexb scores compared to level 1.
aA positive change score is associated with greater fatigue comparing the level to the referent level.
bA positive change score is associated with worse sleep quality comparing the level to the referent level.
PSQI
Mean changes in PSQI scores are shown in Figure 2. There was essentially no change for men in Level 4 (0.14, 95% CI: −1.01, 1.29), Level 3 (0.31, 95% CI: −0.87, 1.49) or Level 2 (0.70, 95% CI: −0.47, 1.88) compared to Level 1 participants. The within-level mean change from baseline to 3-months is provided in Supplemental Table 1.
Given the lack of substantial change observed between baseline and 3 months, we did not analyze 6-month follow-up data.
Discussion
The primary aim of this pilot randomized trial was to assess the feasibility and acceptability of the web-based diet and exercise intervention. Findings from that analysis showed that the intervention was feasible and men were satisfied with the intervention, particularly those randomized to level 4. We also reported small improvements in diet and physical activity at 3 months for men randomized to level 4 versus level 1. Physical activity improvements were strongest among men who were insufficiently active at enrollment. 47 In this analysis of secondary outcomes, we observed little change in cancer-specific QoL outcomes among prostate cancer survivors participating in a pilot, 3-month, web-based intervention offering varying levels of educational information and behavioral support to improve diet and exercise behaviors. There may be several reasons for these results. First, it is possible that the intervention, though modestly effective at improving diet and exercise habits associated with improved prostate cancer outcomes, 47 may not improve QoL. Second, study participants were mostly diagnosed with localized prostate cancer. Prior studies have reported that QoL is notably better in men diagnosed with localized versus advanced disease. 59 Indeed, our lowest global QoL scores were observed among the 40 men diagnosed with T3-T4 disease and our baseline scores suggest that, overall, men had relatively high global QoL and functioning scores and low symptom burden. These high initial values could create a ceiling effect that may explain the limited influence of the intervention. A related reason may be that men in this study were a median of 3 years (IQR: 1, 9) out from their primary treatment for prostate cancer. There are both short- and long-term side effects associated with primary therapy, and these may differ in timing of effect, depending on type of therapy received.6-9,60,61 Given this variability, it is also possible that clear patterns of change could not be observed, given our limited sample size and inability to examine participants based on these factors.
Some trends were observed within the 3 QoL metrics examined. Compared to Level 1, Level 2 participants demonstrated some decreases in QoL over the course of the intervention. There was also some evidence of a meaningful increase in QoL for Level 3 and a (nonmeaningful) decrease in QoL for Level 4 participants, though these trends were not consistent across all metrics. Given this lack of consistency and the possibility of chance findings due to multiple comparison, we caution against drawing conclusions from these noted trends. It is necessary to determine population-specific thresholds for meaningful clinical change. The EORTC 62 Quality of Life Group (QLG) supported the minimally important difference (MID) project whose aim was to establish MIDs for QLQ-C30 questionnaires according to cancer site.Notably, MID guidelines for prostate cancer have not yet been published, nor have MIDs been published for other scores within similar populations of prostate cancer survivors. Thus, general guidelines for meaningful change were used, as noted in the methods. However, previous literature suggest that these thresholds may vary by population and cancer type, in addition to QLQ-C30 sub-scale, and even the direction of change (ie, whether a positive score reflects better or worse QoL).51,63-65 QLG MID project investigators have announced future plans to develop MIDs specific to prostate cancer, which may provide an opportunity to re-evaluate findings from this study. As a related example, although the change in PROMIS fatigue score did not reach the a priori threshold of meaningful change, Yost et al 66 noted a change score of 3 to 5 points using the 7-item PROMIS fatigue questionnaire in men with prostate cancer was a MID. A similar analysis to clarify MID for the 8-item PROMIS fatigue questionnaire used in this study may better inform these findings.
There are several limitations of this study to consider. The intervention was of limited duration and we did not select men with low baseline QoL scores. Men were diagnosed with different stages of disease, varied in their time since diagnosis, and were at varying points in their treatment pathway, all of which may influence QoL. We also acknowledge that men who self-select to participate in a lifestyle study may have better QoL than men who opt out. Further, the intervention was not specifically designed to improve QoL, nor powered to detect differences for this secondary outcome; any statistically significant results should be evaluated cautiously. Attrition may be more common among men experiencing increased symptom burden and decreased QoL. To the extent this is true, we may have missed a decline in QoL due to attrition, though rate of attrition was balanced across Levels. Lastly, individuals who volunteered were predominately white and highly educated which limits generalizability. Despite these potential shortcomings, given limited data on the impact of web-enhanced interventions to improve QoL in PC survivors, this report may be informative for future studies. Such studies may wish to consider a longer intervention period, inclusion of additional tools targeted at modifying specific QoL metrics (eg, meditation to reduce anxiety), and focus on men with worse disease severity or a greater burden of treatment.
Conclusion
This 3-month web-based intervention did not appear to have a meaningful impact on prostate cancer survivors’ health-related QoL, sleep quality, or fatigue levels in the pilot sample. However, given the relative healthiness of the study population at enrollment, conclusions about the potential of this approach to reduce symptoms and improve QoL in men with more advanced disease or those more proximal to their primary treatment may still be warranted. Additionally, Black/African-American men bear a greater burden of prostate cancer morbidity and mortality and are often under-represented in lifestyle intervention trials.20,67,68 To address this, our team is conducting a qualitative assessment of preferences regarding diet and exercise resources for behavioral change, among non-white men with prostate cancer, to inform future tailored interventions. Overall, this pilot study suggests that in a population of more educated, white men with early-stage prostate cancer, more comprehensive (and/or longer) interventions may be needed to modify behavior to improve meaningfully QoL, sleep, and fatigue.
Supplemental Material
Supplemental material, sj-docx-1-ict-10.1177_15347354211063500 for Quality of Life of Prostate Cancer Survivors Participating in a Remotely Delivered Web-Based Behavioral Intervention Pilot Randomized Trial by Crystal S. Langlais, Yea-Hung Chen, Erin L. Van Blarigan, Stacey A. Kenfield, Elizabeth R. Kessler, Kimi Daniel, Justin W. Ramsdill, Tomasz M. Beer, Rebecca E. Graff, Kellie Paich, June M. Chan and Kerri M. Winters-Stone in Integrative Cancer Therapies
Acknowledgments
We would like to thank the participants of the trial who made this research possible.
Footnotes
Authors’ Note: Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: SAK is associated with Fellow Health Inc. TMB is associated with AbbVie, Alliance Foundation Trials, Arvinas Inc, Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Clovis Oncology, Corcept Therapeutics, Endocyte Inc, GlaxoSmithKline, Janssen Biotech, Janssen Japan, Janssen Research & Development, Medivation, Inc, Merck, OncoGenex, Pfizer, Salarius Pharmaceuticals, Sotio, Theraclone Sciences/OncoResponse. JMC’s spouse is employed by GRAIL Inc. KP is employed by Movember. The authors are the developers/sponsors of the intervention evaluated in this report.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Movember Foundation; Prostate Cancer Foundation Young Investigator Award; the National Center for Advancing Translational Sciences [Grant Numbers UL1 TR001872 and UL1TR002369]; and other NIH grants [P30DK098722, P30CA069533-21, P30CA069533, NCI K07CA197077, and NCI F31CA247093].
Ethics Approval and Consent to Participate: All participants provided written consent, and all study-related activities were performed in accordance with and under the supervision of the institutional review board of each study site.
ORCID iDs: Crystal S. Langlais  https://orcid.org/0000-0001-5654-5542
https://orcid.org/0000-0001-5654-5542
Kerri M. Winters-Stone  https://orcid.org/0000-0003-1706-8020
https://orcid.org/0000-0003-1706-8020
Supplemental Material: Supplemental material for this article is available online.
Availability of Data and Materials: The datasets used during the current study may be available from the corresponding author on reasonable request.
References
- 1. American Cancer Society. Cancer facts and figures 2020. 2020. Accessed November 9, 2020. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf.
- 2. SEER*Explorer. An interactive website for SEER cancer statistics. Surveillance Research Program, National Cancer Institute. 2020. Accessed November 9, 2020. https://seer.cancer.gov/explorer/ [Google Scholar]
- 3. Winters-Stone KM, Moe E, Graff JN, et al. Falls and frailty in prostate cancer survivors: current, past, and never users of Androgen deprivation therapy. J Am Geriatr Soc. 2017;65:1414-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Iadeluca L, Mardekian J, Chander P, Hopps M, Makinson GT. The burden of selected cancers in the US: health behaviors and health care resource utilization. Cancer Manag Res. 2017;9:721-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leach CR, Bellizzi KM, Hurria A, Reeve BB. Is it my cancer or am i just getting older? Impact of cancer on age-related health conditions of older cancer survivors. Cancer. 2016;122:1946-1953. [DOI] [PubMed] [Google Scholar]
- 6. Chang D, Joseph DJ, Ebert MA, et al. Effect of androgen deprivation therapy on muscle attenuation in men with prostate cancer. J Med Imaging Radiat Oncol. 2014;58:223-228. [DOI] [PubMed] [Google Scholar]
- 7. Galvão DA, Taaffe DR, Spry N, Joseph D, Turner D, Newton RU. Reduced muscle strength and functional performance in men with prostate cancer undergoing androgen suppression: a comprehensive cross-sectional investigation. Prostate Cancer Prostatic Dis. 2009;12:198-203. [DOI] [PubMed] [Google Scholar]
- 8. Prostate Cancer Foudation. Prostate cancer side effects. Prostate Cancer Foundation. 2020. Accessed March 9, 2020. https://www.pcf.org/about-prostate-cancer/prostate-cancer-side-effects/ [Google Scholar]
- 9. Koskderelioglu A, Gedizlioglu M, Ceylan Y, Gunlusoy B, Kahyaoglu N. Quality of sleep in patients receiving androgen deprivation therapy for prostate cancer. Neurol Sci. 2017;38:1445-1451. [DOI] [PubMed] [Google Scholar]
- 10. Eton DT, Lepore SJ. Prostate cancer and health-related quality of life: a review of the literature. Psychooncology. 2002;11:307-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lubeck DP, Litwin MS, Henning JM, Stoddard ML, Flanders SC, Carroll PR. Changes in health-related quality of life in the first year after treatment for prostate cancer: results from CaPSURE. Urology. 1999;53:180-186. [DOI] [PubMed] [Google Scholar]
- 12. Litwin MS, McGuigan KA, Shpall AI, Dhanani N. Recovery of health related quality of life in the year after radical prostatectomy: early experience. J Urol. 1999;161:515-519. [PubMed] [Google Scholar]
- 13. Department of Health and Human Services. Physical Activity Guidelines for Americans. 2nd ed. U.S. Department of Health and Human Services; 2018. [Google Scholar]
- 14. Winters-Stone KM, Dobek JC, Bennett JA, Maddalozzo GF, Ryan CW, Beer TM. Skeletal response to resistance and impact training in prostate cancer survivors. Med Sci Sports Exerc. 2014;46:1482-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Winters-Stone KM, Dieckmann N, Maddalozzo GF, Bennett JA, Ryan CW, Beer TM. Resistance exercise reduces body fat and insulin during androgen-deprivation therapy for prostate cancer. Oncol Nurs Forum. 2015;42:348-356. [DOI] [PubMed] [Google Scholar]
- 16. Winters-Stone KM, Dobek JC, Bennett JA, et al. Resistance training reduces disability in prostate cancer survivors on androgen deprivation therapy: evidence from a randomized controlled trial. Arch Phys Med Rehabil. 2015;96:7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Richman EL, Kenfield SA, Stampfer MJ, Paciorek A, Carroll PR, Chan JM. Physical activity after diagnosis and risk of prostate cancer progression: data from the cancer of the prostate strategic urologic research endeavor. Cancer Res. 2011;71:3889-3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Winters-Stone KM, Lyons KS, Dobek J, et al. Benefits of partnered strength training for prostate cancer survivors and spouses: results from a randomized controlled trial of the exercising together project. J Cancer Surviv. 2016;10:633-644. [DOI] [PubMed] [Google Scholar]
- 19. Mishra SI, Scherer RW, Geigle PM, et al. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst Rev. 2012;2012:CD007566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Langlais CS, Graff RE, Van Blarigan EL, et al. Post-Diagnostic dietary and lifestyle factors and prostate cancer recurrence, progression, and mortality. Curr Oncol Rep. 2021;23:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guy DE, Vandersluis A, Klotz LH, et al. Total energy expenditure and vigorous-intensity physical activity are associated with reduced odds of reclassification among men on active surveillance. Prostate Cancer Prostatic Dis. 2018;21:187-195. [DOI] [PubMed] [Google Scholar]
- 22. Kenfield SA, Stampfer MJ, Giovannucci E, Chan JM. Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J Clin Oncol. 2011;29:726-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang Y, Jacobs EJ, Gapstur SM, et al. Recreational physical activity in relation to prostate cancer-specific mortality among men with nonmetastatic prostate cancer. Eur Urol. 2017;72:931-939. [DOI] [PubMed] [Google Scholar]
- 24. Friedenreich CM, Wang Q, Neilson HK, Kopciuk KA, McGregor SE, Courneya KS. Physical activity and survival after prostate cancer. Eur Urol. 2016;70:576-585. [DOI] [PubMed] [Google Scholar]
- 25. Bonn SE, Sjölander A, Lagerros YT, et al. Physical activity and survival among men diagnosed with prostate cancer. Cancer Epidemiol Biomarkers Prev. 2015;24:57-64. [DOI] [PubMed] [Google Scholar]
- 26. Dai JY, Wang B, Wang X, et al. Vigorous physical activity is associated with lower risk of metastatic-lethal progression in prostate cancer and hypomethylation in the CRACR2A gene. Cancer Epidemiol Biomarkers Prev. 2019;28:258-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ottenbacher A, Yu M, Moser RP, Phillips SM, Alfano C, Perna FM. Population estimates of meeting strength training and aerobic guidelines, by gender and cancer survivorship status: findings from the Health Information National Trends Survey (HINTS). J Phys Act Health. 2015;12:675-679. [DOI] [PubMed] [Google Scholar]
- 28. Yang M, Kenfield SA, Van Blarigan EL, et al. Dietary patterns after prostate cancer diagnosis in relation to disease-specific and total mortality. Cancer Prev Res. 2015;8:545-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Van Blarigan EL, Kenfield SA, Yang M, et al. Fat intake after prostate cancer diagnosis and mortality in the physicians’ health study. Cancer Causes Control. 2015;26:1117-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Richman EL, Stampfer MJ, Paciorek A, Broering JM, Carroll PR, Chan JM. Intakes of meat, fish, poultry, and eggs and risk of prostate cancer progression. Am J Clin Nutr. 2010;91:712-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Richman EL, Carroll PR, Chan JM. Vegetable and fruit intake after diagnosis and risk of prostate cancer progression. Int J Cancer. 2012;131:201-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Peisch SF, Van Blarigan EL, Chan JM, Stampfer MJ, Kenfield SA. Prostate cancer progression and mortality: a review of diet and lifestyle factors. World J Urol. 2017;35:867-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chan JM, Holick CN, Leitzmann MF, et al. Diet after diagnosis and the risk of prostate cancer progression, recurrence, and death (United States). Cancer Causes Control. 2006;17:199-208. [DOI] [PubMed] [Google Scholar]
- 34. Pettersson A, Kasperzyk JL, Kenfield SA, et al. Milk and dairy consumption among men with prostate cancer and risk of metastases and prostate cancer death. Cancer Epidemiol Biomarkers Prev. 2012;21:428-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Richman EL, Kenfield SA, Stampfer MJ, Giovannucci EL, Chan JM. Egg, red meat, and poultry intake and risk of lethal prostate cancer in the prostate-specific antigen-era: incidence and survival. Cancer Prev Res. 2011;4:2110-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chavarro JE, Stampfer MJ, Hall MN, Sesso HD, Ma J. A 22-y prospective study of fish intake in relation to prostate cancer incidence and mortality. Am J Clin Nutr. 2008;88:1297-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang W, Yang M, Kenfield SA, et al. Nut consumption and prostate cancer risk and mortality. Br J Cancer. 2016;115:371-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vajdi M, Farhangi MA. A systematic review of the association between dietary patterns and health-related quality of life. Health Qual Life Outcomes. 2020;18:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Drewnowski A, Evans WJ. Nutrition, physical activity, and quality of life in older adults: summary. J Gerontol A Biol Sci Med Sci. 2001;56:89-94. [DOI] [PubMed] [Google Scholar]
- 40. Morley JE. Decreased food intake with aging. J Gerontol A Biol Sci Med Sci. 2001;56:81-88. [DOI] [PubMed] [Google Scholar]
- 41. Amarantos E, Martinez A, Dwyer J. Nutrition and quality of life in older adults. J Gerontol A Biol Sci Med Sci. 2001;56:54-64. [DOI] [PubMed] [Google Scholar]
- 42. Bluethmann SM, Basen-Engquist K, Vernon SW, et al. Grasping the 'teachable moment': time since diagnosis, symptom burden and health behaviors in breast, colorectal and prostate cancer survivors. Psychooncology. 2015;24:1250-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Demark-Wahnefried W, Aziz NM, Rowland JH, Pinto BM. Riding the crest of the teachable moment: promoting long-term health after the diagnosis of cancer. J Clin Oncol. 2005;23:5814-5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McBride CM, Clipp E, Peterson BL, Lipkus IM, Demark-Wahnefried W. Psychological impact of diagnosis and risk reduction among cancer survivors. Psychooncology. 2000;9:418-427. [DOI] [PubMed] [Google Scholar]
- 45. Demark-Wahnefried W, Peterson B, McBride C, Lipkus I, Clipp E. Current health behaviors and readiness to pursue life-style changes among men and women diagnosed with early stage prostate and breast carcinomas. Cancer. 2000;88:674-684. [PubMed] [Google Scholar]
- 46. Winters-Stone KM, Kenfield SA, Van Blarigan EL, et al. Effect of increasing levels of web-based behavioral support on changes in physical activity, diet, and symptoms in men with prostate cancer: protocol for a randomized controlled trial. JMIR Res Protoc. 2018;7:e11257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chan JM, Van Blarigan EL, Langlais CS, et al. Feasibility and acceptability of a remotely delivered, web-based behavioral intervention for men with prostate cancer: four-arm randomized controlled pilot trial. J Med Internet Res. 2020;22:e19238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lubeck DP, Litwin MS, Henning JM, et al. The CaPSURE database: a methodology for clinical practice and research in prostate cancer. CaPSURE research panel. Cancer of the prostate strategic urologic research endeavor. Urology. 1996;48:773-777. [DOI] [PubMed] [Google Scholar]
- 49. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365-376. [DOI] [PubMed] [Google Scholar]
- 50. Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A; EORTC Qulaity of Life Group. The EORTC QLQ-C30 Scoring Manual. 3rd ed. European Organisation for Research and Treatment of Cancer; 2001. [Google Scholar]
- 51. Cocks K, King MT, Velikova G, et al. Evidence-based guidelines for interpreting change scores for the European Organisation for the Research and Treatment of cancer quality of life questionnaire core 30. Eur J Cancer. 2012;48:1713-1721. [DOI] [PubMed] [Google Scholar]
- 52. Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139-144. [DOI] [PubMed] [Google Scholar]
- 53. Garcia SF, Cella D, Clauser SB, et al. Standardizing patient-reported outcomes assessment in cancer clinical trials: a patient-reported outcomes measurement information system initiative. J Clin Oncol. 2007;25:5106-5112. [DOI] [PubMed] [Google Scholar]
- 54. System P-ROMI. Patient-reported outcomes measurement information system. Fatigue: a brief guide to the PROMIS fatigue instruments. 2019. Accessed September 3, 2020. http://www.healthmeasures.net/images/PROMIS/manuals/PROMIS_Fatigue_Scoring_Manual.pdf
- 55. HealthMeasures. Meaningful change for PROMIS. 2020. Accessed September 3, 2020. https://www.healthmeasures.net/score-and-interpret/interpret-scores/promis/meaningful-change
- 56. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193-213. [DOI] [PubMed] [Google Scholar]
- 57. Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: a systematic review and meta-analysis. Sleep Med Rev. 2016;25:52-73. [DOI] [PubMed] [Google Scholar]
- 58. Eldridge SM, Chan CL, Campbell MJ, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ. 2016;355:i5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hamaya T, Hatakeyama S, Momota M, et al. Association between the baseline frailty and quality of life in patients with prostate cancer (FRAQ-PC study). Int J Clin Oncol. 2021;26:199-206. [DOI] [PubMed] [Google Scholar]
- 60. Boeve L, Hulshof M, Verhagen P, et al. Patient-reported quality of life in patients with primary metastatic prostate cancer treated with Androgen deprivation therapy with and without concurrent radiation therapy to the prostate in a prospective randomised clinical trial; data from the HORRAD trial. Eur Urol. 2021;79:188-197. [DOI] [PubMed] [Google Scholar]
- 61. Lardas M, Liew M, van den Bergh RC, et al. Quality of life outcomes after primary treatment for clinically localised prostate cancer: a systematic review. Eur Urol. 2017;72:869-885. [DOI] [PubMed] [Google Scholar]
- 62. EORTC. Quality of life MID. 2020. Accessed January 20, 2021. https://qol.eortc.org/projectqol/mid/
- 63. Cocks K, King MT, Velikova G, Martyn St-James M, Fayers PM, Brown JM. Evidence-based guidelines for determination of sample size and interpretation of the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. J Clin Oncol. 2011;29:89-96. [DOI] [PubMed] [Google Scholar]
- 64. King MT. The interpretation of scores from the EORTC quality of life questionnaire QLQ-C30. Qual Life Res. 1996;5:555-567. [DOI] [PubMed] [Google Scholar]
- 65. Musoro JZ, Bottomley A, Coens C, et al. Interpreting European Organisation for Research and Treatment for Cancer Quality of life Questionnaire core 30 scores as minimally importantly different for patients with malignant melanoma. Eur J Cancer. 2018;104:169-181. [DOI] [PubMed] [Google Scholar]
- 66. Yost KJ, Eton DT, Garcia SF, Cella D. Minimally important differences were estimated for six patient-reported outcomes measurement information system-cancer scales in advanced-stage cancer patients. J Clin Epidemiol. 2011;64:507-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zuniga KB, Borno H, Chan JM, et al. The problem of underrepresentation: black participants in lifestyle trials among patients with prostate cancer. J Racial Ethn Health Disparities. 2020;7:996-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. American Cancer Society. Cancer Facts & Figures for African Americans 2019-2021. American Cancer Society; 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ict-10.1177_15347354211063500 for Quality of Life of Prostate Cancer Survivors Participating in a Remotely Delivered Web-Based Behavioral Intervention Pilot Randomized Trial by Crystal S. Langlais, Yea-Hung Chen, Erin L. Van Blarigan, Stacey A. Kenfield, Elizabeth R. Kessler, Kimi Daniel, Justin W. Ramsdill, Tomasz M. Beer, Rebecca E. Graff, Kellie Paich, June M. Chan and Kerri M. Winters-Stone in Integrative Cancer Therapies