Abstract
Background: In the mid-1990s, the development of combination antiretroviral therapy converted HIV infection into a chronic condition, with newly diagnosed patients now living longer than the general population. HIV affects both the central and peripheral nerve systems, resulting in a variety of clinical problems, including peripheral neuropathy, which is a common neurological consequence. Despite this, there is a scarcity of data on the extent of peripheral sensory neuropathy and its underlying factors in Ethiopia, necessitating this study. Objective: The primary goal of this study is to assess the degree of peripheral sensory neuropathy and its related factors among HIV/AIDS clients on follow up at public health institutions in Northwest Ethiopia. Methodology: Institution based cross-sectional study was conducted from November 1 to 30 December 2020 at selected south Gondar zone public health institutions ART clinic. Multistage sampling technique was used to select the study participants. Standardized Questioner adapted from other study was used to collect the data. Moreover, Brief Peripheral Neuropathy Screening tool (BPNS) was used to assess peripheral sensory neuropathy. The data were entered with epi-data manager version 4.4 and analyzed using STATA version 16. Result: A total of 555 adult PLWHIV agreed to participate in the study, resulting in a response rate of 96.8%. The prevalence of Peripheral sensory neuropathy was 32.25, 95% CI (28.28, 36.26). The participant’s age, DM comorbidity, viral load level, and disease clinical stage were all found to have a statistically significant association with peripheral sensory neuropathy. Conclusion: Peripheral sensory neuropathy was incredibly common. Accordingly, peripheral sensory neuropathy was found considerably associated with age, viral load level, stage of the disease, and DM comorbidity. It is vital to integrate routine peripheral sensory neuropathy screening strategies for clients who are on ART follow up for prevention and early identification of the problem.
Keywords: Peripheral neuropathy, associated factors, HIV/AIDS, Ethiopia
Introduction
The current global HIV prevalence is estimated to be over 34 million. 1 HIV infection was changed from a high-mortality illness to a chronic disease with the development of combination antiretroviral treatment (cART) in the mid-1990s, with newly diagnosed patients now living longer than the general population. 2
The central nervous system (CNS) and the peripheral nervous system (PNS) are both affected by HIV-1 (PNS). HIV infection of the nervous system causes a variety of clinical problems, including peripheral neuropathy, which is a common neurological consequence.
There are at least six clinical patterns of HIV-related peripheral neuropathy. The most prevalent kind of peripheral neuropathy seen with chronic HIV infection or with neurotoxic ART is distal sensory polyneuropathy (DSP), which manifests as distal numbness and paresthesia’s, as well as evidence of absent or reduced deep tendon reflexes. Exact HIV-DSP’s etiology is uncertain. Macrophage activation and pro-inflammatory cytokines are involved in the development of neurological diseases and have been linked to HIV-DSP8 immunopathogenesis. TNF-, IFN-, and IL-6, pro-inflammatory cytokines, have been found in the dorsal root ganglia (DRG) of HIV-positive patients, implying inflammation-mediated neuronal injury.1,3-5
Many disabling consequences associated with HIV infection and antiretroviral therapy (ART) have recently come to light. Antiretroviral toxic neuropathy (ATN) is the most common antiretroviral therapy-related toxicity in Sub-Saharan Africa, and HIV-associated sensory neuropathy (HIV-SN) is one of those complications among people living with HIV that is widely under-recognized and under-treated in resource-constrained settings. About 30–67% of people with advanced HIV illness are infected with HIV-DSP and ATN. HIV-associated sensory polyneuropathy (HIV-SN) is a distal symmetrical, primarily sensory polyneuropathy marked by axonal degeneration in a “dying back” pattern. 6
In various HIV-infected populations, the global estimate of HIV-SN ranges from 1.73 to 69.4%. Between 27% and nearly half of ambulatory HIV infected people have HIV-SN, and 38% to 90% of those with HIV-SN experienced pain. 7 The prevalence of HIV–SN was found 42%,15.2%, and 8.6% in Australia, Indonesia, and San Diego, USA, respectively.8-10
Studies conducted in resource-limited settings in Sub-Saharan Africa found a range of estimations for the likelihood of HIV-SN in ART patients, ranging from 30% to as high as 64%, as well as treatment availability constraints in these situations7. It has been found that the magnitude of HIV-SN was 21%, 32.9%, 59% and 13% in Cameroon, Ethiopia, Rwanda, and Uganda, respectively.2,8,11,12
There are few large systematic studies of neuropathy from resource-constrained contexts, and the few that exist reveal vastly disparate neuropathy rates, ranging from 4% in South Africa to 59% in Rwanda. Two-thirds of HIV-SN are subclinical, according to Skopel and associates who utilized a model that included both clinical criteria (symptoms and evidence of neuropathy) and electrophysiological investigations. 13
Chronic neuropathic pain, loss of sensation, paresthesia, foot ulcers, unemployment, poor patient adherence to therapy, missed follow-ups, and poor quality of life are all frequent symptoms of peripheral neuropathy. HIV-PN has a significant social and economic impact. Patients with these symptoms are at risk of losing feeling in their limbs since neuropathy is a degenerative condition. On the other hand, despite successful HAART, the existence of HIV-SN-related neuropathic pain is associated with increased unemployment, higher rates of depression, and more reliance in daily living activities. As a result, painful HIV-SN is one of the leading causes of morbidity and mortality worldwide 14
Because the majority of the world’s HIV1 patients live in Sub-Saharan Africa, a better understanding of neuropathy prevalence and risk factors is critical.
According to the WHO, almost 26 million people in Sub-Saharan Africa possess HIV, accounting for 70% of the world’s 37 million HIV1 patients. 15 The burden of HIV–SN has yet to be determined in Ethiopia, where adult HIV prevalence is predicted to be 1.1%, or about 1.2 million PLWHIV. 11 As a result, the goal of this study is to determine the prevalence of HIV-SN and its underlying factors in HIV-infected patients attending in ART clinics of south Gondar zone public health institutions, North west Ethiopia.
Materials and Methods
Study area and Period
The research has been carried out in ART clinics of South Gondar zone public health institutions. South Gondar zone is one of the administrative zones in Amhara region, Ethiopia. Debre Tabor town, 667 km north of Addis Ababa, is serving as the zone’s administrative center. The zone is divided into 15 districts. There are eight public district hospitals and more than 90 health centers in the zone, including DGH, serving a population of 2.3 million people. More than 31 of those health institutions offer comprehensive services, such as chronic care follow-up for HIV/AIDS patients and other chronic cases. An institution-based cross-sectional study design has been employed and data were collected from.November 1 to December 30, 2020.
Population and Eligibility Criteria
The source population was all HIV/AIDS patients enrolled in health institutions in the South Gondar zone, ART clinic, while our study populations were HIV/AIDS patients getting care at ART clinics of selected health institutions in South Gondar zone, and present during the data collection process. Patients with HIV/AIDS who had a known current opportunistic infection, such as Tuberculosis (TB) or others, neurological problems, renal failure, and hypothyroidism were excluded from the study.
Sample size determination and sampling technique
The sample size for this investigation was calculated using the single population proportion formula, which assumed a prevalence of peripheral neuropathy as 34.6% based on previous research, 16 95% confidence interval, and a 5% margin of error
where N = sample size requiredα = the level of significance (5%)Za/2 = the value of Z (the standard normal distribution value) at the level of significance chosenp = proportion of HIV/AIDS patients with peripheral sensory neuropathy (34.6%) d = Margin of error = 0.05
We used a design effect of 1.5, and adding a 10% non-response rate, the final sample size was 573. The participants for the study were chosen using a multi-stage sampling technique. A total of 11,147 HIV/AIDS patients were registered for follow-up at ART clinics in South Gondar zone public institutions (31 health institutions) throughout the data collection period. Using a simple random sampling procedure, five districts were selected from the 15 districts in the zone to obtain the requisite sample size (573) from the source population. Subsequently, five health institutions (one from each district) were chosen by lottery, and the sample size was proportionally allotted to each health institution depending on the size of the population served (in this case, the number of registered HIV/AIDS patients attending follow up). Finally, using the patient’s MRN obtained from the registration, a simple random sample technique was used to choose participants for the study, and patients with their MRN selected were studied. Patients who were missing on the day of their visit were tolerated for 3 days after their appointment date in order to be included in the study. Those who did not comply after the three dates were classified as non-respondents.
Data collection procedure
A semi-structured questionnaire, which was adapted from previous practices, was delivered by an interviewer to collect the data. The tool comprised phenotypic data such as weight, height, waist size, hip circumference, and blood pressure, as well as sociodemographic characteristics of research participants, and medical record reviews. The Brief Peripheral Neuropathy Screening instrument (BPNS) was used to assess HIV-associated peripheral neuropathy (HIV-SN) after being translated into the local dialect (Amharic version).
Participants were sat with a cuff inserted on their left arm at the level of the heart while their blood pressure was monitored using an adult-sized sphygmomanometer. Brief Peripheral Neuropathy Screening instument (BPNS) is a standardized and an established tool for assessing HIV-related peripheral neuropathy. The BPNS tool evaluates PN-related subjective and objective data. This instrument was used in its standardized version, which is simple to use, practical, and adds less than 5 minutes to the clinical evaluation of HIV patients during follow-up in busy outpatient clinics.
For each leg, patients were asked to score the existence and severity of symptoms on a scale of 1 (mild) to 10 (severe). Pain, aching, or burning in the feet and/or legs; “pins and needles” in the feet and/or legs; and numbness in the feet and/or legs were among the symptoms. The highest of the six scores (three for each leg) will be converted to a subjective grade of peripheral neuropathy. To be classified as ≥ 1, symptoms did not have to be bilateral. The BPNS comprised objective findings such as loss of vibration perception and aberrant ankle-deep tendon reflexes.
A 128-Hz tuning fork was struck maximally and applied to each foot’s great toe distal interphalangeal joint to assess vibration perception. A vibration sense was classified as normal if it lasted more than 10 s, mild loss if it lasted 6–10 s, moderate loss if it lasted less than or equal to 5 s, and severe loss if no feeling of vibration. ankle reflexes were classified as either absent, hypoactive, normal, hyperactive, and clonus.7,11,17.
Twenty data collectors from chronic follow up staff and one supervisor for each institution was recruited and then trained by the principal investigator. Three days before data collection, the questionnaire was pre-tested on 5% of study participants with similar socio-cultural characteristics at one health center, where the actual data were not collected, to ensure its reliability, wording, and to eliminate language barriers and contextual variations on the semi structured questionnaire.
Operational/Term definitions
Peripheral Neuropathy: A subjective neuropathy grade of greater than 0 with at least one bilateral objective result. Normal Vibration sense: A vibration felt by a patient for more than 10 s. Normal deep tendon reflex: is defined as a reflex graded as 2+.
Data analysis procedure
The data collected were validated for correctness, and coded before being entered into Epi data management version 4.4 and then exported to STATA 16 for summary and analysis. For the descriptive analysis of data; frequencies, mean/median, and proportions were used. The association between the primary outcome measure and the explanatory variable was investigated using a binary logistic regression model. After bivariable logistic regression, variables with a p < 0.25 were incorporated into a multivariable logistic regression to determine each explanatory variable’s independent contribution. To analyze the association between different variables and the outcome variable, adjusted odds ratios and their corresponding 95% confidence intervals were presented, with p < 0.05 deemed substantially associated with PSN.
Data Quality management
For the HIV-SN assessment, a validated questionnaire from earlier studies was used, with minor local changes. The goal of the study, interview and measuring methodologies (weight, height, waist circumference, and blood pressure measurement), and ethical considerations during data collection were all discussed in a 2-day training session for data collectors. The questionnaire was translated into Amharic and then retranslated into English by another individual for consistency, and it was examined daily by the supervisors and main investigator for accuracy, consistency, and completeness, with the required corrective actions made as needed. Pretest was done on 5% of the participants in other health institutions where actual data were not collected.
Result
A total of 555 adult PLWHIV agreed to participate in the study, resulting in a response rate of 96.8%. The majority of the respondents were women, with 297 (53.51%) being female. The participants' average age (n = 555) was 42.8 (SD ±13) years. Almost a quarter 359 (64.68%) of them were urban residents, while 157 (28.29) of them had educational status beyond diploma. Almost a quarter 144 (25.95%) of them were married, 107 (19.8%) were governmental employees, and 362 (65.6%) were Orthodox Christian (Table 1).
Table 1.
Socio demographic characteristics of HIV/AIDS clients, South Gondar zone public health institutions, Ethiopia, 2020 (n = 555).
| Characteristics | Category | Frequency | Percent |
|---|---|---|---|
| Age | 18–35 | 250 | 45.05 |
| 36–55 | 224 | 40.36 | |
| Above 56 | 81 | 14.59 | |
| Sex | Female | 297 | 53.51 |
| Male | 258 | 46.49 | |
| Educational status | <grade 8 | 157 | 28.29 |
| Grade 9–12 | 235 | 42.34 | |
| Diploma and above | 163 | 29.37 | |
| Marital status | Single | 163 | 29.37 |
| Married | 144 | 25.95 | |
| Divorced | 176 | 31.71 | |
| Widowed | 72 | 12.97 | |
| Occupation | Gov’t employed | 107 | 19.28 |
| Private | 112 | 20.18 | |
| Merchant | 193 | 74.23 | |
| Farmer | 65 | 11.71 | |
| House wife | 22 | 3.96 | |
| Daily laborer | 56 | 10.09 | |
| Monthly income | <1500 | 335 | 60.36 |
| 1500–3000 | 111 | 20 | |
| >3000 | 109 | 19.64 | |
| Residence | Urban | 359 | 64.68 |
| Rural | 196 | 35.32 |
Association between Sociodemographic characteristics and peripheral sensory neuropathy
Binary logistic regression was used to explore the association between peripheral sensory neuropathy and the individuals' sociodemographic variables. The participant’s age, sex, occupation, marital status, and monthly income were all suggested to be associated to peripheral sensory neuropathy. Among the participants, 258 (7.7%) of them were males and 165 (4.3%) of them had peripheral sensory neuropathy in binary logistic regression analysis [COR = 1.38,95% CI (0.96,1.97) p = 0.001] (Table 2).
Table 2.
Association between peripheral neuropathy and sociodemographic characteristics of the participant in South Gondar Zone public health institutions, 2020, Ethiopia.
| Variable | Peripheral sensory neuropathy | Total | Bi-variable Logistic Regression | ||
|---|---|---|---|---|---|
| Yes (179) | No (376) | n (555) | P value | COR (95% CI) | |
| Age category | |||||
| 18–35 | 164 | 86 | 250 | 1.0 | |
| 36–55 | 164 | 60 | 224 | 0.07* | 0.6 (0.4,1.03) |
| ≥56 | 48 | 33 | 81 | 0.30 | 1.3 (0.78,2.19) |
| Sex | |||||
| male | 165 | 93 | 258 | 0.075 | 1.38 (0.96,1.97) |
| Female | 211 | 86 | 297 | 1.0 | |
| Educational status | |||||
| <grade 8 | 120 | 37 | 157 | 0.23* | 0.7 (0.4,1.2) |
| Grade 9–12 | 141 | 94 | 235 | 0.03* | 1.5 (1.04,2.44) |
| Diploma and above | 115 | 48 | 163 | 1.0 | |
| Occupation | |||||
| Gov’t employ | 94 | 13 | 107 | 1.0 | |
| Private | 68 | 44 | 112 | 0.00 | 4.6 (2.3,9.3) |
| Merchant | 115 | 78 | 193 | 0.00 | 4.9 (2.5,9.5) |
| Farmer | 36 | 29 | 65 | 0.00 | 5.1 (2.3,11.0) |
| Housewife | 16 | 6 | 22 | 0.45 | 1.6 (0.47,5.49) |
| Daily laborer | 43 | 13 | 56 | 0.07 | 2.2 (0.3,5.1) |
| Marital status | |||||
| Married | 102 | 42 | 144 | 1.0 | |
| Single | 99 | 64 | 163 | 0.06* | 1.5 (0.9,2.5) |
| Divorced | 124 | 52 | 176 | 0.07 | 1.0 (0.6,1.6) |
| Widowed | 51 | 21 | 72 | 1* | 1 (0.5,1.8) |
| Monthly income | |||||
| <1500 | 228 | 107 | 335 | 0.05* | 0.64 (0.4,1.0) |
| 1500–3000 | 85 | 26 | 111 | 0.00* | 0.41 (0.2,0.75) |
| >3000 | 63 | 46 | 109 | 1.0 | |
| Residency | |||||
| Urban | 247 | 112 | 359 | 0.47 | (0.87)0.6,1.26 |
| Rural | 129 | 67 | 196 | 1.0 | |
COR = Crude Odds Ratio, *p < 0.25.
Prevalence of Peripheral Neuropathy
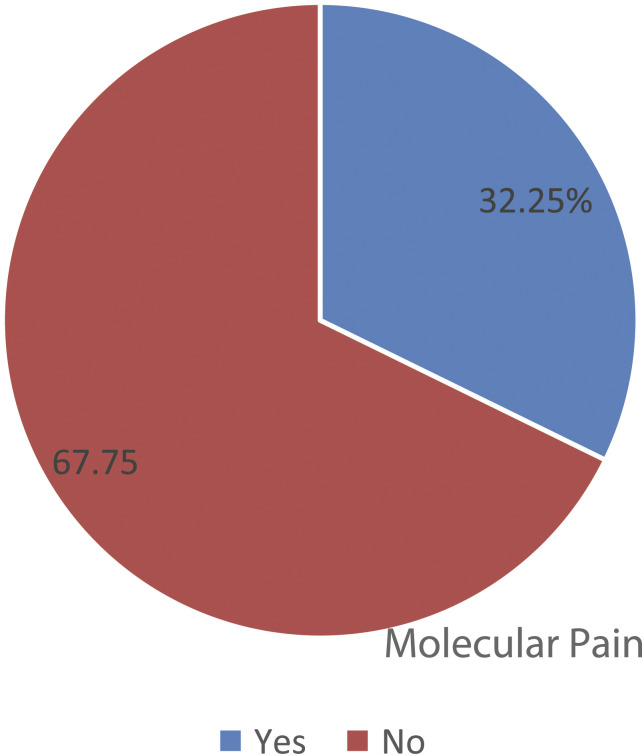
The magnitude of peripheral sensory neuropathy was 32.25, 95% CI (28.28, 36.26). Peripheral neuropathy using symptom report was observed among 276 (49.73%) of the participants. On objective findings, peripheral sensory neuropathy was observed among 331 (59.64%) of the participants (Figure 1).
Figure 1.
A pie chart showing the magnitude of peripheral sensory neuropathy among HIV/AIDS clients receiving care at public health institutions, Northwest Ethiopia, 2020. Peripheral Sensory.
Clinical Characteristics of the Participants
The mean tenure of sickness from diagnosis was 5.48 years (SD ± 7.04), with 256 (46.13%) of the individuals having been sick for longer than 5 years. In addition, according to WHO clinical staging, 276 (49.73%) and 54 (9.73%) of the respondents were in clinical stages I and IV, respectively. Furthermore, around 20% and 54.95% of the patients, their most recent CD4 count (within 3 months of the data collection period) was larger than 500 cells/l and 200–350 cells/l, respectively. In 7.75% of the participants, co-morbid DM was observed.
The participants' plasma viral load status revealed that 58 (10.45%) of them had undetectable viral replication (less than 50 copies/ul of blood), while 329 (59.28%) had viral load between 50 and 1000 copies/ul (Table 3).
Table 3.
Clinical parameters of HIV/AIDS clients, South Gondar zone, Northwest Ethiopia, 2020 (n = 555).
| Clinical factors | Category | Frequency | Percent (%) |
|---|---|---|---|
| Co morbid HT | Yes | 74 | 13.33 |
| No | 481 | 86.67 | |
| Co morbid DM | Yes | 43 | 7.75 |
| No | 512 | 92.25 | |
| Duration of illness since diagnosis (yr.) | <2 | 72 | 12.97 |
| 2–5 | 227 | 40.9 | |
| >5 | 256 | 46.13 | |
| Current (most recent) CD4 count (cell/dl) | <200 | 55 | 9.91 |
| 200–350 | 305 | 54.95 | |
| 351–500 | 84 | 15.14 | |
| >500 | 111 | 20 | |
| Viral load (copies/ul) | <50 | 58 | 10.45 |
| 50–1000 | 329 | 59.28 | |
| >1000 | 168 | 30.27 | |
| Clinical stage of the disease (WHO) | Stage I | 276 | 49.73 |
| Stage II | 115 | 20.72 | |
| Stage III | 110 | 19.82 | |
| Stage IV | 54 | 9.73 |
Association between Clinical Characteristics of the Participants and Peripheral Sensory Neuropathy
Association between peripheral sensory neuropathy and clinical factors of HIV/AIDS were assessed using binary logistic regression. Among the variables; co-morbid DM, duration of the disease since diagnosis, clinical stage of the disease, CD4 cell count and plasma viral load level had been found associated with peripheral sensory neuropathy. In binary logistic regression analysis, 43 (7.7%) of the partcipants had co-morbid DM, while 24 (4.3%) of them had peripheral sensory neuropathy [COR = 2.9, 95% CI, (1.5,5.4), p = 0.001] (Table 4).
Table 4.
Association between peripheral neuropathy and HIV/AIDS clinical factors in South Gondar Zone public health institutions, 2020, Ethiopia.
| Variable | Peripheral sensory neuropathy | Total (n = 555) | Bivariable logistic regression | ||
|---|---|---|---|---|---|
| Yes (n = 179) | No (n = 376) | p value | COR (95% CI) | ||
| Presence of co morbid DM | |||||
| Yes | 24 | 19 | 43 | 0.001 | 2.9 (1.5,5.4) |
| No | 155 | 357 | 512 | 1.0 | |
| Duration of disease | |||||
| <2 | 28 | 44 | 72 | 1.0 | |
| 2–5 | 67 | 160 | 227 | 0.13* | 0.6 (0.38,1.14) |
| >5 | 84 | 172 | 256 | 0.33 | 0.7 (0.4,1.3) |
| Current CD4 count | |||||
| <200 | 23 | 32 | 55 | 0.08* | 0.59 (0.3,1.06) |
| 201–350 | 91 | 214 | 305 | 0.02* | 0.3 (0.14.0.65) |
| 351–500 | 15 | 69 | 84 | 0.69* | 1.1 (0.6,2.2) |
| >500 | 50 | 61 | 111 | 1.0 | |
| Viral load(copies/ul) | |||||
| <50 | 6 | 52 | 58 | 1.0 | |
| 50–1000 | 100 | 229 | 329 | 0.009* | 1.6 (1.1,2.8) |
| >1000 | 73 | 95 | 168 | 0.000* | 2.04 (1.01,4.02) |
| Comorbid HTN | |||||
| Yes | 22 | 52 | 74 | 0.62 | 0.87 (0.5,1.4) |
| No | 157 | 324 | 481 | 1.0 | |
| Stage of the disease | |||||
| Stage I | 95 | 181 | 276 | 1.0 | |
| Stage II | 36 | 79 | 115 | 0.55* | 1.15 (0.72,1.83) |
| Stage III | 30 | 80 | 110 | 0.82* | 1.46 (0.46,2.46) |
| Stage IV | 18 | 36 | 54 | 0.79* | 1.7 (0.55,2.2) |
HTN = Hypertension, DM = Diabetes Mellitus, HAART = Highly Active Anti-Retroviral Therapy * p value < 0.25.
In the multivariable analysis for backward logistic regression, all variables with a p value less than 0.25 in the bivariate analysis were included. In the final model, five variables from the total variables included in the multivariable logistic regression model exhibited statistically significant association at the level of p value 0.05. The Hosmer and Lemeshow test was 23%, indicating that the model was fit and five of the 11 variables were substantially associated, according to the model summary. Accordingly, the study participants' age, viral load level, clinical stage of the disease, monthly income, and occupation were all found to have a statistically significant association with peripheral sensory neuropathy. Furthermore, participants in greater than 55-years age group were three times more likely than those in the 18–35-years age group to have peripheral sensory neuropathy [AOR = 3.2,95% CI (2.4,4.2), p = 0.001]. Participants with DM comorbidity were found to be approximately four times more likely than those without DM comorbidity to develop peripheral sensory neuropathy (AOR= 3.65, 95% CI (1.7,7.9), p = 0.001). Clients with viral loads larger than 1000 copies/ul and between 50 and 1000 copies/ul were found to suffer peripheral sensory neuropathy in comparison to viral loads less than 50 copies/ul. Participants with a viral load of more than 1000 copies/ul were nearly four times more likely to develop peripheral sensory neuropathy than those with a viral load of less than 50 copies/ul [AOR = 4.2,95% CI (2.1,7.3), p 0.0001], while those with a viral load of 50–1000 copies/ul were nearly four times more likely to develop peripheral sensory neuropathy than those with a viral load of less than 50 copies/ul [AOR = 2.2,95% CI(1.2,6.7) p < 0.0001]. The stage of the disease, on the other hand, was revealed to be substantially associated to peripheral sensory neuropathy. Participants in stage IV of the disease were 6 times more likely than those in stage I to have peripheral sensory neuropathy (AOR = 6.5, 95% CI(2.5, 7.8), p = 0.018). (Table 5).
Table 5.
Final model (Multivariable logistic regression analysis) for predictor variables independently associated with peripheral sensory neuropathy at South Gondar zone public health institutions, Northwest Ethiopia, 2020 (n = 555).
| Variable | Peripheral neuropathy | Total | Multivariable logistic regression | |||
|---|---|---|---|---|---|---|
| Yes (n = 179) | No (n = 376) | 555 | COR (95% CI) | AOR | p Value | |
| Age category | ||||||
| 18–35 | 164 | 86 | 250 | 1.0 | 1.0 | |
| 36–55 | 164 | 60 | 224 | 0.6 (0.4,1.03) | 1.7 (1.2,2.9) | 0.003** |
| ≥56 | 48 | 33 | 81 | 1.3 (0.78,2.19) | 3.2 (2.4,4.2) | 0.001** |
| Sex | ||||||
| Male | 165 | 93 | 258 | 1.38 (0.96,1.97) | 3.9 (1.5,9.8) | 0.004** |
| Female | 211 | 86 | 297 | 1.0 | 1.0 | |
| Disease stage | ||||||
| Stage I | 95 | 181 | 276 | 1.0 | 1.0 | |
| Stage II | 36 | 79 | 115 | 1.15 (0.72,1.83) | 0.65 (0.22,1.9) | 0.46 |
| Stage III | 30 | 80 | 110 | 1.46 (0.46,2.46) | 2.8 (1.3,4.6) | 0.015 |
| Stage IV | 18 | 36 | 54 | 1.7 (0.55,2.2) | 6.5 (2.5, 7.8) | 0.018 |
| Comorbid DM | ||||||
| Yes | 24 | 19 | 43 | 2.9 (1.5,5.4) | 3.65 (1.7,7.9) | 0.001** |
| No | 155 | 357 | 512 | 1.0 | 1.0 | |
| Occupation | ||||||
| Gov’t employee | 94 | 13 | 107 | 1.0 | 1.0 | |
| Private | 68 | 44 | 112 | 4.6 (2.3,9.3) | 0.3 (0.07,1.2) | 0.07 |
| Merchant | 115 | 78 | 193 | 4.9 (2.5,9.5) | 2.6 (0.7,6) | 0.24 |
| Farmer | 36 | 29 | 65 | 5.1 (2.3,11.0) | 1.65 (0.5,7.5.5) | 0.43 |
| Housewife | 16 | 6 | 22 | 1.6 (0.47,5.49) | 0.5 (0.1,2.1) | 0.56 |
| Daily laborer | 43 | 13 | 56 | 2.2 (0.3,5.1) | 0.48 (0.06,3.7) | 0.48 |
| Monthly income | ||||||
| <1500 | 228 | 107 | 335 | 0.64 (0.4,1.0) | 0.27 (0.08,0.88) | 0.31 |
| 1500–3000 | 85 | 26 | 111 | 0.41 (0.2,0.75) | 0.16 (0.05,0.55) | 0.25 |
| >3000 | 63 | 46 | 109 | 1.0 | 1.0 | |
| Viral load/ul | ||||||
| <50 | 6 | 52 | 58 | 1.0 | 1.0 | |
| 50–1000 | 100 | 229 | 329 | 1.6 (1.1,2.8) | 3.8 (1.2,6.7) | 0.001** |
| >1000 | 73 | 95 | 168 | 2.04 (1.01,4.02) | 4.2 (2.1,7.3) | 0.000** |
AOR = Adjusted Odds Ratio, COR = Crude Odds Ratio, DM = Diabetes Mellitus, ** p value < 0.05.
Discussion
In the ART era, Human Immune Deficiency Virus (HIV) has evolved from a deadly sickness to a chronic condition, and people living with HIV are now living longer. However, living with HIV and using ART has its drawbacks, one of which is PN, which is disabling. Because of the consequences on PLHIV’s quality of life, HIV-associated neuropathy is identified as a cause of morbidity. 18
Damage to nerves in the peripheral nervous system, the network of connections that transmit nerve impulses from the central nervous system (brain and spinal cord) to the rest of the body, 19 causes peripheral neuropathy. The prevalence of PSN and its associated factors among HIV/AIDS patients in North West Ethiopia were investigated in this study. In this study, the prevalence of peripheral sensory neuropathy was 32.25%, 95% CI (28.28, 36.26). The extent of PSN in this study is comparable with previous research in Cameroon (28%), Kenya (36%), and Jimma, Ethiopia (34.6%). 20 The results of this study, however, were lower than those of a Rwandan study (59%), 21 Nigeria (42.5%), 22 Zimbabwe (44%), 23 and Brazil (69.4%). 24 The discrepancy could be due to differences in the peripheral neuropathy assessment tool, unexplained environmental factors, or sample size. The prevalence in our study, on the other hand, is higher than in earlier investigations in Cameroon (21%), 13 South Africa (13%), 15 and Tanzania (20.9%). 25 The significant sample size disparities, the inclusion criteria used, and the type of ART regimen provided to the study cohorts could all contribute to these differences. Comorbid diabetes is associated to peripheral sensory neuropathy in this study. This is because nerves in extremities and other regions of the body are damaged by chronically high blood sugar levels. Furthermore, while the exact etiology of nerve damage in diabetes is unknown, it is likely to be the outcome of poorly regulated blood sugar over time.
Furthermore, researchers discovered a statistically significant association between peripheral neuropathy and late-stage HIV/AIDS, male sex, and higher viral load. Peripheral sensory neuropathy was found to be considerably related with late-stage HIV/AIDS. Participants in stages IV (AOR = 6.5, 95% CI (2.5, 7.8), p = 0.018) and III (AOR = 2.8, 95% CI, (1.3,4.6) 6.5 (2.5, 7.8), p = 0.015) of the disease were approximately 6 and 3 times more likely to develop peripheral sensory neuropathy, respectively, than those in stage I.
Gender was also discovered to be one of the determinants of peripheral sensory neuropathy. Males were four times more likely than females to have peripheral neuropathy (AOR = 3.9,95% CI (1.5,9.8), p = 0.004). This is in contrast to previous research in South Africa, 26 Kenya, 27 and rural South Africa, 28 which found that women were more affected by PN development, albeit this does not necessarily imply that women are more prone to PN development. A plausible explanation for this finding is women’s more cautious health-care seeking behavior when faced with disease, which accounts for a greater detection rate in women. Furthermore, a high viral load level was related with peripheral sensory neuropathy, with participants with viral load levels greater than 1000 being four times more likely to develop the disease (AOR = 4.2,95% CI (2.1,7.3), p < 0.001).
In this study, older age was associated to peripheral sensory neuropathy. This result is consistent with earlier research from the United States, 29 Nigeria, 30 and Rwanda. As one probable explanation, aging increases vulnerability to neuronal toxicity; on the other hand, the HIV population’s median age is rapidly increasing due to continually improved management of infected persons in Sub-Sahara Africa, and the prevalence of PN too is rapidly increasing. This can also be explained by the fact that peripheral nerves are known to become more sensitive with age as a result of continual metabolic stress and toxic substance exposure due to their length and size.
More research with a big sample size representative of clients at a national level, as well as advanced investigation technologies, will be required to fill in the gaps concerning the reason for the correlation of distinct characteristics with sensory neuropathy in this study.
Limitation of the Study
The study addresses the magnitude of peripheral sensory neuropathy among HIV/AIDS clients in North west Ethiopia which had not been studied before in the study area. Various elements that will have a role in the development of peripheral sensory neuropathy are also identified in the study. Despite this, the results of this study had the following limitations: first, the design was cross sectional in nature. Second, it was good if additional tool was used to assess motor peripheral neuropathy.
Conclusion
In this study, 32.25% of HIV/AIDS clients attending at South Gondar public health institutions ART clinic had been found to have a risk for peripheral sensory neuropathy. The independent predictors of peripheral sensory neuropathy among HIV/AIDS clients were advanced age, plasma viral load, sex, and clinical stage of the disease. This study was conducted with the goal of providing information on the severity of peripheral sensory neuropathy and associated factors among HIV/AIDS patients to aid in the development of diagnosis and management strategies, with a special focus on risk factor prevention. Health practitioners should implement and apply peripheral sensory neuropathy screening protocols on a regular basis to check peripheral sensory neuropathy in all HIV/AIDS patients, with a special focus on those in the late stages of the disease, the elderly, and those with a high plasma viral load.
Ethical consideration
This research was carried out in accordance with the Declaration of Helsinki. Ethical approval was acquired from the College of Health Sciences, Debre Tabor University’s Ethical Review Committee (Ref No. CHS/3231/2013). Following the university’s approval, the study’s aims were communicated to the study’s settings via a support letter from Debre Tabor University. The goal of the study was then explained to each participant, and his or her right to refuse to participate was respected. This was recorded using a unique code that was preserved for each participant. Confidentiality and privacy were guaranteed. Clients' informed consent (written) was obtained before data were collected.
Acknowledgments
Debre Tabor University provided us with funds, which we are quite grateful for. Last but not least, we, the authors, acknowledge the study participants.
Footnotes
Authors’ Contributions: GYY,ATM,GA,WE&SA conceptualized the project, gathered data, evaluated it, interpreted the findings, and wrote the manuscript for publication. BDM, FTD, TM, and WD, designed the study and coordinated the data collection. GW, ES, BK, AA, and WA managed to design the study, supervise the research project, and read the very first version of the manuscript. EA and TJ were involved in the study’s conception as well as its analysis. The paper was reviewed and approved for publication by all authors.
Declaration Conflicts of Interest: There are no conflicts of interest between the authors regarding the publishing of this manuscript.
Funding: The research was funded by Debre Tabor University
Data Availability: The authors confirm that all data used to justify their findings are freely available. The manuscript contains all relevant information.
ORCID iD
Getachew Y Yitbarek https://orcid.org/0000-0001-9823-9981
References
- 1.Robertson K, Fuchs D, Sinclair E. Peripheral Neuropathy in Primary HIV Infection Associates with Systemic and CNS Immune Activation. J Acquir Immune Defic Syndr 2015; 66(3): 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cettomai D, Kwasa JK, Birbeck GL, Price RW, Cohen CR, Bukusi EA, Kendi C, Meyer AC. Screening for HIV-Associated Peripheral Neuropathy in Resource-Limited Settings. Muscle & Nerve 2014; 48(4): 516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pardo CA, Mcarthur JC, Griffin JW. HIV neuropathy: Insights in the pathology of HIV peripheral nerve disease. J Peripher Nervous Syst 2001; 6: 21–27. [DOI] [PubMed] [Google Scholar]
- 4.Yoshioka M, Shapshak P, Srivastava AK, Stewart R. V., Nelson S. J., Bradley W. G., Berger J. R., Rhodes RH, Sun NCJ, Nakamura S. Expression of HIV-1 and interleukin-6 in lumbosacral dorsal root ganglia of patients with AIDS. Neurology 1994; 44: 1120–1120. [DOI] [PubMed] [Google Scholar]
- 5.Nagano I, Shapshak P, Yoshiok M, Xin K, Nakamura S, Bradley WG. Increased NADPH-diaphorase reactivity and cytokine expression in dorsal root ganglia in acquired immunodeficiency syndrome. J Neurol Sci 1996; 136: 117–128. [DOI] [PubMed] [Google Scholar]
- 6.Phillips TJC, Brown M, Ramirez JD, Perkins J., Woldeamanuel YW, Williams ACdC, Orengo C, Bennett DLH, Bodi I, Cox S, Maier C, Krumova EK, Rice ASC. Sensory, psychological, and metabolic dysfunction in HIV-associated peripheral neuropathy: A cross-sectional deep profiling study. Pain 2014; 155(9): 1846–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta SA, Mendillo M, Laverty M, Holzman R, Valentine F, Ahmed A, Sivapalasingam S, Kariuki BW, Said S, Omasete F. Implementation of a Validated Peripheral Neuropathy Screening Tool in Patients Receiving Antiretroviral Therapy in Mombasa, Kenya. Am J Trop Med Hyg 2010; 83(3): 565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smyth K, Affandi JS, Mcarthur JC, Mijch AM, Watson K, Costello K. Prevalence of and Risk Factors for HIV-Associated Neuropathy in Melbourne, 367–373. [DOI] [PubMed]
- 9.Estiasari R, Sp SK, Imran D, Sp SK. Early detection of peripheral neuropathy using stimulated skin wrinkling test in human immunode fi ciency virus infected patients. 2018:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu K, Bosch RJ, Mcarthur JC, Simpson DM. Peripheral neuropathy in HIV : prevalence and risk factors. AIDS 2012; 25(7): 919–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adem KS, Gebremeskel BF. Epidemiology and Factors Associated with Peripheral Neuropathy Among HIV Infected Patients in Gondar , Ethiopia : A Cross-Sectional Study, 2019, pp. 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manuscript A. Peripheral Neuropathy – Clinical and Electrophysiological Considerations. Neuroimaging Clin N Am 2015; 24(1): 49–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luma HN, Tchaleu BC, Doualla MS, Temfack E, Sopouassi VN, Mapoure YN, Djientcheu VD. HIV-associated sensory neuropathy in HIV-1 infected patients at the Douala General Hospital in Cameroon: a cross-sectional study. AIDS Research Therapy 2012; 9: 35–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson-papp J, Schütz SG. HIV-related Neuropathy : Current Perspectives, 2013, pp. 243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saylor D, Nakigozi G, Nakasujja N, Robertson K., Gray R. H., Wawer M. J., Sacktor N. Peripheral neuropathy in HIV-infected and uninfected patients in Rakai, Uganda. Neurology 2017; 89(5): 485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.SJS AD. Assessment of the prevalence of distal symmetrical polyneuropathy and its risk factors among HAART-treated and untreated HIV infected individuals vol. 48, no. 2. Ethiop Med J 2010; 48(2): 8585–9393. [PubMed] [Google Scholar]
- 17.Cherry CL, Wesselingh SL, Lal L, Mcarthur JC. Evaluation of a clinical screening tool for HIV-associated sensory neuropathies. Neurology 2005; 65(4): 1778–1781. [DOI] [PubMed] [Google Scholar]
- 18.Tumusiime DK. Prevalence of Peripheral Neuropathy and Effects of Physiotherapeutic Exercises on Peripheral Neuropathy in People Living With Hiv on Antiretroviral Therapy in Rwanda. BMC Public Health 2014; 14(1): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Sousa EM. AVALIAÇÃO DA RESPOSTA IMUNOLÓGICA AGUDA DE CAMUNDONGOS C57BL/6 E BALB/c FRENTE À INFECÇÃO POR Mycobacterium massiliense. Annu Rev Neurosci 2009; 32: 1–32, [Internet]Available from:, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2768555/pdf/nihms-110677.pdf.19400724 [Google Scholar]
- 20.Shurie JS, Deribew A. Assessment of the prevalence of distal symmetrical polyneuropathy and its risk factors among HAART-treated and untreated HIV infected individuals. Ethiopian Medical Journal 2010; 48(April): 85–93. [PubMed] [Google Scholar]
- 21.Tumusiime DK, Venter F, Musenge E, Stewart A. Prevalence of peripheral neuropathy and its associated demographic and health status characteristics, among people on antiretroviral therapy in Rwanda. BMC Public Health 2014; 14(1): 1306–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ekenze OS, Nwosu CM, Ogunniyi A. Frequency and risk factors for distal sensory polyneuropathy in HIV infection in a developing country. Int J STD AIDS 2014; 25(3): 178–183. [DOI] [PubMed] [Google Scholar]
- 23.Parry O, Mielke J, Latif AS, Ray S, Levy LF, Siziya S. Peripheral neuropathy in individuals with HIV infection in Zimbabwe. Acta Neurologica Scand 1997; 96(4): 218–222. [DOI] [PubMed] [Google Scholar]
- 24.Zanetti C, Manzano GM, Gabbai AA. The frequency of peripheral neuropathy in a group of HIV positive patients in Brazil. Arquivos de neuro-psiquiatria 2004; 62(2): 253–256. [DOI] [PubMed] [Google Scholar]
- 25.Mullin S., Temu A., Kalluvya S., Grant A., Manji H. High prevalence of distal sensory polyneuropathy in antiretroviral-treated and untreated people with HIV in Tanzania. Trop Med Int Health 2011; 16(10): 1291–1296. [DOI] [PubMed] [Google Scholar]
- 26.Collins PY, Berkman A, Mestry K, Pillai A. HIV prevalence among men and women admitted to a South African public psychiatric hospital. AIDS Care 2009; 21(1): 863–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimanga DO, Ogola S, Umuro M, Ng'ang'a A, Kimondo L, Murithi P, Muttunga J, Waruiru W, Mohammed I, Sharrif S, De Cock KM, Kim AA. Prevalence and incidence of HIV infection, trends, and risk factors among persons aged 15-64 years in Kenya: results from a nationally representative study. J Acquired Immune Deficiency Syndromes (1999) 2014; 66 (Supplement 1): S13–S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gómez-Olivé FX, Angotti N, Houle B, Klipstein-Grobusch K, Kabudula C, Menken J, Williams J, Tollman S, Clark SJ. Prevalence of HIV among those 15 and older in rural South Africa. AIDS Care 2013; 25(9): 1122–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evans SR, Clifford DB, Kitch DW, Goodkin K, Schifitto G, McArthur JC, Simpson DM. Simplification of the research diagnosis of HIV-associated sensory neuropathy. HIV Clin Trials 2008; 9(6): 434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwelunmor J, Ezeanolue EE, Airhihenbuwa CO, Obiefune MC, Ezeanolue CO, Ogedegbe GG. Socio-cultural factors influencing the prevention of mother-to-child transmission of HIV in Nigeria: A synthesis of the literature. BMC Public Health 2014; 14(1): 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]