Abstract
Voriconazole (VRC) was evaluated in an immunosuppressed-guinea pig model of invasive aspergillosis. VRC was more effective than amphotericin B or similar doses of itraconazole in the clearance of Aspergillus from tissues. VRC treatment regimens improved survival and significantly reduced tissue colony counts compared with those of controls.
Among immunocompromised patients, morbidity and mortality due to invasive aspergillosis remain high (21). Limited effective therapeutic choices are available. Amphotericin B and itraconazole, each with shortcomings, are the antifungal agents most often used against Aspergillus spp. (10); however, successful treatment is seen with only 34% of patients (21). The drug of choice for the treatment of invasive aspergillosis is amphotericin B, but drug toxicity persists as a problem with amphotericin B, and therapy may be ineffective, particularly in immunosuppressed patients (10). Itraconazole, while less toxic than amphotericin B, has variable bioavailability in certain patient groups, and recently, itraconazole resistance in Aspergillus fumigatus has been described (6, 14). One approach to improving antifungal therapies has been the development of the newer azoles (7, 9). These agents offer several potential advantages over amphotericin B, including oral therapy, reduced toxicity, and a broad therapeutic index (8). One of the newer azoles is voriconazole (VRC), a triazole compound with a broad spectrum of antifungal activity that is tolerated well following oral administration (2, 11, 12).
We used a guinea pig model of invasive aspergillosis to evaluate the efficacy of antifungal therapy in this disease (18–20). In this lethal model, guinea pigs are made leukopenic and are further immunocompromised with steroid therapy. Extensive infection develops in liver, kidney, lung, and brain, which is similar to clinical manifestations of disseminated invasive aspergillosis (16, 17). We assessed the activity of VRC in experimental invasive aspergillosis and compared its efficacy to those of amphotericin B and itraconazole. In addition, we compared the in vitro activity of VRC with those of amphotericin B and itraconazole against several Aspergillus spp. isolates.
Male Hartley guinea pigs (0.5 kg) were immunosuppressed with daily subcutaneous triamcinolone acetonide (20 mg/kg of body weight; Steris Laboratories, Inc., Phoenix, Ariz.) beginning 4 days prior to challenge and made neutropenic with a single intraperitoneal dose of cyclophosphamide (300 mg/kg; Pharmacia Inc., Kalamazoo, Mich.). With this temporarily immunosuppressive regimen, guinea pigs have total white blood cell counts reduced to <1,000 mm3, with immunosuppression lasting through day 7. Twenty-four hours after induction of neutropenia, five groups of 8 to 10 guinea pigs were sedated with ketamine HCl (44 mg/kg; Fort Dodge Laboratories Inc., Fort Dodge, Iowa), atropine (0.04 mg/kg; Elkin-Sinn, Inc., Cherry Hill, N.J.), and xylazine (5 mg/kg; Agriculture Division, Bayer Corporation, Shawnee Mission, Kans.) and challenged intravenously through the saphenous vein with a lethal inoculum of 106 A. fumigatus conidia. Each group contained at least one untreated control guinea pig, for which the lethal challenge was fatal within 6 days of challenge, with a mean survival time of 4.9 ± 0.4 days (range, 3 to 6 days) after challenge. Ceftazidime (100 mg/kg; SmithKline Beecham Pharmaceuticals, Philadelphia, Pa.) was administered intramuscularly daily beginning on the day of challenge to prevent intercurrent bacterial infection.
A. fumigatus isolate P171, a clinical isolate which had been used in previous animal studies, was grown on Sabouraud-dextrose (Sab-Dex) slants at 37°C for 24 h. For injection into the guinea pigs, conidia were harvested by a sterile saline wash of the slant surface, with conidia being dislodged by gentle rubbing with a sterile glass rod. The resultant conidial suspension was adjusted to the desired concentration of 106 conidia/ml by hemacytometer counting, which was verified by duplicate serial plating on Sab-Dex plates for colony counts.
Antifungal therapy using amphotericin B (Fungizone; Bristol-Myers Squibb Co., Princeton, N.J.), VRC (Pfizer, Inc., Groton, Conn.), or itraconazole cyclodextrin solution (Janssen Research Foundation, Beerse, Belgium) was begun 24 h after challenge with A. fumigatus conidia and continued for 5 days. Amphotericin B was diluted with 5% dextrose in sterile water at a ratio of 1.25 mg/ml of diluent and was given intraperitoneally at a dose of 1.25 mg/kg/day. VRC was suspended in polyethylene glycol 200 (Sigma Chemical, St. Louis, Mo.) and administered orally twice a day as a 10-mg/ml suspension at 5 or 10 mg/kg/day. Itraconazole (10 mg/ml) was also administered orally twice a day at 5 or 10 mg/kg/day.
Organ cultures and histopathology were performed at the time of sacrifice (96 h after completion of therapy in the treated guinea pigs). Guinea pigs were sacrificed by terminal exsanguination after being anesthetized with 44 mg of ketamine HCl per kg and 10 mg of xylazine per kg. Organs (brain, lung, liver, and kidneys) were removed aseptically following sacrifice and were cultured using two different techniques to determine the degree of infection with A. fumigatus. Organs were considered positive when three or more colonies of A. fumigatus were present on minced tissues planted directly on Sab-Dex plates (Becton Dickinson and Company, Cockeysville, Md.) or when semiquantitative cultures of tissue homogenates contained over 30 CFU/g of tissue (18). Tissue burdens of Aspergillus were evaluated with semiquantitative cultures in which 30 to 20,000 CFU could be detected per g of tissue (9). Samples of each organ were finely chopped (manually), weighed, diluted 1:10 (wt/vol) with sterile saline, and homogenized for 25 s with an electric tissue homogenizer (IKA-Works, Inc., Cincinnati, Ohio). Duplicate 0.1- and 1.0-ml samples of the organ homogenate were plated on Sab-Dex and incubated at 37°C for 48 h, and colonies were counted. In combination, these two methods detected A. fumigatus at 3 to 20,000 CFU/g of tissue.
Additionally, broth macrodilution MICs and minimal lethal concentrations (MLCs) of amphotericin B, itraconazole, and VRC were determined for 28 clinical isolates of Aspergillus spp. according to National Committee for Clinical Laboratory Standards (NCCLS) guidelines (13). Included in this study were A. fumigatus (16 isolates), A. niger (5 isolates), A. flavus (5 isolates), and A. terreus (2 isolates). Testing was performed by the Fungus Testing Laboratory, University of Texas Health Science Center at San Antonio.
The Fisher exact test and the Wilcoxon rank sum test were used where appropriate. Statistical significance was defined as a P of <0.05, after adjustment for multiple-dose comparisons. Specifically, 15 drug group comparisons were made for each organ evaluated so that the level of significance was a P of <0.003.
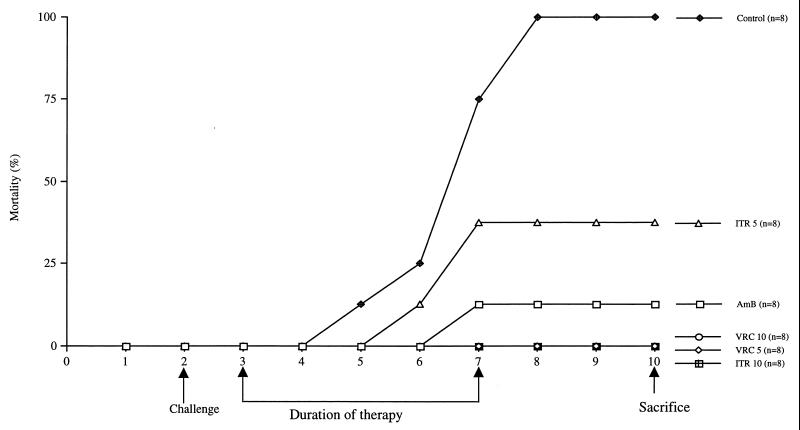
In this series of experiments, antifungal therapy with amphotericin B, itraconazole, and VRC begun 24 h after lethal A. fumigatus challenge improved survival compared to that of untreated infected controls as shown in Fig. 1. By the sixth day following challenge, guinea pigs receiving VRC at either 5 or 10 mg/kg/day or itraconazole at 10 mg/kg/day showed increases in their survival rates over those of guinea pigs treated with either amphotericin B or 5 mg of itraconazole and that of controls; death occurred in all eight control guinea pigs (mean survival of 4.9 ± 0.4 days), one of eight (12.5%) treated with amphotericin B (mean survival of 7.6 ± 0.4 days), and three of eight (37.5%) treated with 5 mg of itraconazole (mean survival of 6.8 ± 0.6 days). All guinea pigs receiving therapy with VRC at either 5 or 10 mg/kg/day or itraconazole at 10 mg/kg/day survived to the eighth day after challenge. In this model of lethal infection, all antifungal treatment regimens used in these experiments increased the mean number of days of survival versus that of control guinea pigs. VRC therapy at either 5 or 10 mg/kg/day also prolonged survival compared to that of guinea pigs treated with either itraconazole at 5 mg/kg/day or amphotericin B at 1.25 mg/kg/day. Either dose of VRC was as effective as the high dose of itraconazole in terms of reductions in mortality in these animals. Tissue or serum VRC levels were not measured in this study, and the efficacy of VRC may be related to higher concentrations of drug. Pharmacokinetic study results vary according to species used in animal models of invasive aspergillosis. Guinea pigs have been shown to approximate humans with respect to the rate of metabolism of VRC, which enhances the utility of this model (12).
FIG. 1.
Cumulative mortality of guinea pigs treated with VRC, itraconazole (ITR), and amphotericin B (AmB). Guinea pigs were challenged on day 2. Controls received no antifungal therapy. Guinea pigs were treated with amphotericin B at 1.25 mg/kg/day, with itraconazole at 5 or 10 mg/kg/day, or with VRC at 5 or 10 mg/kg/day, initiated 24 h after challenge, daily for 5 days. Guinea pigs were sacrificed 96 h after the end of treatment.
Semiquantitative results of organ cultures of liver, lung, kidney, and brain are shown in Table 1. In all untreated control animals, these organs were extensively infected. VRC at 5 and 10 mg reduced the tissue burden of Aspergillus in brain, kidney, and liver 1,000-fold compared with that in controls and also showed an up to 10-fold improvement over results of the other drug therapies used. In lung tissue, reductions in colony counts were obtained with 10 mg of VRC, 10 mg of itraconazole, and amphotericin B relative to colony counts of controls (P < 0.003), yet neither 5 mg of VRC nor 5 mg of itraconazole produced counts that differed from those of controls in this tissue.
TABLE 1.
Semiquantitative results of organ cultures of guinea pigs treated with antifungal therapy begun 24 h after challenge and sacrificed 96 h after completion of therapy
| Treatment groupd (no. of animals) [mg/kg/day] | Colony count (mean log10 CFU/g of tissue) ± SE (no. of positive cultures/no. of guinea pigs cultured)
|
|||
|---|---|---|---|---|
| Liver | Lung | Kidney | Brain | |
| Control (8) | 3.44 ± 0.4 (8/8) | 1.00 ± 0.2 (8/8) | 3.35 ± 0.2 (8/8) | 3.11 ± 0.2 (8/8) |
| VRC [10] (8) | 0.18 ± 0.1ab (3/8) | 0.18 ± 0.2ab (2/8)c | 0.26 ± 0.1ab (6/8) | 0.09 ± 0.1ab (2/8)c |
| VRC [5] (8) | 0.16 ± 0.1ab (3/8) | 0.51 ± 0.1 (6/8) | 0.38 ± 0.1a (6/8) | 0.25 ± 0.1a (3/8) |
| ITR [10] (8) | 0.65 ± 0.4a (6/8) | 0.11 ± 0.1a (5/8) | 0.85 ± 0.3a (7/8) | 0.30 ± 0.2a (3/8) |
| ITR [5] (8) | 1.36 ± 0.3a (8/8) | 0.73 ± 0.2 (8/8) | 1.62 ± 0.4a (8/8) | 1.09 ± 0.4a (6/8) |
| AmB [1.25] (8) | 0.89 ± 0.5a (6/8) | 0.25 ± 0.2a (4/8) | 0.53 ± 0.4a (4/8) | 0.89 ± 0.6a (2/8)c |
P < 0.003 versus values obtained with control animals, calculated by Wilcoxon rank sum test.
P < 0.003 versus values obtained with 5 mg of itraconazole, calculated by Wilcoxon rank sum test.
P < 0.003 versus values obtained with control animals.
ITR, itraconazole; AmB, amphotericin B.
Culture results from guinea pigs treated with VRC, itraconazole, and amphotericin B are also shown in Table 1. VRC at 10 mg/kg/day and amphotericin B were more effective than the other agents examined at sterilizing brain and lung tissues compared to conditions in the brain and lung tissues of controls (P < 0.003). Additionally, VRC at 10 and 5 mg/kg/day was more effective in reducing the number of positive cultures (A. fumigatus was undetectable in five of eight cultures) in liver tissue than were amphotericin B and 5 and 10 mg of itraconazole relative to what occurred with controls. In this study, culture results from guinea pigs treated with 5 mg of itraconazole were indistinguishable from results for controls in kidney, liver, and lung tissue. The burden in tissue was reduced as much as 1,000-fold by VRC therapy, which was as effective as the maximally tolerated doses of amphotericin B at reducing the burden in tissue in this experimental model. The ability of VRC to approximate the ability of amphotericin B to reduce fungal burden in this model, coupled with the reduced mortality attributed to either dose of VRC, suggests that this compound may have clinical usefulness in invasive aspergillosis.
Antifungal susceptibility testing with amphotericin B, itraconazole, and VRC was performed according to the NCCLS M-38P broth macrodilution protocol on 28 Aspergillus spp. clinical isolates, representing four species, including one A. fumigatus isolate used to infect the guinea pigs in this model (13). All isolates examined showed susceptibility to each of the three antifungal drugs tested. The geometric mean 48-h MICs of amphotericin B, itraconazole, and VRC for 16 isolates of A. fumigatus were 0.569, 0.229, and 0.403 μg/ml, respectively. For five isolates of A. niger, the geometric mean MICs of amphotericin B, itraconazole, and VRC were 0.500, 0.435, and 0.500 μg/ml, respectively; for A. flavus (five isolates), the MICs of amphotericin B, itraconazole, and VRC were 1.1, 0.080, and 0.330 μg/ml, respectively; and for two isolates of A. terreus, the MICs of amphotericin B, itraconazole, and VRC were 1.4, 0.042, and 0.500 μg/ml, respectively. For A. fumigatus and A. niger, the geometric mean 48-h MICs of VRC approximated the MICs of amphotericin B and itraconazole, and for A. flavus and A. terreus, MICs fell between those of amphotericin B and itraconazole, indicating the excellent potency of this new drug. These results support other reports of in vitro Aspergillus spp. susceptibility to VRC (1, 3), with similar MIC ranges and geometric mean MICs.
Additionally, the geometric mean 48-h MLCs for these isolates were determined using NCCLS methodology. MLCs of amphotericin B, itraconazole, and VRC for 16 isolates of A. fumigatus were 2.1, 6.7, and 23.6 μg/ml, respectively. For five isolates of A. niger, the geometric mean MLCs of amphotericin B, itraconazole, and VRC were 1.3, 6.1, and 27.9 μg/ml, respectively; for five isolates of A. flavus, the MLCs of amphotericin B, itraconazole, and VRC were 16.0, 5.3, and 21.1 μg/ml, respectively; and for two isolates of A. terreus, the MLCs of amphotericin B, itraconazole, and VRC were 8.0, 5.7, and 22.6 μg/ml, respectively. At 24 h, fungicidal activity was seen with amphotericin B in 24 of 24 isolates, with itraconazole in 26 of 28 isolates, and with VRC in 28 of 28 isolates. At 48 h, fungicidal activity was seen with amphotericin B in 20 of 24 isolates, with itraconazole in 20 of 28 isolates, and with VRC in 28 of 28 isolates tested. In vitro fungicidal activities of amphotericin B, itraconazole, and VRC against these four Aspergillus species were demonstrated in this study. By NCCLS criteria, fungicidal activity at 24 h was documented for VRC and amphotericin B against all isolates tested and for itraconazole against all but two isolates. However, at 48 h, itraconazole lacked fungicidal activity against 8 of 28 isolates (range, 2 to >8 μg/ml), as did amphotericin B against 4 of 24 isolates (range, 1 to >16 μg/ml), in contrast to the fungicidal activity shown by VRC against all 28 isolates. However, the MLCs of VRC were high and ranged from 8 to 64 μg/ml. It should be noted that the upper limits used in testing for amphotericin B and itraconazole were according to NCCLS guidelines. While formal recommendations for testing VRC have not been established, higher levels in serum are achievable with VRC, so that the range of concentrations used in this study was chosen based on that recommended for fluconazole (2, 3). Higher MICs and MLCs of amphotericin B were noted for the two A. terreus strains tested, with good activity being found against these isolates with both VRC and itraconazole, which may indicate an important clinical niche for these compounds. With Aspergillus spp. and other filamentous fungi, reliable correlations of in vitro MIC data with in vivo clinical responses remain controversial and should be validated (7, 15).
Effective therapeutic options for invasive aspergillosis remain limited (1, 3). Amphotericin B, the standard in antifungal therapy, is often ineffective in certain patients and may cause many serious adverse events (4, 6, 10), while itraconazole, which has activity against Aspergillus, has erratic oral bioavailability and has only recently become available in an intravenous formulation (12, 14). Thus, newer antifungal drugs have been developed with activity against Aspergillus spp. VRC is a new triazole antifungal with potent activity against Aspergillus spp. that has excellent oral bioavailability and is also administered intravenously and was evaluated in this new guinea pig model of invasive aspergillosis. In this guinea pig model, VRC demonstrated significant efficacy against experimental invasive aspergillosis in a dose-response fashion. VRC at 5 mg/kg/day was more effective than similar doses of itraconazole and was as effective as amphotericin B in prolonging survival and in reducing tissue burden of the organism. VRC has potent activity against Aspergillus spp. and may be an important advance in the therapy of this disease.
Acknowledgments
This work was supported by a grant from Pfizer, Inc.
We thank the Fungus Testing Laboratory at UTHSCSA for performing antifungal susceptibility testing.
REFERENCES
- 1.Abraham O C, Manavathu E K, Cutright J L, Chandrasekar P H. In vitro susceptibilities of Aspergillus species to voriconazole, itraconazole and amphotericin B. Diagn Microbiol Infect Dis. 1999;33:7–11. doi: 10.1016/s0732-8893(98)00102-3. [DOI] [PubMed] [Google Scholar]
- 2.Barry A L, Brown S D. In vitro studies of two triazole antifungal agents (voriconazole [UK-109,496] and fluconazole) against Candida species. Antimicrob Agents Chemother. 1996;40:1948–1949. doi: 10.1128/aac.40.8.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clancy C J, Nguyen M H. In vitro efficacy and fungicidal activity of voriconazole against Aspergillus and Fusarium species. Eur J Clin Microbiol Infect Dis. 1998;17:573–575. doi: 10.1007/BF01708622. [DOI] [PubMed] [Google Scholar]
- 4.Cuenca-Estrella M, Rodríguez-Tudela J L, Mellado E, Martínez-Suárez J V, Monzón A. Comparison of the in-vitro activity of voriconazole (UK-109,496), itraconazole and amphotericin B against clinical isolates of Aspergillus fumigatus. J Antimicrob Chemother. 1998;42:531–533. doi: 10.1093/jac/42.4.531. [DOI] [PubMed] [Google Scholar]
- 5.Denning D W. Invasive aspergillosis. Clin Infect Dis. 1998;26:781–805. doi: 10.1086/513943. [DOI] [PubMed] [Google Scholar]
- 6.Denning D W, Radford S A, Oakley K L, Hall L, Johnson E M, Warnock D W. Correlation between in-vitro susceptibility testing to itraconazole and in-vivo outcome of Aspergillus fumigatus infection. J Antimicrob Chemother. 1997;40:401–414. doi: 10.1093/jac/40.3.401. [DOI] [PubMed] [Google Scholar]
- 7.Espinel-Ingroff A. Comparison of in vitro activities of the new triazole SCH56592 and the echinocandins MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J Clin Microbiol. 1998;36:2950–2956. doi: 10.1128/jcm.36.10.2950-2956.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graybill J R. New antifungal agents. Eur J Clin Microbiol Infect Dis. 1989;5:402–412. doi: 10.1007/BF01964056. [DOI] [PubMed] [Google Scholar]
- 9.Graybill J R, Kaster S R. Experimental murine aspergillosis: comparison of amphotericin B and a new polyene antifungal drug, SCH 28191. Am Rev Respir Dis. 1984;129:292–295. [PubMed] [Google Scholar]
- 10.Latgé J-P. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev. 1999;12:310–350. doi: 10.1128/cmr.12.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lozano-Chiu M, Arikan S, Paetznick V L, Anaisse E J, Rex J H. Optimizing voriconazole susceptibility testing of Candida: effects of incubation time, endpoint rule, species of Candida, and level of fluconazole susceptibility. J Clin Microbiol. 1999;37:2755–2759. doi: 10.1128/jcm.37.9.2755-2759.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin M V, Yates J, Hitchcock C A. Comparison of voriconazole (UK-109,496) and itraconazole in prevention and treatment of Aspergillus fumigatus endocarditis in guinea pigs. Antimicrob Agents Chemother. 1997;41:13–16. doi: 10.1128/aac.41.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi: proposed standard. Document M38-P. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1998. [Google Scholar]
- 14.Oakley K L, Morrissey G, Denning D W. Efficacy of SCH-56592 in a temporarily neutropenic murine model of invasive aspergillosis with an itraconazole susceptible and an itraconazole-resistant isolate of Aspergillus fumigatus. Antimicrob Agents Chemother. 1997;41:1504–1507. doi: 10.1128/aac.41.7.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Odds T C, Van Gerven F, Espinel-Ingroff A, Bartlett M S, Ghannoum M A, Lancaster M V, Pfaller M A, Rex J H, Rinaldi M G, Walsh T J. Evaluation of possible correlations between antifungal susceptibilities of filamentous fungi in vitro and antifungal treatment outcomes in animal infection models. Antimicrob Agents Chemother. 1998;42:282–288. doi: 10.1128/aac.42.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patterson T F, Fothergill A W, Rinaldi M G. The efficacy of itraconazole solution in experimental invasive aspergillosis. Antimicrob Agents Chemother. 1993;37:2307–2310. doi: 10.1128/aac.37.11.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patterson T F, George D, Ingersoll R, Miniter P, Andriole V T. The efficacy of SCH-39304 in experimental invasive aspergillosis. Antimicrob Agents Chemother. 1991;35:1985–1988. doi: 10.1128/aac.35.10.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patterson T F, Miniter P, Dijkstra J, Szoka F C, Ryan J L, Andriole V T. Treatment of experimental invasive aspergillosis with novel amphotericin B/cholesterol-sulfate complexes. J Infect Dis. 1989;159:717–724. doi: 10.1093/infdis/159.4.717. [DOI] [PubMed] [Google Scholar]
- 19.Patterson T F, Miniter P, Ryan J L, Andriole V T. Effect of immunosuppression and amphotericin B on Aspergillus antigenemia in an experimental model. J Infect Dis. 1988;158:415–422. doi: 10.1093/infdis/158.2.415. [DOI] [PubMed] [Google Scholar]
- 20.Sabetta J R, Miniter P, Andriole V T. The diagnosis of invasive aspergillosis by an enzyme-linked immunosorbent assay for circulating antigen. J Infect Dis. 1985;152:946–953. doi: 10.1093/infdis/152.5.946. [DOI] [PubMed] [Google Scholar]
- 21.Verweij P E, Mensink M, Rijs A J M M, Donnelly J P, Meis J F G, Denning D W. In vitro activities of amphotericin B, itraconazole and voriconazole against 150 clinical and environmental Aspergillus fumigatus isolates. J Antimicrob Chemother. 1998;42:389–392. doi: 10.1093/jac/42.3.389. [DOI] [PubMed] [Google Scholar]