Abstract
Pneumocystis jiroveci pneumonia is a common pathology in HIV-infected but also in uninfected immunocompromised individuals. The pandemic coronavirus disease 2019 (COVID-2019) is a new type of coronavirus disease caused by SARS-COV-2, and the chest imaging is often used as complementary tool in patients’ evaluation. The imaging finding is similar with many pulmonary pathologies. Chest computed tomography scan is gold standard imaging and shows a central and diffuse distribution, ground- glass pattern with septal thickening with “crazy paving pattern.” We reported a case of 57-year-old man patient, followed in oncology for laryngeal cancer who presented of Pneumocystis jiroveci pneumonia during his follow-up. The diagnosis is confirmed by polymerase chain reaction with bronchoalveolar lavage fluid. Other immunochemical tests can be performed but are less specific. Both curative and preventive treatment in subjects at risk remains trimethoprim-sulfamethoxazole. Corticosteroid therapy may be associated depending on the case.
Keywords: COVID-19, ground-glass, Pneumocystis jiroveci pneumonia, radiology, respiratory medicine
Introduction
Human pneumocystis is a deep mycosis secondary to a fungus, Pneumocystis jiroveci, which is responsible for interstitial lung disease. It generally occurs in a context of the deterioration of the immune status of patients, particularly the action of lymphocytes T. Pneumocystis jiroveci pneumonia (PJP) remains an opportunistic infection in HIV-infected individuals and non-HIV-infected individuals with immunosuppression, particularly in cancer patients, transplant patients, and patients undergoing immunosuppressive treatment. 1 The etiological diagnosis of respiratory infections with common ground-glass patterns remains difficult during this COVID-19 pandemic. PJP can be life-threatening. It can cause respiratory failure that can lead to death. It requires better investigation to ensure better management of these patients with COVID-19 mimicking infections. For moderate to severe PJP in people with HIV/AIDS, the short-term use of corticosteroids has decreased the incidence of death. 2
Case report
We report the case of a 57-year-old male patient, followed in oncology for 3 years for laryngeal cancer operated with a tracheotomy in place and having benefited from a complementary radio-chemotherapy for 8 weeks. There is no history of possible HIV risk or contamination.
During his follow-up, he presented episodes of fever, non-productive cough evolving intermittently for about 1 week. His condition worsened with the onset of dyspnea. He went to the emergency room for treatment. Blood test was done (hemogram, white blood cell count, and C-reactive protein (CRP)). Chest computed tomography (CT) scan was performed.
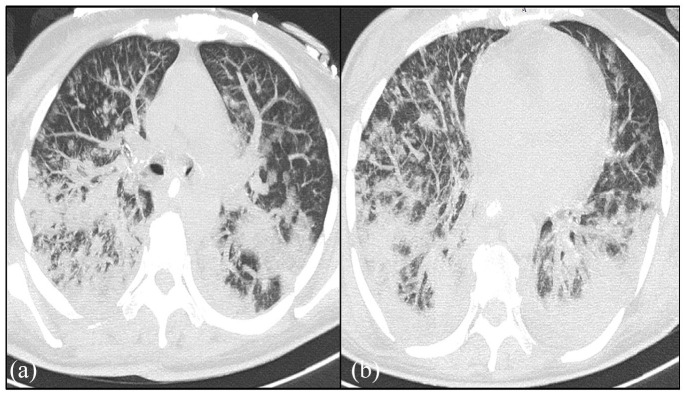
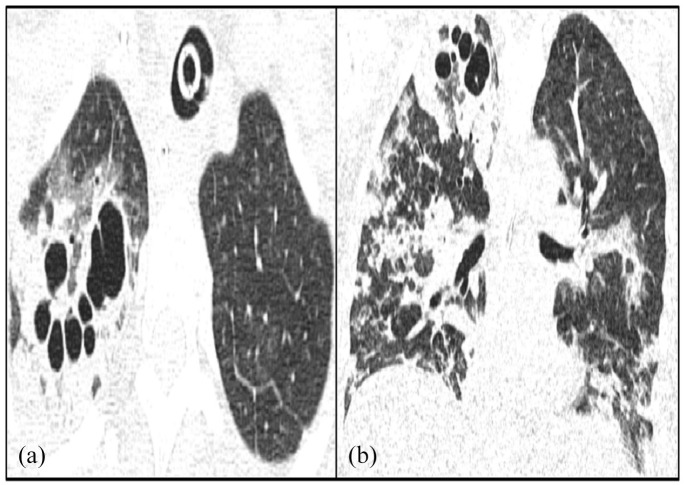
On these axial slices in the parenchymal window, reveals bilateral postero basal ground-glass images associated with peribronchovascular thickening and diffuse central and peripheral distribution are evident. It is associated with interlobular septal thickening realizing a crazy paving appearance. It is associated with pulmonary cysts with variable shape, wall thickness (Figures 1 and 2).
Figure 1.
Axial-CT image of the chest (a and b) on lung window shows bilateral septal thickening in addition to the ground-glass pattern and tree-in-bud opacities.
Figure 2.
Axial-CT (a) and coronal (b) image of the chest on lung window shows bilateral septal thickening in addition to the ground-glass pattern, tree-in-bud opacities, and right apical segment cysts.
A reverse transcription polymerase chain reaction (RT-PCR) of COVID-19 returned negative twice and a complete blood balance found a lymphopenia and a CD4+ T lymphocytes count below of 400, CRP at 60 UI. HIV test was done and returned negative.
PCR on bronchoalveolar lavage fluid confirms the presence of P. jiroveci. The diagnosis of PJP was retained. He received a treatment based on trimethoprim-sulfamethoxazole, combined with corticosteroids. The evolution was favorable with an improvement of the state, the disappearance of the dyspnea.
Discussion
PJP is an atypical pulmonary infection and the most common opportunistic infection in immunocompromised human hosts, particularly those with a deficiency in cell-mediated immunity lower than 200 CD4+. P. jiroveci lives almost exclusively in the pulmonary alveoli and contributes to inflammatory lung damage. 1 Pneumocystis was originally thought to be a member of the protozoan class but was reclassified as an ascomycetous fungus in 1988. It first appeared in Europe following World War II. 1 It can occur in patients without HIV infection (PLHIV), including immunosuppressed patients with malignant diseases, hemopathies, transplant patients, and autoimmune diseases. In Alanio et al.’s 3 Europe multicentre observational study, the number of annual cases of PJP worldwide is currently estimated at 500,000 cases per year:400,000 in PLHIV and 100,000 in non-HIV, while mortality from this condition is about 15% in PLHIV but 50% in non-HIV.
Clinically patients present with progressive dyspnea and/or non-productive cough especially in immunocompromised ones, and confirmed by bronchoalveolar lavage which has a sensitivity of 85%–90%. 3 Confirmation of the diagnosis is based on serological confirmation by PCR on bronchoalveolar lavage fluid. It can be correlated with elevated serum β-(1-3)-d-glucan (BG) levels.3,4
Chest radiographs of patients suffering from PJP are abnormal, but nonspecific they show small pneumatoceles, subpleural blebs, and fine reticular thickening with the perihilar distribution. At chest CT, the most common and specific finding is the ground-glass pattern predominantly involving perihilar or mid zones, besides of reticular opacities, septal thickening, and crazy paving. Pneumatocele, pleural effusion, and adenopathy may be present but rare.1,5 The distribution is central and diffuse with relative subpleural sparing. It is associated with some degree of consolidations, interlobular septal thickening, and “crazy paving pattern.” Thirteen percent of PJP cases present pulmonary cysts with variable shape, wall thickness. Nodules and tree-in-bud opacities are uncommon.1,4
In front of these images, certain differential diagnosis can be evoked: COVID-19 pneumonia (on chest CT, there are predominance ground-glass opacities with subpleural distribution in one, most commonly, both lungs. In late phase, most patients have confluent lesions with mixed consolidation. PCR confirms the diagnosis), non-specific interstitial pneumonia (bilateral ground-glass opacities, bilateral reticular opacities, basal predominance, diffuse or subpleural, honeycombing minimal, and with or without traction bronchiectasis), infectious pulmonary granulomatous disease, and pulmonary embolism (diagnosis confirms by CT pulmonary angiography). PJP diagnosis is confirmed by PCR with bronchoalveolar lavage fluid.3,4
Most patients with acute infection are treated with trimethoprim-sulfamethoxazole, combined with corticosteroids in patients with moderate to severe infections. The same agent may be used as prophylaxis. Survival is good (50%–95%), although relapses are common.5,6
Preventive therapy is recommended for the following: 2
People with HIV/AIDS who have CD4 counts below 200 cells/mL or 200 cells/cubic mL.
Bone narrow transplant recipients.
Organ transplant recipients.
People who take long-term, high-dose corticosteroids.
People who have had previous episodes of this infection.
People who take long-term immunomodulatory drugs.
Conclusion
PJP is a common pathology in HIV-infected but also in uninfected immunocompromised individuals. It must be known to clinicians in order to make an early diagnosis.
Chest CT is gold standard imaging and shows a central and diffuse distribution, ground glass pattern with septal thickening with “crazy paving pattern.” The diagnosis is confirmed by PCR with bronchoalveolar lavage fluid. Other immunochemical tests can be performed but are less specific.
Both curative and preventive treatment in subjects at risk remains trimethoprim-sulfamethoxazole. Corticosteroid therapy may be associated depending on the case.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
ORCID iDs: Romeo Thierry Yehouenou Tessi  https://orcid.org/0000-0002-9037-879X
https://orcid.org/0000-0002-9037-879X
Behyamet Onka  https://orcid.org/0000-0002-6204-7977
https://orcid.org/0000-0002-6204-7977
References
- 1. Kanne JP, Yandow DR, Meyer CA. Pneumocystis jiroveci pneumonia: high-resolution CT findings in patients with and without HIV infection. Am J Roentgenol 2012; 198(6): W555–W561. [DOI] [PubMed] [Google Scholar]
- 2. Kovacs JA. Pneumocystis pneumonia (chapter 321). In: Goldman L, Schafer Al. (eds) Goldman-Cecil medicine. 26th ed. Philadelphia, PA: Elsevier, 2020: 2247–2252. [Google Scholar]
- 3. Alanio A, Gits-Muselli M, Guigue N, et al. Diversity of Pneumocystis jiroveci across Europe: a multicentre observational study. EBioMedicine 2017; 22: 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hochhegger B, Zanon M, Altmayer S, et al. COVID-19 mimics on chest CT: a pictorial review and radiologic guide. Br J Radiol 2021; 94(1118): 20200703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ghosh AK. Mayo Clinic internal medicine review. 8th ed. Florence, KY: Informa HealthCare, 2008. [Google Scholar]
- 6. Maffessanti M, Polverosi R, Dalpiaz G, et al. Diffuse lung diseases, clinical features, pathology, HRCT. Cham: Springer Verlag, 2006. [Google Scholar]