Abstract
Climate and weather directly impact plant phenology, affecting airborne pollen. The objective of this systematic review is to examine the impacts of meteorological variables on airborne pollen concentrations and pollen season timing. Using PRISMA methodology, we reviewed literature that assessed whether there was a relationship between local temperature and precipitation and measured airborne pollen. The search strategy included terms related to pollen, trends or measurements, and season timing. For inclusion, studies must have conducted a correlation analysis of at least 5 years of airborne pollen data to local meteorological data and report quantitative results. Data from peer-reviewed articles were extracted on the correlations between seven pollen indicators (main pollen season start date, end date, peak date, and length, annual pollen integral, average daily pollen concentration, and peak pollen concentration), and two meteorological variables (temperature and precipitation). Ninety-three articles were included in the analysis out of 9,679 articles screened. Overall, warmer temperatures correlated with earlier and longer pollen seasons and higher pollen concentrations. Precipitation had varying effects on pollen concentration and pollen season timing indicators. Increased precipitation may have a short-term effect causing low pollen concentrations potentially due to “wash out” effect. Long-term effects of precipitation varied for trees and weeds and had a positive correlation with grass pollen levels. With increases in temperature due to climate change, pollen seasons for some taxa in some regions may start earlier, last longer, and be more intense, which may be associated with adverse health impacts, as pollen exposure has well-known health effects in sensitized individuals.
Keywords: Pollen, Climate, Aeroallergens, Temperature, Precipitation, Meteorology
Introduction
Exposure to pollen has increasingly been identified as a potential health impact related to climate change (USGCRP 2016). Studies indicate that exposure to airborne pollen may exacerbate asthma (Cirera et al. 2012; Galán et al. 2010), and is associated with chronic obstructive pulmonary disease (COPD) (Hanigan and Johnston 2007), respiratory tract infections (Hanigan and Johnston 2007), allergic rhinitis (Grammer and Greenberger 2009; Kim et al. 2011), and some dermatological conditions (Carracedo-Martinez et al. 2008; Díaz et al. 2007; Kim et al. 2011; Mesa et al. 2005; Orazzo et al. 2009). An estimated 19.2 million people are diagnosed with allergic rhinitis in the USA, about 7.7% of the total population, with 5.2 million being under the age of 18 (Black and Benson 2018; Villarroel et al. 2018). Globally, allergic respiratory disease increased over the second half of the last century, and may still be increasing in some populations (Leth-Møller et al. 2020; Wallace et al. 2008; Zhang and Zhang, 2019). Higher pollen concentrations and longer pollen seasons can increase the incidence of allergic sensitization and incidence of asthma exacerbations, diminishing work productivity and school days attended (Bastl et al. 2018; Fann et al. 2016; Luber et al. 2014). Vulnerable groups such as the young (Cakmak et al. 2002), uninsured (Bunyavanich et al. 2003), and people with pre-existing cardiopulmonary conditions (D’Amato et al. 2014; Rice et al. 2014) are disproportionately impacted by pollen. Additionally, pollen has increased health impacts in urban areas with high concentrations of air pollution (Beck et al. 2013; D’Amato et al. 2001; Zhao et al. 2016). Recent studies have tied shifts in some of these health effects directly to climate factors (Silverberg et al. 2015).
Temperature and precipitation, in both the short term and long term, have direct impacts on plant phenology with the potential to impact airborne pollen concentrations and distribution (García-Mozo et al. 2015; Hajkova et al. 2015; Menzel et al. 2006; Walther et al. 2002). Under a changing climate, more frost-free days and warmer temperatures contribute to changes in plant phenology (Luber et al. 2014; Stennett and Beggs 2004). Global increases in atmospheric CO2 concentration, temperature, and changes in precipitation can result in shifts in the pollen season and greater airborne concentrations of pollen (Bernard et al. 2001; D’Amato et al. 2014), an increase in allergenicity (Rice et al. 2014), longer periods of exposure (D’Amato et al. 2014; Rice et al. 2014), exposure to new pollen types (Bernard et al. 2001; D’Amato et al. 2014), and increased exposure to pollen from more extreme weather events (D’Amato et al., 2007a; Rice et al. 2014). The impact of climate change varies across regions (USGCRP 2016). Numerous studies show local or regional increases in allergenic pollen concentrations from plants such as ragweed, oak, alder, and birch (Corden and Millington 1999; Emberlin et al. 2002; Frei and Gassner 2008; Ziska and Beggs 2012), as well as earlier and longer allergenic pollen seasons for multiple species (Ariano et al. 2010; García-Mozo et al. 2006; Ziello et al. 2012). The result is a potential increase in pollen exposure, resulting in adverse health impacts. The impact on human health can be modified by factors such as land use, access to healthcare, and the demographics and health status of the exposed population. Species type may also play an important role; for example, ragweed (Ambrosia spp.) has high allergenicity (Shah and Grammer 2012) as well as a large geographic range (D’Amato et al. 2007b; Mothes et al. 2004; White and Bernstein 2003), and is sensitive to climatic factors such as temperature and precipitation (Ghiani et al. 2016; Jato et al. 2015; Ziska et al. 2011).
Although observations in many regions have captured changes in pollen concentrations and pollen season timing, there has yet to be a systematic review of the impacts of temperature and precipitation on measured atmospheric pollen. Due to the known effect of pollen on human health, it is important to understand the extent to which changes in temperature and climate affects pollen concentrations and pollen season timing. Thus, we conducted, to our knowledge, the first systematic literature review to determine the association between temperature and precipitation and measured atmospheric pollen trends.
Methods
Systematic review and search strategy
A systematic review was conducted of peer-reviewed literature utilizing a protocol following the PRISMA methodology (Moher et al. 2009) and adapted from the Cochrane Review methodology (Higgins et al. 2016), The Guide to Community Preventive Services (Guide to Community Preventive Servces 2017), and Khan et al. (2003). The review strategy was adapted to assess whether there was an association between temperature and precipitation and airborne pollen concentrations and pollen season timing. The literature search was conducted using the electronic databases Environmental Science Collection, Scopus, and CAB Abstracts.
The search period included articles published before January 1, 2018. The search identified peer-reviewed journal articles with a title or abstract that contained a key term related to pollen within three words of a term related to trends or measurement and a term related to season timing. Table 1 outlines key terms used in the search; relevant terms from all three categories were needed for an article to be identified by the search. While the search was not limited to articles written in English, only journal articles with full-text availability in English were selected for inclusion in the analysis. The search did not exclude review/summary articles but did not include gray literature or conference abstracts.
Table 1.
Terms used for search strategy. The search identified journal articles that contained a term related to pollen within three words of a term related to measurement and season timing in either the title or abstract
| Pollen terms | Trend and measurement terms | Season timing terms |
|---|---|---|
|
| ||
| Pollen, aeroallerg*, aeroallerg* | Quantit*, producti*, increase*, release*, amount*, concentration*, elevat*, measure*, detection, trend*, count*, change*, variation*, data, cubic meter*, cubic metre*, scale, level*, index, indices, grains, records |
Season*, Phenolog*, Spring, Autumn, Fall, flowering, start, peak, onset, temperature*, climate, aerobiolog*, forecast*, pattern* |
Articles identified in the search were reviewed by two researchers and selected for inclusion if they satisfied three inclusion criteria: at least 5 years of airborne pollen data (to establish trends), analysis of the relationship between pollen and meteorological variables, and each study’s reporting of quantitative results. Inclusion criteria are detailed in Table 2. The research questions were examined and answered by pooling each study’s findings and reporting them in a table. The data were then examined to identify trends.
Table 2.
Criteria for inclusion
| Data collection | Analysis | Reporting |
|---|---|---|
|
| ||
| Study length: At least 5 years of measured pollen data Pollen data: Must use airborne pollen data (collected using Rotorod, Burkard sampler, Hirst pollen trap, etc.) |
Correlation with meteorological data: Must analyze relationship between pollen and locally/regionally relevant meteorological data (temperature, precipitation, humidity, or wind) | Quantitative results: Must report quantitative pollen or trend results |
Data extraction and tabulation
Of the articles that met the inclusion criteria, analyses within each article were disaggregated based on the pollen species or grouping (e.g., trees, weeds, grasses) and location. A single article may have data collected at several locations for a single species, or multiple species analyzed within a single location. For this reason, results are presented based on “analyses” rather than “studies” as there were often multiple analytic results within a study.
Data for each analysis were extracted based on pollen species or grouping, location, magnitude of pollen trends, and direction (positive or negative) of correlation of specific pollen indicators to temperature and precipitation, and changes in pollen and meteorological indicators across the study period. Pollen indicators and operational definitions (definitions used to extract data from articles) are outlined in Table 3. Temperature and precipitation were the most consistently assessed meteorological variables and were selected as the focus of this study, though there were a small number of studies that assessed meteorological factors such as sunlight hours, humidity, and wind effects.
Table 3.
Pollen indicators and definitions used to extract data from study population
| Pollen indicator | Operational definition |
|---|---|
|
| |
| Main pollen season (MPS) start date | First day of the main pollen season in which pollen is present in the atmosphere in significant concentrations at a location |
| Main pollen season (MPS) end date | Last day of the main pollen season in which pollen is present in the atmosphere in significant concentrations at a location |
| Main pollen season (MPS) length | Duration of time in which pollen is present in the atmosphere in significant concentrations at a location |
| Peak pollen date | Day of the main pollen season in which the pollen concentration was the highest |
| Annual pollen integral (API) | Cumulative daily pollen concentrations for that year |
| Average daily pollen concentration | Average of daily pollen concentrations for that year |
| Peak pollen concentration | The highest daily pollen concentration for that year |
Results were not assessed by time series as studies used a wide variety of lag timescales and measurements for meteorological variables. For example, temperature data used for the correlation analysis may be from the day the pollen was measured, an average of the season, or even the previous season. Temperature data across studies was also inconsistent in terms of weather maximum temperature, minimum temperature, and average temperature, or another indicator was used. Controls were not assessed, as many studies did not report controlling for factors such as day of the year or confounding weather patterns. This variation in the type of meteorological indicator made it impossible to compare lag across studies. Additionally, a majority of studies did not report regression results (e.g., a one degree temperature increase correlated with a 1 day delay in the start of pollen season); thus, we were unable to assess variations in correlations beyond direction and significance, and unable to perform a meta-analysis.
Results
Literature search results
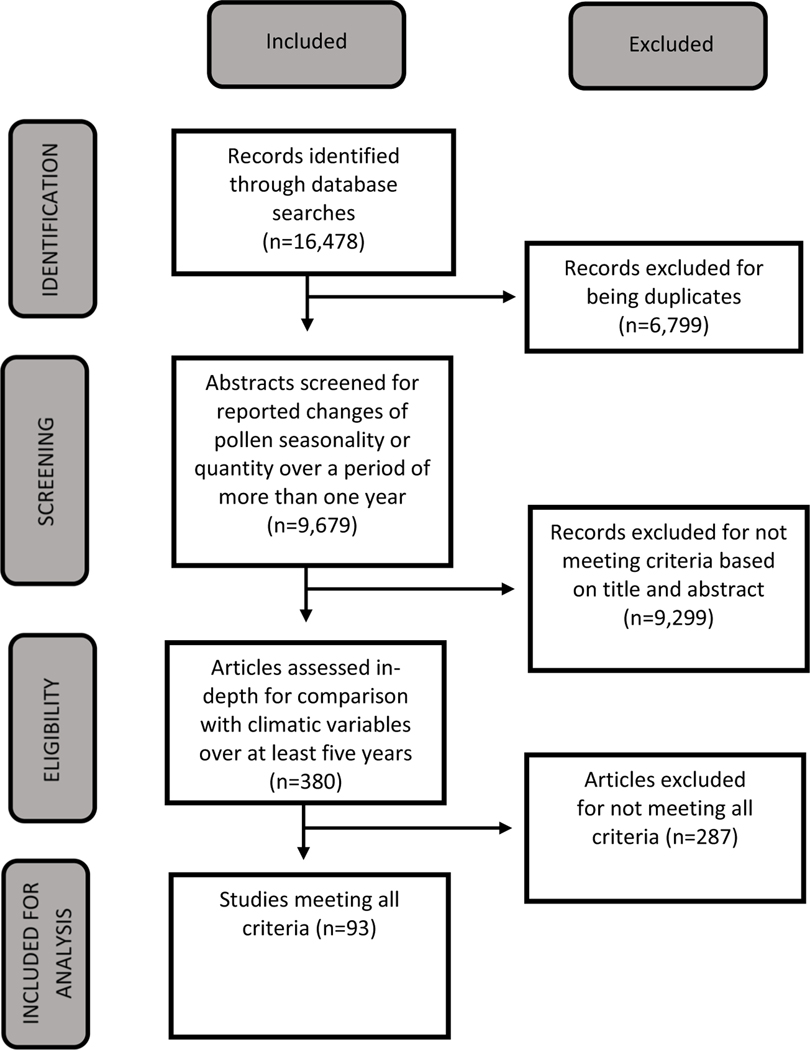
The systematic search identified 16,478 articles. Database results are outlined in the Supplementary information. After removal of duplicates, 9,679 articles remained. Most articles did not analyze pollen concentrations or pollen season timing but instead focused on unrelated topics such as bees (Bromenshenk et al. 1985), plant physiology (Koubouris et al. 2009), reconstructing historical climates (Whitlock and Bartlein 1997), and health impacts (Li et al. 2008). A total of 380 articles were identified to be screened for final inclusion based on the three criteria of interest outlined in the methods section in Table 2. An additional 287 studies were removed due to not having 5 years of pollen data, not reporting a relationship between meteorological variables and pollen, or not reporting quantitative results. A total of 93 studies met the required criteria for final inclusion (outlined in Supplementary information 1). The selection procedure, following PRISMA methodology, is summarized in Fig. 1.
Fig. 1.
PRISMA flow diagram of articles excluded and included in systematic review
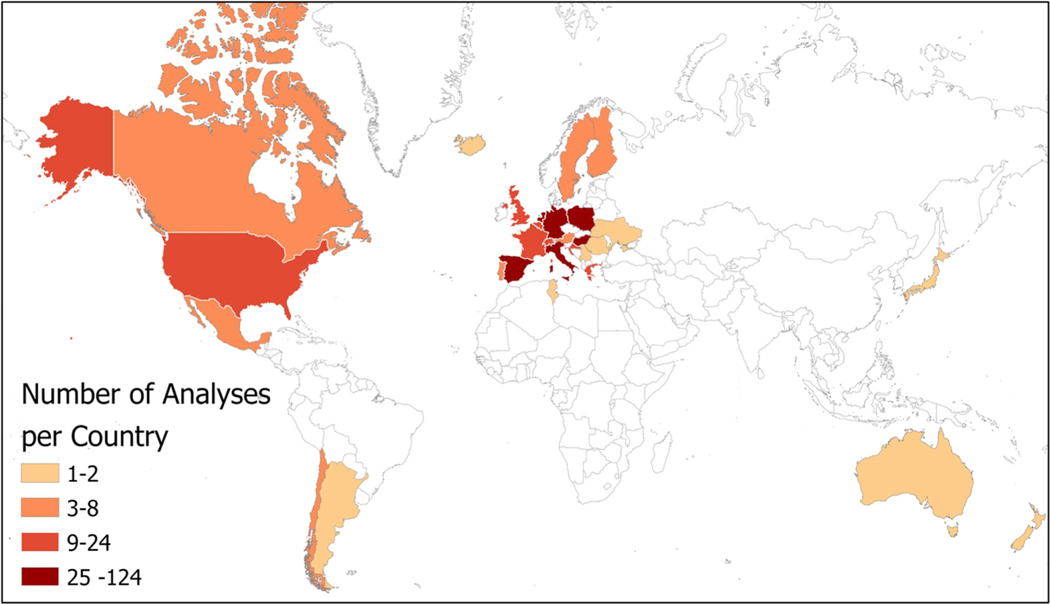
Of the included studies, there was little representation from the southern hemisphere. Studies that matched our inclusion criteria were mostly conducted in Europe (90.1% of the 93 studies) and North America (7.9%). There were two relevant studies in Asia (both in Japan) (Teranishi et al. 2006; Teranishi et al. 2000) and South America (Argentina and Chile)(Murray and Galan 2016; Toro et al. 2015), and one relevant study in Africa (Tunisia) (Aguilera et al. 2015) and Oceania (Australia and New Zealand) (Medek et al. 2016), as demonstrated in Fig. 2. Pollen measurements across the studies took place from 1954 to 2015. Datasets ranged from 5 years (Toro et al. 2015) to 46 years (Donders et al. 2014) with an average length of 18 years, and a median length of 16 years. North and South American analyses had few years of data. European sites had the longest and earliest pollen records, with studies that used pollen data starting as early as 1954 (Emberlin et al. 1997) and collected for as long as 46 years (Donders et al. 2014). The earliest datasets in North America started in 1994 (Breton et al. 2006; Zhang et al. 2014), Asia in 1983 (Teranishi et al. 2000), Africa in 1993 (Aguilera et al. 2015), and South America in 2001 (Murray and Galan 2016). Geographic clustering of trends was not assessed, as there were few analyses outside of Europe.
Fig. 2.
Map of the geographic distribution of analyses included in the study. The map uses a quantile classification with each group containing an equal number of countries
Summary of correlation between grouped pollen indicators, temperature, and precipitation
Table 4 outlines the correlation of all seven pollen indicators (definitions in Table 3) with temperature and precipitation. The correlations where more than half of analyses across all studies had the same directionality were higher temperatures correlated with an earlier main pollen season (MPS) start date; higher temperatures correlated with higher average daily pollen concentrations; higher precipitation correlated with lower average daily pollen concentrations; and higher precipitation correlated with a higher peak pollen concentration. In cases where there were not a majority of analyses demonstrating a directional correlation, results may still indicate a possible correlation. For example, 55 analyses examined the correlation between temperature and peak pollen date with 58.2% not reporting a correlation, 40% finding a negative correlation, and only 1.8% demonstrating a positive correlation. Although in this case a majority of analyses saw no correlation, the results still indicate a possible negative correlation.
Table 4.
Correlation results between pollen indicators and meteorological variables (temperature and precipitation). Number of analyses and percent of analyses that reported a significant correlation between the pollen indicator and meteorological variable are shown. Correlation results with less than five studies for comparison are grayed out to indicate the low number of analyses and limitations to the results
| ConcentrationTemperature Correlation - Percent (n) | Precipitation Correlation - Percent (n) | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Positive | Negative | No Correlation | Total (n) | Positive | Negative | No Correlation | Total (n) | |
|
| ||||||||
| Main Pollen Season Start Date | ||||||||
| Trees | 12.2%(10) | 73.2%(60) | 14.6%(12) | 82 | 41.2%(7) | 11.8%(2) | 47.1%(8) | 17 |
| Weeds | 13.3%(2) | 53.3%(8) | 33.3%(5) | 15 | 33.3%(1) | 0.0%(0) | 66.7%(2) | 3 |
| Grasses | 4.2%(1) | 37.5%(9) | 58.3%(14) | 24 | 5.3%(1) | 15.8%(3) | 78.9%(15) | 19 |
| Total | 10.7%(13) | 63.9%(78) | 25.4%(31) | 122 | 23.1%(9) | 12.8%(5) | 64.1%(25) | 39 |
| Main Pollen Season Peak Date | ||||||||
| Trees | 4.3%(1) | 52.2%(12) | 43.5%(10) | 23 | 16.7%(1) | 33.3%(2) | 50.0%(3) | 6 |
| Weeds | 0.0%(0) | 18.2%(2) | 81.8%(9) | 11 | 0.0%(0) | 0.0%(0) | 100.0%(2) | 2 |
| Grasses | 0.0%(0) | 38.1%(8) | 61.9%(13) | 21 | 5.3%(1) | 0.0%(0) | 94.7%(18) | 19 |
| Total | 1.8%(1) | 40.0%(22) | 58.2%(32) | 55 | 7.4%(2) | 7.4%(2) | 85.2%(23) | 27 |
| Main Pollen Season End Date | ||||||||
| Trees | 5.6%(1) | 50.0%(9) | 44.4%(8) | 18 | 25.0%(1) | 0.0%(0) | 75.0%(3) | 4 |
| Weeds | 27.3%(3) | 0.0%(0) | 72.7%(8) | 1 | 50.0%(1) | 0.0%(0) | 50.0%(1) | 2 |
| Grasses | 40.0%(2) | 20.0%(1) | 40.0%(2) | 5 | 0.0%(0) | 0.0%(0) | 100.0%(2) | 2 |
| Total | 17.6%(6) | 29.4%(10) | 52.9%(18) | 34 | 25.0%(2) | 0.0%(0) | 75.0%(6) | 8 |
| Main Pollen Season Length | ||||||||
| Trees | 38.9%(14) | 22.2%(8) | 38.9%(14) | 36 | 10.0%(1) | 0.0%(0) | 90.0%(9) | 10 |
| Weeds | 50.0%(7) | 7.1%(1) | 42.9%(6) | 14 | 0.0%(0) | 50.0%(1) | 50.0%(1) | 2 |
| Grasses | 7.4%(2) | 14.8%(4) | 77.8%(21) | 27 | 20.8%(5) | 0.0%(0) | 79.2%(19) | 24 |
| Total | 30.8%(24) | 16.7%(13) | 52.6%(41) | 78 | 16.7%(6) | 2.8%(1) | 80.6%(29) | 36 |
| Annual Pollen Integral (API) | ||||||||
| Trees | 44.8%(61) | 19.3%(28) | 35.9%(52) | 141 | 17.7%(11) | 21.0%(13) | 61.3%(38) | 62 |
| Weeds | 38.6%(17) | 20.5%(9) | 40.9%(18) | 3 | 18.2%(4) | 13.6%(3) | 68.2%(15) | 22 |
| Grasses | 20.0%(7) | 17.1%(6) | 62.9%(22) | 35 | 48.3%(14) | 17.2%(5) | 34.5%(10) | 29 |
| Total | 40.3%(87) | 19.0%(43) | 40.7%(92) | 222 | 26.3%(30) | 18.4%(21) | 55.3%(63) | 114 |
| Average Daily Pollen Concentration | ||||||||
| Trees | 63.8%(30) | 25.5%(12) | 10.6%(5) | 47 | 2.5%(1) | 67.5%(27) | 30.0%(12) | 40 |
| Weeds | 82.6%(19) | 17.4%(4) | 0.0%(0) | 23 | 13.6%(3) | 59.1%(13) | 27.3%(6) | 22 |
| Grasses | 50.0%(4) | 37.5%(3) | 12.5%(1) | 8 | 22.2%(2) | 66.7%(6) | 11.1%(1) | 9 |
| Total | 67.7%(65) | 22.9%(22) | 9.4%(9) | 96 | 12.4%(11) | 59.6%(53) | 28.1%(25) | 89 |
| Peak Pollen Concentration | ||||||||
| Trees | 35.3%(6) | 29.4%(5) | 35.3%(6) | 17 | 66.7%(4) | 16.7%(1) | 16.7%(1) | 6 |
| Weeds | 27.3%(3) | 9.1%(1) | 63.6%(7) | 11 | 50.0%(1) | 0.0%(0) | 50.0%(1) | 2 |
| Grasses | 5.3%(1) | 10.5%(2) | 84.2%(16) | 19 | 70.6%(12) | 0.0%(0) | 29.4%(5) | 17 |
| Total | 21.3%(10) | 17.0%(8) | 61.7%(29) | 47 | 68.0%(17) | 4.0%(1) | 28.0%(7) | 25 |
Correlations between pollen season timing and meteorological variables for trees, grass, and weeds
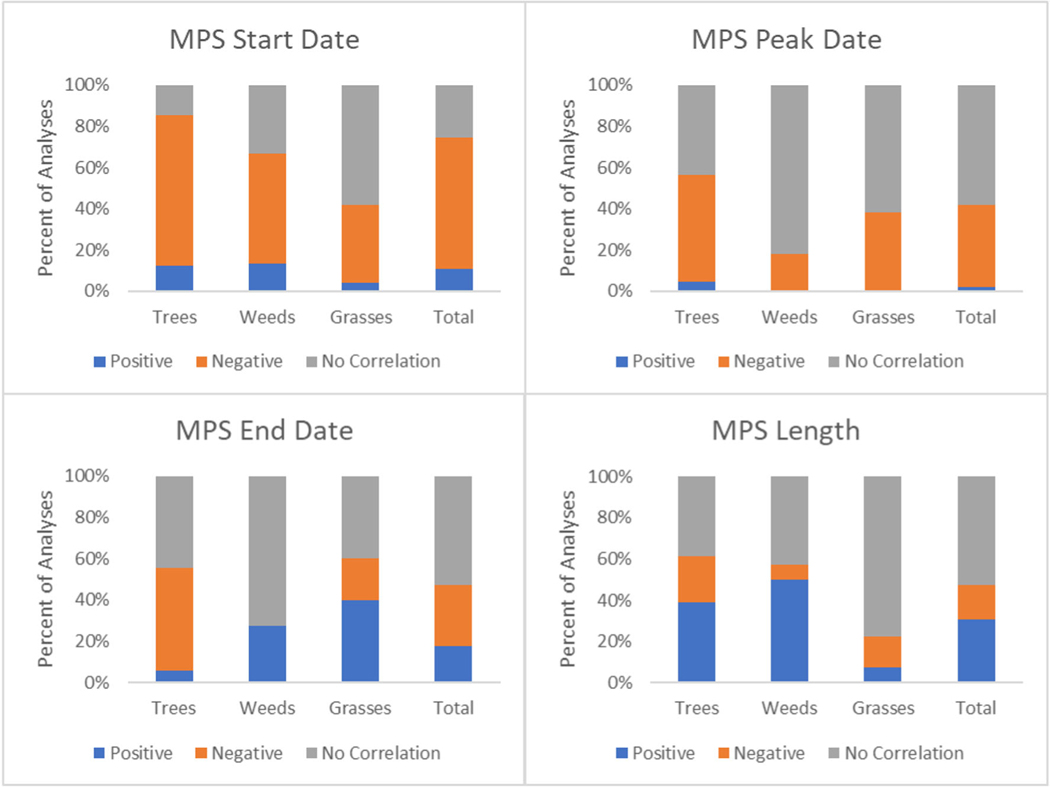
The correlation results between temperature and pollen season timing indicators are outlined in Fig. 3. The MPS start date was the season timing indicator that had the most frequent correlation with temperature. A majority of analyses found significantly negative correlations between MPS start date and temperature (78 of 122 analyses), indicating that earlier pollen seasons were correlated with warmer temperatures. This is evident among tree pollen analyses (60 of 82 analyses), and less common for grass pollen, which had a majority of analyses demonstrating no significant correlation (14 out of 24 analyses).
Fig. 3.
Correlation of temperature with pollen season timing indicators
The correlation between peak pollen date and temperature was less clear. A slight majority of analyses for trees had a negative correlation with temperature (12 of 23 analyses), indicating warmer temperatures correlated with an earlier peak pollen date. While weeds and grasses had a majority of analyses demonstrate no significant correlation, there were no analyses that demonstrated a positive correlation.
The correlation between MPS end date and temperature was less clear. Half of analyses considering tree pollen reported a negative correlation between MPS end date and temperature (9 of 18 analyses); more than half of weed analyses demonstrated no correlation and there were not significant results for grass analyses.
MPS length had a majority of analyses demonstrate no significant correlation with temperature, though 39% of tree analyses and 50% of weed analyses indicated a positive correlation, indicating warmer temperatures correlated with longer pollen seasons.
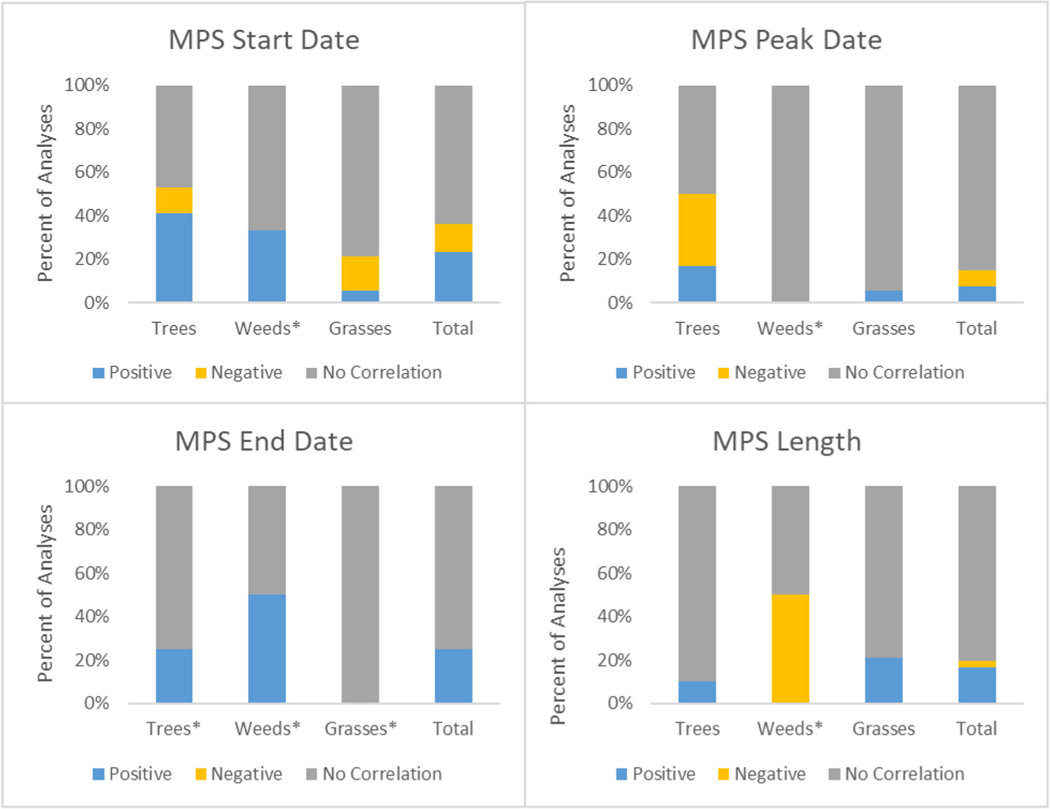
In terms of pollen season timing and precipitation, outlined in Fig. 4, a majority of analyses found no significant correlation between the various pollen season timing indicators and precipitation. However, 41% of tree analyses found a positive correlation between precipitation and MPS start date, meaning that with increases in precipitation, there is a later season start, while grass had three negative and one positive correlation between precipitation and MPS start date. Additionally, five grass analyses had a positive correlation between MPS length and precipitation and no negative, meaning that with increases in precipitation, grass pollen season was longer. Weeds have a very small number of analyses (under five) that looked at precipitation and season timing, so no conclusions can be drawn.
Fig. 4.
Correlation of precipitation with pollen season timing indicators. Asterisks (*) indicate less than five analyses and thus limited results
Correlations between airborne pollen concentrations and meteorological variables for trees, grass, and weeds
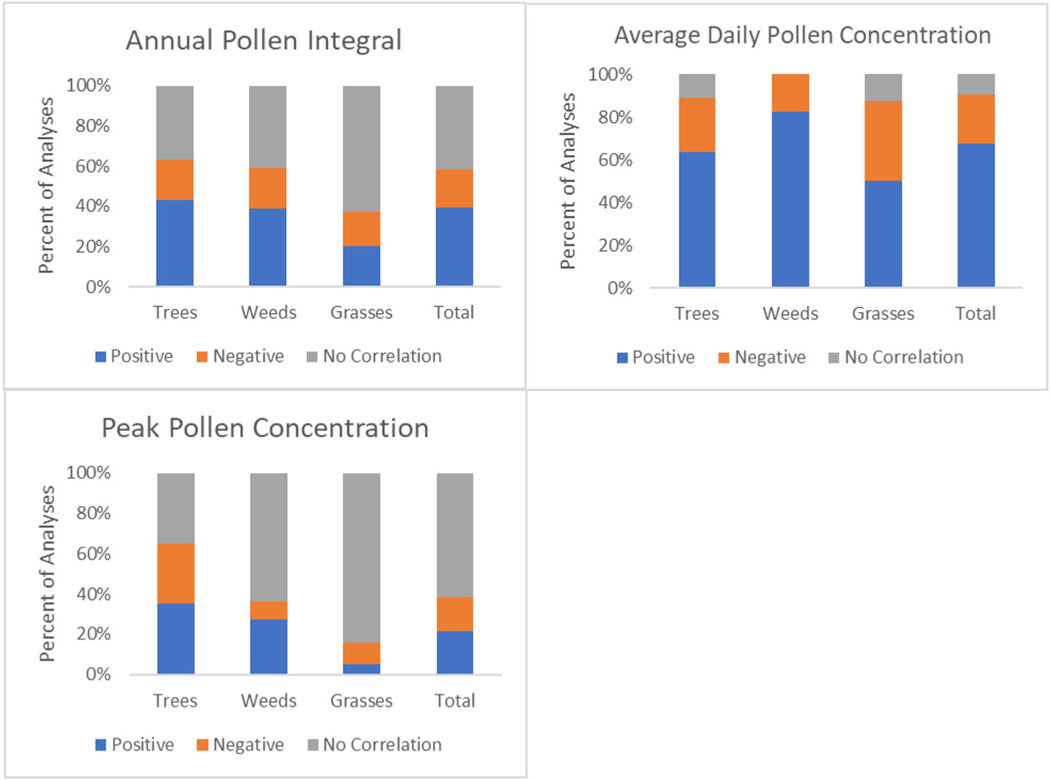
In analyzing the correlation results between airborne pollen concentrations and temperature (Fig. 5), a majority of analyses (65 of 96) found a positive correlation between temperature and average daily pollen concentrations, which suggests that higher daily pollen concentrations occur with warmer temperatures. For API, the number of analyses that found a positive correlation and no significant correlation was almost the same (87 and 92 respectively), with a much lower number of analyses that found a negative correlation (43). Tree analyses demonstrated a mostly positive correlation between API and temperature (61 of 141).
Fig. 5.
Correlation of temperature with airborne pollen concentration indicators
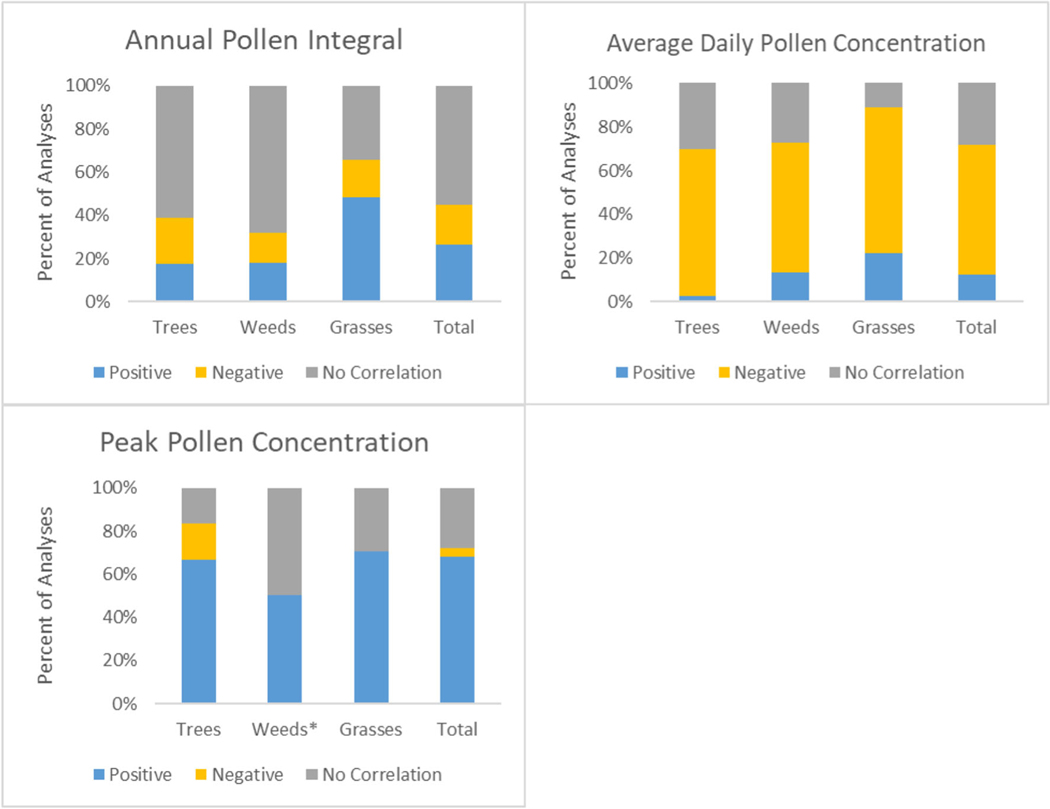
For airborne pollen concentrations and precipitation correlation (Fig. 6), a majority of analyses across trees, weeds, and grasses had a significantly negative correlation between average daily pollen concentrations and precipitation. This means that with increased precipitation, the average daily pollen concentration was lower. However, the definition of precipitation varied across studies, making it difficult to determine if this is indicative of a “wash out” effect or a result of seasonal precipitation amounts. For API, 48% of the grass analyses had a significantly positive correlation with precipitation; so with increased precipitation, there was increased grass API, though there was not a clear trend for trees and weeds. There was a significantly positive correlation between tree and grass peak pollen concentration and precipitation, meaning that with increased precipitation, there were increased peak pollen concentrations.
Fig. 6.
Correlation of precipitation with airborne pollen concentration indicators. Asterisks (*) indicate less than five analyses and thus limited results
Discussion
This systematic review is the first to summarize the effects of temperature and precipitation on airborne pollen concentrations and pollen season timing. Our results confirm previous reports that in general higher temperatures and shifts in precipitation timing and intensity extend the pollen season and increase airborne pollen concentrations (Beggs 2004; Beggs and Bambrick 2006; Bielory et al. 2012; D’Amato et al. 2013; Shea et al. 2008). Our review of 93 articles that explicitly linked meteorological variables to quantitative changes in airborne pollen concentrations and pollen season timing found that, with warmer temperatures, pollen seasons are generally starting earlier, and may be ending later and lasting longer, while airborne pollen concentrations are increasing.
Results were disaggregated by trees, weeds, and grasses, as the seasonal characteristics of these grouped species vary, as does their sensitivity to temperature and precipitation. Trees tend to be the first species to produce pollen in the spring in temperate climates, with weeds being the predominant pollen-producing species in the fall, and grass consistently produces pollen during the frost-free season (Kosisky 2010). Because trees are early pollen producers, the effects of a shorter winter due to warmer temperatures have a more distinct impact on an earlier start of the pollen season.
The correlation of pollen trends with precipitation was not as apparent, and more research is needed in this area. Increased precipitation can promote plant growth and increase aeroallergens (Breton et al. 2006; D’Amato et al., 2007a), though precipitation can also diminish airborne pollen particles through a process called “wash out” (McDonald 1962;Pérez et al. 2009). There was not a standard definition of precipitation used across the included studies; different scales of measuring precipitation (both spatially and temporally) further complicate analysis. It was thus not possible to separate the impacts of seasonal, weekly, and daily precipitation on pollen or to determine lag effects. However, the short-term effect of precipitation was likely seen in the results for average daily pollen concentrations. Multiple studies have also found a relationship between thunderstorms and increases in hospital admissions for asthma from pollen (Bellomo et al. 1992; D’Amato et al., 2007b; Grundstein et al. 2008). Because of the varying roles precipitation can play on pollen and the lack of studies that have looked at changes in precipitation on pollen, more research could help elucidate this complex relationship. That both woody and herbaceous allergenic species are undergoing similar changes in pollen lends further validity to our results particularly that pollen concentrations and season timing is changing globally across a variety of species. Because magnitude of correlation between pollen indicators and meteorological variables were not reported in most studies, and were not consistently reported for comparable meteorological variables or the same species, we were unable to assess or perform a meta-analysis of correlation magnitude.
Challenges and limitations
Data sources, study methodology, and analyses
Synthesizing pollen and climate data across studies introduces several challenges. The research included in this analysis varied widely in terms of the specifics of analysis in determining climatic influences on pollen. There was variation in the temperature and precipitation variables (e.g., climate variables defined with varying timeframes prior to and during the pollen season) as well as variation in the correlation methods used (e.g., Spearman rank correlation, Pearson’s rank correlation, correlation method not reported). This makes analyzing the impact of precipitation particularly difficult, as definitions of precipitation ranged from measured precipitation on the day of pollen measurements to impact of total seasonal precipitation from the previous season on pollen timing. This was further impacted by the relatively few studies assessing precipitation effects on pollen. In addition, there is inconsistency across studies in the methods used to define pollen season timing, including season length.
Multiple methods and various instruments are used to collect and speciate pollen (Frenz 1999), limiting direct comparisons of distinct pollen concentrations between counting stations. The studies included in this analysis reported pollen indicators for at least one pollen type, though some studies reported multiple individual species of pollen. In some cases, differentiation across pollen types was not possible: some analyses grouped pollen into categories (e.g., “grasses”), while others only reported total pollen inclusive of all species.
The varying dates and lengths of studies also introduced bias. The combination of data across place and time, as well as the shorter pollen record in non-European locations, likely results in our estimates being most reflective of pollen relationships in Europe. Our ability to generalize the results globally is limited as most included studies were conducted in Europe and North America. While some recent research has expanded the geographic scope of weather-related pollen research (e.g., Pakistan (Hamid et al. 2015)), pollen data are scarce, especially in developing countries. Most pollencounting stations are self-funded and require technical expertise (e.g., pollen identification through microscopy) that is both expensive and difficult to acquire. Further pollen research using datasets in new locations could aid understanding of global trends outside of Europe and North America.
Additionally, because pollen data are scarce, one dataset is often used for numerous analyses. In this systematic review, it is possible that more than one study relied on the same dataset for separate analyses (e.g., looking at different endpoints or different time periods), which would cause concern for data overlap, further introducing bias as a result of inappropriately weighting such studies. Likewise, publication bias could have influenced the results. Researchers may be less likely to publish if their data does not indicate a trend, or if their pollen data does not appear to be correlated with meteorological variables. However, many studies reported no correlation for some analyses, potentially allaying the fear of publication bias. Our results may underestimate potential correlations, as we focus on correlations where a majority of analyses indicated a positive or negative correlation. In some cases, a plurality of analyses indicated no correlation, but many analyses still indicated a correlation. For example, 58.2% of analyses reported no correlation between temperature and peak pollen date, but 40% indicated a negative correlation and only 1.8% indicated a positive correlation. Even though the plurality of analyses indicated no correlation, given the wide gap between analyses finding a negative correlation versus a positive correlation, it is still likely that increasing temperatures are leading to an earlier peak pollen season date globally.
Varying methodologies across studies make comparing results, at times, impossible. Where studies did report statistical significance, the standards were often inconsistent (e.g., textual language indicating significance versus quantitatively reported confidence intervals). Some studies did not indicate any test of significance. All studies were included in our analysis regardless of the author’s chosen method of reporting significance.
Because many of the European datasets were much longer, more recent data, while still mostly from European sites, is more weighted toward other locations such as North America. These more recent studies utilized data during more accelerated periods of climate change–related warming (e.g., the 2000s had a steeper trend in temperature increases than the 1970s). If newer studies systematically took place in one location (e.g., North America) or at higher or lower latitudes, this would introduce bias.
Climate attribution
Although studies have shown that shifting pollen concentrations and season timing are related to climatic factors such as temperature, we cannot say for certain that these changes in pollen concentrations and season timing are due to long-term changes in climate, principally because many of the included studies did not have sufficiently long time series to assess a shift in climate. In addition, there are many factors that affect plant growth, pollen production, and pollen dispersion. Increasing atmospheric CO2 at ground level (independent of temperature) results in larger pollen grains (Wan et al. 2002) with more allergenic proteins (Albertine et al. 2014; Singer et al. 2005) and earlier pollen seasons with increased pollen concentrations (Ziska et al. 2003). Ground-level CO2 concentrations were not reported in most studies and were not analyzed in this review. Furthermore, global shifts to urbanization, regional suburbanization, and subsequent land use changes in the last few decades may have contributed to increased pollen concentrations from allergenic species, as CO2 concentrations and temperatures are higher in urban areas than rural ones. Growing urban heat islands as well as ongoing suburbanization and planting of ornamental trees could also affect pollen trends. Differences in pollen sources also impact the ability of this study to synthesize across multiple sites. For example, urban green space and broadleaf forest contribute most to pollen load in central Spain (Rojo et al. 2015); in other areas, various topography and vegetation distribution could have a controlling effect on pollen concentrations and distribution. It is likely that a complex variety of factors are influencing changes in airborne pollen concentrations and pollen season timing (Tseng and Kawashima 2019).
Potential implications for human health
Our findings that warmer temperatures were associated with earlier and longer pollen seasons and higher airborne pollen concentrations have potential implications for human health. Previous studies have established a positive dose-response relationship between pollen concentrations and acute allergy–related illness, particularly among those with pre-existing conditions such as asthma (Darrow et al. 2011; Heguy et al. 2008). Additionally, allergy medication sales also coincide with high pollen concentration days (Sheffield et al. 2011), underscoring the acute health burden associated with high pollen concentrations. Given that pollen allergies are strongly associated with absenteeism and presenteeism, the correlation likely extends to a significant economic burden as well. Our results align with an observed increase in health impacts over the last several decades. One study reported that allergic rhinitis prevalence in the USA increased from 10% in 1970 to 30% in 2000 (Grammer and Greenberger 2009). European populations have also seen an increase in allergic rhinitis and other allergy-related disease (Åberg et al. 1995; Linneberg et al. 1999). Although there is an intuitive relationship between allergenic pollen, human health, and changes in climatic factors, the extent to which we fully understand the impacts is limited by study design and data availability. Other potential trends (e.g., ozone and allergenicity of birch pollen (Beck et al. 2013)) have been suggested as possible drivers of the increase in allergies, and neither this study nor those we reviewed were designed to evaluate the relative contributions of these potential drivers on disease burden.
Future climate projections show increased CO2 levels and ambient temperatures, which are expected to increase future allergenic pollen concentrations from ragweed (Rogers et al. 2006) and other clinically relevant species (Emberlin et al. 2002; Ziska and Beggs 2012). Other recent studies have projected future changes in pollen under various climate change scenarios (Hamaoui-Laguel et al. 2015; Lake et al. 2016; Zhang et al. 2015). Incorporating our current understanding of the health impacts of allergenic pollen with our ability to project the changes to climate and, thus, the changes in population exposure to allergenic pollen is important for current and future allergy sufferers.
Steps to protect health
A host of strategies are in place to help patients with pollen allergies to limit exposure and reduce associated symptoms, ranging from pollen forecasts and early warnings to filtering systems to symptomatic therapy and immune modulation drugs. The skill of pollen forecasting efforts could be enhanced, and more effective targeted health communications could be developed to further reduce the burden of respiratory allergic disease. Efforts could also be focused more tightly on those who are most susceptible (e.g., children or those who suffer from asthma). To the extent that immunotherapy needs to be timed in relation to the onset and peak of the allergy season, improved forecasting could facilitate timing of therapies for those with moderate to severe disease. There are data limitations to developing more accurate forecasts for the USA, however. Current pollen monitoring is limited, and there is a need for centralized, coordinated data collection and archiving as well as broader monitoring coverage and data accessibility for public health applications (Council for State and Territorial Epidemiologists (CSTE) 2016). As the geographic and temporal distribution of allergenic plants (floristic zones) changes, the health effects associated with pollen exposure will likely occur earlier and potentially in new areas. Improved pollen-monitoring networks can aid observation of changes in pollen, which can then provide early warning for periods of high pollen exposure.
Conclusion
This is the first systematic review of the correlation between temperature and precipitation with allergenic pollen. We found that warmer temperatures and changes in precipitation correlate with pollen seasons starting earlier and lasting longer, higher peak pollen concentrations, and increasing overall intensity of the pollen season. These shifts are expected to have net adverse health impacts, which may already be occurring. Measuring, monitoring, and analyzing pollen data are crucial steps toward reducing the burden of allergenic pollen on human health, especially as temperatures rise and precipitation patterns change. As the climate changes, establishing baseline trends for allergenic pollen can inform the development of anticipatory public health response and plans to reduce pollen-sensitive health outcomes.
Supplementary Material
Acknowledgements
The authors would like to acknowledge Emmanuelle Hines, Leila Atalla, and Katy Gerber for their assistance.
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Funding This work was supported by the Climate and Health Program at the Centers for Disease Control and Prevention.
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00484-021-02128-7.
Data availability All data extracted from the systematic review is maintained by the CDC and available to the public.
Code availability Analysis was completed in Microsoft Excel; spreadsheets are available to the public.
Declarations
Competing interests The authors declare no competing interests.
References
- Åberg N, Hesselmar B, Åberg B, Eriksson B (1995) Increase of asthma, allergic rhinitis and eczema in Swedish schoolchildren between 1979 and 1991. Clin Exp Allergy 25:815–819 [DOI] [PubMed] [Google Scholar]
- Aguilera F, Orlandi F, Ruiz-Valenzuela L, Msallem M, Fornaciari M (2015) Analysis and interpretation of long temporal trends in cumulative temperatures and olive reproductive features using a seasonal trend decomposition procedure. Agric For Meteorol 203:208–216 [Google Scholar]
- Albertine JM, Manning WJ, DaCosta M, Stinson KA, Muilenberg ML, Rogers CA (2014) Projected carbon dioxide to increase grass pollen and allergen exposure despite higher ozone levels. PLoS One 9: e111712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariano R, Canonica GW, Passalacqua G (2010) Possible role of climate changes in variations in pollen seasons and allergic sensitizations during 27 years. Ann Allergy Asthma Immunol 104:215–222. 10.1016/j.anai.2009.12.005 [DOI] [PubMed] [Google Scholar]
- Bastl K, Kmenta M, Berger M, UJWAOJ B (2018) The connection of pollen concentrations and crowd-sourced symptom data: new insights from daily and seasonal symptom load index data from 2013 to 2017 in Vienna. World Allergy Organ 11:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck I et al. (2013) High environmental ozone levels lead to enhanced allergenicity of birch pollen. PLoS One 8:e80147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs PJ (2004) Impacts of climate change on aeroallergens: past and future. Clin Exp Allergy 34:1507–1513 [DOI] [PubMed] [Google Scholar]
- Beggs PJ, Bambrick HJ (2006) Is the global rise of asthma an early impact of anthropogenic climate change? Ciência & Saúde Coletiva 11:745–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellomo R, Gigliotti P, Treloar A, Holmes P, Suphioglu C, Singh M, Knox B (1992) Two consecutive thunderstorm associated epidemics of asthma in the city of Melbourne The possible role of rye grass pollen. Med J Aust 156:834–837 [DOI] [PubMed] [Google Scholar]
- Bernard SM, Samet JM, Grambsch A, Ebi KL, Romieu I (2001) The potential impacts of climate variability and change on air pollution-related health effects in the United States. Environ Health Perspect 109:199–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielory L, Lyons K, Goldberg R (2012) Climate change and allergic disease. Curr Allergy Asthma Rep 12:485–494 [DOI] [PubMed] [Google Scholar]
- Black LI, Benson V (2019) Tables of Summary Health Statistics for U.S. Children: 2018 National Health Interview Survey. Available from: https://www.cdc.gov/nchs/nhis/SHS/tables.htm
- Breton M-C, Garneau M, Fortier I, Guay F, Louis J (2006) Relationship between climate, pollen concentrations of Ambrosia and medical consultations for allergic rhinitis in Montreal, 1994–2002. Sci Total Environ 370:39–50. 10.1016/j.scitotenv.2006.05.022 [DOI] [PubMed] [Google Scholar]
- Bromenshenk JJ, Carlson SR, Simpson JC, Thomas JM (1985) Pollution monitoring of Puget Sound with honey bees. Science, USA 227: 632–634 [DOI] [PubMed] [Google Scholar]
- Bunyavanich S, Landrigan CP, McMichael AJ, Epstein PR (2003) The impact of climate change on child health. Ambul Pediatr 3:44–52. [DOI] [PubMed] [Google Scholar]
- Cakmak S, Dales RE, Burnett RT, Judek S, Coates F, Brook JR (2002) Effect of airborne allergens on emergency visits by children for conjunctivitis and rhinitis. Lancet 359:947–948. 10.1016/S0140-6736(02)08045-5 [DOI] [PubMed] [Google Scholar]
- Carracedo-Martinez E, Sanchez C, Taracido M, Saez M, Jato V, Figueiras A (2008) Effect of short-term exposure to air pollution and pollen on medical emergency calls: a case-crossover study in Spain. Allergy 63:347–353 [DOI] [PubMed] [Google Scholar]
- Cirera L et al. (2012) Daily effects of air pollutants and pollen types on asthma and COPD hospital emergency visits in the industrial and Mediterranean Spanish city of Cartagena. Allergol Immunopathol 40:231–237. 10.1016/j.aller.2011.05.012 [DOI] [PubMed] [Google Scholar]
- Corden J, Millington W (1999) A study of Quercus pollen in the Derby area, UK. Aerobiologia 15:29–37 [Google Scholar]
- Council for State and Territorial Epidemiologists (CSTE) (2016) Developing a national aeroallergen tracking network. doi:http://c.ymcdn.com/sites/www.cste.org/resource/resmgr/2016PS/16_EH_01.pdf. Accessed 16 Apr 2021
- D’Amato G, Liccardi G, Frenguelli G (2007a) Thunderstorm-asthma and pollen allergy. Allergy 62:11–16 [DOI] [PubMed] [Google Scholar]
- D’Amato G et al. (2007b) Allergenic pollen and pollen allergy in Europe. Allergy 62:976–990. 10.1111/j.1398-9995.2007.01393.x [DOI] [PubMed] [Google Scholar]
- D’Amato G et al. (2013) Climate change, air pollution and extreme events leading to increasing prevalence of allergic respiratory diseases. Multidiscip respir med 8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amato G, Cecchi L, D’Amato M, Annesi-Maesano I (2014) Climate change and respiratory diseases. Eur Respir Rev 23:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amato G, Liccardi G, D’Amato M, Cazzola M (2001) The role of outdoor air pollution and climatic changes on the rising trends in respiratory allergy. Respir Med 95:606–611. 10.1053/rmed.2001.1112 [DOI] [PubMed] [Google Scholar]
- Darrow L, Hess J, Rogers CA, Tolber PE, Klein M, Sarnat SE (2011) Ambient pollen concentrations and emergency department visits for asthma and wheeze. J Allergy Clin Immunol 130:630–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz J, Linares C, Tobías A (2007) Short-term effects of pollen species on hospital admissions in the city of Madrid in terms of specific causes and age. Aerobiologia 23:231–238. 10.1007/s10453-007-9067-x [DOI] [Google Scholar]
- Donders TH, Hagemans K, Dekker SC, de Weger LA, De Klerk P, Wagner-Cremer F (2014) Region-specific sensitivity of anemophilous pollen deposition to temperature and precipitation. PLoS One 9: e104774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emberlin J, Mullins J, Corden J, Millington W, Brooke M, Savage M, Jones SJG (1997) The trend to earlier birch pollen seasons in the UK: a biotic response to changes in weather conditions? Grana 36: 29–33 [Google Scholar]
- Emberlin J, Detandt M, Gehrig R, Jaeger S, Nolard N, Rantio-Lehtimäki A (2002) Responses in the start of Betula (birch) pollen seasons to recent changes in spring temperatures across Europe. Int J Biometeorol 46:159–170. 10.1007/s00484-002-0139-x [DOI] [PubMed] [Google Scholar]
- Fann N et al. (2016) Ch. 3: Air quality impacts. U.S. Global Change Research Program, Washington. 10.7930/J0GQ6VP6 [DOI] [Google Scholar]
- Frei T, Gassner E (2008) Climate change and its impact on birch pollen quantities and the start of the pollen season an example from Switzerland for the period 1969–2006. Int J Biometeorol 52:667–674. 10.1007/s00484-008-0159-2 [DOI] [PubMed] [Google Scholar]
- Frenz DA (1999) Comparing pollen and spore counts collected with the Rotorod Sampler and Burkard spore trap. Ann Allergy Asthma Immunol 83:341–349. 10.1016/S1081-1206(10)62828-1 [DOI] [PubMed] [Google Scholar]
- Galán I et al. (2010) Association between airborne pollen and epidemic asthma in Madrid, Spain: a case–control study. Thorax 65:398–402 [DOI] [PubMed] [Google Scholar]
- García-Mozo H et al. (2006) Quercus pollen season dynamics in the Iberian Peninsula: response to meteorological parameters and possible consequences of climate change. Ann Agric Environ Med 13: 209. [PubMed] [Google Scholar]
- García-Mozo H, Oteros J, Galán C (2015) Phenological changes in olive (Ola europaea L.) reproductive cycle in southern Spain due to climate change. Ann Agric and Environ Med 22(3):421–428 [DOI] [PubMed] [Google Scholar]
- Ghiani A, Ciappetta S, Gentili R, Asero R, Citterio S (2016) Is ragweed pollen allergenicity governed by environmental conditions during plant growth and flowering? Sci Rep 6:30438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammer L, Greenberger P (eds) (2009) Patterson’s allergic diseases, 7th edn. Wolters Kluwer, New York [Google Scholar]
- Grundstein A, Sarnat SE, Klein M, Shepherd M, Naeher L, Mote T, Tolbert P (2008) Thunderstorm associated asthma in Atlanta. Georgia Thorax 63:659–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guide to Community Preventive Servces (2017) Our methodology. The Community Guide. https://www.thecommunityguide.org/about/our-methodology. Accessed April 1, 2016
- Hajkova L, Koznarova V, Mozny M, Bartosova L (2015) Changes in flowering of birch in the Czech Republic in recent 25 years (1991–2015) in connection with meteorological variables. Acta Agrobot 68(4):285–302 [Google Scholar]
- Hamaoui-Laguel L et al. (2015) Effects of climate change and seed dispersal on airborne ragweed pollen loads in Europe. Nat Clim Chang 5:766–771 [Google Scholar]
- Hamid N, Ali SM, Talib F, Sadiq I, Ghufran MA (2015) Spatial and temporal variations of pollen concentrations in Islamabad (Pakistan): effect of meteorological parameters and impact on human health. Grana 54:53–67 [Google Scholar]
- Hanigan IC, Johnston FH (2007) Respiratory hospital admissions were associated with ambient airborne pollen in Darwin, Australia, 2004–2005. Clin Exp Allergy 37:1556–1565. 10.1111/j.1365-2222.2007.02800.x [DOI] [PubMed] [Google Scholar]
- Heguy L, Garneau M, Goldberg MS, Raphoz M, Guay F, Valois M-F (2008) Associations between grass and weed pollen and emergency department visits for asthma among children in Montreal. Environ Res 106:203–211. 10.1016/j.envres.2007.10.005 [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Lasserson T, Chandler J, Tovey D, Churchill R (2016) Methodological expectations of Cochrane intervention reviews. London: Cochrane, 5. Chicago [Google Scholar]
- Jato V, Rodríguez-rajo FJ, Fernandez-gonzález M, Aira MJ (2015) Assessment of Quercus flowering trends in NW Spain. Int J Biometeorol 59:517–531. 10.1007/s00484-014-0865-x [DOI] [PubMed] [Google Scholar]
- Khan KS, Kunz R, Kleijnen J, Antes G (2003) Five steps to conducting a systematic review. J R Soc Med 96:118–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S-H, Park H-S, Jang J-Y (2011) Impact of meteorological variation on hospital visits of patients with tree pollen allergy. BMC Public Health 11:890. 10.1186/1471-2458-11-890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosisky SE (2010) Pollen aeroallergens in the Washington, DC, metropolitan area: a 10-year volumetric survey (1998–2007) (vol 104, pg 223, 2010). Ann Allergy Asthma Immunol 104:545–545 [DOI] [PubMed] [Google Scholar]
- Koubouris GC, Metzidakis IT, Vasilakakis MD (2009) Impact of temperature on olive (Olea europaea L.) pollen performance in relation to relative humidity and genotype. Environ Exp Bot 67:209–214. 10.1016/j.envexpbot.2009.06.002 [DOI] [Google Scholar]
- Lake I et al. (2016) Climate change and future pollen allergy in Europe. Environ Health Perspect [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leth-Møller KB, Skaaby T, AJA L (2020) Allergic rhinitis and allergic sensitisation are still increasing among Danish adults. Allergy 75: 660–668 [DOI] [PubMed] [Google Scholar]
- Li J, Wang HY, Zhang ZG (2008) Association between airborne pollen concentrations and human respiratory diseases. J Environ Health 25: 510–513 [Google Scholar]
- Linneberg A, Jørgensen T, Nielsen N, Madsen F, Frølund L, Dirksen A (1999) Increasing prevalence of allergic rhinitis symptoms in an adult Danish population. Allergy 54:1194–1198 [DOI] [PubMed] [Google Scholar]
- Luber G et al. (2014) Ch. 9: Human health. doi: 10.7930/J0PN93H5 [DOI]
- McDonald JE (1962) Collection and washout of airborne pollens and spores by raindrops. Science 135:435–437 [DOI] [PubMed] [Google Scholar]
- Medek DE et al. (2016) Regional and seasonal variation in airborne grass pollen levels between cities of Australia and New Zealand. Aerobiologia 32:289–302. 10.1007/s10453-015-9399-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel A et al. (2006) European phenological response to climate change matches the warming pattern. Glob Chang Biol 12:1969–1976. 10.1111/j.1365-2486.2006.01193.x [DOI] [Google Scholar]
- Mesa JS, Brandao R, Lopes L, Galan C (2005) Correlation between pollen counts and symptoms in two different areas of the Iberian Peninsula: Cordoba (Spain) and Evora (Portugal). J Investig Allergol Clin Immunol 15:112–116 [PubMed] [Google Scholar]
- Moher D et al. (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement (Chinese edition). J Chin Integr Med 7:889–896 [PMC free article] [PubMed] [Google Scholar]
- Mothes N, Horak F, Valenta R (2004) Transition from a botanical to a molecular classification in tree pollen allergy: implications for diagnosis and therapy. Int Arch Allergy Immunol 135:357–373 [DOI] [PubMed] [Google Scholar]
- Murray MG, Galan C (2016) Effect of the meteorological parameters on the Olea europaea L. pollen season in Bahia Blanca (Argentina). Aerobiologia 32:541–553. 10.1007/s10453-016-9431-9 [DOI] [Google Scholar]
- Orazzo F et al. (2009) Air pollution, aeroallergens, and emergency room visits for acute respiratory diseases and gastroenteric disorders among young children in six Italian cities. Environ Health Perspect 117(11):1780–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez CF, Gassmann MI, Covi MJA (2009) An evaluation of the airborne pollen–precipitation relationship with the superposed epoch method. Aerobiologia 25:313–320 [Google Scholar]
- Rice MB, Thurston GD, Balmes JR, Pinkerton KE (2014) Climate change. A global threat to cardiopulmonary health. Am J Respir Crit Care Med 189:512–519. 10.1164/rccm.201310-1924PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers CA, Wayne PM, Macklin EA, Muilenberg ML, Wagner CJ, Epstein PR, Bazzaz FA (2006) Interaction of the onset of spring and elevated atmospheric CO2 on ragweed (Ambrosia artemisiifolia L.) pollen production. Environ Health Perspect 114(6):865–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo J, Rapp A, Lara B, Fernández-González F, Pérez-Badia R (2015) Effect of land uses and wind direction on the contribution of local sources to airborne pollen. Sci Total Environ 538:672–682 [DOI] [PubMed] [Google Scholar]
- Shah R, Grammer LC (2012) An overview of allergens. Allergy Asthma Proc 33:S2–S5 [DOI] [PubMed] [Google Scholar]
- Shea KM, Truckner RT, Weber RW, Peden DB (2008) Climate change and allergic disease. J Allergy Clin Immunol 122:443–453 [DOI] [PubMed] [Google Scholar]
- Sheffield P, Weinberger K, Ito K, Matte T, Mathes R, Robinson G, Kinney P (2011) The association of tree pollen concentration peaks and allergymedication sales in New York City: 2003–2008. Allergy 2011:537194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg JI, Braunstein M, Lee-Wong M (2015) Association between climate factors, pollen counts, and childhood hay fever prevalence in the United States. J Allergy Clin Immunol 135:463–469. 10.1016/j.jaci.2014.08.003 [DOI] [PubMed] [Google Scholar]
- Singer BD, Ziska LH, Frenz DA, Gebhard DE, Straka JG (2005) Increasing Amb a 1 content in common ragweed (Ambrosia artemisiifolia) pollen as a function of rising atmospheric CO 2 concentration. Funct Plant Biol 32:667–670 [DOI] [PubMed] [Google Scholar]
- Stennett PJ, Beggs PJ (2004) Pollen in the atmosphere of Sydney, Australia, and relationships with meteorological parameters. Grana 43:209–216. 10.1080/00173130410000758 [DOI] [PubMed] [Google Scholar]
- Teranishi H, Kenda Y, Katoh T, Kasuya M, Oura E, Taira H (2000) Possible role of climate change in the pollen scatter of Japanese cedar Cryptomeria japonica in Japan. Clim Res 14:65 [Google Scholar]
- Teranishi H, Katoh T, Kenda K, Hayashi SJA (2006) Global warming and the earlier start of the Japanese-cedar (Cryptomeria japonica) pollen season in Toyama, Japan. Aerobiologia 22:90–94 [Google Scholar]
- Toro R, Córdova A, Canales M, Mardones P (2015) Trends and threshold exceedances analysis of airborne pollen concentrations in Metropolitan Santiago Chile. PLoS One 10:e0123077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng Y-T, Kawashima SJAE (2019) Applying a pollen forecast algorithm to the Swiss Alps clarifies the influence of topography on spatial representativeness of airborne pollen data. Atmos Environ 212:153–162 [Google Scholar]
- USGCRP (2016) The impacts of climate change on human health in the United States: a scientific assessment. US Global Change Research Program, Washington, p 2016 [Google Scholar]
- Villarroel MA, Blackwell DL, Jen A (2019) Tables of Summary Health Statistics for U.S. Adults: 2018 National Health Interview Survey. National Center for Health Statistics. Available from: http://www.cdc.gov/nchs/nhis/SHS/tables.htm [Google Scholar]
- Wallace DV et al. (2008) The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol 122:S1–S84. 10.1016/j.jaci.2008.06.003 [DOI] [PubMed] [Google Scholar]
- Walther G-R, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Fromentin JM, Hoegh-Guldberg O, Bairlein F (2002) Ecological responses to recent climate change. Nature 416:389–395 [DOI] [PubMed] [Google Scholar]
- Wan S, Yuan T, Bowdish S, Wallace L, Russell SD, Luo Y (2002) Response of an allergenic species, Ambrosia psilostachya (Asteraceae), to experimental warming and clipping: implications for public health. Am J Bot 89:1843–1846 [DOI] [PubMed] [Google Scholar]
- White JF, Bernstein DI (2003) Key pollen allergens in North America. Ann Allergy Asthma Immunol 91:425–435. 10.1016/S1081-1206(10)61509-8 [DOI] [PubMed] [Google Scholar]
- Whitlock C, Bartlein PJ (1997) Vegetation and climate change in Northwest America during the past 125 kyr. Nature 388:57–61 [Google Scholar]
- Zhang Y, Zhang LJA (2019) Increasing prevalence of allergic rhinitis in China. Allergy, Asthma Immunol Res 11:156–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Bielory L, Georgopoulos P (2014) Climate change effect on Betula (birch) and Quercus (oak) pollen seasons in the United States. Int J Biometeorol 58:909–919. 10.1007/s00484-013-0674-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Bielory L, Mi Z, Cai T, Robock A, Georgopoulos P (2015) Allergenic pollen season variations in the past two decades under changing climate in the United States. Glob Chang Biol 21:1581–1589. 10.1111/gcb.12755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F et al. (2016) Common ragweed (Ambrosia artemisiifolia L.): allergenicity and molecular characterization of pollen after plant exposure to elevated NO2. Plant Cell Environ 39:147–164 [DOI] [PubMed] [Google Scholar]
- Ziello C, Böck A, Estrella N, Ankerst D, Menzel A (2012) First flowering of wind-pollinated species with the greatest phenological advances in Europe. Ecography 35:1017–1023. 10.1111/j.1600-0587.2012.07607.x [DOI] [Google Scholar]
- Ziska LH, Beggs PJ (2012) Anthropogenic climate change and allergen exposure: the role of plant biology. J Allergy Clin Immunol 129:27–32. 10.1016/j.jaci.2011.10.032 [DOI] [PubMed] [Google Scholar]
- Ziska L, Gebhard D, Frenz D, Faulkner S, Singer B, Straka J (2003) Cities as harbingers of climate change: common ragweed, urbanization, and public health. J Allergy Clin Immunol 111:290–295. 10.1067/mai.2003.53 [DOI] [PubMed] [Google Scholar]
- Ziska L et al. (2011) Recent warming by latitude associated with increased length of ragweed pollen season in central North America. Proc Natl Acad Sci 108:4248–4251. 10.1073/pnas.1014107108 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.