Abstract
Indomethacin, a non-steroidal anti-inflammatory drug (NSAID), has been presented as a broad-spectrum antiviral agent. This randomised clinical trial in a hospital setting evaluated the efficacy and safety of this drug in RT-PCR-positive coronavirus disease 2019 (COVID-19) patients. A total of 210 RT-PCR-positive COVID-19 patients who provided consent were allotted to the control or case arm, based on block randomisation. The control arm received standard of care comprising paracetamol, ivermectin, and other adjuvant therapies. The patients in the case arm received indomethacin instead of paracetamol, with other medications retained. The primary endpoint was the development of hypoxia/desaturation with SpO2 ≤ 93, while time to become afebrile and time for cough and myalgia resolution were the secondary endpoints. The results of 210 patients were available, with 103 and 107 patients in the indomethacin and paracetamol arms, respectively. We monitored patient profiles along with everyday clinical parameters. In addition, blood chemistry at the time of admission and discharge was assessed. As no one in either of the arms required high-flow oxygen, desaturation with a SpO2 level of 93 and below was the vital goal. In the indomethacin group, none of the 103 patients developed desaturation. On the other hand, 20 of the 107 patients in the paracetamol arm developed desaturation. Patients who received indomethacin also experienced more rapid symptomatic relief than those in the paracetamol arm, with most symptoms disappearing in half the time. In addition, 56 out of 107 in the paracetamol arm had fever on the seventh day, while no patient in the indomethacin group had fever. Neither arm reported any adverse event. The fourteenth-day follow-up revealed that the paracetamol arm patients had faced several discomforts; indomethacin arm patients mostly complained only of tiredness. Indomethacin is a safe and effective drug for treating patients with mild and moderate covid-19.
Subject terms: Drug safety, Epidemiology, Infectious diseases
Introduction
SARS-Cov-2, a member of the coronavirus family, has been ravaging the world for the past 18 months. Although an effective treatment has eluded the medical community, there have been several registered trials on finding new or repurposed drugs. Several studies have discussed the mechanism of the virus-host interaction and possible treatments1, but safe and effective treatment for the disease is yet to emerge. Drug repurposing seems to be an immediate solution, and various drugs have been suggested for the COVID-19 treatment2,3.
The drugs required to combat the pathogen may fall into one or more of the following categories: antivirals, anti-inflammatory agents, and supportive therapies4,5. According to V’Kovski1 the antiviral action can be based on the stages of viral-host interactions. These include attachment and virus neutralisation, host protease inhibitors that stop the entry of the virus, viral protease inhibitors, viral RdRp inhibitors, and viral maturation inhibitors. Frediansyah et al.6 enumerated the possible antiviral solutions at various stages of interactions. The role of cathepsin L in cleavage of the S protein complex and subsequent release of virus genome is well documented. Inhibiting cathepsin L inhibits the entry of SARS-Cov-2 by 76%7.
Pro-inflammatory cytokine production is natural during the immune response. The elimination of virus-infected cells is an essential step in disease control. If this step, which naturally follows virus entry and replication, is defective or prolonged, it can result in a cytokine storm8, an uncontrolled release of pro-inflammatory cytokines. Several interleukins are involved in a cytokine storm, the foremost being interleukin 6 (IL-6), IL-18, and IL-179. IL-17 also seems to have a role as the interaction partner of SARS-Cov-2. Therefore, anti-inflammatory drugs targeting the production of these interleukins are necessary for COVID-19 treatment.
Indomethacin as a drug for SARS-Cov-2
Amici et al.10 were the first to identify the antiviral activity of indomethacin. They recorded the antiviral activities of indomethacin against SARS-Cov-1 in vitro. Xu et al.11 presented evidence of its antiviral activity against SARS-Cov-2. Their investigations covered the antiviral effect of indomethacin in vitro, cellular, corona-infected canine models. They also stated that indomethacin does not reduce infectivity, binding, or entry into target cells. This conclusion is based on the results of Amici et al.10 for SARS-Cov-1, although computer models have indicated otherwise12. Downregulation of ACE2 and TMPRSS2 is important to reduce infectivity. Using an open-source code, Gene2Drug, Napolitano et al.12 showed in a computer model that indomethacin downregulates ACE2 by suppressing the genes in the ACE2 pathway. Raghav et al.13 depicted the role of indomethacin in inhibiting cathepsin L activity required for fusion. Interestingly, no other non-steroidal anti-inflammatory drugs13 projected this quality.
Non-Structural Protein7 (Nsp7), along with Nsp12, is essential for RNA synthesis, as highlighted by Frediansyah et al.6, Gordan et al.9 recognised that prostaglandin E synthase 2 (PGES-2) is an “interactor” with Nsp7, and indomethacin inhibits PGES-2. Hence, indomethacin is an essential candidate for blocking RNA synthesis. Amici et al.10 reported this block of RNA synthesis. Amici et al.14 also demonstrated that protein kinase R (PKR) activation by indomethacin inhibits virus protein translation. This follows the work of Brunelli et al.15, where the role of indomethacin in activating PKR directly has been demonstrated.
Several publications16,17 have highlighted the importance of preventing inflammation in Covid-19 patients. For example, indomethacin downregulates IL-6 by inhibiting the synthesis of PGES-218. In addition, indomethacin has successfully contained cytokine reactions in kidney transplant patients receiving OKT3 therapy19,20.
Rajan et al.21 conducted one of the first indomethacin trials. The objective was to compare the data from open-label single-arm data for indomethacin; they showed the effect of indomethacin as a treatment option by matching propensity score with retrospectively collected data on paracetamol. Gordon et al.9 indicated by retrospective data analysis that indomethacin markedly reduces the need for hospitalisation. Two studies22,23 have shown the effectiveness of indomethacin in treating a small number of SARS-Cov-2 patients with severe comorbidities. However, these were small case series, and a larger controlled trial is required to validate these findings.
The primary objective of this study is to determine the percentage of desaturating patients as a quantitative criteria SpO2 ≤ 93 has been used as a measure. The secondary outcome was symptomatic relief. Time to become afebrile, relief from cough, and myalgia are the significant symptoms for the secondary outcome. The safety profile of indomethacin was also monitored as a secondary outcome.
Methods
Study design
This open-label randomized clinical trial consisted of two parallel groups; the control group with paracetamol and standard of care (SOC) and the indomethacin group with SOC. We recruited RT-PCR positive covid-19 patients using four and six-block randomisation parallel-group protocol24. The protocol was computer-generated, and all care was taken to conceal the allocation of patients from the treating physicians. They were not involved either in the randomisation or the allocation. The randomisation was handled by a third person, not involved either in the treatment or in data collection.
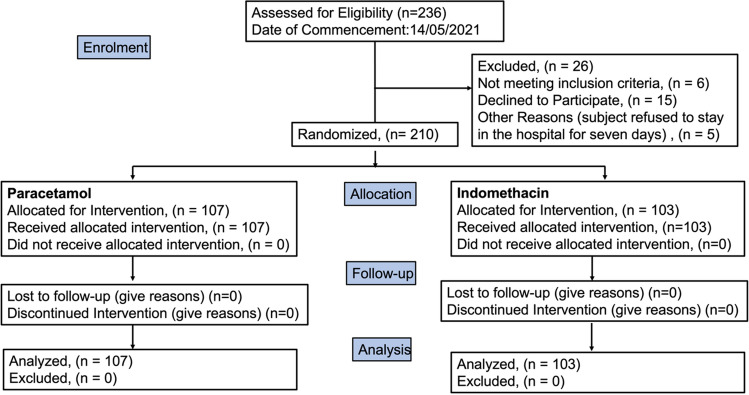
To understand the impact of the sample size, we assumed the response rate for paracetamol and indomethacin to be 0.82 and 0.95, respectively. These believed numbers were based on our earlier study21. The sample size was calculated using R with an alpha value of 0.05. The marginal power was 0.825. The sample size worked out to be 95 in each group. Nevertheless, we registered for 300 patients to have a safety margin on the size. The study was conducted at Panimalar Medical College, Chennai, India, in a designated Covid ward. Figure 1 describes the study design.
Figure 1.
Flow diagram (created in Office 365: PowerPoint as an image and pasted).
Study cohort
The patients recruited were RT-PCR positive. The clearly defined inclusion and exclusion criteria are given below.
Inclusion
Age between 20 and 90 years
RT-PCR positive
Hospitalised patients
The case criteria for the study:
Oxygen saturation—94 or more
Exclusion
Hypersensitivity/allergy to drugs
Gastritis
Recent heart attack
Severe asthma
Acute kidney injury
Patients on immunosuppressants
Pregnant and lactating mothers
Indomethacin allergy
The trial was approved by the Panimalar Medical College Hospital & Research Institute—Institutional Human Ethics Committee with a CDSCO Registration Number: ECR/1399/INST/2020. The approval Number for the trial is PMCH&RI/IHEC/2021/051. The trial was also registered with Clinical Trials Registry—India (CTRI/2021/05/033544) on 11/05/2021. The trial was conducted strictly according to a clinical trial's approved guidelines and regulations. The patients were apprised of the trial and the background before obtaining their informed consent in English and vernacular. No changes were made in the treatment protocol or eligibility protocol after the commencement of the trial.
Treatment
Indomethacin replaced paracetamol and was given along with the hospital standard care, which included doxycycline and ivermectin. The government-regulated protocol decided the standard of care, followed in many hospitals throughout India. However, studies have shown that ivermectin may not effectively treat covid-19 patients26. We also added a proton pump inhibitor along with indomethacin. The drugs and their dosage are listed in Table 1.
Table 1.
Drug chart.
| S. No | Drug | Dose and duration | Timing before/after food (BF/AF) |
|---|---|---|---|
| 1 | C. Doxycycline | 100 mg BD | AF |
| 2 | T. Ivermectin | 12 mg OD | AF |
| 3 | T. Vitamin C | 500 mg BD | AF |
| 4 | T. Zinc | 50 mg OD | AF |
| 5a | T. Indomethacin | 75 mg | 0–0-1 for BMI < 30@@1–0-1 for BMI > 30 |
| 5b | In the paracetamol arm (Dolo 650), instead of indomethacin | 650 mg | 1–1–1–1 |
| 6 | Cough syrup—(Lupituss) | 5 ml TDS | 1–1–1 |
| 7 | T. Pantoprazole | 40 mg BD | 1–0–1 |
T: Tablet; C: Capsule.
The treatment regimen was for five days.
Measurements
The following investigations were conducted on admission: CT scan of the lungs, liver function test (LFT), kidney function test (KFT), C-reactive protein (CRP), and D-dimer as well regular blood tests such as complete blood count. In addition, we repeated blood chemistry on discharge and monitored the well-being of the patients after discharge for 14 days through telephonic communication. We also monitored the patients for clinical symptoms such as oxygen saturation, fever, cough, and myalgia for seven days at the hospital. Patients were deemed symptomatically recovered if the temperature dropped below 99° F for two days and cough reduced to a score of 1 on a one-to-ten scale (1: no cough; 2–3: cough sometimes; 4–6: coughing with the ability to do things; 7–8 persistent cough; 9–10: a great deal of discomfort). RT-PCR was mandatory on admission, and for 122 patients, RT-PCR was repeated on the seventh day, before discharge.
Statistical analysis
We used standard statistical parameters to analyze the recruited patients. The patients in the two arms were also compared based on mean/median, interquartile range (IQR), and the Wilcoxon test to estimate the p-values. Wherever appropriate 95% confidence interval (CI) was calculated. The test of significance for p-value was based on a p-value of 0.01. We followed the dictum “once randomized, always analyzed”. The primary endpoint was examined using the chi-squared test, and the secondary endpoints were also studied based on mean, IQR, and p-values calculated using the Wilcoxon test.
We used linear regression and non-linear regression to analyze the reduction in C-Reactive protein. In addition, to comprehend the time for symptomatic relief clearly, we also carried out Kaplan-Meir survival analysis and used Cox regression to understand the effect of covariates. These two results are presented in Supplementary Section.
Results
Our goal in this study was to recruit 300 patients. However, the results presented here are for 210 patients as the study could not continue due to a drop of cases in India and no hospitalization. Randomisation resulted in 107 patients in the paracetamol group and 103 patients in the indomethacin group.
Patient characteristics and disposition
Patient profiles are shown in Fig. 2 and Table 2. The age profile and gender-wise enumeration match closely in both groups. The temperature on admission had a marginal bias, being higher for patients in the indomethacin group. In addition, more patients in the indomethacin group had a severe cough (above Scale 7). The comorbidity distribution was similar. No patient was lost in the follow-up.
Figure 2.
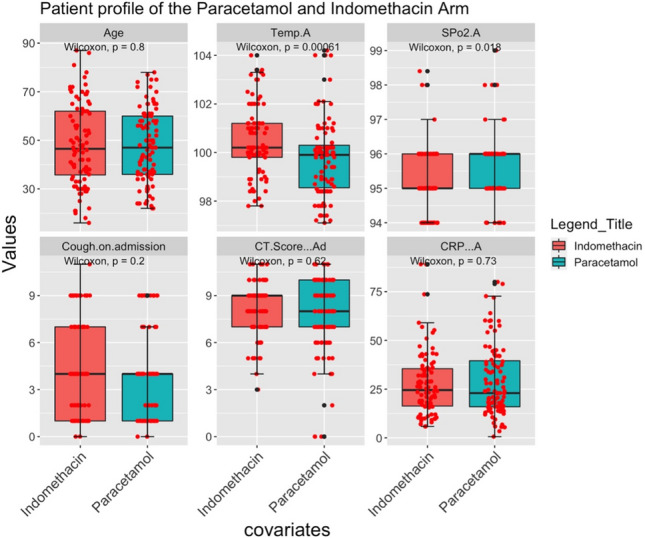
Patient profile on admission.
Table 2.
The profile of the recruited patients.
| Characteristics | All (N = 210) | Paracetamol (N = 107) | Indomethacin (N = 103) |
|---|---|---|---|
| Gender (Female/male) | (86/124) | (44/63) | (42/61) |
| Age (mean ± sd) | 47.04 ± 16.0 | 47.0 ± 15.21 | 47.07 ± 16.86 |
| Age (Male) (mean ± sd) | 48.21 ± 15.47 | 48.04 ± 14.75 | 48.4 ± 16.36 |
| Age (Female) (mean ± sd) | 45.33 ± 16.66 | 45.41 ± 15.93 | 45.25 ± 17.55 |
| Temperature on admission | 100.1 ± 1.5 | 99.8 ± 1.5 | 100.5 ± 1.4 |
| Cough on admission | |||
| a. Scale—7 to 10 | 57 | 21 | 36 |
| b. Scale—2 to 6 | 88 | 54 | 34 |
| Comorbidities | |||
| a. Diabetes | 61 | 28 | 33 |
| b. Hypertension | 40 | 25 | 15 |
Efficacy analysis
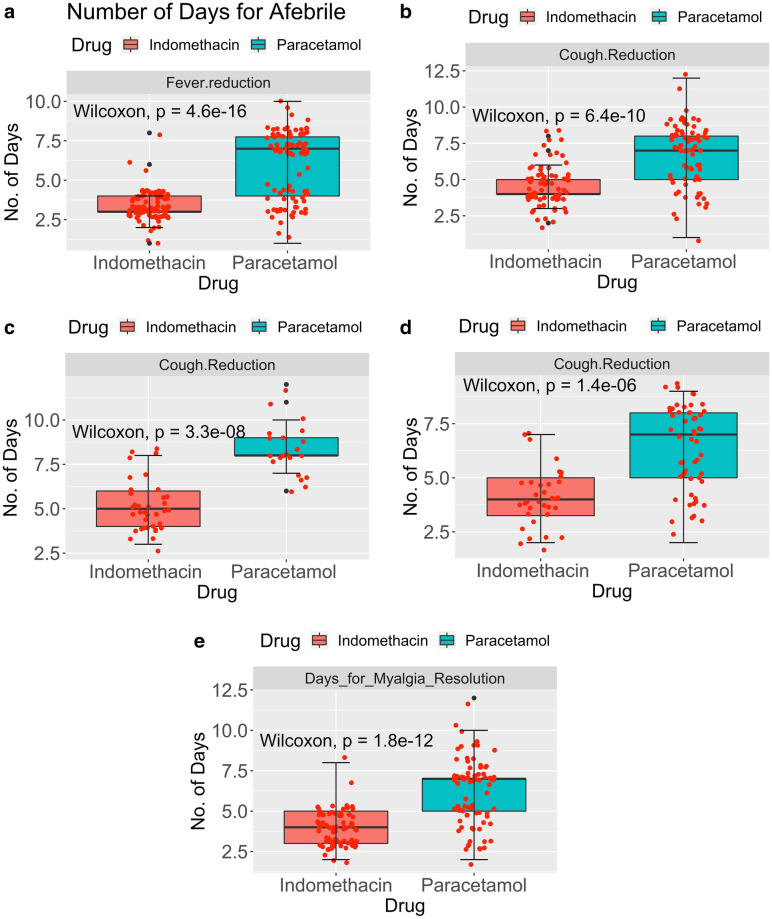
Symptomatic relief is very significant for the psychological and physiological well-being of patients. We monitored the number of days to become afebrile, reduced cough and relieved myalgia. The results are presented in Fig. 3a for afebrile, Fig. 3b for the number of days for cough reduction, Fig. 3c for cough reduction with a higher score on admission, Fig. 3d with a lower cough score on admission, and Fig. 3e for myalgia reduction.
Figure 3.
(a) Number of days for Afebrile. NIndomethacin = 95; NParacetamol = 98. (b) Number of days for cough reduction. NIndomethacin = 70; NParacetamol = 75. (c) Number of days for Cough Reduction—Cough on Admission 7 to 10. (d) Number of days for Cough Reduction—Cough on Admission 2 to 6. (e) Number of days for myalgia resolution. NIndomethacin = 77; NParacetamol = 82.
Median (dark line) and interquartile ranges are shown as boxes. The symptomatic recovery from fever and cough in terms of median values is given in Table 3. Two points are significant from the table and figure. Symptomatic relief with indomethacin takes only half the time compared to paracetamol. Additionally, the IQR, a measure of statistical dispersion, is very small with indomethacin compared to paracetamol and is significant because the action of indomethacin is almost independent of the patient's condition on admission. The Wicoxon p-value in Fig. 3a–e is less than 0.0001. This indicates that the results are statistically significant.
Table 3.
Symptomatic relief due to various treatments.
| Treatment | Days to become afebrile | Days for cough reduction | Days for myalgia reduction | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Median | 95% CI | IQR | Median | 95% CI | IQR | Median | 95% CI | IQR | |
| Indomethacin | 3 | 3.0, 3.0 | 1 | 4 | 4.0, 5.0 | 1 | 4 | 3.0, 4.0 | 2 |
| Paracetamol | 7 | 6.0, 7.0 | 2.75 | 7 | 7.0, 8.0 | 3 | 7 | 5.0, 7.0 | 2 |
Further results are provided in the supplementary appendix. They give the Kaplan–Meir estimator for relief of three symptoms, namely fever, cough, and myalgia, in Fig. S1 to S3. The Cox regression analysis is also given in the Supplementary Appendix (Tables S1 to S3).
The primary objective is the Number of patients desaturating (SpO2 ≤ 93) in both arms. The patients were admitted with a median SpO2 of 95 (IQR = 1) and SpO2 of 96 (IQR = 1) in the indomethacin arm and paracetamol arm, respectively, with a minimum SpO2 of 94. Out of 107 patients, 20 were desaturated in the paracetamol arm, while none were desaturated in the indomethacin arm (p < 0.01). No patient required high-flow oxygen; prone position and occasional low-flow oxygen were sufficient. In the indomethacin arm, saturation improved in one or two doses. The results are presented in Fig. 4.
Figure 4.
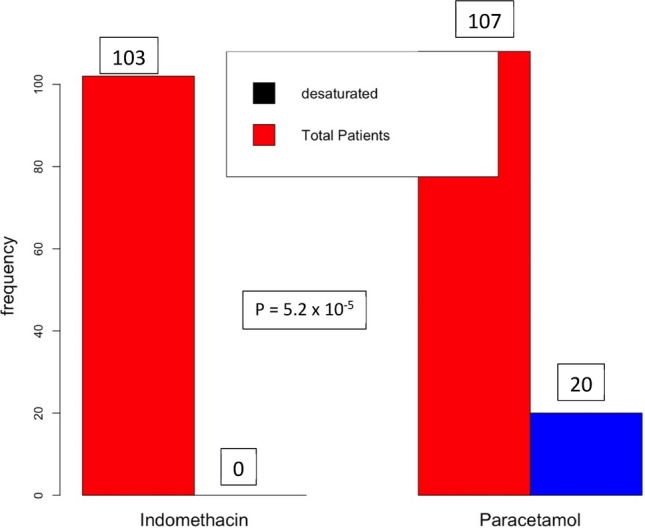
Number of patients desaturated.
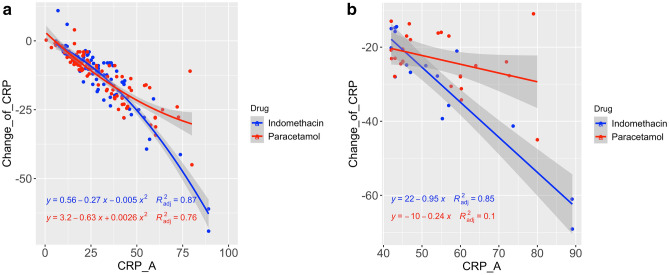
CRP is a well-known inflammatory marker, implicating covid-19 as a severe disease. According to Liu et al.16, CRP score greater than 41.8 may lead to severe illness. Figure 5a shows the relationship between the CRP level on admission and the decrease in the CRP level on the seventh day. Figure 5b shows the relationship between CRP on admission with a value greater than 41 and CRP reduction. Although the information in Fig. 5b is available in Fig. 5a, the separation provides clarity of data.
Figure 5.
(a) Change in CRP vs. CRP on admission. (b) Change in CRP vs. CRP on admission: a closer look of higher CRP on admission.
The results of the RT-PCR test conducted on the seventh day are presented in Fig. 6. As can be seen from the figure, 40.3% of patients in the indomethacin arm became RT-PCR negative on the seventh day compared to 28.3% in the paracetamol arm, although the p-value was 0.42.
Figure 6.
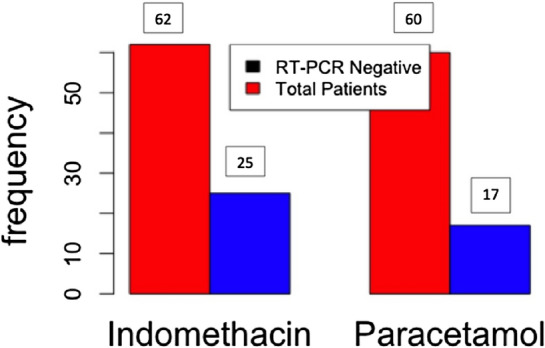
RT-PCR results at seventh day in both treatment and control arms.
Post-hoc calculations based on actual results yielded a marginal power of 0.99. This upholds the sample size selection.
Safety analysis
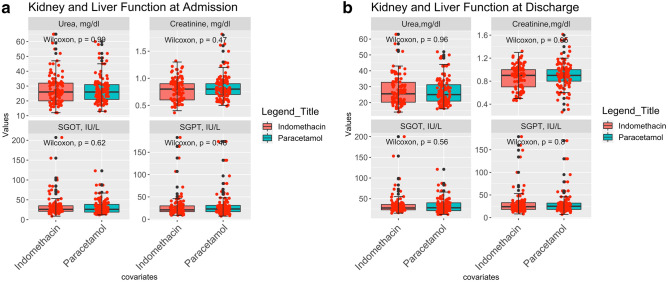
Indomethacin was approved in 1965. However, there have been concerns regarding its safety27. The Number of prescriptions for indomethacin was 2.16 million28 in 2018, in the US alone. Patients were tested for serum urea and creatinine, SGOT, and SGPT before and after the treatment, and the results are shown in Fig. 7a, b.
Figure 7.
(a) Kidney and liver function test on admission. (b) Kidney and liver function test on discharge.
Figure 7a, b show statistical similarities between both arms. Neither the liver nor kidney function deteriorated after treatment in either arm. Neither the Patients nor the attending physicians reported any other side effects.
Discussion
The study's primary aim was to understand the efficacy of indomethacin in preventing desaturation (SpO2 ≤ 93) and deterioration in mild and moderate covid-19 patients and compare this with a paracetamol-based arm. The secondary aim was to evaluate symptomatic relief in indomethacin patients compared to paracetamol patients. The results are striking: Indomethacin arm patients did not develop desaturation, while 20 patients out of 107 of the paracetamol arm patients developed desaturation. When the SpO2 level dips below 93, we managed the patient with low-flow oxygen or placed them in a prone position to enhance breathing. No patient in this study showed further deterioration. Notably, SpO2 improved after one or two doses in the indomethacin arm. Patients with a marginal SpO2 level of 94 showed an improvement. At the end of the seventh day, 13 patients in the paracetamol arm were at a SpO2 level of 94. In the indomethacin arm, only two patients had SpO2 levels of 94, while 97 of the 103 patients had a higher SpO2 level, higher than 97. In the paracetamol arm, only 51 patients (out of 107 patients) had a SpO2 level higher than 97.
Symptomatic relief was even more salient. The median time for becoming afebrile was three and seven days in the indomethacin and paracetamol arm, respectively. The median time for cough reduction was four days and seven days in the indomethacin and paracetamol arm, respectively. 59 out of 107 patients in the paracetamol arm had fever on the seventh day, while none of the indomethacin arm patients did. 49 of 75 patients taking paracetamol took seven or more days to recover from cough; only nine out of 70 patients in the indomethacin arm took seven days or more to recover. They were at level 2 on the ordinal scale. No patient in the indomethacin arm required continuation of indomethacin after the five-day regimen.
On the other hand, patients who were not afebrile after the treatment regimen of five days in the paracetamol arm were continued with the paracetamol at the treating physician's discretion. One of the most critical conclusions came from analysing the IQR. Figure 3a, b show a thin IQR band for fever and cough reduction in indomethacin patients, along with a small error bar, compared to paracetamol patients. A marginally broader IQR brand in myalgia may indicate the subjective nature of the relief.
Kaplan-Meir estimator reinforces the conclusion of the benefit of indomethacin strikingly. All the figures in the supplementary appendix show a big gap between the two treatments. Cox regression results indicate that the hazard ratio with indomethacin therapy is highly significant compared to other covariates. Indomethacin improves the recovery time by 85 to 90% for symptomatic relief. The other covariates that affect cough recovery are the CT Score on admission and cough on admission. Fever reduction and myalgia resolution are not affected by the covariates.
The results are similar to our earlier study, which used propensity score matching21. In that study, the median time for becoming afebrile, cough reduction, and myalgia relief in the indomethacin arm was four, three, and four days, respectively. However, the median in the paracetamol arm was seven and eight and 6.5 days, respectively.
We monitored CRP on admission and discharge. Indomethacin effectively reduces CRP in patients with higher CRP levels on admission (> 41 mg/L). In addition, the R2 value for indomethacin (0.85) was much higher than paracetamol (0.1). Thus, we can conclude that the consistency of indomethacin in reducing inflammation is very high.
A fourteen-day follow-up further revealed the efficacy of the drug. In the indomethacin arm, nearly 50% of patients had fully recovered compared to 28% in the paracetamol arm. The major complaint of the 50% of patients who took indomethacin was tiredness. In addition, only 14% of the patients had myalgia, while 10% complained of joint pain. On the other hand, in the paracetamol arm, the recovery was slower, with 47% complaining of myalgia, 48% of tiredness, 39% of joint pain, and 33% complaining of other ailments.
Alkotaji et al.29 had hypothesised the importance of indomethacin in reducing cough and myalgia through its inhibitive action on bradykinin, and the mechanism and ill-effects of bradykinin are well documented. The theory proposed in this study explains the difference in symptomatic relief between the two groups.
We had hypothesised that early symptomatic relief is vital for recovery and prevents desaturation. However, 17 of the 20 patients who were desaturated in the paracetamol arm had fever beyond seven days, and eleven had cough beyond seven days. None in the indomethacin arm was desaturated. This was also reflected in the fourteenth-day state of the patients.
The viral load reduction was better for indomethacin. However, the difference was not significant. Therefore, seven days may be too early for an RT-PCR test.
Limitation of the study
The limitation of the study is that indomethacin was administered with standard care and not as a stand-alone treatment. With this result, indomethacin may be tried as a stand-alone treatment. In addition, this is not a double-blinded study. The trial involved only hospitalized patients and not under home isolation.
Conclusions
Indomethacin use alongside standard treatment protocol in hospitalised covid-19 patients was associated with significant symptomatic relief and improved oxygen saturation level. Twenty of the 107 patients were desaturated in the paracetamol group, while out of 103 patients none in the indomethacin group was desaturated. The median days for the patients in the indomethacin group to become afebrile was three, while for those in the paracetamol group was seven days. The median days for the resolution of cough and myalgia for the indomethacin group was four days, while it was seven days for the paracetamol group.
We did not observe any adverse effects.
Supplementary data
Anonymised patient data are given in supplementary data file 1. If further data is required, please contact the corresponding author.
Supplementary Information
Acknowledgements
The authors acknowledge the generous funding for this study by Mr. Kris Gopalakrishnan, Alumnus, Indian Institute of Technology Madras.
Author contributions
Author RR: Conception and lead resource and lead clinician. Authors KS, DK, SSD: Clinical study conduction, coordination and data collection. Author SS: Clinical help to A. Author SO: Test technician. Author SV: Protocol Design and data interpretation. Author KR: Data-analysis, interpretation, and manuscript preparation—Dr. Ramarathnam Krishna Kumar.
Funding
Funding for this study was provided by Mr Kris Gopalakrishnan, an Alumnus of the Indian Institute of Technology Madras. He is also the Chairman of Axilor Ventures. However, he did not participate in the study in any way.
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this Article was revised: The original version of this Article contained an error. Supplementary data file 1 containing anonymised patient data was inadvertently omitted.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
6/20/2022
A Correction to this paper has been published: 10.1038/s41598-022-15107-8
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-10370-1.
References
- 1.V’kovski P, Kratzel A, Steiner S, Stalder H, Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021;19(3):155–170. doi: 10.1038/s41579-020-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, White KM, et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583(7816):459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X, Guan Y. COVID-19 drug repurposing: A review of computational screening methods, clinical trials, and protein interaction assays. Med. Res. Rev. 2021;41(1):5–28. doi: 10.1002/med.21728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao M-M, Yang W-L, Yang F-Y, Zhang L, Huang W-J, Hou W, et al. Cathepsin L plays a key role in SARS-CoV-2 infection in humans and humanized mice and is a promising target for new drug development. Signal Transd. Target. Therapy. 2021;6(1):134. doi: 10.1038/s41392-021-00558-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomes CP, Fernandes DE, Casimiro F, da Mata GF, Passos MT, Varela P, et al. Cathepsin L in COVID-19: from pharmacological evidences to genetics. Front. Cell. Infect. Microbiol. 2020;2020:10. doi: 10.3389/fcimb.2020.589505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frediansyah A, Tiwari R, Sharun K, Dhama K, Harapan H. Antivirals for COVID-19: a critical review. Clin. Epidemiol. Glob. Health. 2021;9:90–98. doi: 10.1016/j.cegh.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gil C, Ginex T, Maestro I, Nozal V, Barrado-Gil L, Cuesta-Geijo MÁ, et al. COVID-19: drug targets and potential treatments. J. Med. Chem. 2020;63(21):12359–12386. doi: 10.1021/acs.jmedchem.0c00606. [DOI] [PubMed] [Google Scholar]
- 8.Soy M, Keser G, Atagündüz P, Tabak F, Atagündüz I, Kayhan S. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020;39(7):2085–2094. doi: 10.1007/s10067-020-05190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon DE, Gordon DE, Hiatt J, Bouhaddou M, Rezelj VV, Ulferts S. Comparative host-coronavirus protein interaction networks reveal pan-viral disease mechanisms. Science. 2020;9403(10):1–38. doi: 10.1126/science.abe9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amici C, Di Caro A, Ciucci A, Chiappa L, Castilletti C, Martella V, et al. Indomethacin has a potent antiviral activity against SARS coronavirus. Antivir. Ther. 2006;11(8):1021–1030. doi: 10.1177/135965350601100803. [DOI] [PubMed] [Google Scholar]
- 11.Xu T, Gao X, Wu Z, Selinger DW, Zhou Z. Indomethacin has a potent antiviral activity against SARS CoV-2 in vitro and canine coronavirus in vivo. bioRxiv. 2020;12:8. [Google Scholar]
- 12.Napolitano, F., Gambardella, G., Carrella, D., Gao, X., & di Bernardo, D. Computational drug repositioning and elucidation of mechanism of action of compounds against SARS-CoV-2 (2020)
- 13.Raghav N, Kamboj RC, Singh H. Effect of some steroidal and non-steroidal anti-inflammatory drugs on purified goat brain cathepsin L. Indian J. Med. Res. B Biomed. Res. Other Infect. Dis. 1993;98:188–192. [PubMed] [Google Scholar]
- 14.Amici C, La Frazia S, Brunelli C, Balsamo M, Angelini M, Santoro MG. Inhibition of viral protein translation by indomethacin in vesicular stomatitis virus infection: role of eIF2α kinase PKR. Cell. Microbiol. 2015;17(9):1391–1404. doi: 10.1111/cmi.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunelli C, Amici C, Angelini M, Fracassi C, Belardo G, Santoro MG. The non-steroidal anti-inflammatory drug indomethacin activates the eIF2αkinase PKR, causing a translational block in human colorectal cancer cells. Biochem. J. 2012;443(2):379–386. doi: 10.1042/BJ20111236. [DOI] [PubMed] [Google Scholar]
- 16.Liu F, Li L, Xu M, Wu J, Luo D, Zhu Y, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J. Clin. Virol. 2020;127:104370. doi: 10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahmudpour M, Roozbeh J, Keshavarz M, Farrokhi S, Nabipour I. COVID-19 cytokine storm: the anger of inflammation. Cytokine. 2020;133:155151. doi: 10.1016/j.cyto.2020.155151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bour AMJ, Westendorp RG, Laterveer J, Bollen ELE, Remarque E. Interaction of indomethacin with cytokine production in whole blood. Potential mechanism for a brain-protective effect. Exp. Gerontol. 2000;35(8):1017–1024. doi: 10.1016/S0531-5565(00)00128-5. [DOI] [PubMed] [Google Scholar]
- 19.First MR, Schroeder TJ, Hariharan S, Alexander JW, Weiskittel P. The effect of indomethacin on the febrile response following OKT3 therapy1. Transplantation. 1992;53(1):91–93. doi: 10.1097/00007890-199201000-00017. [DOI] [PubMed] [Google Scholar]
- 20.Gaughan WJ, Francos BB, Dunn SR, Francos GC, Burke JF. A retrospective analysis of the effect of indomethacin on adverse reactions to orthoclone OKT3 in the therapy of acute renal allograft rejection. Am. J. Kidney Dis. 1994;24(3):486–490. doi: 10.1016/S0272-6386(12)80906-1. [DOI] [PubMed] [Google Scholar]
- 21.Ravichandran R, Purna P, Vijayaragavan S, Kalavakollu RT, Gaidhane S, Kumar RK. Use of indomethacin in COVID-19 patients: experience from two medical centres. J. Indian Med. Assoc. 2021;119(7):42–46. [Google Scholar]
- 22.Rajan R, Subramanian S, Clark C. Low dose indomethacin for symptomatic treatment of COVID-19. Int. J. Med. Rev. Case Rep. 2020;2020:1. doi: 10.5455/IJMRCR.LOW-DOSE-INDOMETHACIN. [DOI] [Google Scholar]
- 23.Kanakaraj A, Ravichandran R. Low dose indomethacin in the outpatient treatment of COVID-19 in kidney transplant recipients—a case series. OALib. 2020;07(10):1–8. doi: 10.4236/oalib.1106860. [DOI] [Google Scholar]
- 24.Saghaei M. Random allocation software for parallel group randomized trials. BMC Med. Res. Methodol. 2004;4(1):26. doi: 10.1186/1471-2288-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakpal T. Sample size estimation in clinical trial. Perspect. Clin. Res. 2010;1(2):67. [PMC free article] [PubMed] [Google Scholar]
- 26.Momekov G, Momekova D. Ivermectin as a potential COVID-19 treatment from the pharmacokinetic point of view: antiviral levels are not likely attainable with known dosing regimens. Biotechnol. Biotechnol. Equip. 2020;34(1):469–474. doi: 10.1080/13102818.2020.1775118. [DOI] [Google Scholar]
- 27.Donnelly P, Lloyd K, Campbell H. Indomethacin in rheumatoid arthritis: an evaluation of its anti-inflammatory and side effects. BMJ. 1967;1(5532):69–75. doi: 10.1136/bmj.1.5532.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Indomethacin drug usage statistics, United States, 2018 [Internet]. 2018. https://clincalc.com/DrugStats/Drugs/Indomethacin
- 29.Alkotaji M, Al-Zidan RN. Indomethacin: can it counteract bradykinin effects in COVID-19 patients? Curr. Pharmacol. Rep. 2021;7(3):102–106. doi: 10.1007/s40495-021-00257-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.