Abstract
In the present study we showed by molecular analysis that the inhibition of motility by macrolides in Proteus mirabilis and Pseudomonas aeruginosa was well correlated with the loss of the expression of flagellin. Erythromycin, clarithromycin, and azithromycin at subinhibitory concentrations (sub-MICs) suppressed the expression of flagellin dose dependently. Azithromycin had the strongest inhibitory effect on the expression of P. aeruginosa flagellin, whereas 16-membered rokitamycin had only a weak inhibitory effect. These results indicate the potential effectiveness of sub-MICs of erythromycin, clarithromycin, and azithromycin for the treatment of patients with P. mirabilis and P. aeruginosa infections.
Macrolide antibiotics have been widely used to treat various infections. They bind to the 50S ribosomal subunit, resulting in blockage of transpeptidation and/or translocation. The antimicrobial activity of macrolides is broad in spectrum, being exhibited against gram-positive and some gram-negative bacteria, such as Neisseria spp., Campylobacter spp., Haemophilus spp., and Legionella spp. (4). In contrast, most species of Enterobacteriaceae and nonglucose-fermenting gram-negative rods such as Pseudomonas spp. are innately resistant to macrolides (15).
Some reports have demonstrated that the treatment of bacteria with subinhibitory concentrations (sub-MICs) of macrolide antibiotics suppressed the expression of bacterial virulence factors in various gram-negative rods (5, 8). Although Pseudomonas aeruginosa is generally highly resistant to macrolides, erythromycin (ERY) suppressed the production of exotoxin A and proteases by this organism at concentrations well below the MIC (6). It was also demonstrated that sub-MICs of macrolide antibiotics decreased protein synthesis (18), enhanced the sensitivity of P. aeruginosa in serum (17), and suppressed biofilm formation through inhibition of alginic acid (7). Based on these reports, certain macrolides at sub-MICs were expected to have clinical effects on patients with respiratory infections caused by gram-negative rods such as P. aeruginosa, and in fact long-term low-dose administration of ERY produced clinical improvement in patients with diffuse pulmonary panbronchiolitis associated with P. aeruginosa infection (16).
The present study was therefore undertaken to clarify the in vitro effectiveness of sub-MICs of macrolides as assessed by the inhibition of flagellin expression in P. aeruginosa and Proteus mirabilis. Flagella are among the virulence factors of gram-negative rods and have a role in the initiation of biofilm formation (14). Early studies showed that exposure to sub-MICs of the macrolide azithromycin (AZM) resulted in loss of motility due to the absence of flagella in P. mirabilis and P. aeruginosa (10, 11). However, these observations were based on the conventional light-microscopic examinations and therefore were not quantitative. We describe here the results of molecular analysis of the flagellin inhibition by sub-MICs of macrolides.
Nonmucoid P. aeruginosa NGM111 isolated from the sputum of a patient with respiratory infection and P. mirabilis NGM007 isolated from a patient with urinary tract infection were used in this study.
The antibiotics ERY (Shionogi Pharmaceutical Co., Ltd., Osaka, Japan), clarithromycin (CLR) (Taisho Pharmaceutical Co., Ltd., Tokyo, Japan), AZM (Pfizer Laboratories, Groton, Conn.), rokitamycin (ROM) (Asahi Chemical Industry Co., Ltd., Tokyo, Japan), chloramphenicol (CHL) (Sigma, St. Louis, Mo.), minocycline (MIN) (Lederle Japan, Ltd., Tokyo, Japan), tetracycline (TET) (Sigma), and gentamicin (GEN) (Schering Plough. K.K., Osaka, Japan) were used. The MICs of the antibiotics were determined by the agar dilution method in Mueller-Hinton agar (Difco Laboratories, Detroit, Mich.). Approximately 105 log-phase organisms were inoculated onto the antibiotic-containing agar, and the MICs were defined as the lowest concentration of antibiotics that inhibited the visible growth of bacteria after 18 h of incubation at 35°C (13). The MICs were in good agreement with those measured on 0.3% agar plates, which were used for motility assays.
Motility assays were performed according to the method described by Umemura et al. (20), with some modification. Bacteria grown in Luria broth (LB) at 37°C to an optical density at 660 nm of 0.1 were inoculated onto 0.3% agar LB plates in the presence or absence of 0.25 times the MICs of the antibiotic and incubated at 30°C for 18 h to optimize flagellin protein synthesis. The diameters of the growth zone were measured in the control and antibiotic-containing plates and were expressed as degree of bacterial motility. The experiments were repeated three times, and the results were expressed as the mean values ± standard deviations.
Flagellin protein was prepared by the method of Montie et al. (12) with a slight modification. Cells were cultured on heart infusion (HI) agar at 37°C for 18 h. The cells were then gently scraped from the agar surface and suspended in phosphate-buffered saline (PBS) (pH 7.4). The cell suspension was centrifuged for 15 min at 5,000 × g and 4°C. The resulting pellet was resuspended in PBS and blended in a commercial blender for 3 min to shear off the flagella. The suspension was centrifuged for 15 min at 16,000 × g and 4°C, and the resulting supernatant was centrifuged again for 3 h at 40,000 × g and 4°C. The supernatant was carefully removed, and the pellet was suspended in a small amount of PBS and used for further experiments as a preparation of flagellin. The purity of flagellin preparations was demonstrated to be about 90% by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The total protein concentrations in these samples were determined using a Micro BCA protein assay reagent kit (Pierce, Rockford, Ill.). A portion of each flagellin preparation was boiled for 3 min in Laemmli buffer (9) containing 0.1% β-mercaptoethanol and was separated by SDS–10% PAGE. The separated sample was transferred onto a Protoblot membrane (Applied Biosystems, Foster City, Calif.) by electrophoresis using a semidry blotting system (transfer apparatus model NA-1512; Nihon Eido, Co., Ltd., Tokyo, Japan) and visualized with Coomassie blue according to the manufacturer's instructions. Protein spots were excised from the membrane and sequenced in a Perkin-Elmer Biosystems (Foster City, Calif.) 492cLC protein sequencer. GENETYX-MAC/DB was used to conduct a computer-assisted homology search against the NBRF protein database.
The MICs of macrolide antibiotics for P. mirabilis NGM007 and P. aeruginosa NGM111 were higher than 64 mg/liter for ERY, CLR, AZM, and ROM, and these values were somewhat higher than those of the other classes of antibiotics (Table 1). In contrast to their high MICs, ERY, CLR, and AZM exhibited strong swarming inhibition against P. mirabilis and P. aeruginosa at 0.25 times the MIC, concentrations at which these organisms showed no apparent growth inhibition. The inhibitory effect of AZM on the swarming of P. aeruginosa under these assay conditions was highest (Table 1). Additionally, the pigment production of P. aeruginosa was also suppressed on the plates containing 0.25 times the MIC of ERY, CLR, and AZM, but not other classes of antibiotics (data not shown).
TABLE 1.
MICs and effects of sub-MIC of various antibiotics on bacterial motility
| Antibiotic | MIC (mg/liter) for:
|
Motility inhibitiona (%)
|
||
|---|---|---|---|---|
| P. mirabilis | P. aeruginosa | P. mirabilis | P. aeruginosa | |
| ERY | 512 | 128 | 94 ± 1.3b | 32 ± 1.6b |
| CLR | 256 | 64 | 98 ± 0.7b | 24 ± 3.0b |
| AZM | 128 | 128 | 84 ± 2.2b | 83 ± 2.5b |
| ROM | 256 | 256 | 0 | 0 |
| CHL | 256 | 16 | 33 ± 2.6b | 12 ± 5.1b |
| GEN | 2 | 1 | 0 | 2 ± 0.6 |
| TET | 64 | 8 | 0 | 0 |
| MIN | 16 | 16 | 0 | 0 |
Results are expressed as percent of inhibition of the circular zone of growth on soft agar plates containing 0.25 times the MICs of antibiotics (means ± standard deviations of three experiments).
The Student t test was used to determine differences between each value and its mean. A significant difference was seen between this treatment and the control (P < 0.05).
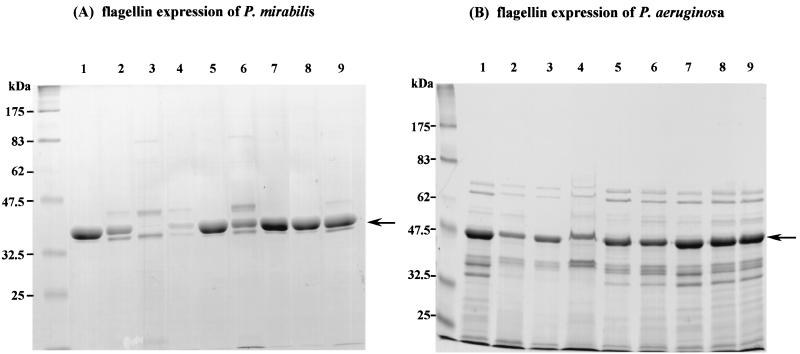
To examine whether the inhibition of motility by the antibiotics was related to flagellin production, the flagellin fraction of each strain was prepared by differential centrifugation. Flagellin proteins appeared as single major bands on SDS-PAGE gels. The identity of these bands as flagellin was confirmed by the fact that their N-terminal amino acid sequences were identical with previously published sequences of P. mirabilis and P. aeruginosa flagellin proteins (2, 19). The effect of the antibiotics on the expression of flagellin was evaluated as the change in intensity of flagellin band on SDS-PAGE gels. Cells cultured on plates containing 0.25 times the MIC of the antibiotics for 18 h were carefully collected, and the flagellin fraction was prepared and analyzed by SDS-PAGE. Macrolides ERY, CLR, and AZM, as well as CHL, suppressed the expression of flagellin (Fig. 1).
FIG. 1.
Effect of antibiotics on flagellin expression. P. mirabilis (A) and P. aeruginosa (B) were grown on HI plates containing 0.25 times the MICs of various antibiotics for 18 h, and the flagellin preparation of each bacterium was analyzed by SDS-PAGE as described in the text. Arrows indicate flagellin bands. Lane 1, no antibiotic; lane 2, ERY; lane 3, CLR; lane 4, AZM; lane 5, ROM; lane 6, CHL; lane 7, GEN; lane 8, TET; lane 9, MIN.
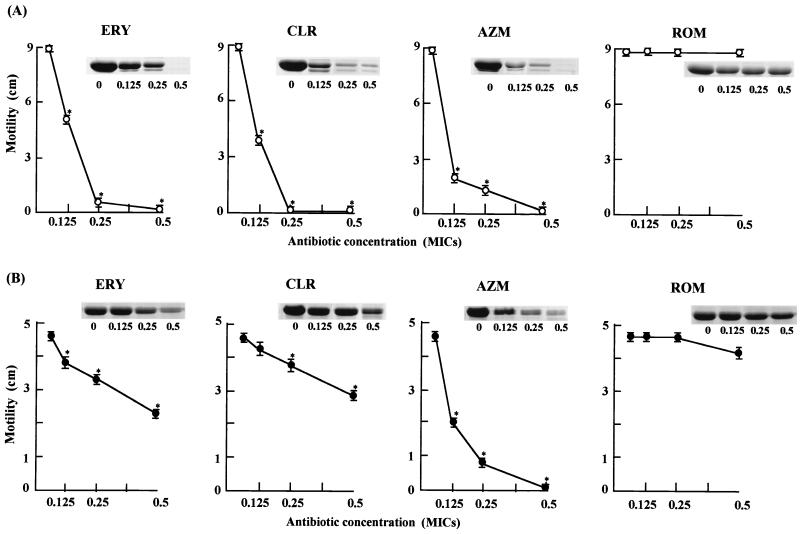
To evaluate the dose effect of macrolides on bacterial motility, cells were incubated on 0.3% agar LB plates containing 0.125, 0.25, and 0.5 times the MICs of the various macrolides for 18 h at 30°C. Similarly, expression of flagellin in the same concentrations of macrolides was measured. Cells were collected from HI plates containing 0.125, 0.25, and 0.5 times the MICs of macrolides after incubation for 18 h at 30°C, and flagellin was extracted and analyzed as described above. The suppression effects on bacterial motility were dose-dependent and were roughly correlated with the inhibitory effects on the expression of flagellin (Fig. 2). The 16-membered macrolide antibiotic ROM had only poor inhibitory effects on bacterial motility and expression of flagellin. These results suggest that the motility-inhibiting effects of macrolides may be due to the suppression of the flagellin expression. This conclusion was consistent with an early observation that some macrolides at sub-MICs induced the loss of flagella and thereby caused a reduction of motility (10). It is noteworthy that AZM showed a much stronger inhibitory effect on the motility of P. aeruginosa than the other macrolides.
FIG. 2.
Effect of macrolide antibiotics on bacterial motility and flagellin expression. P. mirabilis (A) and P. aeruginosa (B) were grown on plates with increasing doses of macrolide antibiotics for 18 h, and the motility and the flagellin preparation of each bacterium were analyzed as described in the text. Results are expressed as means ± standard deviations (error bars) of three experiments. Asterisks indicate statistical difference (P < 0.05) from the control growth without antibiotics.
Recent studies have provided evidence that macrolide antibiotics suppressed the expression of some virulence factors in gram-negative rods at concentrations which were subinhibitory for bacterial growth (5, 8). Motility in P. aeruginosa, which contributes to the biofilm formation, was also suppressed by certain macrolides (10, 11). The inhibition of bacterial motility can be caused by inhibition of two major steps: the production of flagellin and the energy-dependent flagellar movement. Our preliminary observations using phase-contrast microscopy showed that macrolides did not have suppressive effects on the bacterial movement at any dose (data not shown), suggesting that the movement of already-expressed flagella was not inhibited by macrolides. The present study indicated that the production of flagellin protein was suppressed dose dependently by sub-MICs of 14- and 15-membered macrolides but not by the 16-membered macrolide antibiotic ROM. It is noteworthy that the inhibitory effect of AZM on the expression of flagellin in P. aeruginosa was much stronger than the effects of other macrolides.
These results indicate that sub-MICs of ERY, CLR, and AZM may be clinically useful for the treatment of patients with P. aeruginosa and P. mirabilis infections through inhibition of the biofilm formation. Although the MICs of macrolide antibiotics for P. mirabilis and P. aeruginosa were 64 mg/liter or higher, we showed that ERY and AZM even at 0.125 times the MIC for P. aeruginosa and ERY, CLR, and AZM at 0.125 times the MIC for P. mirabilis were effective in motility assay (Fig. 2). These concentrations may be clinically achievable, because recent investigations showed that the concentration of CLR in lung epithelial lining fluid reached 39.6 ± 41.1 mg/liter (3) and the concentration of AZM in alveolar macrophages reached 23 mg/liter (1).
REFERENCES
- 1.Baldwin D R, Wise R, Andrews J M, Ashby J P, Honeybourne D. Azithromycin concentrations at the sites of pulmonary infection. Eur Respir J. 1990;3:886–890. [PubMed] [Google Scholar]
- 2.Belas R, Flaherty D. Sequence and genetic analysis of multiple flagellin-encoding genes from Proteus mirabilis. Gene. 1994;148:33–41. doi: 10.1016/0378-1119(94)90230-5. [DOI] [PubMed] [Google Scholar]
- 3.Conte J E, Jr, Golden J, Duncan S, Mckenna E, Lin E, Zurlinden E. Single-dose intrapulmonary pharmacokinetics of azithromycin, clarithromycin, ciprofloxacin, and cefuroxime in volunteer subjects. Antimicrob Agents Chemother. 1996;40:1617–1622. doi: 10.1128/aac.40.7.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gribble M J, Chow A W. Erythromycin. Med Clin N Am. 1982;66:79–89. doi: 10.1016/s0025-7125(16)31443-2. [DOI] [PubMed] [Google Scholar]
- 5.Grimwood K, To M, Rabin H R, Woods D E. Inhibition of Pseudomonas aeruginosa exoenzyme expression by subinhibitory antibiotic concentrations. Antimicrob Agents Chemother. 1989;33:41–47. doi: 10.1128/aac.33.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirakata Y, Kaku M, Mizukane R, Ishida K, Furuya N, Matsumoto T, Tateda K, Yamaguchi K. Potential effects of erythromycin on host defense systems and virulence of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1992;36:1922–1927. doi: 10.1128/aac.36.9.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ichimiya T, Takeoka K, Hiramatsu K, Hirai K, Yamasaki T, Nasu M. The influence of azithromycin on the biofilm formation of Pseudomonas aeruginosa in vitro. Chemotherapy. 1996;42:186–191. doi: 10.1159/000239440. [DOI] [PubMed] [Google Scholar]
- 8.Kita E, Sawaki M, Oku D, Hamuro A, Mikasa K, Konishi M, Emoto M, Takeuchi S, Narita N, Kashiba S. Suppression of virulence factors of Pseudomonas aeruginosa by erythromycin. J Antimicrob Chemother. 1991;27:273–284. doi: 10.1093/jac/27.3.273. [DOI] [PubMed] [Google Scholar]
- 9.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 10.Molinari G, Paglia P, Schito G C. Inhibition of motility of Pseudomonas aeruginosa and Proteus mirabilis by subinhibitory concentrations of azithromycin. Eur J Clin Microbiol Infect Dis. 1992;11:469–471. doi: 10.1007/BF01961867. [DOI] [PubMed] [Google Scholar]
- 11.Molonari G, Guzman C A, Pesce A, Schito G C. Inhibition of Pseudomonas aeruginosa virulence factors by subinhibitory concentrations of azithromycin and other macrolide antibiotics. J Antimicrob Chemother. 1993;31:681–688. doi: 10.1093/jac/31.5.681. [DOI] [PubMed] [Google Scholar]
- 12.Montie T C, Craven R C, Holder I A. Flagellar preparations from Pseudomonas aeruginosa: isolation and characterization. Infect Immun. 1982;35:281–288. doi: 10.1128/iai.35.1.281-288.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. Approved standard M2-A6. 1997. Villanova, Pa. [Google Scholar]
- 14.O'Toole G A, Kolter R. Flagella and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol. 1998;30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- 15.Rozgonyi F, Papp-Falusi E, Varga J, Rozgonyi-Szitha K. In-vitro activity of cefetamet (Ro15-8074) compared with other oral agents. J Antimicrob Chemother. 1989;24:539–546. doi: 10.1093/jac/24.4.539. [DOI] [PubMed] [Google Scholar]
- 16.Sawaki M, Mikami R, Mikasa K, Kunimatsu M, Ito S, Narita N. The long term chemotherapy with erythromycin in chronic lower respiratory tract infections. Second report, including cases with pseudomonas infections. Kansenshogaku Zasshi. 1986;60:45–50. doi: 10.11150/kansenshogakuzasshi1970.60.45. . (In Japanese.) [DOI] [PubMed] [Google Scholar]
- 17.Tateda K, Hirakawa Y, Furuya N, Ohno A, Yamaguchi K. Effects of sub-MICs of erythromycin and other macrolide antibiotics on serum sensitivity of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1993;37:675–680. doi: 10.1128/aac.37.4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tateda K, Ishii Y, Matsumoto T, Furuya N, Nagashima M, Matsunaga T, Ohno A, Miyazaki S, Yamaguchi K. Direct evidence for antipseudomonal activity of macrolides: exposure-dependent bactericidal activity and inhibition of protein synthesis by erythromycin, clarithromycin, and azithromycin. Antimicrob Agents Chemother. 1996;40:2271–2275. doi: 10.1128/aac.40.10.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Totten P A, Lory S. Characterization of the type a flagellin gene from Pseudomonas aeruginosa PAK. J Bacteriol. 1990;172:7188–7199. doi: 10.1128/jb.172.12.7188-7199.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Umemura T, Tatsuno I, Shibasaki M, Honma M, Kawagishi I. Intersubunit interaction between transmembrane helices of the bacterial aspartate chemoreceptor homodimer. J Biol Chem. 1998;273:30110–30115. doi: 10.1074/jbc.273.46.30110. [DOI] [PubMed] [Google Scholar]