Abstract
Patients with bipolar disorder (BP) often report subjective mood improvements after smoking marijuana (MJ); however, empirical studies supporting this claim have not been conducted. We conducted this study to determine if marijuana has an impact on mood in bipolar patients who smoke marijuana (MJBP), hypothesizing MJBP participants would experience improved mood after smoking MJ. All participants completed electronic mood ratings three times daily and recorded episodes of MJ use using Palm Pilot devices in their own environments in order to examine the impact of MJ use on mood in MJ-smoking bipolar patients (n = 12) and pure MJ smokers (MJ; n = 20). Difference scores were calculated between pre and post-MJ scales. Patients with BP (n = 11) who did not smoke MJ were also included as a comparison group. Significant mood improvement was observed in the MJBP group on a range of clinical scales after smoking MJ, while the MJ group reported a slight worsening of symptoms. Notably, total mood disturbance, a composite of the Profile of Mood States, was significantly reduced in the MJBP group, but increased in the MJ group after smoking. Further, while the MJBP group reported generally worse mood ratings than the BP group prior to smoking MJ, they demonstrated improvement on several scales post-MJ use as compared to BP participants. These data provide empirical support for anecdotal reports that MJ acts to alleviate mood-related symptoms in at least a subset of bipolar patients and underscore the importance of examining MJ use in this population.
Keywords: marijuana, mood, bipolar disorder, ecological momentary assessment, comorbidity
Introduction
Bipolar disorder (BP) affects approximately 2.3 million individuals in the United States alone (National Institute of Public Health, 2008) and is considered one of the most debilitating of the mood disorders. In those affected, BP can be a significant source of distress, disability, and burden on relatives and caregivers (Woods, 2000). It is not surprising, therefore, that BP carries high risks of comorbidity, particularly with substance abuse. In fact, among psychiatric patients, those with bipolar I disorder have been reported to have the highest lifetime rates of drug use disorders compared to those with other Axis I disorders (Grant & Harford, 1995; Reiger et al., 1990), and lifetime rates of drug abuse or dependence in bipolar patients ranges from 14% to 65% as compared to the rate of 6%–12% in the general population (Brown, Suppes, Adinoff, & Thomans, 2001). Additionally, individuals with mania are more than eight times more likely to suffer from drug dependence within the past 12 months and nearly nine times more likely to have lifetime drug dependence compared to the general population (Kessler et al., 1996).
Patients with co-occurring BP and substance use disorder often experience poor treatment response, relapse of mood symptoms, psychosocial difficulties, and less adherence to medication regimens (Strakowski et al., 1998; Tohen, Greenfield, Weiss, Zarate, & Vagge, 1998; van Rossum et al., 2009). Several hypotheses have been suggested regarding the high rates of substance use by patients with BP, however, the precise reasons for this comorbidity remain unknown. While some have posited a self medication theory, which states that drugs are used to relieve specific psychiatric symptoms that results in repetitive use (Khantzian, 1997), others maintain that no single hypothesis can account for all cases of comorbidity (Strakowski & DelBello, 2000). Although a large percentage of patients comorbid for BP and substance use report using drugs to improve mood related symptoms (Sonne, Brady, & Morton, 1994), reports of whether the patients viewed the use as successful is rarely assessed. Weiss et al. (2004) examined the reasons for substance use and the perception of improvement following substance use among the bipolar patients themselves; the authors reported that nearly all patients initiated substance use as the result of at least one bipolar symptom, most commonly depression or racing thoughts, and that the majority of patients reported improvement in at least one symptom as a result of the substance use.
Marijuana (MJ) is the most commonly abused illicit substance in BP (Strakowski et al., 2007), with 20%–50% of patients reporting some form of MJ-related problems (Cerullo & Strakowski, 2007). In previous studies of BP, rates of MJ use disorders have even been found to equal or exceed those of alcohol abuse or dependence, particularly in younger patients (Strakowski et al., 2007). Agrawal, Nurnberger, Lynskey, and The Bipolar Genome Study (2011) recently reported that individuals with BP were 6.8 times more likely to report a lifetime history of MJ use relative to healthy controls. Importantly, in those participants who endorsed MJ-related problems, 63.7% reported disability, as compared to only 44.5% of those not meeting criteria for MJ use disorders, supporting previous findings that patients with BP who engage in MJ use exhibit lower levels of compliance with prescribed medications and higher levels of illness severity (Henquet, Krabbendam, de Graaf, ten Have, & van Os, 2006; Strakowski et al., 2007; van Rossum et al., 2009). The use of MJ has been associated with a fivefold increase in the risk of a first episode of BP, while the risk for a major depressive episode is only modestly increased (van Laar, van Dorsselaer, Monshouwer, & de Graaf, 2007). Despite these findings, bipolar patients frequently report subjective improvement in both manic and depressive symptoms as a result of MJ use (Ashton, Moore, Gallagher, & Young, 2005; Grinspoon & Bakalar, 1998; Gruber, Pope, & Brown, 1996), which raises the question of whether MJ acts to alleviate mood-related symptoms for at least a subset of patients and underscores the importance of examining MJ use in those with BP.
In the current study, we hypothesized that marijuana smokers with bipolar I disorder would exhibit significant mood improvements after smoking MJ relative to marijuana smokers without BP. In order to assess the impact of marijuana on mood, we utilized an ecological momentary assessment (EMA) method in which participants used portable electronic devices to report their mood at multiple points throughout the day as well as episodes of marijuana use. EMAs have been successfully applied in studies of substance use, including both nicotine and MJ (Bucker et al., 2011; Carter et al., 2010; Hopper et al., 2006; van Zundert, Booger, Vermulst, & Engels, 2009). This method has also been shown to provide enhanced reliability from frequent sampling, provide real-time information from participants in their own natural environments, and generate greater adherence and willingness to respond honestly compared to traditional reporting methods (Stone, Shiffman, Schwartz, Broderick, & Hufford, 2003; Turner et al., 1998).
Materials and methods
Participants
Selected from an ongoing neuroimaging study, 12 MJ-smoking patients with bipolar disorder (MJBP), 20 MJ smokers without Axis 1 pathology (MJ), and 11 patients with BP who did not smoke MJ were included in this investigation. Both the MJ and BP groups served as comparison groups for the MJBP group in order to determine the specific relationship between mood and marijuana use in bipolar participants. Participants were recruited from the greater Boston, MA area by posting flyers for marijuana smokers and bipolar participants in both downtown and suburban locations. Recruitment sites included the McLean Hospital campus, local colleges and universities, sports clubs and athletic centers, supermarkets, community centers, other public locations, and clinical referrals. As marijuana is an illegal substance, a Certificate of Confidentiality was obtained for this study from the National Institute of Drug Abuse (NIDA), which provides an additional level of privacy for participants. With this certificate in place, researchers cannot be forced to disclose information that may identify study participants even by a court subpoena, in any federal, state, or local civil, criminal, administrative, legislative, or other proceedings. It is also of note that subjects were not asked to change their MJ smoking behavior (i.e. to smoke more or less MJ or to smoke at certain times); participants were simply asked to record episodes of MJ use on the palm pilot devices as they naturally occurred. Prior to participation, study procedures were explained, and all participants were required to read and sign an informed consent form approved by the McLean Hospital Institutional Review Board, which described in detail the procedures of the study and explained that participation in the study was voluntary.
All participants received the Structured Clinical Interview for DSM-IV, Patient Edition (SCID-P – First, Spitzer, Gibbon, & Williams, 1994) to ensure that no Axis I pathology was present other than bipolar I disorder in both bipolar groups (MJBP and BP) and that they did not meet criteria for current or previous drug or alcohol abuse or dependence, excluding MJ for the MJBP and MJ groups. Additionally, general intellectual functioning was assessed using the four-factor Wechsler Abbreviated Scale of Intelligence (WASI – Wechsler, 1999).
In order to qualify for study entry, participants in the MJBP and MJ groups had to have smoked MJ a minimum of 2500 times in their lives, used MJ at least five of the last seven days, test positive for urinary cannabinoids, and meet DSM-IV criteria for MJ abuse or dependence. Comprised entirely of outpatients, the MJBP and BP groups were required to meet DSM-IV criteria for bipolar I disorder. Participants were excluded if they reported more than 15 lifetime uses of any category of illicit drugs (excluding MJ for the smoking groups), routinely had more than 15 drinks per week, were non-native English speakers, or if they had ever experienced a head injury or received electroconvulsive therapy. Urine samples were tested for MJ, amphetamines, opioids, phencyclidine, barbiturates, benzodiazepines, and cocaine (Triage® Drugs of Abuse Panel: Immediate Response Diagnostics, San Diego, CA), and a positive urine screen for any drug of abuse other than MJ in the smoking groups led to exclusion from the study. This procedure also ensured that participants in the MJ smoking groups had used MJ recently enough to have a positive urine screen. An aliquot of the urine sample was sent to an outside laboratory for quantification of urinary cannabinoid concentration via gas chromatography–mass spectrometry (GC–MS).
Assessments and procedures
All participants were issued a Palm Pilot (Palm Tungsten T5 PalmOne PDA) and instructed to use the device to rate their mood three times daily for four weeks using a custom-designed, preloaded application containing electronic versions of several clinical rating scales, including the Hamilton Anxiety Rating Scale (HAM-A – Hamilton, 1959), Montgomery–Asberg Depression Rating Scale (MADRS – Montgomery & Asberg, 1979), and Young Mania Rating Scale (YMRS – Young, Biggs, Ziegler, & Meyer, 1978). Additionally, participants completed the Profile of Mood States (POMS – Pollock, Cho, Reker, & Volavka, 1979), which yields subscores for vigor, anger, confusion, tension, fatigue, depression, and a composite score for total mood disturbance (TMD). Each subject selected three times throughout the day (at least five hours apart), which were tailored to their typical daily schedule, to rate their mood. This allowed for the division of each day into three epochs for analysis purposes: morning, afternoon, and evening. Participants who smoked MJ were also asked to use the device to record episodes of MJ use in order to calculate mood changes pre- and post-MJ use. For each episode of MJ use, participants recorded amount (in grams), frequency and mode of MJ use (bong, bowl, joint, etc.). Date and time were automatically recorded for each scale and episode of MJ use in order to assist with accurate pre- and post-smoking determinations. To ensure that participants were not arbitrarily answering clinical rating questions, ‘quality control’ questions were interspersed throughout the scales, with such questions as ‘who is the current US president?’ and ‘how thoughtfully are you answering these questions?’ Palm Pilot data were downloaded during each of the subsequent weekly visits in order to monitor whether completion of mood scales over the previous week had been adequate.
In order to determine the effect of MJ on mood, change scores were obtained for each clinical rating scale within four hours of MJ smoking. Participants were asked to complete their clinical rating scales before their first MJ use of the day in order to obtain a daily baseline rating. Each subject’s pre-smoking scores and post-smoking scales were compiled and averaged to obtain pre-smoking and post-smoking means for each group. Determining these values allowed for within-group mood changes to be assessed for statistical significance as well. Individual delta or change scores were also calculated for each instance of smoking for each subject by subtracting baseline (pre-smoking) ratings from post-smoking ratings reported within four hours of MJ use. Delta scores were compiled to obtain averages for both smoking groups (MJBP and MJ) in order to assess between group differences in MJ-related mood changes. In order to explore the clinical significance of MJ use on mood within the MJBP group, we also compared average mood ratings from the BP group with both the pre- and post-MJ smoking mood ratings from the MJBP group.
Results
Demographics
As seen in Table 1, the MJBP group was well-matched to both the MJ and BP groups in terms of general intelligence, as measured by Verbal and Performance IQ scores of the WASI, although a significant difference in age was detected between the MJBP and MJ groups and between the MJBP and BP groups. Additionally, MJBP and BP groups were well-matched for age of BP onset. Table 2 highlights similarities between the MJBP and MJ groups in terms of marijuana use characteristics, which include calculations for age of onset of regular MJ use, urinary tetrahydrocannabinol (THC)/creatinine level, number of smoking episodes (smokes) per week, grams of marijuana used (grams) per week, and duration of MJ use (years).
Table 1.
Subject demographics.
| Characteristics | Mean (SD) | 2-Tailed t-test p values | |||
|---|---|---|---|---|---|
| MJBP (n = 12) |
MJ (n = 20) | BP (n = 11) | MJBP vs. MJ | MJBP vs. BP | |
| Age | 24.25 (4.29) | 20.75 (2.67) | 29.45 (7.19) | 0.01 | 0.05 |
| Age of MJ Onset | 16.67 (2.61) | 15.95 (1.61) | – | NS | – |
| Age of BP Onset | 14.92 (2.94) | – | 17.36 (4.65) | – | NS |
| VIQ | 119.00 (11.31) | 124.11 (13.36) | 119.91 (9.39) | NS | NS |
| PIQ | 111.36 (9.59) | 115.68 (7.52) | 115.45 (11.72) | NS | NS |
Note: VIQ, verbal IQ; PIQ, performance IQ.
Table 2.
Marijuana use characteristics by group.
| MJ-related variables | Mean (SD) | 2-Tailed t-test p values | |
|---|---|---|---|
| MJBP (n = 12) | MJ (n = 20) | MJBP vs. MJ | |
| Age of MJ onset | 16.67 (2.61) | 15.95 (1.61) | NS |
| Urinary THC/creatinine level (ng/mL) | 397.20 (526.30) | 347.87 (357.27) | NS |
| Smokes per week | 13.69 (14.20) | 14.14 (7.12) | NS |
| Grams per week | 4.58 (3.25) | 6.53 (5.66) | NS |
| Duration of MJ use (years) | 7.58 (2.64) | 4.85 (3.13) | 0.02 |
Note: THC, tetrahydrocannabinol.
In order to determine whether pharmacological regimen may have affected the differences in mood noted between the MJBP and BP groups at baseline, we assessed the frequency of use of mood stabilizers, antidepressants, antipsychotics, and benzodiazepines in both BP groups. Within the groups, 92% of MJBP participants and 82% of BP participants were maintained on a daily regimen that included one or more of the aforementioned drug classes. More specifically, in the MJBP group 64% of participants were treated with mood stabilizers, 36% with antidepressants, 64% with antipsychotics, and 18% with benzodiazepines. In the BP group, 75% of participants were treated with a mood stabilizer, 17% with antidepressants, 50% with antipsychotics, and 17% with benzodiazepines. No significant differences were found between groups for the number of participants maintained on each class of drugs, suggesting that pharmacological treatment did not affect between-group assessments of mood.
Mood ratings pre vs post MJ use
Sufficient mood data were available for analysis in 17 participants in the MJ group, nine in the MJBP group, and 10 in the BP group. Mood change was assessed in both MJBP and MJ participants after episodes of MJ use that occurred within four hours of a mood rating. Pre-MJ smoking mood scores were compared to post-smoking scores, revealing significant changes in mood in both the MJBP and MJ groups after MJ use (See Figure 1). As noted in Table 3, results indicated a statistically significant improvement within the MJBP group with lower HAM-A (p = 0.01) and MADRS (p = 0.005) scores after smoking MJ. MJBP participants also reported improvement on several measures of the POMS, including higher vigor (p = 0.003) and lower tension (p < 0.001), depression (p = 0.02) and notably, TMD (p = 0.001) scores. Decreases in anger (p = 0.09) and confusion (p = 0.09) after MJ use approached significance for the MJBP group. In contrast, after smoking MJ, the pure MJ group displayed a slight worsening of mood, with statistically significantly higher ratings on the HAM-A (p = 0.02) as well as confusion (p = 0.004), fatigue (p < 0.001), and TMD (p = 0.009) sub-scores of the POMS. The MJ group also demonstrated a trend for lower vigor on the POMS (p = 0.06) and higher YMRS (p = 0.09) scores.
Figure 1.
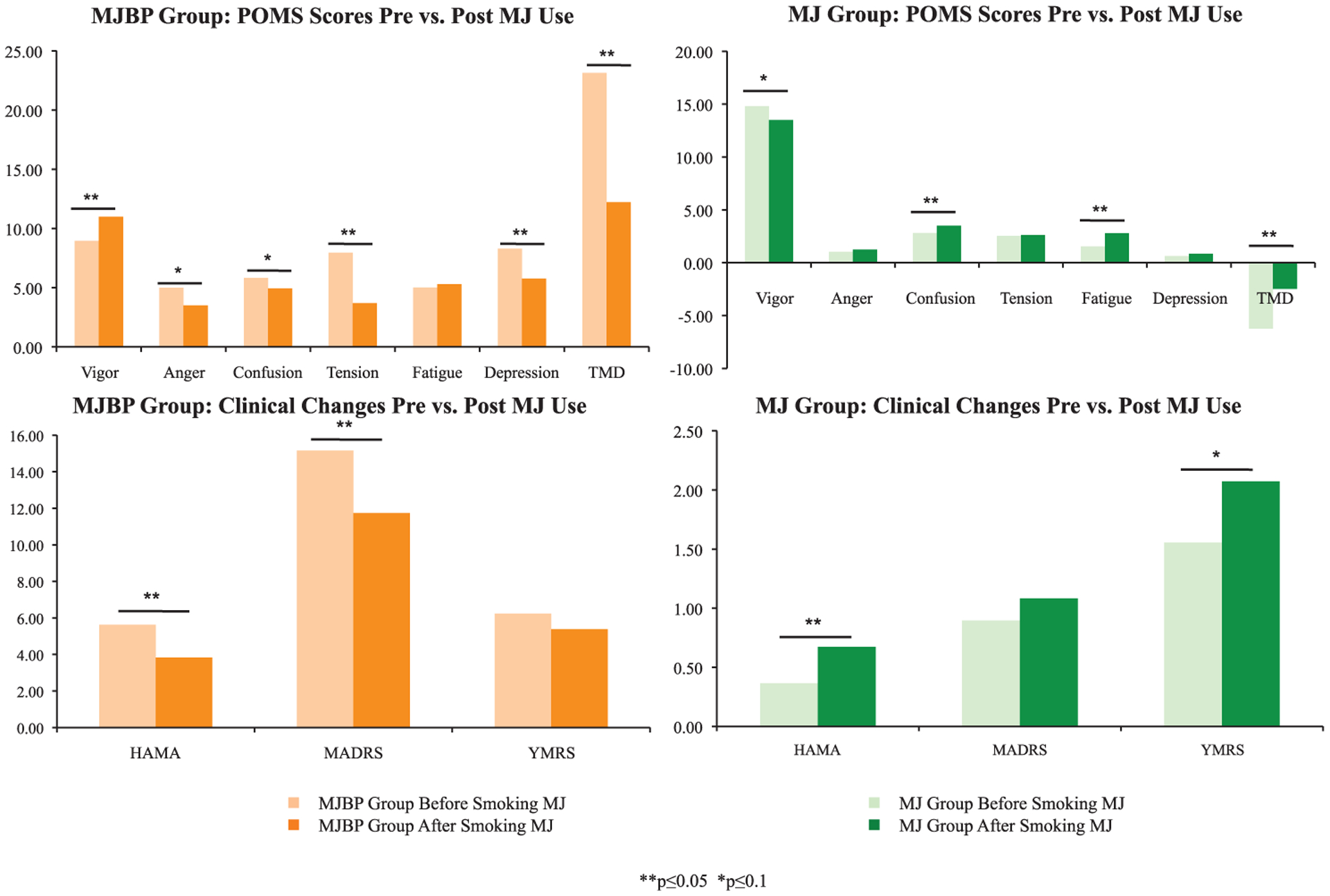
Mood changes: within-group differences for pre vs. post MJ use. Mood change was assessed in both MJBP and MJ participants after episodes of MJ smoking that occurred within four hours of a mood rating; this revealed significant mood improvement in the MJBP group after smoking MJ and a slight worsening of symptoms in the MJ group after smoking MJ. **p ≤ 0.05, *p ≤ 0.10.
Table 3.
Average mood ratings from the PDA devices for the study groups.
| MJBP (n = 12) | MJ (n = 20) | BP (n = 11) | |||||
|---|---|---|---|---|---|---|---|
| Pre-MJ use | Post-MJ use | p-value | Pre-MJ Use | Post-MJ use | p-value | Average ratings | |
| POMS | |||||||
| Vigor | 8.96 (4.59) | 10.99 (4.86) | 0.003 | 14.82 (6.88) | 13.50 (6.49) | 0.06 | 10.35 (6.05) |
| Anger | 4.99 (6.79) | 3.50 (5.44) | 0.09 | 1.04 (1.99) | 1.24 (2.48) | NS | 3.85 (4.96) |
| Confusion | 5.83 (3.93) | 4.94 (3.26) | 0.09 | 2.80 (2.11) | 3.51 (2.39) | 0.004 | 7.41 (4.20) |
| Tension | 7.97 (6.14) | 3.70 (4.21) | >0.001 | 2.53 (3.64) | 2.61 (2.82) | NS | 6.58 (4.96) |
| Fatigue | 5.01 (4.24) | 5.30 (4.82) | NS | 1.54 (2.77) | 2.78 (3.47) | <0.001 | 6.96 (5.69) |
| Depression | 8.29 (7.82) | 5.76 (7.28) | 0.02 | 0.63 (1.69) | 0.85 (2.08) | NS | 6.15 (7.08) |
| TMD | 23.13 (25.01) | 12.22 (21.32) | 0.001 | −6.24 (12.96) | −2.47 (14.12) | 0.009 | 20.60 (26.19) |
| HAM-A | 5.62 (5.41) | 3.83 (4.29) | 0.01 | 0.37 (0.85) | 0.67 (1.44) | 0.02 | 4.62 (4.82) |
| MADRS | 15.16 (8.44) | 11.73 (8.27) | 0.005 | 0.90 (1.73) | 1.08 (2.27) | NS | 6.65 (6.32) |
| YMRS | 6.23 (4.95) | 5.38 (4.37) | 0.001 | 1.55 (2.53) | 2.07 (3.09) | 0.09 | 3.70 (4.41) |
Notes: all p-values reported are 2-tailed. Data are reported as means with standard deviations in parentheses.
POMS, profile of mood states; TMD, total mood disturbance; HAM-A, Hamilton Anxiety Scale; MADRS, Montgomery–Asberg Depression Scale; YMRS, Young Mania Rating Scale.
As seen in Figure 2, change scores (deltas), were also compared between groups, illustrating striking differences in the direction of mood change between the pure MJ group and the MJBP group after MJ use. On every measure, differences between groups were statistically significant, with MJBP smokers showing improvement in mood symptoms and pure MJ smokers reporting a slight worsening of mood symptoms.
Figure 2.
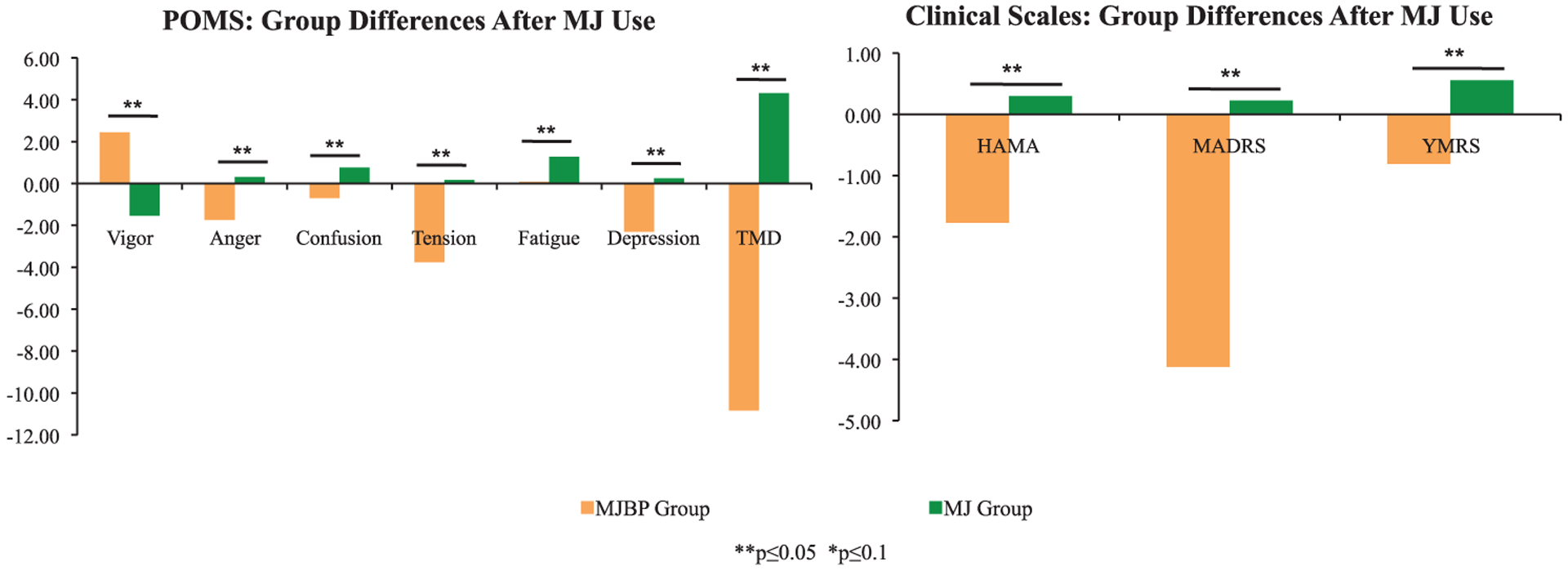
Change scores in mood after MJ use. Change scores (deltas) for both the MJBP and MJ groups illustrate striking differences in the direction of mood change between the two groups after MJ use, as differences in change scores were statistically significant for every measure. **p ≤ 0.05, *p ≤ 0.10.
We also compared the MJBP group to the BP group in order to assess differences in mood symptoms between BP participants who smoke MJ compared to those who do not (See Figure 3). Prior to smoking, the MJBP group reported significantly worse mood than average BP group ratings in several areas, including vigor (p = 0.04), anger (p = 0.05), confusion (p = 0.001), tension (p = 0.02), fatigue (p = 0.002), and depression (p = 0.008) subscores of the POMS, as well as higher MADRS (p < 0.001) and YMRS (p < 0.001) scores and a trend for higher HAMA scores (p = 0.07). Interestingly, post MJ use ratings scales indicate generally better overall mood in the MJBP group relative to the BP group. In particular, MJBP participants reported significantly lower scores for the confusion (p < 0.001), tension (p < 0.001), fatigue (p = 0.005) and TMD (p = 0.002) subscales of the POMS. Prior to smoking, the MJBP group reported higher HAM-A scores relative to the BP group which approached statistical significance, but lower HAM-A scores than the BP group after smoking MJ. It is of note, however, that although MJBP participants exhibited lower scores on several clinical scales post MJ use relative to the BP group, they continued to report significantly higher MADRS (p < 0.001) and YMRS (p < 0.001) ratings compared to the BP group, even after MJ use.
Figure 3.
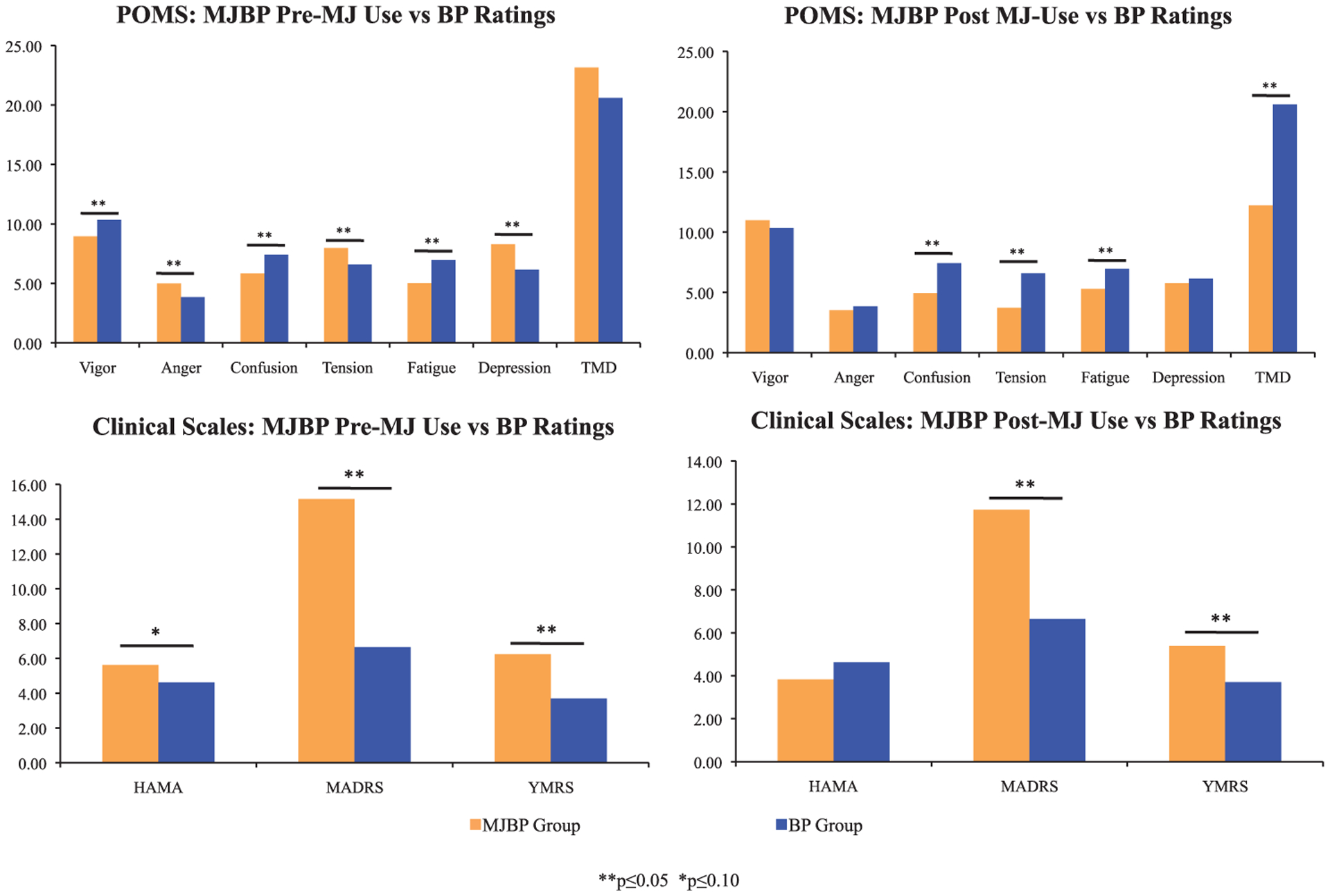
Mood ratings for pre vs. post MJ use for MJBP vs BP participants. Prior to smoking, the MJBP group reported significantly worse mood than average BP group ratings on almost every measure; conversely, post MJ use ratings scales indicate generally better overall mood in the MJBP group relative to the BP group. **p ≤ 0.05, *p ≤ 0.10.
Discussion
As hypothesized, a statistically significant improvement in mood-related symptoms was noted in the MJBP group for each of the POMS, HAM-A and MADRS scales, while the MJ group appeared to have a slight worsening of symptoms after smoking MJ. Of note, for the MJBP participants, TMD, a composite summary score of the POMS, was significantly reduced from 23.1 to 12.2 after smoking MJ, while it increased significantly for the MJ participants after smoking MJ. Consistent with previous reports of bipolar patients using drugs to improve mood-related symptoms, these data underscore the likelihood that MJ may act to partially stabilize and alleviate mood related symptoms for at least a subset of patients and highlights the need for further investigation in this area.
While the MJBP group experienced improvement in mood after smoking MJ, PDA data from the MJ group suggests that these participants actually experienced a slight worsening of clinical state after using MJ. This is particularly interesting, as all participants in the MJ group routinely reported feeling ‘good’ or ‘great’ after smoking MJ, and cited this feeling as the reason for continued heavy use during their weekly check-in visits. This finding underscores the benefit of utilizing a PDA device within participants’ own environments. Previous studies have demonstrated that questions presented on computer screens may be answered with more candor than those presented in paper-and-pencil or interview formats (Turner et al., 1998) and that improved adherence may be achieved through electronic vs. written report formats (Stone et al., 2003). This is particularly important to the study of individuals who misuse substances, given the sensitivity of the information to be gathered and the nature of these participants to be somewhat noncompliant (Goldberg, Garno, Leon, Kocsis, & Portera, 1999). It is likely that participants in the current study felt comfortable reporting their actual mood state via PDA and that ‘real time’ reporting of mood state is more reliable than the retrospective method utilized in a weekly clinical check in visit, with participants reporting their best recollection of their own mood state for the previous week.
It is of note that the improvement in clinical state noted in the MJBP group after smoking MJ takes them from being worse than the BP group pre-MJ use on several clinical scales to better than the BP group averages post-MJ use for all of the POMS subscores and the HAM-A total. In particular TMD, a composite measure of the POMS, shifts from being higher in the MJBP group relative to the BP group prior to smoking MJ to statistically significantly lower after smoking MJ. These data suggest that MJBP participants experience better mood after smoking MJ relative to BP patients who do not smoke. Despite the improvements noted in POMS ratings after smoking for the MJBP group, scores on the MADRS and YMRS remain higher in this group relative to the BP group, while anxiety, as measured by the HAM-A, is reduced in the MJBP group after smoking relative to the BP group. These findings highlight the importance of assessing mood with multiple scales and provide some evidence for improvement in the MJBP group as directly compared to a pure, non-MJ smoking BP group. It appears that prior to smoking MJ, the MJBP group is slightly worse with regard to mood symptoms than the BP group, a pattern that is at least partially reversed after smoking MJ. The precise nature, duration, and time-course of this improvement remain unknown, however, and require further investigation.
While findings from the current study are compelling, and suggest that bipolar patients experience improved mood following MJ use, several issues must be considered. First, the current study is a pilot investigation, and as a result, includes a relatively small number of participants in each of the three study groups. Findings provide a strong justification for further investigations of this kind, which should include larger sample sizes for better generalizability. Further, while the MJBP and MJ participants reported frequency, magnitude and mode (i.e. joint, bong, bowl, vaporizer) of MJ used, it was not possible to collect data on the potency of MJ smoked by participants. Numerous strains of MJ that vary in potency are currently available, and it is therefore possible that the groups differed with regard to the potency of MJ used. Given the within-subjects design of the study, however, it is unlikely that a potential between-group difference in MJ potency would have impacted the study findings. The directional difference in mood ratings after smoking MJ in both of these groups suggests a differential impact of MJ on patients with BP relative to psychiatrically healthy participants who smoke MJ which bears further examination.
Findings from the current study are consistent with previous reports of MJ acting as a partial mood stabilizer in patients with BP. As previously stated, patients with BP who use MJ have been shown to have higher illness severity and poorer outcome, yet report subjective improvement in symptoms after using MJ. Taken together with data from the current investigation, these findings provide strong evidence that bipolar patients using MJ may derive a clinical benefit from smoking MJ, yet raise several possibilities for the poorer outcome often experienced and reported by these individuals. First, if patients’ clinical symptoms are even partially addressed by MJ, then the pharmacotherapeutic regimen prescribed by their physicians may be different than from what would normally be prescribed, especially if clinicians are unaware of the MJ use by their patients. This may be problematic for several reasons, especially if the frequency of MJ use is intermittent in nature, or the magnitude of MJ use varies at all as a function of other psychosocial factors, as the benefit experienced from MJ use would then be more variable. Further, an immediate, short term improvement in clinical state following MJ use might occur, which could result in overt non-adherence to a prescribed medication regimen, which may ultimately result in a poorer, long term outcome. Given the rates of comorbidity of MJ use in BP, and the significant impact of inadequate treatment, additional research is needed to clarify potential correlates and predictors of MJ use in these patients. Further, attempts to educate clinicians regarding the widespread use of MJ in patients with BP may help facilitate appropriate assessment of marijuana use, which could significantly improve treatment planning.
Although there are increasing advances in the treatment of BP, between 20% and 40% of these patients exhibit inadequate or poor response (Muzina & Calabrese, 2005), even with newer atypical antipsychotic medications. Perhaps not surprisingly, patients often turn to illicit substances in an attempt to manage their symptoms. Marijuana use is extremely common among bipolar patients, and anecdotal reports have suggested improved clinical state in these patients after MJ use. Data from the current study, the first to examine the specific impact of MJ use on clinical mood rating scales using a naturalistic, ecologically relevant method of assessment in patients with BP, provide further evidence for symptomatic mood improvement and partial mood stabilization after MJ use. Given the impact of MJ on mood in patients with BP, the development of novel, cannabinoid-based therapies should be explored, in addition to trials of currently available cannabinoid-based treatments, as these may prove to be effective as adjunctive therapy for symptom relief.
Conclusions
MJ appears to alleviate mood-related symptoms in some BP patients, however, further research is necessary in order to determine the long-term impact of MJ use on mood in these individuals.
Acknowledgements
This project was supported by the National Institute of Drug Abuse (NIDA) 5 R21 DA021241.
References
- Agrawal A, Nurnberger JI, Lynskey MT, & The Bipolar Genome Study. (2011). Cannabis involvement in individuals with bipolar disorder. Psychiatry Research, 185, 459–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton CH, Moore PB, Gallagher P, & Young AH (2005). Cannabinoids in bipolar affective disorder: A review and discussion of their therapeutic potential. Journal of Psychopharmacology, 19, 293–300. [DOI] [PubMed] [Google Scholar]
- Brown E, Suppes T, Adinoff B, & Thomans N (2001). Drug abuse and bipolar disorder: Comborbidity or misdiagnosis? Journal of Affective Disorders, 65, 105–115. [DOI] [PubMed] [Google Scholar]
- Bucker JD, Zvolensky MJ, Smits JA, Norton PJ, Crosby RD, Wonderlich SA, & Schmidt NB (2011). Anxiety sensitivity and marijuana use: An analysis from ecological momentary assessment. Depression and Anxiety, 28, 420–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BL, Paris MM, Lam CY, Robinson JD, Traylor AC, Waters AJ, …Cinciripini PM (2010). Real–time craving differences between black and white smokers. American Journal on Addictions, 19, 136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerullo MA, & Strawkoski SM (2007). The prevalence and significance of substance use disorders in bipolar type I and II disorder. Substance Abuse Treatment, Prevention, and Policy, 2, 29. doi: 10.1186/1747-597X-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams BW (1994). Structured clinical interview for axis I DSM IV disorder, patient edition (SCID–I/P). Version 2.0 New York, NY: Biometric Research Department, NY State Psychiatric Institute. [Google Scholar]
- Goldberg JF, Garno JL, Leon AC, Kocsis JH, & Portera L (1999). A history of substance abuse complicates remission from acute mania in bipolar disorder. Journal of Clinical Psychiatry, 60, 733–740. [DOI] [PubMed] [Google Scholar]
- Grant BF, & Harford TC (1995). Comorbidity between DSM–IV alcohol use disorders and major depression: Results of a national survey. Drug and Alcohol Dependence, 39, 197–206. [DOI] [PubMed] [Google Scholar]
- Grinspoon L, & Bakalar JB (1998). The use of cannabis as a mood stabilizer in Bipolar Disorder: Anecdotal evidence and the need for clinical research. Journal of Psychoactive Drugs, 30, 171–177. [DOI] [PubMed] [Google Scholar]
- Gruber AJ, Pope HG, & Brown ME (1996). Do patients use marijuana as an antidepressant? Depression, 4, 77–80. [DOI] [PubMed] [Google Scholar]
- Hamilton M (1959). The assessment of anxiety states by rating. British Journal of Medical Psychology, 32, 50–55. [DOI] [PubMed] [Google Scholar]
- Henquet C, Krabbendam L, de Graaf R, ten Have M, & van Os J (2006). Cannabis use and expression of mania in the general population. Journal of Affective Disorders, 95, 103–110. [DOI] [PubMed] [Google Scholar]
- Hopper JW, Su Z, Looby AR, Ryan ET, Penetar DM, Palmer CM, & Lukas SE (2006). Incidence and patterns of polydrug use and craving for ecstasy in regular ecstasy users: An ecological momentary assessment study. Drug and Alcohol Dependence, 85, 221–235. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Nelson CB, McGonagle KA, Edlund MJ, Frank RG, & Leaf PJ (1996). The epidemiology of co-occurring addictive and mental disorders: Implications for prevention and service utilization. The American Journal of Orthopsychiatry, 66, 17–31. [DOI] [PubMed] [Google Scholar]
- Khantzian EJ (1997). The self–medication hypothesis of substance use disorders: A reconsideration and recent applications. Harvard Review of Psychiatry, 4, 231–244. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, & Asberg M (1979). A new depression scale designed to be sensitive to change. The British Journal of Psychiatry, 134, 561–571. [DOI] [PubMed] [Google Scholar]
- Muzina DJ, & Calabrese JR (2005). Maintenance therapies in bipolar disorder: Focus on randomized controlled trials. The Australia and New Zealand Journal of Psychiatry, 39, 652–661. [DOI] [PubMed] [Google Scholar]
- National Institute of Public Health. (2008). NIH Publication No. 01–4584, The numbers count: Mental disorders in America. A summary of statistics describing the prevalence of mental disorders in America. Retrieved from www.nimh.nih.gov/statistics/index.shtml [Google Scholar]
- Pollock V, Cho DW, Reker D, & Volavka J (1979). Profile of Mood States: The factors and their physiological correlates. The Journal of Nervous and Mental Disease, 167, 612–614. [DOI] [PubMed] [Google Scholar]
- Reiger DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, & Goodwin FK (1990). Comorbidity of mental disorders with alcohol and other drug abuse. Results from the epidemiologic catchment area (ECA) study. The Journal of the American Medical Association, 264, 2511–2518. [PubMed] [Google Scholar]
- Sonne SC, Brady KT, & Morton WA (1994). Substance abuse and bipolar affective disorder. Journal of Nervous and Mental Disease, 182, 349–352. [DOI] [PubMed] [Google Scholar]
- Stone AA, Shiffman S, Schwartz JE, Broderick JE, & Hufford MR (2003). Patient compliance with paper and electronic diaries. Controlled Clinical Trials, 24, 182–199. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, & DelBello MP (2000). The co-occurrence of bipolar and substance use disorders. Clinical Psychology Review, 20, 191–206. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, DelBello MP, Fleck DE, Adler CM, Anthenelli RM, Keck PE, … Amicone J (2007). Effects of co-occurring cannabis use disorders on the course of bipolar disorder after a first hospitalization for mania. Archives of General Psychiatry, 64, 57–64. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, Sax KW, McElroy SL, Keck PE, Hawkins JM, & West SA (1998). Course of psychiatric and substance abuse syndromes co-occurring with bipolar disorder after a first psychiatric hospitalization. Journal of Clinical Psychiatry, 59, 465–471. [DOI] [PubMed] [Google Scholar]
- Tohen M, Greenfield SF, Weiss RD, Zarate CA, & Vagge LM (1998). The effect of comorbid substance use disorders on the course of bipolar disorder: A review. Harvard Review of Psychiatry, 6, 133–141. [DOI] [PubMed] [Google Scholar]
- Turner CF, Ku L, Rogers SM, Lindberg LD, Pleck JH, & Sonenstein FL (1998). Adolescent sexual behavior, drug use, and violence: Increased reporting with computer survey technology. Science, 280, 867–873. [DOI] [PubMed] [Google Scholar]
- van Laar M, van Dorsselaer S, Monshouwer K, & de Graaf R (2007). Does cannabis use predict the first incidence of mood and anxiety disorders in the adult population? Addiction, 102, 1251–1260. [DOI] [PubMed] [Google Scholar]
- van Rossum I, Boomsma M, Tenback MD, Reed C, van Os J, & the EMBLEM Advisory Board. (2009). Does cannabis use affect treatment outcome in bipolar disorder? A longitudinal analysis. The Journal of Nervous and Mental Disease, 197, 35–40. [DOI] [PubMed] [Google Scholar]
- van Zundert RM, Booger EA, Vermulst AA, & Engels RC (2009). Nicotine withdrawal symptoms following a quit attempt: An ecological momentary assessment study among adolescents. Nicotine & Tobacco Research, 11, 722–729. [DOI] [PubMed] [Google Scholar]
- Wechsler D (1999). Wechsler abbreviated scale of intelligence. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Weiss RD, Kolodziej M, Griffin ML, Najavits LM, Jacobson LM, & Greenfield SF (2004). Substance use and perceived symptom improvement among patients with bipolar disorder and substance dependence. Journal of Affective Disorders, 79, 279–283. [DOI] [PubMed] [Google Scholar]
- Woods SW (2000). The economic burden of bipolar disease. Journal of Clinical Psychiatry, 61, 38–41. [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, & Meyer DA (1978). A rating scale for mania: Reliability, validity and sensitivity. British Journal of Psychiatry, 133, 429–435. [DOI] [PubMed] [Google Scholar]


