Abstract
OBJECTIVE
In cross-sectional U.S. studies, patients with diabetes had twice the prevalence of latent tuberculosis infection (LTBI) compared with those without diabetes. However, whether LTBI contributes to diabetes risk is unknown. We used longitudinal data to determine if LTBI is associated with increased diabetes incidence.
RESEARCH DESIGN AND METHODS
We conducted a retrospective cohort study among U.S. Veterans receiving care in the Veterans Health Administration from 2000 to 2015. Eligibility included all patients without preexisting diabetes who received a tuberculin skin test (TST) or interferon-γ release assay (IGRA). We excluded patients with a history of active TB and those diagnosed with diabetes before or within 2 years after LTBI testing. Patients were followed until diabetes diagnosis, death, or 2015. LTBI was defined as TST or IGRA positive. Incident diabetes was defined by use of ICD-9 codes in combination with a diabetes drug prescription.
RESULTS
Among 574,113 eligible patients, 5.3% received both TST/IGRA, 79.1% received TST only, and 15.6% received IGRA only. Overall, 6.6% had LTBI, and there were 2,535,149 person-years (PY) of follow-up after LTBI testing (median 3.2 years). The diabetes incidence rate (per 100,000 PY) was greater in patients with LTBI compared with those without (1,012 vs. 744; hazard ratio [HR] 1.4 [95% CI 1.3–1.4]). Increased diabetes incidence persisted after adjustment for covariates (adjusted HR [aHR] 1.2 [95% CI 1.2–1.3]) compared with those without LTBI. Among patients with LTBI, diabetes incidence was similar in those treated for LTBI compared with those who were not treated (aHR 1.0 [95% CI 0.9–1.1]).
CONCLUSIONS
Comprehensive longitudinal data indicate that LTBI is associated with increased diabetes incidence. These results have implications for people with LTBI, ∼25% of the global population.
Introduction
Growing evidence suggests an interdependent relationship between infections and type 2 diabetes, which may provide new opportunities to substantially expand current diabetes prevention efforts. The rapid rise of diabetes into regions with high tuberculosis (TB) endemicity has renewed interest in the intersection between type 2 diabetes and TB (1,2). Historically, the focus of research has centered on the impact of type 2 diabetes on TB, with numerous studies finding that patients with diabetes have increased rates of TB disease; recent studies indicate that type 2 diabetes increases the risk of TB disease by two to three times (3,4). The synergistic relationship between TB and diabetes already accounts for 15% of TB cases globally; as the diabetes epidemic expands further, diabetes could account for >35% of all TB cases in the coming decade (5,6).
Clinical and epidemiologic evidence, however, suggests the relationship between type 2 diabetes and TB may be bidirectional, in which infection with Mycobacterium tuberculosis also affects diabetes (7,8). Inflammation is a hallmark of active TB disease and can cause stress hyperglycemia, potentially increasing the risk of post-TB metabolic disease (9,10). Observational data from patients with active TB indicate that TB may increase the risk of hyperglycemia and type 2 diabetes (7,10,11). Both active TB and latent TB infection (LTBI), a noncommunicable TB infection without symptoms, modulate human adipose tissue function via inflammatory immune responses that are linked to early type 2 diabetes pathogenesis (12–14). Because the host response to LTBI exists on a spectrum of immune activity (15), it is plausible that LTBI promotes chronic inflammation and may increase the risk of developing type 2 diabetes. In the U.S., population-based studies have demonstrated the prevalence of type 2 diabetes among adults with LTBI (22%) was twice the prevalence of diabetes among those without LTBI (11%) (16). However, data on the relationship between LTBI and type 2 diabetes to date have largely been limited to cross-sectional analyses, and few studies assessing the association defined both LTBI and diabetes used validated measurement criteria (17,18).
The global prevalence of LTBI is ∼25%, reaching 50–70% by adolescence or early adulthood in high-TB-burden settings (e.g., India and South Africa) (19–21). Because of LTBI’s high prevalence, even small increases in type 2 diabetes risk among those infected would have vast implications for type 2 diabetes prevention. We tested the hypothesis that having LTBI increases the future incidence of developing type 2 diabetes. Further, among those with prevalent LTBI, we determined the incidence of type 2 diabetes in patients who received treatment for LTBI compared with those who did not to examine whether eradication of TB infection alters any observed increased incidence of diabetes. We used a large longitudinal cohort of patients (n = 574,113) from the U.S. Veterans Affairs (VA) Health Administration from 2000 to 2015 to estimate the association between LTBI with incidence rates (IRs) of type 2 diabetes.
Research Design and Methods
We conducted a retrospective cohort study using medical records, laboratory results, and prescription information assembled from all U.S. VA Medical Care Centers nationally. Longitudinal study data came from inpatient and outpatient VA Medical Centers from 2000 to 2015, during which time ICD codes were standardized across the VA. Our primary objective was to determine the increased risk of developing type 2 diabetes as a result of having had LTBI.
Study Participants
Eligible participants included adult (aged ≥18 years) patients who received at least one inpatient or outpatient care visit at any VA Medical Center nationwide and who received a tuberculin skin test (TST) or interferon-γ release assay (IGRA) during 2000 to 2015. We excluded patients with history of type 2 diabetes (referred to hereafter as diabetes) before the TST/IGRA test date, those with any history of active TB, and those with newly diagnosed type 2 diabetes within 2 years after TST/IGRA test date. Patients were followed from TST/IGRA test date until date of diabetes diagnosis, death, or 2015.
Data Source and Measures
All study data for this study came from the VA’s Corporate Data Warehouse, an infrastructure to centrally compile VA patients’ clinical and health care service information using standardized data systems nationwide. The primary outcome for this study was incident type 2 diabetes, defined by a filled prescription for a diabetes medication (Supplementary Table 1) in combination with use of a diabetes ICD-9 code (in conjunction with one face-to-face outpatient primary care visit or any two uses of the code 250.xx). The primary exposure of interest was LTBI status based on either IGRA or TST results and was defined as positive or negative based on laboratory records. A secondary exposure of interest, history of LTBI treatment, was determined among participants with a positive IGRA or TST result. History of LTBI treatment (i.e., TB preventive therapy) was defined by a prescription fill of rifampin or isoniazid in combination with an LTBI-positive result (participants with any history of active TB were excluded). Among those with LTBI treatment, treatment time was defined by number of days between IGRA/TST result and LTBI medication prescription fill as early (≤7 days), middle (8–71 days), and later.
Other participant characteristics were measured from medical records, ICD-9 codes, laboratory results, and prescription fills. These covariates of interest included demographic characteristics (age, sex, and race/ethnicity), smoking history as previously validated (22) (current, former, or never), statin use, total cholesterol, HIV status, and hepatitis B and C status.
Analytic Methods
We calculated prevalence differences (PDs) and 95% CIs to compare characteristics among participants with and without LTBI. We determined type 2 diabetes incidence using proportions for cumulative incidence and person-years (PY) for IR density calculations. PY were calculated as the years between date of LTBI test result and date of first indication of type 2 diabetes or censorship. Participants were censored at the end of September 2015 or on the date of death.
To compare incidence of type 2 diabetes by LTBI status, we calculated unadjusted rate ratios and used Cox proportional regression to compare adjusted hazard ratios (aHRs) and 95% CIs. Cox proportional regression was also used to compare diabetes incidence by LTBI treatment history (secondary exposure) among participants with LTBI. Proportional hazards assumptions were evaluated graphically using log-log survival curves and statistically using Schoenfeld residuals. Covariates for multivariable models were selected based on observed bivariate associations with LTBI and diabetes, previously published studies, and directed acyclic graph theory (23). Missing values among covariates were coded as a separate category and included in multivariable models. We assessed statistical interaction between LTBI and statin use and LTBI and race/ethnicity using product terms in Cox proportional regression models. All analyses were performed using SAS (SAS Institute, Cary, NC) within the VA’s Informatics and Computing Infrastructure.
Subgroup and Sensitivity Analyses
In a subgroup analyses, we compared the incidence of type 2 diabetes by LTBI status only among those patients with a continuum of care. First, to increase the likelihood that existing diabetes would be diagnosed before IGRA/TST, only patients with one or more primary care visits per year for 2 consecutive years and who received either a hemoglobin A1c or glucose measurement during that interval were included in a continuum of care subgroup analysis. Second, to increase the likelihood that any new diabetes would be diagnosed after IGRA/TST in those with or without LTBI, only patients with one or more primary care visits per year for 3 consecutive years and who received either a hemoglobin A1c or glucose measurement during that interval were included in a continuum of care subgroup analysis.
We also performed a sensitivity analysis to determine if receiving an LTBI test was associated with altered diabetes risk. We compared the incidence of diabetes among a set of patient control subjects who did not receive an IGRA/TST. Control subjects had the same eligibility criteria as those who did receive an IGRA/TST and were matched by sex, year of birth, and race/ethnicity. Diabetes incidence was compared in the control subjects after an index date that was defined as the date of IGRA/TST among those who were tested and included in our primary analysis.
Ethical Approval
This study was approved by the Institutional Review Board at Emory University/Atlanta VA Medical Center.
Results
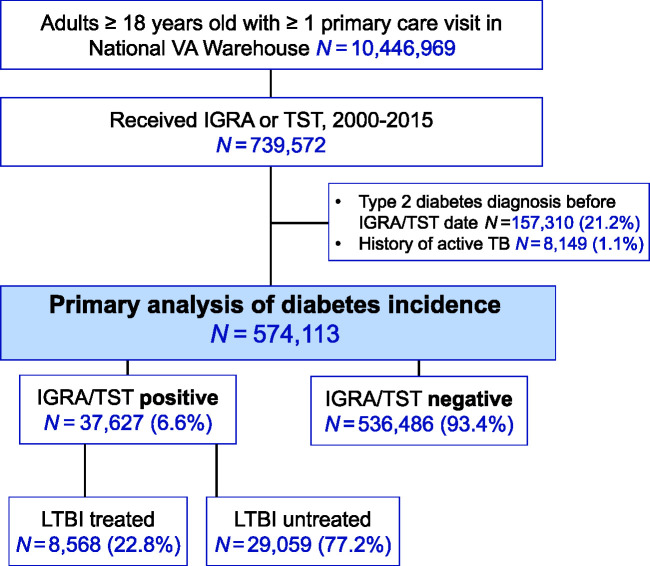
During 2000 to 2015, there were 10.4 million adult patients with at least one primary care visit recorded in the U.S. National VA Corporate Data Warehouse (Fig. 1). Among these, a total of 739,572 patients received either an IGRA or TST during 2000 to 2015 and were potentially eligible for this study. In the primary cohort analysis, those with a history of active TB disease (n = 8,149; 1.1%) and diabetes (n = 157,310; 21%) at the time of LTBI test (or within 2 years) were not eligible and excluded (Fig. 1). Thus, a total of 574,113 participants were included in final analyses. Among participants included in final analyses, a majority were male (n = 483,743, 84%) and overweight (n = 304,946; 53% with BMI ≥25.0 kg/m2) and had a median age of 62 (interquartile range [IQR] 51–71) years.
Figure 1.
Cohort inclusion criteria for patients who received an LTBI test result, 2000–2015.
The study’s primary exposure was LTBI status. The baseline prevalence of LTBI was 6.6%, and our study included 37,627 participants with LTBI and 536,486 without LTBI (Fig. 1 and Table 1). The proportion of participants with LTBI differed based on numerous demographic and comorbid factors. A higher prevalence of LTBI was found among participants with male sex (PD 3.1% [95% CI 3.0–3.3]), increased age (≥65 years vs. 20–34 years, 4.0% [95% CI 3.8–4.3]), Black (vs. White, 7.2% [95% CI 7.0–7.4]) and Asian race/ethnicity (vs. White, 10.9% [95% CI 9.9–11.9]), and hepatitis C coinfection (6.1% [95% CI 5.8–6.4]). Prevalence of LTBI did not differ meaningfully by participants’ HIV status (HIV-positive vs. HIV negative/unknown 0.2% [95% CI −0.2 to 0.6]) or history of statin use (current vs. never, −0.1% [95% CI −0.3 to 0.0]).
Table 1.
Prevalence of LTBI among patients at risk for diabetes, 2000–2015
| Characteristics | Total (N = 574,113)* | LTBI positive** (N = 37,627)‡ | LTBI negative (N = 536,486)‡ | PD (%) (95% CI) |
|---|---|---|---|---|
| Sex | ||||
| Male | 483,743 (84.3) | 34,101 (7.0) | 449,642 (93.0) | 3.1 (3.0–3.3) |
| Female | 90,347 (15.7) | 3,525 (3.9) | 86,822 (96.1) | Reference |
| Age (years) | ||||
| 20–34 | 31,615 (5.5) | 1,093 (3.5) | 30,522 (96.5) | Reference |
| 35–64 | 298,489 (52.0) | 18,217 (6.1) | 280,272 (93.9) | 2.6 (2.4–2.9) |
| ≥65 | 244,009 (42.5) | 18,317 (7.5) | 225,692 (92.5) | 4.0 (3.8–4.3) |
| Race/ethnicity€ | ||||
| White | 314,564 (54.8) | 14,485 (4.6) | 300,079 (95.4) | Reference |
| Black | 117,205 (20.4) | 13,809 (11.8) | 103,396 (88.2) | 7.2 (7.0–7.4) |
| Asian | 4,905 (1.0) | 760 (15.5) | 4,145 (84.5) | 10.9 (9.9–11.9) |
| Other | 7,620 (1.3) | 592 (7.8) | 7,028 (92.2) | 3.2 (2.6–3.8) |
| Region | ||||
| Midwest | 112,608 (19.6) | 5,570 (4.9) | 107,038 (95.1) | Reference |
| Continental | 79,975 (13.9) | 5,886 (7.4) | 74,089 (92.6) | 2.4 (2.2–2.6) |
| North Atlantic | 138,193 (24.1) | 9,820 (7.1) | 128,373 (92.9) | 2.2 (2.0–2.3) |
| Pacific | 153,862 (26.8) | 11,566 (7.5) | 142,296 (92.5) | 2.6 (2.4–2.8) |
| Southeast | 89,427 (15.6) | 4,783 (5.3) | 84,644 (94.7) | 0.4 (0.2–0.6) |
| Smoking status£ | ||||
| Current | 117,380 (20.4) | 9,225 (7.9) | 108,155 (92.1) | 2.8 (2.6–3.0) |
| Former | 297,009 (51.7) | 20,453 (6.9) | 276,556 (93.1) | 1.8 (1.7–2.0) |
| Never | 88,835 (15.5) | 4,476 (5.0) | 84,359 (95.0) | Reference |
| BMI (kg/m2)¥ | ||||
| <25.0 | 156,772 (27.3) | 10,526 (6.7) | 146,246 (93.3) | Reference |
| 25.0–29.9 | 167,034 (29.1) | 11,578 (6.9) | 155,456 (93.1) | 0.2 (0.0–0.4) |
| ≥30.0 | 137,912 (24.0) | 8,620 (6.2) | 129,292 (93.7) | −0.5 (−0.3, −0.6) |
| Total cholesterol (mg/dL)ϴ | ||||
| <200 | 320,940 (55.9) | 21,946 (6.8) | 298,994 (93.2) | Reference |
| >200 | 135,294 (23.6) | 8,987 (6.6) | 126,307 (93.4) | −0.2 (−0.0, −0.4) |
| Hypertension | ||||
| Yes | 260,437 (45.4) | 18,681 (7.2) | 241,756 (92.8) | 1.1 (1.0–1.3) |
| No/unknown | 313,676 (54.6) | 18,946 (6.0) | 294,730 (94.0) | Reference |
| Statins | ||||
| Use | 150,392 (26.2) | 9,708 (6.5) | 140,684 (93.5) | Reference |
| No use | 423,721 (73.8) | 27,919 (6.6) | 395,802 (93.4) | 0.1 (−0.1, 0.3) |
| HIV status | ||||
| Positive | 16,435 (2.9) | 1,103 (6.7) | 15,332 (93.3) | 0.2 (−0.2, 0.6) |
| Negative/unknown | 557,678 (97.1) | 36,524 (6.5) | 521,154 (93.5) | Reference |
| Hepatitis C antibody | ||||
| Positive | 48,755 (8.5) | 5,903 (12.1) | 42,852 (87.9) | 6.1 (5.8–6.4) |
| Negative/unknown | 525,358 (91.5) | 31,724 (6.0) | 493,634 (94.0) | Reference |
| Hepatitis B antibody | ||||
| Positive | 5,722 (1.0) | 686 (12.0) | 5,036 (88.0) | 5.5 (4.6–6.3) |
| Negative/unknown | 568,391 (99.0) | 36,941 (6.5) | 531,450 (93.5) | Reference |
Data are N (%) unless otherwise indicated.
Column percentages.
Row percentages.
Among patients who received either an IGRA or TST result.
Total of 23% had missing race/ethnicity.
Total of 12% had missing smoking status.
Total of 20% had missing BMI and 2% had BMI <18.5 kg/m2.
Total of 21% had no cholesterol measure.
Incident Diabetes During Follow-up
There were 2.5 million PY of follow-up in our study. We found that 19,408 (3.4%) participants were diagnosed with incident type 2 diabetes during follow-up (IR 781/100,000 PY) (Table 2). Median time from LTBI test result to diabetes incidence was 2.6 years (IQR 2.1–4.8). The IR of diabetes among participants with LTBI (1,012/100,000 PY) was higher compared with those without LTBI (744/100,000 PY; rate ratio 1.4 [95% CI 1.2–1.4]). Median time to diabetes incidence was similar among those with LTBI (2.8 years [IQR 2.1–5.2]) compared with those without LTBI (2.6 years [IQR 2.1–4.7]).
Table 2.
Incidence of diabetes among patients previously screened for LTBI, 2000–2015
| Characteristics (n) | Diabetes incidence risk, N (%) | IR/100,000 PY | IR ratio (95% CI) |
|---|---|---|---|
| Total (n = 574,113) | 19,408 (3.4) | 781 | — |
| LTBI (IGRA/TST)* | |||
| Positive (n = 37,627) | 2,072 (5.5) | 1,012 | 1.4 (1.3–1.4) |
| Negative (n = 536,486) | 17,336 (3.2) | 744 | Reference |
| Sex | |||
| Male (n = 483,743) | 18,108 (3.7) | 880 | 2.9 (2.7–3.1) |
| Female (n = 90,347) | 1,299 (1.4) | 303 | Reference |
| Age (years) | |||
| 20–34 (n = 31,564) | 37 (0.1) | 44 | Reference |
| 35–64 (n = 298,489) | 8,812 (2.9) | 682 | 15.5 (11.2–21.4) |
| ≥65 (n = 244,009) | 10,559 (4.3) | 952 | 21.6 (15.7–29.9) |
| Race/ethnicity‡ | |||
| White (n = 314,564) | 10,783 (3.4) | 809 | Reference |
| Black (n = 117,205) | 5,752 (4.9) | 1,091 | 1.3 (1.3–1.4) |
| Asian (n = 4,905) | 130 (2.6) | 754 | 0.9 (0.8–1.1) |
| Other (n = 7,620) | 291 (3.8) | 918 | 1.1 (1.0–1.3) |
| BMI (kg/m2)¥ | |||
| <25.0 (n = 156,772) | 2,185 (1.4) | 342 | Reference |
| 25.0–29.9 (n = 167,034) | 5,455 (3.2) | 740 | 2.2 (2.1–2.3) |
| ≥30.0 (137,912) | 9,706 (7.0) | 1,666 | 4.9 (4.7–5.1) |
| Statins | |||
| Current use (n = 134,785) | 7,924 (5.9) | 1,482 | 2.6 (2.5–2.7) |
| Former use (n = 15,607) | 615 (3.9) | 1,011 | 1.8 (1.6–1.9) |
| Never use (n = 423,721) | 10,869 (2.6) | 574 | Reference |
| HIV status | |||
| Positive (n = 16,435) | 290 (1.8) | 570 | 0.7 (0.6–0.8) |
| Negative/unknown (n = 557,678) | 19,118 (3.4) | 785 | Reference |
| Hepatitis C antibody | |||
| Positive (n = 48,755) | 2,302 (4.7) | 1,057 | 1.4 (1.3–1.5) |
| Negative/unknown (n = 525,358) | 17,106 (3.3) | 754 | Reference |
Either IGRA or TST positive and either IGRA/TST negative with no positive LTBI test.
Total of 23% had missing race/ethnicity.
Total of 20% had missing BMI, and 2% had BMI <18.5 kg/m2.
In a hazard model adjusted for the known confounders of age and sex (Table 3), participants who had LTBI at baseline continued to have a greater rate of developing incident diabetes compared with those without LTBI (aHR 1.3 [95% CI 1.2–1.3]). Further, this relationship persisted (aHR 1.2 [95% CI 1.2–1.3]) even when adjusting more broadly for age, sex, BMI, race, region, smoking status, HIV, hepatitis B, hepatitis C, hypertension, total cholesterol level, and statin use.
Table 3.
HR of incident diabetes comparing patients with and without LTBI
| HR (95% CI) | Model 1 HR* (95% CI) | Model 2 HR** (95% CI) | |
|---|---|---|---|
| LTBI‡ | |||
| IGRA/TST positive | 1.4 (1.3–1.4) | 1.3 (1.2–1.3) | 1.2 (1.2–1.3) |
| IGRA/TST negative | Reference | Reference | Reference |
Among N = 574,113 with IGRA/TST results, N = 37,627 had LTBI.
Either IGRA or TST positive and either IGRA/TST negative with no positive LTBI test.
Model 1 adjusted for age and sex.
Model 2 adjusted for age, sex, BMI, race, region, smoking status, HIV, hepatitis C, hepatitis B, hypertension, cholesterol, and statin use.
Diabetes Incidence Among Those Treated for LTBI Versus Untreated LTBI
Overall, 22.8% (n = 8,568) of the 37,627 participants with LTBI received LTBI treatment during or prior to follow-up (Fig. 1). The aHR of type 2 diabetes was similar among those who received LTBI treatment compared with those who did not receive LTBI treatment (aHR 1.0 [95% CI 0.9–1.1]) (Table 4). Among those with early LTBI treatment relative to the date of IGRA/TST, the adjusted association between LTBI treatment and rate of incident diabetes was modestly lower compared with those with no LTBI treatment (aHR 0.9 [95% CI 0.8–1.1]).
Table 4.
HR for incident diabetes among patients with LTBI, according to TB treatment status
| Diabetes incidence, new cases/total n (%) | HR (95% CI) | aHR* (95% CI) | aHR** (95% CI) | |
|---|---|---|---|---|
| LTBI treatment† | ||||
| No treatment | 1,586/29,059 (5.5) | Reference | Reference | Reference |
| LTBI treated | 486/8,568 (5.7) | 1.0 (0.9–1.1) | 1.02 (0.9–1.1) | 1.0 (0.9–1.1) |
| LTBI treatment time‡ | ||||
| No treatment | 1,586/29,059 (5.5) | Reference | Reference | Reference |
| Early | 149/2,926 (5.1) | 1.0 (0.8–1.2) | 1.0 (0.8–1.2) | 0.9 (0.8–1.1) |
| Middle | 217/3,808 (5.7) | 1.0 (0.9–1.2) | 1.0 (0.9–1.2) | 1.0 (0.8–1.1) |
| Late | 120/1,834 (6.5) | 1.1 (0.9–1.3) | 1.1 (0.9–1.3) | 1.0 (0.8–1.2) |
Among N = 37,627 with positive IGRA/TST results, N = 8,568 received LTBI treatment.
Adjusted for age and sex.
Adjusted for age, sex, BMI, race, region, smoking status, HIV, hepatitis C, hepatitis B, hypertension, cholesterol, and statin use.
LTBI treatment defined as those with any prescription for LTBI medication (before, on the date of IGRA/TST test, after, or both before and after date of IGRA/TST). Participants with no history of treatment or those with history of ethambutol or pyrazinamide defined as no treatment.
Early: LTBI treatment time defined as those with prescription for LTBI medication ≤7 days from IGRA/TST (including those with LTBI medication before IGRA/TST date); middle: LTBI treatment defined as those with prescription for LTBI medication 8–71 days from IGRA/TST; and late: LTBI treatment defined as those with prescription for LTBI medication >71 days from IGRA/TST.
We assessed whether the relationship between LTBI and type 2 diabetes incidence was different by participants’ statin use history or race/ethnicity (Supplementary Table 2). The relative increase in diabetes incidence among participants with LTBI was modestly greater in participants without history of statin use (HR 1.5 [95% CI 1.4–1.6]) compared with those with statin use (HR 1.3 [95% CI 1.2–1.4]); however, this difference was not observed in an adjusted model. We did not observe meaningful interaction differences in the association between LTBI and diabetes by race/ethnicity.
Continuum of Care Subgroup
A subgroup of 183,470 patients met the criteria of continuous care before IGRA/TST date. The IR (per 100,000 PY) of type 2 diabetes among those with LTBI (1,201) and no LTBI (943) was similar compared with the full cohort. In the continuous care before IGRA/TST subgroup, the hazard of diabetes among those with LTBI was 1.3 (95% CI 1.1–1.4) times the hazard of those without LTBI (Supplementary Table 3). A subgroup of 199,846 patients met the criteria of continuous care after IGRA/TST date. In the continuous care after IGRA/TST subgroup, the hazard of diabetes among those with LTBI was 1.1 (95% CI 1.0–1.2) times the hazard of those without LTBI (Supplementary Table 3).
Diabetes Incidence Among Patients Without LTBI Testing
Among patient-matched control subjects who did not receive LTBI testing, the incidence of diabetes after the index date was 794/100,000 PY (Supplementary Table 4). The rate of diabetes incidence among control subjects was similar to the rate among those with the LTBI test (781/100,000 PY).
Conclusions
In this large cohort study among U.S. Veterans, we found that having LTBI was associated with increased incidence of type 2 diabetes. During ∼2.5 million PY of follow-up time, the relative rate of diabetes incidence among participants with LTBI was 20% greater in those with LTBI after adjusting for key confounders. We also found that among patients with LTBI, receiving treatment for LTBI was not associated with a reduction in type 2 diabetes incidence. Although findings from this study should be replicated, our large longitudinal cohort and rigorous analytic design suggest that preventing LTBI may reduce the IR of diabetes. These findings have substantial implications in high-burden LTBI settings in which TB and diabetes epidemics are colliding.
In our study, we used national VA data to establish a large cohort of patients who received a LTBI test but were known to be free of active TB. We then excluded those with existing diabetes and followed patients forward in time to determine incident diabetes using validated measures. To enhance the study’s validity, we ensured diabetes was not prevalent at time of LTBI measurement by excluding patients with diabetes incidence within a 2-year lag time after LTBI testing. Our subgroup analysis of those in a continuum of care (i.e., those with annual visits and HbA1c or glucose measures in the VA system) showed that even if we restricted to those who had a greater “opportunity” to be diagnosed with diabetes, the results were nearly identical to the main results, underscoring the robustness of our findings. Given the massive person-time required to adequately compare diabetes incidence by LTBI status, our design using VA records overcame the limitations of a prospective design while addressing a fundamental but an unanswered question about the nature of the relationship between M. tuberculosis and diabetes.
To date, studies that examined the relationship between LTBI and diabetes have primarily had cross-sectional designs (18). One cohort study from Spain followed 198 household contacts of active TB index cases who were initially LTBI negative (24). Among these contacts, 25% of those with diabetes (n = 4) had LTBI compared with 9% among household contacts without diabetes (n = 194; P = 0.31) (24). However, we are unaware of any previous studies that longitudinally compared the incidence of diabetes in persons with and without LTBI. Findings from several cross-sectional studies (16,25–27) that reported increased diabetes prevalence in persons with LTBI (and vice versa) are consistent with our results that suggest LTBI may increase diabetes IRs. Other studies that are consistent with LTBI impacting metabolic disease risk include case-control and cross-sectional studies from Peru and Uganda that suggest LTBI may increase the risk of coronary artery disease (28,29). The cross-sectional study of 204 adults from Lima and Kampala reported that LTBI was associated (adjusted odds ratio 5.0 [95% CI 1.1–23.4]) with obstructive coronary artery disease after adjusting for confounders (29).
Two shifts in premise regarding M. tuberculosis activity in human hosts may explain how LTBI could increase the risk of diabetes. First, animal models and positron emission tomography/computed tomography imaging techniques in humans demonstrate that the phrase “latent” TB infection encompasses a spectrum of metabolic and immune M. tuberculosis activity within granulomas in the absence of clinically evident TB disease (15,30). Granulomas may be characterized by dormant hypoxia, or conversely, they may have functional cell proliferation and angiogenesis (31). Second, TB causes hepatic and adipose tissue inflammation, which promotes immune activity and disrupts homeostasis of glucose, insulin, and lipid metabolism (12). Murine models have demonstrated that M. tuberculosis can reside within adipocytes and impacts adipose tissue physiology by inducing an influx of NK cells and TB-specific CD8+ T cells (13,32,33). Moreover, M. tuberculosis can enter primary human adipocytes and survive in a nonreplicating state (34). Given M. tuberculosis infection persists in adipose tissue, there is an opportunity for loss of immunologic adipocyte homeostasis due to TB-attributable inflammation. Obesity-induced adipose tissue inflammation similarly results in a cascade of immune activation and leads to insulin resistance, the mechanism by which obesity causes diabetes. Thus, activated macrophages and T-cell infiltration in adipose tissue due to LTBI may induce inflammation and lead to insulin resistance followed by hyperglycemia (14,35,36). There are potential pathophysiologic and molecular mechanisms, when taken in context with findings from our large cohort, that provide compelling evidence that LTBI may increase the risk of diabetes.
Additional work is needed to determine whether treating LTBI could reduce the risk of diabetes incidence. Among patients with LTBI in our study, we did not observe a reduced IR of diabetes in patients who initiated LTBI treatment compared with those who did not initiate treatment. However, we were unable to adequately measure LTBI treatment adherence. Until 2020, most LTBI treatment regimens in the U.S. included 6 to 9 months of isoniazid therapy, and completion rates were historically very low (∼10%) in routine clinical settings (37,38). It is plausible that in some patients with LTBI, completion of LTBI treatment would reduce their risk of diabetes. For patients with LTBI, key gaps in knowledge remain about whether LTBI treatment completion, age at initiation, time between LTBI infection and its treatment, or underlying metabolic health at time of LTBI treatment differentially impact diabetes risk.
This study was subject to limitations. First, we relied on existing laboratory results to determine which patients received an LTBI test; only those with documented results were eligible. Patients who received an LTBI test likely had a clinical indication for increased TB risk and therefore may not be generalizable to all U.S. patients. Nonetheless, this limitation of including patients with existing LTBI results did not impact the internal validity of our estimates, and the principal finding that LTBI is associated with increased diabetes risk remains a key result that should be evaluated in other settings. Second, there may have been misclassification of the type 2 diabetes incidence and misclassification of the measurement of LTBI treatment. For diabetes incidence, we relied on a combination of prescription fill information and ICD-9 codes. If after testing IGRA or TST positive, those patients with LTBI received more frequent metabolic laboratory testing, those patients may also be more likely to be diagnosed with incident diabetes. It is also possible that some patients had undiagnosed diabetes at the time of LTBI testing. However, our subgroup analysis of patients with continuous primary care and either HbA1c or glucose testing before and after LTBI testing indicated findings similar to our primary analyses (increased adjusted rate of diabetes incidence among those with LTBI). Moreover, due to the glucose-lowering medication requirement in our diabetes incidence definition, patients with diet-controlled diabetes would not be captured using our methods. Therefore, our study is only measuring incidence of type 2 diabetes treated with medications. Nonetheless, our measure of diabetes incidence has been validated previously (39,40) and is likely subject to less misclassification compared with ICD alone. Third, because some patients with LTBI may have received treatment outside of the VA medical system, it is also plausible that we were unable to measure their LTBI treatment history and misclassified some patients as not receiving LTBI treatment. Among those who did have a prescription fill for LTBI treatment, we were unable to determine if the regimen was completed. Fourth, we did not have information on TST induration size or quantitative results from IGRA tests. Therefore, we were unable to determine if extent of response to LTBI tests was associated with increased or decreased diabetes incidence.
In summary, our results have important implications for type 2 diabetes prevention. The primary result from this cohort of more than half a million patients suggests that LTBI increases rates of diabetes incidence. Although our findings need to be validated in other populations, in regions with high TB burdens, and with different study designs that include life-course approaches, we provide rigorous epidemiologic evidence to suggest that subclinical infections like LTBI contribute to diabetes risk. If the LTBI–diabetes relationship at the population level is similar to what we observed, an estimated >40 million cases of diabetes globally may be attributable to LTBI, including 670,000 U.S. adults. This study adds to a growing body of evidence that M. tuberculosis infection and disease have metabolic sequela in humans. Findings from this study could be used to test new type 2 diabetes prevention programs that seek to expand the diagnosis and treatment of LTBI both in the U.S. and in high-burden TB settings.
Article Information
Funding. This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID) at the National Institutes of Health (grants R01AI153152 and R03AI133172 to M.J.M.). This work was also supported in part by grants from the NIAID (K24AI114444 to N.R.G., U19AI111211 to the Emory-led TB Research Unit, and P30AI051519 to the Emory Center for AIDS Research) as well as VA awards CSP #2008, I01 CX001899, and I01 CX001737, National Institutes of Health awards R21DK099716, R18DK066204, and R21AI156161, and Cystic Fibrosis Foundation award PHILLI12A0.
The sponsors had no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data, and preparation, review, or approval of the manuscript.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.J.M., A.K., C.L.D., M.K.R., and L.S.P. conceived the study design and analytic plan. N.R.G. and H.K. provided interpretation of data. M.J.M. drafted the manuscript. A.K., N.R.G., C.L.D., H.K., M.K.R., and L.S.P. revised the manuscript critically and provided final approval for publication. M.J.M. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.19082576.
References
- 1. Harries AD, Lin Y, Satyanarayana S, et al. The looming epidemic of diabetes-associated tuberculosis: learning lessons from HIV-associated tuberculosis. Int J Tuberc Lung Dis 2011;15:1436–1444, i [DOI] [PubMed] [Google Scholar]
- 2. Harries AD, Murray MB, Jeon CY, et al. Defining the research agenda to reduce the joint burden of disease from diabetes mellitus and tuberculosis. Trop Med Int Health 2010;15:659–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med 2008;5:e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Al-Rifai RH, Pearson F, Critchley JA, Abu-Raddad LJ. Association between diabetes mellitus and active tuberculosis: a systematic review and meta-analysis. PLoS One 2017;12:e0187967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Awad SF, Dargham SR, Omori R, Pearson F, Critchley JA, Abu-Raddad LJ. Analytical exploration of potential pathways by which diabetes mellitus impacts tuberculosis epidemiology. Sci Rep 2019;9:8494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Odone A, Houben RM, White RG, Lönnroth K. The effect of diabetes and undernutrition trends on reaching 2035 global tuberculosis targets. Lancet Diabetes Endocrinol 2014;2:754–764 [DOI] [PubMed] [Google Scholar]
- 7. Pearson F, Huangfu P, Pearce M, McNally R, Unwin N, Critchley J. Exploring the association between tuberculosis and diabetes in a UK primary care dataset. J Epidemiol Community Health 2016;70:A31–A32 [DOI] [PubMed] [Google Scholar]
- 8. Magee MJ, Salindri AD, Gujral UP, et al. Convergence of non-communicable diseases and tuberculosis: a two-way street? Int J Tuberc Lung Dis 2018;22:1258–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Magee MJ, Salindri AD, Kyaw NTT, Auld SC, Haw JS, Umpierrez GE. Stress hyperglycemia in patients with tuberculosis disease: epidemiology and clinical implications. Curr Diab Rep 2018;18:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kornfeld H, West K, Kane K, et al. High prevalence and heterogeneity of diabetes in patients with TB in South India: a report from the Effects of Diabetes on Tuberculosis Severity (EDOTS) Study. Chest 2016;149:1501–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aftab H, Christensen DL, Ambreen A, et al. Tuberculosis-related diabetes: is it reversible after complete treatment? Am J Trop Med Hyg 2017;97:1099–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martinez N, Cheng CY, Ketheesan N, et al. mTORC2/Akt activation in adipocytes is required for adipose tissue inflammation in tuberculosis. EBioMedicine 2019;45:314–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beigier-Bompadre M, Montagna GN, Kühl AA, et al. Mycobacterium tuberculosis infection modulates adipose tissue biology. PLoS Pathog 2017;13:e1006676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature 2017;542:177–185 [DOI] [PubMed] [Google Scholar]
- 15. Barry CE 3rd, Boshoff HI, Dartois V, et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol 2009;7:845–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barron MM, Shaw KM, Bullard KM, Ali MK, Magee MJ. Diabetes is associated with increased prevalence of latent tuberculosis infection: findings from the National Health and Nutrition Examination Survey, 2011-2012. Diabetes Res Clin Pract 2018;139:366–379 [DOI] [PubMed] [Google Scholar]
- 17. Lee PH, Fu H, Lee MR, Magee M, Lin HH. Tuberculosis and diabetes in low and moderate tuberculosis incidence countries. Int J Tuberc Lung Dis 2018;22:7–16 [DOI] [PubMed] [Google Scholar]
- 18. Lee MR, Huang YP, Kuo YT, et al. Diabetes mellitus and latent tuberculosis infection: a systematic review and metaanalysis. Clin Infect Dis 2017;64:719–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Teklu T, Legesse M, Medhin G, et al. Latent tuberculosis infection and associated risk indicators in pastoral communities in southern Ethiopia: a community based cross-sectional study. BMC Public Health 2018;18:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shivakumar SVBY, Chandrasekaran P, Kumar AMV, et al. Diabetes and pre-diabetes among household contacts of tuberculosis patients in India: is it time to screen them all? Int J Tuberc Lung Dis 2018;22:686–694 [DOI] [PubMed] [Google Scholar]
- 21. Mahomed H, Hawkridge T, Verver S, et al.; SATVI Adolescent Study Team . Predictive factors for latent tuberculosis infection among adolescents in a high-burden area in South Africa. Int J Tuberc Lung Dis 2011;15:331–336 [PubMed] [Google Scholar]
- 22. McGinnis KA, Brandt CA, Skanderson M, et al. Validating smoking data from the Veteran’s Affairs Health Factors dataset, an electronic data source. Nicotine Tob Res 2011;13:1233–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology 1999;10:37–48 [PubMed] [Google Scholar]
- 24. Arnedo-Pena A, Juan-Cerdán JV, Romeu-García MA, et al. Vitamin D status and incidence of tuberculosis infection conversion in contacts of pulmonary tuberculosis patients: a prospective cohort study. Epidemiol Infect 2015;143:1731–1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Martinez L, Zhu L, Castellanos ME, et al. Glycemic control and the prevalence of tuberculosis infection: a population-based observational study. Clin Infect Dis 2017;65:2060–2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hensel RL, Kempker RR, Tapia J, Oladele A, Blumberg HM, Magee MJ. Increased risk of latent tuberculous infection among persons with pre-diabetes and diabetes mellitus. Int J Tuberc Lung Dis 2016;20:71–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee EH, Lee JM, Kang YA, et al. Prevalence and impact of diabetes mellitus among patients with active pulmonary tuberculosis in South Korea. Lung 2017;195:209–215 [DOI] [PubMed] [Google Scholar]
- 28. Huaman MA, Ticona E, Miranda G, et al. The relationship between latent tuberculosis infection and acute myocardial infarction. Clin Infect Dis 2018;66:886–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huaman MA, De Cecco CN, Bittencourt MS, et al. Latent tuberculosis infection and subclinical coronary atherosclerosis in Peru and Uganda. Clin Infect Dis 2021;73:e3384–e3390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Via LE, Schimel D, Weiner DM, et al. Infection dynamics and response to chemotherapy in a rabbit model of tuberculosis using [18F]2-fluoro-deoxy-D-glucose positron emission tomography and computed tomography. Antimicrob Agents Chemother 2012;56:4391–4402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Matty MA, Roca FJ, Cronan MR, Tobin DM. Adventures within the speckled band: heterogeneity, angiogenesis, and balanced inflammation in the tuberculous granuloma. Immunol Rev 2015;264:276–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ayyappan JP, Ganapathi U, Lizardo K, et al. Adipose tissue regulates pulmonary pathology during TB infection. MBio 2019;10:e02771-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Agarwal P, Khan SR, Verma SC, et al. Mycobacterium tuberculosis persistence in various adipose depots of infected mice and the effect of anti-tubercular therapy. Microbes Infect 2014;16:571–580 [DOI] [PubMed] [Google Scholar]
- 34. Neyrolles O, Hernández-Pando R, Pietri-Rouxel F, et al. Is adipose tissue a place for Mycobacterium tuberculosis persistence? PLoS One 2006;1:e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 2007;117:175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nikolajczyk BS, Jagannathan-Bogdan M, Shin H, Gyurko R. State of the union between metabolism and the immune system in type 2 diabetes. Genes Immun 2011;12:239–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Alsdurf H, Hill PC, Matteelli A, Getahun H, Menzies D. The cascade of care in diagnosis and treatment of latent tuberculosis infection: a systematic review and meta-analysis. Lancet Infect Dis 2016;16:1269–1278 [DOI] [PubMed] [Google Scholar]
- 38. Sterling TR, Njie G, Zenner D, et al. Guidelines for the treatment of latent tuberculosis infection: recommendations from the National Tuberculosis Controllers Association and CDC, 2020. MMWR Recomm Rep 2020;69:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jackson SL, Long Q, Rhee MK, et al. Weight loss and incidence of diabetes with the Veterans Health Administration MOVE! lifestyle change programme: an observational study. Lancet Diabetes Endocrinol 2015;3:173–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rhee MK, Ho YL, Raghavan S, et al. Random plasma glucose predicts the diagnosis of diabetes. PLoS One 2019;14:e0219964. [DOI] [PMC free article] [PubMed] [Google Scholar]