Abstract
BACKGROUND
The epidemiology of adult-onset type 1 diabetes (T1D) incidence is not well-characterized due to the historic focus on T1D as a childhood-onset disease.
PURPOSE
We assess the incidence of adult-onset (≥20 years) T1D, by country, from available data.
DATA SOURCES
A systematic review of MEDLINE, Embase, and the gray literature, through 11 May 2021, was undertaken.
STUDY SELECTION
We included all population-based studies reporting on adult-onset T1D incidence and published from 1990 onward in English.
DATA EXTRACTION
With the search we identified 1,374 references of which 46 were included for data extraction. Estimates of annual T1D incidence were allocated into broad age categories (20–39, 40–59, ≥60, or ≥20 years) as appropriate.
DATA SYNTHESIS
Overall, we observed the following patterns: 1) there is a paucity of data, particularly in low- and middle-income countries; 2) the incidence of adult-onset T1D is lowest in Asian and highest in Nordic countries; 3) adult-onset T1D is higher in men versus women; 4) it is unclear whether adult-onset T1D incidence declines with increasing age; and 5) it is unclear whether incidence of adult-onset T1D has changed over time.
LIMITATIONS
Results are generalizable to high-income countries, and misclassification of diabetes type cannot be ruled out.
CONCLUSIONS
From available data, this systematic review suggests that the incidence of T1D in adulthood is substantial and highlights the pressing need to better distinguish T1D from T2D in adults so that we may better assess and respond to the true burden of T1D in adults.
Introduction
The incidence of type 1 diabetes (T1D) is highest in children, though T1D onset can occur at any age (1,2). The global epidemiology of childhood-onset T1D is well characterized, with estimates updated biannually in the International Diabetes Federation (IDF) Diabetes Atlas (3). The epidemiology of adult-onset T1D incidence is, in contrast, less well characterized due to the historic focus on T1D as a common childhood-onset disease (2), challenges in distinguishing adult-onset T1D from type 2 diabetes (T2D), and a lack of national diabetes registries that include T1D incidence across the life span. Recognition of adult-onset T1D, and assessment of trends in its incidence, is important, as this incurable condition is commonly misclassified as T2D (4) and often requires treatment very different from that for T2D (5).
In an earlier systematic review (6) investigators reported on the epidemiology of T1D incidence in young adults (age >15 years) as compared with childhood-onset T1D (age <15 years). Key findings of this earlier review included the following: 1) there is a general paucity of data on adult-onset T1D incidence; 2) country-to-country variations in incidence in those aged >15 years paralleled those of children, with highest estimates in Nordic countries (e.g., Finland); 3) T1D incidence was higher in male (vs. female) young adults; and 4) T1D incidence decreased after the age of 14 years (6).
In the current Systematic Review, our primary objective is to complement and extend the earlier work by Diaz-Valencia et al. (6) by exclusively examining population-based studies reporting on T1D incidence among adults aged ≥20 years and incorporating data from the gray literature (e.g., registries, national health surveys) in an attempt to better capture the epidemiology of adult-onset T1D by country. Our secondary objective is to assess the quality of the epidemiological evidence pertaining to adult-onset T1D incidence, including diagnostic methods.
Methods
This Systematic Review adheres to the Preferring Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. This Systematic Review has been registered with the PROSPERO International prospective register of systematic reviews (reg. no. CRD42021238967).
Data Sources and Searches
A literature search was performed in MEDLINE and Embase on 11 May 2021 without any restrictions of time. We used Medical Subject Headings (MeSH) related to “type 1 diabetes,” “incidence,” and “study design” combined with the operator “AND” and restricted the search strategy to studies published in English. A description of the final search strategies for MEDLINE and Embase are can be found in Supplementary Tables 1 and 2, respectively. To identify additional studies or data sources not captured in the traditional literature search, we also searched the gray literature including national health and diabetes registry websites (see Supplementary Table 3 for a full list of the 54 countries searched), contacted experts and IDF Diabetes Atlas collaborators to identify country-specific estimates of adult-onset T1D incidence, and screened the reference lists of included studies.
Study Selection
We included all full-text research articles in which investigators reported on the incidence of adult-onset T1D (aged ≥20 years) in population-based studies, including occupation- and insurance-based populations, published from 1990 onward. We considered latent autoimmune diabetes in adults as a subtype of T1D and included studies in which incidence of latent autoimmune diabetes in adults was reported. We excluded the following: 1) articles where described T1D incidence was among a non-population-based sample (e.g., a trial or hospital-based population); 2) editorials/commentaries, case studies, or abstracts; 3) articles where investigators described prevalence of T1D; 4) articles where investigators reported on the incidence of maturity-onset diabetes of the young; and 5) studies with a focus on specific populations such as T1D postpancreatectomy. For some studies, the age range included adolescents (i.e., 15–34 years). In these instances, and where data were not reported in age-specific groups ≥20 years, we contacted authors of the original studies to provide age-specific estimates. Where age-specific estimates were not provided, the broader age range including adolescents was reported.
All identified articles from the literature search were entered into Covidence for screening. Each article was title and abstract screened by two reviewers (any combination of J.L.H., P.L.W., X.Z., X.L., and R.C.W.M.), and conflicts were resolved by a third reviewer. Studies subsequently included in the full-text screen were also screened by two reviewers (J.L.H., X.Z., R.C.W.M.), and conflicts were resolved by a third reviewer. In the event that conflicts could not be resolved by a third reviewer, a discussion by the full team was conducted until a final disposition was reached. Where multiple studies reported on the same data source, we included the most recently published study only.
Data Extraction and Quality Assessment
Microsoft Excel was used to extract the following data from included articles: publication characteristics (i.e., year of publication, author names, PubMed identifier [PMID], journal, data source), study characteristics (i.e., study design [cohort, cross-sectional], country/region/territory, study year), sample characteristics (i.e., sample size, description of the sample, age range), and diabetes definition and incidence (overall, sex-stratified, age-stratified, and calendar year stratified where appropriate, with 95% CIs and unit of measurement, i.e., person-years). Data extraction was performed by J.L.H.
The methodological quality of each study was critically appraised by two authors (P.L.W. and X.Z.) using a modified version of the Newcastle-Ottawa Scale (7), and conflicts were resolved by a third reviewer (J.L.H.). This modified tool, previously used in studies of T2D incidence (8), includes items to assess the representativeness of the study population, the sample size, and the method of assessing diabetes status. For the current review, we further tailored the scale of outcome assessment for T1D. Specifically, we scored the quality of the diagnostic criteria using the following algorithm where a higher score indicates higher quality: no description (score 0), patient self-report (score 1), record linkage (clinical diagnosis or ICD code) (score 2), administrative algorithm including where two or more clinical criteria are used (score 3), and use of one or more biomarkers (e.g., anti-GAD, other antibodies, C-peptide, genetic scores) supplemented with clinical criteria (score 4) (Supplementary Table 4). We acknowledge that for earlier studies, the use of biomarker data was not standard practice, and thus these studies may score lower based on current quality metrics. The maximum score was 11, and final scores were categorized as low (score 0–4), medium (score 5–7), or high (score 8–11) quality.
Data Synthesis and Analysis
Estimates of adult-onset T1D are provided per study and overall patterns across studies described. To present results graphically, we allocated T1D incidence estimates, per data source, into broad age categories (20–39, 40–59, ≥60, or ≥20 years) as appropriate. Where one study reported on more than one age-group per category (i.e., 25–29 and 30–34 years), we took the unweighted average of these two estimates and ascribed this to the 20–39 years category. This same approach was used where incidence was reported by sex. The majority of studies (n = 35 [76%]) reported T1D incidence per 100,000 persons per year, while 11 (24%) reported T1D incidence per 100,000 PY. For this review, we have assumed that PY approximates per person per year and have reported this across all studies. Last, the reporting of geographical regions (e.g., Europe, Western Pacific) in this review is in accordance with standard guidelines used for the IDF Diabetes Atlas and does not reflect the views of the authors regarding geographical boundaries or legal status of territories.
Results
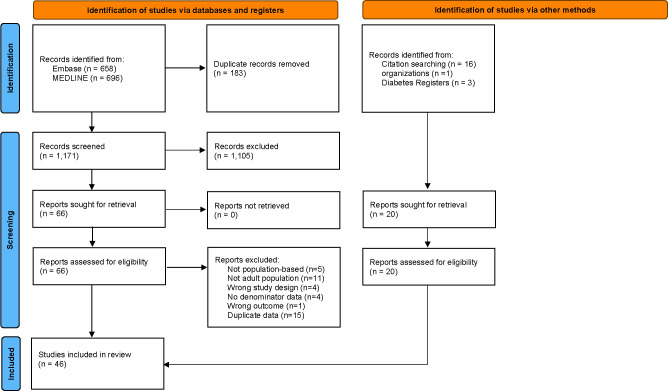
The literature search yielded 1,354 articles (658 from MEDLINE and 696 from Embase). Sixteen additional articles were identified from the reference list of included studies, and four reports were identified from the gray literature (three from diabetes registries and one from an organization). Among 86 studies assessed for eligibility, 40 were excluded including 15 that were excluded due to duplicate results reported from the same data source. At the conclusion of the search, 46 articles or reports were included in the final review (see Figs. 1 and 2).
Figure 1.
PRISMA 2020 flow diagram for new systematic reviews including searches of databases, registers, and other sources. Adapted from Page et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71.
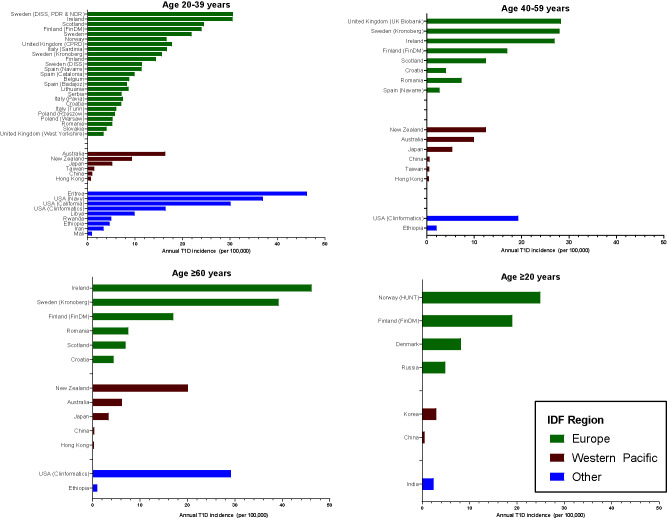
Figure 2.
Incidence of adult-onset T1D diabetes by age-group, IDF region, and country. Clinformatics, Clinformatics Data Mart database; HUNT, Nord-Trøndelag Health Study (The HUNT Study).
The 46 articles or reports included in this review describe the incidence of adult-onset T1D across 32 countries or regions between 1973 and 2019 (Table 1). The majority (36 of 46 [78%]) of studies were from Europe and the Western Pacific, 3 (7%) were from North America (the U.S. only), 4 (8%) were from Africa (Eritrea, Ethiopia, Mali, and Rwanda), 2 (4%) were from the Middle East and North Africa (Iran and Libya), and 1 (2%) was from Southeast Asia (India).
Table 1.
Summary of studies included in Systematic Review of annual T1D incidence (per 100,000 people) in adults by IDF region
| First author (reference no.) | Country (region) | Cohort name | Study year | Population at risk, n (age) | Sex | Age at onset, years (#) | Incidence (95% CI) |
|---|---|---|---|---|---|---|---|
| Europe | |||||||
| Weets (44) | Belgium (Antwerp) | Belgian Diabetes Registry | 1989–2000 | 488,457 (0–39 years) | M+F | 15–39 (1) | 8.8 (7.9–9.8)a |
| M | 15–39 (1) | 10.6 (9.2–12.2)a | |||||
| F | 15–39 (1) | 7.0 (5.9–8.3)a | |||||
| Roglić (45) | Croatia (Zagreb) | N/A | 1988–1992 | 933,914 (all ages) | M | 15–24 (1) | 9.8 (6.5–14.3)c |
| M | 25–34 (1) | 7.8 (5.2–11.2)c | |||||
| M | 35–44 (1) | 7.2 (4.7–10.6)c | |||||
| M | 45–54 (2) | 3.0 (1.4–5.7)c | |||||
| M | ≥55 (3) | 4.4 (2.6–7.0)c | |||||
| F | 15–24 (1) | 9.3 (6.1–13.6)c | |||||
| F | 25–34 (1) | 5.1 (3.2–7.8)c | |||||
| F | 35–44 (1) | 3.8 (2.1–6.3)c | |||||
| F | 45–54 (2) | 5.1 (2.9–8.3)c | |||||
| F | ≥55 (3) | 4.5 (3.0–6.6)c | |||||
| Mølbak (46) | Denmark (Copenhagen and Freseriskborg) | N/A | 1973–1977 | 457,281 (>29 years) | M+F | >29 (4) | 8.2 (7.1–9.4)c |
| M | >29 (4) | 9.1 (7.3–10.8)c | |||||
| F | >29 (4) | 7.5 (6.0–9.0)c | |||||
| Lammi (47) | Finland | N/A | 1992–1996 | Not reported | M+F | 20–24 (1) | 16.1 (14.2–18.3)c |
| M+F | 25–29 (1) | 16.2 (14.4–18.2)c | |||||
| M+F | 30–34 (1) | 15.2 (13.5–17.0)c | |||||
| M+F | 35–39 (1) | 10.1 (8.8–11.6)c | |||||
| M | 20–24 (1) | 19.9 (16.9–23.2)c | |||||
| M | 25–29 (1) | 20.9 (18.0–24.1)c | |||||
| M | 30–34 (1) | 19.8 (17.1–22.8)c | |||||
| M | 35–39 (1) | 12.6 (10.5–15.0)c | |||||
| F | 20–24 (1) | 12.2 (9.8–14.9)c | |||||
| F | 25–29 (1) | 11.3 (9.2–13.8)c | |||||
| F | 30–34 (1) | 10.3 (8.4–12.6)c | |||||
| F | 35–39 (1) | 7.5 (5.9–9.5)c | |||||
| Arffman (18) | Finland | FinDM | 2010–2017T* | Not reported | M+F | ≥20 (4) | 19 (18–20)a |
| M+F | 20–29 (1) | 25 (21–29)c | |||||
| M+F | 30–39 (1) | 23 (20–27)c | |||||
| M+F | 40–49 (2) | 17 (14–20)c | |||||
| M+F | ≥50 (3) | 17 (15–18) | |||||
| Gajewska (48) | Ireland | N/A | 2011–2016 | 3.1 million (≥25 years) | M+F | 25–34 (1) | 29.9 (25.7–34.1)c |
| M+F | 35–44 (1) | 31.2 (27.2–35.2)c | |||||
| M+F | 45–54 (2) | 24.0 (20.1–27.8)c | |||||
| M+F | 55–64 (2) | 29.9 (25.1–34.6)c | |||||
| M+F | 65–74 (3) | 41.2 (34.7–47.7)c | |||||
| M+F | ≥75 (3) | 51.2 (45.3–63.0)c | |||||
| Tenconi (49) | Italy (Pavia) | N/A | 1988–1992 | 71,974 (20–29 years) | M | 20–24 (1) | 7.9 (3.2–16.2)c |
| M | 25–29 (1) | 8.3 (3.6–16.4)c | |||||
| F | 20–24 (1) | 4.8 (1.3–12.2)c | |||||
| F | 25–29 (1) | 8.8 (3.8–17.4)c | |||||
| Muntoni (50) | Italy (Sardinia) | N/A | 1989–1990 | 290,334 (20–29 years) | M+F | 20–24 (1) | 17.0 (12.4–21.6)c |
| M+F | 25–29 (1) | 16.4 (11.6–21.3)c | |||||
| M | 20–24 (1) | 18.9 (12.2–25.7)c | |||||
| M | 25–29 (1) | 25.3 (16.8–33.9)c | |||||
| F | 20–24 (1) | 14.9 (8.8–21.0)c | |||||
| F | 25–29 (1) | 7.5 (2.8–12.1)c | |||||
| Bruno (51) | Italy (Turin) | Turin type 1 diabetes registry | 1984–2000 | Not reported | M+F | 20–24 (1) | 6.6 (5.7–7.6)c |
| M+F | 25–29 (1) | 5.5 (4.7–6.5)c | |||||
| M | 20–24 (1) | 7.9 (6.6–9.5)c | |||||
| M | 25–29 (1) | 6.6 (5.4–8.1)c | |||||
| F | 20–24 (1) | 5.3 (4.2–6.6)c | |||||
| F | 25–29 (1) | 4.4 (3.4–5.6)c | |||||
| Ostrauskas (52) | Lithuania | N/A | 1991–2008 | 798,367 (20–34 years) | M+F | 20–24 (1) | 7.1 (6.4–7.9)c |
| M+F | 25–29 (1) | 8.9 (8.1–9.8)c | |||||
| M+F | 30–34 (1) | 9.9 (9.0–10.7)c | |||||
| M | 20–24 (1) | 8.7 (7.6–9.9)c | |||||
| M | 25–29 (1) | 11.9 (10.6–13.3)c | |||||
| M | 30–34 (1) | 13.7 (12.3–15.3)c | |||||
| F | 20–24 (1) | 5.5 (4.6–6.5)c | |||||
| F | 25–29 (1) | 5.6 (4.7–6.7)c | |||||
| F | 30–34 (1) | 5.9 (5.0–6.9)c | |||||
| Joner (53) | Norway | N/A | 1978–1982 | 613,293 (20–29 years) | M+F | 20–24 (1) | 14.5c |
| M+F | 25–29 (1) | 18.8c | |||||
| M | 20–24 (1) | 15.7c | |||||
| M | 25–29 (1) | 21.1c | |||||
| F | 20–24 (1) | 13.2c | |||||
| F | 25–29 (1) | 16.4c | |||||
| Olsson (25) | Norway | The HUNT Study 1 and 2 | 1995–2008 | 64,264 | M+F | ≥18 (4) | 24.9c |
| Wysocki (19) | Poland (Warsaw) | N/A | 1983–1988T | 623,000 (0–29 years) | M | 20–24 (1) | 4.2 (1.1–10.2)c |
| M | 25–29 (1) | 8.2 (3.5–15.8)c | |||||
| F | 20–24 (1) | 3.3 (0.6–8.8)c | |||||
| F | 25–29 (1) | 5.3 (1.6–11.7)c | |||||
| Sobel-Maruniak (54) | Poland (Rzeszow) | N/A | 1980–1999 | 167,012 (15–29) | M+F | 15–29 (1) | 5.8 (5.0–6.6)a |
| M | 15–29 (1) | 6.8 (5.6–8.1)a | |||||
| F | 15–29 (1) | 4.7 (3.7–5.8)a | |||||
| Ionescu-Tîrgovişte (55) | Romania (Bucharest) | Bucharest Diabetes Registry | 1981–1991 | 1.7 million (20–84 years) | M+F | 20–24 (1) | 5.1 |
| M+F | 25–29 (1) | 7.9 | |||||
| M+F | 30–34 (1) | 3.5 | |||||
| M+F | 35–39 (1) | 4.4 | |||||
| M+F | 40–44 (2) | 5.5 | |||||
| M+F | 45–49 (2) | 8.0 | |||||
| M+F | 50–54 (2) | 7.8 | |||||
| M+F | 55–59 (2) | 7.1 | |||||
| M+F | 60–64 (2) | 8.3 | |||||
| M+F | 65–69 (3) | 10.1 | |||||
| M+F | 70–74 (3) | 8.7 | |||||
| M+F | 75–79 (3) | 8.4 | |||||
| M+F | 80–84 (3) | 3.1 | |||||
| Dedov (56) | Russia | SRDP | 2016 | Not reported | M+F | Adultsd (4) | 4.9c |
| Scottish Diabetes Data Group (21) | Scotland | Scottish Diabetes Survey | 2012–2019T* | 4 million (≥20 years) | M+F | 20–29 (1) | 28.0c |
| M+F | 30–39 (1) | 21.0c | |||||
| M+F | 40–49 (2) | 15.0c | |||||
| M+F | 50–59 (2) | 10.0c | |||||
| M+F | 60–69 (3) | 8.0c | |||||
| M+F | ≥70 (3) | 6.0c | |||||
| Vojislav (9) | Serbia | Serbian Diabetes Registry | 2006–2017T* | Not reported | M+F | 20–24 (1) | 7.5a |
| M+F | 25–29 (1) | 6.9a | |||||
| Kyvik (57) | Slovakia | EURODIAB TIGER | 1996–1997 | Not reported | M | 20–24 (1) | 5.9 (3.8–8.6)c |
| M | 25–29 (1) | 5.0 (3.0–7.8)c | |||||
| F | 20–24 (1) | 3.3 (1.8–5.4)c | |||||
| F | 25–29 (1) | 1.9 (0.8–4.0)c | |||||
| Goday (58) | Spain (Catalonia) | N/A | 1987–1990 | 1.3 million (15–29 years) | M+F | 20–24 (1) | 11.3 (9.7–13.0)c |
| M+F | 25–29 (1) | 8.5 (7.2–9.9)c | |||||
| Forga (15) | Spain (Navarre) | N/A | 2009–2016T* | 508,601 (≥20 years) | M+F | 20–29 (1) | 17.3 (8.6–30.9)c |
| M+F | 30–44 (1) | 5.5 (2.4–10.8)c | |||||
| M+F | ≥45 (2) | 2.7 (1.2–5.3)c | |||||
| M | 20–29 (1) | 21.6 (8.7–44.5)c | |||||
| M | 30–45 (1) | 5.4 (1.5–13.7)c | |||||
| M | ≥45 (2) | 3.5 (1.1–8.2)c | |||||
| F | 20–29 (1) | 12.8 (3.5–32.8)c | |||||
| F | 30–45 (1) | 5.6 (1.5–14.4)c | |||||
| F | ≥45 (2) | 1.9 (0.4–5.6)c | |||||
| Morales-Pérez (59) | Spain (Badajoz) | N/A | 1992–1996 | 107,980 (20–29 years) | M+F | 20–24 (1) | 10.7 (7.1–15.4)c |
| M+F | 25–29 (1) | 5.9 (3.3–9.6)c | |||||
| M | 20–24 (1) | 13.0 (7.6–20.5)c | |||||
| M | 25–29 (1) | 6.6 (3.0–12.4)c | |||||
| F | 20–24 (1) | 8.3 (4.1–14.9)c | |||||
| F | 25–29 (1) | 5.2 (1.7–10.8)c | |||||
| Crump (60) | Sweden | N/A | 1973–2015 | 4.2 million (all ages) | M+F | 18–43 (1) | 21.9c |
| M | 18–43 (1) | 25.6c | |||||
| F | 18–43 (1) | 18.1c | |||||
| Dahlquist (10) | Sweden | DISS | 1983–2007T* | Not reported | M | 20–24 (1) | 17.4c |
| M | 25–29 (1) | 14.6c | |||||
| M | 30–34 (1) | 10.6c | |||||
| F | 20–24 (1) | 11.3c | |||||
| F | 25–29 (1) | 8.6c | |||||
| F | 30–34 (1) | 6.3c | |||||
| Thunander (23) | Sweden (Kronoberg) | N/A | 1998–2001 | 138,000 (≥18 years) | M+F | 20–29 (1) | 19.7 (18.0–21.7)c |
| M+F | 30–39 (1) | 11.7 (10.4–13.2)c | |||||
| M+F | 40–49 (2) | 20.0 (18.2–21.9)c | |||||
| M+F | 50–59 (2) | 36.1 (33.8–38.6)c | |||||
| M+F | 60–69 (3) | 35.3 (32.5–38.1)c | |||||
| M+F | 70–79 (3) | 55.0 (51.1–58.7)c | |||||
| M+F | 80–100 (3) | 27.3 (24.6–30.7)c | |||||
| M | 20–29 (1) | 26.2 (23.3–29.2)c | |||||
| M | 30–39 (1) | 16.9 (14.7–19.4)c | |||||
| M | 40–49 (2) | 16.9 (14.7–19.4)c | |||||
| M | 50–59 (2) | 46.0 (42.3–49.8)c | |||||
| M | 60–69 (3) | 32.1 (28.4–36.0)c | |||||
| M | 70–79 (3) | 38.3 (33.9–43.0)c | |||||
| M | 80–100 (3) | 27.0 (22.0–32.8)c | |||||
| F | 20–29 (1) | 12.7 (10.7–15.0)c | |||||
| F | 30–39 (1) | 6.1 (4.8–7.7)c | |||||
| F | 40–49 (2) | 23.2 (20.5–26.0)c | |||||
| F | 50–59 (2) | 25.7 (22.9–28.8)c | |||||
| F | 60–69 (3) | 38.3 (34.4–42.5)c | |||||
| F | 70–79 (3) | 65.1 (55.9–66.4)c | |||||
| F | 80–100 (3) | 27.4 (23.4–31.8)c | |||||
| Rawshani (20) | Sweden | DISS, PDR, and NDR | 2007–2009T* | Not reported | M+F | 20–24 (1) | 31.2 (26.7–35.7)c |
| M+F | 25–29 (1) | 30.4 (25.8–34.9)c | |||||
| M+F | 30–34 (1) | 30.2 (25.7–34.7)c | |||||
| Abbasi (16) | U.K. | CPRD | 1994–2013T* | 369,362 (2–25 years) | M+F | 16–25 (1) | 17.8 (13.8–22.6)a |
| Thomas (22) | U.K. | UK Biobank | 2006–2010 | 379,511 (37–73 years) | M+F | 31–60 (2) | 28.3c |
| Feltbower (61) | U.K. (West Yorkshire) | Yorkshire Regional Childhood Diabetes Register | 1991–1999 | 2.1 million (all ages) | M+F | 15–29 (1) | 3.4c |
| Western Pacific | |||||||
| Diabetes Australia (62) | Australia | NDSSb | 2020 | 18.8 million (≥21 years) | M+F | 21–29 (1) | 17.7c |
| M+F | 30–39 (1) | 15.1c | |||||
| M+F | 40–49 (2) | 10.1c | |||||
| M+F | 50–59 (2) | 9.8c | |||||
| M+F | 60–69 (3) | 7.2c | |||||
| M+F | 70–79 (3) | 7.5c | |||||
| M+F | ≥80 (3) | 4.0c | |||||
| Weng (63) | China | N/A | 2010–2013 | 133 million PY (≥20 years) | M+F | ≥30 (4) | 0.51 (0.49–0.53)c |
| M+F | 20–24 (1) | 1.11 (1.03–1.19)c | |||||
| M+F | 25–29 (1) | 1.19 (1.11–1.29)c | |||||
| M+F | 30–34 (1) | 1.02 (0.94–1.12)c | |||||
| M+F | 35–39 (1) | 0.73 (0.66–0.81)c | |||||
| M+F | 40–44 (2) | 0.54 (0.48–0.61)c | |||||
| M+F | 45–49 (2) | 0.54 (0.47–0.61)c | |||||
| M+F | 50–54 (2) | 0.60 (0.52–0.69)c | |||||
| M+F | 55–59 (2) | 0.54 (0.47–0.62)c | |||||
| M+F | 60–64 (3) | 0.44 (0.36–0.53)c | |||||
| M+F | 65–69 (3) | 0.38 (0.29–0.49)c | |||||
| M+F | 70–74 (3) | 0.32 (0.23–0.43)c | |||||
| M+F | ≥75 (3) | 0.37 (0.27–0.51)c | |||||
| Luk (17) | Hong Kong | HKDSD | 2002–2015T* | 7.3 million (all ages) | M | 20–39 (1) | 0.53c |
| M | 40–59 (2) | 0.59c | |||||
| M | ≥60 (3) | 0.39c | |||||
| F | 20–39 (1) | 0.84c | |||||
| F | 40–59 (2) | 0.33c | |||||
| F | ≥60 (3) | 0.23c | |||||
| Nishioka (64) | Japan | NDB | 2014–2017 | 65.3 million (≥20 years) | M | 20–39 (1) | 5.6 (5.2–5.9)c |
| M | 40–59 (2) | 5.7 (5.4–6.0)c | |||||
| M | ≥60 (3) | 3.5 (3.3–3.7)c | |||||
| F | 20–39 (1) | 4.8 (4.6–5.1)c | |||||
| F | 40–59 (2) | 5.0 (4.8–5.2)c | |||||
| F | ≥60 (3) | 3.3 (3.1–3.5)c | |||||
| Lee (11) | Korea | NHIS | 2007–2013T* | 51.3 million (all ages) | M+F | ≥20 (4) | 3.0c |
| Scott (65) | New Zealand (Canterbury) | Canterbury Diabetes Registry | 1981–1986 | 345,768 (all ages) | M+F | 20–29 (1) | 8.1 (5.4–11.7)c |
| M+F | 30–39 (1) | 10.6 (7.3–14.9)c | |||||
| M+F | 40–49 (2) | 11.8 (7.8–17.2)c | |||||
| M+F | 50–59 (2) | 13.1 (8.6–19.3)c | |||||
| M+F | 60–69 (3) | 18.6 (12.9–26.0)c | |||||
| M+F | ≥70 (3) | 21.7 (15.2–30.0)c | |||||
| M | 20–29 (1) | 11.1 (6.8–17.1)c | |||||
| M | 30–39 (1) | 11.7 (6.9–18.5)c | |||||
| M | 40–49 (2) | 16.7 (10.1–26.1)c | |||||
| M | 50–59 (2) | 17.0 (9.9–27.2)c | |||||
| M | 60–69 (3) | 25.9 (15.2–39.1)c | |||||
| M | ≥70 (3) | 25.3 (14.5–41.0)c | |||||
| F | 20–29 (1) | 5.1 (2.3–9.7)c | |||||
| F | 30–39 (1) | 9.5 (5.3–15.7)c | |||||
| F | 40–49 (2) | 7.0 (3.0–13.8)c | |||||
| F | 50–59 (2) | 9.1 (4.2–17.3)c | |||||
| F | 60–69 (3) | 12.3 (6.4–21.5)c | |||||
| F | ≥70 (3) | 19.5 (11.9–30.0)c | |||||
| Sheen (12) | Taiwan | NHIRD | 2005–2014T* | Not reported | M+F | 20–39 (1) | 1.4c |
| M+F | ≥40 (2) | 0.5 | |||||
| All other regions | |||||||
| Gorham (66) | U.S. | U.S. Navye | 1974–1988 | 1.6 million (17–34 years) | M (W) | 20–24 (1) | 18.0 (16.3–20.0)c |
| M (W) | 25–29 (1) | 24.1 (20.9–27.7)c | |||||
| M (W) | 30–34 (1) | 32.4 (28.0–37.6)c | |||||
| F (W) | 20–24 (1) | 26.2 (19.5–34.6)c | |||||
| F (W) | 25–29 (1) | 29.8 (19.1–44.4)c | |||||
| F (W) | 30–34 (1) | 33.2 (15.9–61.1)c | |||||
| M (B) | 20–24 (1) | 17.1 (12.7–22.6)c | |||||
| M (B) | 25–29 (1) | 39.4 (30.3–51.2)c | |||||
| M (B) | 30–34 (1) | 88.1 (67.9–114.0)c | |||||
| F (B) | 20–24 (1) | 25.9 (12.9–46.4)c | |||||
| F (B) | 25–29 (1) | 15.9 (3.3–46.3)c | |||||
| F (B) | 30–34 (1) | 92.9 (34.1–202.0)c | |||||
| Rogers (13) | U.S. | Clinformatics Data Mart databasee | 2001–2015T | 61 million (all ages) | M+F | 20–24 (1) | 18.0 (17.2–18.9)c |
| M+F | 25–29 (1) | 16.6 (15.8–17.3)c | |||||
| M+F | 30–34 (1) | 15.3 (14.6–16.0)c | |||||
| M+F | 35–39 (1) | 15.9 (15.2–16.6)c | |||||
| M+F | 40–44 (2) | 16.0 (15.3–16.7)c | |||||
| M+F | 45–49 (2) | 17.8 (17.1–18.5)c | |||||
| M+F | 50–54 (2) | 20.0 (19.2–20.8)c | |||||
| M+F | 55–59 (2) | 23.4 (22.5–24.3)c | |||||
| M+F | 60–64 (3) | 29.2 (28.0–30.4)c | |||||
| Lawrence (24) | U.S. (California) | Kaiser Permanentee | 2017 | 2.4 million (20–45 years) | M+F | 20–45 (1) | 30.1 (23.5–36.8)a |
| M+F | 20–29 (1) | 15.2 (10.2–20.1)c | |||||
| M+F | 30–45 (1) | 38.2 (28.6–47.8)c | |||||
| M | 20–45 (1) | 32.5 (22.2–42.8)a | |||||
| F | 20–45 (1) | 27.2 (21.0–34.5)a | |||||
| Mebrahtu (67) | Eritrea | N/A | 2019 | 316,118 (20–24) | M+F | 20–24 (1) | 46.2 (39.0–53.3)c |
| M | 20–24 (1) | 55.0 (44.1–68.0)c | |||||
| F | 20–24 (1) | 37.2 (28.2–48.0)c | |||||
| Alemu (68) | Ethiopia | N/A | 1995–2008 | 2.5 million (all ages) | M | 21–25 | 7.2c |
| M | 26–30 | 8.9c | |||||
| M | 31–35 | 6.6c | |||||
| M | 36–40 | 3.9c | |||||
| M | 41–50 | 2.8c | |||||
| M | 46–60 | 1.8c | |||||
| M | 61–70 | 0.5c | |||||
| M | 71–80 | 1.3c | |||||
| F | 21–25 | 2.4c | |||||
| F | 26–30 | 3.1c | |||||
| F | 31–35 | 2.9c | |||||
| F | 36–40 | 2.3c | |||||
| F | 41–50 | 2.4c | |||||
| F | 46–60 | 1.2c | |||||
| F | 61–70 | 1.6c | |||||
| F | 71–80 | 0.7c | |||||
| Sandy (14) | Mali | N/A | 2007–2016T* | 12.1 million (<25 years) | M+F | 20–24 (1) | 1.1 (0.6–1.8)c |
| M+F | 20–24 (1) | 0.7 (0.5–0.9)c | |||||
| Marshall (69) | Rwanda | LFAC program registry | 2007–2011 | Not reported | M+F | 20–24 (1) | 5.0 (2.8–8.6)c |
| Pishdad (70) | Iran (Fars) | N/A | 1991–1996 | 587,000 (20–29 years) | M | 20–24 (1) | 3.3 (2.0–4.6)c |
| M | 25–29 (1) | 3.1 (1.8–4.4)c | |||||
| F | 20–24 (1) | 3.4 (2.1–4.7)c | |||||
| F | 25–29 (1) | 3.6 (2.1–5.0)c | |||||
| Kadiki (71) | Libya (Benghazi) | N/A | 1981–1990 | 9,635 (20–34 years) | M+F | 20–24 (1) | 7.0 (4.7–10.2)c |
| M+F | 25–29 (1) | 10.4 (7.0–14.8)c | |||||
| M+F | 30–34 (1) | 12.4 (8.4–17.7)c | |||||
| M | 20–24 (1) | 10.9 (6.8–16.5)c | |||||
| M | 25–29 (1) | 11.4 (6.8–18.0)c | |||||
| M | 30–34 (1) | 16.7 (10.3–25.6)c | |||||
| F | 20–24 (1) | 2.9 (1.1–6.3)c | |||||
| F | 25–29 (1) | 9.4 (5.1–15.8)c | |||||
| F | 30–34 (1) | 7.7 (3.5–14.6)c | |||||
| Kumar (72) | India | Military personnele | 1990–2015 | 51,217 (≥18 years) | M | ≥18 (4) | 2.4c |
B, Black adults; CPRD, Clinical Practice Research Datalink; EURODIAB TIGER, EUROpe and DIABetes Type I Genetic Epidemiology Resource; F, females; HKDSD, The Hong Kong Diabetes Surveillance Database; LFAC, Life For a Child; M, males; N/A, not applicable; NDB, National Database of Health Insurance Claims and Specific Health Checkups of Japan; NDSS, National Diabetes Service Scheme; NHIRD, National Health Insurance Research Database; NHIS, National Health Insurance Service; SRDP, State Registry of DM Patients; W, White adults.
Time trends available.
Most recent year/period reported.
Assigned categories for age-specific analysis (1, 20–40 years; 2, 40–60 years; 3, >60 years; 4, ≥20 years).
Age-standardized rates.
For estimation of Australian rates, the number of new cases of T1D, reported by the National Diabetes Service Scheme, was divided by the 2019 Australian estimated resident population, obtained from the Australian Bureau of Statistics.
Crude rates.
Age range not defined.
Insurance- or occupation-based population.
The incidence of adult-onset T1D varied considerably, and the following general patterns were observed: 1) the incidence of adult-onset T1D is lowest in predominantly Asian countries, regions, or territories (China, Taiwan, and Hong Kong) and highest in Nordic countries (Sweden, Finland, Norway, and Denmark); 2) the majority of studies stem from high-income countries in Europe and the Western Pacific, with a clear gap in data from low- and middle-income countries; 3) among studies with reporting of T1D incidence across all age categories (20–39, 40–59, and ≥60 years), in 7 of 12 (58%) a decrease was reported in T1D incidence with increasing age, and in 5 of 12 (42%) an increase was reported in T1D incidence with increasing age (Supplementary Fig. 1); and 4) among 26 studies with reporting of sex-specific estimates, in 92% there was a higher incidence of T1D reported among men compared with women, excluding in Iran and in the U.S. Navy study where women had a similar or higher incidence of T1D relative to men (Supplementary Fig. 2).
Time Trends
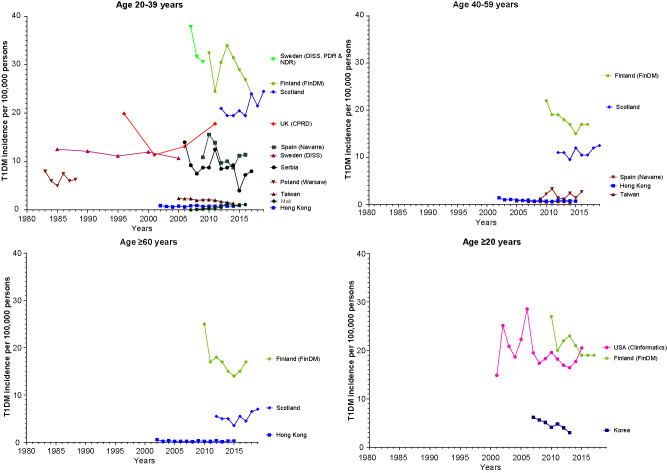
In 13 studies investigators reported trends in adult-onset T1D incidence over time (Fig. 3), with mixed findings. For five (38%) studies, including data from Serbia (9), Sweden (Diabetes Incidence Study in Sweden [DISS]) (10), Korea (11), Taiwan (12), and the U.S. (13), a decrease was reported in adult-onset T1D incidence over time, ranging from 1983 to 2017. In contrast, in one study from Mali (14) an increase was reported in adult-onset T1D incidence between 2007 and 2016, while data from Spain (2009–2016) (15), the U.K. (1994–2013) (16), and Hong Kong (2002–2015) (17) showed no change over time. These trends were consistent across age-groups. Four studies did not include formal assessment of changes in incidence over time, though data from Finland (Diabetes in Finland [FinDM]) (18) suggest a decline in adult-onset T1D incidence between 2010 and 2017, data from Poland (1983–1988) (19) and Sweden (DISS, National Diabetes Register [NDR], and Prescribed Drug Register [PDR], from 2007–2009) (20) indicate T1D incidence was stable over time, and data from Scotland (2012–2019) (21) indicate an increase in T1D incidence among the 20–39 and ≥60 years age-groups.
Figure 3.
Trends in the incidence of adult-onset T1D by age-group and country/region. Note: U.S. trend data are based on an insurance population. As such, it cannot be determined whether declines in adult-onset T1D are true changes over time or due to changes in the underlying study population. Clinformatics, Clinformatics Data Mart database.
Diagnosis of T1D and Quality Assessment
Among all studies, seven (15%) included use of biomarkers (in conjunction with clinical features) for definition of T1D. These studies were from the U.K. (UK Biobank) (22), Sweden (10,20,23), the U.S. (California) (24), Spain (Navarre) (15), and Norway (Nord-Trøndelag Health Study [The HUNT Study]) (25). (See Supplementary Table 5 for detailed description of T1D definition and Supplementary Table 6 for scores related to diagnosis of T1D.) For the large majority, record linkage (n = 19 [41%]) or an administrative algorithm (n = 20 [43%]) was used to define T1D.
Based on the modified Newcastle-Ottawa Scale, the quality of studies ranged from a score of 5 (Ethiopia) to a perfect score of 11 (Sweden). No studies were deemed low quality (score 0–4), 13 (28%) studies were deemed moderate quality (score 5–7), and 33 studies (72%) were deemed high-quality studies (score 8–11) (Supplementary Table 6).
Discussion
In this Systematic Review, we identified five key messages from 46 studies across 32 countries and regions reporting on adult-onset T1D incidence. First, there is a general paucity of data on adult-onset T1D, particularly from low- and middle-income countries, despite our attempts to include data from the gray literature, limiting our ability to make a truly global assessment on the burden of adult-onset T1D. Second, adult-onset T1D generally reflects patterns seen in childhood-onset T1D in that incidence is higher in men (vs. women) and rates are highest in Nordic populations and lowest in Asian populations. Third, though more data are needed, we found no clear relationship between adult-onset T1D incidence and age, with 42% of studies showing an increase in T1D incidence with increasing age, while the remaining 58% of studies showed a decline in T1D incidence with increasing age. Regardless, we found that the incidence of T1D onset in older adults remained substantial. Fourth, there are no clear trends in adult-onset T1D over time due to a paucity of data. Among 13 studies reporting on trends over time, 46% (n = 6), 15% (n = 2), and 38% (n = 5) reported decreasing, increasing, and stable trends, respectively, over varying time periods between 1983 and 2019. Although various organizations such as the World Health Organization and the American Diabetes Association have provided consensus-based guidelines for the diagnosis and classification of diabetes since 1979 (26), we found varying approaches to defining adult-onset T1D with as yet no internationally adopted consensus. Given that the findings of this Systematic Review highlight a substantial burden of adult-onset T1D, there is a pressing need to define, test, and compare diagnostic criteria in multiple high-quality studies to better distinguish T1D from T2D in adults so that we may better assess the true burden of T1D in adults.
This is an updated and comprehensive overview of the current knowledge on adult-onset T1D, an area with significant knowledge gaps and seemingly slowly advancing knowledge. In comparison with an earlier systematic review of T1D in young adults >15 years (6), we included fewer studies, given our focus on reporting estimates from population-based studies only and our stricter age criteria (≥20 years). The earlier systematic review by Diaz-Valencia et al. (6) included several data sources that were not population based. Hospital-based or other clinic-based cohorts are more likely to represent severe cases of T1D, a form of selection bias that might lead to underestimates of T1D incidence, hence the alternative approach we have undertaken. Nevertheless, we believe the incidence rates of adult-onset T1D are still likely to be in general underestimates, given the likelihood of missed cases among those presenting with diabetes in adulthood (5).
Similar to findings by Diaz-Valencia et al. (6), patterns of adult-onset T1D generally mirror those of childhood-onset T1D, whereby countries and regions with high incidence of adult-onset T1D are also the areas with high incidence of childhood T1D incidence. This suggests that similar patterns of underlying genetic predisposition and environmental exposures, or the interaction between genes and environment, may operate in increased risk of T1D, regardless of age (27). Similar to Diaz-Valencia et al., we also noted that incidence of adult-onset T1D is in general higher among men as compared with women. As an extension to the study by Diaz-Valencia, we searched the gray literature to identify data on adult-onset T1D that may appear in national diabetes registries, or national health surveys, but not in the published literature. From our search of data from 54 countries, we were able to add information from three countries (Australia, Scotland, and Finland) using this approach. To truly capture the burden of adult-onset T1D, existing diabetes registries, and national health surveys, need to incorporate metrics of adult-onset T1D, and not just childhood-onset T1D, and to make this data publicly available.
In comparison with the analysis of adult-onset T1D in the current study, the results of most studies in children and adolescents suggest a global increase in the incidence of T1D, at a rate of approximately 3–4% per year over past decades, with the increase in general steeper in low-incidence countries (1,27–29). Several studies posit that the increases in childhood-onset T1D may be due to changes in dietary patterns, and other environmental factors associated with T1D such as increases in maternal age, obesity, and low vitamin D (27). Whether the incidence of adult-onset T1D is also increasing remains unclear due to a limited number of studies including reports on trends over time. Among these, mixed findings are likely a result of differences in T1D classification as well as different calendar periods. This is coupled with issues relating to missed diagnosis of autoimmune diabetes among those presenting with adult-onset diabetes, and comparatively high and changing background incidence of T2D, which may render any potential increase in adult-onset T1D difficult to detect.
Data from the available studies suggest that T1D incidence in adults does not decline with increasing age. In a U.S. study with use of electronic health records, T1D incidence peaked at age 10–14 years and a stable and gradual increase in T1D incidence was observed throughout adulthood (13). This differs from the common school of thought that T1D is a disease with onset in childhood or adolescence. Results of a recent UK Biobank study with incorporation of genetic markers suggest that the incidence of genetically defined T1D remains stable through different age-groups up to the oldest age-group (51–60 years) studied within the UK Biobank, although T1D cases represent a much smaller proportion of total diabetes cases in the older age-groups, as the number of incident T2D cases increase with age (22). In this same study, patients with genetically identified T1D had clinical features distinct from those with T2D such as being more likely to use insulin within the first year of diagnosis (22).
In most of the studies included in this Systematic Review, physician diagnosis of T1D was used, and some misclassification is likely present. In sub-Saharan African populations where severe undernutrition is prevalent, T1D identified by an administrative algorithm is strongly associated with poverty or markers of undernutrition (30), with a strong male predominance (30). Whether T1D identified in this setting results from β-cell loss due to autoimmunity or other mechanisms remains a topic of inquiry (31). Given the much higher prevalence of T2D in adults, a significant proportion of cases of T1D are likely to be missed and managed as T2D (32–34). Indeed, >40% of those developing T1D after age 30 years are initially treated as having T2D (5,35,36), highlighting the need for assessment of autoimmunity or β-cell function in the evaluation of adult-onset diabetes. In the current Systematic Review, only 15% of included studies had biomarkers incorporated for the diagnosis of T1D, though it should be noted that biomarkers other than blood glucose are often not available, particularly for studies conducted in the 1970–1990s. This is similar to findings from an earlier systematic review, in which only 14 of 70 studies (20%) included measurements of autoantibodies or C-peptide in the assessment of T1D, with most studies relying on clinical symptoms or early initiation of insulin therapy to diagnose T1D (6). Though autoantibody testing among all people with adult-onset diabetes may aid identification of T1D, it is important to remember that in a setting of high prior odds of T2D, and known background population autoantibody positivity, this could also lead to overestimation of T1D in adults. Therefore, combining clinical features that increase odds of T1D, prior to autoantibody measurement, or a combined diagnostic model including clinical features such as low BMI may in the future be the most accurate way to identify and classify individuals with adult-onset T1D (37–39). Indeed, in a 2021 joint consensus statement (published after our Systematic Review was conducted) (40), the American Diabetes Association and the European Association for the Study of Diabetes recommend the use of islet antibodies, in conjunction with clinical features and a C-peptide test (particularly after >3 years’ diabetes duration [41]), to distinguish T1D from T2D in adults with suspected T1D.
Our work has highlighted the paucity of data on adult-onset T1D. Given the nature of the disease and the difficulty in establishing an accurate diagnosis, national registries provide an invaluable source of data to chart the burden of adult-onset T1D. National diabetes registries provide population-based data that can offer insights into diagnosis, complications, treatment, and burden of diabetes (42). In this analysis, we have included data from several national registries that provided estimates of incidence of adult-onset T1D. Nevertheless, identifying relevant national registries and extracting the relevant information are not necessarily straightforward. Further, some registries are restricted to childhood-onset T1D only and do not capture data on adult-onset T1D. Development of a globally implemented standard for the implementation and maintenance of national diabetes registries is needed to assess the true burden of adult-onset T1D and to determine where resources and interventions are most needed.
This is a comprehensive Systematic Review of adult-onset T1D with incorporation of both a traditional literature search (e.g., MEDLINE) and an extensive assessment of the available gray literature. There are, however, some limitations. First, with our MEDLINE and Embase search strategies we considered only MeSH terms (not text words) and thus may have missed some studies that had not been indexed accordingly; however, by virtue of searching the reference lists of 1) included studies and 2) previous reviews on T1D incidence (6,43), we believe the likelihood that we have missed any relevant studies is low. Second, as discussed above, misclassification of diabetes type cannot be ruled out. Third, given the paucity of data particularly in low- and middle-income countries, our results are generalizable mainly to high-income countries. Last, our data are limited in terms of the time period covered by some data sources.
Conclusion
The findings of this Systematic Review demonstrate a substantial burden of adult-onset T1D incidence and a pressing need to improve the quality and quantity of information on adult-onset T1D, particularly in low- and middle-income countries, through well-designed and maintained diabetes registries with biomarker data. Such data are essential to better understanding of the epidemiology and natural history of adult-onset T1D and, more importantly, ensuring the planning and provision of appropriate clinical care.
Article Information
Acknowledgments. This work was initiated by the IDF as an extension of the 10th edition of the IDF Diabetes Atlas.
Funding. R.C.W.M. acknowledge support from the Research Grants Council Research Impact Fund (R4012-18) and a Croucher Foundation Senior Medical Research Fellowship. R.A.O. acknowledges support from a Diabetes UK Harry Keen Fellowship (16/0005529). P.L.W. acknowledges support from VA Puget Sound. A.J.J. acknowledges support from an Australian National Health and Medical Research Council (NHMRC) Practitioner Fellowship. A.J.J. has received research grants from JDRF and the NHMRC.
Duality of Interest. R.A.O. has received consulting fees from Janssen Research & Development (R&D), and funding from Janssen R&D as a co-investigator on the CASCADE Study (Combined Antibody Screening for Celiac and Diabetes Evaluation). R.A.O. has received U.K. Medical Research Council funding to develop a T1D genetic risk score diagnostic test in collaboration with Randox Laboratories R&D. A.J.J. has served on advisory boards for Medtronic Australia, Abbott Diabetes Australia, and Sanofi, has received research grants from Abbott Europe, Mylan, and Sanofi and has received speaker honorarium from Amgen. R.C.W.M. has received research grants for clinical trials from AstraZeneca, Bayer, Merck Sharp & Dohme (MSD), Novo Nordisk, Sanofi, and Tricida, Inc., and honoraria for consultancy or lectures from AstraZeneca and Boehringer Ingelheim. All proceeds were donated to The Chinese University of Hong Kong to support diabetes research. The 10th edition of the IDF Diabetes Atlas was supported by an educational grant from the Pfizer-MSD alliance, with additional support from Sanofi and Novo Nordisk. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. J.L.H. contributed to study design, conducted the literature search, screened eligible studies, extracted and summarized data, and wrote the manuscript. P.L.W. and X.Z. contributed to study design, screened eligible studies, conducted the quality review, and reviewed and edited the final manuscript. S.K., D.J.M., and A.J.J. contributed to the grey literature search and reviewed and edited the final manuscript. X.L. contributed to screening and reviewed and edited the final manuscript. S.K., H.C., H.S. Y.X., R.A.O., and Z.Z. provided intellectual input and reviewed and edited the final manuscript. R.C.W.M. oversaw the project, contributed to study design, screened eligible studies, and reviewed and edited the final manuscript.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.18409844.
References
- 1. DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet 2018;391:2449–2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am 2010;39:481–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. International Diabetes Federation . IDF Diabetes Atlas. 9th ed. Belgium, International Diabetes Federation, 2019 [Google Scholar]
- 4. Shields BM, Peters JL, Cooper C, et al. Can clinical features be used to differentiate type 1 from type 2 diabetes? A systematic review of the literature. BMJ Open 2015;5:e009088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thomas NJ, Lynam AL, Hill AV, et al. Type 1 diabetes defined by severe insulin deficiency occurs after 30 years of age and is commonly treated as type 2 diabetes. Diabetologia 2019;62:1167–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Diaz-Valencia PA, Bougnères P, Valleron AJ. Global epidemiology of type 1 diabetes in young adults and adults: a systematic review. BMC Public Health 2015;15:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wells G, Shea B, O’connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses, 2021. Accessed 16 June 2021. Available from https://www.ohri.ca/programs/clinical_ epidemiology/oxford.asp
- 8. Magliano DJ, Islam RM, Barr ELM, et al. Trends in incidence of total or type 2 diabetes: systematic review. BMJ 2019;366:l5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vojislav C, Natasa R, Milica P, et al. Incidence trend of type 1 diabetes mellitus in Serbia. BMC Endocr Disord 2020;20:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dahlquist GG, Nyström L; Swedish Childhood Diabetes Study Group; Diabetes Incidence in Sweden Study Group . Incidence of type 1 diabetes in Sweden among individuals aged 0-34 years, 1983-2007: an analysis of time trends. Diabetes Care 2011;34:1754–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee YB, Han K, Kim B, et al. High proportion of adult cases and prevalence of metabolic syndrome in type 1 diabetes mellitus population in Korea: a nationwide study. Diabetes Metab J 2019;43:76–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sheen YJ, Hsu CC, Jiang YD, Huang CN, Liu JS, Sheu WH. Trends in prevalence and incidence of diabetes mellitus from 2005 to 2014 in Taiwan. J Formos Med Assoc 2019;118(Suppl. 2):S66–S73 [DOI] [PubMed] [Google Scholar]
- 13. Rogers MAM, Kim C, Banerjee T, Lee JM. Fluctuations in the incidence of type 1 diabetes in the United States from 2001 to 2015: a longitudinal study. BMC Med 2017;15:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sandy JL, Besançon S, Sidibé AT, Minkailou M, Togo A, Ogle GD. Rapid increases in observed incidence and prevalence of type 1 diabetes in children and youth in Mali, 2007-2016. Pediatr Diabetes 2021;22:545–551 [DOI] [PubMed] [Google Scholar]
- 15. Forga L, Tamayo I, Chueca M, Ibáñez B, Sainz de Los Terreros A; en representación del Grupo de Estudio de Diabetes tipo 1 de Navarra . Incidence of type 1 diabetes mellitus in Navarre stabilized in the last eight years. Endocrinol Diabetes Nutr (Engl Ed) 2018;65:274–279 [DOI] [PubMed] [Google Scholar]
- 16. Abbasi A, Juszczyk D, van Jaarsveld CHM, Gulliford MC. Body mass index and incident type 1 and type 2 diabetes in children and young adults: a retrospective cohort study. J Endocr Soc 2017;1:524–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Luk AOY, Ke C, Lau ESH, et al. Secular trends in incidence of type 1 and type 2 diabetes in Hong Kong: a retrospective cohort study. PLoS Med 2020;17:e1003052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arffman M, Ilanne-Parikka P, Keskimäki I. FinDM database on diabetes in Finland, Discussion Paper 19/2020. Finnish Institute for Health and Welfare (THL). Accessed 10 February 2022. Available from https://urn.fi/URN:ISBN:978-952-343-492-9
- 19. Wysocki MJ, Chanska M, Bak M, Czyzyk AS. Incidence of insulin-dependent diabetes mellitus in Warsaw, Poland, in children and young adults, 1983-1988. World Health Stat Q 1992;45:315–320 [PubMed] [Google Scholar]
- 20. Rawshani A, Landin-Olsson M, Svensson AM, et al. The incidence of diabetes among 0-34 year olds in Sweden: new data and better methods. Diabetologia 2014;57:1375–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Scottish Diabetes Data Group . Scottish Diabetes Survey 2019. Scotland, National Health Service. Accessed 10 July 2021. Available from https://www.diabetesinscotland.org.uk/wp-content/uploads/2020/10/Diabetes-Scottish- Diabetes-Survey-2019.pdf
- 22. Thomas NJ, Jones SE, Weedon MN, Shields BM, Oram RA, Hattersley AT. Frequency and phenotype of type 1 diabetes in the first six decades of life: a cross-sectional, genetically stratified survival analysis from UK Biobank. Lancet Diabetes Endocrinol 2018;6:122–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thunander M, Petersson C, Jonzon K, et al. Incidence of type 1 and type 2 diabetes in adults and children in Kronoberg, Sweden. Diabetes Res Clin Pract 2008;82:247–255 [DOI] [PubMed] [Google Scholar]
- 24. Lawrence JM, Slezak JM, Quesenberry C, et al. Incidence and predictors of type 1 diabetes among younger adults aged 20-45 years: The diabetes in young adults (DiYA) study. Diabetes Res Clin Pract 2021;171:108624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Olsson L, Grill V, Midthjell K, Ahlbom A, Andersson T, Carlsson S. Mortality in adult-onset autoimmune diabetes is associated with poor glycemic control: results from The HUNT Study. Diabetes Care 2013;36:3971–3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. National Diabetes Data Group . Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039–1057 [DOI] [PubMed] [Google Scholar]
- 27. Norris JM, Johnson RK, Stene LC. Type 1 diabetes-early life origins and changing epidemiology. Lancet Diabetes Endocrinol 2020;8:226–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Patterson CC, Karuranga S, Salpea P, et al. Worldwide estimates of incidence, prevalence and mortality of type 1 diabetes in children and adolescents: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract 2019;157:107842. [DOI] [PubMed] [Google Scholar]
- 29. Tuomilehto J, Ogle GD, Lund-Blix NA, Stene LC. Update on worldwide trends in occurrence of childhood type 1 diabetes in 2020. Pediatr Endocrinol Rev 2020;17(Suppl. 1):198–209 [DOI] [PubMed] [Google Scholar]
- 30. Fekadu S, Yigzaw M, Alemu S, et al. Insulin-requiring diabetes in Ethiopia: associations with poverty, early undernutrition and anthropometric disproportion. Eur J Clin Nutr 2010;64:1192–1198 [DOI] [PubMed] [Google Scholar]
- 31. Balcha SA, Phillips DIW, Trimble ER. Type 1 diabetes in a resource-poor setting: malnutrition related, malnutrition modified, or just diabetes? Curr Diab Rep 2018;18:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu B, Xiang Y, Liu Z, Zhou Z. Past, present and future of latent autoimmune diabetes in adults. Diabetes Metab Res Rev 2020;36:e3205. [DOI] [PubMed] [Google Scholar]
- 33. Turner R, Stratton I, Horton V, et al.; UK Prospective Diabetes Study Group . UKPDS 25: autoantibodies to islet-cell cytoplasm and glutamic acid decarboxylase for prediction of insulin requirement in type 2 diabetes. Lancet 1997;350:1288–1293 [DOI] [PubMed] [Google Scholar]
- 34. Luk AOY, Lau ESH, Lim C, et al. Diabetes-related complications and mortality in patients with young-onset latent autoimmune diabetes: a 14-year analysis of the prospective Hong Kong Diabetes Register. Diabetes Care 2019;42:1042–1050 [DOI] [PubMed] [Google Scholar]
- 35. Muñoz C, Floreen A, Garey C, et al. Misdiagnosis and diabetic ketoacidosis at diagnosis of type 1 diabetes: patient and caregiver perspectives. Clin Diabetes 2019;37:276–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hope SV, Wienand-Barnett S, Shepherd M, et al. Practical classification guidelines for diabetes in patients treated with insulin: a cross-sectional study of the accuracy of diabetes diagnosis. Br J Gen Pract 2016;66:e315–e322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jones AG, McDonald TJ, Shields BM, Hagopian W, Hattersley AT. Latent autoimmune diabetes of adults (LADA) is likely to represent a mixed population of autoimmune (type 1) and nonautoimmune (type 2) diabetes. Diabetes Care 2021;44:1243–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lynam A, McDonald T, Hill A, et al. Development and validation of multivariable clinical diagnostic models to identify type 1 diabetes requiring rapid insulin therapy in adults aged 18-50 years. BMJ Open 2019;9:e031586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Leslie RD, Evans-Molina C, Freund-Brown J, et al. Adult-onset type 1 diabetes: current understanding and challenges. Diabetes Care 2021;44:2449–2456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Holt RIG, DeVries JH, Hess-Fischl A, et al. The management of type 1 diabetes in adults. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2021;64:2609–2652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Foteinopoulou E, Clarke CAL, Pattenden RJ, et al. Impact of routine clinic measurement of serum C-peptide in people with a clinician-diagnosis of type 1 diabetes. Diabet Med 2021;38:e14449. [DOI] [PubMed] [Google Scholar]
- 42. Bak JCG, Serné EH, Kramer MHH, Nieuwdorp M, Verheugt CL. National diabetes registries: do they make a difference? Acta Diabetol 2021;58:267–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mobasseri M, Shirmohammadi M, Amiri T, Vahed N, Hosseini Fard H, Ghojazadeh M. Prevalence and incidence of type 1 diabetes in the world: a systematic review and meta-analysis. Health Promot Perspect 2020;10:98–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weets I, De Leeuw IH, Du Caju MV, et al.; Belgian Diabetes Registry . The incidence of type 1 diabetes in the age group 0-39 years has not increased in Antwerp (Belgium) between 1989 and 2000: evidence for earlier disease manifestation. Diabetes Care 2002;25:840–846 [DOI] [PubMed] [Google Scholar]
- 45. Roglić G, Pavlić-Renar I, Sestan-Crnek S, et al. Incidence of IDDM during 1988-1992 in Zagreb, Croatia. Diabetologia 1995;38:550–554 [DOI] [PubMed] [Google Scholar]
- 46. Mølbak AG, Christau B, Marner B, Borch-Johnsen K, Nerup J. Incidence of insulin-dependent diabetes mellitus in age groups over 30 years in Denmark. Diabet Med 1994;11:650–655 [DOI] [PubMed] [Google Scholar]
- 47. Lammi N, Taskinen O, Moltchanova E, et al. A high incidence of type 1 diabetes and an alarming increase in the incidence of type 2 diabetes among young adults in Finland between 1992 and 1996. Diabetologia 2007;50:1393–1400 [DOI] [PubMed] [Google Scholar]
- 48. Gajewska KA, Biesma R, Sreenan S, Bennett K. Prevalence and incidence of type 1 diabetes in Ireland: a retrospective cross-sectional study using a national pharmacy claims data from 2016. BMJ Open 2020;10:e032916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tenconi MT, Devoti G, Albani I, et al. IDDM in the province of Pavia, Italy, from a population-based registry: a descriptive study. Diabetes Care 1995;18:1017–1019 [DOI] [PubMed] [Google Scholar]
- 50. Muntoni S; Sardinian Collaborative Group for Epidemiology of IDDM . High incidence rate of IDDM in Sardinia. Diabetes Care 1992;15:1317–1322 [DOI] [PubMed] [Google Scholar]
- 51. Bruno G, Cerutti F, Merletti F, et al.; Piedmont Study Group for Diabetes Epidemiology . Residual β-cell function and male/female ratio are higher in incident young adults than in children: the registry of type 1 diabetes of the province of Turin, Italy, 1984–2000. Diabetes Care 2005;28:312–317 [DOI] [PubMed] [Google Scholar]
- 52. Ostrauskas R, Žalinkevičius R, Jurgevičienė N, Radzevičienė L, Lašaitė L. The incidence of type 1 diabetes mellitus among 15-34 years aged Lithuanian population: 18-year incidence study based on prospective databases. BMC Public Health 2011;11:813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Joner G, Søvik O. The incidence of type 1 (insulin-dependent) diabetes mellitus 15-29 years in Norway 1978-1982. Diabetologia 1991;34:271–274 [DOI] [PubMed] [Google Scholar]
- 54. Sobel-Maruniak A, Grzywa M, Orłowska-Florek R, Staniszewski A. The rising incidence of type 1 diabetes in south-eastern Poland. A study of the 0-29 year-old age group, 1980-1999. Endokrynol Pol 2006;57:127–130 [PubMed] [Google Scholar]
- 55. Ionescu-Tîrgovişte C, Paterache E, Cheţa D, Farcaşiu E, Serafinceanu C, Mincu I. Epidemiology of diabetes in Bucharest. Diabet Med 1994;11:413–417 [DOI] [PubMed] [Google Scholar]
- 56. Dedov SM II, Vikulova OK. Epidemiology of diabetes mellitus in the Russian Federation: clinical and statistical report for 2016 according to the federal diabetes registry. Diabetes Mellitus. 2017;20:13–41. Accessed 10 July 2021. Available from 10.14341/DM8664 [DOI] [Google Scholar]
- 57. Kyvik KO, Nystrom L, Gorus F, et al. The epidemiology of type 1 diabetes mellitus is not the same in young adults as in children. Diabetologia 2004;47:377–384 [DOI] [PubMed] [Google Scholar]
- 58. Goday A, Castell C, Tresserras R, Canela J, Taberner JL; The Catalan Epidemiology Diabetes Study Group . Incidence of type 1 (insulin-dependent) diabetes mellitus in Catalonia, Spain. Diabetologia 1992;35:267–271 [DOI] [PubMed] [Google Scholar]
- 59. Morales-Pérez FM, Barquero-Romero J, Pérez-Miranda M. Incidence of type I diabetes among children and young adults (0-29 years) in the province of Badajoz, Spain during 1992 to 1996. Acta Paediatr 2000;89:101–104 [DOI] [PubMed] [Google Scholar]
- 60. Crump C, Sundquist J, Sundquist K. Preterm birth and risk of type 1 and type 2 diabetes: a national cohort study. Diabetologia 2020;63:508–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Feltbower RG, McKinney PA, Parslow RC, Stephenson CR, Bodansky HJ. Type 1 diabetes in Yorkshire, UK: time trends in 0-14 and 15-29-year-olds, age at onset and age-period-cohort modelling. Diabet Med 2003;20:437–441 [DOI] [PubMed] [Google Scholar]
- 62. Diabetes Australia . National Diabetes Service Scheme Facts and Figures, 2020. Accessed 10 February 2022. Available from https://www.ndss.com.au/about-the-ndss/diabetes-facts-and-figures/
- 63. Weng J, Zhou Z, Guo L, et al.; T1D China Study Group . Incidence of type 1 diabetes in China, 2010-13: population based study. BMJ 2018;360:j5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nishioka Y, Noda T, Okada S, et al. Incidence and seasonality of type 1 diabetes: a population-based 3-year cohort study using the National Database in Japan. BMJ Open Diabetes Res Care 2020;8:e001262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Scott RS, Brown LJ. Prevalence and incidence of insulin-treated diabetes mellitus in adults in Canterbury, New Zealand. Diabet Med 1991;8:443–447 [DOI] [PubMed] [Google Scholar]
- 66. Gorham ED, Garland FC, Barrett-Connor E, Garland CF, Wingard DL, Pugh WM. Incidence of insulin-dependent diabetes mellitus in young adults: experience of 1,587,630 US Navy enlisted personnel. Am J Epidemiol 1993;138:984–987 [DOI] [PubMed] [Google Scholar]
- 67. Mebrahtu G, Maniam J, James S, Ogle GD. High incidence of type 1 diabetes in adolescents and young adults in Eritrea. Diabet Med 2021;38:e14544. [DOI] [PubMed] [Google Scholar]
- 68. Alemu S, Dessie A, Seid E, et al. Insulin-requiring diabetes in rural Ethiopia: should we reopen the case for malnutrition-related diabetes? Diabetologia 2009;52:1842–1845 [DOI] [PubMed] [Google Scholar]
- 69. Marshall SL, Edidin D, Arena VC, et al. Prevalence and incidence of clinically recognized cases of type 1 diabetes in children and adolescents in Rwanda, Africa. Diabet Med 2015;32:1186–1192 [DOI] [PubMed] [Google Scholar]
- 70. Pishdad GR. Low incidence of type 1 diabetes in Iran. Diabetes Care 2005;28:927–928 [DOI] [PubMed] [Google Scholar]
- 71. Kadiki OA, Reddy MR, Marzouk AA. Incidence of insulin-dependent diabetes (IDDM) and non-insulin-dependent diabetes (NIDDM) (0-34 years at onset) in Benghazi, Libya. Diabetes Res Clin Pract 1996;32:165–173 [DOI] [PubMed] [Google Scholar]
- 72. Kumar KVSH, Patnaik SK. A long term follow-up study from India assessing the risk of diabetes mellitus in service population. Diabetes Metab Syndr 2018;12:87–90 [DOI] [PubMed] [Google Scholar]