Abstract
BACKGROUND
Due to the rapidly increasing availability of metabolomics data in prospective studies, an update of the meta evidence on metabolomics and type 2 diabetes risk is warranted.
PURPOSE
To conduct an updated systematic review and meta-analysis of plasma, serum, and urine metabolite markers and incident type 2 diabetes.
DATA SOURCES
We searched PubMed and Embase until 6 March 2021.
STUDY SELECTION
We selected prospective observational studies where investigators used high-throughput techniques to investigate the relationship between plasma, serum, or urine metabolites and incident type 2 diabetes.
DATA EXTRACTION
Baseline metabolites per-SD risk estimates and 95% CIs for incident type 2 diabetes were extracted from all eligible studies.
DATA SYNTHESIS
A total of 61 reports with 71,196 participants and 11,771 type 2 diabetes cases/events were included in the updated review. Meta-analysis was performed for 412 metabolites, of which 123 were statistically significantly associated (false discovery rate–corrected P < 0.05) with type 2 diabetes risk. Higher plasma and serum levels of certain amino acids (branched-chain, aromatic, alanine, glutamate, lysine, and methionine), carbohydrates and energy-related metabolites (mannose, trehalose, and pyruvate), acylcarnitines (C4-DC, C4-OH, C5, C5-OH, and C8:1), the majority of glycerolipids (di- and triacylglycerols), (lyso)phosphatidylethanolamines, and ceramides included in meta-analysis were associated with higher risk of type 2 diabetes (hazard ratio 1.07–2.58). Higher levels of glycine, glutamine, betaine, indolepropionate, and (lyso)phosphatidylcholines were associated with lower type 2 diabetes risk (hazard ratio 0.69–0.90).
LIMITATIONS
Substantial heterogeneity (I2 > 50%, τ2 > 0.1) was observed for some of the metabolites.
CONCLUSIONS
Several plasma and serum metabolites, including amino acids, lipids, and carbohydrates, are associated with type 2 diabetes risk.
Introduction
Metabolomics is the comprehensive identification, using high-throughput techniques, of small molecules, including amino acids, carbohydrates, lipids, peptides, and organic acids, among others (1). Simultaneous assessment of disease risk associated with a broad spectrum of metabolites can further highlight the critical metabolic pathways in type 2 diabetes etiology.
In 2016, we published a systematic review and meta-analysis of observational studies identifying metabolites associated with prediabetes and type 2 diabetes (2). Evidence from 27 cross-sectional studies and 19 prospective cohort studies suggested that several amino acids (branched-chain amino acids [BCAAs], aromatic amino acids [AAAs], glycine, and glutamine), carbohydrates (glucose and fructose), and lipid metabolites from various classes (phospholipids, sphingomyelins [SMs], and triglycerides) were associated with type 2 diabetes risk. The meta-analysis of prospective studies indicated that a 1-SD increase in circulating levels of BCAAs (isoleucine, leucine, and valine) and AAAs (tyrosine and phenylalanine) was associated with a 26–36% higher risk of incident type 2 diabetes. Moreover, inverse associations between glycine and glutamine and type 2 diabetes risk were observed.
At that time, many identifiable metabolites, especially lipid metabolites, were unavailable for meta-analyses because there were few studies with available data (2). Since then, the number of prospective metabolomics profiling studies on type 2 diabetes risk has substantially increased (3–8). Furthermore, the progress in the metabolomics field allowed for annotation of previously unknown signals to known metabolites. In the majority of new studies, investigators focused on plasma and serum metabolite profiles and type 2 diabetes risk. However, in some studies urine metabolomics was also investigated in the context of type 2 diabetes risk (9,10).
The recent publications of state-of-the-art metabolomics data in relation to type 2 diabetes risk, including geographically and ethnically diverse prospective cohorts, biospecimens (plasma, serum, and urine), and distinct metabolomics platforms, merit consideration in updating the prior evidence. Therefore, we aimed to conduct an updated systematic review and meta-analysis of prospective studies where, using high-throughput metabolomics, investigators evaluate the association of single metabolite concentrations in plasma, serum, or urine with subsequent risk of type 2 diabetes.
Methods
This updated review is based on the protocol of its primary report (2), registered in the International Prospective Register of Systematic Reviews (PROSPERO) (identifier [ID] CRD42015023439). All changes to the protocol are summarized in Supplementary Table 1. We followed the Cochrane Handbook for Systematic Reviews of Interventions and guidelines from the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) to report the methods and results of this review (11).
Data Sources and Searches
We conducted a systematic search of published literature in two electronic databases, PubMed and Embase, to identify studies published between 1 August 2015 (date of completion for original report search) and 6 March 2021 (date of completion for the search for the current update). The search was not restricted by any database filter. Details of search strategies used for both databases are presented in Supplementary Table 2. We further hand searched references from retrieved articles to identify other potentially eligible studies. The primary study search was completed by one author (J.M.).
Study Selection
Titles and abstracts were independently screened in duplicate by two authors (J.M. and A.D.) for eligibility. Any disagreements were resolved through a discussion with a third author (M.G.-F.). Full-text versions of articles identified at this step were further read and assessed for eligibility to be included. Studies were eligible for inclusion if they met the following criteria: 1) were prospective observational studies in humans (cohort, case-cohort, or nested case-control); 2) were conducted in adults (aged ≥18 years); 3) used high-throughput metabolomics techniques (i.e., liquid or gas chromatography coupled with mass spectrometry [MS] or proton nuclear magnetic resonance [1H NMR] spectroscopy); 4) assessed metabolites in plasma, serum, or urine samples; and 5) reported associations between metabolite markers and risk of incident type 2 diabetes. We excluded cross-sectional and retrospective case-control studies; studies conducted in patients with type 1 or gestational diabetes mellitus; studies conducted in children, adolescents, and pregnant women; and nonoriginal articles (reviews, commentaries, editorials, or letters). Moreover, we excluded studies that did not provide risk estimates between specific metabolites and type 2 diabetes risk (studies focused solely on multivariate analyses, metabolite scores, or prediction models). We evaluated potential overlap between reports based on the same study, and if present, we selected the one with longer follow-up or more incident type 2 diabetes cases. Two authors completed the study eligibility assessment (J.M. and A.D.), with conflicts discussed with a third author (M.G.-F.).
Data Extraction and Quality Assessment
From each study, we extracted the following information: first author, year of publication, study location and name, study design, number of included participants, number of type 2 diabetes cases, the average length of follow-up, metabolomics assessment details (platform provider/name), technique (MS/1H NMR), targeting (targeted/non-targeted assay), number and type of analyzed metabolites/metabolic features, biological sample (biospecimen, fasting status), list of metabolites, covariate adjustment set, and multivariable-adjusted risk estimates for type 2 diabetes (odds ratio [OR], risk ratio [RR], or hazard ratio [HR]) with 95% CI. If the authors presented several estimates, we selected the one with adjustment for the highest number of covariates. When results were provided with stratification by sex, we pooled them with fixed-effects models. If the main results were pooled analyses of several studies or external validation, we extracted all parameters separately from each underlying study. We contacted the corresponding author of studies with missing or incomplete data (authors who provided additional data are listed in Supplementary Table 3).
Metabolites were identified according to their database ID or provided names. Where a metabolite could not be matched to a specific compound (i.e., a mixture of isomers), we have interpreted it as its most common variant in humans. To avoid simultaneous use of synonymous names of different metabolites, we have annotated names and IDs from the Human Metabolome Database (HMDB) (https://hmdb.ca/metabolites) or, if not found, from the Chemical Entities of Biological Interest (ChEBI) (https://www.ebi.ac.uk/chebi/). Additionally, when possible, we have assigned metabolites to the primary pathway in which they are involved.
Consistent with our original systematic review and meta-analysis (2), we assessed the methodological quality of included studies independently and in duplicate using a previously published scoring tool (12), with evaluation of study quality in six domains: study participation, study attrition, exposure measurement, outcome ascertainment, confounding, and statistical analysis appropriateness. Each domain is scored up to 1 point (maximum of 6 points), and the summary score represents overall quality (≤3 points denotes low quality).
Data Synthesis and Analysis
Due to a limited number of metabolomics studies using urine, we provided only a qualitative summary of the findings. We extracted the relevant information, as detailed above, and described and summarized the findings of these reports in a qualitative manner. For plasma and serum metabolites, findings were summarized using meta-analysis. We have performed a meta-analysis for type 2 diabetes risk estimates of all metabolites for which evidence from at least two independent, eligible studies was available. We made additional assumptions for grouping and performing meta-analysis for lipid metabolites due to varying analytical resolution of the molecular species on different metabolomics platforms (Supplementary Table 4).
We pooled risk estimates for the association between specific metabolites and risk of incident type 2 diabetes using random-effects models. For the meta-analyses, we interpreted ORs, RRs, and HRs as relative risk. We fitted models using a restricted maximum likelihood approach, which is recommended over the traditional DerSimonian-Laird method (13). We corrected P values for multiple testing via false discovery rate (FDR) and considered FDR-corrected P < 0.05 to be statistically significant.
Heterogeneity between studies was assessed using τ2 and I2 statistics (14), with an I2 value of 50% indicating substantial heterogeneity. As no guideline for interpretation of τ2 exists in literature, we selected a cutoff point of 0.10 based on a previous empirical study (15). To examine potential sources of heterogeneity, we stratified meta-analyses of ≥10 studies by biospecimen (plasma/serum), study location (U.S./Europe/Asia), fasting status (fasted/nonfasted), metabolomics platform (MS-based/NMR), fasting glucose adjustment (yes/no), and follow-up length (≤7/>7 years). Additionally, we used meta-regressions to examine the number of carbons and double bonds in lipid metabolites as potential determinants of the type 2 diabetes risk association. According to Cochrane Handbook for Systematic Reviews of Interventions recommendations, we evaluated the presence of publication bias through visual inspection of funnel plots and Egger’s linear regression test for comparisons where ≥10 studies were available (14). All analyses were conducted in R (version 3.6.0; R Foundation for Statistical Computing).
Data and Resource Availability
The data set supporting results of meta-analyses presented in this study is available in Supplementary Material.
Results
Literature Search Results
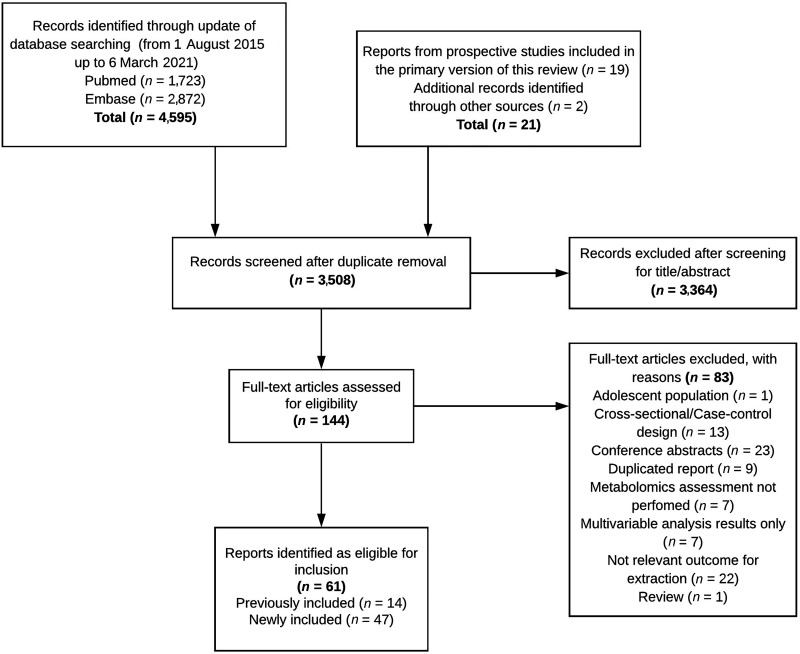
The study selection process is outlined in Fig. 1. The database search revealed 4,595 records. Nineteen prospective studies from the primary version of this review and two additional studies were identified by hand search of references. After deduplication of searches, 3,508 records were screened and 3,364 records were removed based on title and abstract, leaving 144 reports for full-text examination. We excluded 83 reports for reasons indicated in Supplementary Table 5. The present systematic review finally included 61 reports (3–10,16–68); 14 reports were already included in the previous version of this review, and 47 new reports have been added to the current update.
Figure 1.
Flowchart presenting information on the search and selection of studies included in the updated systematic review on metabolomics and incident type 2 diabetes.
Study Characteristics
We summarized the characteristics of the included studies in Supplementary Table 6. We identified 61 reports with data from 44 original prospective observational studies with 71,196 participants, of whom 11,771 developed type 2 diabetes. Twenty-six of the included studies were conducted in Europe, 10 in Asia, and 8 in North America. The average follow-up time ranged from 3.2 to 21.2 years (median 7.0 years). Most reports (n = 60) were of high quality (median quality score 5.0), and one of the included studies was judged to be of low quality (≤3 points).
In most studies investigators used MS coupled to different chromatography techniques to generate the metabolomics data (n = 37). In six studies an NMR platform was used and in one study a combination of MS and NMR platforms. Respectively, in 30 and 14 studies targeted and nontargeted approaches were used for metabolite measurements. Plasma samples were used in 21 studies and serum in 24 studies (two studies included evaluation of metabolites in both biospecimens). Urine samples were used in two studies.
Studies on Plasma and Serum Metabolites
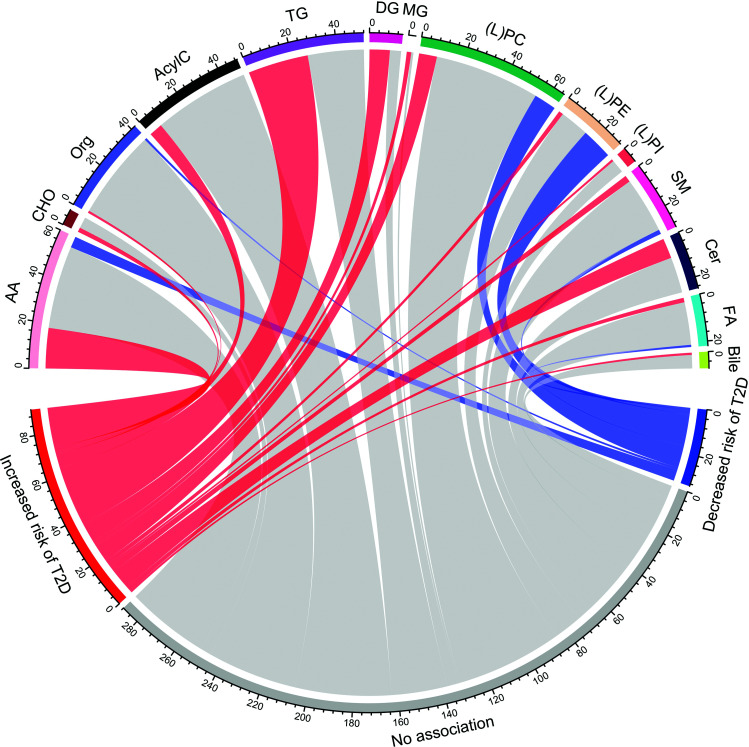
Overall, we extracted the relative risk estimates for 4,416 metabolites or metabolic features measured in plasma or serum (n = 60 reports). We identified and pooled estimates for 412 unique metabolites from 37 metabolic pathways (Fig. 2).
Figure 2.
Chord diagram illustrating groups of metabolites identified in the current review. The thickness of ribbons and sectors is proportional to the number of metabolites. Red, gray, and blue colors correspond, respectively, with metabolites positively, not, and inversely associated with type 2 diabetes risk. AA, amino acids; AcylC, acylcarnitines; Bile, bile acids; CHO, carbohydrates; FA, fatty acids; (L)PC, (lyso)phosphatidylcholine; (L)PE, (lyso)phosphatidylethanolamine; L(PI), (lyso)phosphatidylinositol; Org, organic compounds; T2D, type 2 diabetes.
Amino Acids
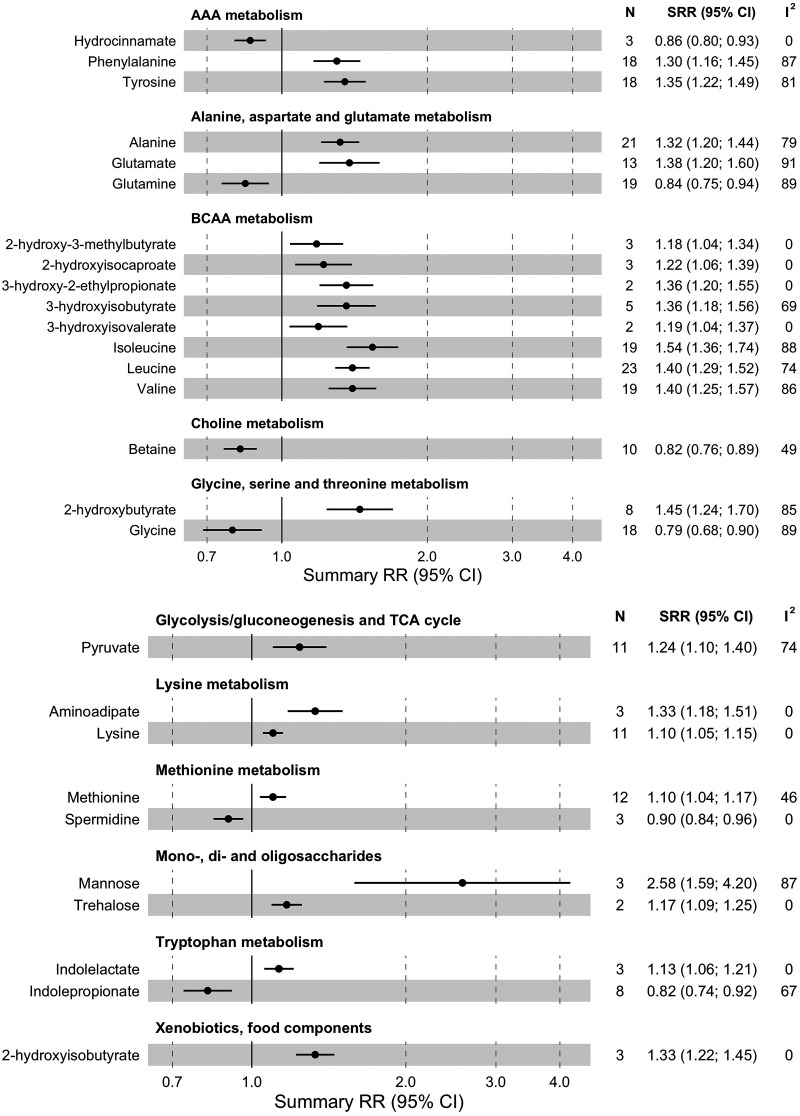
We performed a meta-analysis for 62 amino acids and amino acid derivatives (Fig. 3 and Supplementary Table 7); 22 were associated with type 2 diabetes risk (FDR-corrected P < 0.05). Higher circulating levels of BCAAs, including isoleucine (RR for a 1-SD increase [RR1-SD] 1.54 [95% CI 1.36–1.74], I2 = 88%, τ2 = 0.058, n = 19 studies), leucine (RR1-SD 1.40 [1.29–1.52], I2 = 74%, τ2 = 0.027, n = 23), and valine (RR1-SD 1.40 [1.25–1.57], I2 = 86%, τ2 = 0.047, n = 19), were associated (FDR-corrected P < 0.05) with higher risk of type 2 diabetes. Also, five BCAA-related metabolites (2-hydroxy-3-methylbutyrate, 2-hydroxyisocaproate 3-hydroxy-2-ethylpropionate, 3-hydroxyisobutyrate, and 3-hydrooxyvalerate) were associated with higher type 2 diabetes risk (increase of 18–36%, I2 = 0–69%, τ2 = 0.000–0.016, n = 2–5).
Figure 3.
Summary relative risk (SRR) with corresponding 95% CIs for the association between 1 SD increase in levels of amino acids and other organic compounds and risk of incident type 2 diabetes. N, number of studies; TCA, tricarboxylic acid.
Two AAAs, phenylalanine (RR1-SD 1.30 [95% CI 1.16–1.45], I2 = 87%, τ2 = 0.044, n = 18) and tyrosine (RR1-SD 1.35 [1.22–1.49], I2 = 81%, τ2 = 0.031, n = 18), were associated with a higher type 2 diabetes risk. Moreover, higher alanine (RR1-SD 1.32 [1.20–1.44], I2 = 79%, τ2 = 0.030, n = 21) and glutamate (RR1-SD 1.38 [1.20–1.60], I2 = 91%, τ2 = 0.058, n = 13) levels were associated with higher type 2 diabetes risk. An inverse association was observed for glutamine and type 2 diabetes risk (RR1-SD 0.84 [0.75–0.94], I2 = 89%, τ2 = 0.051, n = 19). Higher levels of glycine were also linked to lower risk (RR1-SD 0.79 [0.68–0.90], I2 = 89%, τ2 = 0.071, n = 18), while 2-hydroxybutyrate was associated with higher risk (RR1-SD 1.45 [1.24–1.70], I2 = 85%, τ2 = 0.040, n = 8). Methionine was associated with higher risk (RR1-SD 1.10 [1.04–1.17], I2 = 46%, τ2 = 0.005, n = 12) and its metabolite, spermidine, with lower risk (RR1-SD 0.90 [0.84–0.96], I2 = 0%, τ2 = 0.000, n = 3). Both lysine (RR1-SD 1.10 [1.05–1.15], I2 = 0%, τ2 = 0.000, n = 11) and 2-aminoadipate (RR1-SD 1.33 [1.18–1.51], I2 = 0%, τ2 = 0.000, n = 3) were associated with higher type 2 diabetes risk. Two metabolites of tryptophan metabolism were associated with type 2 diabetes: indoleproprionate, with lower risk (RR1-SD 0.82 [0.74–0.92], I2 = 67%, τ2 = 0.014, n = 8), and indolelactate (RR1-SD 1.13 [1.06–1.21], I2 = 0%, τ2 = 0.000, n = 3), with higher risk.
Carbohydrates and Energy Metabolism
In total, 19 carbohydrates and energy-related metabolites were included in meta-analysis (Fig. 3 and Supplementary Table 7), and 3 of them were associated with type 2 diabetes risk (FDR-corrected P < 0.05). For carbohydrate metabolites, we found that higher mannose (RR1-SD 2.58 [95% CI 1.59–4.20], I2 = 87%, τ2 = 0.157, n = 3) and trehalose (RR1-SD 1.17 [1.09–1.25], I2 = 0%, τ2 = 0.000, n = 2) levels were associated with higher type 2 diabetes risk. One glycolysis/gluconeogenesis metabolite, pyruvate (RR1-SD 1.24 [1.10–1.40], I2 = 74%, τ2 = 0.027, n = 11), was also associated with higher type 2 diabetes risk.
Acylcarnitines
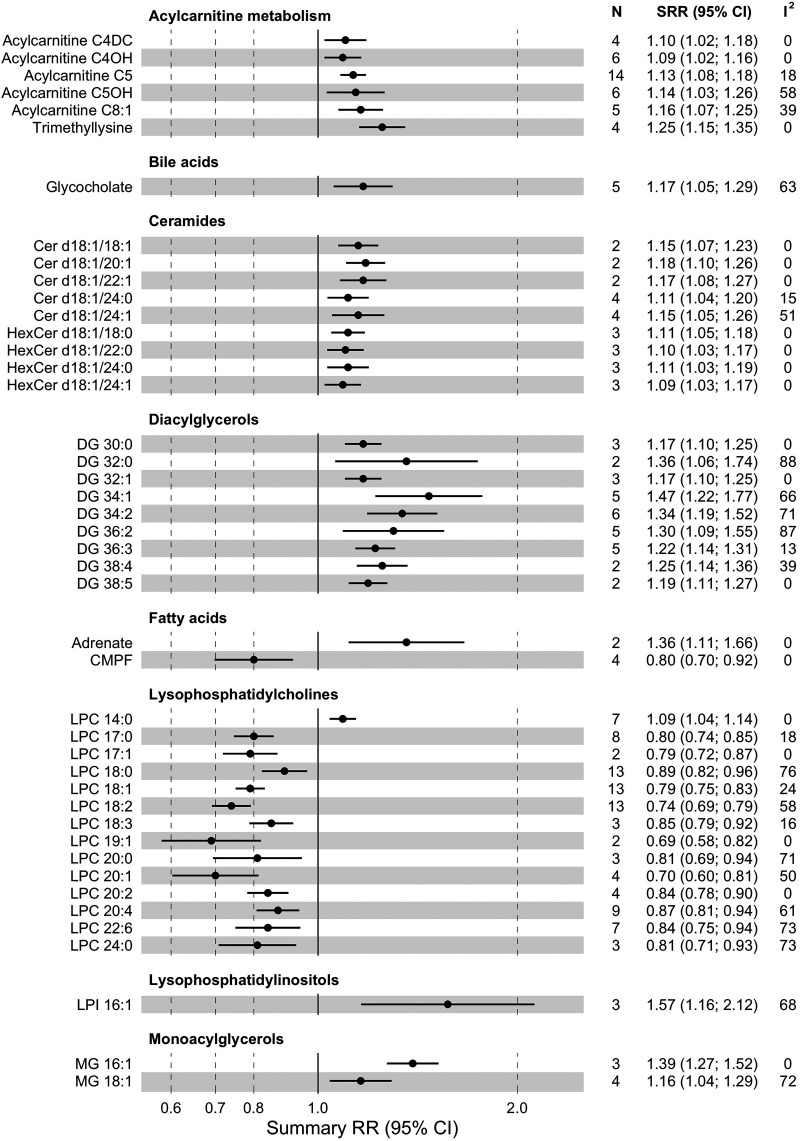
We performed meta-analysis for 49 acylcarnitines and related metabolites (Fig. 4 and Supplementary Table 7), and 6 of them were significantly associated with type 2 diabetes risk (FDR-corrected P < 0.05). Trimethyllysine, a precursor of carnitine, was associated with higher type 2 diabetes risk (RR1-SD 1.25 [95% CI 1.15–1.35], I2 = 0%, τ2 = 0.000, n = 4). Higher levels of short-chain acylcarnitines C4-DC (RR1-SD 1.10 [1.02–1.18], I2 = 0%, τ2 = 0.000, n = 4), C4-OH (RR1-SD 1.09 [1.02–1.16], I2 = 0%, τ2 = 0.000, n = 6), C5 (RR1-SD 1.13 [1.08–1.18], I2 = 18%, τ2 = 0.001, n = 14), and C5-OH (RR1-SD 1.14 [1.03–1.26], I2 = 58%, τ2 = 0.008, n = 6) were associated with higher type 2 diabetes risk. Regarding medium-chain acylcarnitines, higher C8:1 was associated with higher type 2 diabetes risk (RR1-SD 1.16 [1.07–1.25], I2 = 39%, τ2 = 0.003, n = 5). After pooling of specific compounds to length groups, higher levels of short-chain and long-chain acylcarnitines were associated with 6% and 7% higher type 2 diabetes risk (P = 0.049 and P = 0.016, respectively), and no association was found for the medium-chain group (Supplementary Table 8).
Figure 4.
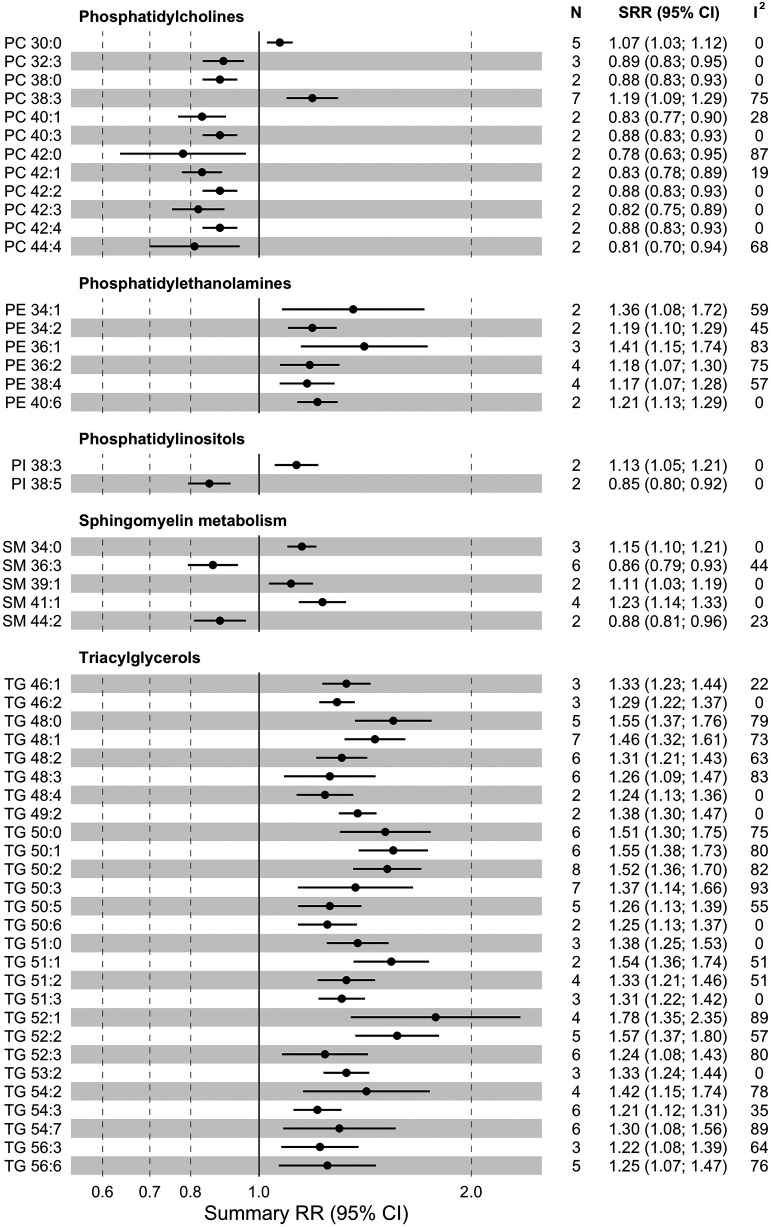
Summary relative risk (SRR) with corresponding 95% CIs for the association between 1-SD increase in levels of lipid metabolites and risk of incident type 2 diabetes. N, number of studies; CMPF, 3-carboxy-4-methyl-5-propyl-2-furanpropanoic acid; HexCer, hexosylceramide.
Glycerolipids
From the triacylglycerol (TG) class, we performed meta-analysis for 52 compounds (Fig. 4 and Supplementary Table 7). Higher levels of 27 of them were associated (FDR-corrected P < 0.05) with higher type 2 diabetes risk (increase of 21–78%, I2 = 0–93%, τ2 = 0.000–0.068, n = 2–8). Among 14 diacylglycerols (DGs) included in meta-analysis, 9 were associated with higher type 2 diabetes risk (increase of 17–46%, I2 = 0–88%, τ2 = 0.000–0.029, n = 2–9). Two out of three monoacylglycerols were linked to higher type 2 diabetes risk (increase of 16–39%, I2 = 0–72%, τ2 = 0.000–0.008, n = 2–3). Moreover, there was a significant trend of lower type 2 diabetes risk with a higher number of carbon atoms in TGs (P < 0.001) and double bonds in TGs and DGs (P < 0.001 and P = 0.008) (Supplementary Table 8).
Glycerophospholipids
We performed meta-analysis for 53 phosphatidylcholines (PCs), out of which 2, PC 38:3 (RR1-SD 1.19 [95% CI 1.09–1.29], I2 = 75%, τ2 = 0.008, n = 7) and PC 30:0 (RR1-SD 1.07 [1.03–1.12], I2 = 0%, τ2 = 0.000, n = 5), were associated (FDR-corrected P < 0.05) with a higher type 2 diabetes risk (Fig. 4 and Supplementary Table 7). Inverse associations with type 2 diabetes risk were found for higher levels of 10 PCs (decrease of 11–22%, I2 = 0–87%, τ2 = 0.000–0.019, n = 2–3). Of 20 lysophosphatidylcholines (LPCs) included in meta-analysis, 13 showed an association with lower type 2 diabetes risk (decrease of 11–31%, I2 = 0–76%, τ2 = 0.000–0.015, n = 2–13). Out of 12 pooled phosphatidylethanolamines (PEs), 6 were associated with higher type 2 diabetes risk (increase of 17–41%, I2 = 0–83%, τ2 = 0.000–0.026, n = 2–4). In the phosphatidylinositol (PI) class (nine metabolites included for meta-analysis), PI 38:3 was associated with higher (RR1-SD 1.13 [1.05–1.21], I2 = 0%, τ2 = 0.000, n = 2) and PI 38:5 with lower (RR1-SD 0.85 [0.80–0.92], I2 = 0%, τ2 = 0.000 n = 2) type 2 diabetes risk. The risk estimates tended to be lower with higher numbers of carbon atoms in PCs (Ptrend < 0.001) and LPCs (Ptrend = 0.006) and higher numbers of double bonds in PCs (Ptrend <0.001) and PIs (Ptrend = 0.034) (Supplementary Table 8).
Sphingolipids
We performed meta-analysis for 30 SMs, of which 5 were significantly (FDR-corrected P < 0.05) associated with type 2 diabetes risk (Fig. 4 and Supplementary Table 7). An association with lower type 2 diabetes risk was observed for two compounds, SM 36:3 (RR1-SD 0.86 [95% CI 0.79–0.93], I2 = 44%, τ2 = 0.004, n = 6) and SM 44:2 (RR1-SD 0.88 [0.81–0.96], I2 = 23%, τ2 = 0.001, n = 2). Three SMs, 34:0 (RR1-SD 1.15 [1.10–1.21], I2 = 0%, τ2 = 0.000, n = 3), 39:1 (RR1-SD 1.11 [1.03–1.19], I2 = 0%, τ2 = 0.000, n = 2), and 41:1 (RR1-SD 1.23 [1.14–1.33], I2 = 0%, τ2 = 0.000, n = 4), were associated with higher type 2 diabetes risk. Among 25 ceramides (Cer) included in meta-analysis, 9 were positively associated with incident type 2 diabetes (increase of 9–18%, I2 = 0–51%, τ2 = 0.000–0.004, n = 2–4). The SM-associated type 2 diabetes risk tended to decrease with higher numbers of double bonds (Ptrend < 0.001) (Supplementary Table 8).
Nonesterified Fatty Acids
Among 23 nonesterified fatty acids included in meta-analysis (Fig. 4 and Supplementary Table 7), two were associated with type 2 diabetes risk (FDR-corrected P < 0.05). Adrenate (C22:4) (RR1-SD 1.36 [95% CI 1.11–1.66], I2 = 0%, τ2 = 0.000, n = 2) was associated with higher type 2 diabetes risk, and 3-carboxy-4-methyl-5-propyl-2-furanpropanoate was associated with lower type 2 diabetes risk (RR1-SD 0.80 [0.70–0.92], I2 = 0%, τ2 = 0.000, n = 4).
Other Metabolites
Among seven bile acids included in meta-analysis (Figs. 3 and 4 and Supplementary Table 7), only glycocholate level was linked (FDR-corrected P < 0.05) to a higher type 2 diabetes risk (RR1-SD 1.17 [95% CI 1.05–1.29], I2 = 63%, τ2 = 0.008, n = 5). Among four choline-related metabolites, betaine was associated with a lower type 2 diabetes risk (RR1-SD 0.82 [0.76–0.89], I2 = 49%, τ2 = 0.007, n = 10).
Inconsistency and Publication Bias
We detected notable differences in some of the metabolite-associated type 2 diabetes risk estimates in analysis stratified by geographical location (studies based in the U.S., Europe, or Asia) or fasting status. Other subgroup analyses were generally consistent with the results from the primary analyses (Supplementary Tables 9–14). For BCAAs, AAAs, betaine, pyruvate, and tryptophan, moderate funnel plot asymmetry (Supplementary Fig. 1), confirmed by Egger’s test (Supplementary Table 15), was observed, suggesting possible publication bias.
Studies on Urine Metabolites
Friedrich et al. (9) conducted a targeted metabolomics assessment using 1H NMR in urine samples of 2,709 subjects from northeastern Germany and evaluated the association between measured metabolites and type 2 diabetes incidence after 5 years of follow-up. Results indicated a marked difference in the type 2 diabetes risk associations of the urinary metabolites between men and women. Only urine glucose was robustly associated with a higher type 2 diabetes risk in both sexes. Svingen et al. (10) measured urine levels of five choline-related metabolites in a hospital-based cohort of patients with suspected stable angina. Three metabolites, betaine, N,N-dimethylglycine, and sarcosine, were associated with a 19–25% increased risk of incident type 2 diabetes after an average of 7.5 years of follow-up.
Discussion
This systematic review and meta-analysis provides an updated overview and quantitative summary of the associations between single baseline metabolite levels and subsequent risk of type 2 diabetes from high-throughput metabolomics approaches in prospective human population studies. In comparison with the previous version of this meta-analysis published in 2016, we included a total of 61 reports, including 47 additional reports (14 reports were included in the original version), and performed meta-analysis for 403 additional metabolites (vs. 9 metabolites in the original version), representing many metabolic pathways, including carbohydrate, acylcarnitine, and lipid metabolism. Our updated meta-analysis reflects impressive technological advances and the increasing availability of metabolomics applications in prospective cohorts, generating a multitude of putative biomarkers of type 2 diabetes risk.
Mechanisms Explaining the Link Between Metabolome and Diabetes
The findings of the present meta-analysis confirm our previous findings on the strong positive prospective associations between BCAAs and type 2 diabetes risk. Moreover, we observed similar results for branched-chain keto acids and further derivates of BCAA turnover. BCAAs were the first and, so far, the most widely investigated group of metabolites in the context of type 2 diabetes development (27,65). BCAAs might impair insulin signaling by interaction with mTOR kinase, and accumulation of their metabolites may lead to increased insulin secretion and pancreatic β-cell exhaustion (69,70). Moreover, a possible causal role of BCAAs in type 2 diabetes development was also recently supported by findings from Mendelian randomization studies (27,71). BCAAs are critical markers of the metabolic alterations that precede type 2 diabetes incidence, and our meta-results corroborate their robust and strong associations with type 2 diabetes risk.
In addition, our meta-analysis indicated robust associations of other amino acids with increased (AAAs, alanine, glutamate, lysine, and methionine) or decreased (glutamine and glycine) type 2 diabetes risk. AAAs, phenylalanine, and tyrosine are precursors of dopamine and levodopa, which might have an anti-incretin effect, decreasing cell uptake of glucose. The observed inverse associations of glycine with type 2 diabetes risk can potentially be explained by improved insulin sensitivity (59). Glutamate might promote oxidative damage and dysfunction of β-cells—the opposite of glutamine effects, including reduction of postprandial glycemia and secretion of glucagon-like peptide 1 (26). Both lysine and its metabolite, 2-aminoadipate, were previously linked with unfavorable cardiometabolic status and impaired insulin sensitivity (66). A deteriorating effect of 2-aminoadipate on insulin signaling through interaction with AKT kinase was proposed in a recent study using a mouse model (72). Moreover, results of studies in rodent models suggested that dietary methionine restriction can improve insulin sensitivity through elevated hepatic secretion of FGF-21 (73).
To our knowledge, this is the first comprehensive meta-analysis of lipid metabolites in relation to type 2 diabetes risk. Meta-analysis results showed a higher risk of type 2 diabetes associated with higher levels of lipid metabolites in the classes of glycerolipids (DGs and TGs), Cer, PEs, and selected acylcarnitines. Lower type 2 diabetes risk was primarily associated with lipid metabolites in the PC and LPC classes. Excessive lipids supply and reduction in mitochondrial oxidative function impair β-oxidation, leading to accumulation of DGs, Cer, and acylcarnitines (74). Studies reported correlations of Cer with obesity, insulin resistance, and disturbed glucose metabolism (16,19). Moreover, inhibition of Cer synthase-6 improved glucose tolerance and insulin sensitivity in genetically modified mouse models (75). The role of acylcarnitines in the development of diabetes may depend on their acyl chain length. Short-chain acylcarnitines are linked to BCAA catabolism, while long-chain compounds may reflect incomplete fatty acid oxidation (5). Indeed, in our meta-analysis, we observed that BCAA catabolism–related individual acylcarnitines and the sum of short-chain acylcarnitines were associated with higher type 2 diabetes risk. Overall, metabolic profiling captures the complex relationship between dysregulated lipid metabolism and type 2 diabetes risk.
While risk estimates included in meta-analyses of lipid metabolites were derived from prospective studies with sufficient statistical power, the small number of studies available per comparison (two to five on average) precluded exploring potential sources of heterogeneity (i.e., study population, analytical platform, biospecimen, or covariate adjustment set). Moreover, due to the use of different lipidomic protocols, studies provided different depths of lipid profiling. Therefore, we generalized annotation of lipid metabolites to the lipid species level, i.e., the total number of carbon atoms and double bonds, and disregarded the more detailed information on single fatty acids and isomers provided by the most advanced platforms. Altogether, our meta-analysis confirms significant advances as well as critical gaps in the evidence base on comprehensive lipid profiles and type 2 diabetes risk.
In this meta-analysis, we observed/detected considerable between-study heterogeneity for type 2 diabetes risk associations of many metabolites. We considered biospecimen, study location, fasting status, metabolomics platform, fasting glucose adjustment, and follow-up length as potential sources of heterogeneity. The results of these subgroup analyses were largely consistent with the primary analyses results. However, stratified analyses were limited to comparisons with ≥10 available studies, and these factors may still explain heterogeneous risk associations of metabolites with fewer available studies. Race and ethnicity can influence metabolite levels through genetic factors. We found a nonequal representation of studies from different geographical locations and potential differences in metabolite associations across those locations. Therefore, studies investigating potential heterogeneity of metabolite–type 2 diabetes risk associations across different races and ethnicities are warranted.
With respect to biospecimens, simultaneous analysis of some metabolites in serum and plasma revealed higher concentrations in serum samples (76). However, we observed a general consistency of associations for the same metabolites assessed in plasma and serum. Most included studies used overnight fasted blood samples. For most metabolites, the biological stability within the same individuals over months to years was moderately higher in fasted than nonfasted samples (77,78). Specifically, the use of nonfasting samples for lipid measurements is frequently debated; however, the performance in risk prediction was comparable with that of using lipid measurements from fasting samples (79). Application of different metabolomics platforms leads to some differences in the list of analyzed metabolites. This issue influences the comparability across platforms and therefore may have an impact on the number of studies per comparison as well as the degree of statistical heterogeneity. Due to the small number of studies, we could not stratify our analysis on platform providers, and a systematic examination of the cross-platform comparability is warranted (80).
Over the years, the research framework evolved from studies on a limited number of selected metabolites and pathways into comprehensive metabolome-wide investigations. Some studies suggested that the addition of metabolites to models including traditional risk factors moderately improves the ability to predict incident type 2 diabetes (30,33,39–41). However, such risk prediction models involving metabolomics measures require further optimization and validation before they can be introduced into clinical practice. While some groups of metabolites, like BCAAs and AAAs, have been widely studied and consistently associated with type 2 diabetes risk, others (like lipid metabolites) still lack larger numbers of comparable high-quality cohort studies. Future pooled analyses of cohorts with individual-level data may allow examination of other potential sources of heterogeneity, such as sex, age, race/ethnicity, and time to disease incidence, that we could not address based on published results. Future studies combining metabolomics with genome, proteome, and gut microbiota will likely further elucidate the role of the identified metabolites in the pathogenesis of type 2 diabetes. In our systematic review, we identified only two studies investigating individual urine metabolites and type 2 diabetes risk, indicating the need for further sufficiently powered studies.
Strengths and Limitations
To the best of our knowledge, our study provides the most comprehensive meta-evidence on the associations of single metabolites with type 2 diabetes risk. This systematic review’s particular strength is a large number of included studies conducted in different populations and adopting metabolomics profiling using different biospecimens and analytic techniques. We have performed meta-analysis for a large number of metabolites and conducted several stratified analyses. In addition, we corrected the P values for multiple testing. Narrowing the review’s focus to prospective studies allowed us to limit the influence of reverse causality and selection bias, and most of the included studies were evaluated to be of high quality.
There are several limitations to be considered. First, our search covered only two databases. However, relevant studies are usually indexed in those databases. Second, the majority of metabolites for which we performed meta-analysis showed considerable between-study heterogeneity. As discussed above, we explored potential sources of heterogeneity by conducting extensive subgroup analyses, which were largely in line with the primary analysis results. However, we could not systematically explore the effect of different adjustment sets because a standardized modeling strategy across the underlying studies was lacking, and we also could not examine sex as a potential effect modifier due to the scarcity of sex-specific risk estimates. Third, the observational nature of the studies included in meta-analysis impedes inferences about potential causal mechanisms underlying the observed type 2 diabetes risk associations. However, Mendelian randomization studies supported a possible causal role of BCAAs in type 2 diabetes, but similar investigations have not been conducted for other metabolites (27,71). We only considered evidence from metabolomics studies, which implies that we disregarded potentially relevant data from targeted assays for selected metabolites by design. Finally, misclassification of the reports during study selection and publication bias are essential sources of bias for evidence summaries, which we mitigated through independent review of the included studies by three authors and detailed analyses of publication bias.
Summary
Taken together, the present systematic review and meta-analysis provides an updated overview of the associations between a large number of metabolites and incident type 2 diabetes. We performed meta-analysis for >400 serum and plasma metabolites in relation to the risk of type 2 diabetes, detecting 123 significant risk associations. The type 2 diabetes risk–associated compounds reflect dysregulation of a variety of metabolic pathways and processes, such as proteolysis, gluconeogenesis, mitochondrial function, de novo lipogenesis, and fatty acid oxidation. Low availability of studies applying urine metabolomics suggests a gap for future studies. Further research is also warranted to understand the underlying biological processes and translate the robust type 2 diabetes risk associations of multiple metabolites into clinically applicable biomarkers.
Article Information
Funding. This work was supported by National Institutes of Health research grants R21AG070375-01A1, R01DK112940, and R01DK127601. M.G.-F. is supported by American Diabetes Association grant 1-18-PMF-029. C.W. is supported by the German Research Foundation’s (DFG) individual fellowship no. WI5132/1-1, and the Boston Nutrition Obesity Research Center (P30 DK46200).
The funding sources played no role in the design, collection, analysis, or interpretation of the data or in the decision to submit the manuscript for publication.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. J.M., L.S., A.R., F.B.H., and M.G.-F. designed the research. J.M., C.W., L.S., A.D., and M.G.-F. conducted the research. J.M., C.W., L.S., A.D., and M.G.-F. analyzed data. J.M. and M.G.-F. drafted the manuscript. J.M., C.W., L.S., A.D., A.R., F.B.H., and M.G.-F. made critical revisions to the manuscript for important intellectual content. C.W., F.B.H., and M.G.-F. obtained funding. All authors read and approved the final manuscript. J.M. and M.G.-F. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.18857807.
References
- 1. Wishart DS. Metabolomics for investigating physiological and pathophysiological processes. Physiol Rev 2019;99:1819–1875 [DOI] [PubMed] [Google Scholar]
- 2. Guasch-Ferré M, Hruby A, Toledo E, et al. Metabolomics in prediabetes and diabetes: a systematic review and meta-analysis. Diabetes Care 2016;39:833–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ahola-Olli AV, Mustelin L, Kalimeri M, et al. Circulating metabolites and the risk of type 2 diabetes: a prospective study of 11,896 young adults from four Finnish cohorts. Diabetologia 2019;62:2298–2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fernandez C, Surma MA, Klose C, et al. Plasma lipidome and prediction of type 2 diabetes in the population-based Malmö Diet and Cancer Cohort. Diabetes Care 2020;43:366–373 [DOI] [PubMed] [Google Scholar]
- 5. Guasch-Ferré M, Ruiz-Canela M, Li J, et al. Plasma acylcarnitines and risk of type 2 diabetes in a Mediterranean population at high cardiovascular risk. J Clin Endocrinol Metab 2019;104:1508–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guasch-Ferré M, Santos JL, Martínez-González MA, et al. Glycolysis/gluconeogenesis- and tricarboxylic acid cycle-related metabolites, Mediterranean diet, and type 2 diabetes. Am J Clin Nutr 2020;111:835–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Razquin C, Ruiz-Canela M, Clish CB, et al. Lysine pathway metabolites and the risk of type 2 diabetes and cardiovascular disease in the PREDIMED study: results from two case-cohort studies. Cardiovasc Diabetol 2019;18:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yu E, Papandreou C, Ruiz-Canela M, et al. Association of tryptophan metabolites with incident type 2 diabetes in the PREDIMED trial: a case-cohort study. Clin Chem 2018;64:1211–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Friedrich N, Budde K, Suhre K, et al. Sex differences in urine metabolites related with risk of diabetes using NMR spectroscopy: results of the study of health in pomerania. Metabolomics 2015;11:1405–1415 [Google Scholar]
- 10. Svingen GFT, Schartum-Hansen H, Pedersen ER, et al. Prospective associations of systemic and urinary choline metabolites with incident type 2 diabetes. Clin Chem 2016;62:755–765 [DOI] [PubMed] [Google Scholar]
- 11. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–2012 [DOI] [PubMed] [Google Scholar]
- 12. Hayden JA, Côté P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med 2006;144:427–437 [DOI] [PubMed] [Google Scholar]
- 13. Langan D, Higgins JPT, Jackson D, et al. A comparison of heterogeneity variance estimators in simulated random-effects meta-analyses. Res Synth Methods 2019;10:83–98 [DOI] [PubMed] [Google Scholar]
- 14. Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. Chichester, U.K., John Wiley & Sons, 2019 [Google Scholar]
- 15. Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP. Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane Database of Systematic Reviews. Int J Epidemiol 2012;41:818–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen GC, Chai JC, Yu B, et al. Serum sphingolipids and incident diabetes in a US population with high diabetes burden: the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Am J Clin Nutr 2020;112:57–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen T, Ni Y, Ma X, et al. Branched-chain and aromatic amino acid profiles and diabetes risk in Chinese populations. Sci Rep 2016;6:20594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen ZZ, Liu J, Morningstar J, et al.; Diabetes Prevention Program Research Group . Metabolite profiles of incident diabetes and heterogeneity of treatment effect in the diabetes prevention program. Diabetes 2019;68:2337–2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chew WS, Torta F, Ji S, et al. Large-scale lipidomics identifies associations between plasma sphingolipids and T2DM incidence. JCI Insight 2019;5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Mello VD, Paananen J, Lindström J, et al. Indolepropionic acid and novel lipid metabolites are associated with a lower risk of type 2 diabetes in the Finnish Diabetes Prevention Study. Sci Rep 2017;7:46337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fall T, Salihovic S, Brandmaier S, et al. Non-targeted metabolomics combined with genetic analyses identifies bile acid synthesis and phospholipid metabolism as being associated with incident type 2 diabetes. Diabetologia 2016;59:2114–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gängler S, Waldenberger M, Artati A, et al. Exposure to disinfection byproducts and risk of type 2 diabetes: a nested case-control study in the HUNT and Lifelines cohorts. Metabolomics 2019;15:60. [DOI] [PubMed] [Google Scholar]
- 23. Gunther SH, Khoo CM, Tai ES, et al. Serum acylcarnitines and amino acids and risk of type 2 diabetes in a multiethnic Asian population. BMJ Open Diabetes Res Care 2020;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hang D, Zeleznik OA, He X, et al. Metabolomic signatures of long-term coffee consumption and risk of type 2 diabetes in women. Diabetes Care 2020;43:2588–2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu J, Semiz S, van der Lee SJ, et al. Metabolomics based markers predict type 2 diabetes in a 14-year follow-up study. Metabolomics 2017;13:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu X, Zheng Y, Guasch-Ferré M, et al. High plasma glutamate and low glutamine-to-glutamate ratio are associated with type 2 diabetes: case-cohort study within the PREDIMED trial. Nutr Metab Cardiovasc Dis 2019;29:1040–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lotta LA, Scott RA, Sharp SJ, et al. Genetic predisposition to an impaired metabolism of the branched-chain amino acids and risk of type 2 diabetes: a Mendelian randomisation analysis. PLoS Med 2016;13:e1002179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lu J, Lam SM, Wan Q, et al. High-coverage targeted lipidomics reveals novel serum lipid predictors and lipid pathway dysregulation antecedent to type 2 diabetes onset in normoglycemic Chinese adults. Diabetes Care 2019;42:2117–2126 [DOI] [PubMed] [Google Scholar]
- 29. Lu Y, Wang Y, Liang X, et al. Serum amino acids in association with prevalent and incident type 2 diabetes in a Chinese population. Metabolites 2019;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lu Y, Wang Y, Ong CN, et al. Metabolic signatures and risk of type 2 diabetes in a Chinese population: an untargeted metabolomics study using both LC-MS and GC-MS. Diabetologia 2016;59:2349–2359 [DOI] [PubMed] [Google Scholar]
- 31. Lu Y, Wang Y, Zou L, et al. Serum lipids in association with type 2 diabetes risk and prevalence in a Chinese population. J Clin Endocrinol Metab 2018;103:671–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mamtani M, Kulkarni H, Wong G, et al. Lipidomic risk score independently and cost-effectively predicts risk of future type 2 diabetes: results from diverse cohorts. Lipids Health Dis 2016;15:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Merino J, Leong A, Liu CT, et al. Metabolomics insights into early type 2 diabetes pathogenesis and detection in individuals with normal fasting glucose. Diabetologia 2018;61:1315–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Muilwijk M, Goorden SMI, Celis-Morales C, et al. Contributions of amino acid, acylcarnitine and sphingolipid profiles to type 2 diabetes risk among South-Asian Surinamese and Dutch adults. BMJ Open Diabetes Res Care 2020;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Noerman S, Kärkkäinen O, Mattsson A, et al. Metabolic profiling of high egg consumption and the associated lower risk of type 2 diabetes in middle-aged Finnish men. Mol Nutr Food Res 2019;63:e1800605. [DOI] [PubMed] [Google Scholar]
- 36. Ottosson F, Smith E, Gallo W, Fernandez C, Melander O. Purine metabolites and carnitine biosynthesis intermediates are biomarkers for incident type 2 diabetes. J Clin Endocrinol Metab 2019;104:4921–4930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ottosson F, Smith E, Melander O, Fernandez C. Altered asparagine and glutamate homeostasis precede coronary artery disease and type 2 diabetes. J Clin Endocrinol Metab 2018;103:3060–3069 [DOI] [PubMed] [Google Scholar]
- 38. Papandreou C, Bulló M, Zheng Y, et al. Plasma trimethylamine-N-oxide and related metabolites are associated with type 2 diabetes risk in the Prevención con Dieta Mediterránea (PREDIMED) trial. Am J Clin Nutr 2018;108:163–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Peddinti G, Cobb J, Yengo L, et al. Early metabolic markers identify potential targets for the prevention of type 2 diabetes. Diabetologia 2017;60:1740–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Qiu G, Zheng Y, Wang H, et al. Plasma metabolomics identified novel metabolites associated with risk of type 2 diabetes in two prospective cohorts of Chinese adults. Int J Epidemiol 2016;45:1507–1516 [DOI] [PubMed] [Google Scholar]
- 41. Rebholz CM, Yu B, Zheng Z, et al. Serum metabolomic profile of incident diabetes. Diabetologia 2018;61:1046–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Savolainen O, Lind MV, Bergström G, Fagerberg B, Sandberg AS, Ross A. Biomarkers of food intake and nutrient status are associated with glucose tolerance status and development of type 2 diabetes in older Swedish women. Am J Clin Nutr 2017;106:1302–1310 [DOI] [PubMed] [Google Scholar]
- 43. Shi L, Brunius C, Bergdahl IA, et al. Joint analysis of metabolite markers of fish intake and persistent organic pollutants in relation to type 2 diabetes risk in Swedish adults. J Nutr 2019;149:1413–1423 [DOI] [PubMed] [Google Scholar]
- 44. Shi L, Brunius C, Johansson I, et al. Plasma metabolites associated with healthy Nordic dietary indexes and risk of type 2 diabetes-a nested case-control study in a Swedish population. Am J Clin Nutr 2018;108:564–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shi L, Brunius C, Johansson I, et al. Plasma metabolite biomarkers of boiled and filtered coffee intake and their association with type 2 diabetes risk. J Intern Med 2020;287:405–421 [DOI] [PubMed] [Google Scholar]
- 46. Shi L, Brunius C, Lehtonen M, et al. Plasma metabolites associated with type 2 diabetes in a Swedish population: a case-control study nested in a prospective cohort. Diabetologia 2018;61:849–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Strand E, Rebnord EW, Flygel MR, et al. Serum carnitine metabolites and incident type 2 diabetes mellitus in patients with suspected stable angina pectoris. J Clin Endocrinol Metab 2018;103:1033–1041 [DOI] [PubMed] [Google Scholar]
- 48. Sun L, Liang L, Gao X, et al. Early prediction of developing type 2 diabetes by plasma acylcarnitines: a population-based study. Diabetes Care 2016;39:1563–1570 [DOI] [PubMed] [Google Scholar]
- 49. Vangipurapu J, Fernandes Silva L, Kuulasmaa T, Smith U, Laakso M. Microbiota-related metabolites and the risk of type 2 diabetes. Diabetes Care 2020;43:1319–1325 [DOI] [PubMed] [Google Scholar]
- 50. Vangipurapu J, Stancáková A, Smith U, Kuusisto J, Laakso M. Nine amino acids are associated with decreased insulin secretion and elevated glucose levels in a 7.4-year follow-up study of 5,181 Finnish men. Diabetes 2019;68:1353–1358 [DOI] [PubMed] [Google Scholar]
- 51. Yang SJ, Kwak SY, Jo G, Song TJ, Shin MJ. Serum metabolite profile associated with incident type 2 diabetes in Koreans: findings from the Korean Genome and Epidemiology Study. Sci Rep 2018;8:8207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yu D, Moore SC, Matthews CE, et al. Plasma metabolomic profiles in association with type 2 diabetes risk and prevalence in Chinese adults. Metabolomics 2016;12:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yu E, Ruiz-Canela M, Razquin C, et al. Changes in arginine are inversely associated with type 2 diabetes: a case-cohort study in the PREDIMED trial. Diabetes Obes Metab 2019;21:397–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yun H, Sun L, Wu Q, et al. Associations among circulating sphingolipids, β-cell function, and risk of developing type 2 diabetes: a population-based cohort study in China. PLoS Med 2020;17:e1003451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cheng S, Rhee EP, Larson MG, et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation 2012;125:2222–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Drogan D, Dunn WB, Lin W, et al. Untargeted metabolic profiling identifies altered serum metabolites of type 2 diabetes mellitus in a prospective, nested case control study. Clin Chem 2015;61:487–497 [DOI] [PubMed] [Google Scholar]
- 57. Ferrannini E, Natali A, Camastra S, et al. Early metabolic markers of the development of dysglycemia and type 2 diabetes and their physiological significance. Diabetes 2013;62:1730–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fizelova M, Miilunpohja M, Kangas AJ, et al. Associations of multiple lipoprotein and apolipoprotein measures with worsening of glycemia and incident type 2 diabetes in 6607 non-diabetic Finnish men. Atherosclerosis 2015;240:272–277 [DOI] [PubMed] [Google Scholar]
- 59. Floegel A, Stefan N, Yu Z, et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes 2013;62:639–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mahendran Y, Cederberg H, Vangipurapu J, et al. Glycerol and fatty acids in serum predict the development of hyperglycemia and type 2 diabetes in Finnish men. Diabetes Care 2013;36:3732–3738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mahendran Y, Vangipurapu J, Cederberg H, et al. Association of ketone body levels with hyperglycemia and type 2 diabetes in 9,398 Finnish men. Diabetes 2013;62:3618–3626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Palmer ND, Stevens RD, Antinozzi PA, et al. Metabolomic profile associated with insulin resistance and conversion to diabetes in the Insulin Resistance Atherosclerosis Study. J Clin Endocrinol Metab 2015;100:E463–E468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rhee EP, Cheng S, Larson MG, et al. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J Clin Invest 2011;121:1402–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tillin T, Hughes AD, Wang Q, et al. Diabetes risk and amino acid profiles: cross-sectional and prospective analyses of ethnicity, amino acids and diabetes in a South Asian and European cohort from the SABRE (Southall And Brent REvisited) Study. Diabetologia 2015;58:968–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med 2011;17:448–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wang TJ, Ngo D, Psychogios N, et al. 2-aminoadipic acid is a biomarker for diabetes risk. J Clin Invest 2013;123:4309–4317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang-Sattler R, Yu Z, Herder C, et al. Novel biomarkers for pre-diabetes identified by metabolomics. Mol Syst Biol 2012;8:615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhao J, Zhu Y, Hyun N, et al. Novel metabolic markers for the risk of diabetes development in American Indians. Diabetes Care 2015;38:220–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yoon MS. The emerging role of branched-chain amino acids in insulin resistance and metabolism. Nutrients 2016;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Giesbertz P, Daniel H. Branched-chain amino acids as biomarkers in diabetes. Curr Opin Clin Nutr Metab Care 2016;19:48–54 [DOI] [PubMed] [Google Scholar]
- 71. Wang Q, Holmes MV, Davey Smith G, Ala-Korpela M. Genetic support for a causal role of insulin resistance on circulating branched-chain amino acids and inflammation. Diabetes Care 2017;40:1779–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lee HJ, Jang HB, Kim W-H, et al. 2-aminoadipic acid (2-AAA) as a potential biomarker for insulin resistance in childhood obesity. Sci Rep 2019;9:13610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Stone KP, Wanders D, Orgeron M, Cortez CC, Gettys TW. Mechanisms of increased in vivo insulin sensitivity by dietary methionine restriction in mice. Diabetes 2014;63:3721–3733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Schooneman MG, Vaz FM, Houten SM, Soeters MR. Acylcarnitines: reflecting or inflicting insulin resistance? Diabetes 2013;62:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Raichur S, Brunner B, Bielohuby M, et al. The role of C16:0 ceramide in the development of obesity and type 2 diabetes: CerS6 inhibition as a novel therapeutic approach. Mol Metab 2019;21:36–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Liu X, Hoene M, Wang X, et al. Serum or plasma, what is the difference? Investigations to facilitate the sample material selection decision making process for metabolomics studies and beyond. Anal Chim Acta 2018;1037:293–300 [DOI] [PubMed] [Google Scholar]
- 77. Carayol M, Licaj I, Achaintre D, et al. Reliability of serum metabolites over a two-year period: a targeted metabolomic approach in fasting and non-fasting samples from EPIC. PLoS One 2015;10:e0135437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Townsend MK, Clish CB, Kraft P, et al. Reproducibility of metabolomic profiles among men and women in 2 large cohort studies. Clin Chem 2013;59:1657–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Rahman F, Blumenthal RS, Jones SR, Martin SS, Gluckman TJ, Whelton SP. Fasting or non-fasting lipids for atherosclerotic cardiovascular disease risk assessment and treatment? Curr Atheroscler Rep 2018;20:14. [DOI] [PubMed] [Google Scholar]
- 80. Yu B, Zanetti KA, Temprosa M, et al. The Consortium of Metabolomics Studies (COMETS): metabolomics in 47 prospective cohort studies. Am J Epidemiol 2019;188:991–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]