Abstract

Cenotes are habitats with unique physical, chemical, and biological features. Unexplored microorganisms from these sinkholes represent a potential source of bioactive molecules. Thus, a series of cultivable fungi (Aspergillus spp. NCA257, NCA264, and NCA276, Stachybotrys sp. NCA252, and Cladosporium sp. NCA273) isolated from the cenote Tza Itzá were subjected to chemical, coculture, and metabolomic analyses. Nineteen compounds were obtained and tested for their antimicrobial potential against ESKAPE pathogens, Mycobacterium tuberculosis, and nontuberculous mycobacteria. In particular, phenylspirodrimanes from Stachybotrys sp. NCA252 showed significant activity against MRSA, MSSA, and mycobacterial strains. On the other hand, the absolute configuration of the new compound 17-deoxy-aspergillin PZ (1) isolated from Aspergillus sp. NCA276 was established via single-crystal X-ray crystallography. Also, the chemical analysis of the cocultures between Aspergillus and Cladosporium strains revealed the production of metabolites that were not present or were barely detected in the monocultures. Finally, molecular networking analysis of the LC-MS-MS/MS data for each fungus was used as a tool for the annotation of additional compounds, increasing the chemical knowledge on the corresponding fungal strains. Overall, this is the first systematic chemical study on fungi isolated from a sinkhole in Mexico.
Introduction
One of the most famous geological features in the Yucatan peninsula is the ring of cenotes (from the Maya word ts’onot), which is a group of sinkholes formed by the process of karstification.1 The origin of cenotes is related to the impact of the Chicxulub asteroid around 66 millions of years ago, which caused changes in the karst features.2,3 These habitats are physically and chemically unique due to the phototrophic activity of its microbial communities. In particular, the carbon and sulfur cycles completed by different microbes affect the sulfate reduction into aqueous sulfide, accelerating the limestone dissolution, and increasing alkalinity.4,5 In addition, most of the cenotes have hydraulic connections or networks across the region, and the microbial communities, especially freshwater fungi and bacteria, play a key role in keeping the balance of the entire ecosystem.6
Metagenomic analysis of soil samples from freshwater environments in the Yucatan peninsula revealed a remarkable microbial diversity, where fungi (ascomycetes) prevail over bacteria (actinobacteria) in terms of biomass production and enzymatic substrate degradation.7 Furthermore, the biosynthetic potential of these communities of microbes was assessed using bioinformatics tools coupled with direct amplification of environmental DNA.8−10 The number of studies demonstrating the potential of microorganisms from cenotes for the discovery of bioactive natural products is scarce. Such studies have focused on the biological activity of extracts, exhibiting a high percentage of hits (∼81%). About half of the extracts showed varied activities, including insecticidal and nematoxic.11−13 To date, the only formal chemical study reported from a fungal strain isolated from plant litter submerged in a sinkhole in Merida, Yucatan, led to the discovery of the novel hexahydroacremonintriol. This compound showed moderate insecticidal activity against phytophagous Myzus persicae, and Rhopalosiphum padi.(14)
The number of microorganisms from this region seems to be underexplored. The Yucatan peninsula thus remains an invaluable source of biological diversity for bioprospecting purposes.15,16 In this study, the chemical diversity and antimicrobial properties of a series of cultivable fungi isolated from the Tza Itzá cenote were explored by combining conventional chemical studies, cocultures analysis, and metabolomics (Figure 1). The isolation of several compounds from single-strain cultures demonstrated their potential to produce interesting chemistry. In addition, the analysis of cocultures led to the identification of compounds that were not present, or were barely detected, in the monocultures. Moreover, molecular networking (MN) analysis of the liquid chromatography coupled to high-resolution mass spectrometry (LC-HRMS) data of each fungus, allowed the annotation of additional compounds beyond to the isolated. Finally, several isolated compounds showed significant antibacterial activity against ESKAPE pathogens, Mycobacterium tuberculosis, and nontuberculous mycobacterias.
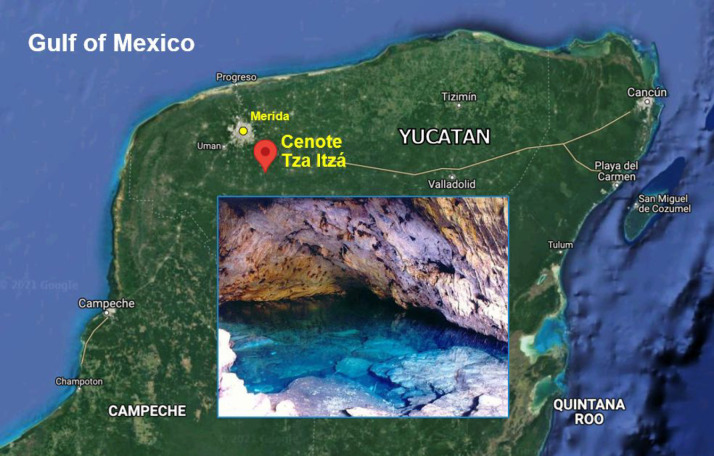
Figure 1.
Location of cenote Tza Itzá (20°43′50.27″ N, 89°27′56.82″ W) in the Yucatan peninsula.
Results and Discussion
Fungal Strains Isolation and Identification
Five culturable fungal strains were isolated from sediments samples collected in the cenote Tza Itzá. The taxonomic identity of the strains was determined by molecular sequencing of the ITS rDNA, followed by BLAST search and maximum likelihood analysis.17,18 The strains were identified as Aspergillus (NCA257, NCA264, and NCA276), Stachybotrys (NCA252), and Cladosporium (NCA273) (Figure 2 and Table S1, Supporting Information). Next, and as part of a program to explore the chemical diversity and antimicrobial potential of fungal species isolated from unexplored areas of Mexico, isolated strains were subjected to chemical, biological, and metabolomic analyses.
Figure 2.
Fungal isolates from cenote Tza Itzá on PDA medium: Aspergillus spp. NCA257, NCA264, and NCA276; Stachybotrys sp. NCA252; and Cladosporium sp. NCA273.
Chemical Study and Molecular Networking Analysis of Aspergillus spp
The chemical study of the defatted extracts from moist rice cultures of the Aspergillus spp. yielded a new compound, 17-deoxy-aspergillin PZ (1),19 and the known aspergillin PZ (2),20 aspochalasin D (3),21 asperphenamate (4),22N-benzoyl-l-phenylalaninol (5),23 and 2-O-methylbutyrolactone II (6)24 from strain NCA276; diorcinol (7),25 sydonic acid (8),26 and 11-dehydrosydonic acid (9)27 from NCA264; and asterriquinol D dimethyl ether (10)28 from NCA257 (Figure 3). All known compounds were elucidated by comparison with reported spectroscopic data (Supporting Information). Compound 1 was originally isolated from a jar fermentation of a presumably Aspergillus species, and its planar structure was established by NMR analysis.19 However, its absolute configuration had not been determined before. Suitable colorless crystals (Figure S1, Supporting Information) of 1 were obtained for X-ray structural determination and its absolute configuration was established as 3S,3aR,4S,6aS,8aS,9R,13S,13aR,13bR-1 (Figure 4).
Figure 3.
Isolated compounds from Aspergillus spp. NCA257, NCA264, and NCA276.
Figure 4.

Displacement ellipsoid plot (50% probability level) of 1 at 100(2) K.
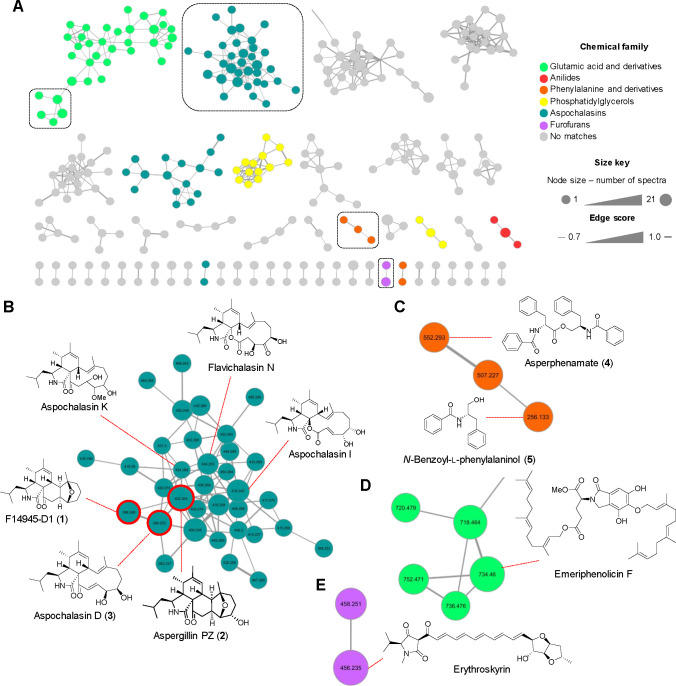
Next, the organic extracts of these strains were analyzed by ultrahigh-performance liquid chromatography tandem high-resolution electrospray mass spectrometry (UPLC-HRESIMS-MS/MS). Then, metabolomic analyses were performed using an in-house dereplication procedure29,30 and the Global Natural Products Social (GNPS) platform to perform feature-based molecular networking (FBMN) and spectral library search.31−33 The strain NCA276 showed the largest and most chemically diverse MN. Its metabolite features were grouped into 800 nodes arranged in 22 clusters or chemical families with >3 nodes per cluster, 29 with two nodes, and 506 singletons (Figure 5). Chemical ontology analysis by the MolNetEnhancer GNPS tool classified the molecular features in six classes of compounds (Figure 5). Detailed analysis of the aspochalasins (cytochalasans) cluster allowed to manually annotate the isolated compounds 1–3 using their HRMS-MS/MS data along with a series of related compounds (aspochalasins I and K, and flavichalasin N)34,35 annotated by GNPS (Figure 5 and Table 1). In the phenylalanine and derivatives cluster, 4 and its precursor 5 were annotated (Figure 5). Lastly, emeriphenolicin F36 and erythroskyrin37 were found by GNPS in the glutamic and derivatives and furofuranes nodes (Figure 5). For strain NCA264, FBMN analysis grouped the molecular features (591 nodes) into 9 classes of compounds clustered in 29 chemical families with >3 nodes per cluster, 29 with two nodes, and 542 singletons (Figure 6). The biggest family in the network belongs to the sesquiterpenoids, where 8, 9, and its derivative (S)-10-hydroxysydonic acid38 were annotated (Figure 6). In addition, 7 was successfully annotated by GNPS in the phenylpropanoids and polyketides family (Table 1). Finally, MN of strain NCA257 showed 154 nodes grouped in 11 clusters with >3 nodes per cluster, 3 with two nodes, and 99 singletons. Interestingly, FBMN analysis did not show any structural families, but the annotation of 10 was possible using its HRMS-MS/MS data (Figure 7 and Table 1).
Figure 5.
Metabolomic analysis of Aspergillus sp. NCA276. (A) FBMN (>2 nodes per cluster) and (B–E) selected clusters with nodes showing the compounds manually annotated (red circles) and by GNPS library search. Singletons (no molecular relatives) are not shown in the network.
Table 1. Chemical Annotation of Metabolites from Aspergillus spp.
| compound | observed ion (m/z)d | adduct | molecular formula | exact mass (m/z)e | mass accuracy (ppm) |
|---|---|---|---|---|---|
| Aspergillus sp. NCA276 | |||||
| 17-deoxy-aspergillin PZ (1)a,b | 386.268 | [M+H]+ | C24H36NO3 | 386.2682 | –2.0 |
| aspergillin PZ (2)a,b | 402.263 | [M+H]+ | C24H36NO4 | 402.2630 | –2.2 |
| aspochalasin D (3)a,b | 384.253 | [M–H2O+H]+ | C24H34NO3 | 384.2523 | –2.7 |
| asperphenamate (4)a,c | 507.228 | [M+H]+ | C32H31N2O4 | 507.2279 | +0.1 |
| N-benzoyl-l-phenylalaninol (5)a,c | 256.133 | [M+H]+ | C16H18NO2 | 256.1328 | +1.6 |
| aspochalasin Ic | 416.240 | [M+H]+ | C24H34NO5 | 416.2437 | +1.3 |
| aspochalasin Kc | 434.289 | [M+H]+ | C25H40NO5 | 434.2906 | +1.2 |
| flavichalasin Nc | 434.253 | [M+H]+ | C25H40NO5 | 434.2906 | +1.2 |
| emeriphenolicin Fc | 734.459 | [M+H]+ | C44H64NO8 | 734.4632 | +0.8 |
| erythroskyrinc | 456.235 | [M+H]+ | C26H34NO6 | 456.2386 | +1.2 |
| Aspergillus sp. NCA264 | |||||
| diorcinol (7)a,c | 229.086 | [M–H]− | C14H13O3 | 229.0863 | –3.1 |
| sydonic acid (8)a,c | 265.144 | [M–H]− | C15H21O4 | 265.1442 | –1.3 |
| 11-dehydrosydonic acid (9)a,c | 263.129 | [M–H]− | C15H19O4 | 263.1286 | –1.1 |
| (S)-10-hydroxysydonic acidc | 281.139 | [M–H]− | C15H21O5 | 281.1389 | –1.9 |
| Aspergillus sp. NCA257 | |||||
| Asterriquinol D dimethyl ether (10)a,b | 429.180 | [M+H]+ | C26H25N2O4 | 429.1800 | –2.1 |
Isolated compound.
Manual annotation.
GNPS annotation.
Values from GNPS.
Values from UPLC-HRESIMS-MS/MS analysis.
Figure 6.
Metabolomic analysis of Aspergillus sp. NCA264. (A) FBMN and (B,C) selected clusters with nodes showing the compounds annotated by GNPS library search.
Figure 7.
Metabolomic analysis of Aspergillus sp. NCA257 and selected node showing manually annotated 9 (red circle).
Chemical Study and Molecular Networking Analysis of Stachybotrys sp. NCA252 and Cladosporium sp. NCA273
From the organic extract of Stachybotrys sp. NCA252 culture, the isocoumarin, O-methylmellein (11),39 the dolabellane-type diterpenoids, atranones A and B (12 and 13),40 and the phenylspirodrimanes, stachybotrolide acetate (14), stachybotrydial acetate (15), and stachybotrolide (16),41,42 were isolated (Figure 8 and Supporting Information). Compounds 12 and 13 are commonly observed in the chemotype A of Stachybotrys spp., and no compounds from chemotype S (e.g., macrocyclic trichothecenes such as satratoxins and roridins) were detected in the NCA252 strain.43,44 In addition, the interconversion of 15 into lactone 14 was observed by NMR45 (Figure S44). Finally, this is the first report of the isolation of 11 in a fungus of the Stachybotrys genera.
Figure 8.

Isolated compounds from Stachybotrys sp. NCA252.
GNPS analysis of this strain grouped the metabolite features into 325 nodes arranged in 12 clusters with >3 nodes per cluster, 17 with two nodes, and 103 singletons. Chemical ontology analysis showed the presence of seven classes of compounds (Figure 9). Detailed analysis of the benzofurans and phtalides nodes allowed the manual annotation of 14 and 16, and by GNPS the annotation of a series of related phenylspyrodrimanes, including 15, asperugin,46 and stachybysbin A47 (Figure 9 and Table 2).
Figure 9.
Metabolomic analysis of Stachybotrys sp. NCA252. (A) FBMN and (B–F) selected clusters with nodes showing the compounds manually annotated (red circles) and by GNPS library search.
Table 2. Chemical Annotation of Metabolites from Stachybotrys sp. NCA252.
| compound | observed ion (m/z)d | adduct | molecular formula | exact mass (m/z)e | mass accuracy (ppm) |
|---|---|---|---|---|---|
| O-methylmellein (11)a,b | 215.068 | [M+Na]+ | C11H12O3Na | 215.0675 | +1.7 |
| atranone A (12)a,b | 461.218 | [M+HCOO]− | C25H33O8 | 461.2182 | +0.2 |
| atranone B (13)a,b | 445.223 | [M–H]− | C25H33O7 | 445.2226 | –1.3 |
| stachybotrolide acetate (14)a,b | 429.227 | [M+H]+ | C25H33O6 | 429.2272 | +0.1 |
| stachybotrydial acetate (15)a | 369.206 | [M–AcO+H]+ | C23H29O4 | 369.2061 | +0.2 |
| stachybotrolide (16)a,b | 387.217 | [M+H]+ | C23H31O5 | 387.2160 | –1.6 |
| stachybotramidec | 430.258 | [M+H]+ | C25H36NO5 | 430.2593 | +1.2 |
| K-76 diacetatec | 487.233 | [M+H]+ | C27H35O8 | 487.2332 | +1.1 |
| stachybotrysin Hc | 471.238 | [M–H2O+H]+ | C27H35O7 | 471.2383 | +1.2 |
| myrothecisin Dc | 371.222 | [M–H2O+H]+ | C23H31O4 | 371.2222 | +1.4 |
| F1839Ec | 446.254 | [M+H]+ | C25H36NO6 | 446.2542 | +1.1 |
| asperuginc | 401.232 | [M+H]+ | C24H33O5 | 401.2328 | +1.4 |
| stachybysbin Ac | 385.201 | [M+H]+ | C23H29O5 | 385.2015 | +1.4 |
Isolated compound.
Manually annotation.
GNPS annotation.
Values from GNPS.
Values from UPLC-HRESIMS-MS/MS analysis.
The chemical study of the culture of Cladosporium sp. NCA273 did not yield any pure compound due to the scarcity of the organic extract obtained from the solid culture (poor growth in this medium). In addition, the MN analysis of this strain grouped the metabolite features into 77 nodes arranged in 6 clusters with >3 nodes per cluster, 8 with two nodes, and 63 singletons. No matches were found using the in-house dereplication procedure nor the spectral library search in the GNPS, and only two chemical families (O-glycosyl compounds and glycerophosphocholines) were observed by FBMN (Figure S45).
Aspergillus spp. NCA257 and NCA276 and Cladosporium sp. NCA273 Coculture Analysis
During the isolation process of the fungal strains from the cenote sediment samples, a series of antagonistic interactions between Aspergillus spp. NCA257 and NCA276 and Cladosporium sp. NCA273 were observed (Figure 10). It is known that coculture fermentation can trigger the expression of silent biosynthetic gene clusters to produce new secondary metabolites, increase the amounts of others, or in some cases inhibit the production of some compounds.48−50 This strategy was thus employed to analyze the chemical variation of these strains when cocultivated in solid media (Supporting Information).
Figure 10.
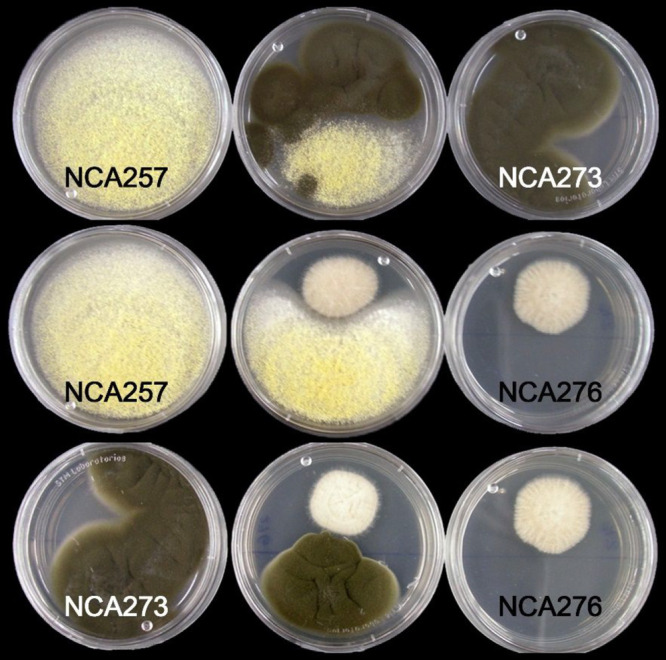
Fungal interaction between Aspergillus spp. NCA257 and NCA276 and Cladosporium sp. NCA273 in PDA plates.
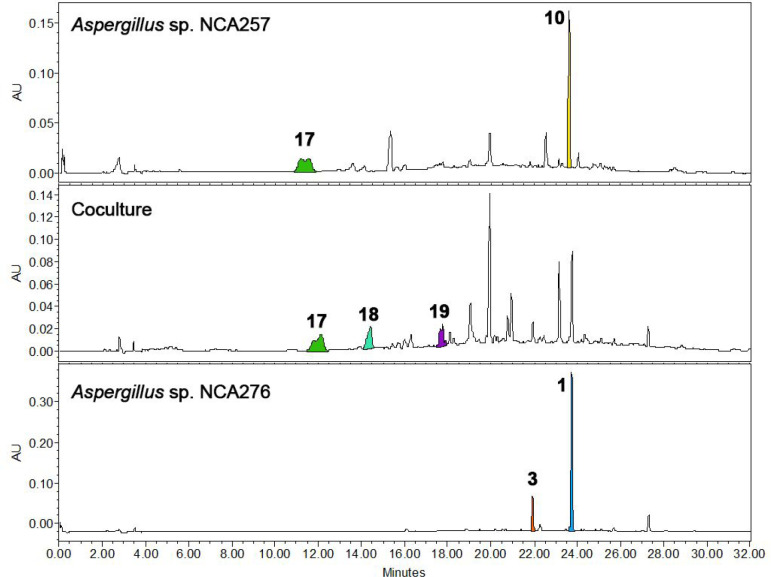
High-performance liquid chromatography (HPLC) analysis of the organic extract of the coculture of Aspergillus sp. NCA257 and Cladosporium sp. NCA273 revealed the presence of penicillic acid (17),51 5,6-dihydropenicillic acid (18),52 and 3-isobutyl-6-(1-hydroxy-2-methylpropyl)-pyrazin-2(1H)-one (19)53 (Figure 11). Compound 17 was not isolated from the monoculture of Aspergillus sp. NCA257 due to its low abundance. However, the induction of 17–19 by coculture allowed its isolation and structural elucidation (Supporting Information). Interestingly, 17 abundance (AUC by HPLC analysis) was 250% times higher in the coculture compared to the monoculture of Aspergillus sp. NCA257, whereas 10 was totally suppressed. On the other hand, the analysis of the Aspergillus spp. NCA257 and NCA276 coculture revealed similar results than those of NCA257 and NCA273 coculture, that is, 17–19 were induced in the coculture, and the aspochalasins 1 and 3 were suppressed (Figure 12).
Figure 11.
HPLC traces (PDA UV λ = 254 nm) of monoculture extracts of Aspergillus sp. NCA257 and Cladosporium sp. NCA273 and the coculture extract.
Figure 12.
HPLC traces (PDA UV λ = 254 nm) of monoculture extracts of Aspergillus spp. NCA257 and NCA276 and the coculture extract.
The MN analysis by GNPS of the cocultures of NCA257/NCA273 and NCA257/NCA276, and the single-cultures of all three strains consisted of 1380 nodes, which were grouped into 114 clusters with >2 nodes per cluster (Figure S46). Interestingly, the MN showed a major contribution from strain NCA257 (Aspergillus sp.) in the clusters of the chemical features (Figure S46). This agrees with the HPLC profiles (Figures 11 and 12), where the production of the penicillic acids 17 and 18, and the pyrazinone 19 suppresses the biosynthesis of the main metabolites from the other strains.54 Finally, aspochalasin J, aspochalasinol A, and alterporriol B were annotated by GNPS in MN (Figure S46 and Table S4, Supporting Information).
Biological Activity of Pure Compounds
The antibacterial activity of the isolated compounds was assessed against a panel of ESKAPE pathogens using the microdilution assay55,56 at 100 μg/mL or 10 μg/mL (Table 3). From these, the aspochalasin 3, the amino acid ester 4, the bis-indolyl benzenoid 10, and the phenylspirodrimanes 14–16 showed high activity (>50% inhibition) against MSSA and MRSA strains with 14 as the most potent metabolite (>81% inhibition at test concentration of 10 μg/mL). None of the tested compounds were active against Pseudomonas aeruginosa ATCC 27853, Klebsiella aerogenes ATCC 13048, K. pneumoniae ATCC 700603, Acinetobacter baumannii ATCC 17978, and the multidrug-resistant A. baumannii clinical strain A564.57 Finally, 7, 9, and 19 were not tested due to the scarcity of the isolated material.
Table 3. Antimicrobial Activity of Isolated Compounds against ESKAPE Bacteria at 100 μg/mL.
| growth inhibition (%) |
|||||
|---|---|---|---|---|---|
| compound | VSEFe | VREFe | MSSAe | MRSAe | ECe |
| 17-deoxy-aspergillin PZ (1) | 11.8 | 27.7 | 28.1 | ||
| aspergillin PZ (2) | 23.0 | 9.0 | |||
| aspochalasin D (3) | 37.2 | 23.8 | 81.4 | 87.2 | |
| asperphenamate (4) | 10.4 | 62.9 | 22.5 | ||
| N-benzoyl-l-phenylalaninol (5) | 13.3 | 50.7 | |||
| 2-O-methylbutyrolactone II (6) | |||||
| sydonic acid (8) | 34.0 | 11.9 | |||
| asterriquinol D dimethyl ether (10) | 50.9 | 62.3 | 48.2 | ||
| O-methylmellein (11) | 16.2 | ||||
| atranone A (12) | 13.0 | 6.0 | |||
| atranone B (13) | 12.2 | ||||
| stachybotrolide acetate (14)d | 81.0 | 92.9 | 21.5 | ||
| stachybotrydial acetate (15) | 82.7 | 89.3 | 18.3 | ||
| stachybotrolide (16) | 82.2 | 91.2 | 23.7 | ||
| penicillic acid (17) | 80.4 | 51.4 | |||
| 5,6-dihydropenicillic acid (18) | 38.3 | ||||
| MIC positive control in μg/mL. | 3.75a | 25.0a | 200.0b | 2.5a | 0.5c |
Vancomycin - Inactive at 100 μg/mL.
Ampicillin - Inactive at 100 μg/mL.
Gentamicin - Inactive at 100 μg/mL.
Tested at 10 μg/mL.
VSEF, vancomycin-susceptible E. faecalis ATCC 29212; VREF, vancomycin-resistant E. faecalis ATCC 51299; MSSA, methicillin-susceptible S. aureus ATCC 25923; MRSA, methicillin-resistant S. aureus ATCC 43300; and EC, E. cloacae ATCC 700323.
In addition, the anti-mycobacteria activity of the isolated compounds was assessed using the microplate Alamar blue (MABA) and low oxygen recovery (LORA) assays58,59 against tuberculous (TB) and nontuberculous mycobacterias (NTM), respectively, and a cytotoxicity assay against Vero cell line (ATCC CCL-81)60 (Table 4). Compounds 3, 4, 7, 14, 16, and 17 showed >98% growth inhibition of TB at 50 μg/mL and CC50 values >35 μM against Vero cells. Moreover, 3, 4, 7, 14, and 16, displayed anti-M. avium activity with MIC values of 22.6, 37.0, 20.0, 11.6, and 17.0 μM, respectively, and 7, 14, and 16 against M. marinum with MIC values of 47.2, 23.1, and 47.0 μM, respectively. Compounds 2 and 9 were not tested in these assays due to the paucity of the isolated material.
Table 4. Anti-mycobacteria and Cytotoxic Activities of Isolated Compounds.
|
M.
tuberculosis H37Rv |
M. abscessus ATCC 19977 | M. chelonae ATCC 35752 | M. marinum ATCC 927 | M. avium ATCC 15769 | M. kansasii ATCC 12478 | |||
|---|---|---|---|---|---|---|---|---|
| compound | MABAa | LORAb | MABAc | Vero cell ATCC CCL-81d | ||||
| 17-deoxy-aspergillin PZ (1) | 19 (ND) | ND | ND | ND | ND | ND | ND | ND |
| aspochalasin D (3) | 101 (39.5) | >50 | >50 (0) | >50 (17) | >50 (31) | 22.55 | >50 (16) | 37.5 |
| asperphenamate (4) | 101 (16.6) | >50 | >50 (0) | >50 (6) | >50 (22) | 37.00 | >50 (0) | >50 |
| N-benzoyl-l-phenylalaninol (5) | 20 (ND) | ND | ND | ND | ND | ND | ND | ND |
| 2-O-methylbutyrolactone II (6) | 83 (ND) | ND | ND | ND | ND | ND | ND | ND |
| diorcinol (7) | 100 (47.9) | >50 | >50 (0) | >50 (12) | 47.24 | 19.98 | >50 (0) | 39.9 |
| sydonic acid (8) | 30 (ND) | ND | ND | ND | ND | ND | ND | ND |
| asterriquinol D dimethyl ether (10) | 13 (ND) | ND | ND | ND | ND | ND | ND | ND |
| O-methylmellein (11) | 22 (ND) | ND | ND | ND | ND | ND | ND | ND |
| atranone A (12) | 29 (ND) | ND | ND | ND | ND | ND | ND | ND |
| atranone B (13) | 33 (ND) | ND | ND | ND | ND | ND | ND | ND |
| stachybotrolide acetate (14) | 99 (48.3) | >50 | >50 (0) | >50(0) | 23.15 | 11.63 | >50(0) | 38.2 |
| stachybotrydial acetate (15) | 38 (ND) | ND | ND | ND | ND | ND | ND | ND |
| stachybotrolide (16) | 103 (49.4) | >50 | >50 (0) | >50 (0) | 47.02 | 16.92 | >50(0) | 39.7 |
| penicillic acid (17) | 98 (49.5) | >50 | ND | ND | ND | ND | ND | ND |
| 5,6-dihydropenicillic acid (18) | 42 (ND) | ND | >50 (0) | >50 (73) | >50 (58) | >50 (53) | >50(0) | 35.4 |
| 3-isobutyl-6-(1-hydroxy-2-methylpropyl)-pyrazin-2(1H)-one (19) | 23 (ND) | ND | ND | ND | ND | ND | ND | ND |
| MIC Rifampicin (μg/mL) | 100 (0.03) | 0.08 | >8.0 | 4.25 | 0.09 | 0.05 | 0.41 | >8.0 |
% Inhibition at 50 μg/mL (MIC μM).
MIC μM.
MIC μM (% Inhibition).
CC50, cytotoxic concentration to 50% inhibition of the cell line. ND, not determined since % inhibition of M. tuberculosis H37Rv using MABA was below 90%.
Conclusions
In summary, this work represents the first contribution to the chemical diversity and biology of fungi from sediments in a cenote of the Yucatan peninsula. Strains Aspergillus (NCA257, NCA264, and NCA276), Stachybotrys (NCA252), and Cladosporium (NCA273) were isolated from sediments’ samples collected in the cenote Tza Itzá and taxonomically identified by molecular sequencing of the ITS rDNA. From this, several isolated compounds showed significant activity against MSSA, MRSA, other ESKAPE pathogens and mycobacterial strains and could be further studied for the development of potential drug leads. The absolute configuration of new 17-deoxy-aspergillin PZ (1) was established via single-crystal X-ray crystallography. Finally, cocultures and metabolomics analysis revealed that communication between the strains is needed to produce metabolites that were not present in the monocultures. Overall, this work uncovers the chemical and biological potential of understudied fungal strains to produce biosynthetic bioactive natural products.
Experimental Section
General Experimental Procedures
Optical rotations, and UV data were measured using a Rudolph Research Autopol III polarimeter (Rudolph Research Analytical), and a Varian Cary 100 Bio UV–vis spectrophotometer (Varian Inc.), respectively. NMR experiments were conducted in CDCl3 or methanol-d4, using a JEOL ECA-600 spectrometer (JEOL Ltd.), a Varian VNMRS 400 (Varian Inc.), or a Bruker Avance III 400 MHz (Bruker BioSpin Corp.). HRESIMS data were acquired using a Q Exactive Plus system (Thermo Fisher Scientific), equipped with an electrospray ionization source with an HCD cell. Data were collected in both positive and negative modes via direct injection or through an Acquity UPLC system (Waters Corp.) using a BEH C18 column (50 × 2.1 mm i.d., 1.7 μm; Waters Corp.) with a gradient solvent system from 15:85 to 100:0 CH3CN-0.1% aqueous formic acid for 10 min at a flow rate of 0.3 mL/min. Analytical and preparative HPLC were carried out on a Waters HPLC system equipped with a 2535 quaternary pump, a 2707 autosampler, and 2998 PDA and 2424 ELSD detectors, using Gemini C18 and Kinetex C18 columns (5 μm,110 Å, 250 × 4.6 mm i.d. and 5 μm, 110 Å, 250 × 21.2 mm i.d.; Phenomenex) for analytical and preparative runs, respectively. Data acquisition and management were performed with the Empower 3 software (Waters Corp.). Flash chromatography was conducted on a CombiFlash Rf+ Lumen system (Teledyne Technologies Inc.) equipped with PDA and ELSD detectors using RediSep Rf Gold Si-gel columns (Teledyne Technologies Inc.). Reagent-grade chloroform, n-hexane, and methanol, and HPLC- and MS-grade acetonitrile, methanol, and water were purchased from J.T. Baker (Avantor Performance Materials). Deuterated NMR solvents were acquired from Cambridge Isotope Laboratories, Inc.
Fungal Strains Isolation and Identification
The fungi of interest were isolated from sediment samples collected from cenote Tza Itzá (20°43′50.27″ N, 89°27′56.82″ W), in the state of Yucatán, Mexico in 2018. The cenote dimensions are a maximum depth of 40 m, water mirror of 7.6 × 10.5 m, and dry cave of 30 × 8 × 5 m (L × W × H). The isolation of the strains was made in A1 10% media with peptone (0.4 g), starch (1.0 g), yeast extract (0.2 g), supplemented with rifampicin (5 μg/mL) and cycloheximide (0.1 mg/mL) to reduce the growth of Gram-negative bacteria as previously described.10 The fungal cultures are maintained at −80 °C at the microbial culture collection from Unidad de Química en Sisal, Facultad de Química, UNAM, in Yucatan, Mexico. A copy of each strain used for the chemical studies is also maintained at the Departamento de Farmacia, Facultad de Química, UNAM, Mexico. Axenic cultures of strains NCA252, NCA257, NCA264, NCA273, and NCA276 were subjected to molecular identification by ITS sequencing and BLAST search with the RefSeg database using the methodologies reported.61,62 For designation of taxonomic names, the results of the ITS BLAST search using GenBank were interpreted with caution using modification of outlined criteria.63 Since we sequenced only the ITS region, we chose a rather conservative approach and made identifications only to the genus level even when BLAST sequence homology was ≥99%. Sequences for each strain were deposited in the National Center for Biotechnology Information (NCBI, http://www.ncbi.nlm.nih.gov/BLAST/) and given the GenBank Data Base accession numbers indicated in (Table S1 Supporting Information).
Fungal Cultivation, Extraction, and Isolation and Characterization of Pure Compounds
The methods utilized to culture the fungal strains (mono and cocultures) to prepare their organic extracts and to isolate and structurally elucidate compounds 1–19 from the fungi followed well-established protocols64−67 and are detailed in the Supporting Information.
17-Deoxy-aspergillin PZ (1)
Colorless solid; [α]D18.1 + 7.5 (c 0.1, CHCl3); UV (CHCl3) λmax (log ε) 322 (3.28), 285 (3.34) nm; 1H and 13C NMR, see Table S3 on Supporting Information; HRESIMS m/z 386.2682 [M + H]+ (calcd. for C24H36NO3, 386.2695).
X-ray Crystallographic Analysis of 17-deoxy-aspergillin PZ (1)
Suitable X-ray quality single crystals for 17-deoxy-aspergillin PZ (1) were successfully obtained from MeOH–CHCl3–H2O (20:1:1) recrystallization mixture. The crystallographic data and data collection parameters are mentioned in Table S2. All reflection intensities were measured at 100(2) K using a Gemini R diffractometer (equipped with Atlas detector) with CuKα radiation (λ = 1.54178 Å) under the program CrysAlisPro (Version CrysAlisPro 1.171.38.43f, Rigaku OD, 2015). The refinement of cell dimensions and data reduction were performed using the most recent version of the program (viz. CrysAlisPro 1.171.40.53, Rigaku OD, 2019). The structure was solved with the program SHELXT-2018/2 and was refined on F2 by the full-matrix least-squares method using SHELXL-2018/3.68 An empirical absorption correction was applied using CrysAlisPro 1.171.40.53.69 Non-hydrogen atoms were refined anisotropically. In the refinement, hydrogens attached to carbon, were treated as riding atoms using SHELXL default parameters, while those attached to nitrogen were located with electron difference maps. The crystallographic data of 1 in CIF format was deposited in the Cambridge Structural Database (CSD) (CCDC no. 2142221).
Biological Activity Assays
Pure compounds were tested in vitro for antibacterial activity using the Clinical and Laboratory Standards Institute (CLSI) broth dilution standard method.55,56 Target bacteria used in the assays belong to the ESKAPE group: vancomycin-susceptible Enterococcus faecalis ATCC 29212 (VSEF), vancomycin-resistant E. faecalis ATCC 51299 (VREF), methicillin-susceptible Staphylococcus aureus ATCC 25923 (MSSA), methicillin-resistant S. aureus ATCC 43300 (MRSA), Klebsiella aerogenes ATCC 13048, K. pneumoniae ATCC 700603, Acinetobacter baumannii ATCC 17978, multidrug-resistant A. baumannii strain 564 (clinical isolated),57Pseudomonas aeruginosa ATCC 27853, and Enterobacter cloacae ATCC 700323. The compounds were dissolved in DMSO to obtain a stock solution and then tested at a final concentration of 100 μg/mL or 10 μg/mL. The bioassays were carried out in 96-well plates in triplicate at concentrations of pure compound. In addition, pure compounds were also tested against M. tuberculosis H37Rv (MT) strain under both aerobic (replicating) and anaerobic (nonreplicating; NRMT) conditions using MABA and LORA assays.58 Compounds with activity >90% of inhibition of the growth of MT in MABA were further tested against M. abscessus ATCC 19977, M. chelonae ATCC 35752, M. marinum ATCC 927, M. avium ATCC 15769, and M. kansasii ATCC 12478, using MABA58 and a cytotoxicity assay against Vero cell line ATCC CCL-81.60 For the latter, 0.6 mM of resazurin was used and the absorbance was recorded at 530 nm (excitation) and 590 nm (emission). Positive controls for all assays are indicated in Tables 3 and 4.
Molecular Networking and Metabolomics Analysis
Each extract from the mono and cocultures was analyzed by LC-HRMS-MS/MS using a previously described methodology.66,67 Raw data were converted to mzML format using the ProteoWizard tool MsConvert (version 3.0.20239) and the resulting files uploaded to the Global Natural Products Social (GNPS; https://gnps.ucsd.edu) Web server using the FTP Server Version 5.17.16. Molecular networks were generated following the workflow previously published.31,32 Data was filtered by removing all MS/MS fragment ions within ±17 Da of the precursor m/z. The precursor ion mass tolerance was set to 0.01 Da and a MS/MS fragment ion tolerance of 0.02 Da. A network was then created where edges were filtered to have a cosine score above 0.7 and more than five matched peaks. Furthermore, edges between two nodes were kept in the network if and only if each of the nodes appeared in each other’s respective top 10 most similar nodes. Finally, the maximum size of a molecular family was set to 100, and the lowest scoring edges were removed from molecular families until the molecular family size was below this threshold. The spectra in the network were then searched against GNPS’ spectral libraries. The library spectra were filtered in the same manner as the input data. All matches between network spectra and library spectra were required to have a score above 0.7 and at least five matched peaks. GNPS MolNetEnhancer and Dereplicator+ tools were applied for chemical classification.33,70 Molecular networks were visualized with Cytoscape 3.8.1.71 Finally, manual dereplication was assessed using UV-absorption maxima and HRMS-MS/MS data against MS/MS data of 1–19 and by comparison with those reported in the Dictionary of Natural Products,72 SciFinder,73 and an in-house mycotoxins database. The annotation of isolated compounds was at confidence level 1, according to the metabolomics standards initiative and exact mass accuracy <5 ppm.74
Acknowledgments
This work was supported by grants from UNAM-DGAPA-PAPIIT IN222220 (M.F.) and FQ-PAIP 5000-9145 (M.F.). A.P.-D. thanks to CONACyT, Ciencia Básica Project A1-S-10785 and the Rockefeller University for providing funds for sampling expeditions and to the divers Biol. Luis Arturo Liévano and M.S. Efraín Chávez Solís for collecting the samples from the cenote. C.A.F.-H. thanks to CONACyT for the fellowship (no. 596831) to pursue his Ph.D. studies. A.M.-C. acknowledges the postdoctoral fellowship from DGAPA, UNAM. S.H. and F.S.T.K. gratefully acknowledge the Joint School of Nanoscience and Nanoengineering (JSNN) for providing access to the X-ray diffraction facility. M.F. thanks to Q.F.B. Alejandro Camacho Cruz (Cepario, FQ, UNAM), Dr. Isabel Rivero-Cruz and Ramiro del Carmen (FQ, UNAM), Dr. Norma Angélica Márquez-Velázquez (Unidad de Química en Sisal, FQ, UNAM), and M.S. Rosa Isela del Villar and M.S. Nayeli López-Balbiaux, (USAII, FQ, UNAM) for their valuable technical assistance. The authors are deeply grateful to Professor Nicholas H. Oberlies for providing access to the nuclear magnetic resonance (NMR) and mass spectrometry (MS) facilities at the University of North Carolina at Greensboro.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c00544.
Methods for fungal cultivation, extraction, and isolation and characterization of pure compounds; identity of fungal isolates; NMR and MS spectra of 1–19; crystallographic data of 1; MN and chemical annotation of cocultures (PDF)
Author Contributions
This work was part for the Ph.D. thesis of C.A.F.-H. from the Posgrado en Ciencias Qumicas, UNAM. C.A.F.-H. and M.F. designed the experiments. C.A.F.-H., F.S.T.K., L.F.-B., A.P.-D., B.W., R.M., M.Q., S.G.F., R.V.-S., A.M.-C., M.A.L.-L., and H.A.R. performed the experiments. C.A.F.-H., A.P.-D., S.H., S.G.F., H.A.R., R.G.-C., and M.F. analyzed the data and revised the manuscript. C.A.F.-H. and M.F. wrote, reviewed, and edited the manuscript. All authors have read and agreed to the revised version of the manuscript.
The authors declare no competing financial interest.
Notes
LC-MS/MS data can be accessed at MassIVE (accession no. MSV000088691; accessed on January 26, 2022). The molecular network data set of fungal coculture species Aspergillus spp. NCA257, NCA276 and Cladosporium sp. NCA273 can be accessed at MassIVE (accession no. MSV000088690; accessed on January 26, 2022). The molecular network of Stachybotrys sp. NCA252 at https://gnps.ucsd.edu/ProteoSAFe/status.jsp?task=2e6ed82df2854ee481908c5e7be883e0 (MolNetEnhancer analysis; accessed on January 26, 2022), molecular network of Aspergillus sp. NCA257 at https://gnps.ucsd.edu/ProteoSAFe/status.jsp?task=2aea96c6e47746e28349aa3241c8d065 (MolNetEnhancer analysis; accessed on January 26, 2022), molecular network of Aspergillus sp. NCA264 at https://gnps.ucsd.edu/ProteoSAFe/status.jsp?task=63f8482f83c94d3ea4b34910a972f170 (MolNetEnhancer analysis; accessed on January 26, 2022), molecular network of Cladosporium sp. NCA273 at https://gnps.ucsd.edu/ProteoSAFe/status.jsp?task=5f13c0f6339742879130611c389af5da (MolNetEnhancer analysis; accessed on January 26, 2022) and molecular network of Aspergillus sp. NCA276 at https://gnps.ucsd.edu/ProteoSAFe/status.jsp?task=ee9f321cb43d4e1da6f38fde4cfa696b (MolNetEnhancer analysis; accessed on January 26, 2022). Molecular network of fungal coculture species Aspergillus spp. NCA257, NCA276 and Cladosporium sp. NCA273 at https://gnps.ucsd.edu/ProteoSAFe/status.jsp?task=66c153a4faa4418485b252df22c99023 (accessed on January 26, 2022).
Supplementary Material
References
- Schmitter-Soto J. J.; Comín F. A.; Escobar-Briones E.; Herrera-Silveira J.; Alcocer J.; Suárez-Morales E.; Elías-Gutiérrez M.; Díaz-Arce V.; Marín L. E.; Steinich B. Hydrogeochemical and biological characteristics of cenotes in the Yucatan peninsula (SE Mexico). Hydrobiol. 2002, 467 (1–3), 215–228. 10.1023/A:1014923217206. [DOI] [Google Scholar]
- Gómez-Nicolás M.; Rebolledo-Vieyra M.; Canto-Lugo E.; Huerta-Quintanilla R.; Ochoa-Sandoval P. Connectivity in a karst system using electrical resistivity tomography and network theory. Groundwater 2018, 56 (5), 732–741. 10.1111/gwat.12618. [DOI] [PubMed] [Google Scholar]
- Hildebrand A. R.; Pilkington M.; Connors M.; Ortiz-Aleman C.; Chavez R. E. Size and structure of the Chicxulub crater revealed by horizontal gravity gradients and cenotes. Nature 1995, 376 (6539), 415–417. 10.1038/376415a0. [DOI] [Google Scholar]
- Lian B.; Yuan D.; Liu Z. Effect of microbes on karstification in karst ecosystems. Chin. Sci. Bull. 2011, 56, 3743–3747. 10.1007/s11434-011-4648-z. [DOI] [Google Scholar]
- Stoessell R. K.; Moore Y. H.; Coke J. G. The occurrence and effect of sulfate reduction and sulfide oxidation on coastal limestone dissolution in Yucatan cenotes. Groundwater 1993, 31 (4), 566–575. 10.1111/j.1745-6584.1993.tb00589.x. [DOI] [Google Scholar]
- Hahn M. W. The microbial diversity of inland waters. Curr. Opin. Biotechnol. 2006, 17 (3), 256–261. 10.1016/j.copbio.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Krauss G. J.; Sole M.; Krauss G.; Schlosser D.; Wesenberg D.; Baerlocher F. Fungi in freshwaters: ecology, physiology and biochemical potential. FEMS Microbiol. Rev. 2011, 35 (4), 620–651. 10.1111/j.1574-6976.2011.00266.x. [DOI] [PubMed] [Google Scholar]
- Estrada-Medina H.; Canto-Canché B. B.; De los Santos-Briones C.; O’Connor-Sánchez A. Yucatán in black and red: Linking edaphic analysis and pyrosequencing-based assessment of bacterial and fungal community structures in the two main kinds of soil of Yucatán State. Microbiol. Res. 2016, 188, 23–33. 10.1016/j.micres.2016.04.007. [DOI] [PubMed] [Google Scholar]
- Marfil-Santana M. D.; O’Connor-Sánchez A.; Ramírez-Prado J. H.; De los Santos-Briones C.; Lluvia K.; Rojas-Herrera R.; Lago-Lestón A.; Prieto-Davó A. A computationally simplistic poly-phasic approach to explore microbial communities from the Yucatan aquifer as a potential sources of novel natural products. J. Microbiol. 2016, 54 (11), 774–781. 10.1007/s12275-016-6092-x. [DOI] [PubMed] [Google Scholar]
- Parera-Valadez Y.; Yam-Puc A.; López-Aguiar L. K.; Borges-Argáez R.; Figueroa-Saldivar M. A.; Cáceres-Farfán M.; Márquez-Velázquez N. A.; Prieto-Davó A. Ecological strategies behind the selection of cultivable actinomycete strains from the Yucatan peninsula for the discovery of secondary metabolites with antibiotic activity. Microb. Ecol. 2019, 77 (4), 839–851. 10.1007/s00248-019-01329-3. [DOI] [PubMed] [Google Scholar]
- De la Rosa-García S. C.; Muñoz-García A. A.; Barahona-Pérez L. F.; Gamboa-Angulo M. M. Antimicrobial properties of moderately halotolerant bacteria from cenotes of the Yucatan peninsula. Lett. Appl. Microbiol. 2007, 45 (3), 289–294. 10.1111/j.1472-765X.2007.02185.x. [DOI] [PubMed] [Google Scholar]
- Marcela Gamboa-Angulo Antimicrobial screening of tropical microfungi isolated from sinkholes located in the Yucatan peninsula, Mexico. Afr. J. Microbiol. Res. 2012, 6 (10), 2305–2312. 10.5897/AJMR11.1129. [DOI] [Google Scholar]
- Ruiz-Jiménez A. L.; González-Coloma A.; Andrés-Yeves M. F.; Ruiz-Sánchez E.; Heredia G.; Peraza-Sánchez S. R.; Medina-Baizabal I. L.; Reyes-Estebanez M.; Canto-Caché B.; Gamboa-Angulo M. Insect deterrent and nematicidal screening of microfungi from Mexico and anti-aphid compounds from Gliomastix masseei. Rev. Argent. Microbiol. 2017, 49 (1), 83–92. 10.1016/j.ram.2016.08.009. [DOI] [PubMed] [Google Scholar]
- Ruiz-Jiménez A. L.; Ruiz-Sánchez E.; Heredia G.; Tapia-Tussell R.; González-Coloma A.; Peraza-Jiménez K.; Moo-Koh F. A.; Medina-Baizabal I. L.; Hernández-Romero Y.; Mena-Rejón G. J.; Quijano-Quiñones R. F.; Gamboa-Angulo M. Identification of insect-deterrent metabolites from Acremonium masseei strain CICY026, a saprophytic fungus from a sinkhole in Yucatán. Microorganisms 2019, 7 (12), 712. 10.3390/microorganisms7120712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pech-Puch D.; Pérez-Povedano M.; Lenis-Rojas O. A.; Rodríguez J.; Jiménez C. Marine natural products from the Yucatan peninsula. Mar. Drugs 2020, 18 (1), 59. 10.3390/md18010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Bolio G. I.; Ruiz-Vargas J. A.; Peña-Rodríguez L. M. Natural products from the yucatecan flora: Structural diversity and biological activity. J. Nat. Prod. 2019, 82 (3), 647–656. 10.1021/acs.jnatprod.8b00959. [DOI] [PubMed] [Google Scholar]
- Gardes M.; Bruns T. D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2 (2), 113–118. 10.1111/j.1365-294X.1993.tb00005.x. [DOI] [PubMed] [Google Scholar]
- White T. J.; Bruns T.; Lee S. J. W. T.; Taylor J.. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protocols: a guide to methods and applications; Academic Press: San Diego, 1990; Vol. 18, pp 315–322. [Google Scholar]
- Ding X.; Terui Y.; Chen Y. Separation and structural confirmation of three new compounds with antibacterial activity. Huaxue Yanjiu Yu Yingyong 2006, 18 (9), 1026–1028. [Google Scholar]
- Chen L.; Liu Y. T.; Song B.; Zhang H. W.; Ding G.; Liu X. Z.; Gu Y. C.; Zou Z. M. Stereochemical determination of new cytochalasans from the plant endophytic fungus Trichoderma gamsii. Fitoterapia 2014, 96, 115–122. 10.1016/j.fitote.2014.04.009. [DOI] [PubMed] [Google Scholar]
- Tomikawa T.; Shin-Ya K.; Kinoshita T.; Miyajima A.; Seto H.; Hayakawa Y. Selective cytotoxicity and stereochemistry of aspochalasin D. J. Antibiot. 2001, 54 (4), 379–381. 10.7164/antibiotics.54.379. [DOI] [PubMed] [Google Scholar]
- Hou X. M.; Zhang Y. H.; Hai Y.; Zheng J. Y.; Gu Y. C.; Wang C. Y.; Shao C. L. Aspersymmetide A, a new centrosymmetric cyclohexapeptide from the marine-derived fungus Aspergillus versicolor. Mar. drugs 2017, 15 (11), 363. 10.3390/md15110363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W.; Fan A.; Wang L.; Zhang P.; Liu Z.; An Z.; Yin W. B. Asperphenamate biosynthesis reveals a novel two-module NRPS system to synthesize amino acid esters in fungi. Chem. Sci. 2018, 9 (9), 2589–2594. 10.1039/C7SC02396K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.; Wang K. L.; Liu M.; She Z. G.; Wang C. Y. Bioactive steroid derivatives and butyrolactone derivatives from a Gorgonian-derived Aspergillus sp. fungus. Chem. Biodivers. 2015, 12 (9), 1398–1406. 10.1002/cbdv.201400321. [DOI] [PubMed] [Google Scholar]
- Sanchez J. F.; Chiang Y. M.; Szewczyk E.; Davidson A. D.; Ahuja M.; Oakley C. E.; Bok J. W.; Keller N.; Oakley B. R.; Wang C. C. Molecular genetic analysis of the orsellinic acid/F9775 gene cluster of Aspergillus nidulans. Mol. Biosyst. 2010, 6 (3), 587–593. 10.1039/B904541D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D.; Xu Y.; Shao C. L.; Yang R. Y.; Zheng C. J.; Chen Y. Y.; Fu X. M.; Qian P. Y.; She Z. G.; de Voogd N. J.; Wang C. Y. Antibacterial bisabolane-type sesquiterpenoids from the sponge-derived fungus Aspergillus sp. Mar. drugs 2012, 10 (12), 234–241. 10.3390/md10010234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z.; Zhu H.; Fu P.; Wang Y.; Zhang Z.; Lin H.; Liu P.; Zhuang Y.; Hong K.; Zhu W. Cytotoxic polyphenols from the marine-derived fungus Penicillium expansum. J. Nat. Prod. 2010, 73 (5), 911–914. 10.1021/np100059m. [DOI] [PubMed] [Google Scholar]
- Lacey H. J.; Vuong D.; Pitt J. I.; Lacey E.; Piggott A. M. Kumbicins A–D: bis-indolyl benzenoids and benzoquinones from an Australian soil fungus, Aspergillus kumbius. Aust. J. Chem. 2016, 69 (2), 152–160. 10.1071/CH15488. [DOI] [Google Scholar]
- El-Elimat T.; Figueroa M.; Ehrmann B. M.; Cech N. B.; Pearce C. J.; Oberlies N. H. High-resolution MS, MS/MS, and UV database of fungal secondary metabolites as a dereplication protocol for bioactive natural products. J. Nat. Prod. 2013, 76 (9), 1709–1716. 10.1021/np4004307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paguigan N. D.; El-Elimat T.; Kao D.; Raja H. A.; Pearce C. J.; Oberlies N. H. Enhanced dereplication of fungal cultures via use of mass defect filtering. J. Antibiot. 2017, 70, 553–561. 10.1038/ja.2016.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A. T.; Gentry E. C.; McPhail K. L.; Nothias L. F.; Nothias-Esposito M.; Bouslimani A.; Petras D.; Gauglitz J. M.; Sikora N.; Vargas F.; et al. Reproducible molecular networking of untargeted mass spectrometry data using GNPS. Nat. Protoc. 2020, 15, 1954–1991. 10.1038/s41596-020-0317-5. [DOI] [PubMed] [Google Scholar]
- Wang M.; Carver J. J.; Phelan V. V.; Sanchez L. M.; Garg N.; Peng Y.; Nguyen D. D.; Watrous J.; Kapono C. A.; Luzzatto-Knaan T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. 10.1038/nbt.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M.; Kang K. B.; Caraballo-Rodríguez A. M.; Nothias L. F.; Wandy J.; Chen C.; Wang M.; Rogers S.; Medema M. H.; Dorrestein P. C.; Van Der Hooft J. J. MolNetEnhancer: Enhanced molecular networks by integrating metabolome mining and annotation tools. Metabolites 2019, 9 (7), 144. 10.3390/metabo9070144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G. X.; Wijeratne E. K.; Bigelow D.; Pierson L. S.; VanEtten H. D.; Gunatilaka A. L. Aspochalasins I, J, and K: three new cytotoxic cytochalasans of Aspergillus flavipes from the rhizosphere of Ericameria laricifolia of the Sonoran Desert. J. Nat. Prod. 2004, 67 (3), 328–332. 10.1021/np030353m. [DOI] [PubMed] [Google Scholar]
- Si Y.; Tang M.; Lin S.; Chen G.; Feng Q.; Wang Y.; Hua H.; Bai J.; Wang H.; Pei Y. Cytotoxic cytochalasans from Aspergillus flavipes PJ03–11 by OSMAC method. Tetrahedron Lett. 2018, 59 (18), 1767–1771. 10.1016/j.tetlet.2018.03.077. [DOI] [Google Scholar]
- Zhou H.; Sun X.; Li N.; Che Q.; Zhu T.; Gu Q.; Li D. Isoindolone-containing meroperpenoids from the endophytic fungus Emericella nidulans HDN12–249. Org. Lett. 2016, 18 (18), 4670–4673. 10.1021/acs.orglett.6b02297. [DOI] [PubMed] [Google Scholar]
- Shibata S.; Sankawa U.; Taguchi H.; Yamasaki K. Biosynthesis of Natural Products. III. Biosynthesis of erythroskyrine, a coloring matter of Penicillium islandicum SOPP. Chem. Pharm. Bull. 1966, 14 (5), 474–478. 10.1248/cpb.14.474. [DOI] [PubMed] [Google Scholar]
- Li X. D.; Li X. M.; Xu G. M.; Zhang P.; Wang B. G. Antimicrobial phenolic bisabolanes and related derivatives from Penicillium aculeatum SD-321, a deep sea sediment-derived fungus. J. Nat. Prod. 2015, 78 (4), 844–849. 10.1021/acs.jnatprod.5b00004. [DOI] [PubMed] [Google Scholar]
- Glauser G.; Gindro K.; Fringeli J.; De Joffrey J. P.; Rudaz S.; Wolfender J. L. Differential analysis of mycoalexins in confrontation zones of grapevine fungal pathogens by ultrahigh pressure liquid chromatography/time-of-flight mass spectrometry and capillary nuclear magnetic resonance. J. Agr. Food Chem. 2009, 57 (4), 1127–1134. 10.1021/jf8033539. [DOI] [PubMed] [Google Scholar]
- Hinkley S. F.; Jiang J.; Mazzola E. P.; Jarvis B. B. Atranones: Novel diterpenoids from the toxigenic mold Stachybotrys atra. Tetrahedron Lett. 1999, 40 (14), 2725–2728. 10.1016/S0040-4039(99)00350-0. [DOI] [Google Scholar]
- Jarvis B. B.; Salemme J.; Morals A. Stachybotrys toxins. 1. Nat. Toxins 1995, 3 (1), 10–16. 10.1002/nt.2620030104. [DOI] [PubMed] [Google Scholar]
- Ayer W. A.; Miao S. Secondary metabolites of the aspen fungus Stachybotrys cylindrospora. Can. J. Chem. 1993, 71 (4), 487–493. 10.1139/v93-069. [DOI] [Google Scholar]
- Andersen B.; Nielsen K. F.; Thrane U.; Szaro T.; Taylor J. W.; Jarvis B. B. Molecular and phenotypic descriptions of Stachybotrys chlorohalonata sp. nov. and two chemotypes of Stachybotrys chartarum found in water-damaged buildings. Mycologia 2003, 95 (6), 1227–1238. 10.2307/3761923. [DOI] [PubMed] [Google Scholar]
- Semeiks J.; Borek D.; Otwinowski Z.; Grishin N. V. Comparative genome sequencing reveals chemotype-specific gene clusters in the toxigenic black mold Stachybotrys. BMC Genomics 2014, 15 (1), 590. 10.1186/1471-2164-15-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagels A.; Lindemann V.; Ulrich S.; Gottschalk C.; Cramer B.; Hübner F.; Gareis M.; Humpf H. U. Exploring secondary metabolite profiles of Stachybotrys spp. by LC-MS/MS. Toxins 2019, 11 (3), 133. 10.3390/toxins11030133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballantine J. A.; Hassall C. H.; Jones G. Asperugin, a metabolite associated with abnormal morphology of Aspergillus rugulosus. Tetrahedron Lett. 1964, 5 (49), 3739–3740. 10.1016/S0040-4039(01)89370-9. [DOI] [Google Scholar]
- Bao Y. R.; Chen G. D.; Wu Y. H.; Li X. X.; Hu D.; Liu X. Z.; Li Y.; Yao X. S.; Gao H. Stachybisbins A and B, the first cases of seco-bisabosquals from Stachybotrys bisbyi. Fitoterapia 2015, 105, 151–155. 10.1016/j.fitote.2015.06.022. [DOI] [PubMed] [Google Scholar]
- Arora D.; Gupta P.; Jaglan S.; Roullier C.; Grovel O.; Bertrand S. Expanding the chemical diversity through microorganisms co-culture: Current status and outlook. Biotechnol. Adv. 2020, 40, 107521. 10.1016/j.biotechadv.2020.107521. [DOI] [PubMed] [Google Scholar]
- Knowles S. L.; Raja H. A.; Roberts C. D.; Oberlies N. H. Fungal–fungal co-culture: a primer for generating chemical diversity. Nat. Prod. Rep. 2022, 10.1039/D1NP00070E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. L.; Li X. M.; Yang S. Q.; Cao J.; Li Y. H.; Wang B. G. Induced terreins production from marine red algal-derived endophytic fungus Aspergillus terreus EN-539 co-cultured with symbiotic fungus Paecilomyces lilacinus EN-531. J. Antibiot. 2020, 73 (2), 108–111. 10.1038/s41429-019-0242-4. [DOI] [PubMed] [Google Scholar]
- Nonaka K.; Chiba T.; Suga T.; Asami Y.; Iwatsuki M.; Masuma R.; O̅mura S.; Shiomi K. Coculnol, a new penicillic acid produced by a coculture of Fusarium solani FKI-6853 and Talaromyces sp. FKA-65. J. Antibiot. 2015, 68 (8), 530–532. 10.1038/ja.2015.15. [DOI] [PubMed] [Google Scholar]
- Sassa T.; Hayakari S.; Ikeda M.; Miura Y. Plant growth inhibitors produced by fungi Part I. Isolation and identification of penicillic acid and dihydropenicillic acid. Agr. Biol. Chem. 1971, 35 (13), 2130–2131. 10.1080/00021369.1971.10860199. [DOI] [Google Scholar]
- Li H. J.; Cai Y. T.; Chen Y. Y.; Lam C. K.; Lan W. J. Metabolites of marine fungus Aspergillus sp. collected from soft coral Sarcophyton tortuosum. Chem. Res. Chin. Univ. 2010, 26 (3), 415–419. [Google Scholar]
- Frisvad J. C. A critical review of producers of small lactone mycotoxins: patulin, penicillic acid and moniliformin. World Mycotoxin J. 2018, 11 (1), 73–100. 10.3920/WMJ2017.2294. [DOI] [Google Scholar]
- Balouiri M.; Sadiki M.; Ibnsouda S. K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6 (2), 71–79. 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute, https://clsi.org/standards/?page=1&sort=date&sortdir=desc&subcat=&area=.
- Cruz-Muñiz M. Y.; López-Jacome L. E.; Hernández-Durán M.; Franco-Cendejas R.; Licona-Limón P.; Ramos-Balderas J. L.; Martinéz-Vázquez M.; Belmont-Díaz J. A.; Wood T. K.; García-Contreras R. Repurposing the anticancer drug mitomycin C for the treatment of persistent Acinetobacter baumannii infections. Int. J. Antimicrob. Agents 2017, 49 (1), 88–92. 10.1016/j.ijantimicag.2016.08.022. [DOI] [PubMed] [Google Scholar]
- Cho S.; Lee H. S.; Franzblau S.. Microplate Alamar Blue Assay (MABA) and Low Oxygen Recovery Assay (LORA) for Mycobacterium tuberculosis. In Mycobacteria Protocols; Parish T., Roberts D. M., Eds.; Springer:: New York, 2015; pp 281–292. [DOI] [PubMed] [Google Scholar]
- Gao W.; Kim J.-Y.; Anderson J. R.; Akopian T.; Hong S.; Jin Y.-Y.; Kandror O.; Kim J.-W.; Lee I.-A.; Lee S.-Y.; et al. The cyclic peptide ecumicin targeting ClpC1 is active against Mycobacterium tuberculosis in vivo. Antimicrob. Agents Chemother. 2015, 59, 880–889. 10.1128/AAC.04054-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandikolla A.; Srinivasarao S.; Khetmalis Y. M.; Kumar B. K.; Murugesan S.; Shetye G.; Ma R.; Franzblau S. G.; Sekhar K. V. G. C. Design, synthesis and biological evaluation of novel 1, 2, 3-triazole analogues of Imidazo-[1, 2-a]-pyridine-3-carboxamide against Mycobacterium tuberculosis. Toxicol. In Vitro 2021, 74, 105137. 10.1016/j.tiv.2021.105137. [DOI] [PubMed] [Google Scholar]
- Raja H. A.; Miller A. N.; Pearce C. J.; Oberlies N. H. Fungal identification using molecular tools: a primer for the natural products research community. J. Nat. Prod. 2017, 80 (3), 756–770. 10.1021/acs.jnatprod.6b01085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch C. L.; Seifert K. A.; Huhndorf S.; Robert V.; Spouge J. L.; Levesque C. A.; Chen W.; Bolchacova E.; Voigt K.; Crous P. W.; Miller A. N.; Wingfield M. J.; Aime M. C.; An K.-D.; Bai F.-Y.; Barreto R. W.; Begerow D.; Bergeron M.-J.; Blackwell M.; Boekhout T.; Bogale M.; Boonyuen N.; Burgaz A. R.; Buyck B.; Cai L.; Cai Q.; Cardinali G.; Chaverri P.; Coppins B. J.; Crespo A.; Cubas P.; Cummings C.; Damm U.; de Beer Z. W.; de Hoog G. S.; Del-Prado R.; Dentinger B.; Dieguez-Uribeondo J.; Divakar P. K.; Douglas B.; Duenas M.; Duong T. A.; Eberhardt U.; Edwards J. E.; Elshahed M. S.; Fliegerova K.; Furtado M.; Garcia M. A.; Ge Z.-W.; Griffith G. W.; Griffiths K.; Groenewald J. Z.; Groenewald M.; Grube M.; Gryzenhout M.; Guo L.-D.; Hagen F.; Hambleton S.; Hamelin R. C.; Hansen K.; Harrold P.; Heller G.; Herrera C.; Hirayama K.; Hirooka Y.; Ho H.-M.; Hoffmann K.; Hofstetter V.; Hognabba F.; Hollingsworth P. M.; Hong S.-B.; Hosaka K.; Houbraken J.; Hughes K.; Huhtinen S.; Hyde K. D.; James T.; Johnson E. M.; Johnson J. E.; Johnston P. R.; Jones E.B. G.; Kelly L. J.; Kirk P. M.; Knapp D. G.; Koljalg U.; Kovacs G. M.; Kurtzman C. P.; Landvik S.; Leavitt S. D.; Liggenstoffer A. S.; Liimatainen K.; Lombard L.; Luangsa-ard J. J.; Lumbsch H. T.; Maganti H.; Maharachchikumbura S. S. N.; Martin M. P.; May T. W.; McTaggart A. R.; Methven A. S.; Meyer W.; Moncalvo J.-M.; Mongkolsamrit S.; Nagy L. G.; Nilsson R. H.; Niskanen T.; Nyilasi I.; Okada G.; Okane I.; Olariaga I.; Otte J.; Papp T.; Park D.; Petkovits T.; Pino-Bodas R.; Quaedvlieg W.; Raja H. A.; Redecker D.; Rintoul T. L.; Ruibal C.; Sarmiento-Ramirez J. M.; Schmitt I.; Schußler A.; Shearer C.; Sotome K.; Stefani F. O.P.; Stenroos S.; Stielow B.; Stockinger H.; Suetrong S.; Suh S.-O.; Sung G.-H.; Suzuki M.; Tanaka K.; Tedersoo L.; Telleria M. T.; Tretter E.; Untereiner W. A.; Urbina H.; Vagvolgyi C.; Vialle A.; Vu T. D.; Walther G.; Wang Q.-M.; Wang Y.; Weir B. S.; Weiß M.; White M. M.; Xu J.; Yahr R.; Yang Z. L.; Yurkov A.; Zamora J.-C.; Zhang N.; Zhuang W.-Y.; Schindel D. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. U. S. A. 2012, 109 (16), 6241–6246. 10.1073/pnas.1117018109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves V. N.; Vaz A. B. M.; Rosa C. A.; Rosa L. H. Diversity and distribution of fungal communities in lakes of Antarctica. FEMS Microbiol. Ecol. 2012, 82 (2), 459–471. 10.1111/j.1574-6941.2012.01424.x. [DOI] [PubMed] [Google Scholar]
- Aparicio-Cuevas M. A.; Rivero-Cruz I.; Sánchez-Castellanos M.; Menéndez D.; Raja H. A.; Joseph-Nathan P.; González M. C.; Figueroa M. Dioxomorpholines and derivatives from a marine-facultative Aspergillus species. J. Nat. Prod. 2017, 80 (8), 2311–2318. 10.1021/acs.jnatprod.7b00331. [DOI] [PubMed] [Google Scholar]
- Aparicio-Cuevas M. A.; González M. C.; Raja H. A.; Rivero-Cruz I.; Kurina S. J.; Burdette J. E.; Oberlies N. H.; Figueroa M. Metabolites from the marine-facultative Aspergillus sp. MEXU 27854 and Gymnoascus hyalinosporus MEXU 29901 from Caleta Bay, Mexico. Tetrahedron Lett. 2019, 60 (25), 1649–1652. 10.1016/j.tetlet.2019.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Cárdenas A.; Cruz-Zamora Y.; Fajardo-Hernández C. A.; Villanueva-Silva R.; Cruz-García F.; Raja H. A.; Figueroa M. Genome Mining and Molecular Networking-Based Metabolomics of the Marine Facultative Aspergillus sp. MEXU 27854. Molecules 2021, 26 (17), 5362. 10.3390/molecules26175362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva-Silva R.; Velez P.; Riquelme M.; Fajardo-Hernández C. A.; Martínez-Cárdenas A.; Arista-Romero A.; Wan B.; Ma R.; Qader M.; Franzblau S. G.; Figueroa M. Chemical Diversity and Antimicrobial Potential of Cultivable Fungi from Deep-Sea Sediments of the Gulf of Mexico. Molecules 2021, 26 (23), 7328. 10.3390/molecules26237328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldrick G. M.SHELXL-2018: Program for Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 2018. [Google Scholar]
- Rigaku Oxford Diffraction. CrysAlisPro: Empirical absorption correction using spherical harmonics, implemented in SCALE3 ABSPACK scaling algorithm; Agilent Technologies Ltd.: Yarnton, U.K., 2019. [Google Scholar]
- Mohimani H.; Gurevich A.; Shlemov A.; Mikheenko A.; Korobeynikov A.; Cao L.; Shcherbin E.; Nothias L. F.; Dorrestein P. C.; Pevzner P. A. Dereplication of microbial metabolites through database search of mass spectra. Nat. Commun. 2018, 9, 4035. 10.1038/s41467-018-06082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon P.; Markiel A.; Ozier O.; Baliga N. S.; Wang J. T.; Ramage D.; Amin N.; Schwikowski B.; Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13 (11), 2498–2504. 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dictionary of Natural Products 30.1. Available online at https://dnp.chemnetbase.com/faces/chemical/ChemicalSearch.xhtml (accessed on 26 November 2021).
- SciFinder—CAS. Available online at https://scifinder.cas.org (accessed on 26 November 2021).
- Sumner L. W.; Amberg A.; Barrett D.; Beale M. H.; Beger R.; Daykin C. A.; Fan T.W.-M.; Fiehn O.; Goodacre R.; Griffin J. L.; et al. Proposed minimum reporting standards for chemical analysis: Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. 10.1007/s11306-007-0082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.