Abstract
As the strategies of enzyme immobilization possess attractive advantages that contribute to realizing recovery or reuse of enzymes and improving their stability, they have become one of the most desirable techniques in industrial catalysis, biosensing, and biomedicine. Among them, 3D printing is the emerging and most potential enzyme immobilization strategy. The main advantages of 3D printing strategies for enzyme immobilization are that they can directly produce complex channel structures at low cost, and the printed scaffolds with immobilized enzymes can be completely modified just by changing the original design graphics. In this review, a comprehensive set of developments in the fields of 3D printing techniques, materials, and strategies for enzyme immobilization and the potential applications in industry and biomedicine are summarized. In addition, we put forward some challenges and possible solutions for the development of this field and some possible development directions in the future.
1. Introduction
Enzyme catalysts play an important role in different fields such as food, chemistry, and pharmaceuticals, promoting sustainable production of green manufacturing, fine chemicals, food additives, and medicines.1 Despite the many benefits of enzyme catalysis, free enzymes cannot be used in wide-scale industrial production due to the lack of adaptation to hydrophobic solvents, weak stability at higher reaction temperatures, or an excessively unstable pH range.2 In addition, separating and reusing free enzymes from the reaction system might be tricky. Therefore, it is necessary to immobilize the enzymes to avoid the defects as mentioned above. Enzyme immobilization is the process of combining the enzymes with the carrier in such a way that it is insoluble in the reaction phase containing the substrate, allowing the enzymatic reaction to take place within a specific spatial range. Compared with free enzymes, immobilized enzymes have many indispensable advantages, such as improved operational stability, enhanced enantioselectivity, reusability of the enzymes, and easier reactor operation and product separation, which avoids the waste of enzyme catalyst.3 Multipoint or multisubunit covalent attachment to a support would, in general, increase the operational stability of monomeric or multimeric enzymes by preventing subunit dissociation, aggregation, autolysis or proteolysis by proteases, and rigidification of the enzyme structure.4 In addition, there is no residue of enzyme solution in the reaction system because the immobilized enzymes do not “agglomerate”, making the subsequent purification process easier, and the reaction process becomes more controlled and suited for multienzyme reaction systems.5a5b More importantly, immobilization may increase enzyme selectivity by changing the geometry of the active center, stabilizing enzyme conformation, and enhancing the enzyme environment.5c However, immobilized enzymes typically have lower activity than free enzymes due to partially blocking of the enzymes’ active sites during the immobilization process, increased mass-transfer limitations between the enzymes and substrate, and conformational changes in the enzymes, all of which are detrimental to their catalytic performances.5d,5g Therefore, it is necessary to study and summarize novel enzyme immobilization methods.
Since emerging in the 1910s, enzymes have been immobilized on microspheres,6a metal–organic frameworks,6b,6f nanoparticles,7 and 3D-printed carriers for enzymatic reactions. One of the most promising is enzyme immobilization technology based on 3D printing. Additive manufacturing, often is referred to as 3D printing, is an emerging bottom-up manufacturing technology that can quickly achieve prototyping of complex geometries. It has been widely used in industrial design, construction, tissue engineering, and other fields.8 The enzyme immobilization strategy of 3D printing has the advantage of strong operability that we can quickly print those complex structures with pores and channels at low cost.9 Furthermore, in the process of 3D-printing, the enzyme immobilized scaffold will not cause material loss and use organic chemical reagents harmful to the environment. In addition, many 3D printing materials used in the field of enzyme immobilization are degradable and will not affect the environment. Especially, thermoplastics, such as polylactic acid, are not only degradable but can also be recycled and processed to achieve sustainable use of materials. It is because 3D printing for enzyme immobilization has these advantages that it is widely used in biological power generation, high-throughput screening in laboratories, and biosensing.
There have been many reviews that mainly refer to certain immobilized materials or specific industrial applications on enzyme immobilization,10 but a comprehensive and summarized review about the emerging 3D printing technology for enzyme immobilization and its industrial and biomedical applications are still unavailable, so we have done this work to provide some convenience for future research. In this review, we introduce 3D printing materials and technologies used in enzyme immobilization. Recent progress on advanced strategies of enzyme immobilization on 3D-printed scaffolds is highlighted. Then, we describe the industrial and biomedical applications of enzyme immobilization using 3D printing technology, putting attention to their excellent functions in biological power generation, biotransformation, and biosensing. Finally, a brief prospect about the development of emerging enzyme immobilization is presented.
2. 3D Printing Technologies for Enzyme Immobilization
With the development of people’s research, more and more 3D printing methods have been developed, but not many are suitable for the enzyme immobilization of 3D printing. 3D printing technologies used in enzyme immobilization are divided into stereolithography apparatus (SLA), fused deposition modeling (FDM), and extrusion-based 3D printing, according to the different printing principles.
2.1. Stereolithography Apparatus (SLA)
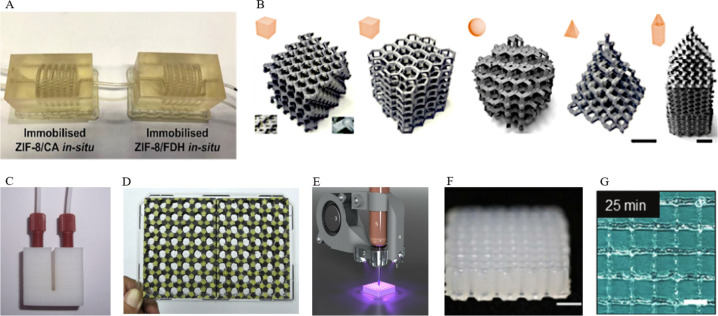
As the earliest method of 3D printing, SLA has been widely used in tissue engineering and tissue scaffolds.11a A laser beam used by SLA machine is above the resin tank and solidifies the liquid resin to the production of a solid mass.11b The model can be swiftly printed thanks to the excellent precision and fast polymerization speed of the SLA technology, indicating that it has a lot of potential in the production of microfluidic devices. As shown in Figure 1A, Chai et al. reported using an SLA printed micromixer with the threaded channel to test formic acid production performance. Two enzymes, carbonic anhydrase and formate dehydrogenase, were coimmobilized via biomineralization in a zeolitic imidazolate framework-8 (ZIF-8) thin film on the micromixer channels that have been modified with polydopamine/polyethylenimine.11c Although this technology can quickly manufacture relatively exquisite equipment, few studies that apply it to immobilize enzymes have been reported because of the relatively high processing temperature.
Figure 1.
(A) Arrangement of two micromixers in series with carbonic anhydrase (CA) and formate dehydrogenase (FDH) enzymes immobilized in separate micromixers (domino immobilization) of SLA 3D printing for the enzymatic cascade reduction of CO2. Reprinted with permission from ref (11c) Copyright 2021 Elsevier. (B) Digital images of FDM 3D-printed scaffolds for enzymes immobilization. Reprinted from ref (12b). Copyright 2019 American Chemical Society. (C) Photograph of the FDM printed reactor. Reprinted from ref (12c). Copyright 2016 American Chemical Society. (D) Photograph of two FDM printed enzyme/substrate-incorporated 48-well plates (side-by-side/layer-by-layer design) glued onto a transparent polystyrene microplate, with glucose oxidase immobilized near the center of each well. Reprinted with permission from ref (12d). Copyright 2018 Elsevier. (E) Photograph of an extrusion-based printer: Cure-on-dispense setup used in combination with a Gesim BioScaffolder 3.1. Reprinted with permission from ref (13b). Copyright 2020 Frontiers Media S.A. (F) Side view of 3D-printed lattice structures. Reprinted with permission from ref (13a). Copyright 2021 Institute of Physics Publishing. (G) The image of three-dimensional printed grid pattern using preheated gelatin 10 represents proper gelation. Reprinted with permission from ref (13c). Copyright 2020 Whioce Publishing Pte. Ltd.
Above all, the SLA approach may be used to make a sophisticated enzyme immobilization carrier that meets the demands of enzyme catalysis industrial production to a degree. However, there are some shortcomings. First, the temperature is the main problem that limits its use because the laser coagulation resin is used to make the enzyme immobilization carrier. The too high temperature will make the immobilized enzyme inactivation, so the effect is not up to expectations. Therefore, this technique is a good choice when it is necessary to fix some heat-resistant enzymes to catalyze the reaction. Second, the carrier material is only a resin material, and the range of enzyme immobilization may be narrow, which cannot meet the needs of diversified industrial production.
2.2. Fused Deposition Modeling (FDM)
FDM is a simple and low-cost manufacturing process that creates 3D objects layer by layer depositing thermoplastic polymers, including acrylonitrile-butadiene-styrene (ABS) and polylactic acid (PLA).12a It is because thermoplastics are easy to recycle and modify that more and more researchers pay their attention to using this technology for immobilized enzyme research. Ye et al. demonstrated a novel type of enzyme immobilization strategy using FDM printing methods. The FDM printed scaffolds, through chemical modification, achieved a high specific surface area with plentiful surface-active groups, which are conducive to the immobilization of enzymes. As a fact, four kinds of enzymes, including penicillin G acylase (PGA), protease, glycosidase, and lipase were immobilized on the 3D scaffolds to obtain good reaction effect and repeatability (Figure 1B).12b This technology can produce high strength and toughness as well as complex pores of enzyme immobilization scaffolds. Then the enzyme is immobilized on the surface of the modified stent, and the mass transfer has little effect on the enzyme activity. Even in solid–liquid reaction systems and high-viscosity systems, the catalytic activity of the immobilized enzyme is still very high. Using the similar printing method, Su and colleagues constructed acrylonitrile-butadiene-styrene (ABS) flow bioreactors which were immobilized glucose oxidase and lactate to online detect the concentration of glucose and lactose in the rat brain (Figure 1C).12c This is because the enzyme-immobilized carrier can be rapidly formed using the FDM technology, and the carrier material used is inexpensive and recyclable. Therefore, FDM is also used in experimental analysis devices requiring rapid preparation. Su and Chen reported the one-step manufacture of enzyme/substrate-incorporated multiwell plates that glucose oxidase immobilized in each well, using multimaterial FDM printing for rapid and high throughput screening of glucose in clinical samples (Figure 1D).12d Owing to the attractive advantages, FDM should be the development tendency of enzyme immobilization based on 3D-printing in the future.
In summary, FDM technology can rapidly form small-sized enzyme immobilized carriers and is an ideal technology for the rapid and accurate manufacturing of small-scale enzyme fuel cells for industrialization. If the simple manufacture of small enzyme fuel cells can be realized, green and sustainable energy production can be realized, and the serious environmental pollution caused by traditional battery scrap processing can be avoided. At the same time, strong acids, heavy metals, and other compounds harmful to the environment will not be used in the production. The most important thing is that the material used in this technology is thermoplastic polymers, which can be easily recycled, to realize the repeated and continuous use of the enzyme-immobilized carriers.
2.3. Extrusion-Based 3D Printing
Extrusion-based printing technologies may be utilized to create constructs in 3D forms that are often packed with live cells or bioactive chemicals. Before printing, the enzymes and materials need to be mixed in advance. Hence, most materials with good rheological properties are used as the substrate for enzyme immobilization. As shown in Figure 1F, Zhou et al. reported a novel composite bioink based on gelatin, bacterial cellulose (BC), and microbial transglutaminase (mTG enzyme) for extrusion-based printing with excellent printing controllability and durable architectural integrity.13a The described extrusion-based 3D printing processes and inks for enzyme immobilization are confined to constructing extremely basic geometries with only a few layers and relatively high wall thicknesses, restricting mass transfer and lowering efficiency. High internal phase emulsions (HIPEs) act as a solid retaining their shape under the yield stress, making HIPEs become ideal candidates for extrusion-based 3D printing. In 2020, Wenger and his colleagues demonstrated the hydrogel-filled high internal phase emulsions (HIPEs) as extrusion-based 3D-printed material. Moreover, HIPEs containing enzymes were employed in biocatalytic reactors that increased enzyme reusability and improved biocatalytic performance (Figure 1E).13b Of course, some other methods can also be used to increase the printability of the material. Tan et al. proposed a novel strategy that used preheated gelatin to extend the duration of the direct ink writing (DIW) printing time and used immobilized transglutaminase to cross-link gelatin chains, avoiding the use of toxic cross-linkers, which is essential for extrusion-based printing (Figure 1G).13c No matter what method is used, the goal is to be able to print more complex structures through extrusion printing of immobilized enzymes to meet the needs of actual production better.
In conclusion, extrusion-based 3D printing has been used in some enzyme immobilization research, but it is mainly in the fields of biological detection and fine catalytic processing. These fields do not need to print the enzyme immobilization carrier with a large volume but only make the desired shape. This is primarily due to material limitations; if you want to use extrusion-based printing, the material must have a specific rheology, which means the material’s mechanical and tensile properties may not be ideal, so the technology cannot be used to manufacture large-scale enzyme immobilization carriers. Possible solutions will be presented in the enzyme immobilization carrier materials discussed below.
Among various 3D printing technologies, the FDM and extrusion-based printing strategies are frequently used printing strategies due to their low-cost and simple operations. Additionally, the used materials of the two methods, thermoplastic polymers and hydrogels, are easily degradable and environmentally friendly. The polylactic acid used in FDM can even be recycled and reused, realizing the sustainable use of carrier materials. Compared with SLA, these two methods have a wider range of immobilized enzymes, only by changing the chemical modification method or changing the type and ratio of the hydrogel. SLA and FDM, on the other hand, can make large-scale enzyme immobilization carriers, which may be more essential for industrial manufacturing. In the extrusion-based printing approach, this is difficult to do.
3. 3D Printing Materials for Enzyme Immobilization
After decades of development, there are already many materials (including organic and inorganic materials) that can be used to immobilize enzymes. However, due to some particularities of 3D printing, many materials, such as magnetic nanoparticles, silica, and ceramics, that can be used in traditional enzyme immobilization methods are not suitable for enzyme immobilization based on 3D printing. The carrier materials suitable for the field of 3D printing generally have good rheology, so various ideal shapes and structures can be produced by the printer. Obviously, the above-mentioned nanoparticles, ceramics, etc. are not suitable for application in this new field. In short, most of the carrier materials used in emerging technologies are readily available and inexpensive materials compared to traditional methods. At the same time, compared with traditionally used small microspheres such as nanoparticles, the 3D-printed carrier is generally larger and easier to separate from the enzyme-catalyzed reaction system. Although the volume is smaller, the specific surface area is larger, and the mass transfer effect is better. However, because many of the materials used in 3D printing are porous materials, or are easily modified into porous substrates, it does not affect the mass transfer of the reaction system, and even the 3D-printed carrier is better than traditional methods. The following is an overview of many materials that have been investigated for the enzyme immobilization of 3D printing in recent years. They are simply divided into three categories: the metal–organic framework (MOF), polylactic acid (PLA), and hydrogel.
3.1. Metal–Organic Framework (MOF)
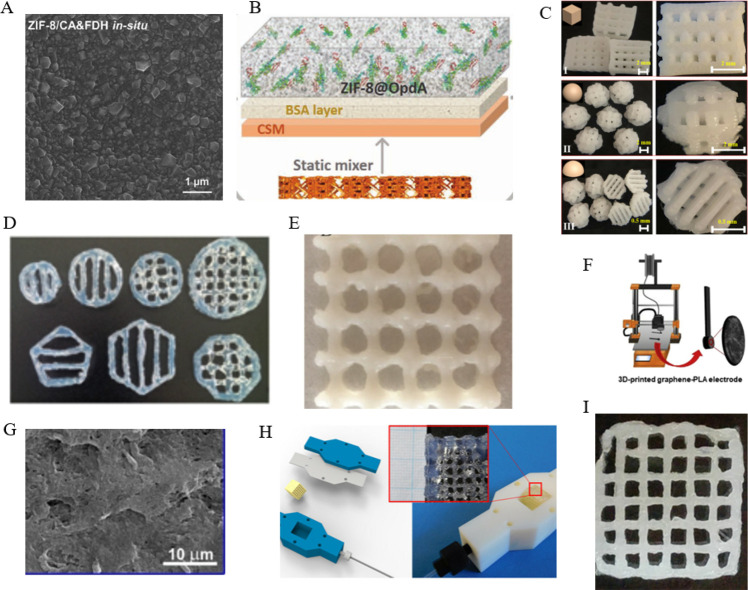
As a porous material, the metal–organic framework cannot only immobilize the enzyme and protect the rigid structure of the enzyme but also make the enzyme and the substrate fully in contact.14 It has been widely used for the research of enzyme immobilization. However, it is difficult to directly use 3D-printing technologies to make complex structures because most of them are synthesized with loose particles. In recent years, researchers have focused their attention on using metal–organic frameworks to encapsulate enzymes attached to the surface of a 3D-printed device. In 2021, Chai et al. reported a novel method that carbonic anhydrase and formate dehydrogenase are coimmobilized via biomineralization in a metal–organic framework (ZIF-8) thin membrane to fabricate the biocatalytic micromixer (Figure 2A).11c MOFs with the larger surface area and tunable porosity properties enable the loading of more enzymes than conventional carrier materials,6b,15a,15b so there is also a great research prospect in the detection of liquids, especially the detection of harmful substances in water. As shown in Figure 2B, Singh et al. also used a porous metal–organic framework to immobilize the organophosphate degrading enzyme. The inline flow device has a sophisticated 3D-printed architecture that maximizes flow and interfacial interactions between the encapsulated organophosphate degrading enzyme (OpdA) and organophosphate-based contaminants.15c Although the MOF should be of its characteristics, it cannot be directly used as a 3D printing material. However, combining the advantages of printing technology and metal–organic framework-immobilized enzymes, attaching the metal–organic framework that encapsulates the enzyme to the 3D-printed device will be a popular direction.
Figure 2.
Various 3D printing materials for enzyme immobilization. (A) SEM image of ZIF-8/carbonic anhydrase (CA) and formate dehydrogenase (FDH) in situ thin film. Reprinted with permission from ref (11c). Copyright 2021 Elsevier. (B) Image of in situ metal–organic framework growth and encapsulation process. Reprinted with permission from ref (15c). Copyright 2020 Elsevier. (C) Digital images of three types of 3D-printed polylactic acid scaffolds with adjustable aperture structures: cube (I), sphere (II), and semicircle (III). Reprinted with permission from ref (16d). Copyright 2021 Elsevier. (D) Agarose hydrogel scaffolds of different shapes and sizes can be printed. Reprinted with permission from ref (17b). Copyright 2018 John Wiley and Sons Ltd. (E) Picture of the 3D bioprinted constructs with the ratio of acrylamide: hydroxyapatite/sodium alginate = 4:1.2:1. Reprinted with permission from ref (17d). Copyright 2020 Elsevier. (F) 3D printing of the graphene-polylactic acid electrode. Reprinted with permission from ref (16a). Copyright 2020 Elsevier. (G) SEM micrographs of the carbon black-polylactic acid electrode surface after electrolysis in 1 M NaOH (−1.4 to +1.2 V vs the Ag|AgCl polarization range). Reprinted with permission from ref (16b). Copyright 2022 Elsevier. (H) Hydrogel structure is inserted in the reactor housing with connection to the fluidic system. Reprinted with permission from ref (17a). Copyright 2018 Frontiers Media S.A. (I) Top view image of the printed hydrogels using the additives Deuteron XG. Reprinted with permission from ref (17c). Copyright 2018 John Wiley and Sons Ltd.
3.2. Polylactic Acid (PLA)
Polylactic acid, as a kind of thermoplastic, has the advantages of low cost and easy recycling. It can be printed into the desired shape easily and quickly through FDM technology. Thus, more and more researchers use PLA and graphene to make bioelectrodes quickly and at a low cost through 3D printing. As shown in Figure 2F, Lopez Marzo and colleagues made a 3D printed graphene-PLA electrode that was modified by gold nanoparticles and immobilized horseradish peroxidase (HRP) to directly detect hydrogen peroxide.16a It is because polylactic acid does not conduct electricity that the printed electrodes need to be etched by enzymes16b or chemical reagents to expose the graphene inside to have electrochemical activity (Figure 2G). For example, Manzanares-Palenzuela et al. used proteinase to digest PLA of the printed graphene-PLA electrode.16c Then the resulting activated surface is used to immobilize alkaline phosphatase (ALP) enzyme for the subsequent electrochemical detection of 1-naphthol. At the same time, polylactic acid, at a predetermined temperature, has good rheological properties, so that it can be printed into various complex scaffolds for enzyme immobilization. In 2021, Zhang et al. prepared several types of 3D-printed scaffolds using carbon fiber reinforced PLA (C-PLA) to discuss the efficient resolution of racemic 1-indanol by immobilized lipase YCJ01 at high substrate concentrations. This method enhanced the performance and stabilities of lipase YCJ01 (Figure 2C).16d The reason for using carbon-reinforced polylactic acid is that it can enhance the mechanical properties of the printing stent without changing the rheology ability to print more complex structures and reduce mass transfer impact. As polylactic acid is easy to print and modify, it will be more used in the field of enzyme immobilization in the future.
In addition, as a kind of thermoplastic, polylactic acid can be easily recycled after one-time use or structural printing errors, which avoids material waste and ensures the sustainable use of materials. Furthermore, polylactic acid has good degradation properties, has been widely used in surgical sutures and packaging bags, and has no environmental pollution. At the same time, it has good rheology and mechanical properties, which enables it to be printed into various complex structures, suitable for the needs of different reactors and reaction systems. These characteristics make polylactic acid an ideal 3D printing material in the field of enzyme immobilization.
3.3. Hydrogel
Hydrogels have excellent rheological properties and are suitable for 3D printing into various complex structures. They can also maintain the spatial specificity of the enzyme by mixing the enzyme and the hydrogel for 3D printing. Therefore, various hydrogels have been developed for enzyme immobilization based on 3D printing. As shown in Figure 2H, Schmieg et al. presented a method for immobilization of enzymes (alcohol dehydrogenase, benzoylformate decarboxylase, and β-galactosidase) in poly(ethylene glycol) diacrylate hydrogel grids based on 3D printing under mild conditions. The hydrogel lattices of immobilized enzymes were put in matching housings that were also manufactured by 3D printing to measure the activity of the immobilized enzymes.17a In the other study, Maier et al. demonstrated 3D printed grid-structured carriers based on 3% (w/v) agarose hydrogels to immobilize two kinds of thermostable enzymes (including esterase and alcohol dehydrogenase from thermophilic organisms), realizing a continuous, two-step sequential biotransformation in a fluidic setup (Figure 2D).17b Although hydrogels are porous materials, long-term immobilized enzyme activity may be affected due to mass transfer limitations. Schmieg et al. developed a poly(ethylene glycol) diacrylate-based hydrogel system that was suitable for enzyme entrapment and 3D printing. Compared with the free enzyme in solution, β-galactosidase immobilized in the long-range stable hydrogel structure retained an effective activity of generally 10% (Figure 2I).17c If we want to optimize the enzyme activity, we can change the composition of the hydrogel and print more detailed and complex structures. Liu et al. developed an optimal immobilization material that is the hydrogel with the ratio of acrylamide/hydroxyapatite/sodium alginate = 4:1.2:1 using 3D printing for laccase immobilization. The immobilized laccase displayed excellent stability and reusability in the biodegradation of phenolic compounds (Figure 2E).17d At present, the hydrogel is an ideal material for immobilization of 3D-printing enzymes, but to better meet the needs of practical applications, more chemically resistant, long-term mechanical stable, and high porosity hydrogels need to be developed in the future.
In general, the great rheological properties of hydrogels make them materials for immobilizing printing enzymes, but poor mechanical properties limit the use of hydrogels. Because of the poor mechanical properties, in actual use, it will cause the leakage of the enzyme and the inability to manufacture a very complex structure, which will result in the restriction of the mass transfer between the immobilized enzyme and the substrate. One of the possible solutions to solve the above problem is to use multiple cross-linking hydrogels as enzyme immobilization carriers and printing materials. The specific idea is that the precross-linked hydrogel maintains good rheological properties so that the hydrogel can be printed and formed and then covalently cross-linked or photopolymerized again to improve the mechanical properties and structural stability of the carrier. The other solution is to optimize the composition and ratio of the hydrogel, which also provides the possibility to control the pore structure and reduce the restriction of mass transfer.
4. Enzyme Immobilization Methods of 3D Printing
In traditional enzyme immobilization, the enzyme can be physically adsorbed or covalently attached to the substrate and nanoparticles. That is because many materials can be used for enzyme immobilization. Compared with traditional methods, printing enzyme immobilization mainly uses two types of materials: polylactic acid and hydrogels. Correspondingly, there are two main methods for printing enzyme immobilization: covalent attachment and physical entrapment.
4.1. Physical Entrapment
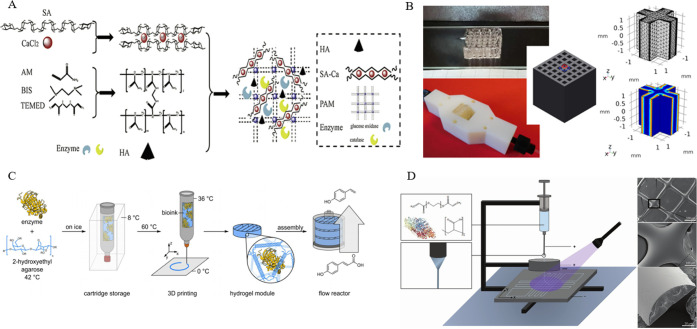
Not only can the process of physical entrapment maintain the spatial configuration of the enzyme in the porous material, but also the enzyme and the material do not need to be complicatedly modified. Therefore, when the hydrogel is used as a printing material for enzyme immobilization, the immobilization method is usually physical entrapment. As shown in Figure 3C, Peng et al. mixed enzymes with a liquid agarose solution. Through 3D printing, the bioink solidified to form biocatalytic modules that phenacrylate decarboxylase enzymes were entrapped in agarose hydrogel.18a The enzyme catalytic effect and the amount of immobilized enzyme are also closely related. When the enzyme catalytic effect is not very good, biocatalytic modules can be physically added to improve the catalytic effect. Of course, it is best to optimize the composition and ratio of the hydrogel and three-dimensional printing technology to increase enzyme loading. At the same time, the structural design of the enzyme immobilization carrier is also a major influencing factor. Schmieg and his co-workers used 3D-printed lattices to entrap enzymes. Then a 3D-simulation approach was developed to estimate, within the available design space, the optimization potential of varying the 3D-printed geometries and the reaction rate (Figure 3B).18b As discussed earlier, although the physical entrapment of the enzyme inside a matrix has the advantages of simplicity and speed, the enzyme activity will be affected due to the limitation of mass transfer. To eliminate this limitation as much as possible, the researchers used more advanced printing technology to create a more complex and better porosity hydrogel network. In 2019, Shen et al. reported a hybrid interpenetrating polymer network hydrogel to entrap glucose oxidase and catalase in suit, which is through one-pot preparation of the immobilized enzymes based on 3D printing. Moreover, the immobilized enzymes exhibited operational stability and repeatability that a high conversion of 97% was maintained after reuse in four batches (Figure 3A).18c In 2020, Steier and cooperators investigated the entrapment of enzymes into polymer hydrogels via 3D jet writing to enhance the stability of enzymes. In fact, the immobilized enzyme does show good activity (21.2% activity relative to the free enzyme) and stability (in a continuous reactor for 76 h with a stable turnover of 50%) (Figure 3D).18d
Figure 3.
(A) Production of immobilized glucose oxidase/catalase by 3D bioprinted hybrid interpenetrating polymer network hydrogel. Reprinted with permission from ref (18c). Copyright 2019 Elsevier. (B) Picture of 3D-printed lattices for the physical entrapment of enzymes. Reprinted with permission from ref (18b). Copyright 2020 Frontiers Media S.A. (C) Manufacturing process of agarose-based, compartmentalized biocatalytic flow reactors. Reprinted with permission from ref (18a). Copyright 2019 John Wiley and Sons Ltd. (D) 3D jet writing of hydrogel fibers allows yielding precisely oriented hydrogel fibers loaded with enzymes. Reprinted with permission from ref (18d). Copyright 2020 John Wiley and Sons Ltd.
To summarize, the ease with which enzymes may be entrapped in hydrogels and their general applicability is appealing, especially if the enzyme-loaded hydrogel can be 3D-printed into geometries that are optimal for the application. However, physical immobilization of enzymes has the drawback of probable leakage if the matrix disintegrates as well as a diffusion constraint that is not present in the solution. In response to this problem, possible solutions have been proposed above.
4.2. Covalent Connection
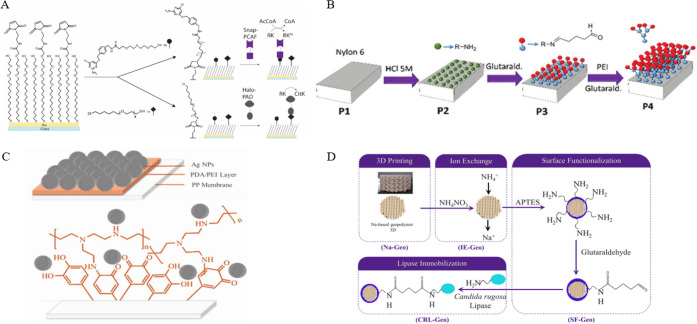
Compared with physical entrapment, the immobilization of the enzyme by covalent connection can make the enzyme more stably attached to the substrate, and the immobilized enzyme will not be leached out due to the fragmentation of the substrate material. Therefore, the covalent connection is used in some continuous flow reactions. Grant et al. fabricated the microfluidic chip that has one enzyme covalently patterned on the chip surface and the second enzyme patterned in a downstream region of the channel from the mold of 3D-printed polydimethylsiloxane (Figure 4A).19a In another study, Changani et al. developed a paper-based microfluidic-membrane based biosensor using the mold of 3D printed polydimethylsiloxane which covalently immobilizes three kinds of enzymes, including α-amylase, glucose oxidase (GOx), and horseradish peroxidase (HRP), to detect starch (Figure 4C).19b Under normal circumstances, the enzyme immobilization of the microfluidic reaction adopts the covalent connection method because the reactor itself is small, and the covalent connection method can be used to immobilize the enzyme at a specific position. The process only needs to be chemically modified at the ideal position to be easily controlled, and the enzyme is closely connected with the substrate and will not leak, which makes postreaction processing simple. Although the covalent connection has high stability, the 3D-printed matrix often needs to be modified and functionalized in multiple steps to be able to immobilize the enzyme. For example, as shown in Figure 4B, Peris et al. presented a method that the 3D-printed nylon-6 generates covalent link points for ω-transaminase (ω-TA) enzymes through the modification of HCl, glutaraldehyde, and polyethylenimine to covalently immobilize enzymes on 3D printed continuous-devices.19c Rewatkar et al. reported lattice-shaped sodium-based geopolymers of 3D printing, via surface functionalization of 3-aminopropyltriethoxysilane and modification of glutaraldehyde, to realize the covalent immobilization of the Candida rugosa lipase (Figure 4D).19d Of course, the free carboxyl groups of some printing materials can also be used, and the enzyme can be immobilized by simple modification. For instance, Wang et al. immobilized the glucose oxidase enzyme on the 3D-printed graphene-PLA electrode by covalent reaction between the carboxyl and amino groups in order to obtain a highly stable biosensor.19e In short, the 3D printing material used corresponds to the enzyme immobilization method. When using hot-melt materials as enzyme immobilization substrates, the surface of the material needs to be modified and functionalized to immobilize the enzyme covalently.
Figure 4.
(A) Strategy for acetyltransferase p300/CBP associated factor (PCAF) and peptidylarginine deiminase type 1 (PAD) covalent immobilization. Reprinted with permission from ref (19a). Copyright 2018 John Wiley and Sons Ltd. (B) 3D-printed nylon part undergoes several modifications to covalently immobilize enzymes. Reprinted with permission from ref (19c). Copyright 2017 Royal Society of Chemistry. (C) Schematic of the silver nanoparticles coated on the polypropylene/polydopamine/polyethylenimine membrane used for covalent immobilization of the glucose oxidase and horseradish peroxidase enzymes. Reprinted with permission from ref (19b). Copyright 2020 John Wiley and Sons Ltd. (D) Sequential stages for covalent immobilization of Candida rugosa lipase on lattice geopolymers. Reprinted with permission from ref (19d). Copyright 2021 Elsevier.
As mentioned above, the two enzyme immobilization methods, including physical entrapment and covalent connection, have their advantages and disadvantages. Physical entrapment is simple and induces less damage to the native rigid structure of enzymes, but there are problems of mass transfer limitation and enzyme leakage. For the covalent connection, printed carriers need to be modified by several chemical reagents, which requires optimization of the immobilization conditions. At the same time, enzymes will stably attach to the substrate with a covalent connection. Moreover, the enzyme immobilization method and the materials used and the 3D printing method make the corresponding under normal circumstances. Therefore, these factors should be considered comprehensively when conducting printing enzyme immobilization research and applications.
5. Enzyme Immobilization Applications of 3D Printing
Enzyme immobilization of 3D printing has become a promising candidate for industrial applications in enzyme cascade and biomedical applications in biosensing. To meet the needs of different applications, different enzymes need to be fixed to various materials of 3D printing in different ways. The experimental conditions involved will affect the final enzyme activity and applications. Table 1 lists some different applications that have been reported in recent years with their using materials, fabricating methods and immobilized enzymes. Based on this, we divide the applications of enzyme immobilization based on 3D printing into industrial and biomedical applications.
Table 1. Examples of Different Applications.
| material | 3D-printed technology | immobilized enzyme | immobilization method | application | refs |
|---|---|---|---|---|---|
| hydrogel | extrusion-based | phenacrylate decarboxylase | entrapment | synthetic p-vinylphenol | (18a) |
| ABS | FDM | glucose oxidase, lactate oxidase | covalent | monitor glucose and lactate | (12c) |
| hydrogel | extrusion-based | laccase | entrapment | biodegradation of phenolic compounds | (17d) |
| ABS | FDM | glucose oxidase | glucose testing | (12d) | |
| polycaprolactone | FDM | amano lipase | entrapment | biodegradable plastics | (20a) |
| graphene/PLA | FDM | glucose oxidase | covalent | biosensing | (21f) |
| nylon-6 | extrusion-based | ω-transaminase | covalent | kinetic resolution of 1-methylbenzylamine | (19c) |
| graphene/PLA | FDM | alkaline phosphatase | covalent | detection of 1-naphthol | (16c) |
5.1. Industrial Applications
The 3D printing operation is fast and straightforward, and the 3D printing materials are usually relatively low-cost and easy to obtain, making the immobilization of printing enzymes very suitable for batch production of some industrial-use items, especially enzyme-catalyzed biofuel cells. In 2019, Prakash and cooperators designed and fabricated a miniaturized microfluidic enzymatic biofuel cell by covering pencil graphite electrodes in carboxylated multiwalled carbon nanotubes, the covalent immobilization of glucose oxidase and laccase enzymes, and encapsulated into Y-shaped microchannel using 3D printing technology (Figure 5A).20b As shown in Figure 5B, Rewatkar et al. also reported a rapid, cost-effective, and novel method to manufacture bioelectrodes for biofuel cells. The method is that 3D-printed bioelectrodes using composite graphene/PLA, via the modification of dimethylformamide solution, immobilize glucose oxidase and laccase as a bioanode and biocathode.20c As discussed above, neither polylactic acid nor graphene alone can be used as a 3D printed bioelectrode. However, when the two materials are mixed and used as a composite material, it cannot only ensure the rheology during printing but also maintain the good electrical conductivity and mechanical properties that must be possessed as an electrode. In addition to using the more researched graphite and polylactic acid as small-scale biofuel cell electrodes, paper-based electrodes can also be used. For example, Rewatkar et al. reported an optimized shelf-stacked paper origami-based glucose biofuel cell with immobilized enzymes (glucose oxidase and laccase).20d By optimizing the stack structure of the electrodes, the volume of the enzymatic fuel cell can be effectively reduced, providing new ideas for the design of miniaturized, portable enzyme-catalyzed biofuels.
Figure 5.
(A) Experimental setup of pencil graphite electrodes based on 3D printed enzymatic biofuel cell. Reprinted with permission from ref (20b). Copyright 2019 Elsevier Ltd. (B) Final structure optimized 3D printed bioelectrodes of glucose oxidase or laccase immobilization. Reprinted with permission from ref (20c). Copyright 2020 Institute of Electrical and Electronics Engineers Inc. (C) Preparation routes of 3D-printed xylanase which is applied to digest the corn cob reaction system enzymatically. Reprinted with permission from ref (21c). Copyright 2020 Elsevier. (D) Preparation routes of 3D-printed aldo-keto reductases-IA. Reprinted with permission from ref (21d). Copyright 2022 Elsevier. (E) Image of 3D-printed labware. Reprinted from ref (21a). Copyright 2020 American Chemical Society. (F) Photograph of the polycaprolactone-modified paper immobilized α-glucosidase after cutting. Reprinted with permission from ref (21b). Copyright 2019 BioMed Central Ltd.
In general, traditional batteries, as a renewable and sustainable power supply method, require the use of strong acids and heavy metal salts in the production process and have greater pollution to the environment. The polylactic acid, graphene, and paper-based electrodes used in producing the printed enzymatic biofuel cell have no impact on the environment. The biofuel cell currently being studied can already power small electronic devices and biosensors, the use of printing rapid prototyping is cost-effective, and the biofuel cell is portable. What needs to be done now is to optimize the electrode material, immobilized enzyme, and structure design to meet the needs of different output powers and volumes.
Because of the easy operability of printing, immobilization of printing has also been applied to rapid screening in the industrial field. In 2020, Spano et al. reported 3D-printed labware for high-throughput immobilization of enzymes (including alkaline phosphatase, glucose dehydrogenase, and laccase) which may solve the screening to optimize the immobilization of each enzyme in continuous flow (Figure 5E).21a One of the difficulties in the industrial application of enzymes is that to obtain the best catalytic efficiency, it is necessary to optimize the immobilization conditions between each enzyme and the immobilized carrier, including the carrier composition, reaction temperature, and protective solution. This work is time-consuming and laborious. The 3D-printing equipment greatly shortens the experiment time through parallel weighing and multichannel solid handling. Similarly, the traditional screening process of natural active ingredients is also very cumbersome. Enzyme immobilization of 3D printing can simplify the screening process through the specificity of the enzyme reaction, the simplicity and low cost of the production process, and reduce the production cost. For instance, Guo et al. designed and fabricated polycaprolactone-chitosan-modified paper prepared with the assistance of 3D printing technology. α-Glucosidase was immobilized on the modified paper to screen bioactive compounds in mulberry leaves and lotus leaves (Figure 5F).21b In other industrial fields, enzyme immobilization of 3D printing also shows exciting application potential. For example, Jiang and colleagues immobilized xylanase on the sodium alginate (SA) microspheres using 3D printing technology, which are applied to decompose lignocellulose of corn cobs. Additionally, the SA/xylanase microspheres showed great catalytic activity and reusability, which demonstrated the excellent potential of the application in industrial production (Figure 5C).21c Corn cobs and crop stalks have lignocellulose, which can be degraded naturally, but it takes a long time and pollutes water resources and the environment. The use of three-dimensional printing technology to immobilize enzymes to degrade lignocellulose to produce xylose has the advantages of low cost and high added value. It can also effectively avoid the high cost and pollution conditions (high temperature and acid conditions) of traditional production methods. At the same time, because of the low 3D-printing cost and the specificity of enzyme catalysis, there are also reports in some industrial chiral catalysis fields. As shown in Figure 5D, Pei et al. fabricated the aldehyde ketone reductase AKR-IA/biocompatible polymer materials using the 3D printing strategy. The biocatalyst not only has excellent recyclability and enzyme activity but also can be printed in various shapes to adapt to various catalytic environments, which showed certain application potential in preparing the antidepressant duloxetine intermediate S–N, N-dimethyl-3-hydroxy-3-(2-thienyl)-1-propanamine.21d In addition to the manufacturing of chiral species, chiral species identification is a difficult task. Muñoz et al. devised an unprecedented electrochemical approach in which the class-enzyme l-amino acid oxidase was immobilized on a 3D-printed nanocomposite carbon electrode and used as the proof chiral selector for fabricating an unprecedented chiral 3D-printed bioelectrode via electrochemical impedance spectroscopy.21e
In the chemical sector, three-dimensional printing enzyme immobilization offers great potential. Three-dimensional printing can produce a variety of sophisticated reactors, allowing it to match the needs of actual production better. Chai et al., for example, created a unique micromixer with a 3D-printed helical, threaded channel immobilized carbonic anhydrase and formate dehydrogenase, resulting in a 3-fold increase in formic acid yield over a standard bubble column.11c The reason for such high production efficiency may be that the 3D-printed micromixer can enhance the mass transfer of reactants and product in an enzymatic cascade reaction converting CO2 to formic acid. Similarly, Ye et al. used 3D printing to create complex spherical scaffolds to immobilize penicillin G acylase and glycosidase, resulting in high reaction yields.12b The results show that the more complex enzyme immobilization carrier can be employed in solid–liquid reaction systems and systems with high viscosity without affecting the mass transfer of the immobilized enzyme. Finally, water pollution is also one of the challenges facing the world today. 3D-printed enzyme immobilization offers the possibility for mild and inexpensive solutions to water pollution due to its customizable, low-cost, and sustainable advantages. Singh et al. 3D-printed a catalytic static mixer (CSM) coated in a MOF@organophosphate degrading enzyme complex which not only detects organophosphorus contamination in water within a few days but also continuously decomposes organic contamination.16a In addition to organic pollutants in water, drug pollution is also severe. Xu et al. designed and developed a novel system for removing drugs from water.21f The device, fabricated with stereolithography (SLA) 3D printing and immobilized laccase, can effectively remove 95% of diclofenac and ethinylestradiol from aqueous solution within 24 and 2 h. The biggest advantage of using the 3D printing method for enzyme immobilization to remove pollutants in water is that different treatment devices can be designed according to the environment (such as pipes or machinery). After all, 3D printing can quickly change the printing structure according to different modeling.
5.2. Biomedical Applications
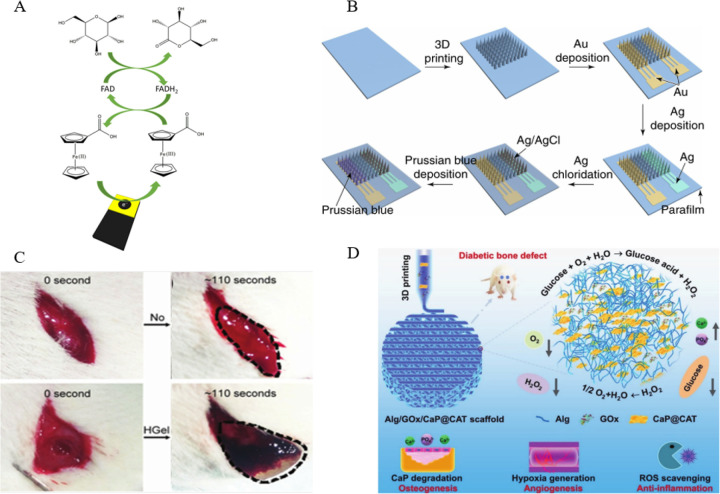
With the improvement of people’s living standards, diabetes has become a major disease endangering human health. Therefore, the convenient detection of glucose level has attracted the attention of more and more researchers.22a The enzyme immobilization based on 3D printing technology can quickly manufacture bioelectrodes at a low cost, which provides a reliable direction for realizing this demand. Gardoso et al. demonstrated 3D-printed graphene/polylactic acid electrodes, immobilized enzymes on the surface, which are used as biosensors to realize biosensing of glucose and simultaneous determination of uric acid and nitrite in biological fluids (Figure 6A).22b In another study, 3D-printed microneedles immobilized glucose oxidase, achieving continuous detection of blood glucose concentration. As shown in Figure 6B, Liu et al. manufactured an integrated and 3D-printed microneedle biosensing device for painless and continuous monitoring of interstitial glucose. This method has been obtained in normal mice and diabetic mice, which is highly correlated with the test results of commercial blood glucose meters.22c The electrochemical strategy of the immobilized enzyme microneedle can continuously monitor the blood glucose level for a long time, effectively avoiding the pain of taking blood from the finger every day. Also using electrochemical methods, Wang et al. prepared a glucose oxidase-based 3D-printed carbon nanoelectrode biosensor to detect two biomarkers of hydrogen peroxide and glucose by chronoamperometry.19e In addition to the above-mentioned electrochemical methods, optical correlation detection methods are also commonly used to detect glucose levels. Su et al. employed fused deposition modeling-type three-dimensional printing and two functionalized ABS filaments incorporating peroxidase-mimicking iron oxide (Fe3O4) nanoparticles, glucose oxidase, and the chromogenic substrate o-phenylenediamine for the one-step manufacture of enzyme/substrate-incorporated multiwell plates.12d When in use, it is only loaded into a microplate reader for colorimetric observation with the naked eye or absorbance measurements. In short, these two methods have their advantages. The electrochemical method has a low detection limit and high sensitivity, while the optical colorimetric method is simpler to make and suitable for areas with limited resources. In disease treatment areas, 3D-printed enzyme fixation scaffolds have also shown promise in the field of diabetic bone regeneration, according to studies.22d,22eFigure 6D shows a multifunctional scaffold composed of alginate, glucose oxidase (GOx), and catalase-assisted biomineralized calcium phosphate nanosheets (CaP@CAT NSs). In diabetic situations, a 3D printed enzyme-functionalized scaffold with numerous bioactivities such as osteogenesis, angiogenesis, and anti-inflammation is proposed by Yang and cooperators.22f The innovative solution fixes two enzymes on the implantable scaffold, and the application of the enzyme cascade reaction can effectively solve the problem of difficult fracture repair in diabetic patients. In other biomedical fields, the enzyme immobilization of 3D printing has also been studied. For example, Wei et al. used dual-enzyme-triggered self-assembly and polymerization to create a hybrid hydrogel of guanidinium-containing oligopeptide. The hybrid hydrogel’s time-dependent strength might allow for an extended time window for in situ 3D printing and molding. As a result, this biodegradable hybrid hydrogel might be used as a printed scaffold material for cell culture, hemostasis, and tissue engineering in the future (Figure 6C).22g
Figure 6.
(A) Representation of the 3D-printed glucose oxidase biosensor. Reprinted with permission from ref (22b). Copyright 2020 Elsevier. (B) Schematic illustration of the preparation process for the biosensor. Reprinted with permission from ref (22c). Copyright 2021 Nature Publishing Group. (C) Hemostatic photo images within 110 s: a negative control group without treatment (upper), the hemostatic effect of hybrid hydrogel (Hgel) (lower). Reprinted with permission from ref (22g). Copyright 2016 Royal Society of Chemistry. (D) Schematic illustration of 3D printed alginate/glucose oxidase/catalase-assisted biomineralized calcium phosphate nanosheets scaffolds. Reprinted with permission from ref (22f). Copyright 2021 Wiley-VCH Verlag.
Currently, there are not many research studies on the immobilization of 3D-printed enzymes in medicine. The main reason is that whether it is an enzyme immobilization stent implanted in the body or an in vitro dressing, patch, etc., the matrix material used must have good biocompatibility. However, there are few materials with good biocompatibility, rheology, printability, and mechanical properties at the same time. Of course, there are other reasons. For example, suitable organisms have a complex microenvironment and have obvious rejection reactions to foreign substances. As a result, there are not many enzymes suitable for application in the field of biomedicine. Therefore, it is necessary to develop more printing materials suitable for medical applications and to screen more enzymes suitable for biomedical applications.
6. Conclusions and Perspectives
The most important part of this review includes examples of immobilized enzymes that were immobilized on the scaffolds of 3D printing and their industrial and biomedical applications. It is because of technological innovations that 3D printing has become a convenient, efficient, and economical tool for enzyme immobilization, thus promoting its functions in biological power generation, biotransformation, and biosensing. Over the past decade, 3D printing has made great progress in enzyme immobilization. However, several challenges also should be dedicated to promoting the technology becoming a powerful tool for enzyme immobilization.
First, although many papers about enzyme immobilization based on 3D printing have been reported, there are relatively few materials with ideal effects for the immobilization of 3D printing enzymes in general. Moreover, it is complicated to modify the enzyme linking group on the surface of polylactic acid (PLA) that was broadly used in the field of enzyme immobilization of 3D printing. These challenges make the scope of application of this technology relatively limited. Therefore, to obtain high protein loading and immobilization efficiency and more applications, the printing materials, the modification methods, and the linkage approaches of 3D printing enzymes should be further investigated and studied.
Second, it will have the best activity when the enzyme is at its optimum reaction temperature. When the temperature is too high or too low, the catalytic activity of the enzyme will decrease. Compared to other 3D printing methods, fused deposition modeling (FDM) technology has the advantages of being simple and low cost that led to the methods being widely used in enzyme immobilization based on 3D printing. However, FDM is based on extrudable thermoplastics, such as acrylonitrile-butadiene-styrene (ABS) and polylactic acid (PLA), which results in the need for very high temperatures. The mechanical of FDM may not only influence enzyme activity but also directly inactivate enzymes. According to the practical applications, on the one hand, we can explore 3D printing methods with low printing temperature (such as direct ink writing and extrusion-based 3D printing); on the other hand, we need to screen more thermostable enzymes for different applications.
Third, the three-dimensional printing enzyme immobilization carrier and enzyme immobilization method can be further improved. Because most 3D-printed immobilized enzymes are less efficient than normal free enzymes. The reason for this could be that the unreasonable printing structure prevents mass transfer between the enzyme and the substrate as well as conformational changes in the enzyme caused by the printing process, and the enzyme’s active site is partially blocked during the incorrect immobilization process. Improved technology for designing and manufacturing finer and more inventive enzyme immobilization carriers, as well as more reasonable enzyme immobilization procedures, can reduce immobilized enzyme and substrate mass transfer and improve immobilized enzyme activity. Therefore, it is necessary to further optimize the 3D printing carrier technology for enzyme immobilization and the enzyme immobilization method.
Fourth, great efforts should be devoted to researching enzymes suitable for biomedical applications in 3D-printed enzyme immobilization. A thorough examination of the published application directions for enzyme immobilization of 3D printing reveals that the majority of them are for industrial use, with only a few studies in medical detection and illness therapy. However, owing to its low cost and ease of manufacture, this developing technology is ideal for rapid and high-throughput screening of disease biomarkers in clinical samples. At the same time, it is ideal for wound care and the creation of implantable medical devices because 3D printing can work with a wide range of biocompatible materials. However, investigations on the aforementioned applications are scarce. One factor could be that there are few studies on enzymes that are acceptable for biological applications. As a result, soon, researchers will need to test new enzymes acceptable for medicinal uses.
In conclusion, the development of 3D printing technology in enzyme immobilization brings exciting opportunities to expand the industrial and biomedical applications of enzyme immobilization. We do believe that with further investigations and optimizations, the enzyme immobilization based on 3D printing will enable higher efficiency of immobilization, lower cost, simpler immobilization step, and more practical applications, which is difficult to achieve in other immobilization approaches.
Acknowledgments
We gratefully acknowledge financial support from the Natural Science Foundation of Jiangsu Province (Grant BK20200703), the National Key R&D Program of China (Grant 2019YFA0905200), the National Natural Science Foundation of China (Grant 32101118), Natural Science Research of Jiangsu Higher Education Institutions of China (Grant 20KJB416011), and the special funds for the introduction of talents of Nanjing Tech University (Grant 39828122).
Biographies
Yun Shao received his B.S. degree from Nanjing Tech University in 2021 and currently is pursuing a Master’s degree in the Nanjing Tech University team led by Prof. Bingfang He. His research interests are enzyme immobilization, 3D printing, and osteoarthritis treatment.
Zhijun Liao is currently studying at Nanjing University of Technology and is on the team of Associate Professor Gao Bingbing. His research interests are electrochemical microneedles, 3D printing, and laser engraving machines.
Bingbing Gao is currently working at Nanjing Tech University as an Associate Professor. In 2013, he joined Prof. Zhongze Gu’s group and received his Ph.D. degree in biomedical engineering at Southeast University in 2017. Then he continued his research work as postdoctoral researcher at Southeast University for 2 years. His current scientific interests are focused on paper microfluidics, paper-based organ chips, flexible electronics, and wearable devices.
Bingfang He earned her B.S. degree in pharmaceutical chemistry and Master’s degree in biochemistry from the China Pharmaceutical University. She received her Ph.D. in microbiology from University of Tsukuba at 2001. Bingfang He is currently a Professor at the School of Pharmaceutical Sciences, Nanjing Tech University. Her research interests include extremophiles, molecular biology, enzyme design and protein engineering, biochemical pharmacy, enzyme catalytic mechanism, biocatalysis, and biotechnological production of natural products.
The authors declare no competing financial interest.
References
- a Xia W.; Zhang K.; Su L.; Wu J. Microbial starch debranching enzymes: Developments and applications. Biotechnol. Adv. 2021, 50, 107786–107805. 10.1016/j.biotechadv.2021.107786. [DOI] [PubMed] [Google Scholar]; b Yang H.; Cai G.; Lu J.; Gomez Plaza E. The production and application of enzymes related to the quality of fruit wine. Crit. Rev. Food Sci. Nutr. 2021, 61 (10), 1605–1615. 10.1080/10408398.2020.1763251. [DOI] [PubMed] [Google Scholar]; c Zhang Y.; He S.; Simpson B. K. Enzymes in food bioprocessing — novel food enzymes, applications, and related techniques. Curr. Opin. Food Sci. 2018, 19, 30–35. 10.1016/j.cofs.2017.12.007. [DOI] [Google Scholar]; d Zhou Z.; Austin G. L.; Shaffer R.; Armstrong D. D.; Gentry M. S. Antibody-Mediated Enzyme Therapeutics and Applications in Glycogen Storage Diseases. Trends Mol. Med. 2019, 25 (12), 1094–1109. 10.1016/j.molmed.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Huang W.-C.; Wang W.; Xue C.; Mao X. Effective Enzyme Immobilization onto a Magnetic Chitin Nanofiber Composite. ACS Sustain. Chem. Eng. 2018, 6 (7), 8118–8124. 10.1021/acssuschemeng.8b01150. [DOI] [Google Scholar]; b Ribeiro E. S.; de Farias B. S.; Sant’Anna Cadaval Junior T. R.; de Almeida Pinto L. A.; Diaz P. S. Chitosan-based nanofibers for enzyme immobilization. Int. J. Biol. Macromol. 2021, 183, 1959–1970. 10.1016/j.ijbiomac.2021.05.214. [DOI] [PubMed] [Google Scholar]; c Verma M. L.; Kumar S.; Das A.; Randhawa J. S.; Chamundeeswari M. Chitin and chitosan-based support materials for enzyme immobilization and biotechnological applications. Environ. Chem. Lett. 2020, 18 (2), 315–323. 10.1007/s10311-019-00942-5. [DOI] [Google Scholar]
- a Farmakes J.; Schuster I.; Overby A.; Alhalhooly L.; Lenertz M.; Li Q.; Ugrinov A.; Choi Y.; Pan Y.; Yang Z. Enzyme Immobilization on Graphite Oxide (GO) Surface via One-Pot Synthesis of GO/Metal-Organic Framework Composites for Large-Substrate Biocatalysis. ACS Appl. Mater. Interfaces 2020, 12 (20), 23119–23126. 10.1021/acsami.0c04101. [DOI] [PubMed] [Google Scholar]; b Gkantzou E.; Govatsi K.; Chatzikonstantinou A. V.; Yannopoulos S. N.; Stamatis H. Development of a ZnO Nanowire Continuous Flow Microreactor with β-Glucosidase Activity: Characterization and Application for the Glycosylation of Natural Products. ACS Sustain. Chem. Eng. 2021, 9 (22), 7658–7667. 10.1021/acssuschemeng.1c02557. [DOI] [Google Scholar]; c Pan Y.; Li H.; Lenertz M.; Han Y.; Ugrinov A.; Kilin D.; Chen B.; Yang Z. One-pot synthesis of enzyme@metal–organic material (MOM) biocomposites for enzyme biocatalysis. Green Chem. 2021, 23 (12), 4466–4476. 10.1039/D1GC00775K. [DOI] [Google Scholar]; d Patel S. K. S.; Choi H.; Lee J.-K. Multimetal-Based Inorganic–Protein Hybrid System for Enzyme Immobilization. ACS Sustain. Chem. Eng. 2019, 7 (16), 13633–13638. 10.1021/acssuschemeng.9b02583. [DOI] [Google Scholar]; e Cui J.; Ren S.; Sun B.; Jia S. Optimization protocols and improved strategies for metal-organic frameworks for immobilizing enzymes: Current development and future challenges. Coord. Chem. Rev. 2018, 370, 22–41. 10.1016/j.ccr.2018.05.004. [DOI] [Google Scholar]
- a Liu D.-M.; Chen J.; Shi Y.-P. Advances on methods and easy separated support materials for enzymes immobilization. TrAC, Trends Anal. Chem. 2018, 102, 332–342. 10.1016/j.trac.2018.03.011. [DOI] [Google Scholar]; b Pinheiro B. B.; Rios N. S.; Rodriguez Aguado E.; Fernandez-Lafuente R.; Freire T. M.; Fechine P. B. A.; Dos Santos J. C. S.; Goncalves L. R. B. Chitosan activated with divinyl sulfone: a new heterofunctional support for enzyme immobilization. Application in the immobilization of lipase B from Candida antarctica. Int. J. Biol. Macromol. 2019, 130, 798–809. 10.1016/j.ijbiomac.2019.02.145. [DOI] [PubMed] [Google Scholar]; c Tang W.; Ma T.; Zhou L.; Wang G.; Wang X.; Ying H.; Chen C.; Wang P. Polyamine-induced tannic acid co-deposition on magnetic nanoparticles for enzyme immobilization and efficient biodiesel production catalysed by an immobilized enzyme under an alternating magnetic field. Catal. Sci. Technol. 2019, 9 (21), 6015–6026. 10.1039/C9CY01350D. [DOI] [Google Scholar]; d Cui J.; Zhao Y.; Liu R.; Zhong C.; Jia S. Surfactant-activated lipase hybrid nanoflowers with enhanced enzymatic performance. Sci. Rep. 2016, 6, 27928–27940. 10.1038/srep27928. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Cui J. D.; Jia S. R. Optimization protocols and improved strategies of cross-linked enzyme aggregates technology: current development and future challenges. Crit. Rev. Biotechnol. 2015, 35 (1), 15–28. 10.3109/07388551.2013.795516. [DOI] [PubMed] [Google Scholar]
- a Liu T.; Yin Y.; Yang Y.; Russell T. P.; Shi S. Layer-by-Layer Engineered All-Liquid Microfluidic Chips for Enzyme Immobilization. Adv. Mater. 2022, 34 (5), 2105386–2105392. 10.1002/adma.202105386. [DOI] [PubMed] [Google Scholar]; b Valikhani D.; Bolivar J. M.; Viefhues M.; McIlroy D. N.; Vrouwe E. X.; Nidetzky B. A Spring in Performance: Silica Nanosprings Boost Enzyme Immobilization in Microfluidic Channels. ACS Appl. Mater. Interfaces 2017, 9 (40), 34641–34649. 10.1021/acsami.7b09875. [DOI] [PubMed] [Google Scholar]; c Barbosa O.; Ortiz C.; Berenguer-Murcia A.; Torres R.; Rodrigues R. C.; Fernandez-Lafuente R. Strategies for the one-step immobilization-purification of enzymes as industrial biocatalysts. Biotechnol. Adv. 2015, 33 (5), 435–456. 10.1016/j.biotechadv.2015.03.006. [DOI] [PubMed] [Google Scholar]; d Obst F.; Mertz M.; Mehner P. J.; Beck A.; Castiglione K.; Richter A.; Voit B.; Appelhans D. Enzymatic Synthesis of Sialic Acids in Microfluidics to Overcome Cross-Inhibitions and Substrate Supply Limitations. ACS Appl. Mater. Interfaces 2021, 13 (41), 49433–49444. 10.1021/acsami.1c12307. [DOI] [PubMed] [Google Scholar]; e Peschke T.; Skoupi M.; Burgahn T.; Gallus S.; Ahmed I.; Rabe K. S.; Niemeyer C. M. Self-Immobilizing Fusion Enzymes for Compartmentalized Biocatalysis. ACS Catal. 2017, 7 (11), 7866–7872. 10.1021/acscatal.7b02230. [DOI] [Google Scholar]; f Cui J.; Ren S.; Lin T.; Feng Y.; Jia S. Shielding effects of Fe3+-tannic acid nanocoatings for immobilized enzyme on magnetic Fe3O4@silica core shell nanosphere. Chem. Eng. J. 2018, 343, 629–637. 10.1016/j.cej.2018.03.002. [DOI] [Google Scholar]; g Cui J.; Jia S. Organic–inorganic hybrid nanoflowers: A novel host platform for immobilizing biomolecules. Coord. Chem. Rev. 2017, 352, 249–263. 10.1016/j.ccr.2017.09.008. [DOI] [Google Scholar]
- a Chen G.; Zhang H.; Wang H.; Wang F. Immune tolerance induced by immune-homeostatic particles. Engineered Regeneration 2021, 2, 133–136. 10.1016/j.engreg.2021.09.007. [DOI] [Google Scholar]; b Feng Y.; Hu H.; Wang Z.; Du Y.; Zhong L.; Zhang C.; Jiang Y.; Jia S.; Cui J. Three-dimensional ordered magnetic macroporous metal-organic frameworks for enzyme immobilization. J. Colloid Interface Sci. 2021, 590, 436–445. 10.1016/j.jcis.2021.01.078. [DOI] [PubMed] [Google Scholar]; c Liu Q.; Chapman J.; Huang A.; Williams K. C.; Wagner A.; Garapati N.; Sierros K. A.; Dinu C. Z. User-Tailored Metal-Organic Frameworks as Supports for Carbonic Anhydrase. ACS Appl. Mater. Interfaces 2018, 10 (48), 41326–41337. 10.1021/acsami.8b14125. [DOI] [PubMed] [Google Scholar]; d Liu X.; Qi W.; Wang Y.; Su R.; He Z. A facile strategy for enzyme immobilization with highly stable hierarchically porous metal-organic frameworks. Nanoscale 2017, 9 (44), 17561–17570. 10.1039/C7NR06019J. [DOI] [PubMed] [Google Scholar]; e Nadar S. S.; Rathod V. K. Magnetic-metal organic framework (magnetic-MOF): A novel platform for enzyme immobilization and nanozyme applications. Int. J. Biol. Macromol. 2018, 120 (Part B), 2293–2302. 10.1016/j.ijbiomac.2018.08.126. [DOI] [PubMed] [Google Scholar]; f Wang X.; Lan P. C.; Ma S. Metal-Organic Frameworks for Enzyme Immobilization: Beyond Host Matrix Materials. ACS Cent. Sci. 2020, 6 (9), 1497–1506. 10.1021/acscentsci.0c00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Conte M. P.; Sahoo J. K.; Abul-Haija Y. M.; Lau K. H. A.; Ulijn R. V. Biocatalytic Self-Assembly on Magnetic Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10 (3), 3069–3075. 10.1021/acsami.7b15456. [DOI] [PubMed] [Google Scholar]; b Ellis G. A.; Diaz S. A.; Medintz I. L. Enhancing enzymatic performance with nanoparticle immobilization: improved analytical and control capability for synthetic biochemistry. Curr. Opin. Biotechnol. 2021, 71, 77–90. 10.1016/j.copbio.2021.06.021. [DOI] [PubMed] [Google Scholar]; c Engelmann C.; Ekambaram N.; Johannsen J.; Fellechner O.; Waluga T.; Fieg G.; Liese A.; Bubenheim P. Enzyme Immobilization on Synthesized Nanoporous Silica Particles and their Application in a Bi-enzymatic Reaction. ChemCatChem. 2020, 12 (8), 2245–2252. 10.1002/cctc.201902293. [DOI] [Google Scholar]; d Kumar S.; Sindhu A.; Venkatesu P. Ionic Liquid-Modified Gold Nanoparticles for Enhancing Antimicrobial Activity and Thermal Stability of Enzymes. ACS Appl. Nano Mater. 2021, 4 (3), 3185–3196. 10.1021/acsanm.1c00401. [DOI] [Google Scholar]; e Ulu A.; Ozcan I.; Koytepe S.; Ates B. Design of epoxy-functionalized Fe3O4@MCM-41 core-shell nanoparticles for enzyme immobilization. Int. J. Biol. Macromol. 2018, 115, 1122–1130. 10.1016/j.ijbiomac.2018.04.157. [DOI] [PubMed] [Google Scholar]; f Vranish J. N.; Ancona M. G.; Oh E.; Susumu K.; Medintz I. L. Enhancing coupled enzymatic activity by conjugating one enzyme to a nanoparticle. Nanoscale 2017, 9 (16), 5172–5187. 10.1039/C7NR00200A. [DOI] [PubMed] [Google Scholar]
- a Goldberg D. M.; Deane J. K.; Rakes T. R.; Rees L. P. 3D Printing Technology and the Market Value of the Firm. Inf. Syst. Front. 2021, 10.1007/s10796-021-10143-7. [DOI] [Google Scholar]; b Tetsuka H.; Shin S. R. Materials and technical innovations in 3D printing in biomedical applications. J. Mater. Chem. B 2020, 8 (15), 2930–2950. 10.1039/D0TB00034E. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Yang E.; Miao S.; Zhong J.; Zhang Z.; Mills D. K.; Zhang L. G. Bio-Based Polymers for 3D Printing of Bioscaffolds. Polym. Rev. (Phila Pa) 2018, 58 (4), 668–687. 10.1080/15583724.2018.1484761. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Yang C.; Yu Y.; Wang X.; Wang Q.; Shang L. Cellular fluidic-based vascular networks for tissue engineering. Engineered Regeneration 2021, 2, 171–174. 10.1016/j.engreg.2021.09.006. [DOI] [Google Scholar]
- a Ali M. A.; Hu C.; Yttri E. A.; Panat R. Recent Advances in 3D Printing of Biomedical Sensing Devices. Adv. Funct. Mater. 2022, 32, 2107671–2107692. 10.1002/adfm.202107671. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Zhang Y.; Xu Y.; Simon-Masseron A.; Lalevee J. Radical photoinitiation with LEDs and applications in the 3D printing of composites. Chem. Soc. Rev. 2021, 50 (6), 3824–3841. 10.1039/D0CS01411G. [DOI] [PubMed] [Google Scholar]; c Zhang Y.; Zhang Y.; Liu G.; Yang Y.; Wu M.; Pang B. Fresh properties of a novel 3D printing concrete ink. Constr Build Mater. 2018, 174, 263–271. 10.1016/j.conbuildmat.2018.04.115. [DOI] [Google Scholar]
- a Cui J.; Feng Y.; Jia S. Silica encapsulated catalase@metal-organic framework composite: A highly stable and recyclable biocatalyst. Chem. Eng. J. 2018, 351, 506–514. 10.1016/j.cej.2018.06.121. [DOI] [Google Scholar]; b Bilal M.; Rasheed T.; Zhao Y.; Iqbal H. M. N.; Cui J. ″Smart″ chemistry and its application in peroxidase immobilization using different support materials. Int. J. Biol. Macromol. 2018, 119, 278–290. 10.1016/j.ijbiomac.2018.07.134. [DOI] [PubMed] [Google Scholar]; c Ren S.; Li C.; Jiao X.; Jia S.; Jiang Y.; Bilal M.; Cui J. Recent progress in multienzymes co-immobilization and multienzyme system applications. Chem. Eng. J. 2019, 373, 1254–1278. 10.1016/j.cej.2019.05.141. [DOI] [Google Scholar]; d Zhong L.; Feng Y.; Wang G.; Wang Z.; Bilal M.; Lv H.; Jia S.; Cui J. Production and use of immobilized lipases in/on nanomaterials: A review from the waste to biodiesel production. Int. J. Biol. Macromol. 2020, 152, 207–222. 10.1016/j.ijbiomac.2020.02.258. [DOI] [PubMed] [Google Scholar]
- a Martinez P. R.; Goyanes A.; Basit A. W.; Gaisford S. Fabrication of drug-loaded hydrogels with stereolithographic 3D printing. Int. J. Pharm. 2017, 532 (1), 313–317. 10.1016/j.ijpharm.2017.09.003. [DOI] [PubMed] [Google Scholar]; b Quan H.; Zhang T.; Xu H.; Luo S.; Nie J.; Zhu X. Photo-curing 3D printing technique and its challenges. Bioact Mater. 2020, 5 (1), 110–115. 10.1016/j.bioactmat.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Chai M.; Razavi Bazaz S.; Daiyan R.; Razmjou A.; Ebrahimi Warkiani M.; Amal R.; Chen V. Biocatalytic micromixer coated with enzyme-MOF thin film for CO2 conversion to formic acid. Chem. Eng. J. 2021, 426, 130856–130866. 10.1016/j.cej.2021.130856. [DOI] [Google Scholar]
- a Rahim T. N. A. T.; Abdullah A. M.; Md Akil H. Recent Developments in Fused Deposition Modeling-Based 3D Printing of Polymers and Their Composites. Polym. Rev. (Phila Pa) 2019, 59 (4), 589–624. 10.1080/15583724.2019.1597883. [DOI] [Google Scholar]; b Ye J.; Chu T.; Chu J.; Gao B.; He B. A Versatile Approach for Enzyme Immobilization Using Chemically Modified 3D-Printed Scaffolds. ACS Sustain. Chem. Eng. 2019, 7 (21), 18048–18054. 10.1021/acssuschemeng.9b04980. [DOI] [Google Scholar]; c Su C. K.; Yen S. C.; Li T. W.; Sun Y. C. Enzyme-Immobilized 3D-Printed Reactors for Online Monitoring of Rat Brain Extracellular Glucose and Lactate. Anal. Chem. 2016, 88 (12), 6265–6273. 10.1021/acs.analchem.6b00272. [DOI] [PubMed] [Google Scholar]; d Su C. K.; Chen J. C. One-step three-dimensional printing of enzyme/substrate-incorporated devices for glucose testing. Anal. Chim. Acta 2018, 1036, 133–140. 10.1016/j.aca.2018.06.073. [DOI] [PubMed] [Google Scholar]
- a Zhou Y.; Liao S.; Chu Y.; Yuan B.; Tao X.; Hu X.; Wang Y. An injectable bioink with rapid prototyping in the air and in-situ mild polymerization for 3D bioprinting. Biofabrication 2021, 13 (4), 045026. 10.1088/1758-5090/ac23e4. [DOI] [PubMed] [Google Scholar]; b Wenger L.; Radtke C. P.; Gopper J.; Worner M.; Hubbuch J. 3D-Printable and Enzymatically Active Composite Materials Based on Hydrogel-Filled High Internal Phase Emulsions. Front. Bioeng. Biotechnol. 2020, 8, 713–729. 10.3389/fbioe.2020.00713. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Tan J. J. Y.; Lee C. P.; Hashimoto M. Preheating of Gelatin Improves its Printability with Transglutaminase in Direct Ink Writing 3D Printing. Int. J. Bioprint. 2020, 6 (4), 296. 10.18063/ijb.v6i4.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Cui J.; Feng Y.; Lin T.; Tan Z.; Zhong C.; Jia S. Mesoporous Metal-Organic Framework with Well-Defined Cruciate Flower-Like Morphology for Enzyme Immobilization. ACS Appl. Mater. Interfaces 2017, 9 (12), 10587–10594. 10.1021/acsami.7b00512. [DOI] [PubMed] [Google Scholar]; b Feng Y.; Zhong L.; Hou Y.; Jia S.; Cui J. Acid-resistant enzyme@MOF nanocomposites with mesoporous silica shells for enzymatic applications in acidic environments. J. Biotechnol. 2019, 306, 54–61. 10.1016/j.jbiotec.2019.09.010. [DOI] [PubMed] [Google Scholar]; c Ren S.; Wang Z.; Bilal M.; Feng Y.; Jiang Y.; Jia S.; Cui J. Co-immobilization multienzyme nanoreactor with co-factor regeneration for conversion of CO2. Int. J. Biol. Macromol. 2020, 155, 110–118. 10.1016/j.ijbiomac.2020.03.177. [DOI] [PubMed] [Google Scholar]; d Zhong L.; Feng Y.; Hu H.; Xu J.; Wang Z.; Du Y.; Cui J.; Jia S. Enhanced enzymatic performance of immobilized lipase on metal organic frameworks with superhydrophobic coating for biodiesel production. J. Colloid Interface Sci. 2021, 602, 426–436. 10.1016/j.jcis.2021.06.017. [DOI] [PubMed] [Google Scholar]
- a Feng Y.; Du Y.; Kuang G.; Zhong L.; Hu H.; Jia S.; Cui J. Hierarchical micro- and mesoporous ZIF-8 with core-shell superstructures using colloidal metal sulfates as soft templates for enzyme immobilization. J. Colloid Interface Sci. 2022, 610, 709–718. 10.1016/j.jcis.2021.11.123. [DOI] [PubMed] [Google Scholar]; b Du Y.; Jia X.; Zhong L.; Jiao Y.; Zhang Z.; Wang Z.; Feng Y.; Bilal M.; Cui J.; Jia S. Metal-organic frameworks with different dimensionalities: An ideal host platform for enzyme@MOF composites. Coord. Chem. Rev. 2022, 454, 214327–214346. 10.1016/j.ccr.2021.214327. [DOI] [Google Scholar]; c Singh R.; Souillard G.; Chassat L.; Gao Y.; Mulet X.; Doherty C. M. Fabricating Bioactive 3D Metal–Organic Framework Devices. Adv. Sustain. Syst. 2020, 4 (12), 2000059–2000066. 10.1002/adsu.202000059. [DOI] [Google Scholar]
- a Lopez Marzo A. M.; Mayorga-Martinez C. C.; Pumera M. 3D-printed graphene direct electron transfer enzyme biosensors. Biosens. Bioelectron. 2020, 151, 111980–111986. 10.1016/j.bios.2019.111980. [DOI] [PubMed] [Google Scholar]; b Koterwa A.; Kaczmarzyk I.; Mania S.; Cieslik M.; Tylingo R.; Ossowski T.; Bogdanowicz R.; Niedziałkowski P.; Ryl J. The role of electrolysis and enzymatic hydrolysis treatment in the enhancement of the electrochemical properties of 3D-printed carbon black/poly(lactic acid) structures. Appl. Surf. Sci. 2022, 574, 151587–151599. 10.1016/j.apsusc.2021.151587. [DOI] [Google Scholar]; c Manzanares-Palenzuela C. L.; Hermanova S.; Sofer Z.; Pumera M. Proteinase-sculptured 3D-printed graphene/polylactic acid electrodes as potential biosensing platforms: towards enzymatic modeling of 3D-printed structures. Nanoscale 2019, 11 (25), 12124–12131. 10.1039/C9NR02754H. [DOI] [PubMed] [Google Scholar]; d Zhang J.; Gao B.; Lv K.; Kumissay L.; Wu B.; Chu J.; He B. Specific immobilization of lipase on functionalized 3D printing scaffolds via enhanced hydrophobic interaction for efficient resolution of racemic 1-indanol. Biochem. Biophys. Res. Commun. 2021, 546, 111–117. 10.1016/j.bbrc.2021.02.003. [DOI] [PubMed] [Google Scholar]
- a Schmieg B.; Dobber J.; Kirschhofer F.; Pohl M.; Franzreb M. Advantages of Hydrogel-Based 3D-Printed Enzyme Reactors and Their Limitations for Biocatalysis. Front. Bioeng. Biotechnol. 2019, 6, 211–222. 10.3389/fbioe.2018.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Maier M.; Radtke C. P.; Hubbuch J.; Niemeyer C. M.; Rabe K. S. On-Demand Production of Flow-Reactor Cartridges by 3D Printing of Thermostable Enzymes. Angew. Chem., Int. Ed. Engl. 2018, 57 (19), 5539–5543. 10.1002/anie.201711072. [DOI] [PubMed] [Google Scholar]; c Schmieg B.; Schimek A.; Franzreb M. Development and performance of a 3D-printable poly(ethylene glycol) diacrylate hydrogel suitable for enzyme entrapment and long-term biocatalytic applications. Eng. Life Sci. 2018, 18 (9), 659–667. 10.1002/elsc.201800030. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Liu J.; Shen X.; Zheng Z.; Li M.; Zhu X.; Cao H.; Cui C. Immobilization of laccase by 3D bioprinting and its application in the biodegradation of phenolic compounds. Int. J. Biol. Macromol. 2020, 164, 518–525. 10.1016/j.ijbiomac.2020.07.144. [DOI] [PubMed] [Google Scholar]
- a Peng M.; Mittmann E.; Wenger L.; Hubbuch J.; Engqvist M. K. M.; Niemeyer C. M.; Rabe K. S. 3D-Printed Phenacrylate Decarboxylase Flow Reactors for the Chemoenzymatic Synthesis of 4-Hydroxystilbene. Chemistry (Easton) 2019, 25, 15998–16001. 10.1002/chem.201904206. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Schmieg B.; Nguyen M.; Franzreb M. Simulative Minimization of Mass Transfer Limitations Within Hydrogel-Based 3D-Printed Enzyme Carriers. Front. Bioeng. Biotechnol. 2020, 8, 365–377. 10.3389/fbioe.2020.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Shen X.; Yang M.; Cui C.; Cao H. In situ immobilization of glucose oxidase and catalase in a hybrid interpenetrating polymer network by 3D bioprinting and its application. Colloids Surf. Physicochem. Eng. Aspects 2019, 568, 411–418. 10.1016/j.colsurfa.2019.02.021. [DOI] [Google Scholar]; d Steier A.; Schmieg B.; Irtel von Brenndorff Y.; Meier M.; Nirschl H.; Franzreb M.; Lahann J. Enzyme Scaffolds with Hierarchically Defined Properties via 3D Jet Writing. Macromol. Biosci. 2020, 20 (9), 2000154–2000163. 10.1002/mabi.202000154. [DOI] [PubMed] [Google Scholar]
- a Grant J.; Modica J. A.; Roll J.; Perkovich P.; Mrksich M. An Immobilized Enzyme Reactor for Spatiotemporal Control over Reaction Products. Small 2018, 14, 1800923–1800933. 10.1002/smll.201800923. [DOI] [PubMed] [Google Scholar]; b Changani Z.; Razmjou A.; Taheri-Kafrani A.; Asadnia M. Domino P-μMB: A New Approach for the Sequential Immobilization of Enzymes Using Polydopamine/Polyethyleneimine Chemistry and Microfabrication. Adv. Mater. Interfaces 2020, 7 (13), 1901864–1901875. 10.1002/admi.201901864. [DOI] [Google Scholar]; c Peris E.; Okafor O.; Kulcinskaja E.; Goodridge R.; Luis S. V.; Garcia-Verdugo E.; O’Reilly E.; Sans V. Tuneable 3D printed bioreactors for transaminations under continuous-flow. Green Chem. 2017, 19 (22), 5345–5349. 10.1039/C7GC02421E. [DOI] [Google Scholar]; d dos Santos L. K.; Botti R. F.; Innocentini M. D. d. M.; Marques R. F. C.; Colombo P.; de Paula A. V.; Flumignan D. L. 3D printed geopolymer: An efficient support for immobilization of Candida rugosa lipase. Chem. Eng. J. 2021, 414, 128843–128851. 10.1016/j.cej.2021.128843. [DOI] [Google Scholar]; e Wang L.; Pumera M. Covalently modified enzymatic 3D-printed bioelectrode. Microchim. Acta 2021, 188 (11), 374–381. 10.1007/s00604-021-05006-6. [DOI] [PubMed] [Google Scholar]
- a Greene A. F.; Vaidya A.; Collet C.; Wade K. R.; Patel M.; Gaugler M.; West M.; Petcu M.; Parker K. 3D-Printed Enzyme-Embedded Plastics. Biomacromolecules 2021, 22 (5), 1999–2009. 10.1021/acs.biomac.1c00105. [DOI] [PubMed] [Google Scholar]; b Rewatkar P.; Bandapati M.; Goel S. Miniaturized additively manufactured co-laminar microfluidic glucose biofuel cell with optimized grade pencil bioelectrodes. Int. J. Hydrogen Energy 2019, 44 (59), 31434–31444. 10.1016/j.ijhydene.2019.10.002. [DOI] [Google Scholar]; c Rewatkar P.; Goel S. 3D Printed Bioelectrodes for Enzymatic Biofuel Cell: Simple, Rapid, Optimized and Enhanced Approach. IEEE Trans Nanobioscience 2020, 19 (1), 4–10. 10.1109/TNB.2019.2941196. [DOI] [PubMed] [Google Scholar]; d Rewatkar P.; U. S J.; Goel S. Optimized Shelf-Stacked Paper Origami-Based Glucose Biofuel Cell with Immobilized Enzymes and a Mediator. ACS Sustain. Chem. Eng. 2020, 8 (32), 12313–12320. 10.1021/acssuschemeng.0c04752. [DOI] [Google Scholar]
- a Spano M. B.; Tran B. H.; Majumdar S.; Weiss G. A. 3D-Printed Labware for High-Throughput Immobilization of Enzymes. J. Org. Chem. 2020, 85 (13), 8480–8488. 10.1021/acs.joc.0c00789. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Guo S.; Lin X.; Wang Y.; Gong X. Fabrication of paper-based enzyme immobilized microarray by 3D-printing technique for screening alpha-glucosidase inhibitors in mulberry leaves and lotus leaves. Chin. Med. 2019, 14, 13–23. 10.1186/s13020-019-0236-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Jiang W.; Pei R.; Zhou S.-F. 3D-printed xylanase within biocompatible polymers as excellent catalyst for lignocellulose degradation. Chem. Eng. J. 2020, 400, 125920–125927. 10.1016/j.cej.2020.125920. [DOI] [Google Scholar]; d Pei R.; Jiang W.; Fu X.; Tian L.; Zhou S.-F. 3D-Printed Aldo-keto reductase within biocompatible polymers as catalyst for chiral drug intermediate. Chem. Eng. J. 2022, 429, 132293–132299. 10.1016/j.cej.2021.132293. [DOI] [Google Scholar]; e Muñoz J.; Redondo E.; Pumera M. Chiral 3D-printed Bioelectrodes. Adv. Funct. Mater. 2021, 31 (16), 2010608–2010616. 10.1002/adfm.202010608. [DOI] [Google Scholar]; f Xu X.; Pose-Boirazian T.; Eibes G.; McCoubrey L. E.; Martinez-Costas J.; Gaisford S.; Goyanes A.; Basit A. W. A customizable 3D printed device for enzymatic removal of drugs in water. Water Res. 2022, 208, 117861–117872. 10.1016/j.watres.2021.117861. [DOI] [PubMed] [Google Scholar]
- a Luo H.; Gao B. Development of smart wearable sensors for life healthcare. Engineered Regeneration 2021, 2, 163–170. 10.1016/j.engreg.2021.10.001. [DOI] [Google Scholar]; b Cardoso R. M.; Silva P. R. L.; Lima A. P.; Rocha D. P.; Oliveira T. C.; do Prado T. M.; Fava E. L.; Fatibello-Filho O.; Richter E. M.; Muñoz R. A. A. 3D-Printed graphene/polylactic acid electrode for bioanalysis: Biosensing of glucose and simultaneous determination of uric acid and nitrite in biological fluids. Sens. Actuators B Chem. 2020, 307, 127621–127629. 10.1016/j.snb.2019.127621. [DOI] [Google Scholar]; c Liu Y.; Yu Q.; Luo X.; Yang L.; Cui Y. Continuous monitoring of diabetes with an integrated microneedle biosensing device through 3D printing. Microsyst. Nanoeng. 2021, 7, 75–86. 10.1038/s41378-021-00302-w. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Yu Y.; Wang Q.; Wang C.; Shang L. Living Materials for Regenerative Medicine. Engineered Regeneration 2021, 2, 96–104. 10.1016/j.engreg.2021.08.003. [DOI] [Google Scholar]; e Cai L.; Xu D.; Chen H.; Wang L.; Zhao Y. Designing bioactive micro-/nanomotors for engineered regeneration. Engineered Regeneration 2021, 2, 109–115. 10.1016/j.engreg.2021.09.003. [DOI] [Google Scholar]; f Yang C.; Zheng Z.; Younis M. R.; Dong C.; Chen Y.; Lei S.; Zhang D. Y.; Wu J.; Wu X.; Lin J.; Wang X.; Huang P. 3D Printed Enzyme-Functionalized Scaffold Facilitates Diabetic Bone Regeneration. Adv. Funct. Mater. 2021, 31 (20), 2101372–2101383. 10.1002/adfm.202101372. [DOI] [Google Scholar]; g Wei Q.; Xu W.; Liu M.; Wu Q.; Cheng L.; Wang Q. Viscosity-controlled printing of supramolecular-polymeric hydrogels via dual-enzyme catalysis. J. Mater. Chem. B 2016, 4 (38), 6302–6306. 10.1039/C6TB01792D. [DOI] [PubMed] [Google Scholar]