Abstract
Background
Esophagogastric junction outflow obstruction (EGJOO) has a variable disease course. Currently, barium swallow (BaS) and manometric parameters are used to characterize clinically significant EGJOO. The esophagogastric junction distensibility index (EGJ-DI) measured via functional lumen imaging probe (FLIP) can provide complementary information. Our aim was to assess symptom response in patients with EGJOO and an abnormal EGJ-DI after botulinum toxin (BT) treatment.
Methods
A prospective cohort study of adults with idiopathic EGJOO was performed from September 2019 to March 2021. Patients with dysphagia underwent upper endoscopy with FLIP. If the EGJ-DI was abnormally low, BT was injected. Data examined included demographics, medical history, endoscopic and FLIP findings, BaS, manometry, and Eckardt score (ES). ES improvement was assessed via paired samples t-test. Pearson’s chi-square tests were used to assess for associations.
Results
Of the 20 patients, 75% had an abnormal EGJ-DI and underwent BT injections. Mean ES for patients with abnormal EGJ-DIs significantly improved from baseline to 1, 3, and 6 month follow-up (P-values: 0.01, 0.05, and 0.02, respectively). There was a significant association between an abnormal EGJ-DI with delayed bolus transit and presence of rapid drink challenge panesophageal pressurization on manometry: P = 0.03 and P = 0.03.
Conclusion
This prospective study revealed that an abnormal EGJ-DI can guide BT as assessed via symptomatic response. Additionally, abnormal EGJ-DI measurements were significantly associated with other parameters used previously to determine clinically relevant EGJOO. Larger follow-up studies are warranted to further elucidate guidance for therapy in EGJOO.
Keywords: esophageal motility, esophagogastric junction, esophagus, esophagogastric junction outflow obstruction, functional lumen imaging probe, patient-reported outcomes
INTRODUCTION
Idiopathic esophagogastric junction outflow obstruction (EGJOO) is a commonly encountered manometric diagnosis of unclear clinical significance. It is increasingly recognized and reported in up to 13.7% of manometries.1–8 There are limited data to guide the optimal management of patients with this finding.9–12 On manometry, EGJOO is defined by an elevated median integrated relaxation pressure (IRP) with intact or weak peristalsis.10,13 Although patients may present with a variety of symptoms including dysphagia, chest pain, regurgitation, and heartburn, the most significant symptoms are dysphagia and chest pain.3–7,9,11–17 The clinical course of EGJOO is highly variable including symptom resolution, persistent symptoms, and, in a minority of patients, progression to achalasia.2,4,6–8,15,18–20 Currently, experts advise the consideration of lower esophageal sphincter (LES) targeted therapy for EGJOO if patients have dysphagia or chest pain and objective evidence of obstruction.11,12,17 Other frequently used tools to assess clinically relevant EGJOO include an abnormal timed barium esophagram and manometric parameters, specifically panesophageal pressurization (PEP) and elevated IRP during the rapid drink challenge (RDC).2,21–23 Treatment for EGJOO can include botulinum toxin injections, pneumatic dilation, and myotomy.
The functional lumen imaging probe (FLIP) has been proposed as another useful test to further characterize the LES.24 It is a catheter-based test that is typically performed under sedation during an upper endoscopy, which can assess esophageal distensibility, compliance, and peristalsis.24,25 In a recent retrospective study on EGJOO, a low esophagogastric distensibility index (EGJ-DI) was predictive of obstruction seen on barium esophagram with symptomatic improvement in the majority of patients who received LES targeted therapy based on EGJ-DI.26 With these promising data, it is recommended in the Chicago Classification version 4 to obtain a timed barium esophagram or FLIP study to support a manometric diagnosis of EGJOO.13
Given that EGJOO is a heterogenous condition with a variable disease course, diagnosis and management can be challenging. FLIP is a promising endoscopic modality to better identify clinically relevant EGJOO in patients in need of LES targeted therapy. To our knowledge, no study to date has prospectively evaluated clinical outcomes based on FLIP in idiopathic EGJOO. The primary aim of this study was to assess symptom response to botulinum toxin injections in patients with manometric EGJOO who also had an abnormal EGJ-DI on FLIP. Secondary aims include assessing the association between EGJ-DI and manometric findings (including RDC) and delayed emptying on barium swallow.
METHODS
Subjects and study design
Adult patients were identified prospectively at the University of Pennsylvania between September 2019 and March 2021. All patients had a high-resolution manometry (HRM) study meeting criteria for EGJOO based on the most current Chicago Classification criteria, namely a median IRP ≥ 15 mm Hg supine (and if applicable, ≥20% elevated intrabolus pressure (supine) and median IRP ≥ 12 mm Hg when upright).10,13 All patients underwent a barium swallow and upper endoscopy with biopsies to rule out secondary causes. Eligible patients for FLIP were those referred for an upper endoscopy due to symptom of dysphagia or evidence of delayed emptying on barium swallow. Eligibility criteria were based on current expert advice for management.11,12,17 Exclusion criteria included: current opiate use, known or suspected contraindication for esophageal intubation, esophageal stricture with inability to pass an endoscope, history of esophageal perforation, history of esophageal resection, esophageal fistula, food impaction, esophageal varices, coagulopathy, active anticoagulation, active GI bleeding, eosinophilic esophagitis, and pregnancy. The electronic medical records were utilized for the following: demographics, medications, past medical history, past surgical history, symptom scores, clinic notes, previous endoscopic evaluation, radiographic evaluation, and HRM data. The study protocol was approved by the University of Pennsylvania Institutional Review Board.
High-resolution manometry
HRM studies were completed within 12 months prior to FLIP measurements. These studies were performed using a catheter with 36 circumferential pressure sensors at 1 cm intervals (Medtronic, Minneapolis, MN, USA) to obtain esophageal pressure topography measurements with impedance also included. After a minimum 6-hour fast, the catheter was placed transnasally with the distal end in the stomach at least 5 cm distal to the gastroesophageal junction. The catheter was calibrated to visualize pressure bands of upper and LES on color contour as well as verifying the diaphragmatic pinch on deep inspiration. A baseline recording in the supine position was performed first, followed by a series of 10 wet swallows using 5 cc of normal saline with at least 30 seconds between swallows. Provocative testing was performed including RDC with 200 mL of water and multiple rapid swallow with five 2 mL wet swallows. After the introduction of the Chicago Classification version 4, an additional five upright swallows were performed.13
Manoview analysis software was used to analyze pressure topography plots. Thermal compensation was performed and the following landmarks manually positioned: upper esophageal sphincter, LES borders, pressure inversion point, and gastric body. The median IRP, distal latency, distal contractile integral, percent bolus clearance, and percent swallows with PEP were automatically calculated by the software and each swallow manually reviewed. The supine intrabolus pressure and RDC were assessed as well, including median IRP and PEP. Delayed bolus transit is defined as fewer than 80% of liquid swallows having complete bolus transit.27 Three gastroenterologists with expertise in esophageal diseases interpreted all of the manometry studies, rotating on a random weekly basis. CAB independently interpreted each study; if there was a discrepancy in interpretation, the study was discussed with the reading expert and KLL for consensus.
Barium swallow
Barium swallow examinations were performed within 12 months prior to the upper endoscopy with FLIP. This involved performing a radiograph of the esophagus after ingestion of 200 mL of low density barium sulfate. If applicable, a barium tablet was used as well. The images and radiology interpretation were assessed. Evidence of delay or obstruction on barium swallow was defined as a delay in the movement of barium liquid or a barium tablet as interpreted by the assigned radiologist. CAB also independently reviewed all of the studies to ensure no discrepancies were noted.
Functional lumen imaging probe
FLIP was performed at the time of a sedated upper endoscopy by three esophageal experts. The proceduralist who performed both the FLIP and endoscopy was based on a predetermined endoscopy schedule. FLIP entails a 16-cm catheter with an attached cylindrical balloon with 16 impedance planimetry sensors to measure intraballoon pressure and cross-sectional area (EndoFLIP EF-322 N; Medtronic Inc, Minneapolis, MN). The catheter was calibrated to atmospheric pressure before transoral placement. It was positioned with 1–3 sensors in the stomach. Landmarks were confirmed when the balloon was filled to 30 mL for 15 seconds. Stepwise balloon distension with saline (40 mL, 50 mL, 60 mL and maximum of 70 mL) were performed with each distension maintained for 30–60 seconds. EGJ-DI was measured intraprocedurally from the FLIP device.28 A value of less than 2 mm2/mm Hg was considered abnormally reduced, 2.1 to 3 mm2/mm Hg was borderline, and 3.1 to 9 mm2/mm Hg was normal.29 In terms of a borderline EGJ-DI, if the maximum diameter was <12 mm with bag pressure > 20 mm Hg, it was considered abnormally reduced. If the EGJ-DI was abnormal, 100 units of botulinum toxin were injected at the LES. This determination was made intraprocedurally as real-time FLIP has excellent agreement with post hoc FLIP analysis.28
For the statistical analysis, we did a post hoc FLIP analysis for which data were filtered to minimize the effect of respirations and contractions using a STATA 16.0 (StataCorp, College Station, TX, USA) based analytical protocol.30 We analyzed pressure geometry measurements and calculated DI which was defined as the minimal cross-sectional area at the esophagogastric junction versus the intrabolus pressure at an inflation volume of 40 mL–70 mL. Esophageal body contractility was also assessed (absent, repetitive antegrade contractions, repetitive retrograde contractions, disordered or diminished contractile response). Post hoc analysis did not significantly alter the DI in that the plan for injection of botulinum toxin would have remained the same.
Patient questionnaires
Questionnaires were done at baseline (within 1 month prior to procedure date) and follow-up, including 1, 3, 6, and 12 months post procedure (upper endoscopy with FLIP). Questionnaires utilized included the Eckardt Score (ES), GerdQ, and PROMIS-10. ES was used as the primary questionnaire to assess response to therapy. ES is a 4-item scale assessing dysphagia, chest pain, and regurgitation based on the frequency of each symptom (0: never, 1: occasional, 2: daily, and 3: with each meal) plus a score based on the degree of weight loss (0: none, 1:<10 lb, 2:10–20 lbs, and 3: >20 lbs).31 Maximum score is 12 with 3 or less considered to be a good symptomatic outcome. GerdQ is a validated questionnaire for gastroesophageal reflux disease that uses a graded Likert scale (0–3) to score frequency of heartburn, regurgitation, sleep disturbance, and use of over the counter medications for symptoms.32 The maximum score is 18 with 9 or higher indicating gastroesophageal reflux disease. PROMIS-10 is a validated 10-question survey that assesses general quality of life, specifically overall physical and mental health.33
Statistical analysis
Descriptive statistics were used. Comparisons using chi-square analysis, t-tests, and paired t-tests were made among groups depending on data type and distribution. Analyses were tested for a 0.05 level of statistical significance. Assuming a type 1 error of 0.05 and power of 80%, we expected to have a within group standard deviation ranging from 0.7 to 3.1 with a distensibility index ranging from 0.5 to 2.5, using achalasia data as a proxy as well as limited EGJOO data.24,34 We calculated that a sample size of 20–32 would allow us to analyze clinical outcomes.
RESULTS
There were 20 patients with manometric EGJOO who underwent EGD with FLIP. On assessment of HRM, there were no discrepancies between the reading esophageal expert and the independent review by CAB. Out of 20 patients, 15 patients (75%) had an abnormal EGJ-DI (mean of 1.43 mm2/mm Hg) and underwent botulinum toxin injections at the LES. Demographics and baseline characteristics are listed in Table 1. There was a mean follow-up of 4.55 ± 3.43 months. Manometry, barium swallow, and FLIP findings of all patients and patients with abnormal DIs are listed in Table 2.
Table 1.
Patient demographics and baseline characteristics
| Age: years mean, SD | 62.9 (11.4) |
|---|---|
| BMI: mean, SD | 26.6 (8.5) |
| Gender: female n (%) | 12 (60.0%) |
| Race: Caucasian n (%) Black n (%) Asian n (%) |
15 (75.0%) 4 (20.0%) 1 (5.0%) |
| Proton pump inhibitor use: yes n (%) | 15 (75.0%) |
Table 2.
Manometry, barium swallow and FLIP findings of all patients and patients with abnormal distensibility index
| Findings | All (n = 20) | Abnormal DIa |
|---|---|---|
| IRPb: mean, SD | 26.1 (11.3) | 27.6 (12.7) |
| Distal contractile integral: mean, SD | 3561.9 (3325.6) | 3648.8 (3357.5) |
| Distal latency: mean, SDc | 6.22 (1.73) | 5.76 (1.24) |
| RDCd IRP: mean, SD | 11.8 (8.05) | 13.2 (9.04) |
| RDC PEPe: present n (%) | 9 (52.9%) | 9 (69.2%) |
| HRMf delayed bolus transit: n (%) | 13 (65.0%) | 12 (80.0%) |
| EGJOOg features Spastic n (%) Hypercontractile n (%) Ineffective motility n (%) No disordered peristalsis n (%) |
6 (30.0%) 2 (10.0%) 5 (25.0%) 7 (35.0%) |
6 (40.0%) 1 (6.60%) 4 (26.7%) 4 (26.7%) |
| BaSh: delay n (%) | 7 (35.0%) | 5 (33.3%) |
| BaS: tablet delayi n (%) | 4 (57.1%) | 4 (57.1%) |
| FLIPf DI: mean, SD | 2.12 (1.60) | 1.43 (0.60) |
| FLIP: contractility pattern Contractility, no RAC/RRCg n (%) RAC n (%) RRC n (%) Absent n (%) |
6 (30.0%) 8 (40.0%) 2 (10.0%) 4 (20.0%) |
6 (40.0%) 4 (26.7%) 2 (13.3%) 3 (20.0%) |
aDistensibility index (DI), n = 15 patients for abnormal DI.
bAverage of median integrated relaxation pressure (IRP) measurements.
c n = 19 due to missing datapoint for all patients.
dRapid drink challenge (RDC), n = 16 for all patients and n = 11 for patients with abnormal DI.
ePanesophageal pressurization (PEP), n = 17 for all patients and n = 13 for patients with abnormal DI.
fHigh-resolution manometry (HRM).
gEsophagogastric junction outflow obstruction (EGJOO).
hBarium swallow (BaS).
i n = 7 patients for tablet for all patients and with abnormal DI.
fFunctional lumen imaging probe (FLIP), DI at maximum fill volume (depending on the patient 50–70 cc).
gRepetitive antegrade contractions (RAC), repetitive retrograde contractions (RRC).
Mean Eckardt scores for patients with normal and abnormal EGJ-DIs at baseline, 1 month, 3 months, 6 months, and 12 months postdistensibility measurements are shown in Fig. 1. In patients with an abnormal EGJ-DI, paired analyses of baseline mean ES (5.40 ± 2.80) to follow-up mean ES at 1 month (3.47 ± 3.09; n = 15), 3 months (3.00 ± 2.83; n = 9), and 6 months (2.56 ± 2.40; n = 9) were significant with P-values: 0.01, 0.05, and 0.02, respectively. Paired analysis at 12 months was not able to be assessed due to a small sample size of 2. In patients with a normal EGJ-DI, paired analyses of baseline mean ES (4.80 ± 2.95) to follow-up mean ES at 1 month (3.00 ± 2.24; n = 5) and 3 months (4.00 ± 2.16; n = 4) were not significant with P-values of 0.11 and 0.39, respectively. Paired analyses at 6 months could not be assessed due to small sample size of 2 and there was no follow-up at 12 months.
Fig. 1.
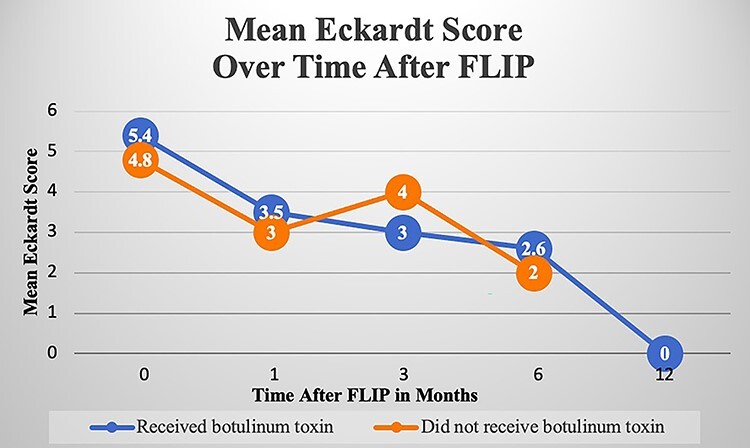
Mean Eckardt Score over time after FLIP. Mean Eckardt scores (ES) for patients with a normal and abnormal esophagogastric junction distensibility index (EGJ-DI) at baseline, 1 month, 3 months, 6 months, and 12 months postdistensibility measurements are shown. EGJ-DI was measured via functional lumen imaging probe (FLIP). In patients with an abnormal EGJ-DI, paired analyses of baseline mean ES to follow-up mean ES at 1 month, 3 months, and 6 months were each statistically significant. In patients with a normal EGJ-DI, paired analyses of baseline mean ES to follow-up mean ES at 1 month and 3 months were each not statistically significant.
In terms of other patient-reported outcomes, there were no meaningful significant differences in GerdQ or PROMIS-10 from baseline to follow-up in those who had an abnormal EGJ-DI and received BT. Mean GerdQ scores were 8.07 ± 3.45 at baseline (n = 15), 8.64 ± 4.58 at 1 month (n = 14), 7.67 ± 2.65 at 3 months (n = 9), 7.89 ± 2.76 at 6 months (n = 9), and 6.00 at 12 months (n = 1). When analyzed, scores were dichotomized to ≥9 for significant symptoms and less than 9 for controlled symptoms with no significant paired improvement over time. Mean PROMIS-10 mental health T-scores were 46.0 ± 9.31 at baseline (n = 15), 48.3 ± 9.61 at 1 month (n = 15), 47.3 ± 11.0 at 3 months (n = 9), 45.0 ± 10.0 at 6 months (n = 9), and 43.6 ± 10.1 at 12 months (n = 2). Mean T-scores were below the mean T-score of the relevant reference population, which is 50. Paired analyses of baseline mean mental health T-scores to follow-up at 1 month was significant with P = 0.016. That being said, PROMIS-10 T-scores are most informative when there is a change of at least 10, corresponding with change in one standard deviation above or below the mean reference population. The mean T-score of the cohort as well as the T-score of each individual patient does not change by 10 or more at each follow-up interval. There was also no significant change in PROMIS-10 physical health mean T-scores over time during follow-up.
Next we assessed association between an abnormal EGJ-DI with HRM and BaS parameters using the Pearson’s chi-square test. There was a significant association between an abnormal DI and delayed bolus transit: χ2 (1) = 5.93, P = 0.03. Based on the odds ratio, the odds of delayed bolus transit were 16 times higher if there was an abnormal EGJ-DI than a normal EGJ-DI. There was also a significant association between an abnormal DI and presence of RDC PEP on HRM: χ2 (1) = 5.89, P = 0.03. The odds ratio was not able to be calculated. There were no significant associations between an abnormal DI and BaS abnormality (tablet and/or liquid delay), RDC IRP relaxation, or spasm. Of note, there were no discrepancies between radiologist interpretation of BaS and the independent review by CAB.
DISCUSSION
EGJOO has a variable disease course with limited guidance regarding treatment. This prospective study revealed that an abnormal EGJ-DI of the LES may guide symptom response to therapy in patients with idiopathic EGJOO. In this study, symptom scores improved after targeted LES treatment with botulinum toxin with mean follow-up of 4.55 months. Additionally, our EGJ-DI measurements were significantly associated with other parameters used previously to determine clinically relevant EGJOO, namely delayed bolus transit and RDC PEP. Associations were not significant for other HRM or BaS parameters.
FLIP is a promising modality to further characterize EGJOO. FLIP has been established in achalasia with EGJ-DIs consistently less than 2.8 mm2/mm Hg and, in majority of patients, less than 2 mm2/mm Hg.34,35 In achalasia patients, EGJ-DI correlates well with symptom scores and significantly increases to a median of 3.4 mm2/mm Hg if patients report a good symptomatic improvement after treatment.34 However, limited data exists on the role of FLIP in EGJOO. One study of 38 EGJOO patients noted that the majority of patients (87%) had an abnormal distensibility index on FLIP.24 The authors postulated that impedance planimetry can more accurately assess for true EGJOO as HRM may be more prone to pressure artifact. However, outcome data were not assessed in this study, making definitive conclusions difficult. In a different retrospective study of 18 patients with EGJOO, a EGJ-DI of less than 2 mm2/mm Hg was predictive of obstruction on timed barium esophagram.26 There were 9 patients who had an EGJ-DI of less than 2 mm2/mm Hg, evidence of obstruction on barium esophagram, and received LES targeted therapy. Eighty percent of these treated patients had a significant improvement in symptom scores. Given these promising data, the Chicago Classification version 4 updated the criteria of EGJOO to include obstruction on barium esophagram or abnormal EGJ-DI.13
In our study, there were 20 patients with idiopathic EGJOO on HRM and dysphagia. The majority of patients (75%) had an abnormal EGJ-DI and thus were considered to have clinically significant EGJOO. These patients all underwent LES targeted therapy, specifically botulinum toxin injections. Out of the patients who underwent LES targeted therapy, there was a significant improvement in symptoms by Eckardt score from baseline to follow-up. There were no significant or meaningful improvements in GerdQ and PROMIS-10 after BT. This may be due to the fact that many patients with EGJOO predominantly have symptoms of chest discomfort or dysphagia over reflux. Moreover, PROMIS-10 is a general quality of life score as opposed to a gastroenterology specific score. Although we were unable to quantify these data as it was not uniformly collected from each patient, many patients unprompted offered that their general quality of life was influenced by the COVID-19 pandemic, which was ongoing for the majority of the study. For future studies, it may be worthwhile assessing a gastroenterology specific quality of life score.
Prior to FLIP, HRM parameters and barium esophagram were frequently used to help delineate clinically significant EGJOO. Specifically, delayed bolus transit and RDC PEP on HRM have been assessed in EGJOO patients. In a study of 169 patients with EGJOO, a combination of delayed bolus transit, dysphagia, and chest pain had the highest predictive value for identifying clinically relevant EGJOO (sensitivity of 90%, specificity of 92.5%, and negative predictive value of 99.3%).22 Clinically relevant EGJOO was defined by delayed passage of contrast on timed barium esophagram, improvement in symptoms after pneumatic dilation, or the eventual development of achalasia. In another study of 70 patients with EGJOO, presence of RDC PEP was significantly associated with more severe dysphagia based on ES compared with absence of RDC PEP.21 In our study, abnormal EGJ-DI were significantly associated with delayed bolus transit and RDC PEP, but not with other HRM parameters (e.g. RDC IRP relaxation) or delay of liquid or tablet on a barium swallow.
There were a few limitations to our study as well as strengths. The criteria for EGJOO shifted during the study based on updates to the Chicago Classification; our protocol shifted accordingly and three manometry studies were done with the updated protocol. Notably, all patients underwent FLIP which is a component of the latest Chicago Classification. Although consistency is preferred, we felt that it was more important to adhere to the most recent recommendations. Our study was limited to assessing botulinum toxin as the sole LES targeted therapy; however, this is a low risk intervention. This study was limited to barium esophagrams and not timed barium esophagrams, but the latter will become available shortly and can be used for future studies. That being said, FLIP is an appropriate alternative to timed barium esophagrams in this clinical setting. The major strength of this study is that it is the first prospective study to our knowledge to assess idiopathic EGJOO and symptomatic outcomes in those who received FLIP. Moreover, we achieved our goal for sample size based on our power calculations.
In this prospective study, the majority of patients had EGJOO based on the most recent Chicago Classification and we demonstrated that an abnormal EGJ-DI can guide LES targeted therapy with appropriate symptomatic response. Moreover, acting on an abnormal EGJ-DI is supported by other frequently used parameters, namely delayed bolus transit and RDC PEP. Continued data for this study and larger follow-up studies assessing FLIP data and different types of therapies are warranted to further elucidate guidance for therapy in idiopathic EGJOO.
ACKNOWLEDGEMENTS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The research was performed at University of Pennsylvania.
Financial disclosure: The authors have no relevant financial disclosures.
Guarantor of the article: Kristle L Lynch, MD.
Contributor Information
Claire A Beveridge, Department of Gastroenterology and Hepatology, Digestive Disease and Surgery Institute, Cleveland Clinic Foundation, Cleveland, OH, USA.
Joseph R Triggs, Department of Medicine, Division of Gastroenterology & Hepatology, University of Pennsylvania, Philadelphia, PA, USA.
Shivani U Thanawala, Department of Medicine, Division of Gastroenterology & Hepatology, University of Pennsylvania, Philadelphia, PA, USA.
Nitin K Ahuja, Department of Medicine, Division of Gastroenterology & Hepatology, University of Pennsylvania, Philadelphia, PA, USA.
Gary W Falk, Department of Medicine, Division of Gastroenterology & Hepatology, University of Pennsylvania, Philadelphia, PA, USA.
Alain J Benitez, Department of Pediatrics, Division of Pediatric Gastroenterology, Hepatology, & Nutrition, Children’s Hospital of Philadelphia, Philadelphia, PA, USA; Department of Pediatrics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Kristle L Lynch, Department of Medicine, Division of Gastroenterology & Hepatology, University of Pennsylvania, Philadelphia, PA, USA.
Conflict of interest
Claire A Beveridge, Joseph R Triggs, Shivani U Thanawala, and Alain J Benitez: None. Nitin K Ahuja, Kristle L Lynch, and Gary W Falk: consultants for Medtronic.
Authors’ contributions
CAB was involved in study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript and critical revision of the manuscript. JRT was involved in study concept and design, analysis and interpretation of data, and critical revision of the manuscript. SUT was involved in interpretation of data and critical revision of the manuscript. NKA involved in study concept and design and critical revision of the manuscript. GWF was involved in study concept and design, interpretation of data, drafting of the manuscript and critical revision of the manuscript. AJB was involved in acquisition of data and critical revision of the manuscript. KLL was involved in study concept and design, analysis and interpretation of the data, drafting of the manuscript and critical revision of the manuscript.
Financial support
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number KL2TR001879 (A.J.B.).
References
- 1. Beveridge C A et al. Low yield of cross-sectional imaging in patients with esophagogastric junction outflow obstruction. Clin Gastroenterol Hepatol 2020;18(7):1643–1644. [DOI] [PubMed] [Google Scholar]
- 2. Clayton S B, Patel R, Richter J E. Functional and anatomic esophagogastic junction outflow obstruction: manometry, timed barium esophagram findings, and treatment outcomes. Clin Gastroenterol Hepatol 2016; 14(6): 907–11. [DOI] [PubMed] [Google Scholar]
- 3. DeLay K, Austin G L, Menard-Katcher P. Anatomic abnormalities are common potential explanations of manometric esophagogastric junction outflow obstruction. Neurogastroenterol Motil 2016; 28(8): 1166–71. [DOI] [PubMed] [Google Scholar]
- 4. Lynch K L et al. Clinical presentation and disease course of patients with esophagogastric junction outflow obstruction. Dis Esophagus 2017; 30(6): 1–6. [DOI] [PubMed] [Google Scholar]
- 5. Okeke F C et al. What is the clinical significance of esophagogastric junction outflow obstruction? Evaluation of 60 patients at a tertiary referral center. Neurogastroenterol Motil 2017; 29(6). doi: 10.1111/nmo.13061. Epub 2017 Apr 9. PMID: 28393437. [DOI] [PubMed] [Google Scholar]
- 6. Ong A M L, Namasivayam V, Wang Y T. Evaluation of symptomatic esophagogastric junction outflow obstruction. J Gastroenterol Hepatol 2018; 33(10): 1745–50. [DOI] [PubMed] [Google Scholar]
- 7. Perez-Fernandez M T et al. Characterization and follow-up of esophagogastric junction outflow obstruction detected by high resolution manometry. Neurogastroenterol Motil 2016; 28(1): 116–26. [DOI] [PubMed] [Google Scholar]
- 8. van Hoeij F B, Smout A J P M, Bredenoord A J. Characterization of idiopathic esophagogastric junction outflow obstruction. Neurogastroenterol Motil 2015; 27(9): 1310–6. [DOI] [PubMed] [Google Scholar]
- 9. Garbarino S et al. Management of functional esophagogastric junction outflow obstruction: a systematic review. J Clin Gastroenterol 2020;54(1):35–42. [DOI] [PubMed] [Google Scholar]
- 10. Kahrilas P J et al. The Chicago classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015; 27(2): 160–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Samo S, Qayed E. Esophagogastric junction outflow obstruction: where are we now in diagnosis and management? World J Gastroenterol 2019; 25(4): 411–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Richter J E, Clayton S B. Diagnosis and management of esophagogastric junction outflow obstruction. Am J Gastroenterol 2019; 114(4): 544–7. [DOI] [PubMed] [Google Scholar]
- 13. Yadlapati R et al. Esophageal motility disorders on high-resolution manometry: Chicago classification version 4.0((c)). Neurogastroenterol Motil 2021; 33(1): e14058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schupack D et al. The clinical significance of esophagogastric junction outflow obstruction and hypercontractile esophagus in high resolution esophageal manometry. Neurogastroenterol Motil 2017; 29(10): 1–9. [DOI] [PubMed] [Google Scholar]
- 15. Triadafilopoulos G, Clarke J O. Clinical and manometric characteristics of patients with oesophagogastric outflow obstruction: towards a new classification. BMJ Open Gastroenterol 2018; 5(1): e000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Triggs J R et al. Upright integrated relaxation pressure facilitates characterization of esophagogastric junction outflow obstruction. Clin Gastroenterol Hepatol 2019; 17(11): 2218–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schlottmann F, Patti M G. Primary esophageal motility disorders: beyond achalasia. Int J Mol Sci 2017; 18(7): 1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shin I S, Min Y W, Rhee P L. Esophagogastric junction outflow obstruction transformed to type II achalasia. J Neurogastroenterol Motil 2016; 22(2): 344–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Song B G et al. Clinicomanometric factors associated with clinically relevant esophagogastric junction outflow obstruction from the Sandhill high-resolution manometry system. Neurogastroenterol Motil 2018; 30(3):e13221. [DOI] [PubMed] [Google Scholar]
- 20. Pandolfino J E, Gawron A J. Achalasia: a systematic review. JAMA 2015; 313(18): 1841–52. [DOI] [PubMed] [Google Scholar]
- 21. Biasutto D et al. Rapid drink challenge test during esophageal high resolution manometry in patients with esophago-gastric junction outflow obstruction. Neurogastroenterol Motil 2018; 30(6): e13293. [DOI] [PubMed] [Google Scholar]
- 22. Song B G et al. Combined multichannel intraluminal impedance and high-resolution manometry improves detection of clinically relevant esophagogastric junction outflow obstruction. J Neurogastroenterol Motil 2019; 25(1): 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ang D et al. Rapid drink challenge in high-resolution manometry: an adjunctive test for detection of esophageal motility disorders. Neurogastroenterol Motil 2017; 29(1):e12902. [DOI] [PubMed] [Google Scholar]
- 24. Carlson D A et al. Evaluation of esophageal motility utilizing the functional lumen imaging probe. Am J Gastroenterol 2016; 111(12): 1726–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen H M et al. Functional lumen imaging probe in gastrointestinal motility diseases. J Dig Dis 2019; 20(11): 572–7. [DOI] [PubMed] [Google Scholar]
- 26. Triggs J et al. Functional luminal imaging probe panometry: a method to identify achalasia-type esophagogastric junction outflow obstruction. Clin Gastroenterol Hepatol 2019; 17(11): 672–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Beveridge C, Lynch K L. Diagnosis and management of esophagogastric junction outflow obstruction. Gastroenterol Hepatol 2020; 16(3): 131–8. [PMC free article] [PubMed] [Google Scholar]
- 28. Carlson D A et al. Esophageal motility classification can be established at the time of endoscopy: a study evaluating real-time functional luminal imaging probe panometry. Gastrointest Endosc 2019; 90(6): 915–923 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pandolfino J E et al. ENDOFLIP impedance Planimetry system protocol and interpretation. Medtronic 2018. [Google Scholar]
- 30. Menard-Katcher C et al. Influence of age and eosinophilic esophagitis on esophageal distensibility in a pediatric cohort. Am J Gastroenterol 2017; 112(9): 1466–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Eckardt V F, Aignherr C, Bernhard G. Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology 1992; 103(6): 1732–8. [DOI] [PubMed] [Google Scholar]
- 32. Jonasson C et al. Validation of the GerdQ questionnaire for the diagnosis of gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2013; 37(5): 564–72. [DOI] [PubMed] [Google Scholar]
- 33. Hays R D et al. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual Life Res 2009; 18: 873–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pandolfino J E et al. Distensibility of the esophagogastric junction assessed with the functional lumen imaging probe (FLIP) in achalasia patients. Neurogastroenterol Motil 2013; 25(6): 496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rohof W O et al. Efficacy of treatment for patients with achalasia depends on the distensibility of the esophagogastric junction. Gastroenterology 2012; 143(2): 328–35. [DOI] [PubMed] [Google Scholar]


